1. Introduction
In recent decades, attention to rare-earth mineral resources has increased worldwide [
1,
2]. The growing demand for green technologies has led to the large-scale application of rare-earth elements in advanced electronic products and increased the demand for rare-earth elements in the world’s major economies, including the United States, the European Union, the United Kingdom, and Japan [
3,
4], with an estimated annual growth rate of 3.7% to 8.6% [
5,
6]. Deposits of high-grade rare-earth elements continue to be depleted. China’s reserves have declined significantly over the past 50 years, despite being the world’s richest country in rare-earth resources [
6]. Therefore, there is an urgent need to exploit practical technology to recover rare-earth resources effectively from large quantities of rare-earth tailings with a high content [
7].
Bayan Obo tailing pond, one of the world’s largest rare-earth tailing ponds [
8], contains tailings generated since the 1960s, at the rate of 386,000 tpa [
9,
10,
11,
12]. Currently, the tailing pond covers approximately 12 km
2 and stores approximately 200 million tons of tailings [
13,
14,
15,
16,
17]. Tailings contain a large amount of iron, rare-earth elements, fluorine, Niobium, and other elements, which are a valuable secondary resource [
18,
19,
20]. However, reprocessing tailings with methods such as recycling is difficult because of their low grade, fine particle sizes, and complex mineralogy among other factors [
21]. Currently, the utilization of Bayan Obo tailings is still in the laboratory research stage. The main treatment options include physical separation, as well as hydrometallurgical and pyrometallurgical treatments [
6,
15,
20,
22,
23,
24,
25,
26,
27]. In a typical Bayan Obo tailing treatment process, physical operations such as grinding, screening, magnetic force, flotation, gravity separation, and density-based separation are used as pretreatments to release and concentrate metallic parts (MFs) and non-metallic fractions (NMFs) [
28].
The effective utilization of tailings and the optimal processing method depend on the mineral species, correlations and interlocking between minerals, and metal distribution characteristics [
29]. The process mineralogy of tailings helps to provide essential information for recovery research [
28,
30]. Therefore, it is necessary to study the process mineralogy of tailings.
Process mineralogy is a widely accepted integrated discipline that combines quantitative (and qualitative) mineralogy and metallurgy [
31,
32,
33]. The aim is to optimize metallurgical processes, reduce operating costs, and improve metal recovery through the comprehensive characterization of precursor ore mineralogy, mineral assemblages, grain size, and texture. Therefore, process mineralogy is commonly applied to grade and recovery optimization in the working process and has been applied to many deposit types, including rare-earth elements.
This study aims to contribute to the current knowledge by providing detailed characteristics of the occurrence states of valuable elements in tailings and their associations with physical enrichment and hydrometallurgical extraction, which may be a possible option for processing these resources. To fully understand the characteristics of the tailings, advanced characterization methods such as MLA, EPMA, XRD, and ICP were used in this study to analyze the process parameters of rare-earth ore, including element content, mineral composition, the occurrence state of elements, mineral particle size distribution, mineral combination, and deionization. The possible combination of Sc in the minerals was obtained by combining the DFT calculation results. Detailed basic theoretical data are provided for further improvement in the relevant beneficiation process, the recovery of other valuable minerals, and the comprehensive utilization of tailings.
2. Materials and Reagents
Bayan Obo rare-earth tailings, provided by Baotou Iron and Steel (Group) Co., Ltd. of China (Baotou, Inner Mongolia, China), were sourced from the tailing pond of Bayan Obo and homogenized. Subsequently, 100 g dried rare-earth tailing sample was screened into different size fractions, namely +200 mesh, 200–250 mesh, 250–325 mesh, 325–400 mesh, 400–500 mesh, and −500 mesh, and placed in an oven at 105 °C for 48 h to reduce the error caused by the moisture in the sample. All dried samples were weighed and stored in zip-lock bags for later use.
Mineral phase analysis of the dried samples, which were ground to less than 250 mesh, was performed using an X-ray powder diffractometer (D8 Advance, Brock AXS GmbH, Bruker, Billerica, MA, USA). The operating conditions were Cu Kα radiation of λ = 1.54060 Å as the excitation source, with 40 kV accelerating voltage, 40 mA current, a constant step of 0.019°, and a counting time of 1 s for each step for 2-theta, varying from 5° to 90°. The diffraction pattern was analyzed using the X Pert HighScore plus analysis software (version 5.1, Malvern Panalytical, Malvern, UK) equipped with the International Centre for Diffraction Data (ICDD) database.
The particle size distribution of Bayan Obo rare-earth tailings was analyzed using a Malvern laser particle size analyzer (Mastersizer 3000, Malvery Instruments Ltd., Alemlo, The Netherlands), which can accurately quantify the morphological characteristics of non-spherical and non-smooth particles by processing the optical signals of different detection windows and obtaining the fractal dimension of sample particles for measurement to describe the effectiveness of the space occupied by the most complex shapes. The powdered sample was added to 50 mL of deionized water and placed in an ultrasonic instrument (20 kW) for 30 s. A 500 mL measuring cup filled with 2/3 deionized water was placed in the analyzer. Subsequently, the fully dispersed samples were slowly added to the measuring cup. The working parameters were as follows: red light was the main light source, blue light was the auxiliary light source, and the injection stirring speed was 3000 rpm.
The dried 1.0 g mineral powder was placed into a cylindrical plastic mold with a diameter of 30 mm. Subsequently, a 10:1 mixture of epoxy resin and curing agent was mixed with the sample, followed by rapid agitation for approximately 3 min to remove air. Subsequently, the processed mold was placed in the oven at 35 °C for 8 h to obtain the MLA resin sample. The curing sample was fixed in the polisher (MP-2G100A) purchased from Shandong Honghong Instrument Equipment Co., Ltd., with 25 N of polishing strength and 500 r/min of revolving speed to make the cut surface of the mineral smooth enough after 600, 800, 1200, 1500, and 2000 abrasive paper in turn. The polished samples were cleaned with distilled water inside a microwave ultrasonic cleaner for 5 min, removed, dried, and used for MLA and EPMA analyses.
Further mineralogical characterization, such as the association and distribution of each mineral and element in Bayan Obo rare-earth tailings, was performed using a Type-250 automatic mineral liberation analyzer (MLA) (FEI Company, Hillsboro, OR, USA). The analyzer was equipped with a suit of back-scattered scanning electron microscopy (SEM) system, an energy-dispersive spectrometry (EDS) probe, and a suit of MLA software. The operation conditions were as follows: the working distance was 11.6 mm, the frame resolution was 500 pixels, the probe current was 10 nA, and the electron beam accelerating voltage was 25 kV.
