Deeper Understanding of the Strength Evolution and Deformation Characteristics of Sodium Silicate–Cemented Paste Tailing Material
Abstract
:1. Introduction
2. Experimental Programs
2.1. Materials
2.1.1. Tailings
2.1.2. Sodium Silicate
2.1.3. Binder and Mixing Waters
2.2. Preparing Specimens and Determining Mix Ratios
Specimen Preparation
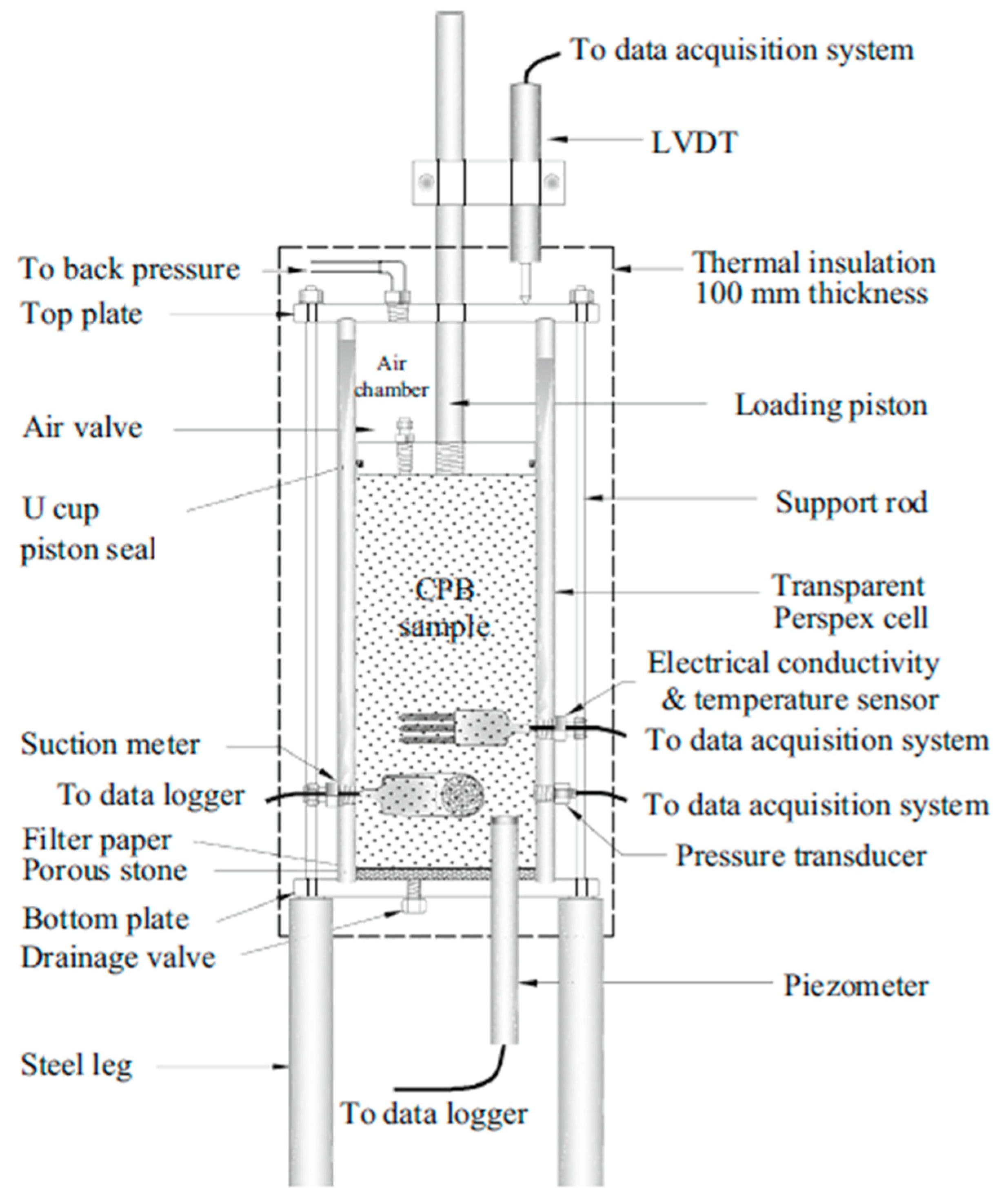
