Abstract
The possibility of extraction of metals from ores of different genesis, containing low-dimensional structures of rare and noble metals, increases their commodity value and, in a deficit for some types of metals, leads to the need to search and develop new nature-like technologies, which can be used to extract from ores of different genesis almost all valuable noble, rare earth and nonferrous metals regardless of their concentration. This article presents the results of studying the processes of comminution and flotation to extract low-dimensional structures of noble and rare metals from carbonaceous ores using low-temperature and energy impacts at successive stages of the ores’ transformation. With the use of modern mineralogical, physical and chemical methods of research of composition, structure and properties of ores, the initial samples, concentrates and tailings after enrichment were studied. During the study, it was established that the difficulty of extraction of strategic metals from carbonaceous hard-enriched ores consists in fine dissemination of valuable components in concentrator minerals, mutual penetration of ore mineralization into each other and into rock-forming minerals, and in proximity of physical, chemical and technological properties of minerals, which complicates selective extraction of valuable components in concentrates. Also, difficulties in enrichment are associated with high flotation activity of waste rock, which significantly reduces the quality of concentrates.
1. Introduction
The depletion of the mineral resource base (including certain types of strategic ores) is a real challenge for the global economy. In order to minimize the negative impact of this process, ongoing research is being conducted to develop technological solutions for involvement in the processing of complex, low-grade and nonconventional ores [1,2,3,4,5,6,7,8]. Examples of such new types of ores are deposits of carbonaceous ores, including black shale, refractory sulfide carbon-bearing ores, impactites, etc., which contain carbon of varying degrees of metamorphism [9,10,11,12]. Carbonaceous ores have several characteristic features that cause problems for their processing on an industrial scale [13,14,15,16,17,18]:
- −
- low grade of valuable components;
- −
- complex ore types and the proximity of the properties of the minerals to be separated;
- −
- fine intergrowth of ore and rock-forming minerals;
- −
- deportment and dimensionality of the valuable component (up to low-dimensional structures), etc.
The extraction of noble and rare metal cluster forms from carbonaceous ores requires a deeper structural transformation of their concentrating minerals than the processing of traditional ores containing finely disseminated valuable components. For the extraction of dispersed forms of noble and rare metals in concentrates, it is necessary to ensure the formation in ore-forming minerals of a system of defective structures of different orders, including those commensurate with the linear parameters of its inclusions. Therefore, various energetic and physical–chemical methods of transformation of ores at successive stages of enrichment should be used for such ore processing. For example, solutions should provide not only the transfer of mineral-forming elements to form oxides or mobile ions but also the chemogenic development of microcracks, pores, dislocations and other even finer defects of the crystal lattices of mineral matrices.
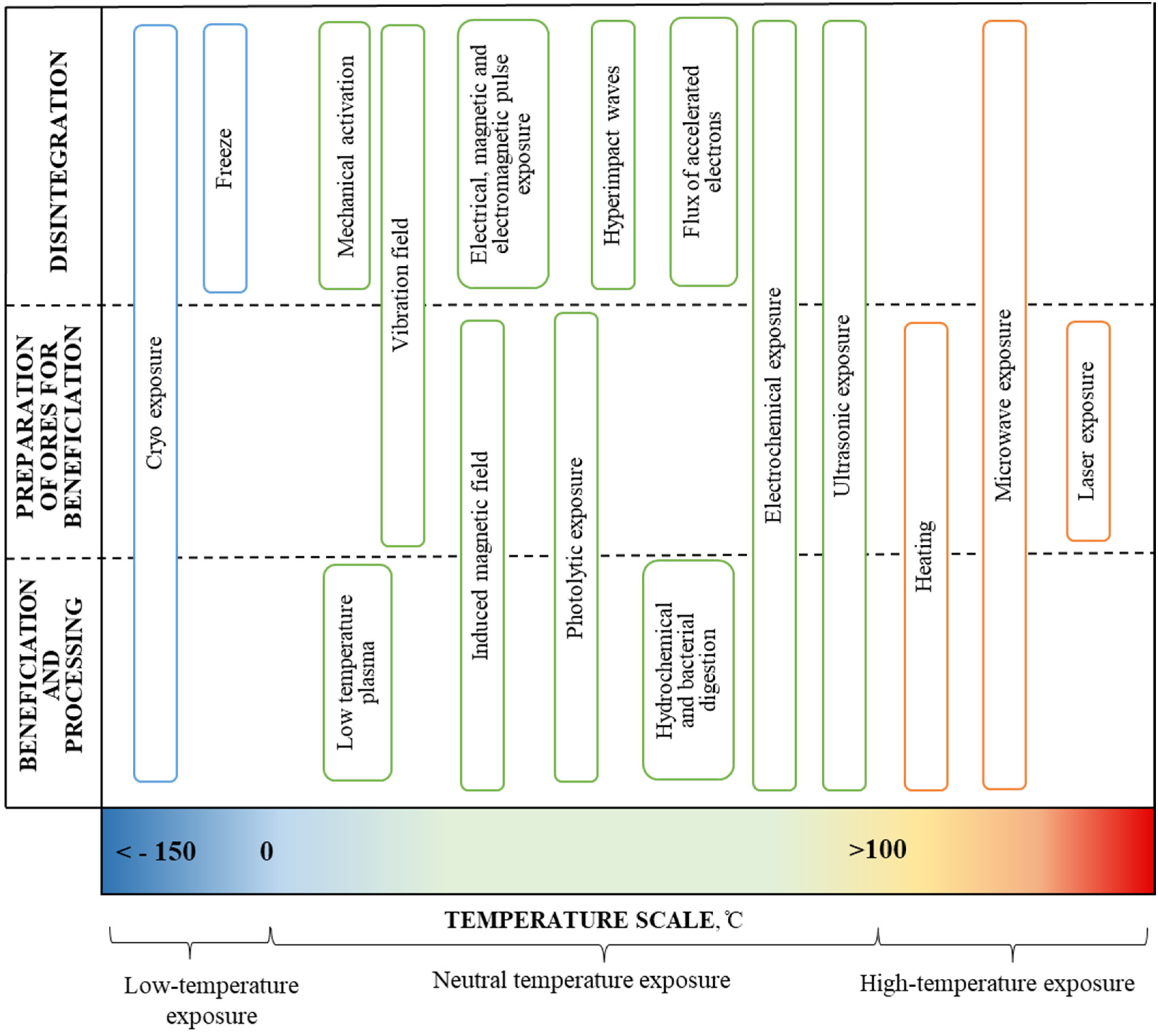
The analysis of ongoing research both in Russia and in the world has shown that the use of physical–mechanical (radiation, ultrasonic, mechanochemical, plasma, etc.) and chemical effects during the enrichment and processing of minerals, including unconventional, make it possible to defragment the initial ores, change the structure and reduce the overall costs of the processing stage in a complex during a single technological operation (Figure 1).
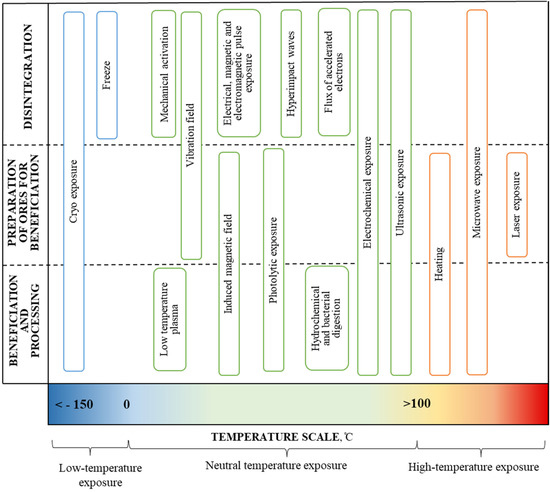
Figure 1.
Energy and physical and chemical exposures at the successive stages of mineral transformation.
Research conducted at the Institute of Comprehensive Exploitation of Mineral Resources Russian Academy of Sciences (ICEMR RAS) showed, for the first time, that the nonthermal action of high-power nanosecond electromagnetic pulses produces high-efficiency disintegration of finely disseminated mineral complexes; at the same time, this starts the physicochemical processes (solid-phase chemical reactions) on the surface of sulfide minerals involving the formation of micro- and nanophases in the form of hydrophobic elemental sulfur and various hydrophilic oxygen-containing compounds, which increases the selectivity of the flotation process of sulfides with similar physical and chemical properties [19,20,21]. The leading scientists of ICEMR RAS have developed the main classifications of the methods of exposure on ores. The classifications presented [20,21] were taken as a basis and supplemented (Table 1). Significant contributions were also made by the leading scientists of the ICEMR RAS to the development of the electrochemistry of the sulphide ore flotation processes [22,23].

Table 1.
Systematization of energy and physical and chemical exposures in preparation, beneficiation and processing of ores.
In addition to the application of exposures, a rather important aspect in the comminution process is the assessment of the mineral liberation during the comminution process. A number of papers are devoted to this aspect from researchers from the JKMRC at the University of Queensland [80,81,82,83,84].
The conducted critical analysis in the field of chemical and physical–mechanical (energy) exposures has shown that properly selected dosed influences on the surface of the whole ore material as well as individual minerals allow for a change in their properties directionally. Also, the impacts contribute to increase the extraction of valuable components in the concentrate and are involved in the processing of nonconventional deposits of ores. Thus, the aim of this work was to substantiate the methods and technologies for the extraction of noble and rare metals from low-grade “nonconventional” carbonaceous ores using directed energy and chemical exposures based on experimental research.
2. Materials and Methods
2.1. Materials
Two types of carbonaceous ores were chosen as objects of the research: dyctionema oil shales (DOS) and carbonaceous gold-bearing ores (CGO).
The objects were selected according to the following criteria:
- ○
- both objects belong to the carbonaceous ores due to the presence of carbonaceous matter in the ore;
- ○
- presence of low-dimensional structures of noble and rare metals;
- ○
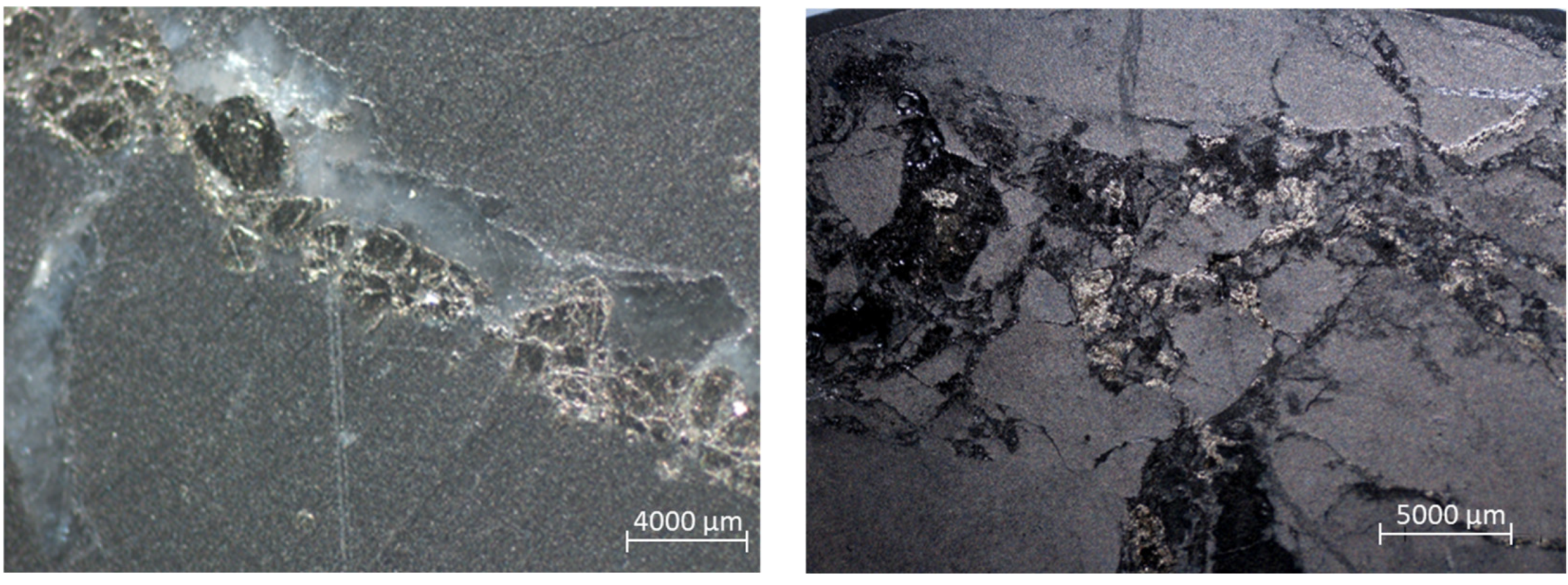
- sulfide mineralization in the thin dissemination form (Figure 2).
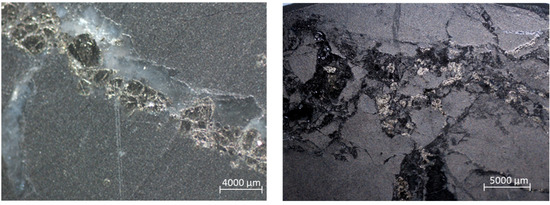 Figure 2. Sulfide dissemination in the samples of CGO (left) and DOS (right).
Figure 2. Sulfide dissemination in the samples of CGO (left) and DOS (right).
In order to determine the chemical composition of the investigated samples, an Energy Dispersive X-ray fluorescence analyzer and laboratory total organic carbon analyzer were used. Inductively coupled plasma mass spectrometry (ICP-MS) was used to determine the grade of the noble and rare metals in the samples (Table 2 and Table 3).

Table 2.
Results of the investigation of the chemical composition of CGO.

Table 3.
Results of the investigation of the chemical composition of DOS.
Carbonaceous sulfide gold ores are classified as refractory ores due to their low processing efficiency using traditional methods, such as cyanidation. These ores are characterized by thin gold phenocrysts in concentrator minerals, which are represented by pyrite and arsenopyrite and the presence of sorption-active in relation to dissolved gold carbonaceous organic matter (at its grade of 1.52% ± 0.07%). Gold is the main valuable component; the average gold grade for the investigated ore samples is 5.99 ± 0.29 g/t. Ore mineralization is 2%–3% of the total ore mass and is represented mainly by pyrite (0.65%) and arsenopyrite (1.10%), chalcopyrite, bornite, antimonite and others. Rock-forming minerals account for 97%–98% of the total ore mass and are represented mainly by quartz (43.30%), muscovite (23.65%), chlorite (7.72%), albite (4.82%), calcite (3.31%), ankerite (3.30%), carbonates (2.61%), siderite (2.60%), dolomite (1.20%), apatite (0.41%), etc.
The dyctionema oil shales belong to the carbonaceous ores of the black-shale type and are a promising source of rare metals (the rhenium grade is 0.081 ± 0.004 g/t) and platinum group metals (the total grade of the platinum group metals in the ore is ΣMPG + Pt = 0.0393 g/t). The presence of silver (0.192 ± 0.009 g/t) was also noted for the dyctionema oil shales. Rhenium is associated mainly with molybdenite. The ore mineralization of the dyctionema oil shales is of the order of 7%–10% and includes pyrite (3.15%), marcasite (3.33%), molybdenite (0.124%) and others. The rock-forming mineralization is represented mainly by quartz, feldspars, microcline, muscovite, apatite, zircon, etc.
The initial ores for the experiments were samples of DOS and SGO after crushing to −3.0 mm grain size.
2.2. Methods
Mineralogical and technological methods of ore investigation in combination with low-temperature and energy exposure methods represent the complex of methods used in this research. The plan of the research includes investigations into the application of
- ○
- low-temperature exposure
- ○
- mechanical and chemical activation
- ○
- microwave treatment
in the ore preparation and beneficiation stages (the experimental scheme).
2.2.1. Comminution and Sieve Analysis
Experimental studies on the comminution of the dyctionema oil shales in a ball mill with ceramic balls (Figure 3 and Figure 4) were carried out to substantiate the use of cryogenic exposure at the stage of the ore preparation of minerals and to choose the most effective method. The particle size of the feed material was −3000 + 0 µm. The mass of the feed sample for each comminution cycle was 100 g. The calculated (finished) grade was taken as −0.14 mm. The comminution conditions were taken according to Table 4 and Table 5. The sieve analysis using a standard set of sieves was carried out after each comminution cycle.