To accurately obtain the chemical composition of trace mineral elements, the polished samples were analyzed with a JEOL JXA-iSP-100 Electron Probe Microanalyzer equipped with five wavelength-dispersive spectrometers (WDSs) at the Laboratory of Guangzhou Tuoyan Analytical Technology Co., Ltd., in Guangdong Province, China. The operating conditions for the quantitative WDS analysis included an accelerating voltage of 15 kV, a probe beam current of 10 nA, and a 5–10 µm beam spot size. The peak counting time was 10 s for F, K, P, Na, Ca, and Fe and 20 s for Al, Si, Mg, Mn, Ti, S, and Cl. The background counting time was half the peak counting time for the high- and low-energy background positions. The following standards were used: BaF2(F), quartz (Si), olivine (Mg), orthoclase (K), BaSO4(S), TiO2(Ti), Fe3O4(Fe), MnO2(Mn), albite (Na), spodumene (Al), NaCl(Cl), and apatite (Ca and P).
The precise quantification of trace elements, such as Sc, in Bayan Obo rare-earth tailings was performed using an inductively coupled plasma emission spectrometer (ICP-OES, PQ9000, Analytik Jena GmbH, Jena, Germany). The solid tailing samples were dissolved in the solution and prepared according to the following steps:
First, 0.2 g of solid dried sample was mixed with 6 mL of HCl solution, 2 mL of HNO3 solution, and 2 mL of HF solution, and the mixture was then placed into a Teflon microwave digestion tank.
The tank was sealed and placed in a digestion instrument (WX-8000, Preekem Scientific Instruments Co., Ltd., Shanghai, China), and digestion was conducted according to the procedure, with the holding temperature set at 120 °C for 3 min under 15 bar pressure, 150 °C for 3 min under 20 bar pressure, 180 °C for 3 min under 30 bar pressure, 200 °C for 3 min under 40 bar pressure, and 220 ℃ for 45 min under 45 bar pressure.
The tank was removed after cooling and lowering pressure, and then the opened tank was heated to 160 °C, the solvent was evaporated to the size of a soybean (~0.25 mL), and finally the solution was moved to the volumetric bottle for the ICP-OES test.
Epoxy resin, curing agent (ethanediamine, NH2CH2CH2NH2), and single-crystal diamond polishing solution (0.5 μm) were analytically pure products and were purchased from Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China.
3. Results and Discussion
3.1. Composition Analysis of Bayan Obo Rare-Earth Tailings
3.1.1. Chemical Composition
The major element compositions of Bayan Obo rare-earth tailings are listed in
Table 1. The main components of the tailings are CaO, Fe
2O
3, SiO
2, and F, accounting for 30.50 wt%, 20.40 wt%, 13.10 wt%, and 14.20 wt%, respectively. The total rare-earth oxides, including La
2O
3, CeO
2, and Nd
2O
3, exceed 2.5 wt%. Because of their high prices, 0.11% of Nb and 137.91 ppm of Sc are the most valuable elements in tailings. The total content of the main impurities, such as MnO, Al
2O
3, MgO, Na
2O, and SiO
2, is greater than 20 wt%, which seriously affects the grade of fluorite and iron concentrates and increases the cost of purification.
3.1.2. Mineral Constituent and Particle Size Distribution
To comprehensively assess the residual value of tailings, it is necessary to know their mineral compositions and their distribution across particle sizes. Mineral composition determines the valuable components of the tailings and the particle size distribution is a proxy for the degree of dissociation of the target minerals. These factors determine the selection of beneficiation methods.
The XRD analysis results of Bayan Obo rare-earth tailings, shown in
Figure 1, reveal that the main mineral phases are bastnasite ((Ce, La)[CO
3]F), quartz (SiO
2), biotite (K
2Mg
6(Al
2Si
6O
20)(OH)
4), aegirine (NaFeSi
2O
6), riebeckite (AB
2C
5T
8O
22W
2, A = Na, K, Ca, Pb, and Li; B = Na, Ca, Mn
2+, Fe
2+, Mg, and Li; C = Mg, Fe
2+, Mn
2+, Al, Fe
3+, Ti
4+, and Li; T = Si, Al, Ti
4+, and Be; W = (OH), F, Cl, and O
2−), barite (BaSO
4), hematite (Fe
2O
3), pyrite (FeS
2), and fluorite (CaF
2). Although XRD is an important means of characterizing mineral phases, it can determine the existence of a phase but not its absence. The limit of detection (LOD) of XRD is influenced by many factors, such as dispersion, crystallinity, and the type of substance being detected. XRD can usually detect mineral phases with more than 5% content, indicating that the tailings contain at least one of the phases shown in
Figure 1. Furthermore, different mineral formation environments result in differences in the elemental content of the same mineral, thus changing the XRD spectrum peaks, and the ICDD database does not contain all spectral peaks. Also, XRD cannot determine the specific elemental content in a mineral. Additional detection methods, such as EPMA and MLA, are required to detect the minerals contained in tailings and the content of each element in them.
The particle size distribution of Bayan Obo rare-earth tailings is shown in
Figure 2, where only one asymmetrical peak is observed. All particles are above 500 μm, but the content in the range of 0.70–140.52 μm is 90%, and that in the range of 0.70–42.82 μm is 50%, indicating that the tailings are dominated by fine tailings.