2.3. Test Methods and Monitoring Program
2.3.1. UCS Test
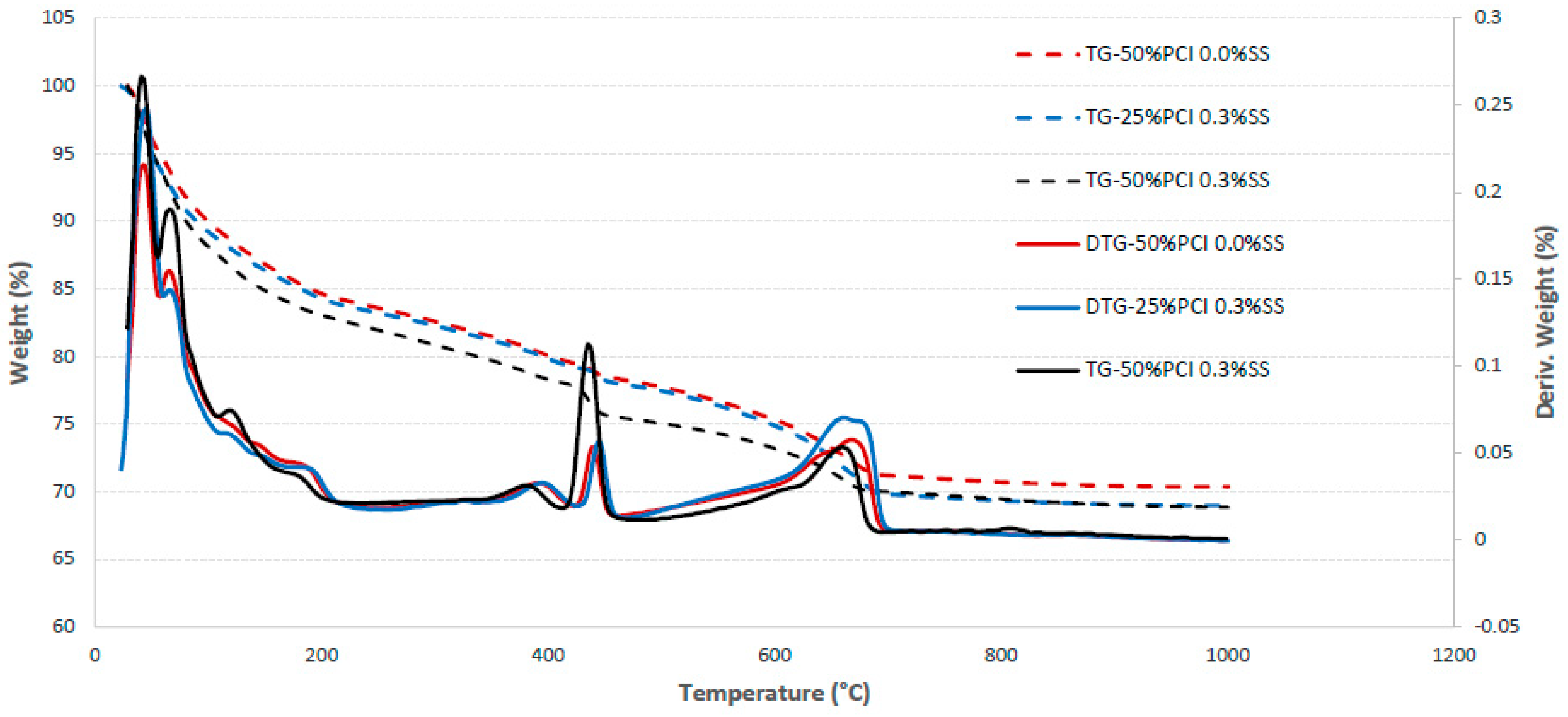
2.3.2. Microstructural Analysis and Tests
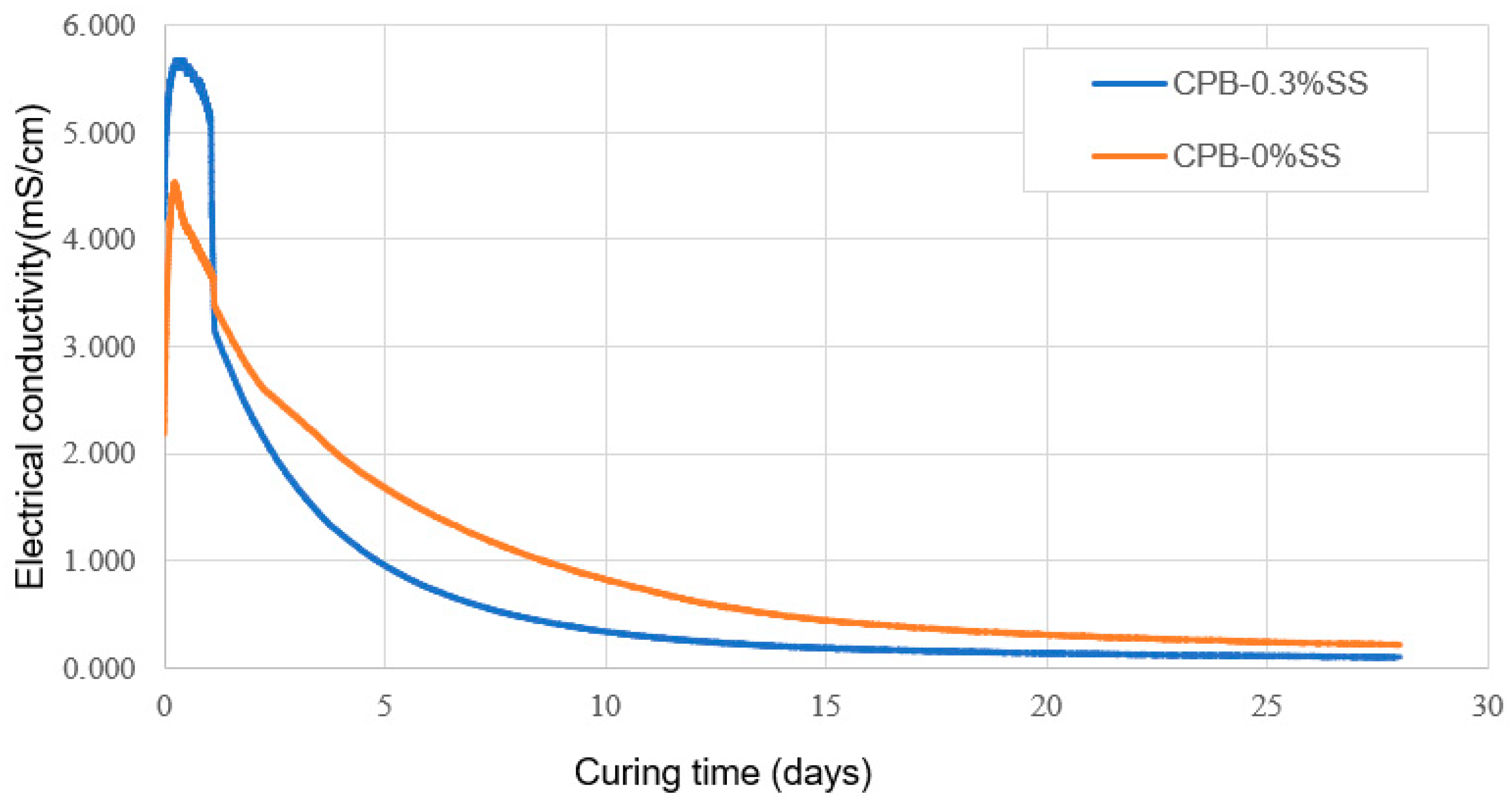
2.3.3. Monitoring Program
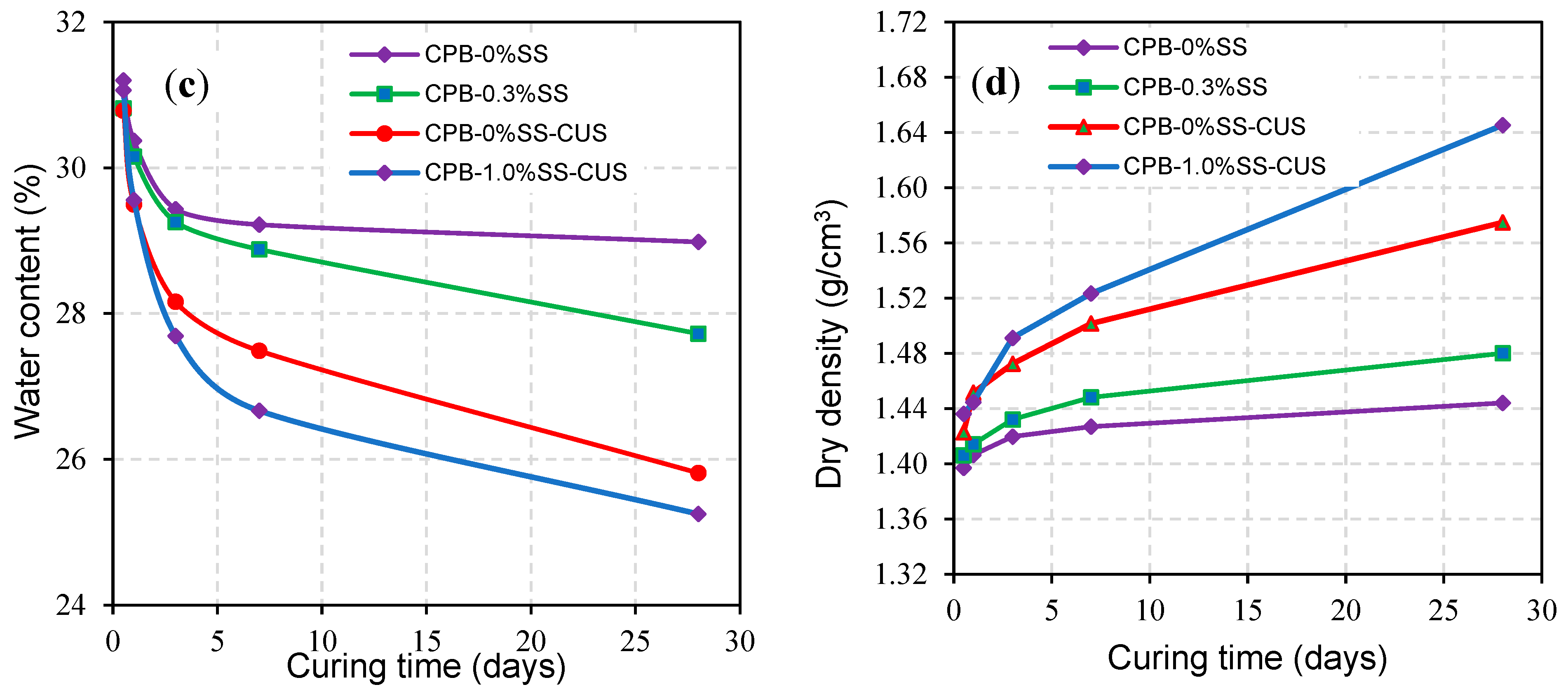
3. Results and Discussion
3.1. Strength Development of SS-CPB
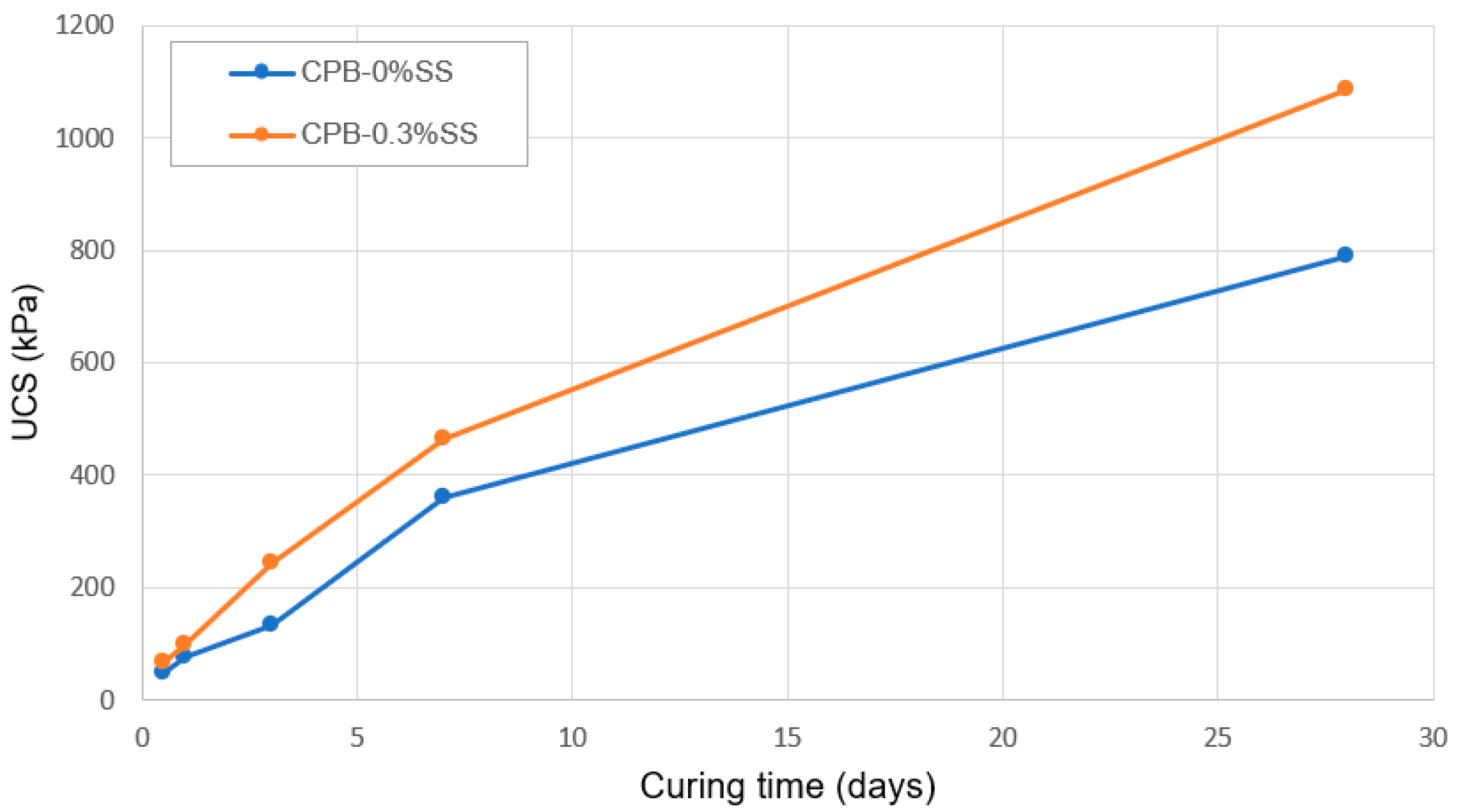
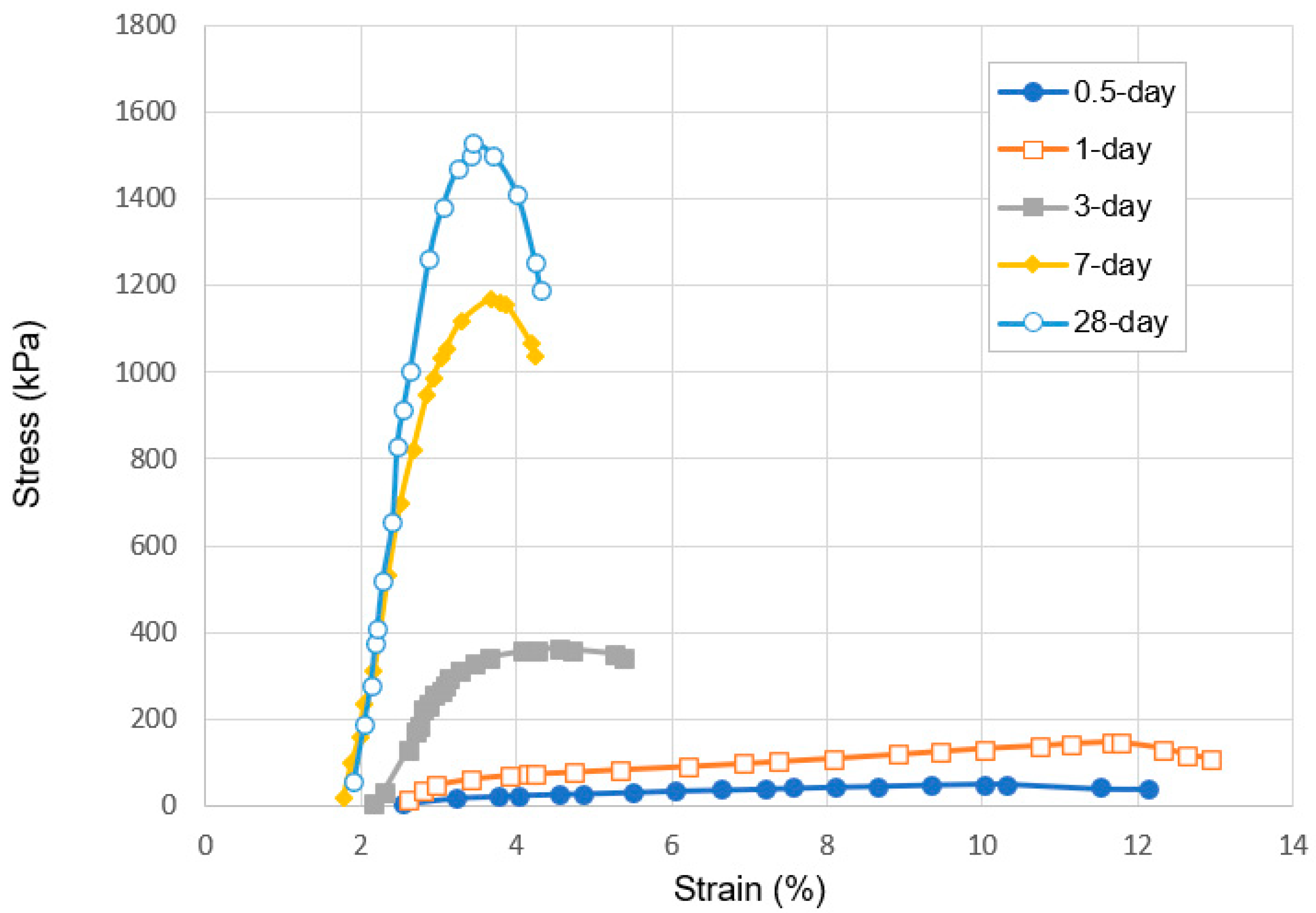