Figure 3.
Comminution media.

Figure 4.
Upgraded mill for exhaust of generated gases.

Table 4.
Comminution conditions.

Table 5.
Ball characteristic.
Research on the effects of cryogenic exposure on the comminution process was carried out in several directions:
- Comminution samples without applying exposures;
- Comminution of the frozen sample (the sample was frozen in a freezer to −50 °C);
- Comminution with freezing medium and cryoagents:
- −
- comminution with the addition of carbon dioxide ice (CO2);
- −
- sample comminution in liquid nitrogen medium (N2).
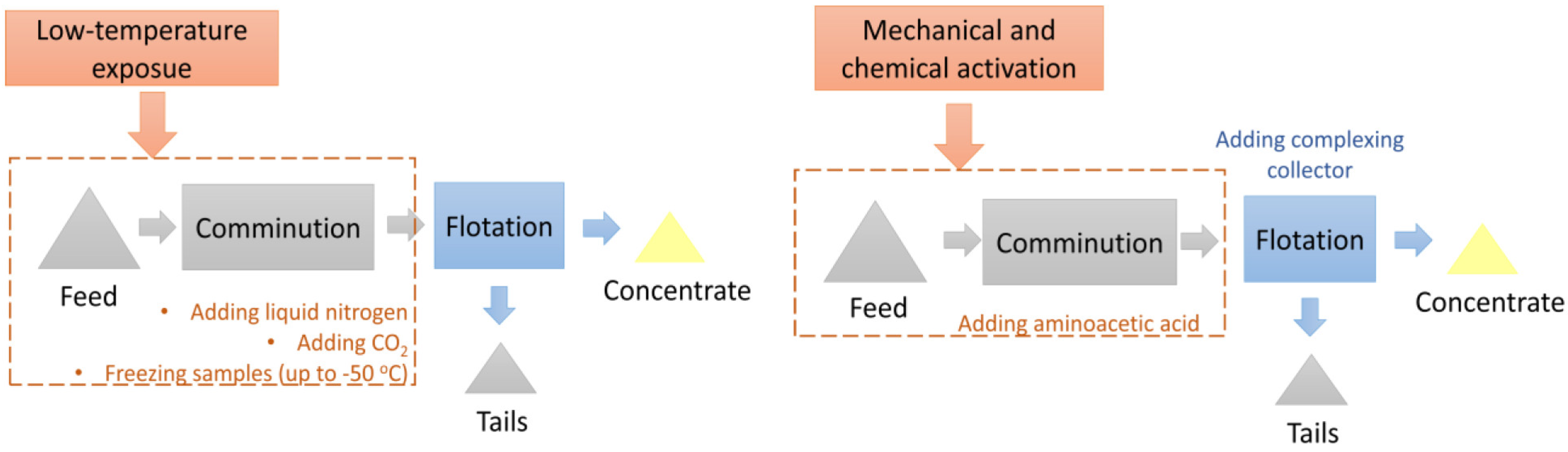
The research on the application of mechanical and chemical activation was carried out during wet comminution with the addition of aminoacetic acid. The experimental scheme of the low-temperature exposure and mechanical and chemical activation investigations is shown in Figure 5.
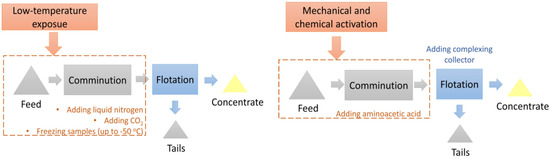
Figure 5.
The scheme of the investigations into the application of low-temperature exposure (left) and mechanical and chemical activation (right).
2.2.2. Flotation Experiments
The flotation experiments were carried out on a Laarmann Flotation Bench Test Machine (Laarmann Group B.V., Roermond, The Netherlands) with a cell volume of 1.5 L. The SGO flotation experiments were carried out according to the following scheme: comminution of the initial ore to the size of 60% of −71 microns class, followed by carbonaceous flotation with the use of a foaming agent with a consumption of 100 g/t to produce a carbonaceous concentrate and carbonaceous flotation tailings. The carbonaceous flotation tailings received comminution to the size of 90% of −71 microns and were fed into the sulphide flotation cycle, which included the rougher, two-cleaner and scavenger stages of flotation. The reagent regime of the flotation included a media regulator (Na2CO3) with a consumption of 350 g/t; carbonate depressor (dextrin) with a consumption of 100 g/t; activator of sulphide minerals (CuSO4) with a consumption of 300 g/t; collectors (potassium butyl xanthate and sodium butyl aeroflot) with consumptions of 150 and 50 g/t, respectively; and foaming agent with a consumption of 50 g/t.
The DOS flotation experiments were carried out according to the following scheme: comminution of the initial ore to the size of 80% of −71 microns in the presence of surfactants followed by the flotation stage with a combination of depressors (Na2SiO3 at 200 g/t as a depressor of gangue minerals represented mainly by silicates and dextrin at 100 g/t as a depressor of the carbonaceous organic component), sodium diisobutyl dithiophosphinate (C8H18PS2∙Na)(its molecule contains electron-donor atoms of sulphur and phosphorus, which predetermines its tendency to complexation and enough long alkyl radicals provide the collecting activity (consumption of 200 g/t)) and a foaming agent with a consumption of 40 g/t.
The experiments on the flotation of the monofractions of gangue minerals were carried out at a cell volume of 0.1 L using a foaming agent with a consumption of 50 g/t as a reagent.
The obtained flotation concentrates were dewatered and dried to a constant weight, after which a representative sample was taken for an analysis of the elemental composition. All flotation experiments were carried out 3 times each in order to minimize the inaccuracy and increase the reproducibility of the results.
2.2.3. Microwave Treatment Experiments
The investigation of the effect of microwave treatment on the samples was carried out using a microwave unit Sineo UWave-2000 (Sineo Microwave Chemistry Technology Co., Shanghai, China) with the ability to adjust the power in the range from 100 to 1000 W. The investigation was carried out with the addition of magnetite of −44 microns, the amount of which varied depending on the given conditions.
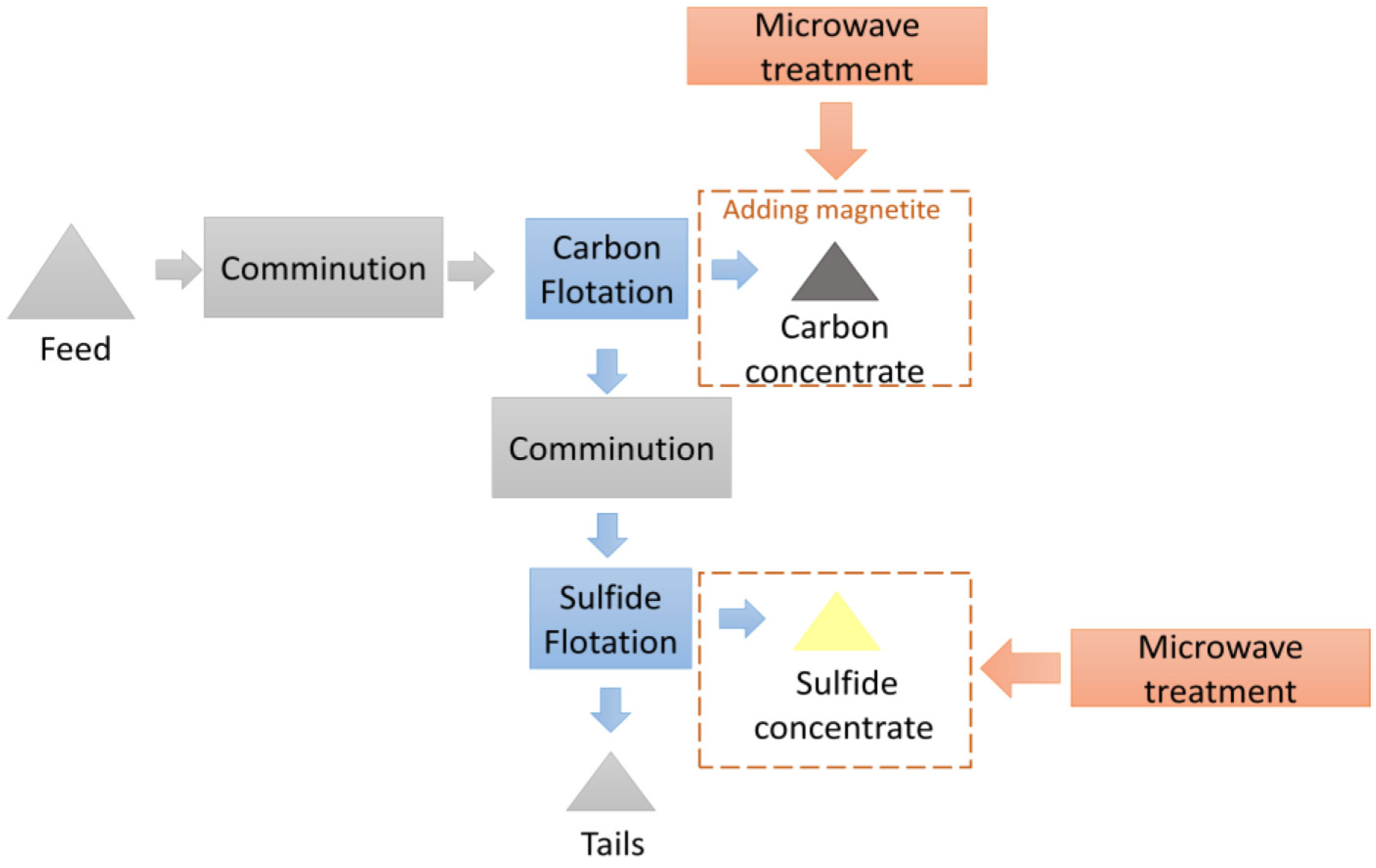
The scheme of the experimental research of the microwave treatment investigation is shown in Figure 6.
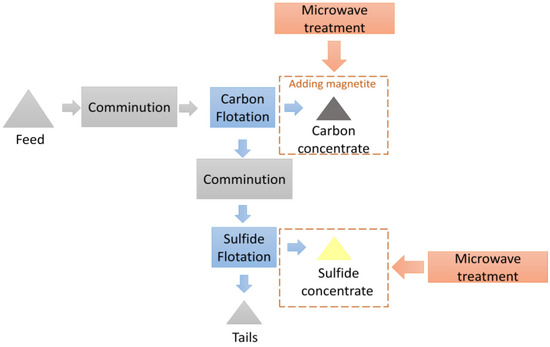
Figure 6.
The scheme of the investigations into the application of microwave treatment.
2.2.4. Preparation of Modeling Samples
Leaf gold (97%–98% gold grade) was used as a source of gold for the preparation of the model solutions. A reagent that was a mixture of amino acids (20%–35%), an iodine complex (30%–45%), sodium chloride (15%–30%), urea (10%–25%) and ammonium chloride (10%–15%) was used as a leaching agent for the gold. The leaching was carried out by contact of a known weight of gold with the leaching agent at a constant agitation for 48 h. After the leaching, activated carbon was added to the prepared gold model solutions, and the contacting was held for three days to adsorb the dissolved gold on the sorbent. After contacting the gold with the sorbent, filtration and subsequent drying of the cakes was carried out. The obtained products were the samples used for the experiments on microwave treatment.
2.2.5. Determination of the Value of the Specific Surface Area of Particles
The effect of comminution time under various conditions on the value of the specific surface of particles was investigated using the particle size analyzer Mastersizer 2000 Malvern (Malvern, Almelo, The Netherlands).
2.2.6. Scanning Electron and Optical Microscopy
The samples before and after treatment were analyzed using a Vega 3 LMH scanning electron microscope with an Oxford Instruments INCA Energy 250/X-max 20 X-ray energy dispersive microanalysis system (TESCAN, Brno, Czech Republic). To exclude the possibility of particle exposure during the analysis, the samples were pre-carbonized using a Q150R E sputtering unit manufactured by Quorum Technologies Ltd (Laughton, UK).
The mineralogical features of the samples and the nature of the sulphide mineral embedding in the waste rock were investigated using a ZEISS Axio Lab A1 microscope (Zeiss, Jena, Germany). The Thixomet PRO software package (Thixomet PRO, Thixomet, St. Petersburg, Russia) was used to evaluate the mineralogical analysis of the samples.
2.2.7. X-ray Fluorescence and Organic Carbon Analysis
Analysis of the elemental composition of the samples was carried out using an X-ray fluorescent energy dispersive analyzer EDX 7000 by Shimadzu (Shimadzu Corporation, Kyoto, Japan) according to the calibration curves. The organic carbon grade of the sample was analyzed using a Shimadzu TOC-L organic carbon analyzer (Shimadzu Corporation, Kyoto, Japan). The elemental composition and organic carbon were measured at least three times to minimize the inaccuracy and increase the reproducibility of the results.
2.2.8. Total Surface Free Energy (SFE) Analysis
