Ion-Exchange Model for the Leaching Process of Ion-Adsorption-Type Rare-Earth Ores Considering the Influence of Anions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore Sample
2.2. Rare-Earth Grade Test
2.3. Shielding Test for Sulfate Ions
2.4. Balanced Leaching Tests
3. Mathematical Model
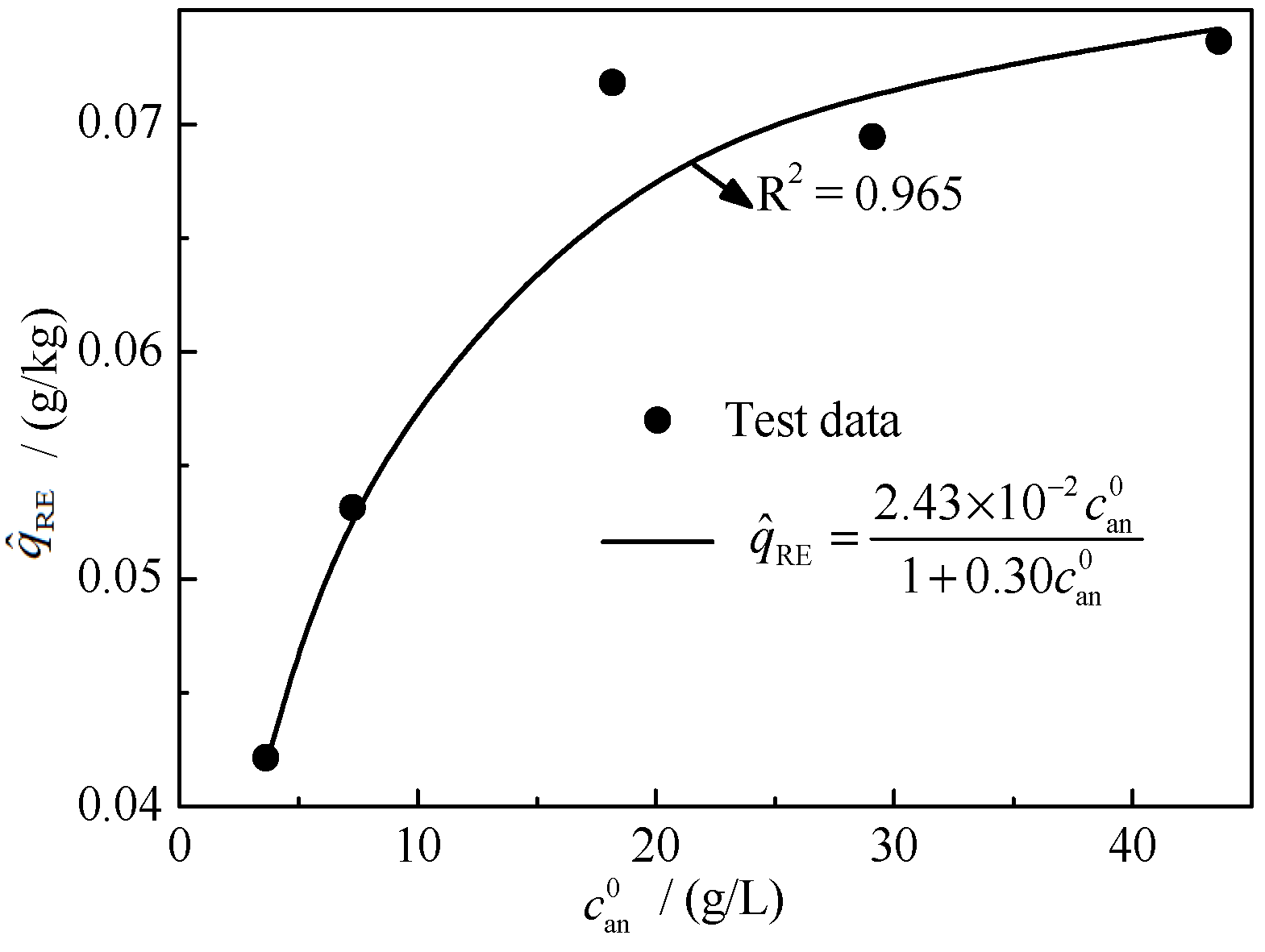
3.1. Calculation Method for the Shielding Amount
3.2. Ion-Exchange Shielding Model
4. Results and Discussion
4.1. Analysis of the Balanced Leaching Test Results
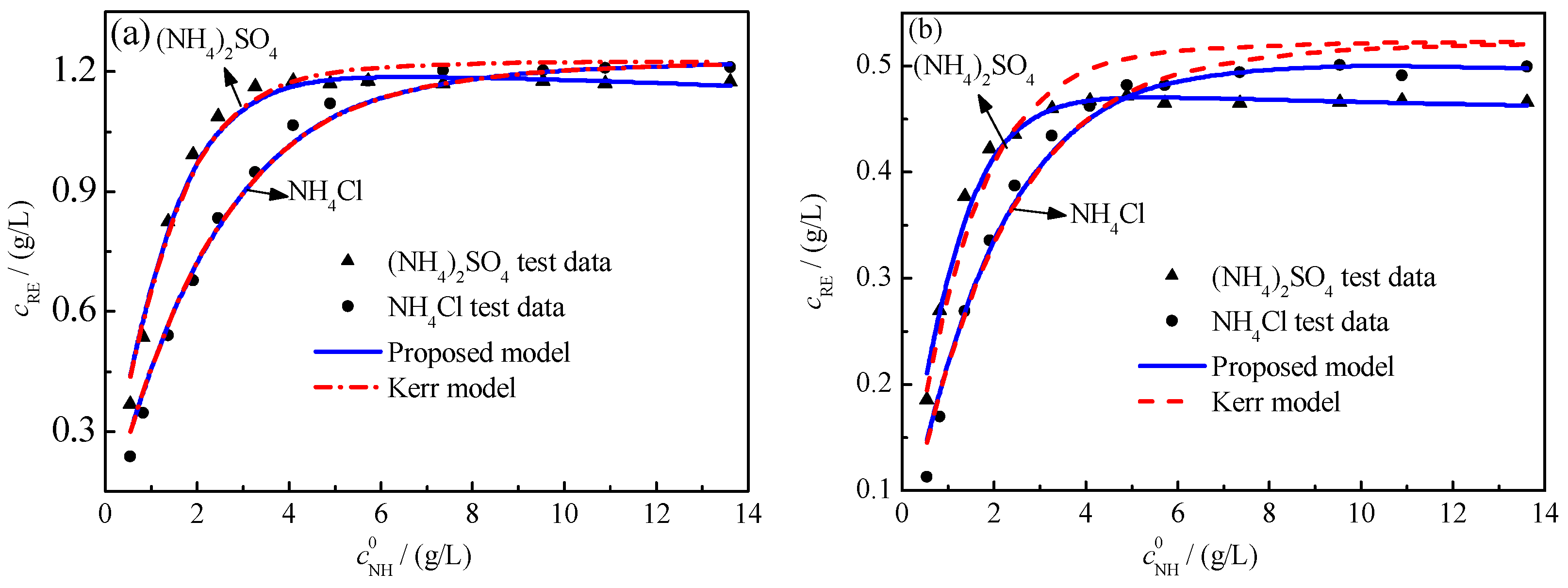
4.2. Model Validation
4.3. Parametric Analysis
5. Conclusions
- (1)
- The amount of shielded rare-earth ions was quantified in Langmuir form with the anion concentration. Describing the ion-exchange process with the Kerr model, an ion-exchange model considering the shielding influence of anions on the leaching of rare-earth ions was established.
- (2)
- At low ammonium ion concentrations—with the same NH4+ concentration—more rare-earth ions were leached when using ammonium sulfate as the leaching agent than when using ammonium chloride. While at high ammonium ion concentrations, compared with ammonium sulfate, using ammonium chloride as the leaching agent extracted more rare-earth ions.