3.1.1. Relationship between Curing Time and Strength Development of SS-CPB
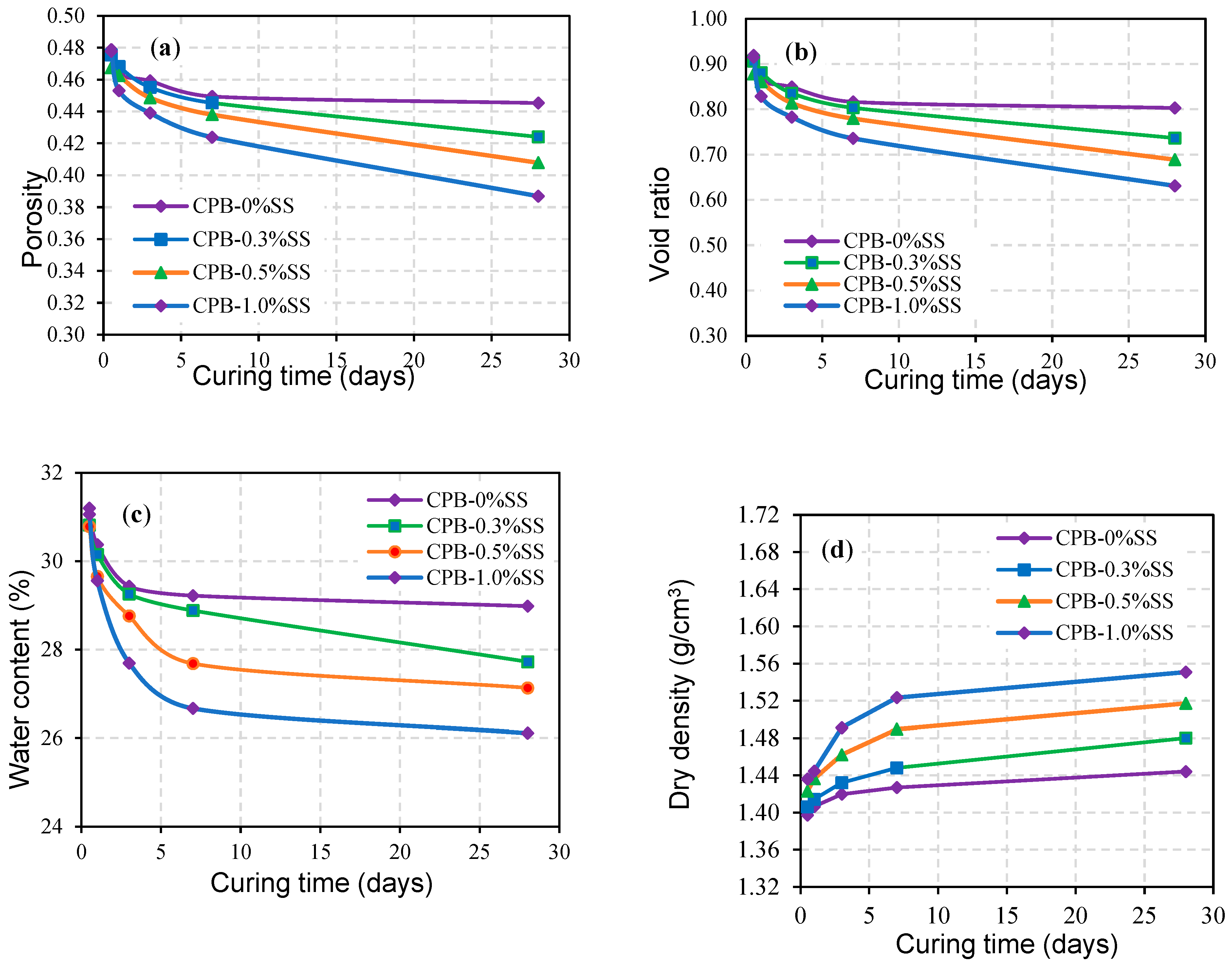
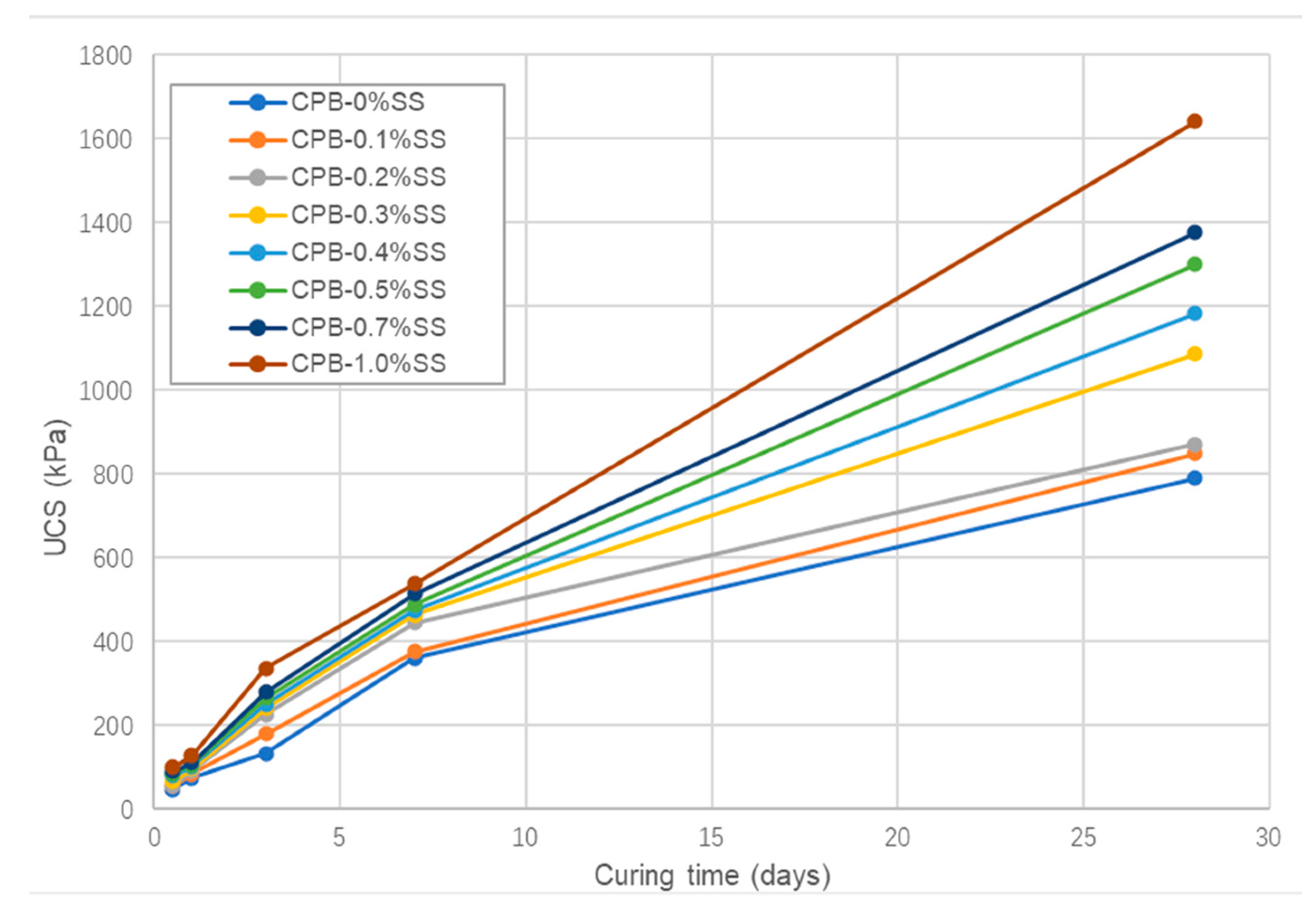
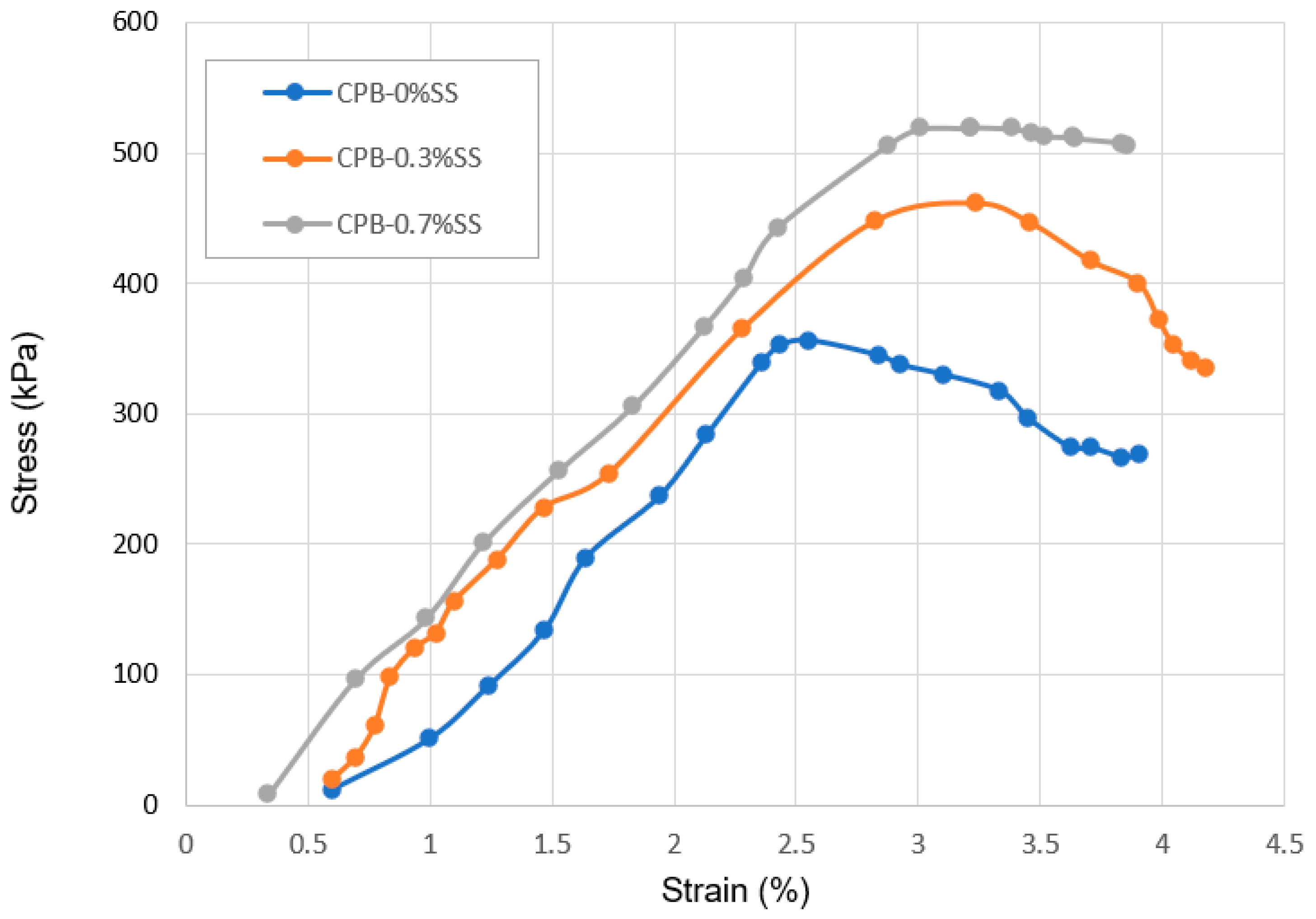
3.1.2. The Role of SS Content in the Strength Growth of CPB
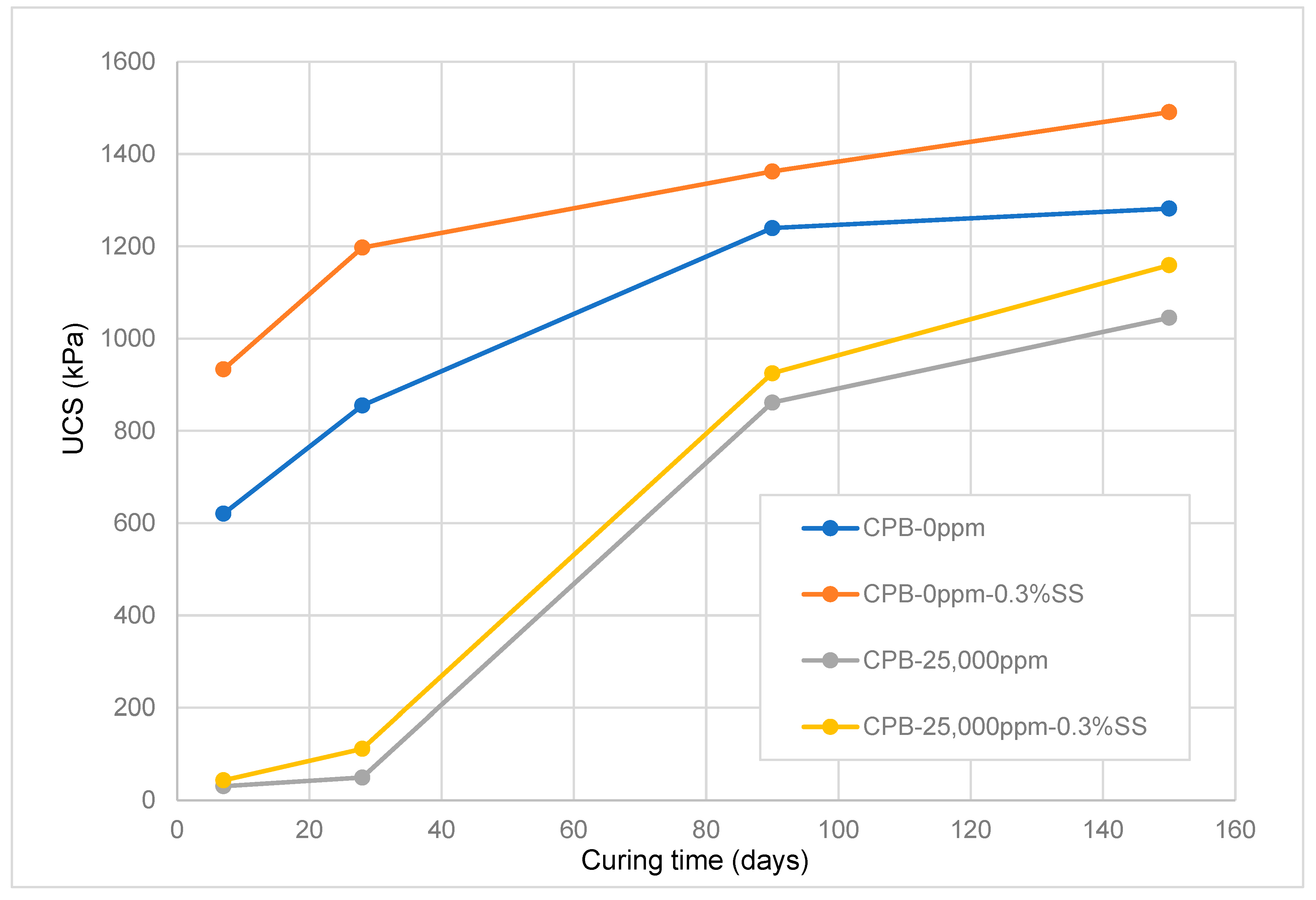
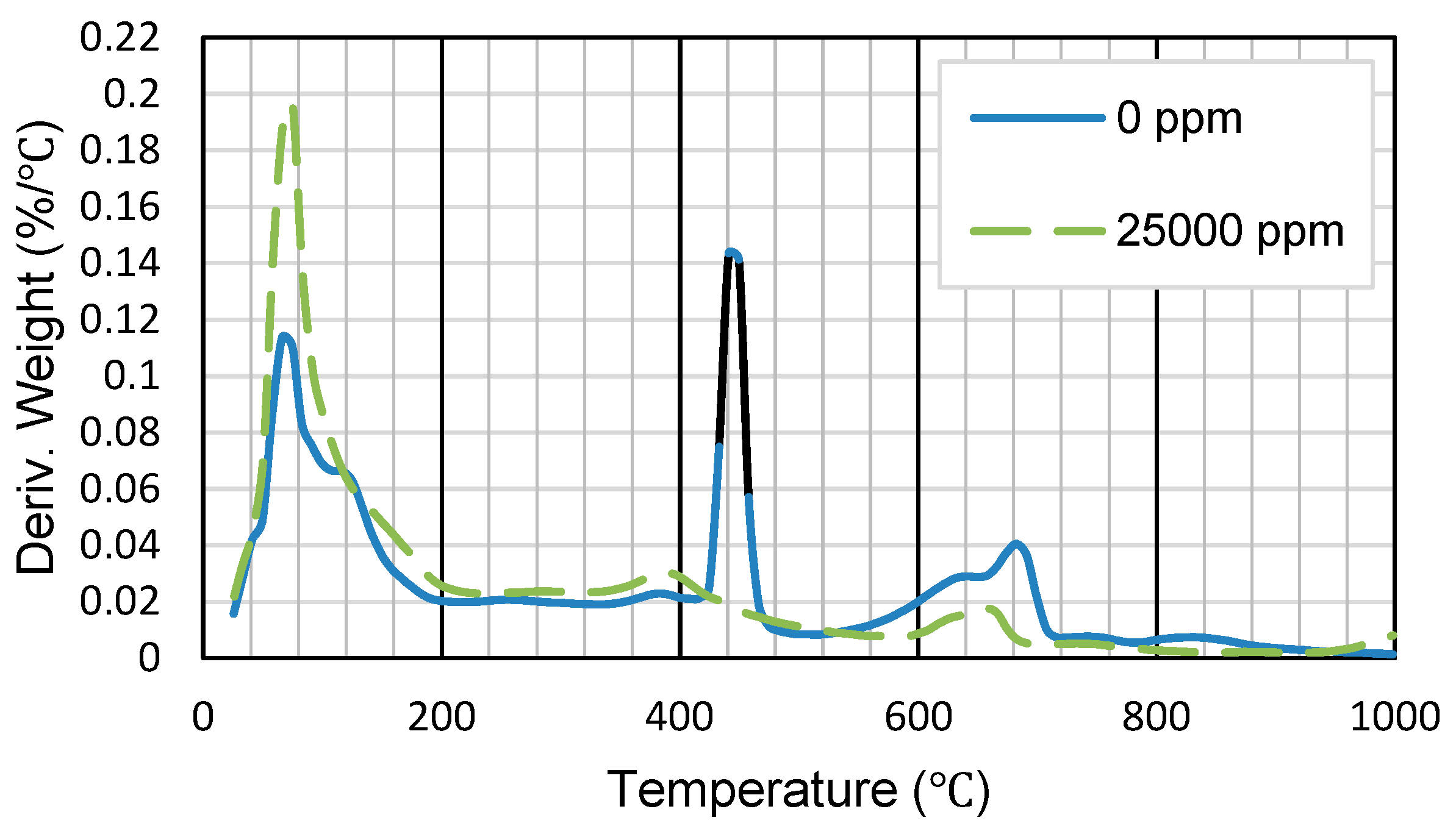
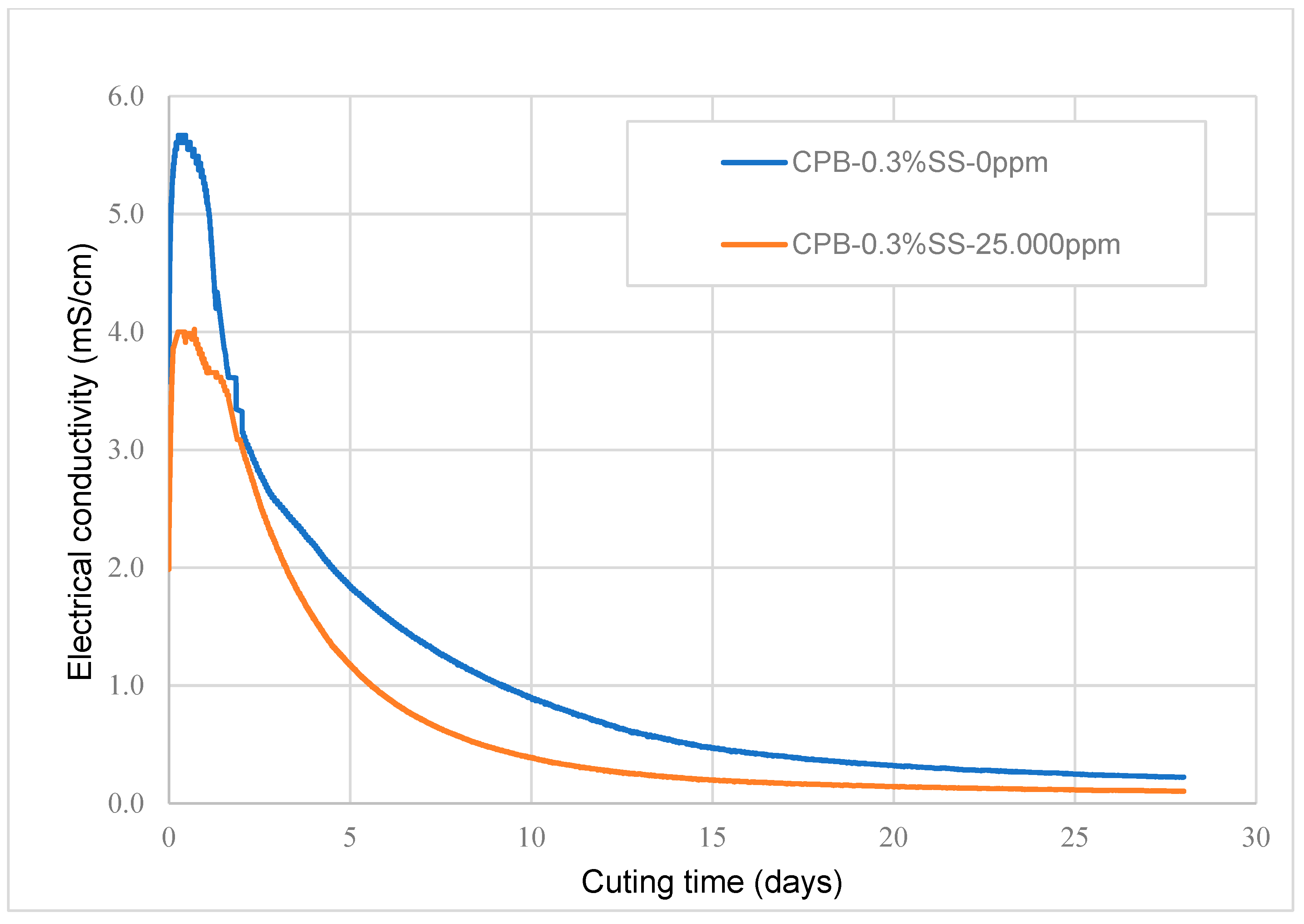