The SFE of the samples was investigated using a DSA 25 by Krüss (Hamburg, Germany). The method consists of dropping two polar and non-polar liquids (water and diiodomethane) onto the test surface and then determining the values of the contact angles. A minimum of 10 drops are measured for each liquid and each sample. The OWRK method was used to calculate the ratio of the components and the total free energy of the surface [85]. The Owens, Wendt, Rabel and Kaelble method is a standard method for calculating the surface free energy of a solid from the contact angle with several liquids. In doing so, the surface free energy is divided into a polar part and a disperse part. According to Young’s equation, there is a relationship between the contact angle, the surface tension of the liquid, the interfacial tension between the liquid and solid and the surface free energy of the solid. In order to be able to calculate the surface free energy from the contact angle, the second unknown variable, interfacial tension, must be determined. The interfacial tension is calculated based on the two surface tensions and the similar interactions between the phases. At least two liquids with known disperse and polar parts of the surface tension are required to determine the surface free energy of the solid, wherein at least one of the liquids must have a polar part > 0.
2.2.9. Calculation of the Effectiveness Ratio of the Finished Size Class (Kef) and Coefficient of Homogeneity (UC)
In order to evaluate the effectiveness of cryogenic exposure on the comminution process for a given comminution time, the effectiveness ratio of the finished size class (Kef) was calculated according to the formula:
where Ci is the mass fraction of the class in the ground product, % (i—type of exposure); and C0 is the mass fraction of the class in the feed, %.
The size homogeneity of the material can be characterized by the coefficient of homogeneity (UC), determined by the following expression on the basis of mathematical statistics:
2.2.10. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
The analysis of noble and rare metals was carried out using a mass spectrometer with an inductively coupled ELAN-6100 DRC plasma mass spectrometer (Perkin-Elmer SCIEX, Wellesley, MA, USA) using the «TOTALQUANT» data processing software (Perkin-Elmer SCIEX, Wellesley, MA, USA)).
3. Results
The results of the research include investigations into the application of
- ○
- low-temperature exposure
- ○
- mechanical and chemical activation
- ○
- microwave treatment.
3.1. Low-Temperature Exposure
“Cryogenics” (hydrics of a low temperature) investigates the production and behavior of materials at extremely low (“cryogenic”) temperatures. This concept is not clearly defined, but many scientists consider that “cryogenics” begins at temperatures below −150 °C (123 K; −238 F). Despite this, in mineral processing and beneficiation, the term “cryogenics” refers to temperatures below 0 °C.
The changes of thermodynamic conditions in time and space determine the intensity and impact of the cryogenic exposure on minerals. Both external (process conditions) and internal (morphometric characteristics, structure and properties of minerals, thermodynamic parameters and physical and chemical properties of ores and media, etc.) factors that lead to changes in the material, mineralogical, chemical and technological properties of ores must be considered when exposing the ore (as a geosystem). If we take into consideration that minerals are a multi-component, heterogeneous system, all processes, including thermodynamic ones, have several specific features, which are observed both in the minerals themselves and at the boundaries of the intergrowths.
3.1.1. Cryogenic Comminution
As an example, cryogenic comminution technology can effectively comminute the strongest materials. As the results of research have shown, destruction with the application of cryogenic exposure can increase the degradation of minerals by several thousand times. The use of cryogenic exposure can solve the problems that arise in the comminution process, such as heat generation, the occurrence of tensile stresses, reduced equipment service life, oxidation, etc.
Several methods of cryogenic comminution can be distinguished:
- (1)
- Cryogenic comminution—the materials to be comminuted are cooled and crisped with liquid nitrogen or carbon dioxide.
- (2)
- Comminution using freeze–thaw cycling.
- (3)
- Cryogenic comminution is a type of mechanical comminution, in which minerals are ground in a cryogenic suspension or at ultra-low temperatures to produce microstructured particles.
The application of a cryogenic exposure leads to the creation of defects, which are microcracks both on the surface and in the volume of ores that is achieved due to the disjoining pressure of ice crystals and a sharp decrease in the temperature. The effect of the intensity of the cryogenic exposure on the changes in the hardness and porosity of minerals depends on the petrographic composition, the free surface area, the cryogenic comminution conditions, etc. Such technologies are especially relevant for fields in the Arctic zone.
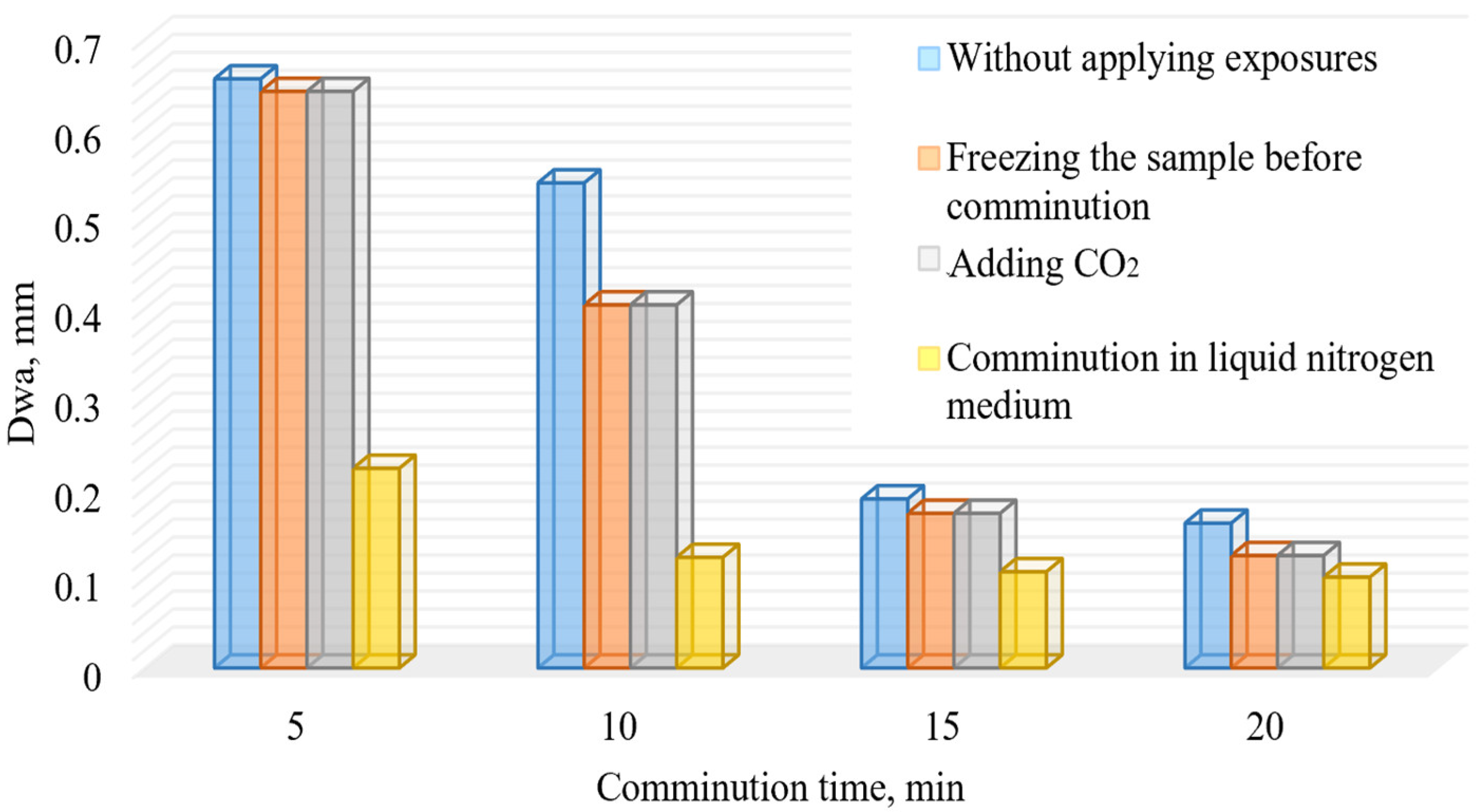
The conducted research of comminution using cryogenic exposure established a number of characteristic features. The results of comminution are visualized in Figure 7 and Figure 8.
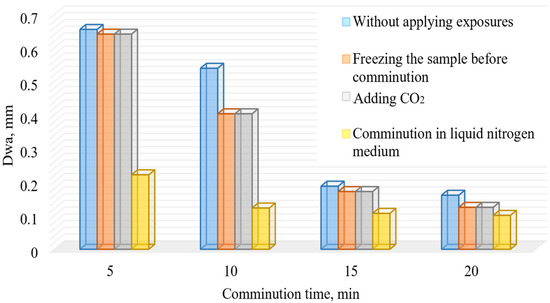
Figure 7.
Dependence of the weighted average diameter of the comminution products of the DOS samples on the comminution time with and without using cryogenic exposure.
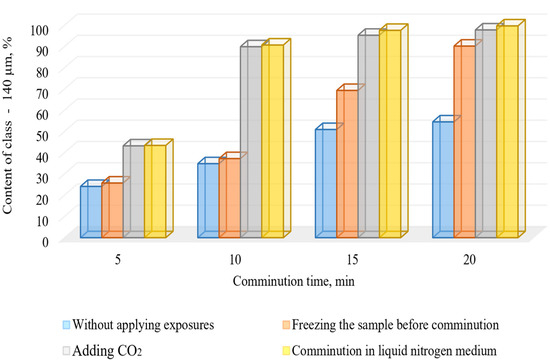
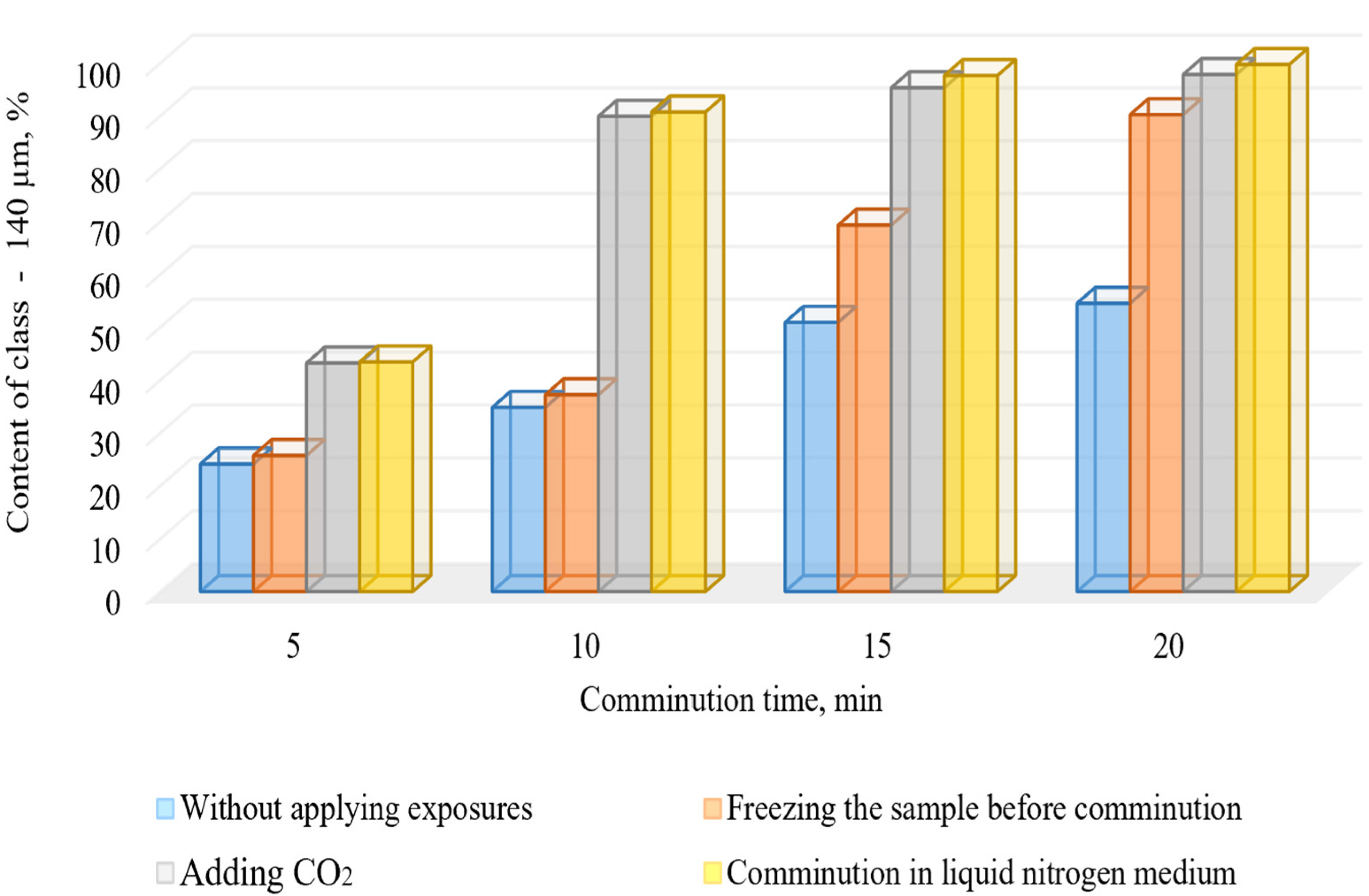
Figure 8.
Dependence of -140 μm grade in the comminution products of the DOS samples on the comminution time with and without cryogenic exposure.
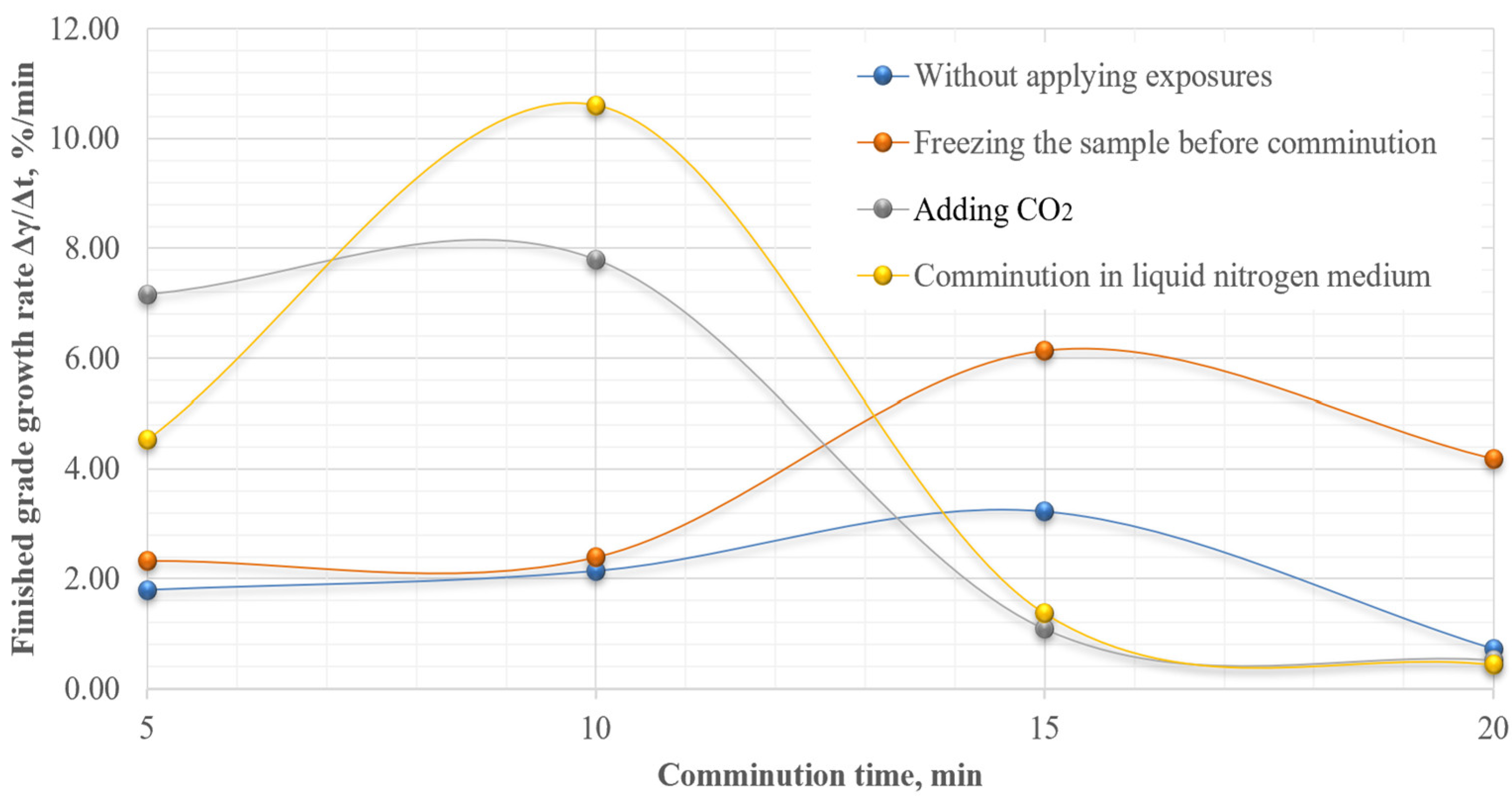
Analysis of the obtained results show that the sample is characterized by a light ability of comminution. However, in order to justify the necessary and sufficient comminution time, the rate of growth of the finished grade (Δγ/Δt) was also calculated (Figure 9).
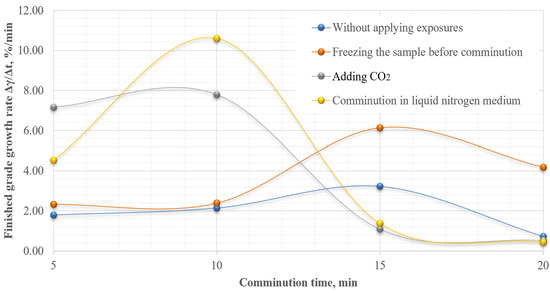
Figure 9.
Dependence of finished grade growth rate on comminution time.
The obtained results show that the maximum increase in the finished grade yield is achieved at 10 min of comminution in liquid nitrogen medium. To evaluate the effectiveness of cryogenic exposure on the comminution process for a given comminution time, the effectiveness ratio of the finished size class (Kef) was calculated.
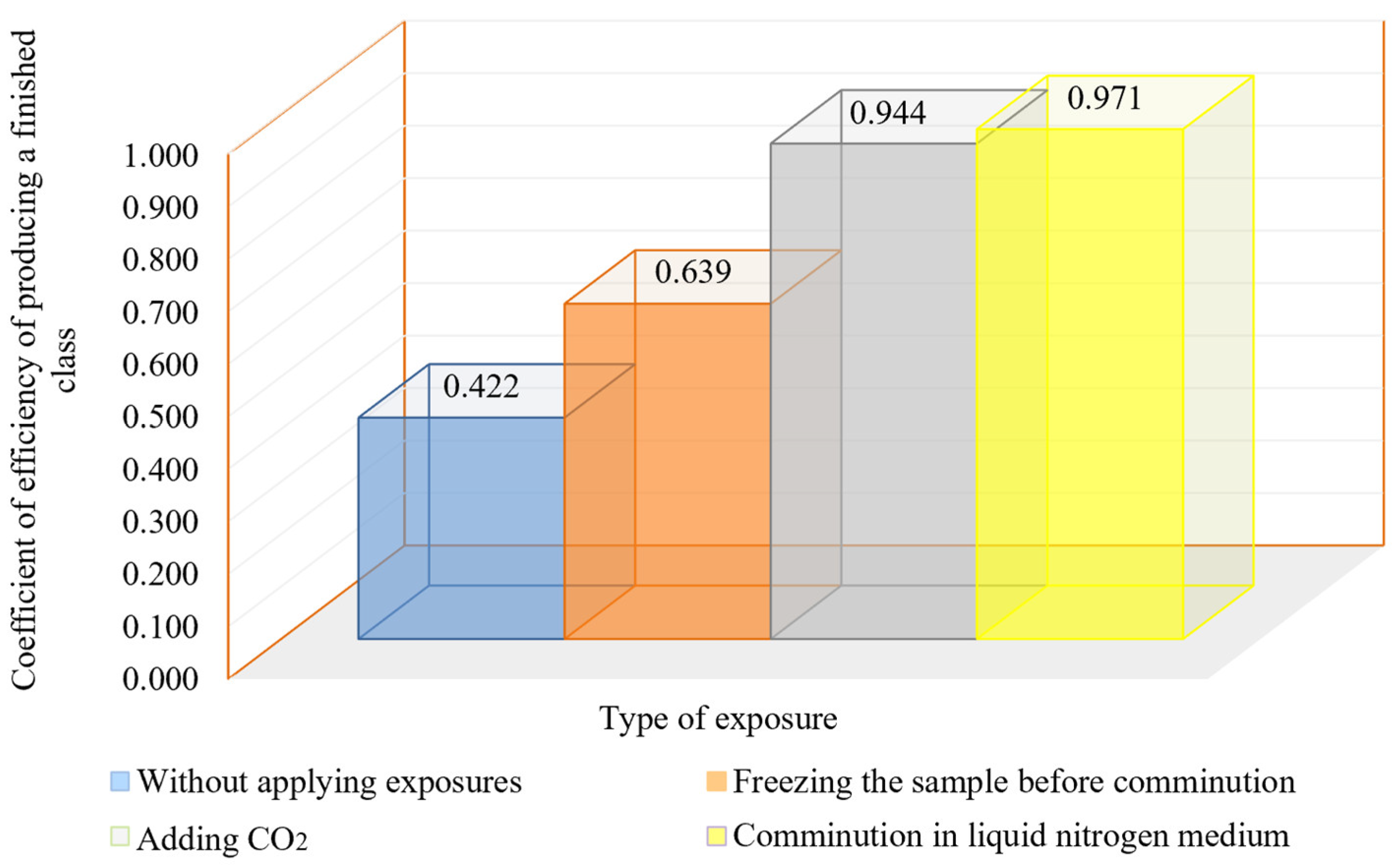
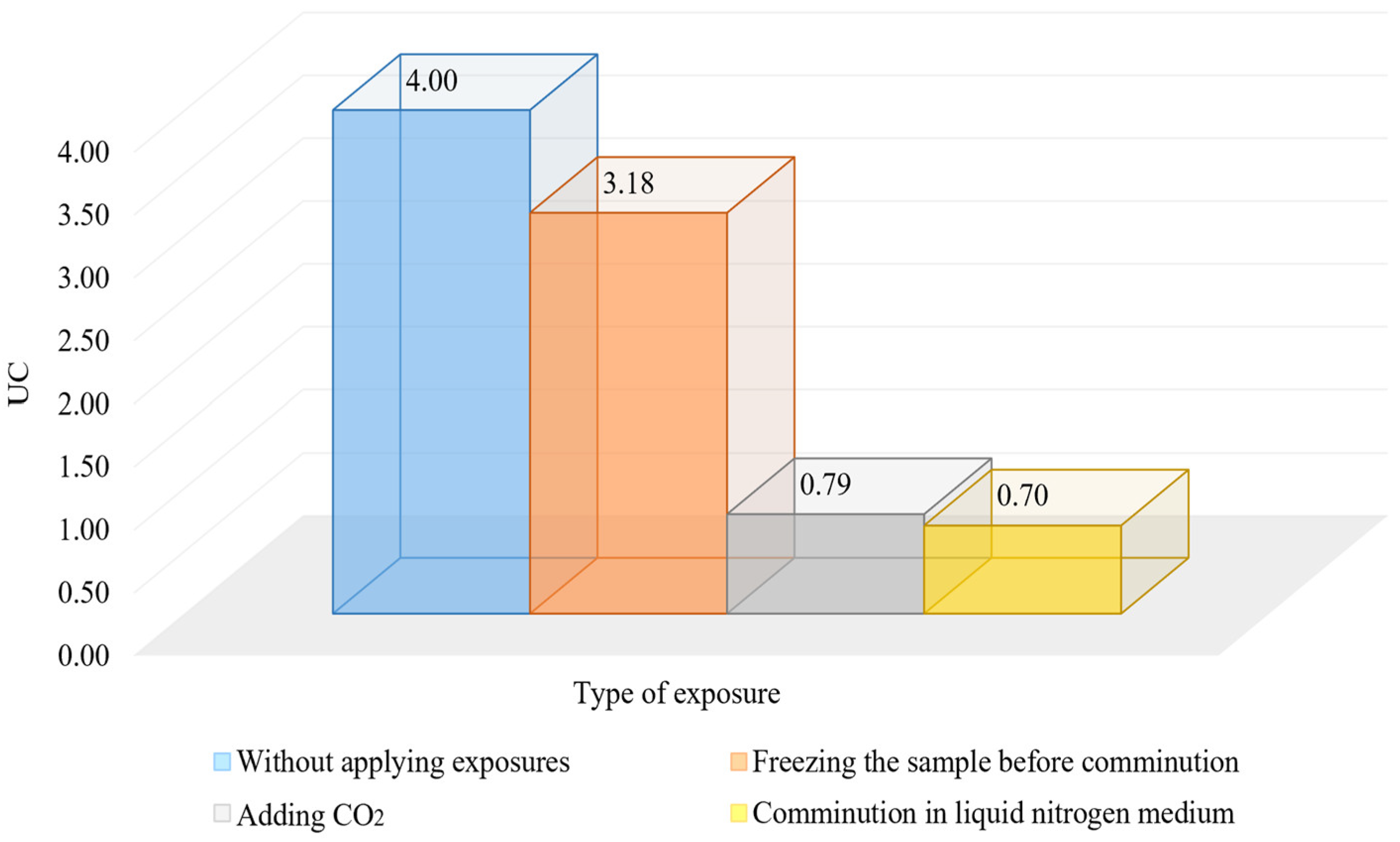
It is important to obtain the most homogeneous material in terms of grain size when preparing material at the comminution stage for the subsequent beneficiation. The more homogeneous the material in the classifier overflow, the better the comminution and classification cycle works, and the better the flotation results will be. The size homogeneity of the material can be characterized by the coefficient of homogeneity (UC). The results of the calculations are shown in Figure 10 and Figure 11 and Table 6.
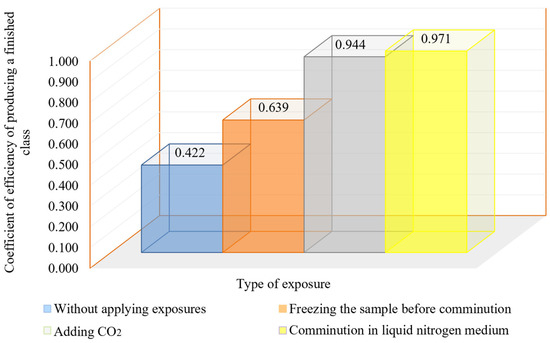
Figure 10.
Dependence of the effectiveness ratio of the finished size class working out on the method of cryogenic exposure to ore.
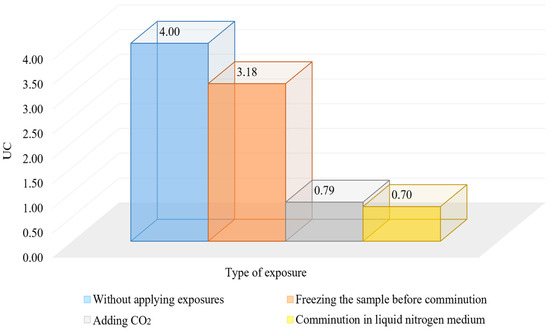
Figure 11.
Influence of cryogenic exposure on the homogeneity of the material.