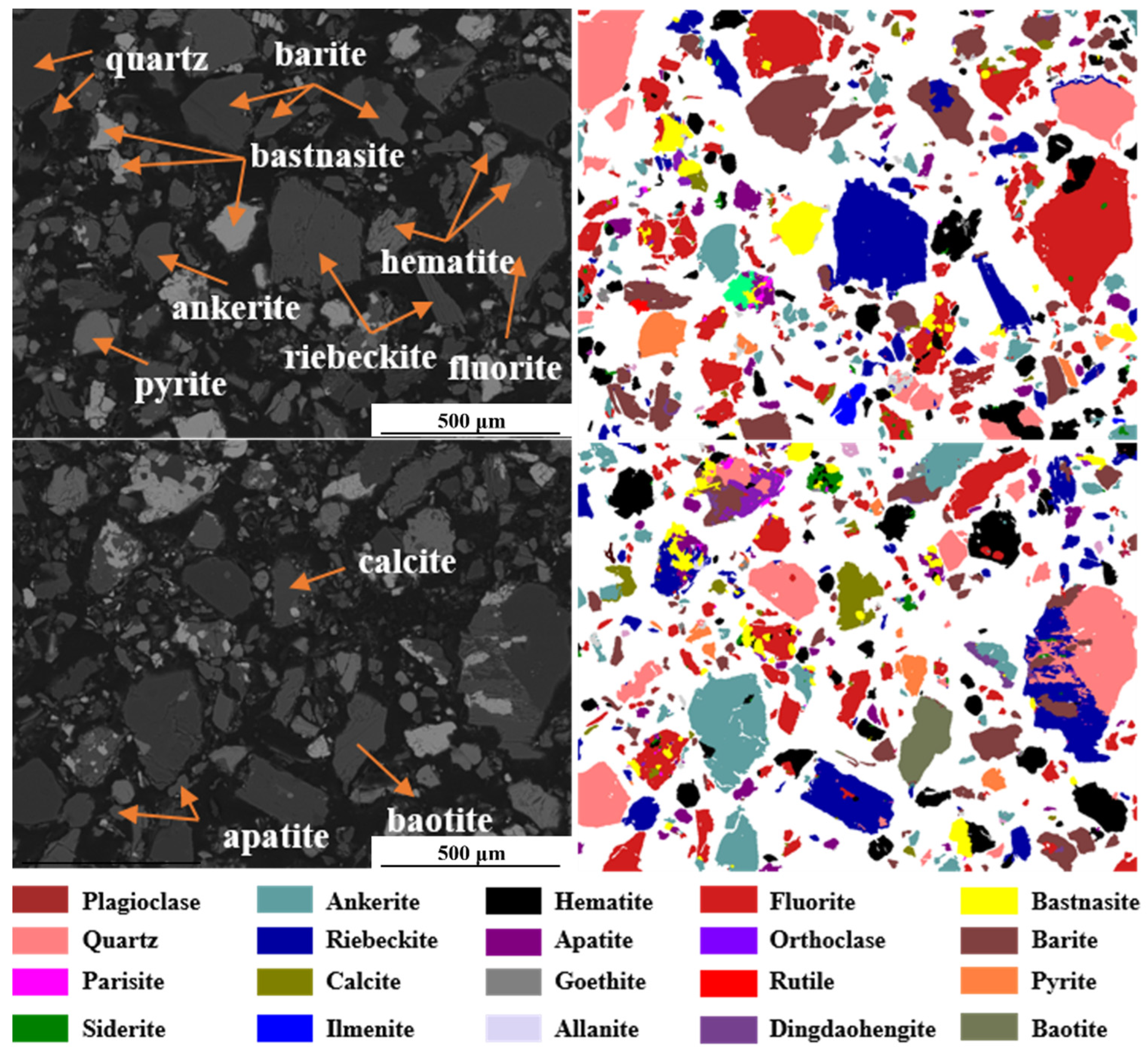
The mineral compositions and contents of the tailings are shown in
Figure 3. The iron minerals in the tailings are mainly hematite, siderite, ankerite, and pyrite, with contents of 11.8%, 1.7%, 8.5%, and 2.0%, respectively. The main rare-earth minerals are bastnasite, apatite, and monazite, with contents of 5.0%, 5.0%, and 1.6%, respectively. The silicate minerals are mainly biotite, riebeckite, and small quantities of albite and phlogopite, with contents of 1.2%, 15.5%, 0.7%, and 0.7%, respectively. The mineral with the highest content in the tailings is fluorite, and there are still large amounts of quartz and barite, with contents of 26.8%, 6.5%, and 5.6%, respectively. Both riebeckite and aegirine are single-chain silicate minerals with similar structures and similar contrast imaging under back-scattered electrons (BSEs). Therefore, they were classified into one category in this study. MLA images of Bayan Obo rare-earth tailings are shown in
Figure 4, which is a detailed visual demonstration of
Figure 3.
3.1.3. Distribution of Valuable Elements in Minerals
The valuable elements in Bayan Obo tailings include F, REE, Fe, Sc, and Nb. Therefore, the occurrence states of these elements in tailings are discussed in this study.
Iron in Bayan Obo tailings mainly occurs in the form of independent minerals, such as hematite, riebeckite, ankerite, siderite, and pyrite, with contents of 50.15 wt%, 27.94 wt%, 8.34 wt%, 4.92 wt%, and 5.59 wt%, respectively. The majority of the F was found in fluorite (97.20%). However, it was also found in bastnasite in trace amounts. Bayan Obo rare-earth deposits mainly consist of light rare-earth elements. In this study, the distributions of La and Ce were used instead of the distribution of rare earth. Cerium mainly occurs in dingdaohengite, monazite, allanite, bastnasite, and wollastonite, with contents of 6.52 wt%, 14.11 wt%, 3.87 wt%, 72.78 wt%, and 2.72 wt%, respectively. Lanthanum mainly occurs in monazite, bastnasite, and wollastonite, with contents of 42.17 wt%, 56.03 wt%, and 1.8 wt%, respectively. Notably, 86.89% of La and 98.20% of Ce in Bayan Obo rare-earth tailings occur in bastnasite and monazite, respectively. Therefore, the states of bastnasite and monazite as the main minerals of rare-earth occurrence are discussed later. Niobium was primarily found in nioboaeschynite (48.47 wt%), pyrochlore (21.70 wt%), dingdaohengite (10.34 wt%), and ilmenorutile (10.28 wt%), and also in baotite in trace amounts, as shown in
Table 2.
The results of the MLA software analysis were based on the qualitative mineral composition of the EDS scans. Regarding the low precision of EDS, which has a detection limit of about 0.5%, Sc has no independent mineral in the Bayan Obo ore and only hosts in the lattice of other minerals in the form of homomorphism, and its content is low. Thus, the analytical detection means of EDS and MLA cannot be used to study the occurrence state of Sc.
An electron probe microanalyzer (EPMA) uses a focused, high-energy electron beam to bombard the solid sample surface and stimulate the characteristic X-rays and a variety of electronic signals (such as secondary electrons and backscattered electrons) to perform qualitative and quantitative analyses of the elements in situ and morphological observations of the sample. It is the most basic in situ microbeam microzone analysis instrument used in the fields of mineralogy, petrology, geochemistry, mineral deposit science, comparative planetology, and materials science. An EPMA, with an analytical region of less than 1 μm and a detection limit of approximately 100 ppm, was used to determine the occurrence of Sc in various minerals in Bayan Obo tailings.
The EPMA test results for the six silicate minerals are presented in
Table 3. The presence of Sc was detected only in chain silicates (aegirine and riebeckite), in which SiO
4 tetrahedrons are connected by sharing oxygen to form a continuous chain, and they are absent in silicates with other structures. Although some EPMA data are not zero, the values’ reliability is deemed low because they are less than the detection limit. Therefore, they were not adopted in this paper.