3.1.3. Impact of the Chemical Composition of Mixing Water (Sulfate Content)
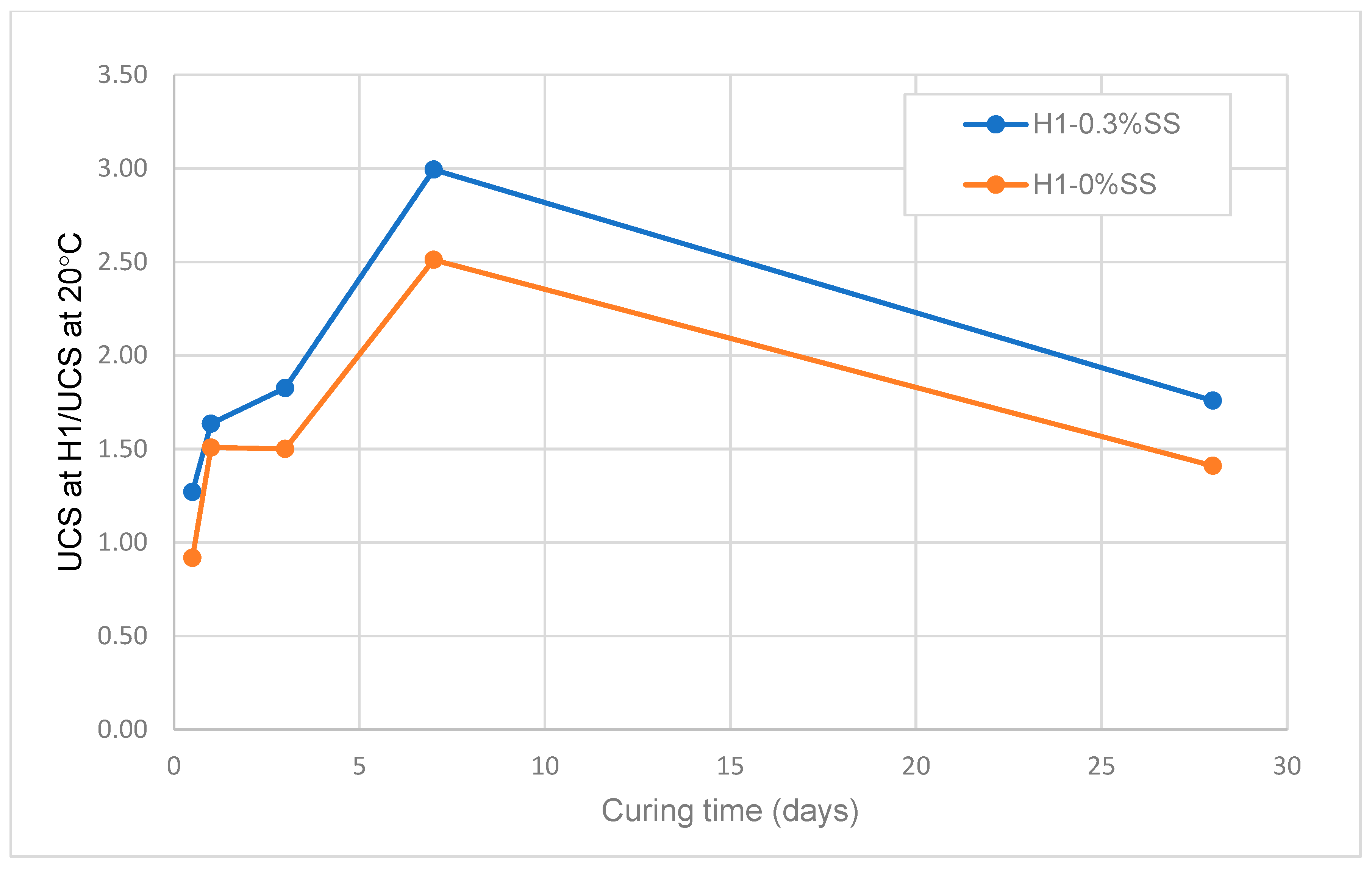
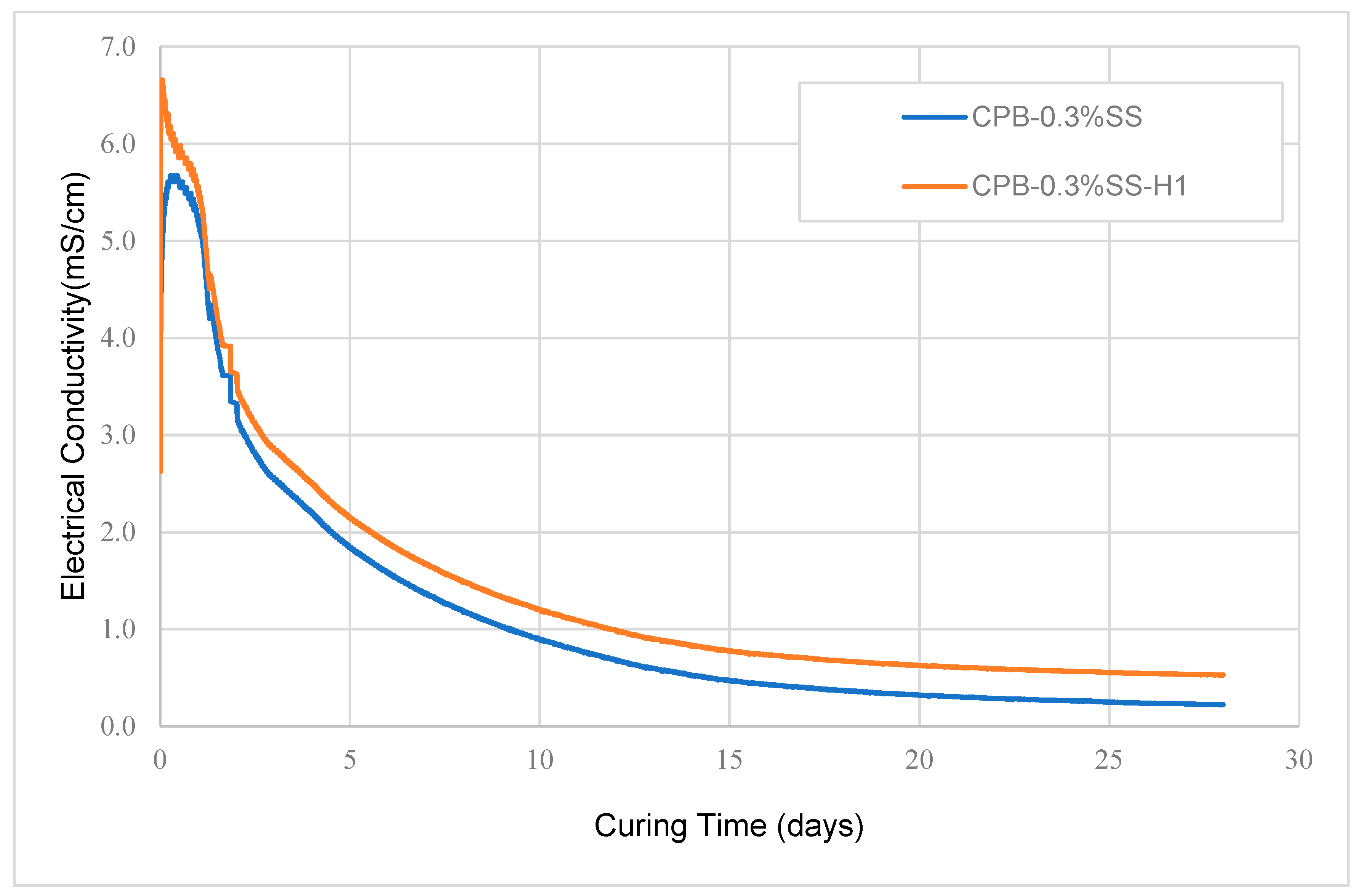
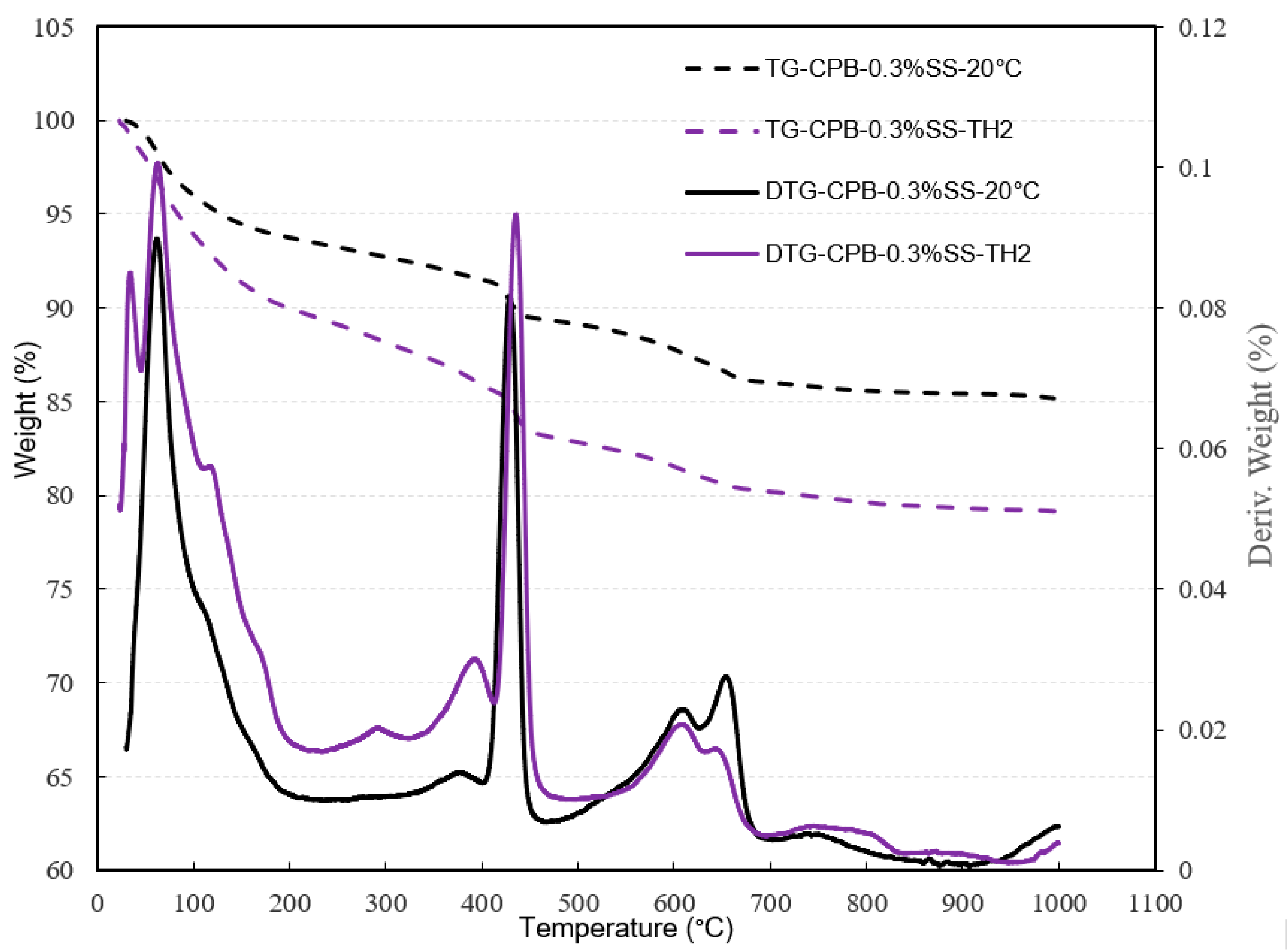
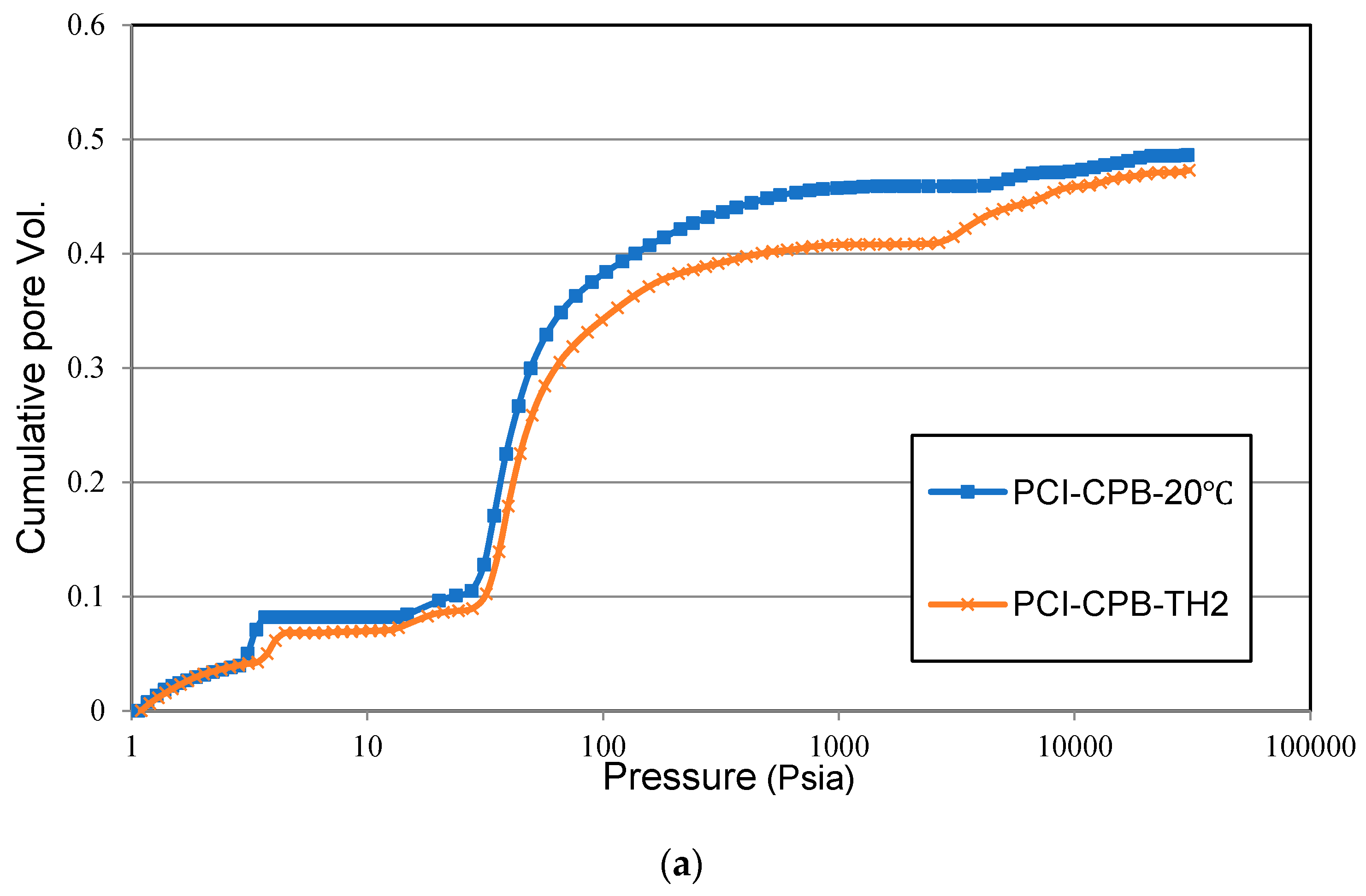
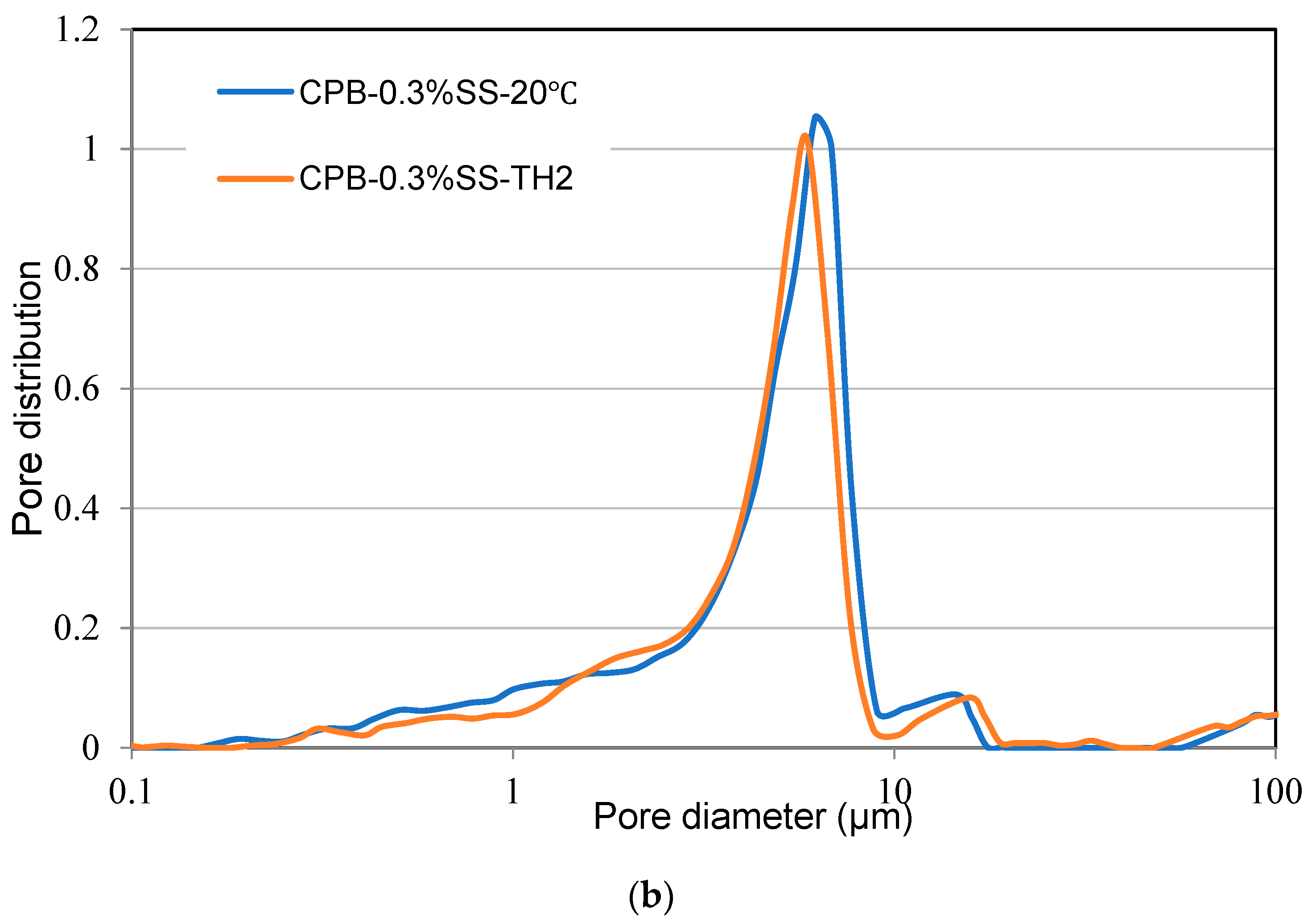
3.1.4. Effect of Non-Isothermal Curing on the Strength Development of SS-CPB