Table 6.
Scale of bulk material homogeneity according to particle size distribution.
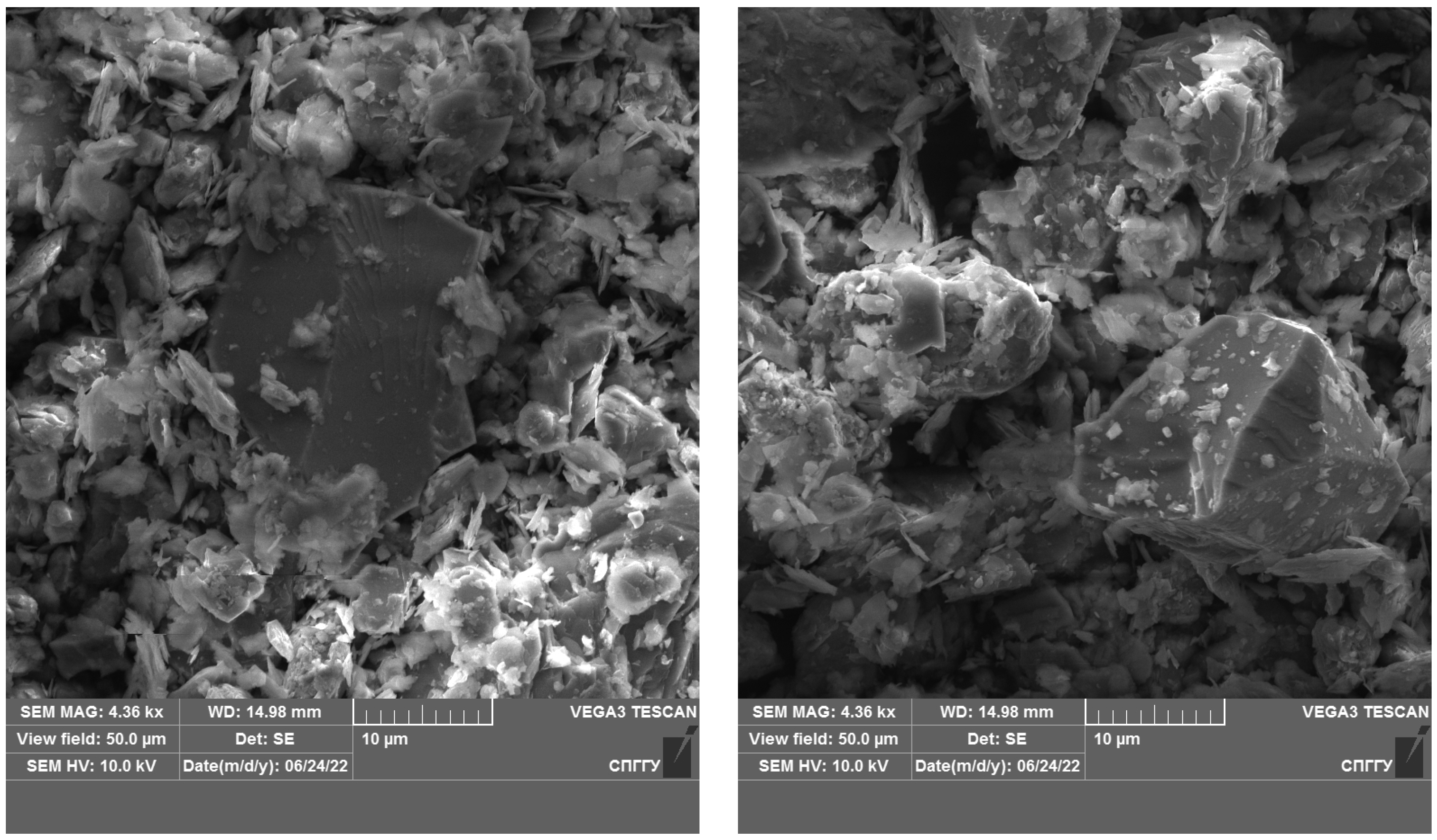
The results of the studies show that the use of cryogenic exposure increases the effectiveness ratio of the finished size class at the comminution of carbonaceous ores by 2.3-times compared with comminution without using cryogenic exposure, as well as obtains a more homogeneous comminution product (to avoid over-slagging). The experimental data show that cryoexposure at the comminution stage has a positive effect by increasing brittleness (sharp decrease in temperature). Depending on their intensity, the application of cryogenic exposures may result in an increase in cracks (both formation and development of existing cracks) (Figure 12 and Figure 13), formation of external and internal defects due to the disjoining effect of ice crystals, etc. The reason for the observed effect is the development of hydration and cryohydration processes that occur both on the surface and in the volume of minerals.
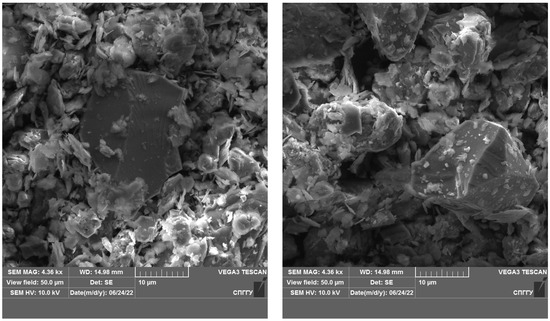
Figure 12.
Microphotograph of DOS sample.
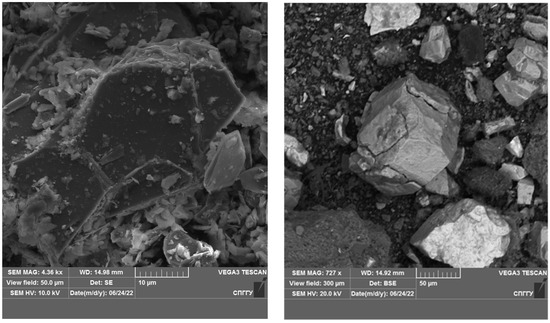
Figure 13.
Microphotograph of DOS sample after treatment with liquid nitrogen.
The most efficient process is comminution in liquid nitrogen medium. Nitrogen cooling provides a high rate of temperature reduction, and an inert atmosphere prevents self-oxidation. It has been suggested that the amount of organic carbon decreases with a sharp decrease in temperature (exposure to ultra-low temperatures). The results are presented in Table 7.