- (3)
- When the leaching agent is ammonium sulfate, the accuracy of the shielding model—based on analysis of the experimental data of the XW and AY samples—was improved by 1.15% and 5.75%, respectively. The shielding model has a high calculation accuracy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chi, R.A.; Tian, J. Weathered Crust Elution-Deposited Rare Earth Ores; Nova Science Publishers: New York, NY, USA, 2008. [Google Scholar]
- Cao, Z.; Cheng, Z.Y.; Wang, J.L.; Cao, Y.D. Synergistic depression mechanism of Ca2+ ions and sodium silicate on bastnaesite flotation. J. Rare Earths 2022, 40, 988–995. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: I. Desorption mechanism. Hydrometallurgy 2012, 117–118, 71–78. [Google Scholar] [CrossRef]
- Deng, Z.; Qin, L.; Wang, G.; Luo, S.; Peng, C.; Li, Q. Metallogenic process of ion adsorption REE ore based on the occurrence regularity of La in kaolin. Ore Geol. Rev. 2019, 112, 103022. [Google Scholar] [CrossRef]
- Long, P.; Wang, G.S.; Tian, J.; Hu, S.L.; Luo, S.H. Simulation of one-dimensional column leaching of weathered crust elution-deposited rare earth ore. Trans. Nonferrous Met. Soc. China 2019, 29, 625–633. [Google Scholar] [CrossRef]
- Tian, J.; Tang, X.; Yin, J.; Luo, X.; Rao, G.; Jiang, M. Process optimization on leaching of a lean weathered crust elution-deposited rare earth ores. Int. J. Min. Process 2013, 119, 83–88. [Google Scholar] [CrossRef]
- Tian, J.; Yin, J.Q.; Chi, R.A.; Rao, G.H.; Jiang, M.T.; Ouyang, K.X. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar]
- Xiao, Y.F.; CHEN, Y.Y.; Feng, Z.Y.; Huang, X.W.; Huang, L.; Long, Z.Q.; CUI, D.L. Leaching characteristics of ion-adsorption type rare earths ore with magnesium sulfate. Trans. Nonferrous Met. Soc. China 2015, 25, 3784–3790. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Feng, Z.Y.; Hu, G.H.; Huang, L.; Huang, X.W.; Chen, Y.Y.; Li, M.L. Leaching and mass transfer characteristics of elements from ion-adsorption type rare earth ore. Rare Metals 2015, 34, 357–365. [Google Scholar] [CrossRef]
- He, Z.Y.; Zhang, Z.Y.; Yu, J.X.; Xu, Z.G.; Chi, R.A. Process optimization of rare earth and aluminum leaching from weathered crust elution-deposited rare earth ore with compound ammonium salts. J. Rare Earths 2016, 34, 413–419. [Google Scholar] [CrossRef]
- He, Z.Y.; Zhang, Z.Y.; Yu, J.X.; Zhou, F.; Xu, Y.L.; Xu, Z.G.; Chen, Z.; Chi, R.A. Kinetics of column leaching of rare earth and aluminum from weathered crust elution-deposited rare earth ore with ammonium salt solutions. Hydrometallurgy 2016, 63, 33–39. [Google Scholar] [CrossRef]
- Yan, H.S.; Liang, T.M.; Liu, Q.S.; Qiu, T.S.; Ai, G.H. Compound leaching behavior and regularity of ionic rare earth ore. Powder Technol. 2018, 333, 106–114. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Xu, Q.H.; Li, Y.X. Leaching kinetics of ion adsorption rare earths using low concentration of ammonium sulfate solution. Chin. Rare Earths 2017, 38, 61–67. [Google Scholar]