The content of Sc
2O
3 in aegirine was 0%–0.156%, and the average of the 19 samples was 0.037%. The chemical formula of aegirine was calculated according to the average content of each element as follows: Na
0.948Ca
0.097Mg
0.038Al
0.031Sc
0.001Fe
3+0.885Si
2O
5.992F
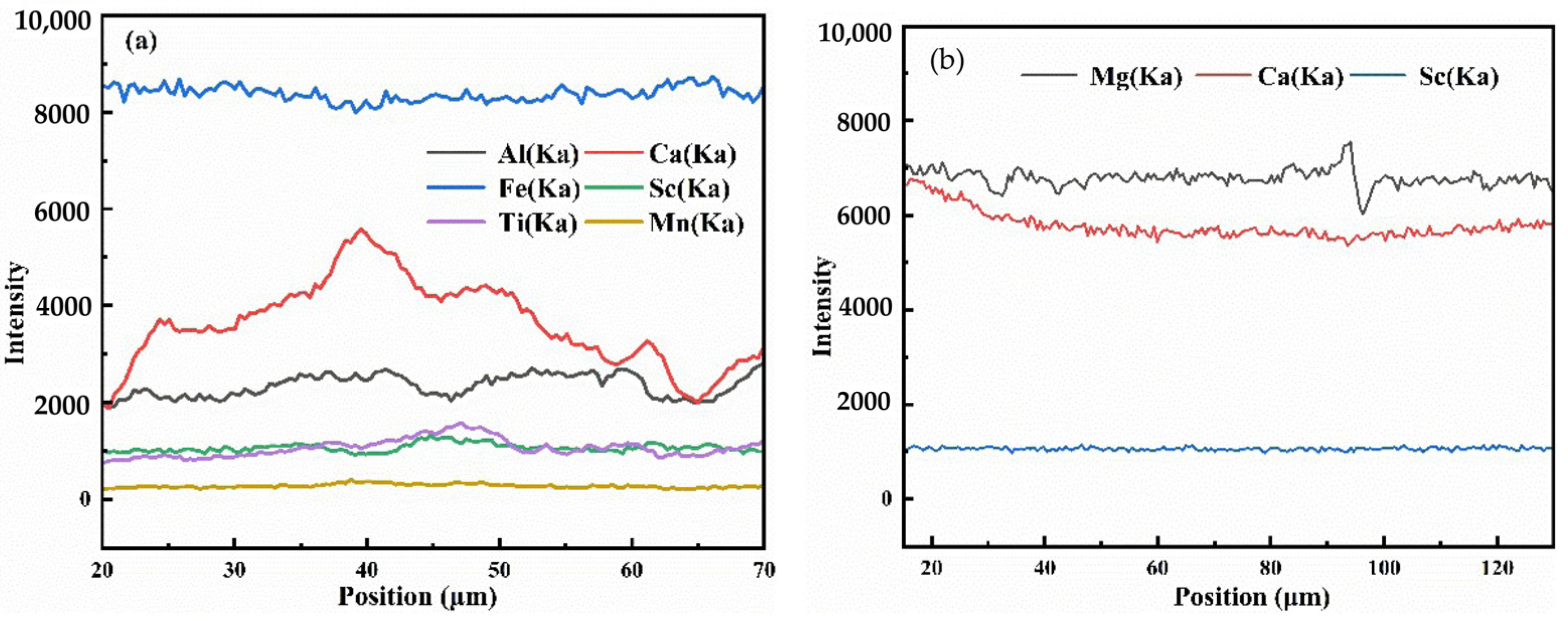
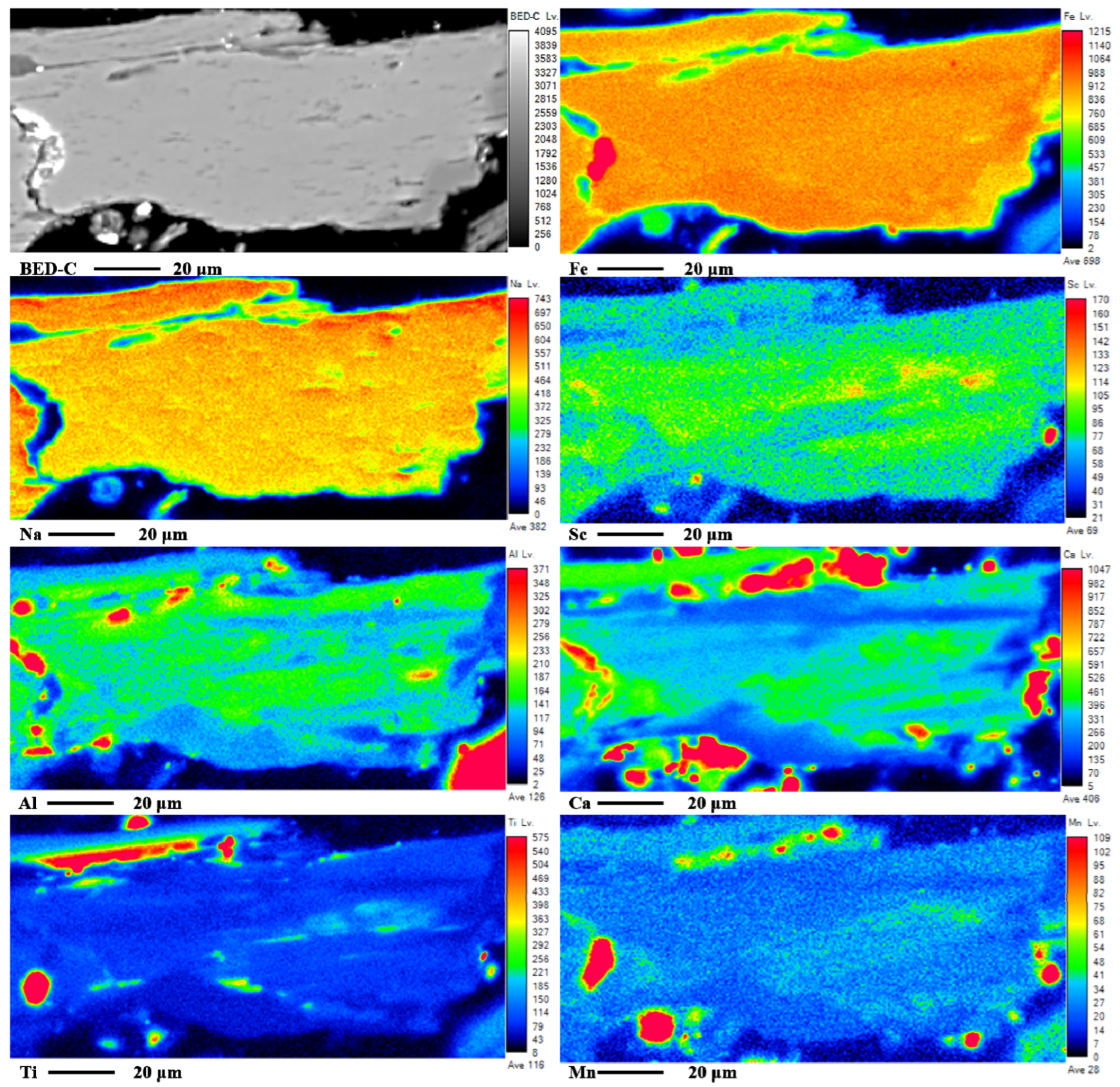
0.016. The distribution of Sc in aegirine was investigated using EPMA line scanning and mapping, as shown in
Figure 5a and
Figure 6. Sc is uniformly distributed in the individual aegirine spots, and the difference in composition is mainly due to the different aegirine spots.