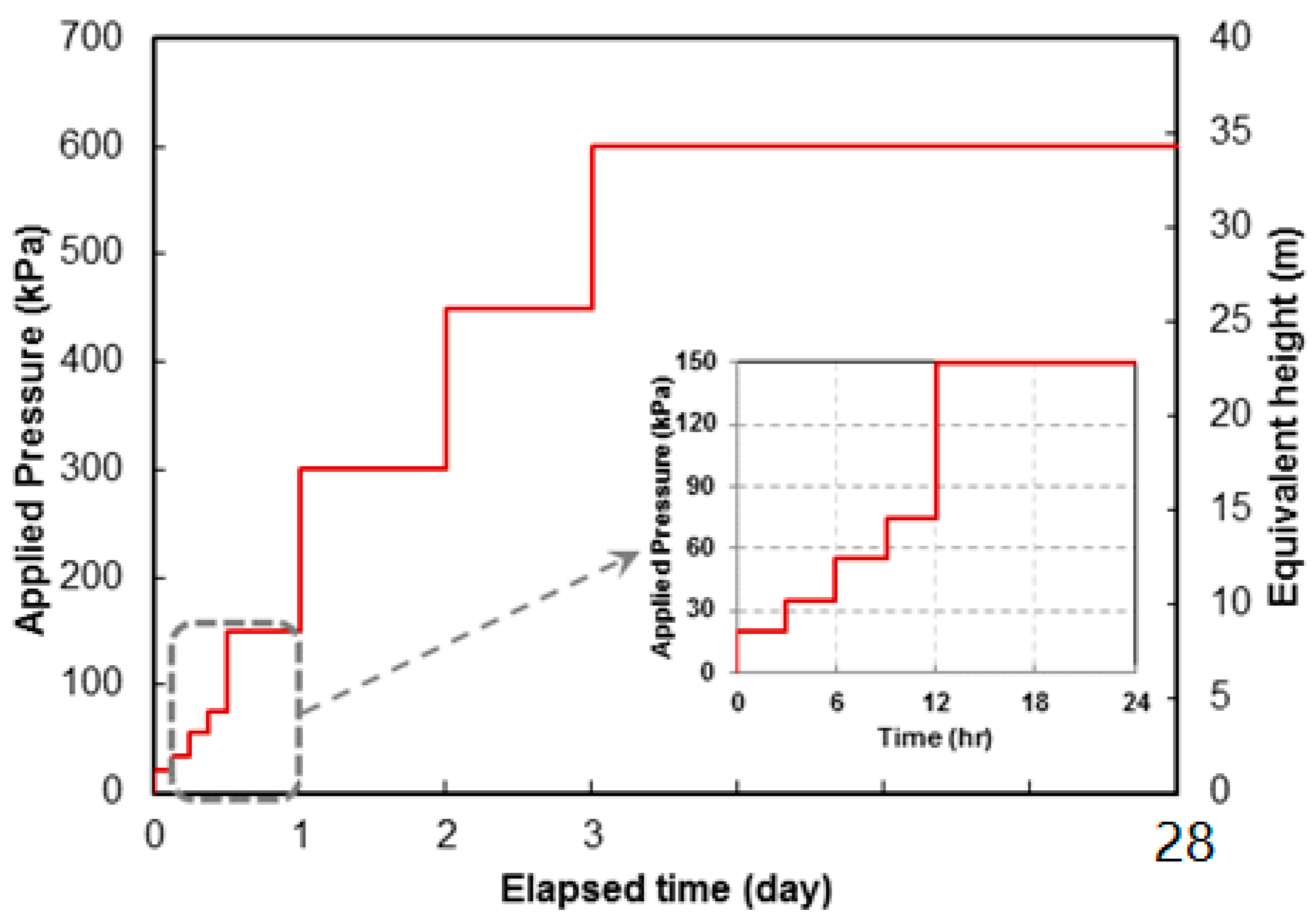
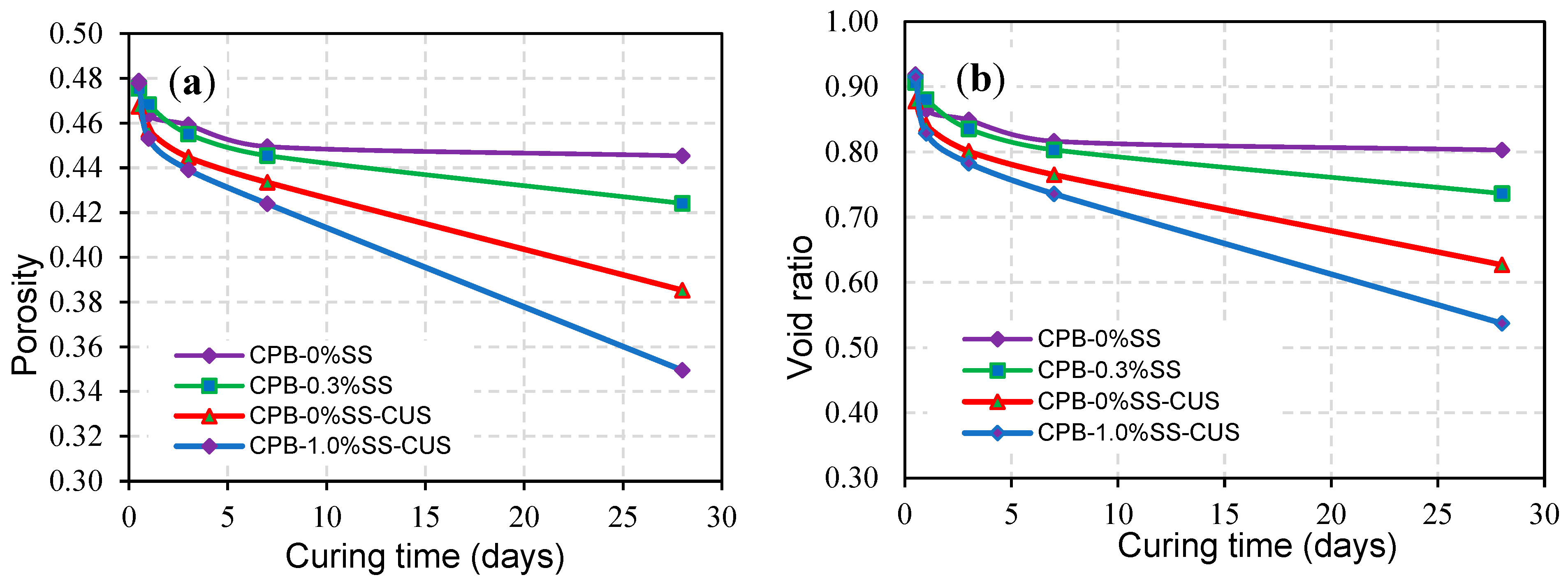
3.1.5. Strength of SS-CPB under the Influence of Curing Stress
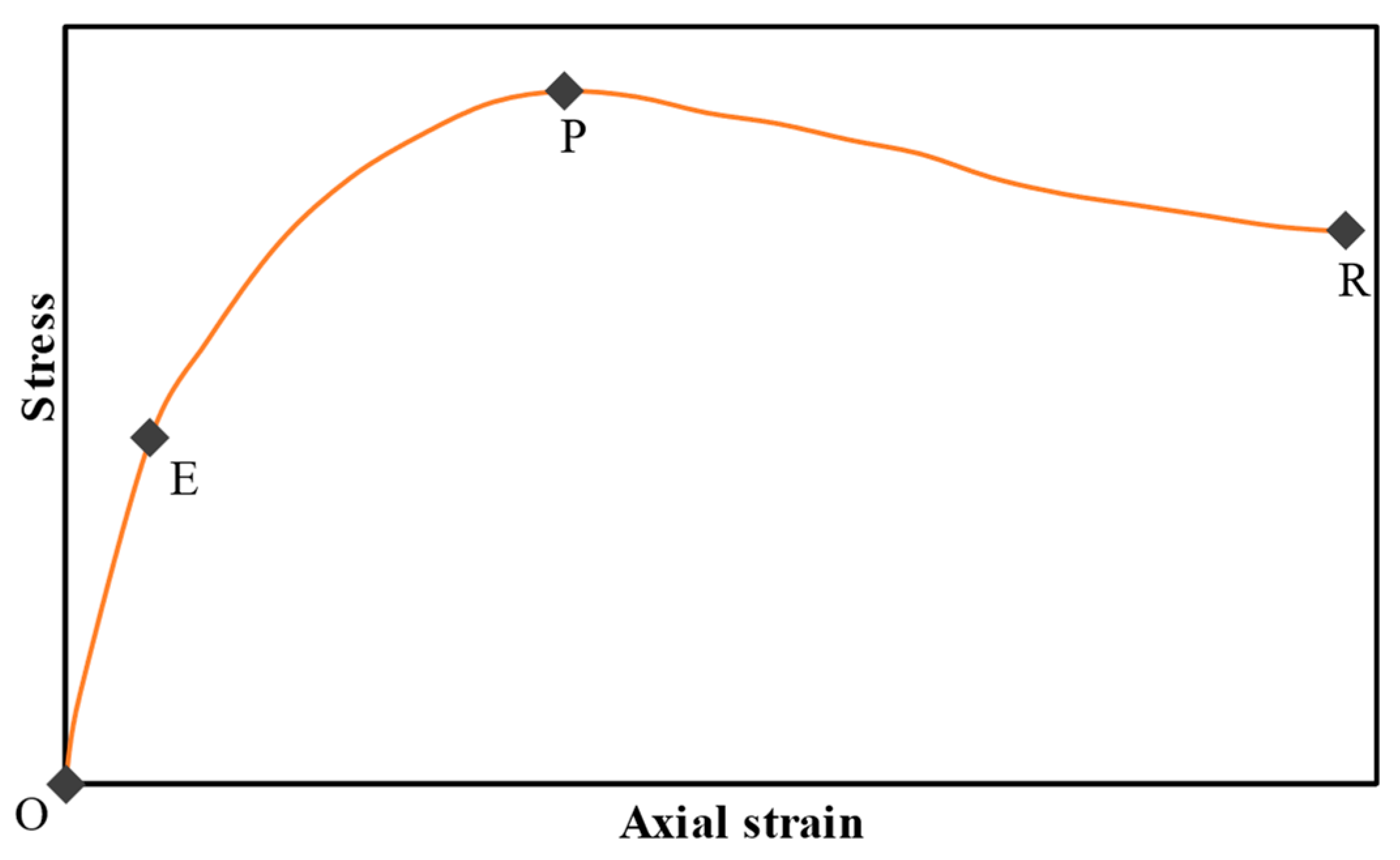
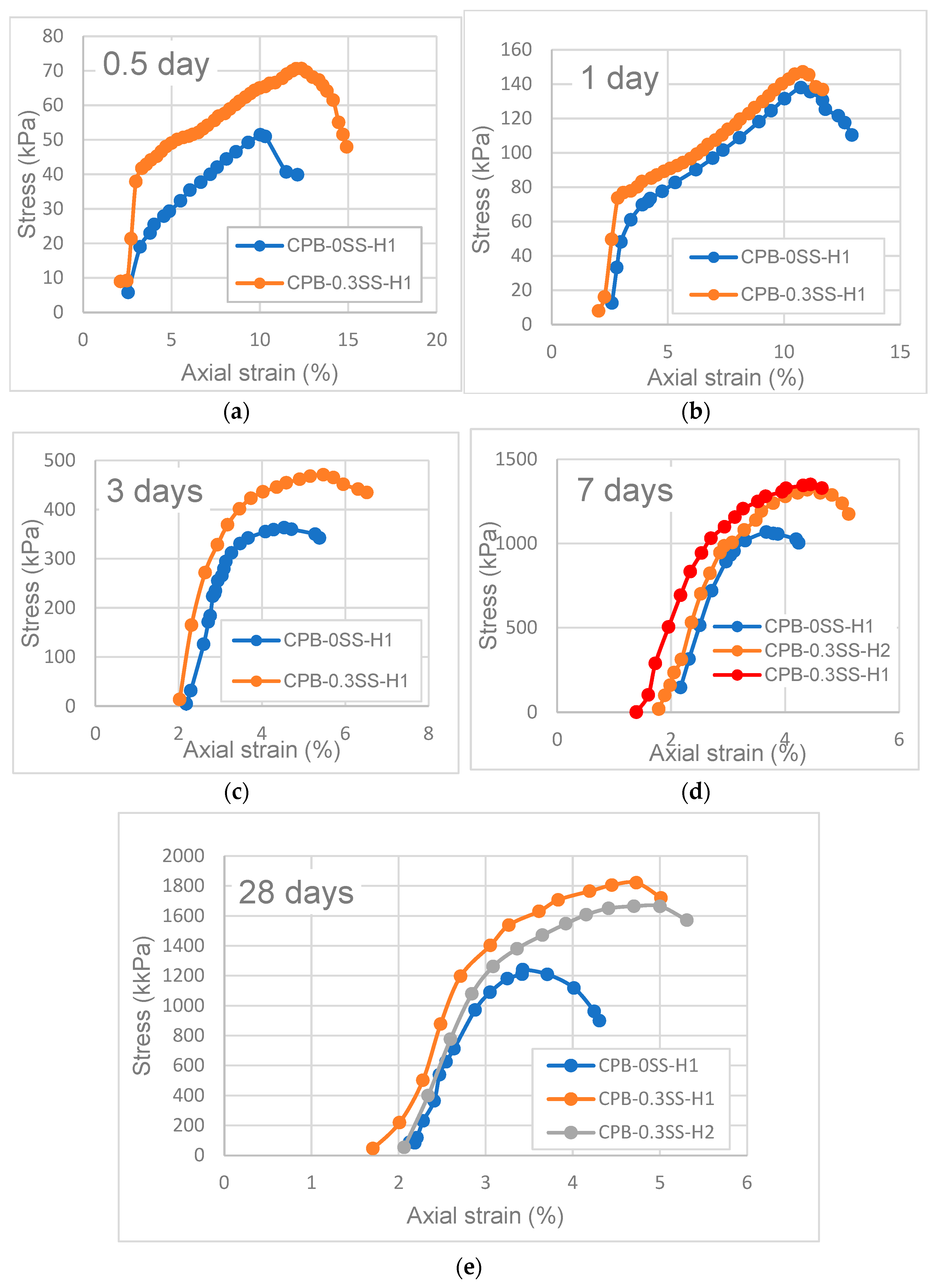
3.2. Deformation Behavior of SS-CPB
4. Conclusions
- The addition of SS to CPB mixtures significantly increases the mechanical strength of the CPB (up to 100%+ compared to the UCS of CPB without SS); in other words, it enhances the mechanical stability of CPB structures. But the extent of the increase depends on the amount of SS added and the length of the curing time. SS acts as an alkaline activator, which results in accelerated hydration of the binder. In addition, it also creates an extra C-S-H gel because of the reaction of SiO2 with CH.