- Long, P.; Wang, G.S.; Zhang, C.; Yang, Y.J.; Cao, X.J.; Shi, Z.B. Kinetics model for leaching of ion-adsorption type rare earth ores. J. Rare Earths 2020, 38, 1354–1360. [Google Scholar] [CrossRef]
- Chi, R.A.; Li, L.F.; Wang, D.Z. Studies of ion exchange equilibrium in clay minerals of adsorbed rare earth. J. Cent. South I Min. Metall. 1991, 22, 142–148. [Google Scholar]
- Long, P.; Wang, G.S.; Zhang, C.; Huang, Y.; Luo, S.H. A two-parameter model for ion exchange process of ion-adsorption type rare earth ores. J. Rare Earths 2020, 38, 1251–1256. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on clay minerals: II. Leaching with ammonium sulfateI. Desorption mechanism. Hydrometallurgy 2013, 131–132, 156–166. [Google Scholar] [CrossRef]
- Shanmuganathan, R.T.; Oades, J.M. Influence of anions on dispersion and physical properties of the A horizon of a Red-brown earth. Geoderma 1983, 29, 257–277. [Google Scholar] [CrossRef]
- Xu, R.K.; Zhao, A.Z.; Ji, G.L. Effect of low-molecular-weight organic anions on surface charge of variable charge soils. J. Colloid Interf. Sci. 2003, 264, 322–326. [Google Scholar] [CrossRef]
- Liao, J.H.; Wang, M.C.; Mannepalli, M.R. Effect of Cl−, SO42−, and fulvate anions on Cd2+ free ion concentrations in simulated rhizosphere soil solutions. J. Hazard. Mater. 2009, 172, 809. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.V. Effects of Salinity on Ion Transport, Water Relations and Oxidative Damage; Springer New York: New York, NY, USA, 2013. [Google Scholar]
- EL-Hefnawy, M.E.; Selim, E.M.; Assaad, F.F.; Ismail, A.I. The Effect of Chloride and Sulfate Ions on the Adsorption of Cd2+ on Clay and Sandy Loam Egyptian Soils. Sci. World J. 2014, 2014, 806252. [Google Scholar] [CrossRef]
- Yu, T.R.; Beyme, B.; Richter, J. Direct determination of potassium-calcium activity ratio in soils with two ion-selective electrodes. I. Method of determination. Z. Pflanzenernaehrung Bodenkd. 1989, 152, 359. [Google Scholar] [CrossRef]
- Zou, X.Z.; Jiang, J.; Zhao, A.Z.; Ji, G.L. Mechanism of anion effect on adsorption of Cu2+ by variable charge soils. Acta Pedol. Sin. 2012, 49, 311–318. [Google Scholar]
- Wang, L.; Wang, C.; Liao, C.F.; Yang, Y.M. Effect of ionic interaction on leaching behavior of ion-adsorption type rare earth ore. Chin. J. Rare Met. 2018, 42, 1002–1008. [Google Scholar]
- Huang, Y.; Long, P.; Wang, G.S.; Luo, S.H. Decoupling method for the convective-dominated leaching process of ion-adsorption-type rare-earth ores. Minerals 2023, 13, 89. [Google Scholar] [CrossRef]
- Wang, L. Study on Leaching Characteristics and Enhanced Leaching Mechanism of Ion Adsorption Type Rare Earth Ore at Ore-Water Interface. Ph.D. Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2018. [Google Scholar]
- Li, Y.X. Ion Adsorption Rare Earth Resources and Their Green Extraction; Chemical Industry Press: Beijing, China, 2014. [Google Scholar]
- Broekmann, P.; Wilms, M.; Kruft, M.; Stuhlmann, C. In-situ STM investigation of specific anion adsorption on Cu(111). Electroanal. Chem. 1999, 467, 307–324. [Google Scholar] [CrossRef]