The content of Sc2O3 in riebeckite was 0%–2.298%, and the average of the 21 samples was 0.222%. The chemical formula of riebeckite was calculated based on the average content of each element as follows:
(Ca0.83Na0.104K0.369Mn0.302)Na2(Fe0.3853+Fe1.3932+Mg2.935Al0.073Sc0.029Ti0.004)[Si8O22](OH)0.852F1.148.
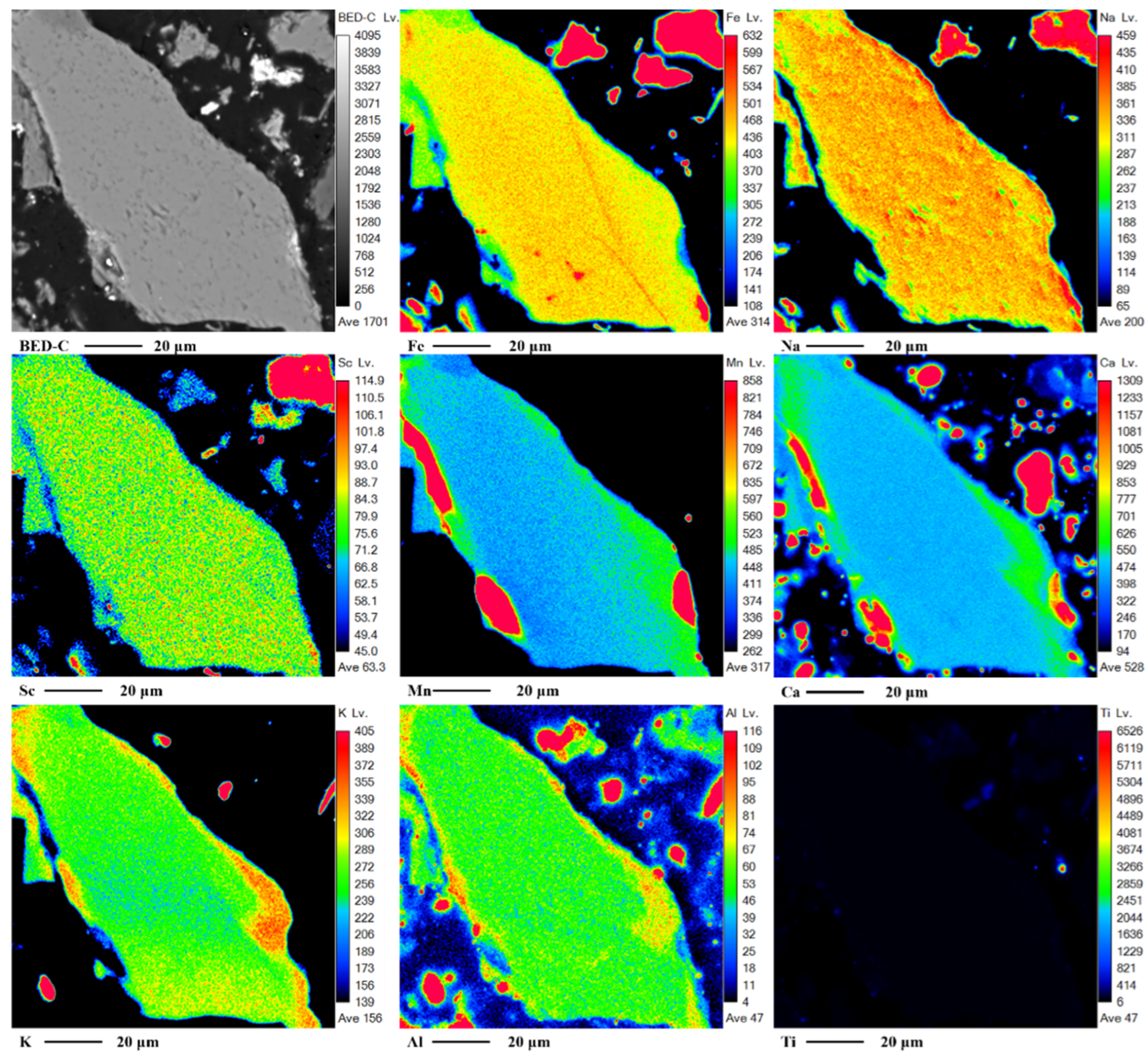
The distribution of Sc in riebeckite was investigated using EPMA line scanning and mapping, as shown in
Figure 5b and
Figure 7. The distribution of Sc in riebeckite is similar to that in aegirine. This may be because the ionic radius of Sc is similar to that of metal ions such as Fe
3+ and Al
3+. Therefore, there is a possible competitive relationship in the formation of minerals. Scandium is in contact with various fluorine-bearing minerals during high-temperature and high-pressure geological activities, resulting in the formation and disruption of complex fluoride of Sc. In the cooling process, F combines with calcium ions to form fluorite (CaF
2) precipitates, whereas Sc forms a solid solution of aegirine and riebeckite with metal ions such as Na
+ and Fe
3+. This could be the reason why Sc exists uniformly in aegirine and riebeckite and Sc-bearing minerals are associated with fluorite [
34]. The complex of aluminum and fluorine ions is also stable during the formation of silicates with high Al content, such as feldspar and mica. However, unlike Sc, aluminum has tetrahedral coordination and can easily enter feldspar and mica. This may explain why Sc does not exist in feldspar and mica.
The EPMA test results for the four rare-earth minerals are listed in
Table 4. Among them, only monazite contained Sc, and the content of Sc
2O
3 was 0%–0.152%. The chemical formula of monazite was calculated according to the average content of each element as follows: (Eu
0.001Sm
0.013Er
0.001Y
0.002Ce
0.495Nd
0.197Th
0.008La
0.244Pr
0.046Gd
0.002Sc
0.002)PO
4. The total value of the highest content of each element was 73.78% higher than that of the lowest content because the contents of the major elements La, Ce, Nd, and Pr were significantly different, with 353.75%, 36.78%, 187.93, and 74.90%, respectively. This finding may be attributed to the presence of two types of monazite. However, the content of Sc
2O
3 in both types of monazite did not seem to have a significant effect.