- The amount of sulfate present in the mixing water notably changes the short- and long-term strength of SS-CPB. The sulfate ions retard the hydration process of the cement, resulting in the production of a smaller amount of hydration products in the specimens with 25,000 ppm sulfate ions, resulting in lower strength. However, it was found that sodium silicate improves the resistance of CPB samples to sulfate attack. This would mean that when tailings and/or mixing waters with relatively high sulfate contents are used in the preparation of CPB, the addition of SS to CPB would result in more stable and safer CPB structures when exposed to sulfate attack, which is beneficial for underground mine work safety and mine productivity.
- The strength of SS-CPB samples cured under simulated field CPB overburden pressure was found to be greater than that of samples cured under stress-free conditions. Curing stress has the effect of rearranging the tailing particles in the SS-CPB samples, which in turn increases packing density, reduces void ratio and porosity, and ultimately results in an increase in compressive strength. This finding means that the field overburden pressure or stress to which SS-CPB is exposed during placement and curing should be considered in the strength evaluation of SS-CPB in order to design more cost-effective cemented backfill structures.
- The strength and rate of strength gain of SS-CPB cured at non-isothermal field curing temperatures is higher than that of SS-CPB cured at constant room temperature (20 °C) due to the enhanced binder hydration by the higher field curing temperature. It was also found that the non-isothermal curing of the backfill and the higher curing temperatures in the field are more favorable for the strength development of the CPB with SS than that of CPB without SS. In other words, a CPB structure with SS would reach the required mechanical stability more quickly, which is advantageous to improve the productivity of the mine.
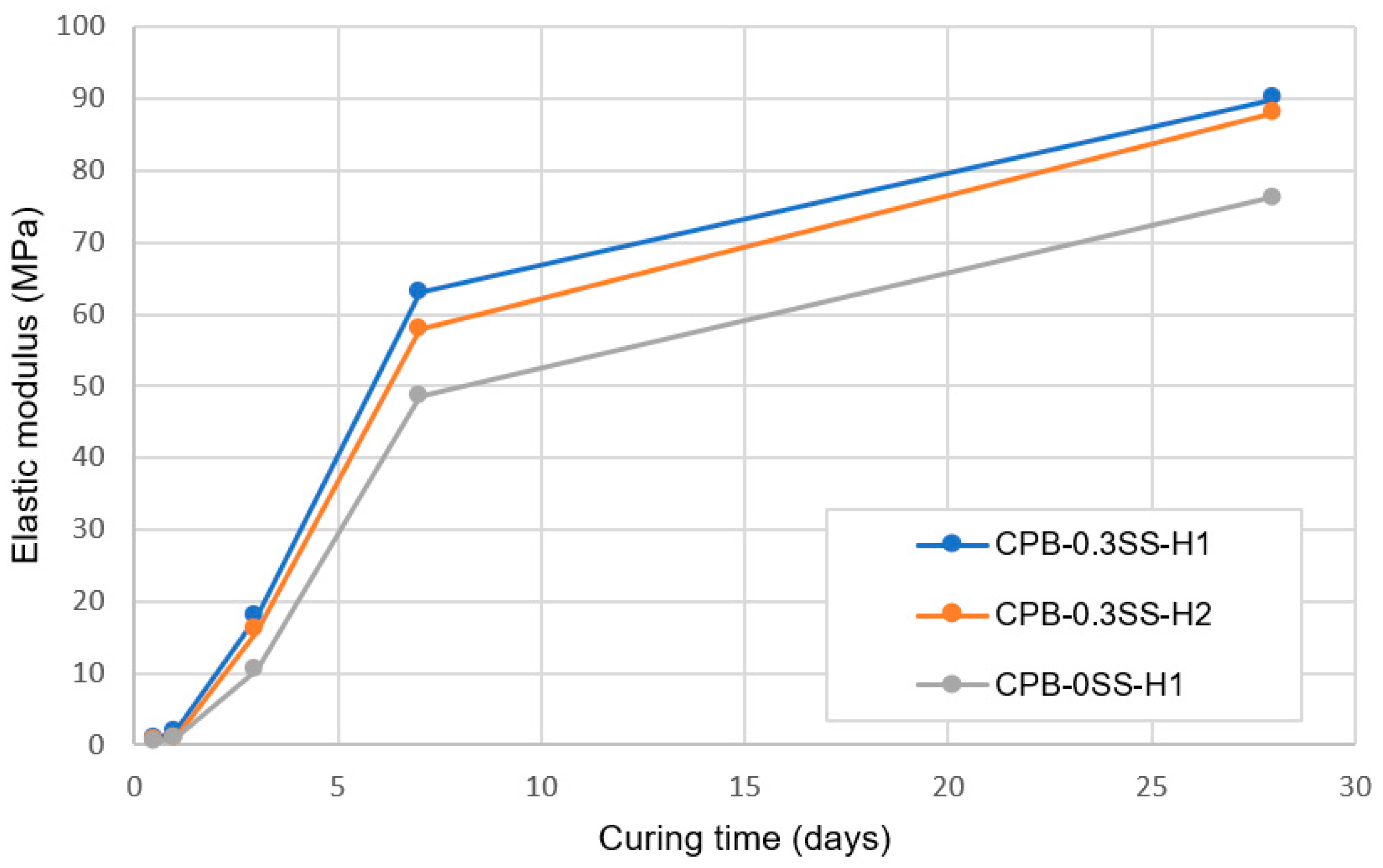
- The stress–strain characteristics (e.g., the shape of the stress–strain curve, maximum stress and strain, modulus of elasticity) of CPB are markedly influenced by the presence of sodium silicate in the CPB composition. CPB’s axial stress–strain responses differ depending on the presence or absence of SS. The presence of SS in CPB leads to an augmentation of peak stress and modulus of elasticity, primarily due to the enhanced hydration of the binder caused by SS. It is also observed that the stress–strain characteristics of SS-CPB are significantly affected by the curing time. For longer curing times, the stress–strain responses of SS-CPB show greater stiffness, higher peak stress, more defined peaks, and subsequent softening. The reason for this is that longer curing times lead to a greater degree of cement hydration, greater self-dehydration, and a finer pore structure of the SS-CPB material.
- The results demonstrate that the stress–strain characteristics (e.g., the shape of the stress–strain curve, maximum stress and strain, and modulus of elasticity) of CPB under field temperature conditions are impacted by the presence of SS in the CPB composition. The maximum stress is enhanced, and the corresponding strain is increased with the addition of SS. Furthermore, under field thermal curing conditions, the modulus of elasticity of CPB with SS is higher than that without SS, irrespective of curing time, i.e., SS-CPB is stiffer.
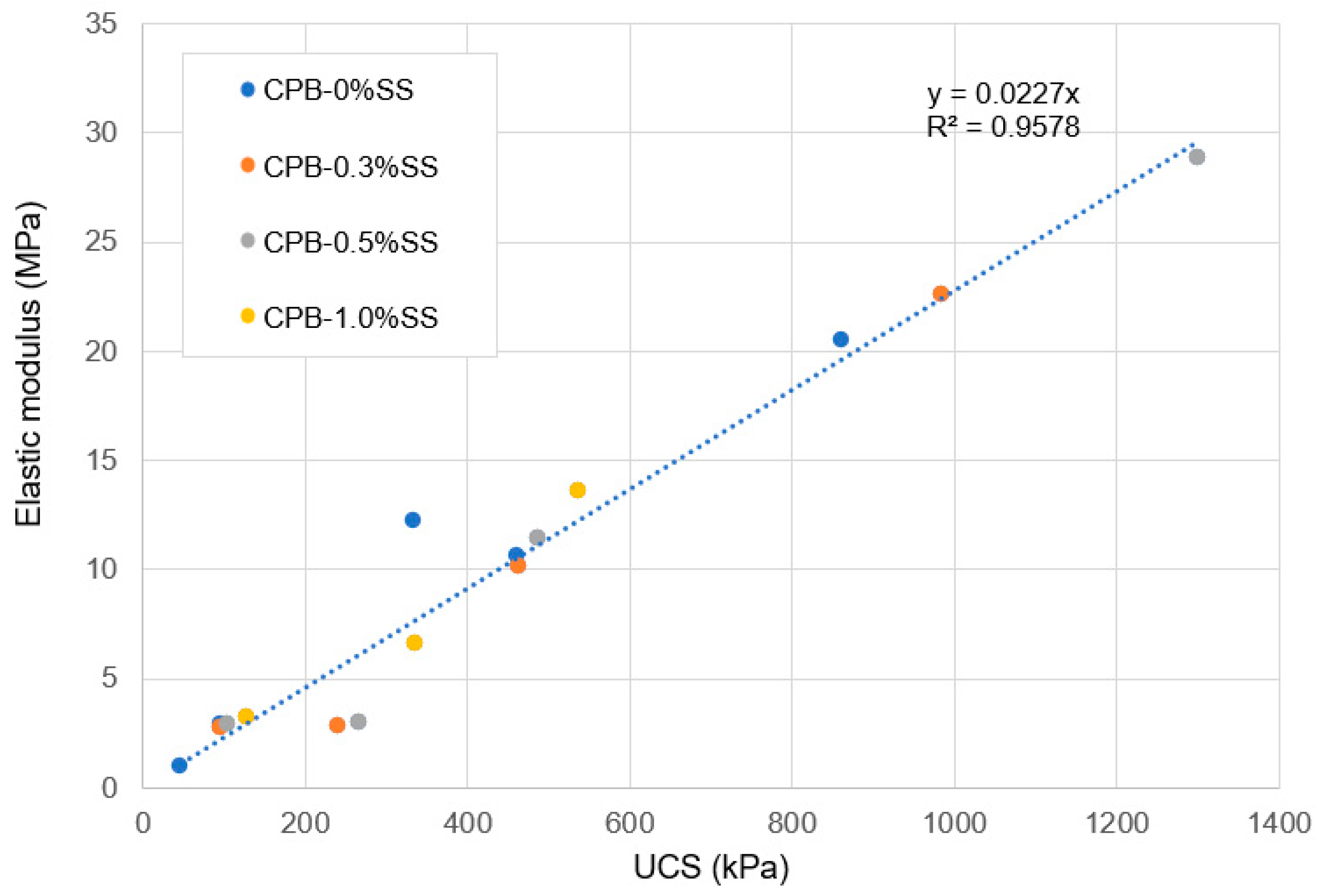
- It was also found that the compressive strength of SS-CPB has a linear relationship with the modulus of elasticity, and SS contents do not significantly affect this relationship.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kasap, T.; Yilmaz, E.; Sari, M. Physico-chemical and micro-structural behavior of cemented mine backfill: Effect of pH in dam tailings. J. Environ. Manag. 2022, 314, 115034. [Google Scholar] [CrossRef]
- Yang, L.H.; Li, J.C.; Jiao, H.Z.; Wu, A.; Yin, S. Research on the homogenization evaluation of cemented paste backfill in the preparation process based on image texture features. Minerals 2022, 12, 1622. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Yilmaz, E.; Han, J.; Qiu, J.; Dong, C. Effectiveness of alkali-activated slag as alternative binder on workability and early age compressive strength of cemented paste backfills. Constr. Build. Mater. 2019, 218, 689–700. [Google Scholar] [CrossRef]
- Fridjonsson, E.O.; Hasan, A.; Fourie, A.B.; Johns, M.L. Pore structure in a gold mine cemented paste backfill. Miner. Eng. 2013, 53, 144–151. [Google Scholar] [CrossRef]
- Yilmaz, E.; Belem, T.; Bussière, B.; Benzaazoua, M. Relationships between microstructural properties and compressive strength of consolidated and unconsolidated cemented paste backfills. Cem. Concr. Compos. 2011, 33, 702–715. [Google Scholar] [CrossRef]
- Vergne, J. Rules of thumb for the hard rock mining industry. In Hard Rock Miner’s Handbook 2000, 3rd ed.; McIntosh Engineering Inc.: Tempe, AZ, USA, 2000. [Google Scholar]
- Fall, M.; Célestin, J.C.; Pokharel, M.; Touré, M. A contribution to understanding the effects of curing temperature on the mechanical properties of mine cemented tailings backfill. Eng. Geol. 2010, 114, 397–413. [Google Scholar] [CrossRef]
- Fall, M.; Pokharel, M. Coupled effects of sulphate and temperature on the strength development of cemented tailings backfills: Portland cement-paste backfill. Cem. Concr. Compos. 2010, 32, 819–828. [Google Scholar] [CrossRef]
- Xue, G.; Yilmaz, E.; Song, W.; Cao, S. Compressive strength characteristics of cemented tailings backfill with alkali-activated slag. Appl. Sci. 2018, 8, 1537. [Google Scholar] [CrossRef]
- Yang, L.; Yilmaz, E.; Li, J.; Liu, H.; Jiang, H. Effect of superplasticizer type and dosage on fluidity and strength behavior of cemented tailings backfill with different solid contents. Constr. Build. Mater. 2018, 187, 290–298. [Google Scholar] [CrossRef]
- Hassani, F.; Archibald, J.F. Mine Backfill; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 1998. [Google Scholar]
- Tariq, A.; Yanful, E.K. A review of binders used in cemented paste tailings for underground and surface disposal practices. J. Environ. Manag. 2013, 131, 138–149. [Google Scholar] [CrossRef]
- Ali, G.A. Temperature Dependency of the Rheological Properties and Strength of Cemented Paste Backfill that Contains Sodium Silicate. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 2021; 134p. [Google Scholar]
- Cavusoglu, I.; Yilmaz, E.; Yilmaz, A.O. Sodium silicate effect on setting properties, strength behavior and microstructure of cemented coal fly ash backfill. Powder Technol. 2021, 384, 17–28. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Turan, A.; Deveci, H. Utilisation of alkali-activated blast furnace slag in paste backfill of high-sulphide mill tailings: Effect of binder type and dosage. Miner. Eng. 2012, 30, 33–43. [Google Scholar] [CrossRef]
- Kermani, M.; Hassani, F.P.; Aflaki, E.; Benzaazoua, M.; Nokken, M. Evaluation of the effect of sodium silicate addition to mine backfill, Gelfill—Part 1. J. Rock Mech. Geotech. Eng. 2015, 7, 266–272. [Google Scholar] [CrossRef]
- Kermani, M.; Hassani, F.P.; Aflaki, E.; Benzaazoua, M.; Nokken, M. Evaluation of the effect of sodium silicate addition to mine backfill, Gelfill—Part 2: Effects of mixing time and curing temperature. J. Rock Mech. Geotech. Eng. 2015, 7, 668–673. [Google Scholar] [CrossRef]
- Chang, S. Strength and Deformation Behaviour of Cemented Paste Backfill in Sub-Zero Environment. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 2016; p. 152. [Google Scholar]
- Wang, Z.; Wang, Y.; Cui, L.; Bi, C.; Wu, A. Insight into the isothermal multiphysics processes in cemented paste backfill: Effect of curing time and cement-to-tailings ratio. Constr. Build. Mater. 2022, 325, 126739. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Properties of cemented paste backfill. In Paste Tailing Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 59–109. [Google Scholar]
- Cui, L.; Fall, M. Mechanical and thermal properties of cemented tailings materials at early ages: Influence of initial temperature, curing stress and drainage conditions. Constr. Build. Mater. 2016, 125, 553–563. [Google Scholar] [CrossRef]
- Wu, D.; Fall, M.; Cai, S.-J. Numerical modelling of thermally and hydraulically coupled processes in hydrating tailings backfill columns. Int. J. Min. Reclam. Environ. 2014, 28, 173–199. [Google Scholar] [CrossRef]