- Weggler, K.; Mclaughlin, M.J.; Graham, R.D. Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J. Environ. Qual. 2004, 33, 496. [Google Scholar]
- Xu, M.G. Soil ion adsorption: 2. Adsorption characteristics of main anions and cations. Soils Fertil. Sci. China 1997, 29, 3–7. [Google Scholar]
- Li, F.H. Physical Chemistry of Soil; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
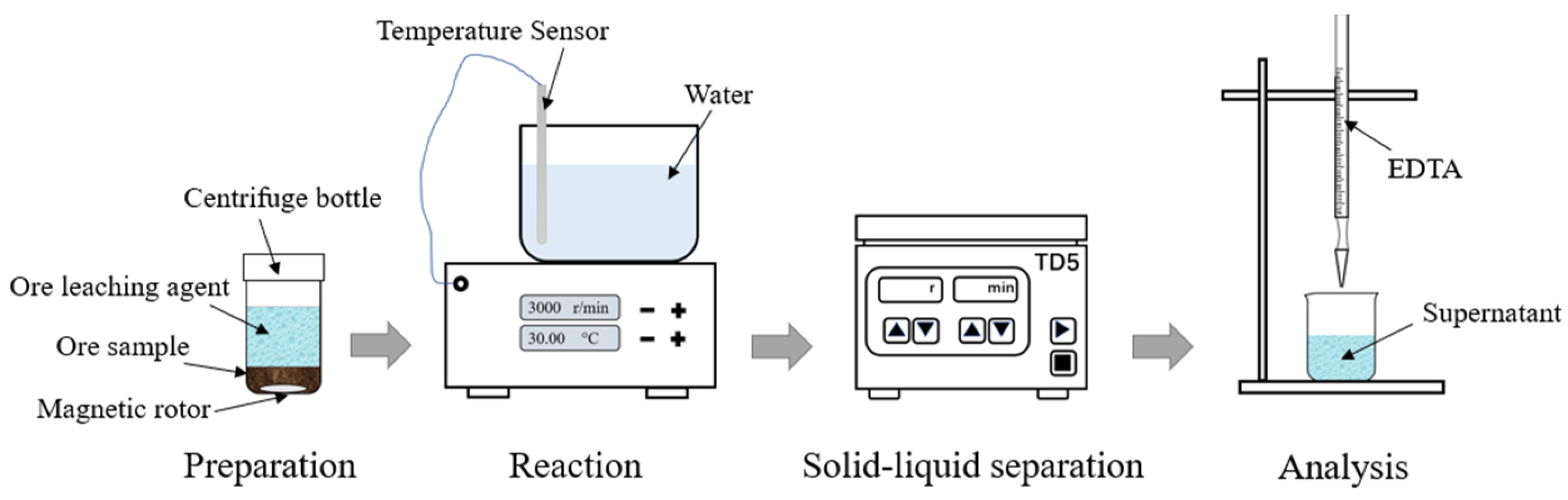



| Type | La2O3 | CeO2 | Pr6O11 | Nd2O3 | Sm2O3 |
| XW sample | 31.57 | 1.02 | 2.41 | 26.32 | 5.29 |
| AY sample | 15.60 | 1.52 | 1.04 | 11.47 | 2.65 |
| Type | Eu2O3 | Gd2O3 | Tb4O7 | Dy2O3 | Ho2O3 |
| XW sample | 0.43 | 4.16 | 0.26 | 2.57 | 0.44 |
| AY sample | 0.56 | 4.19 | 0.38 | 4.41 | 0.85 |
| Type | Er2O3 | Tm2O3 | Yb2O3 | Lu2O3 | Y2O3 |
| XW sample | 1.12 | 0.14 | 0.77 | 0.09 | 23.43 |
| AY sample | 2.38 | 0.29 | 1.79 | 0.25 | 52.57 |
| Type | Leaching Agent | Proposed Model | Kerr Model | ||||
|---|---|---|---|---|---|---|---|
| K/(L2/kg2) | α/(L/g) | β/(L/kg) | ξ/% | Kk/(L2/kg2) | ξ/% | ||
| XW sample | (NH4)2SO4 | 4.18 × 10−1 | 2.10 × 10−5 | 5.04 × 10−3 | 3.53 | 3.91 × 10−1 | 4.68 |
| NH4Cl | 5.16 × 10−2 | 6.57 × 10−1 | 1.00 × 10−7 | 5.43 | 5.16 × 10−2 | 5.42 | |
| AY sample | (NH4)2SO4 | 3.03 × 10−2 | 1.25 × 10−1 | 2.75 × 10−2 | 2.08 | 1.95 × 10−2 | 7.83 |
| NH4Cl | 5.17 × 10−3 | 9.86 × 10−6 | 2.58 × 10−3 | 4.83 | 4.81 × 10−3 | 5.57 | |
| Analyte | O | Na | Mg | Al | Si | K |
| XW sample | 31.748 | 0.424 | 0.172 | 9.507 | 31.367 | 3.373 |
| AY sample | 30.390 | 0.063 | 0.342 | 12.658 | 26.134 | 3.716 |
| Analyte | Ca | Ti | Mn | Fe | Cu | Zn |
| XW sample | 0.076 | 0.180 | 0.026 | 0.988 | 0.004 | 0.008 |
| AY sample | 0.009 | 0.473 | 0.056 | 3.913 | 0.005 | 0.008 |
| Type | Illite | Kaolinite |
|---|---|---|
| XW sample | 0.237 | 0.012 |
| AY sample | 0.177 | 0.135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Long, P.; Wang, G.; Luo, S.; Shi, Y.; Zhang, C.; Lan, X. Ion-Exchange Model for the Leaching Process of Ion-Adsorption-Type Rare-Earth Ores Considering the Influence of Anions. Minerals 2023, 13, 1475. https://doi.org/10.3390/min13121475
Huang Y, Long P, Wang G, Luo S, Shi Y, Zhang C, Lan X. Ion-Exchange Model for the Leaching Process of Ion-Adsorption-Type Rare-Earth Ores Considering the Influence of Anions. Minerals. 2023; 13(12):1475. https://doi.org/10.3390/min13121475
Chicago/Turabian StyleHuang, Ying, Ping Long, Guanshi Wang, Sihai Luo, Yonghui Shi, Chao Zhang, and Xiongdong Lan. 2023. "Ion-Exchange Model for the Leaching Process of Ion-Adsorption-Type Rare-Earth Ores Considering the Influence of Anions" Minerals 13, no. 12: 1475. https://doi.org/10.3390/min13121475
APA StyleHuang, Y., Long, P., Wang, G., Luo, S., Shi, Y., Zhang, C., & Lan, X. (2023). Ion-Exchange Model for the Leaching Process of Ion-Adsorption-Type Rare-Earth Ores Considering the Influence of Anions. Minerals, 13(12), 1475. https://doi.org/10.3390/min13121475