Table 7.
Results of Corg determination.
The treatment of the sample with liquid nitrogen reduced the organic carbon grade by more than 2% compared to the sample without treatment. It can be assumed that the use of cryogenic exposure will reduce the refractoriness of the carbon-bearing ores associated with the high grade of carbonaceous matter.
3.1.2. Investigation of the Effects of Low-Temperature Impact on Gangue Minerals
The effects of low-temperature impacts were investigated on the monomineral fractions of quartz and calcite as the main minerals comprising the gangue.
The particle size distribution of the fractions is shown in Table 8.

Table 8.
Particle size distribution of calcite and quartz.
The particle size distribution of the monomineral fractions is based on data from the objects under investigation. The objects under study are characterized by the presence of gangue minerals in thin classes. The assessment of the SFE of the minerals was carried out before and after low-temperature treatment. Also, the influence of low temperature on the mineral surface was estimated by the results of flotation of the monofractions. The results are presented in Table 9.

Table 9.
Results of research on the effect of low-temperature treatment on the technological properties of quartz and calcite.
Analysis of the data presented in Table 8 shows that the low-temperature treatment leads to an increase in the value of SFE for both samples from 39 to 57 mN/m. It should be noted that an increase in the polar component, which supposedly increases the hydrophilic properties of minerals, leads to a decrease in the recovery into concentrate for calcite from 6.15 to 4.07% and for quartz from 3.75 to 2.02%. This allows the use of low-temperature effects to increase the hydrophilicity of the gangue minerals and reduce their recovery in the concentrate.
3.2. Mechanical and Chemical Activation
The research into the possibility of applying mechanical and chemical activation to the DOS processing was conducted in two directions:
- ○
- Reducing the hardness of the ore in the comminution through penetration of the surfactant into micro-cracks, which has a wedging effect, preventing them from locking together;
- ○
- An increase in the recovery of rare and noble metals in the flotation concentrate due to the formation of chelate compounds with the use of a complex-forming collector.
3.2.1. Reducing the Hardness of the Ore in the Comminution
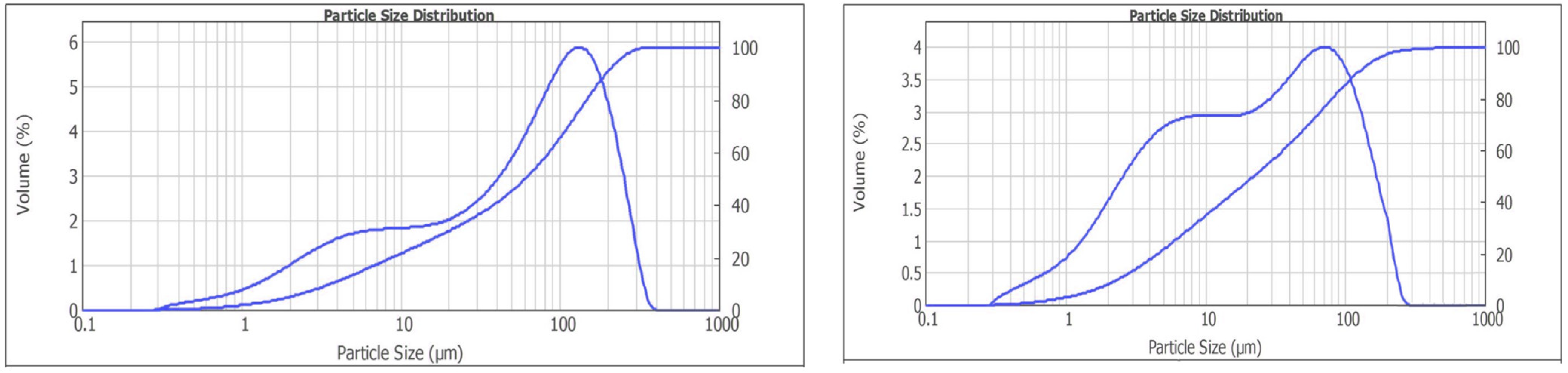
The effect of mechanical and chemical activation to reduce the hardness of the DSO ore in the comminution was investigated with the addition of aminoacetic acid at a rate of 100 g/t. The comminution products were analyzed using a laser particle analyzer to determine the particle size distribution. The results are shown in Figure 14.
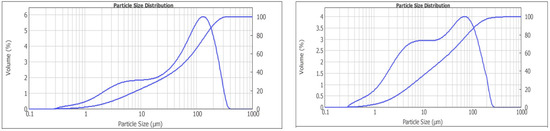
Figure 14.
Results of the particle size distribution investigations for the comminution without (left) and with aminoacetic acid (right).
The interpretation of the results presented in Figure 14 shows that with aminoacetic acid, there is an increase in the fine-size classes and a simultaneous decrease in the percentage of the coarser-size classes. The most probable explanation is the Rebinder effect. Surfactants physically sorb on the mineral surfaces, penetrate microcracks and have a proclinatory effect. The occurrence of new microcracks in the comminution and the contact of the new surface with the aminoacetic acid will enhance the Rebinder effect and lead to a reduction in the hardness of the ore body during the comminution. This leads to a reduction in the time and energy required for the ore-preparation stage.
3.2.2. Increase in Recovery of Rare and Noble Metals in the Flotation Concentrate
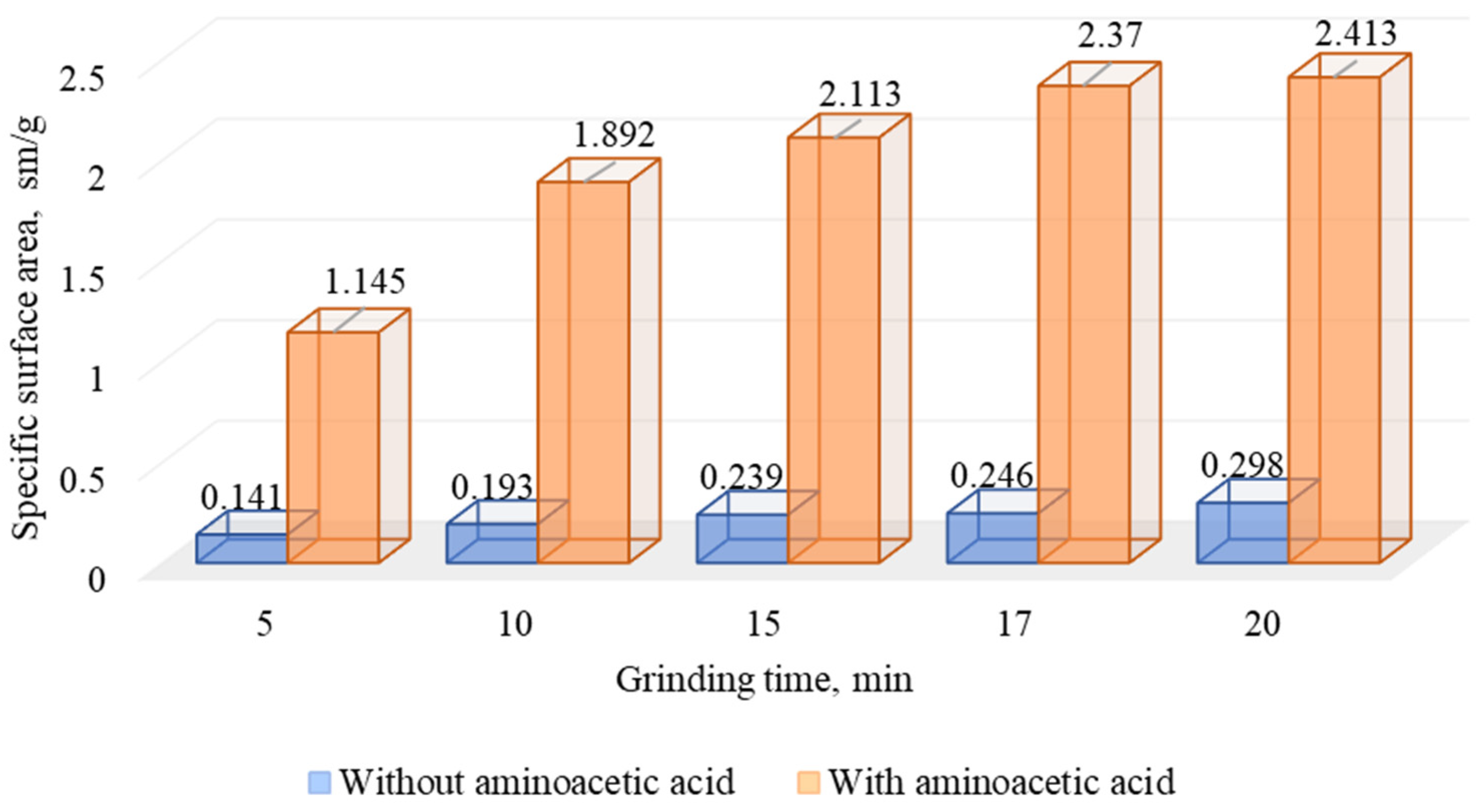
The formation of new surfaces and microcracks also increases the sorption of reagents on the mineral surfaces. In the comminution research, the change of the specific surface area with the use of a particle size analyzer was carried out (Figure 15).
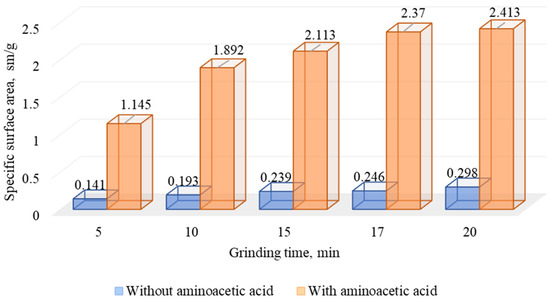
Figure 15.
Results of research on changes in the specific surface area of DOS during comminution without and with aminoacetic acid.
The analysis of the data presented in Figure 16 shows that the use of aminoacetic acid in the comminution leads to a significant increase in the specific surface area of the particles compared to the classic comminution, which agrees well with the data presented in Figure 14.
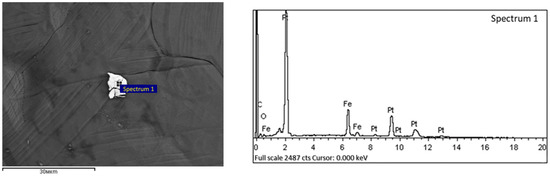
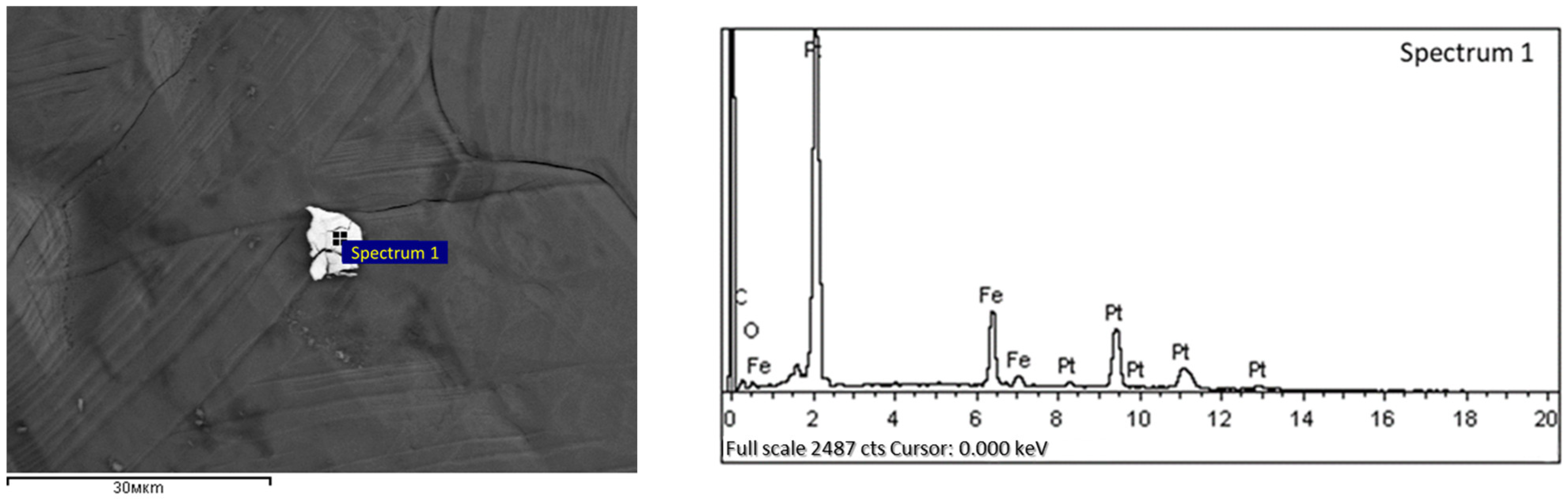
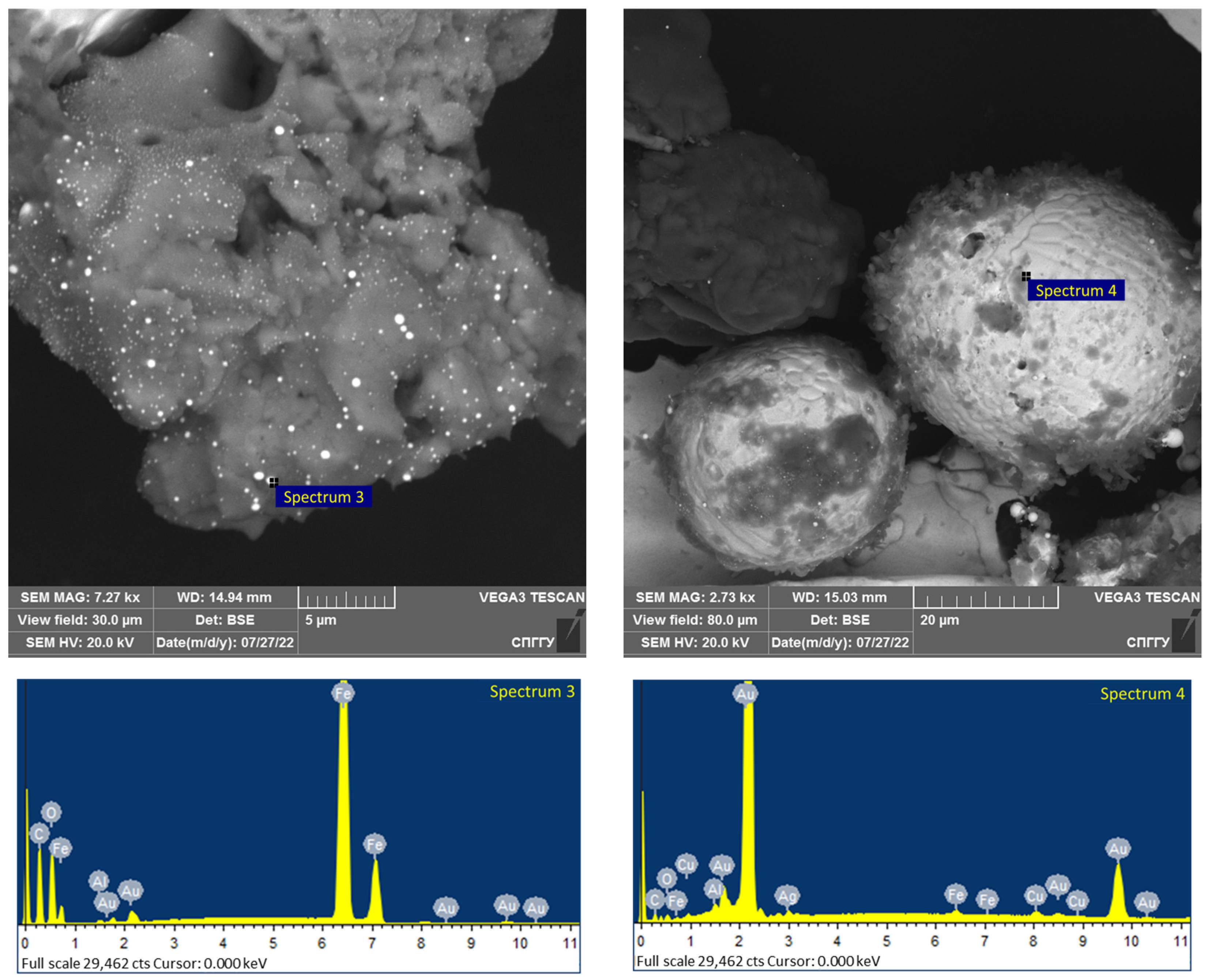
Figure 16.
Tetroferroplatinum observed by scanning electron microscopy in DOS samples.
The platinum group metals, including platinum and rare metals such as rhenium and vanadium, are associated with sulfide minerals in the studied type of carbonaceous ores and are represented by such minerals as tulaminite (Pt2FeCu), tetraferroplatinum (PtFe) (Figure 16, elemental composition is given in Table 10) and nickelferroplatinum (Pt2FeNi).