The EPMA test results for the eight iron-bearing minerals are listed in
Table 5. Among these, Sc
2O
3 was detected in two types of Nb-bearing minerals, namely ilmenorutile and niobite, with contents of 0%–0.122% and 0.575%–2.112%, respectively. The presence of Sc appears to be strongly related to Nd, which is consistent with Yang’s result [
35]. The chemical formula of niobite was calculated according to the average content of each element as follows: (Fe
0.59Mn
0.38Sc
0.02)Nb
2O
6. The chemical formula of ilmenorutile could not be obtained because of its complex composition. Among the other iron-bearing minerals, only one ilmenite sample contained Sc, and its EPMA results were 0.335% MgO, 0.305% Nb
2O
5, 3.821% MnO, 44.307% FeO, 50.611% TiO
2, 0.146% V
2O
3, and 0.038% Sc
2O
3. The composition, except for Sc
2O
3, was not significantly different from other ilmenite samples. Therefore, we speculated that there was no Sc
2O
3 in ilmenite, which is consistent with Yang’s result [
35].
The EPMA test results for fluorite and calcite are listed in
Table 6. No Sc
2O
3 was detected in these two minerals. In this study, the mineral with the highest Sc content in Bayan Obo tailings was niobite, with a content of 1.58%, and the highest content of a single point was riebeckite, with a content of 2.30%. The content of Sc in different minerals and different samples of the same mineral varied greatly, and the content of Sc
2O
3 was less than 0.10% in most samples. Only five minerals were confirmed to contain Sc, and the content of Sc
2O
3 in descending order was niobite, riebeckite, ilmenorutile, monazite, and aegirine. In conclusion, very low amounts of Sc are dispersed in the Bayan Obo deposit, which is consistent with previous findings. However, the results differ from those of previous studies in the following points: (1) Our result regarding niobite is consistent with previous studies, showing a relative richness of Sc. However, the average Sc content was found to be 1.58%, which is higher than that measured by predecessors (0.22%, 0.67%, and 1.26%) [
36]. (2) Previous researchers found ilmenorutile to have the highest scandium content [
37].
However, the average scandium content in ilmenorutile was only 0.06% in this work, whereas Yang did not detect the presence of Sc in ilmenorutile [
35]. (3) Aegirine was considered to be the richest Sc among the silicates [
38], but the Sc content of riebeckite (0.22%) was much higher than that of aegirine (0.037%) in this study. (4) For the same sample, the content of Sc
2O
3 was uniformly distributed, which was not observed in a previous study. This may be related to the conditions of mineral formation.
3.2. Grain Size Distribution of Valuable Minerals
An important factor in mineral flotation is the particle size, and −75 μm is generally taken as a reference. Therefore, with 75 μm as the dividing line, the proportions of fluorite, ilmenorutile, nioboaeschynite, bastnasite, dingdaohengite, monazite, riebeckite, and hematite below 75 μm are 59.11%, 74.4%, 73.38%, 85.50%, 89.04%, 86.56%, 65.08, and 85.62%, respectively. All pyrochlore particles are below −75 μm, and their specific distributions are listed in
Table 7 and
Figure 8. Due to the large particles of Fe- and F-bearing minerals, further grinding is required to recover iron and fluorine via flotation. The proportions of fluorite, ilmenorutile, pyrochlore nioboaeschynite, bastnasite, dingdaohengite, monazite, riebeckite, and hematite below 20 μm are 9.20%, 21.68%, 32.33%, 17.26%, 16.82%, 11.48%, 19.79, 6.45%, and 13.05%, respectively. The proportions of ilmenorutile, pyrochlore, and nioboaeschynite below 10 μm are 6.35%, 12.14%, and 6.66%, respectively. For physical beneficiation, minerals with particle size greater than 10 μm are easy to recover, while minerals with particle size in the range of 5–10 μm are difficult to recover, and the mineral particle size is less than 5 μm, which belongs to mud ore and is extremely difficult to recover. Therefore, Niobium-bearing minerals are generally fine, which seriously affects the recovery of niobium using flotation.
3.3. The Main Valuable Minerals’ Association
The free-exposed surface area method was used to analyze the association of monomer minerals, and the results are shown in
Table 8. Overall, 79.39% of the fluorite was liberated, and non-liberated and partially liberated fluorite contents were closely associated with bastnasite, monazite, barite, riebeckite, hematite, siderite, ankerite, and calcite with associations of 5.32%, 1.01%, 1.38%, 3.39%, 0.74%, 1.68%, 2.28%, and 0.99%, respectively. Hence, 6.33% of the fluorite was associated with rare-earth minerals, and 8.09% of fluorite was dispersed in iron-bearing minerals. The flotation of fluorite significantly influenced the recovery of Fe and rare-earth elements but had minimal influence on the recovery of Nb and Sc. Therefore, further grinding was needed to reduce the association with other minerals and increase the degree of liberation.
Monazite and bastnasite were liberated at 54.14% and 47.85%, respectively. Only 4.65% of the monazite and 7.02% of the bastnasite were associated with the REE-bearing mineral apatite, which could be floated and recovered together with monazite. Notably, 8.56% of monazite and 6.59% of bastnasite were associated with silicate minerals, and 16.64% and 15.05% of monazite were dispersed in fluorite and Fe-bearing minerals, respectively. In addition, 24.36% and 10.78% of bastnasite were dispersed in fluorite and Fe-bearing minerals, respectively. Hence, fluorite and Fe-bearing minerals significantly affected the flotation of monazite and bastnasite. However, bastnasite and monazite with apatite should be extracted separately. Since scandium is mainly present in silicate minerals, 1.27% of riebeckite is bonded with fluorite, so the flotation of fluorite has little influence on the recovery of Sc, and scandium mainly exists in flotation tailings. Nb-bearing minerals mainly include nioboaeschynite, pyrochlore, and ilmenorutile, which are 10.19%, 16.59%, and 11.75% bonded with fluorite, respectively. Therefore, the flotation of fluorite has a certain impact on the recovery of Nb, and part of Nb enters the flotation concentrate.
Fluorite, barite, apatite, bastnasite, and quartz were associated with 9.36%, 1.19%, 1.35%, 2.17%, and 2.37% of hematite, respectively, affecting the grade of the Fe concentrate. Hematite (6.19%) was dispersed in other Fe-bearing minerals, such as riebeckite, siderite, and ankerite. The extraction of hematite had little effect on Sc extraction but did not affect Nb extraction.