- Haruna, S.; Fall, M. Time- and temperature-dependent rheological properties of cemented paste backfill that contains superplasticizer. Power Technol. 2020, 360, 731–740. [Google Scholar] [CrossRef]
- Aldhafeeri, A.; Fall, M.; Pokharel, M.; Pouramini, Z. Temperature dependency of the reactivity of cemented paste backfill. Appl. Geochem. 2016, 72, 10–19. [Google Scholar] [CrossRef]
- Britannica. Water Glass. 2018. Available online: https://www.britannica.com/science/water-glass (accessed on 5 September 2023).
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Genty, T. In situ effectiveness of alkaline and cementitious amendments to stabilize oxidized acid-generating tailings. Minerals 2019, 9, 314. [Google Scholar] [CrossRef]
- Cihangir, F.; Akyol, Y. Effect of desliming of tailings on the fresh and hardened properties of paste backfill made from alkali-activated slag. Adv. Mater. Sci. Eng. 2020, 2020, 4536257. [Google Scholar] [CrossRef]
- Orejarena, L.; Fall, M. Mechanical response of a mine composite material to extreme heat. Bull. Eng. Geol. Environ. 2008, 67, 387–396. [Google Scholar] [CrossRef]
- Diamond, S. The microstructure of cement paste and concrete—A visual primer. Cem. Concr. Compos. 2004, 26, 919–933. [Google Scholar] [CrossRef]
- Jiang, H.; Ren, L.; Gu, X.; Zheng, J.; Cui, L. Synergistic effect of activator nature and curing temperature on time-dependent rheological behavior of cemented paste backfill containing alkali-activated slag. Environ. Sci. Pollut. Res. 2023, 30, 12857–12871. [Google Scholar] [CrossRef]
- Libos, I.L.; Cui, L. Effects of curing time, cement content, and saturation state on mode-I fracture toughness of cemented paste backfill. Eng. Fract. Mech. 2020, 235, 107174. [Google Scholar] [CrossRef]
- Gan, M. Cement and Concrete; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Taylor, H.F.W. The Chemistry of Cements. Academic Press: London, UK, 1993. [Google Scholar]
- Al-Moselly, Z.; Fall, M.; Haruna, S. Further insight into the strength development of cemented paste backfill materials containing polycarboxylate ether-based superplasticizer. J. Build. Eng. 2021, 47, 103859. [Google Scholar] [CrossRef]
- Cui, L.; McAdie, A. Experimental study on evolutive fracture behavior and properties of sulfate-rich fiber-reinforced cemented paste backfill under pure mode-I, mode-II, and mode-III loadings. Int. J. Rock Mech. Min. Sci. 2023, 169, 105434. [Google Scholar] [CrossRef]
- Erçikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H. Practical importance of tailings for cemented paste backfill. In Paste Tailings Management; Springer International Publishing: Cham, Switzerland, 2017; pp. 7–32. [Google Scholar]
- Ercikdi, B.; Kesimal, A.; Cihangir, F.; Deveci, H.; Alp, İ. Cemented paste backfill of sulphide-rich tailings: Importance of binder type and dosage. Cem. Concr. Compos. 2009, 31, 268–274. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E.G. Mix proportioning of underground cemented tailings backfill. Tunn. Undergr. Space Technol. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Wang, Y.; Fall, M.; Wu, A. Initial temperature-dependence of strength development and self-desiccation in cemented paste backfill that contains sodium silicate. Cem. Concr. Compos. 2016, 67, 101–110. [Google Scholar] [CrossRef]
- Nasir, O.; Fall, M. Modeling the heat development in hydrating cemented paste backfill structures. Comput. Geotech. 2009, 36, 1207–1218. [Google Scholar] [CrossRef]
- Wu, D.; Fall, M.; Cai, S.-J. Coupled modeling of temperature distribution and evolution in cemented tailings backfill structures that contains mineral admixtures. J. Geotech. Geol. Eng. 2012, 30, 935–961. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Coupled behavior of cemented paste backfill at early ages. Geotech. Geol. Eng. 2015, 33, 1141–1166. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Long-term coupled behaviour of cemented paste backfill in load cell experiments. Geomech. Geoengin. 2016, 11, 237–251. [Google Scholar] [CrossRef]
- Fall, M.; Belem, T.; Samb, S.; Benzaazoua, M. Experimental characterization of the stress-strain behaviour of cemented paste backfill. J. Mater. Sci. 2007, 42, 3914–3922. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, D.; Tian, Y.; Liu, X. Numerical simulation of stress-strain behaviour of cemented paste backfill in triaxial compression. Eng. Geol. 2017, 231, 165–175. [Google Scholar] [CrossRef]
- Wu, A.; Wang, Y.; Zhou, B.; Shen, J. Effect of initial backfill temperature on the deformation behavior of early age cemented paste backfill that contains sodium silicate. Adv. Mater. Sci. Eng. 2016, 2016, 8481090. [Google Scholar] [CrossRef]
- Cao, S.; Yilmaz, E.; Song, W. Dynamic response of cemented tailings matrix composites under SHPB compression load. Constr. Build. Mater. 2018, 186, 892–903. [Google Scholar] [CrossRef]





| Element | PCI (%) | Slag (%) |
|---|---|---|
| Fe2O3 | 2.7 | - |
| SO3 | 3.8 | 3.9 |
| SiO2 | 18.0 | 34.2 |
| Al2O3 | 4.5 | 9.5 |
| MgO | 2.6 | 11.0 |
| CaO | 62.8 | 41.1 |
| Relative density | 3.2 | 3.9 |
| Sample | Binder Content (%) | Binder | Binder Ratio | Tailing Type | W/C Ratio | Mixing Water | SS (%) | Curing Stress | Curing Times (Days) | Curing Temp (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| A. Impact of curing time on the UCS of SS-CPB | ||||||||||
| CPB-ST-0.3%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.3 | 0 | 0.5, 1, 3, 7, 28 | 20 |
| CPB-NT-0.3%SS | 4.50 | PCI:Slag | 50/50 | GT | 7.6 | TW | 0.3 | 0 | 0.5, 1, 3, 7, 28 | 20 |
| B. Impact of curing time and SS content on the strength of SS-CPB | ||||||||||
| CPB-0%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0 | 0 | 0.5, 1, 3, 7, 28 | 20 |
| CPB-0.1%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.1 | 0 | 0.5, 1, 3, 7, 28 | 20 |
| CPB-0.2%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.2 | 0 | 0.5, 1, 3, 7, 28 | 20 |
| CPB-0.3%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.3 | 0 | 0.5, 1, 3, 7, 28, | 20 |
| CPB-0.4%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.3 | 0 | 0.5, 1, 3, 7, 28, | 20 |
| CPB-0.5%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.5 | 0 | 0.5, 1, 3, 7, 28, | 20 |
| CPB-0.7%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.7 | 0 | 0.5, 1, 3, 7, 28, | 20 |
| CPB-1.0%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 1.0 | 0 | 0.5, 1, 3, 7, 28, | 20 |
| C. Impact of mixing water chemistry on the strength of SS-CPB | ||||||||||
| CPB-0p-0.3%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | DW | 0.3 | 0 | 7, 28, 90, 150 | 20 |
| CPB-25p-0.3%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | 25,000 ppm | 0.3 | 0 | 7, 28, 90, 150 | 20 |
| CPB-25p | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | 25,000 ppm | 0 | 0 | 7, 28, 90, 150 | 20 |
| CPB-0p | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | DW | 0 | 0 | 7, 28, 90, 150 | 20 |
| D. Impact of curing stress on the strength of SS-CPB | ||||||||||
| CPB-0.3%SS-CS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.3 | CSH-1 | 0.5, 1, 3, 7, 28 | 20 |
| CPB-CS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0 | CSH-1 | 0.5, 1, 3, 7, 28 | 20 |
| E. Impact of non-isothermal curing on the strength of SS-CPB | ||||||||||
| CPB-H1-0.3%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.3 | 0 | 0.5, 1, 3, 7, 28 | H1 |
| CPB-H1 | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.0 | 0 | 0.5, 1, 3, 7, 28 | H1 |
| CPB-H2-0.3%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.3 | 0 | 0.5, 1, 3, 7, 28 | H2 |
| CPB-0.3%SS | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0.3 | 0 | 0.5, 1, 3, 7, 28 | 20 |
| CPB | 4.50 | PCI:Slag | 50/50 | ST | 7.6 | TW | 0 | 0 | 0.5, 1, 3, 7, 28 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, J.; Fall, M.; Pour, H.M. Deeper Understanding of the Strength Evolution and Deformation Characteristics of Sodium Silicate–Cemented Paste Tailing Material. Minerals 2023, 13, 1382. https://doi.org/10.3390/min13111382
Meng J, Fall M, Pour HM. Deeper Understanding of the Strength Evolution and Deformation Characteristics of Sodium Silicate–Cemented Paste Tailing Material. Minerals. 2023; 13(11):1382. https://doi.org/10.3390/min13111382
Chicago/Turabian StyleMeng, Jie, Mamadou Fall, and Hoda M. Pour. 2023. "Deeper Understanding of the Strength Evolution and Deformation Characteristics of Sodium Silicate–Cemented Paste Tailing Material" Minerals 13, no. 11: 1382. https://doi.org/10.3390/min13111382
APA StyleMeng, J., Fall, M., & Pour, H. M. (2023). Deeper Understanding of the Strength Evolution and Deformation Characteristics of Sodium Silicate–Cemented Paste Tailing Material. Minerals, 13(11), 1382. https://doi.org/10.3390/min13111382