Table 10.
The elemental composition of the spectrum for the samples shown in Figure 16.
The reagent regime for the flotation experiments is given in the methodology. The results of the experiments with and without aminoacetic acid are shown in Table 11.

Table 11.
Results of DOS flotation with and without aminoacetic acid.
Analysis of the data presented in Table 11 shows that the use of aminoacetic acid at the comminution stage, when using a complexing collector, leads to a significant increase in the recovery of rhenium in the concentrate from 8.92% to 89.32%. Such a strong increase in the recovery is most likely due to the high probability of leaching of rhenium into the liquid phase during the flotation, which leads to significant losses of the rare metal in the tailings, while the use of aminoacetic acid leads to the formation of complex NH4ReO4, which is stable in water and allows the recovery of rhenium in the concentrate during the flotation.
The interpretation of the results presented in Table 11 shows a significant increase in the recovery of palladium and platinum from 41.93% to 61.67% and from 48.62% to 78.03%, respectively. An interaction of elements of the platinum group with aminoacetic acid and the complexing collector, the cyclic groups including a complexing agent and ligand of aminoacetic acid which belongs to the category of bidentate ligands, forms two chemical bonds with a complexing agent—through an oxygen atom of the carboxylic group and through a nitrogen atom of the amino group. This leads to a higher recovery of palladium and platinum in the flotation concentrate.
3.3. Microwave Treatment
Investigation of the impact of microwave treatment in CGO processing was investigated in two directions:
- ○
- coarsening of low-dimensional noble metal structures from tailings after carbonaceous flotation to improve the gold recovery from ore and reduce the environmental load by involving waste in the processing;
- ○
- reducing the refractoriness of the flotation sulfide concentrates to improve the recovery of gold from them after leaching.
3.3.1. Coarsening of Low-Dimensional Noble Metal Structures from Tailings after Carbonaceous Flotation Using Microwave Treatment
To establish the possibility of coarsening of the low-dimensional particles of noble metals, microwave treatment experiments on model samples were carried out. Magnetite was added to the model sample weights in the amounts of 3, 5, 10 and 15% of the sample mass at equal power (0.8–1 W) and treatment time (5 min).
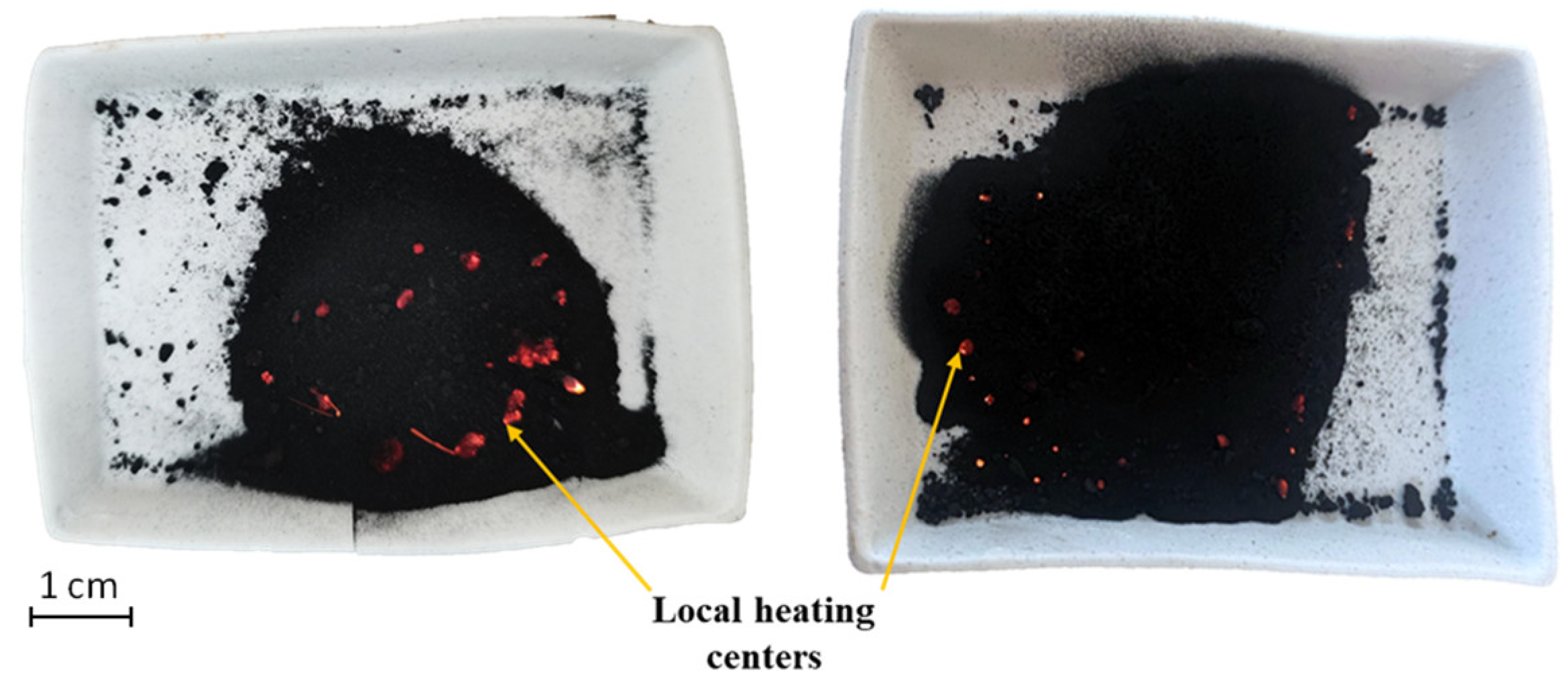
Figure 17 shows images of the samples directly after the treatment, in which the local heating centers are clearly visible.

Figure 17.
Samples directly after microwave treatment.
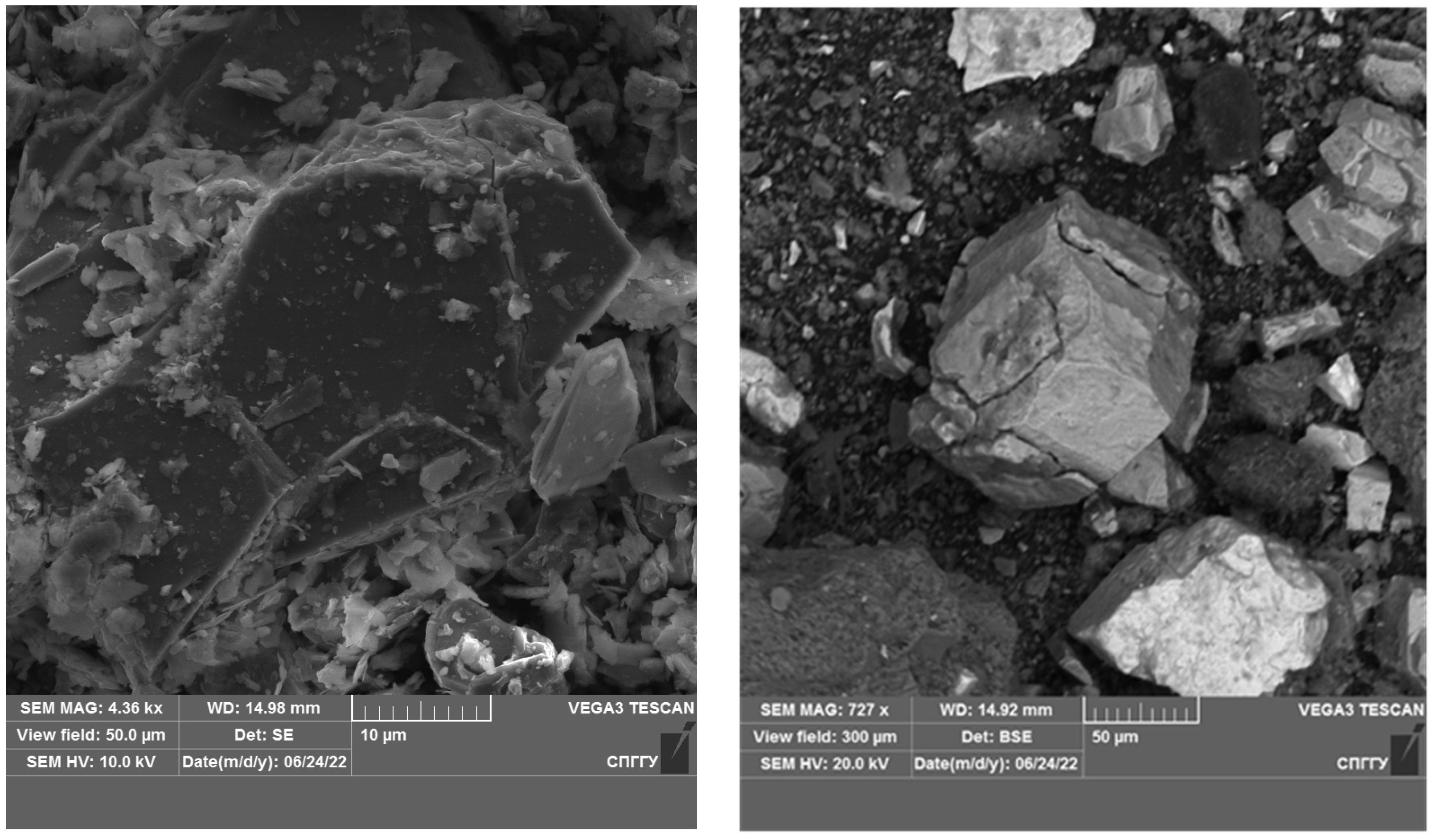
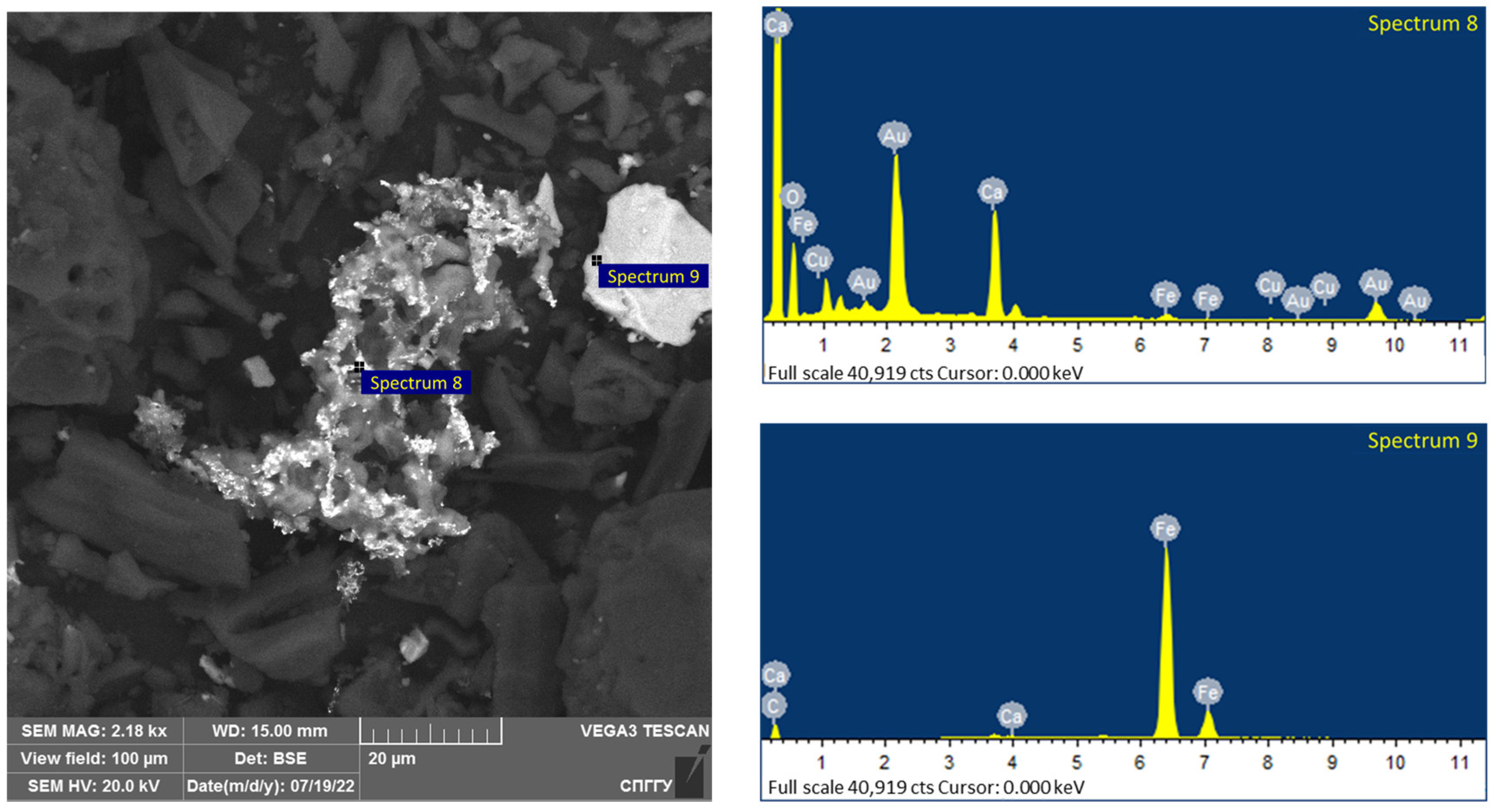
Magnetite, due to its sufficiently high values of dielectric constant, thermal conductivity and specific heat capacity, has a high capacity to absorb microwaves and heat [86,87,88]. The −44 μm magnetite particle size allows for its uniform distribution in the concentrate. The addition of magnetite leads to the formation of active local heating centers, which will subsequently lead to the coarsening of the low-dimensional structures of noble metals. An investigation of the samples using a scanning electron microscope after treatment is shown in Figure 18 and Figure 19.
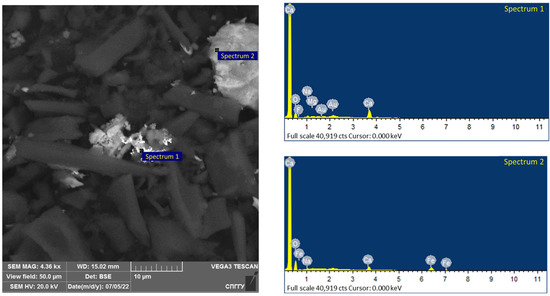
Figure 18.
Results of the investigation of samples after microwave treatment with the addition of 5% magnetite.
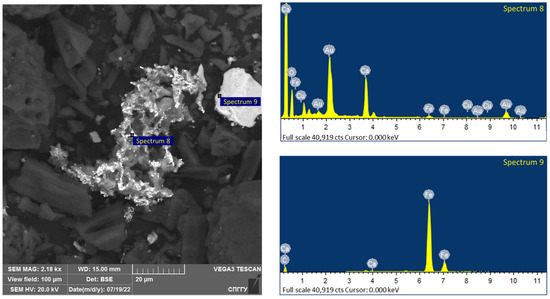
Figure 19.
Results of the investigation of samples after microwave treatment with the addition of 10% magnetite.
The elemental composition of the spectra is shown in Table 12.
Analysis of the data in Table 11 confirms the formation of coarsened gold aggregates for the investigated samples, as shown in spectra 1 and 8. The presence of magnetite in the samples is confirmed by the significant iron grade, including on spectra 2 and 9 together with gold.
To justify the choice of the amount of magnetite in the sample for the coarsening of the low-dimensional structures, the samples were analyzed using scanning electron microscopy, and the particle sizes after treatment were assessed. The treatment experiments were carried out five times for each amount of magnetite. The total number of particles for which the size estimation was carried out was 50 for each amount of magnetite. The results of establishing the average particle size when different amounts of magnetite were added after the treatment are shown in Table 13.