The liberation of Nb-bearing minerals, especially pyrochlore and ilmenorutile, was very low, at 38.98 and 45.72, respectively. Only 1.03%, 2.80%, and 5.42% of nioboaeschynite, pyrochlore, and ilmenorutile were associated with other Nb-bearing minerals, respectively. Hence, Nb enrichment was difficult to achieve. The BSE images of Bayan Obo rare-earth tailings are shown in
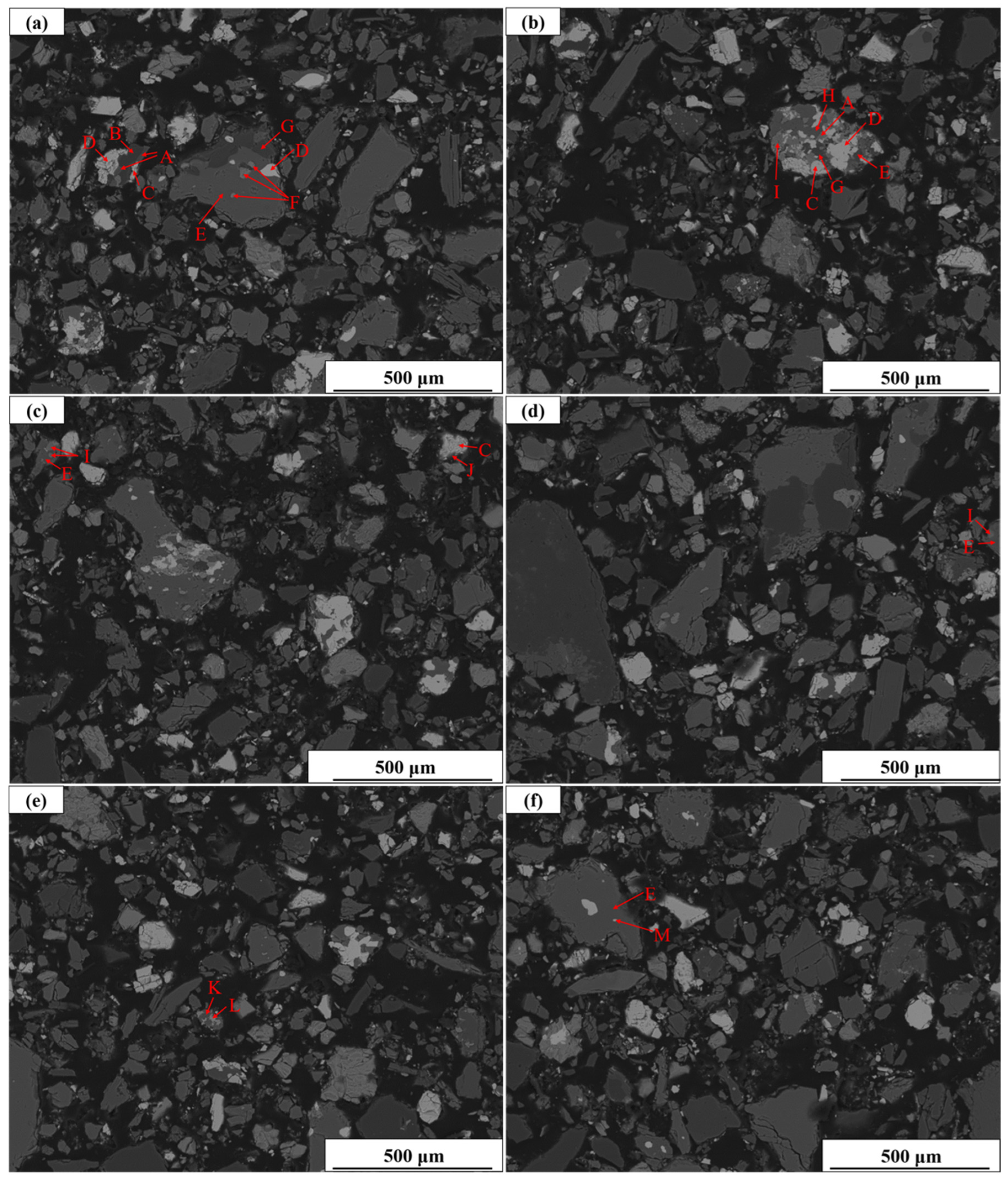
Figure 9, which provide a visual illustration of the complex symbiotic relationships between minerals.
Since both Nb and Sc are partially present in ilmenorutile, Nb enrichment is accompanied by Sc enrichment to a certain extent. In total, 82.67% of the riebeckites are liberated. Therefore, Sc can be enriched by pretreatment. However, the distribution of Sc is highly scattered, and extraction is extremely difficult.
Density functional theory (DFT), which is a universal tool to predict the properties of molecules and condensed matter based on first principles [
39,
40], was used in this work to verify the occurrence state of Sc in aegirine. The simulated computations were conducted with the DFT-based Cambridge Serial Total Energy Package (CASTEP) code of the Materials Studio software (Materials Studio 2016, BIOVIA, San Diego, CA, USA) [
41]. The valence electronic configurations of Na, Fe, O, Si, Mg, and Sc were [Ne]3s
1, [Ar]3d
8, [He]2s
22p
4, [Ne]3S
23p
2, [Ne]3s
2, and [Ar]3d
14s
2, respectively. The generalized gradient approximation with Perdew–Burke–Ernzerhof (GGA-PBE) was selected to calculate the exchange and correlation energies of electron–electron interactions with 400 eV of cut-off energy [
42,
43,
44,
45]. Other parameters were set as 2.0 × 10
−6 eV/atom of energy tolerance, 2 × 2 × 2 grid of Brillouin zone, 0.05 eV/Å of force tolerance, and 0.002 Å of displacement tolerance [
46]. As shown in
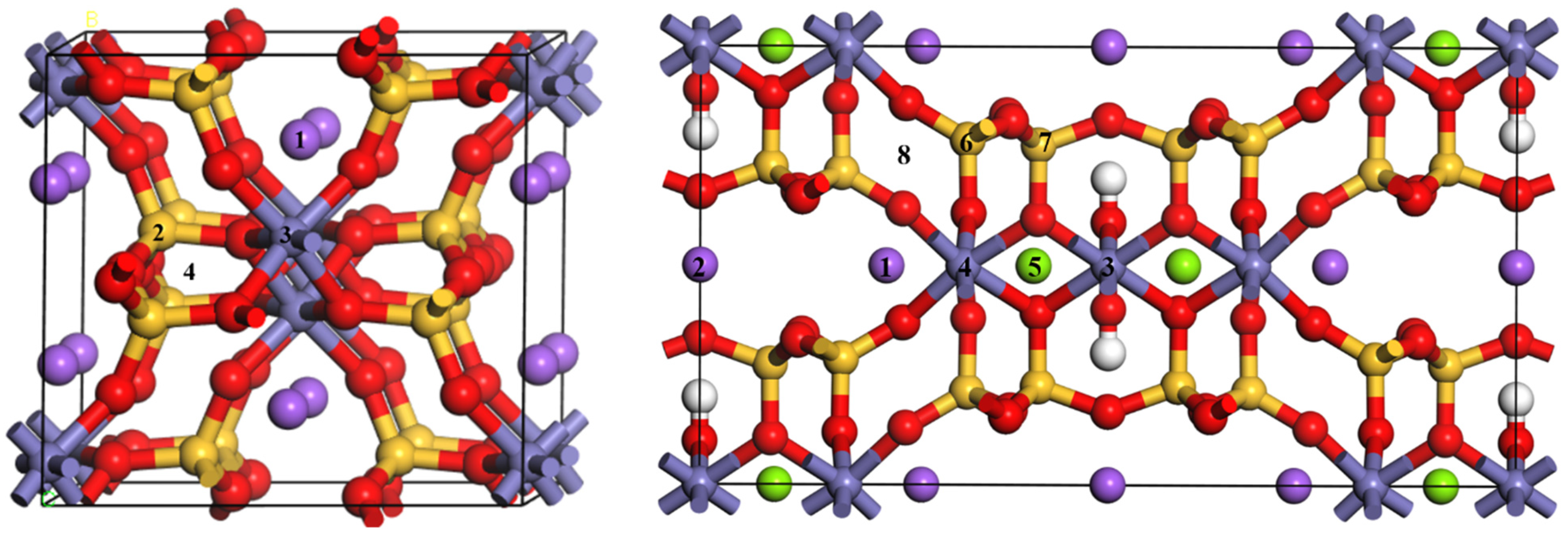
Figure 10, a 1 × 1 × 2 supercell of aegirine and riebeckite is formed, and one Sc atom is substituted with one atom or in the interstitial lattice site. The structures were optimized according to the aforementioned parameters. The structural optimization was deemed complete when these parameters satisfied the convergence standard, and the calculation results were satisfactory.