Table 13.
Average particle size of noble metals after microwave treatment.
The interpretation of the results presented in Table 12 shows that the addition of 10% magnetite to the sample obtains a coarsening of the particles up to the size of 20–50 microns. The increase in the magnetite grade up to 15% does not result in an increase in the particle size, while a reduction in the magnetite grade down to 5% does not allow a significant coarsening of the low-dimensional noble metal structures. Thus, the research on the model suspension makes it possible to substantiate the possibility of the coarsening of the low-dimensional structures of noble metals using microwave treatment and the addition of magnetite to generate the active centers of local heating.
An investigation of the possibility of coarsening of the low-dimensional noble metal structures on the ore was carried out on carbonaceous concentrates obtained after carbonaceous CGO flotation. The elemental composition of the flotation concentrate averaged after three tests is presented in Table 14.

Table 14.
Carbonaceous flotation CGO results.
Analysis of the data presented in Table 14 shows that the use of carbonaceous flotation can significantly reduce the amount of carbonaceous matter in the sulphide flotation feed (carbonaceous flotation tailings) without significant loss of valuable components. The loss of gold with the carbonaceous concentrate is 1.18%. Recovery of organic carbon at the same time is 38.86%. The carbonaceous concentrate after flotation is dewatered and sent to a tailings dump. The proposed technology makes it possible to use this product for processing.
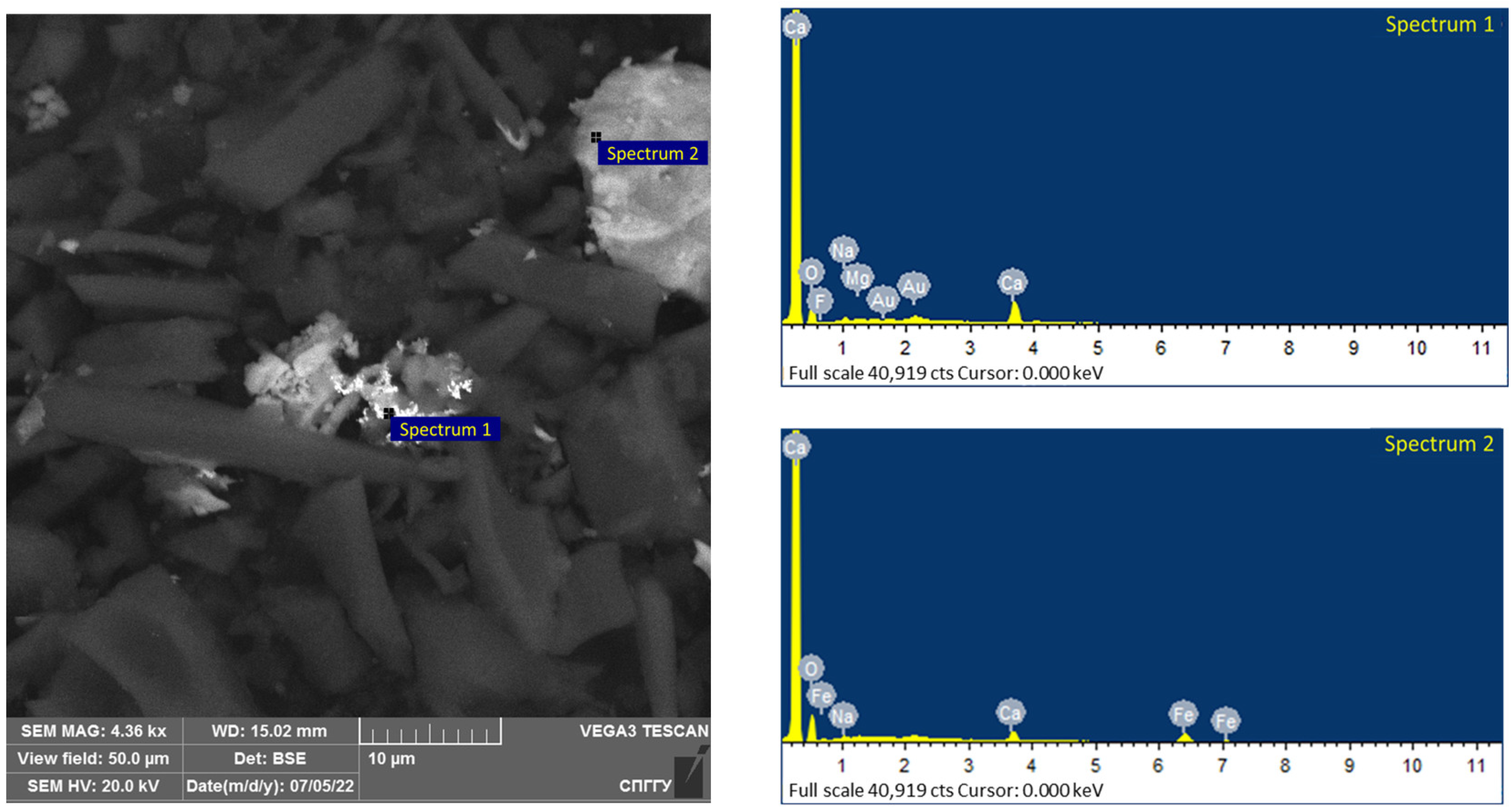
The morphology of the carbonaceous concentrate was examined using a scanning electron microscope (Figure 20). The elemental composition of spectrum 1 is shown in Table 15.
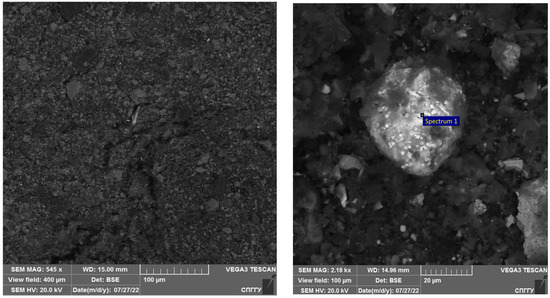
Figure 20.
Results of the investigation of carbonaceous concentrate.