3.4. Formation of Sc in Aegirine Lattice
The formation energy (Δ
E), which is used to analyze and compare the difficulty of formation with different impurity defects, refers to the energy required for an Sc atom to merge into the aegirine crystal and is calculated using the following formula [
41,
47]:
where
Eimpurity is the energy of aegirine with impurity defects;
Eperfect is the energy of the perfect aegirine;
x is the Na atom, Fe atom, or Si atom in aegirine;
y is the Sc atom of the impurity; and
Ex and
Ey are the energies of the
x atom and
y atom, respectively. In the case of doping in a lattice gap,
Ex = 0 eV. Under the same calculation conditions, the smaller the value of Δ
E, the greater the possibility of the corresponding doping form. The four doping forms that represent the four occurrence states of Sc in aegirine and riebeckite are as follows:
The Crystallographic Information File (cif) of aegirine and riebeckite was downloaded from the American Mineralogist Crystal Structure Database (AMCSD) [
48,
49], and the lattice parameters of aegirine were a = 9.6554 Å, b = 8.7952 Å, c = 10.5884 Å, α = 90°, β = 107.396°, and γ = 90°. After optimization, the lattice parameters were a = 9.5727 Å, b = 8.7007 Å, c = 10.6098 Å, α = 89.999°, β = 108.499°, and γ = 90°, which were very close to the experimental values in the literature [
50,
51]. The formation energies of Reactions (2)–(5) are shown in
Table 9. The smallest of the four formation energies was −8.60 eV of Reaction (5), indicating that the most likely Sc doping occurred in the aegirine interstitial lattice site. In addition, the value of −6.88 eV for Reaction (3) was relatively small, indicating that Sc may also have a high probability of being present in aegirine upon Fe doping. The formation energies of Reactions (2) and (4) were −3.72 eV and 0.03 eV, respectively. This was much higher than those of the other reactions, indicating that Sc had a small chance of substituting Na and Si. Notably, the values of Reactions (2), (3) and (5) were negative, indicating that it was not very difficult for Sc to enter the aegirine cells under normal temperature and pressure.
The lattice parameters of riebeckite were a = 9.860 Å, b = 18.070 Å, c = 5.333 Å, α = 90°, β = 105.48°, and γ = 90°. After optimization, the lattice parameters were a = 9.873 Å, b = 17.735 Å, c = 5.307 Å, α = 90.000°, β = 103.390°, and γ = 90°, which were very close to the experimental values in the literature [
52]. The formation energies of Reactions (6)–(10) are shown in
Table 10. Since Na, Fe, and Si in riebeckite had two different sites each, there were a total of eight alternative sites, as shown in
Figure 10. The formation energies of Reaction (7) in two different Fe sites were −5.05 eV and −6.26 eV, respectively, indicating that Sc may also have a high probability of being present in riebeckite upon Fe doping. The formation energies of Reaction (10) was 1.92 eV, indicating that it was very difficult for Sc doping in the riebeckite interstitial lattice site.
The lattice parameters of different cells containing Sc are listed in
Table 9 and
Table 10. The results showed that the cell volumes of aegirine with Sc for Fe, Si, and the interstitial lattice site were 849.78 Å
3, 851.81 Å
3, and 851.724 Å
3, respectively, which were greater than the 838.015 Å
3 of perfect cell volume. This phenomenon also appeared in the Sc doping of riebeckite. This may be because the ionic radius of Sc
3+ (~81 Å) is larger than those of Fe
3+ (~64 Å) and Si
4+ (~42 Å) [
53]. Therefore, the replacement of Sc for Na reduced the cell volume of aegirine because of the smaller ionic radius of Sc
3+ (~81 Å) than Na
+ (~95 Å).
3.5. Proposed Flowsheet for Comprehensive Utilization of Tailings
A feasible process for the comprehensive utilization of Bayan Obo rare-earth tailings was designed based on the study of process mineralogical characterization, as shown in
Figure 11. The flowsheet includes physical, pyrometallurgical, and hydrometallurgical processes.
Since 97.20% of the F element exists in 26.8% of the fluorite in Bayan Obo rare-earth tailings, the monomer dissociation of fluorite is 79.39%, and the fluorite particles above 53 μm account for 57.67%; therefore, further grinding is needed to reduce the size of the fluorite particles and increase the liberation of the fluorite. The ground pulp is floated to produce a fluorite concentrate and tailings that are enriched in Fe, REE, Nb, and Sc. Because Sc mainly occurs in silicate minerals and some Nb-bearing minerals, in which it exists only in minor amounts, Sc and Nb need further enrichment. Hematite in the fluorite flotation tailings was magnetized (transformed to magnetite) by roasting under reducing conditions. Magnetite was then separated from the tailings via magnetic separation, enriching the residual tailings in REE and Nb. REE in tailings is mainly found in bastnasite, which can be decomposed at low temperatures by dilute acid, whereas minerals containing Sc and Nb are not easily decomposed at low temperatures. Therefore, more than 70% of REE can be recovered via low-temperature acid pickling. Finally, the pickled slag is leached under pressure to obtain a solution containing Nb, Sc, and non-toxic Si slag, which can be used as building materials. The entire process makes full use of valuable elements in tailings and is environmentally friendly.