Interpretation of the results presented in Figure 20 shows that the carbonaceous concentrate is characterized by a high organic carbon grade and low sulphide minerals and sulphide nodules with the size range of 20–40 µm.
The obtained flotation concentrates were microwave treated at a power of 0.8–1.0 kW for 5 min with the pre-mixing of the concentrate with a magnetite size of −44 microns. The addition of magnetite during treatment allows the formation of active centers of local heating near where the coarsened structures of the noble metals are formed (Figure 21 and Figure 22). The elemental composition of the CGO samples by spectrum is presented in Table 15.
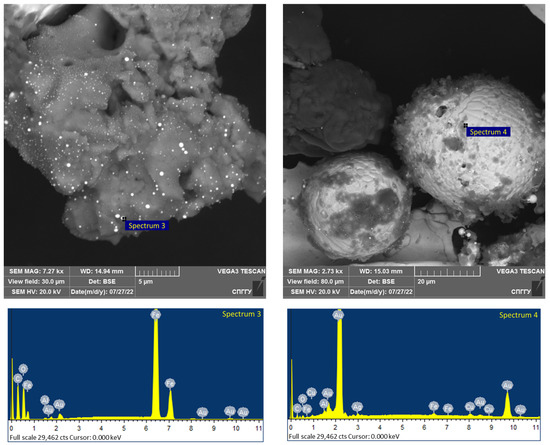
Figure 21.
Results of the investigation of the CGO flotation concentrates after microwave treatment.
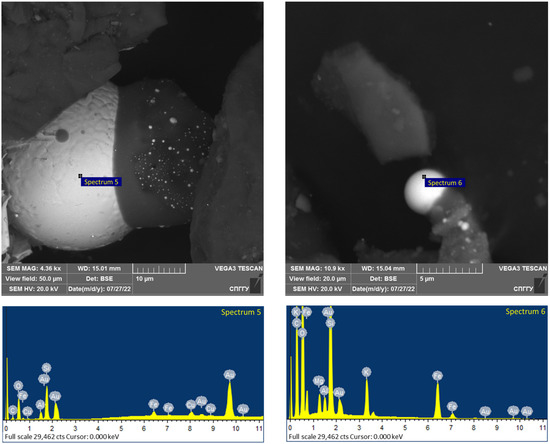
Figure 22.
Results of the investigation of the CGO flotation concentrates after microwave treatment.
The analysis of the investigation of the microwave treatment data presented in Figure 21 and Figure 22 shows the coarsening of low-dimensional noble metals to spherical particles of 20–40 microns in size, which allows their further recovery. At the same time, clustering of formed spherical particles of 1–3 microns in size is registered. It should be noted that for spectra 4, the presence of not only gold but also silver was detected.
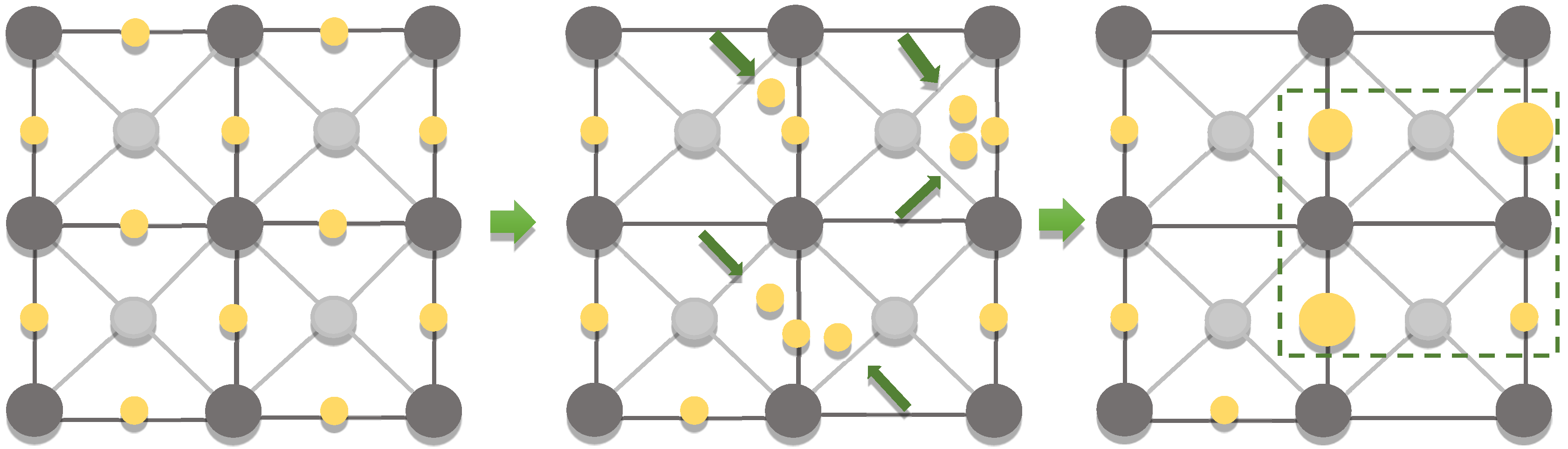
Supposedly, the significant coarsening of the low-dimensional structures of noble metals is due to the addition of magnetite, which allows for the creation of local heated active centers during processing, resulting in the concentration of the noble metals. A possible coarsening mechanism is the Frenkel defect, when the atom leaves its place in the crystal lattice due to the thermal effect and finds itself in an inter-node (cyclic displacement of several atoms, direct exchange of the places of two neighboring atoms, etc.) (Figure 23). In this way, the formation of point defects, i.e., vacancies, occurs, which leads to an increase in the energy of the crystal. To compensate for the increasing energy, the low-dimensional structures of the noble metals are coarsened due to the thermodynamic system tendency towards equilibrium. This is also in good agreement with the decrease in the gold melting point for the low-dimensional structures.
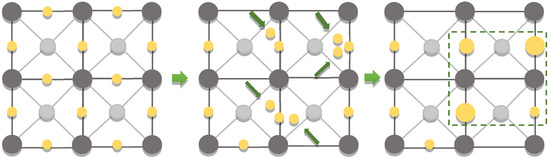
Figure 23.
Schematic representation of the Frenkel defect.
Thus, the possibility of coarsening of the low-dimensional structures of noble metals using microwave treatment by adding magnetite to create active centers of local heating has been substantiated. The coarsening of the low-dimensional structures of noble metals allows an increase in their recovery as well as a reduction of the environmental load due to the involvement in the processing of tailings.
3.3.2. Reducing the Refractoriness of Sulphide Flotation Carbonaceous Concentrates Using Microwave Treatment
The reduction in the flotation concentrate refractoriness was carried out on sulphide concentrates. The flotation concentrates after dewatering and drying were put on microwave treatment for 10–12 min with a furnace power of 0.4–0.6 kW. The microwave treatment enables the thermal destruction of the carbonaceous component, which has a high sorption activity towards the dissolved gold and uncovers the sulphide minerals. As a result, the gold recovery after leaching increased from 46.4 to 60.3% as compared with the classical technology (autoclave oxidation and leaching). The results of the research are presented in the paper [89].
4. Conclusions
This paper presents the results of the application of low-temperature and energetic effects to the recovery of noble and rare metals from carbonaceous ores. The research of the mineralogical features of the objects confirmed the presence of noble metals, such as gold, silver, platinum, platinum group metals and rare metals, such as rhenium, molybdenum and titanium, in the objects. The dyctionema oil shales and sulphide carbonaceous ores are combined due to the fine dissemination of the sulphide minerals in the gangue and the presence of organic carbon.
Cryogenic comminution uses cold energy (cryogenic exposure) to cool and, therefore, increase the brittleness and inertness of materials before or during the comminution process. Cryo-exposure on the comminution stage of carbonaceous ores increases the effectiveness ratio of the finished size class by 2.3-times in comparison with the classical comminution technology, while over-slagging is not observed. It was also noted that the treatment of carbonaceous ores with liquid nitrogen reduced the organic carbon grade by more than 2% compared to the original ore.
Application of low-temperature treatment on the monomineral fraction of gangue leads to a decrease in its recovery into the concentrate for quartz from 3.75% to 2.02% and for calcite from 6.15% to 4.07%, which is proven by the results of the flotation experiments and the measurement of surface free energy and its components before and after low-temperature treatment.
Application of mechanical and chemical activation during the processing of DOS by means of the addition of aminoacetic acid leads to a decrease in the hardness of ore mass during comminution at the expense of surfactant penetration into microcracks, which has a wedging effect, preventing their locking; this is most probably caused by the Rebinder effect and reduces the time and power inputs at the stage of ore preparation and also leads to an increase in the recovery of rare and noble metals in the concentrate of flotation at the expense of the formation of chelate compounds with the use of a complexing collector.
The application of microwave treatment to CGO processing leads to the reduction in sulphide flotation concentrates received for metallurgical processing in order to increase the recovery of gold from them after leaching, and also leads to the recovery of low-sized noble metal structures from carbonaceous flotation tailings to increase gold recovery from the ore and reduce the environmental load by involving waste in processing, which is most likely due to the occurrence of the Frenkel defect. The possibility of coarsening of low-dimensional structures of noble metals is also proved on modeling samples.
Thus, the introduction of low-temperature and energetic effects into the technological circuit at succeeding stages of carbonaceous ores to increase the extraction of noble and rare metals from them is a justified and promising direction.
Author Contributions
T.A.—conceived, designed the experiments and analyzed the data; N.N. and A.R.—implementation and processing of the analysis results; A.A., V.A. and E.P.—performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the grant of the Russian Science Foundation (Project N 19-17-00096).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chanturiya, V.A. Scientific Substantiation and Development of Innovative Processes for the Extraction of Zirconium and Rare Earth Elements in the Deep and Comprehensive Treatment of Eudialyte Concentrate. J. Min. Inst. 2022, 256, 505–516. [Google Scholar] [CrossRef]
- Litvinenko, V.S. Digital Economy as a Factor in the Technological Development of the Mineral Sector. Nat. Resour. Res. 2020, 29, 1521–1541. [Google Scholar] [CrossRef]
- Litvinenko, V.S.; Sergeev, I.B. Innovations as a Factor in the Development of the Natural Resources Sector. Stud. Russ. Econ. Dev. 2019, 30, 637–645. [Google Scholar] [CrossRef]
- Kruk, M.N.; Guryleva, N.S.; Cherepovitsyn, A.E.; Nikulina, A.Y. Opportunities for Improving the Corporate Social Responsibility Programs for Metallurgical Companies in the Arctic. Non-Ferr. Met. 2018, 44, 3–6. [Google Scholar] [CrossRef]
- Litvinenko, V.S.; Petrov, E.I.; Vasilevskaya, D.V.; Yakovenko, A.V.; Naumov, I.A.; Ratnikov, M.A. Assessment of the Role of the State in the Management of Mineral Resources. J. Min. Inst. 2022, 1–17. [Google Scholar] [CrossRef]
- Duryagina, A.M.; Talovina, I.V.; Lieberwirth, H.; Ilalova, R.K. Morphometric Parameters of Sulphide Ores as a Basis for Selective Ore Dressing. J. Min. Inst. 2022, 256, 527–538. [Google Scholar] [CrossRef]
- Litvinenko, V.S.; Tsvetkov, P.S.; Molodtsov, K.V. The Social and Market Mechanism of Sustainable Development of Public Companies in the Mineral Resource Sector. Eurasian Min. 2020, 2020, 36–41. [Google Scholar] [CrossRef]
- Popov, O.; Talovina, I.; Lieberwirth, H.; Duryagina, A. Quantitative Microstructural Analysis and X-Ray Computed Tomography of Ores and Rocks—Comparison of Results. Minerals 2020, 10, 129. [Google Scholar] [CrossRef]
- Aleksandrova, T.N.; Aleksandrov, A.V.; Nikolaeva, N.V.; Romashev, A.O. Noble and Rare Metals in Caustobioliths and Prospects of Their Recovery. J. Min. Sci. 2015, 51, 1254–1261. [Google Scholar] [CrossRef]
- Rasskazova, A.V.; Alexandrova, T.N.; Lavrik, N.A. The increase of effectiveness of power utilization of brown coal of russian far east and prospects of valuable metals extraction. Eurasian Min. 2014, 1, 25–27. [Google Scholar]
- Khanchuk, A.I.; Rasskazov, I.Y.; Aleksandrova, T.N.; Komarova, V.S. Natural and technological typomorphic associations of trace elements in carbonaceous rocks of the kimkan noble metal occurrence, far east. Rus. J. Pac. Geol. 2012, 6, 339–348. [Google Scholar] [CrossRef]
- Aleksandrova, T.N.; Talovina, I.V.; Duryagina, A.M. Gold–sulphide deposits of the russian arctic zone: Mineralogical features and prospects of ore benefication. Chem. Der Er. 2020, 80, 125510. [Google Scholar] [CrossRef]
- Koteleva, N.; Kuznetsov, V.; Vasilyeva, N. A Simulator for Educating the Digital Technologies Skills in Industry. Part One. Dynamic Simulation of Technological Processes. Appl. Sci. 2021, 11, 10885. [Google Scholar] [CrossRef]
- Pashkevich, M.A.; Danilov, A.S.; Matveeva, V.A. Remote Sensing of Chemical Anomalies in the Atmosphere in Influence Zone of Korkino Open Pit Coal Mine. Eurasian Min. 2021, 35, 79–83. [Google Scholar] [CrossRef]
- Rasskazov, I.Y.; Sekisov, A.G.; Rasskazova, A.V. In-Situ Leaching of Molybdenum and Uranium by Percarbonate and Chloride-Hypochlorite Solutions. J. Min. Inst. 2022, 256, 623–631. [Google Scholar] [CrossRef]
- Dzhevaga, N.; Lobacheva, O. Reduction in Technogenic Burden on the Environment by Flotation Recovery of Rare Earth Elements from Diluted Industrial Solutions. Appl. Sci. 2021, 11, 7452. [Google Scholar] [CrossRef]
- Lobacheva, O.; Dzhevaga, N. Method for Removing Valuable Components from Technogenic Solutions by the Example of Rare Earth Elements. J. Phys. Conf. Ser. 2020, 1679, 042016. [Google Scholar] [CrossRef]
- Lobacheva, O.L.; Dzhevaga, N.V.; Danilov, A.S. Understanding the Regularities of Recovering Non-Ferrous and Rare Earth Metals from Standard Test Solutions by Flotation and Solvent Sublation. Tsvetnye Met. 2020, 2020, 14–19. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Bunin, I.Z. Advances in Pulsed Power Mineral Processing Technologies. Minerals 2022, 12, 1177. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Bunin, I.Z.; Ryazantseva, M.V.; Filippov, L.O. Theory and Applications of High-Power Nanosecond Pulses to Processing of Mineral Complexes. Miner. Process. Extr. Metall. Rev. 2011, 32, 105–136. [Google Scholar] [CrossRef]
- Chanturia, V.A.; Bunin, I.Z. Non-Traditional High-Energy Processes for Disintegration and Exposure of Finely Disseminated Mineral Complexes. J. Min. Sci. 2007, 43, 311–330. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Vigdergauz, V.E. Electrochemistry of Sulfides. Theory and Practice of Flotation; Publishing House “Ore and Metals”: Moscow, Russia, 2008. [Google Scholar]
- Chanturiya, V.A.; Lunin, V.D. Electrochemical Methods for Intensifying the Flotation Process; Nauka: Moscow, Russia, 1983; 145p. [Google Scholar]
- Chanturia, V.A.; Bunin, I.Z.; Ryazantseva, M.V.; Filippova, I.V.; Koporulina, E.V. Nanosecond Electromagnetic Pulse Effect on Phase Composition of Pyrite and Arsenopyrite Surfaces, Their Sorption and Flotation Properties. J. Min. Sci. 2011, 47, 506–513. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Bunin, I.Z.; Kovalev, A.T.; Koporulina, E.V. Formation of Micro- and Nanophases on Sulfide Mineral Surfaces under the Effect of Nanosecond Electromagnetic Pulses. Bull. Russ. Acad. Sci. Phys. 2012, 76, 757–760. [Google Scholar] [CrossRef]
- Gholami, H.; Rezai, B.; Mehdilo, A.; Hassanzadeh, A.; Yarahmadi, M. Effect of microwave system location on floatability of chalcopyrite and pyrite in a copper ore processing circuit. Physicochem. Probl. Miner. Process. 2020, 56, 432–448. [Google Scholar] [CrossRef]
- Kingman, S.W.; Rowson, N.A. Microwave treatment of minerals—A review. Miner. Eng. 1998, 11, 1081–1087. [Google Scholar] [CrossRef]
- Pickles, C.A. Microwaves in extractive metallurgy: Part 2—A review of applications. Miner. Eng. 2009, 22, 1112–1118. [Google Scholar] [CrossRef]
- Haque, K.E. Microwave energy for mineral treatment processes—A brief review. Int. J. Miner. Process. 1999, 57, 1–24. [Google Scholar] [CrossRef]
- Kamariah, N.; Kalebic, D.; Xanthopoulos, P.; Blannin, R.; Araujo, F.P.; Koelewijn, S.-F.; Dehaen, W.; Binnemans, K.; Spooren, J. Conventional versus microwave-assisted roasting of sulfidic tailings: Mineralogical transformation and metal leaching behavior. Miner. Eng. 2022, 183, 107587. [Google Scholar] [CrossRef]
- Pickles, C.A. Microwaves in extractive metallurgy: Part 1—Review of fundamentals. Miner. Eng. 2009, 22, 1102–1111. [Google Scholar] [CrossRef]
- Goldbaum, M.W.; Elliott, R.; Forster, J.; Maham, Y.; Bobicki, E.R. Investigating the microwave heating behaviour of pyrrhotite tailings. Miner. Eng. 2020, 146, 106152. [Google Scholar] [CrossRef]
- Valeev, D.; Shoppert, A.; Dogadkin, D.; Romashova, T.; Kuz’mina, T.; Salazar-Concha, C. Extraction of al and rare earth elements via high-pressure leaching of boehmite-kaolinite bauxite using NH4HSO4 and H2SO4. Hydrometallurgy 2023, 215, 105994. [Google Scholar] [CrossRef]
- Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A comprehensive review of high-pressure laser-induced materials processing, part I: Laser-heated diamond anvil cells. J. Manuf. Mater. Process. 2022, 65, 111. [Google Scholar] [CrossRef]
- Cai, J.; Liu, D.; Shen, P.; Zhang, X.; Song, K.; Jia, X.; Su, C. Effects of heating-sulfidation on the formation of zinc sulfide species on smithsonite surfaces and its response to flotation. Miner. Eng. 2021, 169, 106956. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 1: Conventional heating. Miner. Eng. 2016, 98, 71–79. [Google Scholar] [CrossRef]
- Bunin, I.Z.; Ryazantseva, M.V.; Samusev, A.L.; Khabarova, I.A. Composite physicochemical and energy action on geomaterials and aqueous slurries: Theory and practice. Gorn. Zhurnal 2017, 11, 77–83. [Google Scholar] [CrossRef]
- Pavlova Ulyana, M.; Romashev Artyom, O.; Aleksandrova Tatyana, N. Researchment of carbonaceous ores dressability with the use of directional exposure. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, Bulgaria, 2–8 July 2018; Volume 18, pp. 147–154. [Google Scholar] [CrossRef]
- Vorobyov, A.E.; Chekushina, T.V. Methods of electrochemical leaching of metals from ores. Min. Inf. Anal. Bull. 1997, 176–180. [Google Scholar]
- Kharlamova, T.A.; Alaferdov, A.F. Chemical ore processing with electrochemically generated oxidizers. Russ. Min. Ind. J. 2014, 91. [Google Scholar]
- Mitra, S.; Mainul Hoque, M.; Evans, G.; Nguyen, A.V. Direct visualisation of bubble-particle interactions in presence of cavitation bubbles in an ultrasonic flotation cell. Miner. Eng. 2021, 174, 107258. [Google Scholar] [CrossRef]
- Huang, Z.; Kuang, J.; Zhu, L.; Yuan, W.; Zou, Z. Effect of ultrasonication on the separation kinetics of scheelite and calcite. Miner. Eng. 2021, 163, 106762. [Google Scholar] [CrossRef]
- Cilek, E.C.; Ozgen, S. Effect of ultrasound on separation selectivity and efficiency of flotation. Miner. Eng. 2009, 22, 1209–1217. [Google Scholar] [CrossRef]
- Chernykh, S.I.; Rybakova, O.I.; Lebedev, N.M.; Zhirnova, T.I. On the Study of the Effect of Ultrasound, Magnetic Fields and Electric Current on Gold Flotation. Tsvetnye Met. 2003, 15–21. [Google Scholar]
- Huang, W.; Chen, Y. The Application of High Voltage Pulses in the Mineral Processing Industry—A Review. Powder Technol. 2021, 393, 116–130. [Google Scholar] [CrossRef]
- Qin, Y.; Han, Y.; Gao, P.; Li, Y.; Yuan, S. Pre-Weakening Behavior of Magnetite Quartzite Based on High-Voltage Pulse Discharge. Miner. Eng. 2021, 160, 106662. [Google Scholar] [CrossRef]
- Parker, T.; Shi, F.; Evans, C.; Powell, M. The Effects of Electrical Comminution on the Mineral Liberation and Surface Chemistry of a Porphyry Copper Ore. Miner. Eng. 2015, 82, 101–106. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, W.; Han, Y.; Li, Y. Strengthening Liberation and Separation of Magnetite Ore via Magnetic Pulse Pretreatment: An Industrial Test Study. Adv. Powder Technol. 2020, 31, 2101–2109. [Google Scholar] [CrossRef]
- Yu, J.-W.; Han, Y.-X.; Li, Y.-J.; Gao, P. Effect of Magnetic Pulse Pretreatment on Grindability of a Magnetite Ore and Its Implication on Magnetic Separation. J. Cent. South Univ. 2016, 23, 3108–3114. [Google Scholar] [CrossRef]
- Zhuchkov, A.I.; Kurets, V.I.; Lobanova, G.L.; Tarakanovskij, E.N.; Filatov, G.P. Investigation of Destruction of Kimberlites by Electric Pulsed Method. Izv. Vyss. Uchebnykh Zaved. Gorn. Zhurnal 2004, 99–102. [Google Scholar]
- Sekisov, A.G.; Rubtsov, Y.I.; Lavrov, A.Y.; Manzirev, D.V. Heap and Heap Cell Leaching of Gold Using Photoelectroactivated Solutions. Zolotodobyvayushchaya Promyshlennost’ 2013, 18–26. [Google Scholar]
- Lavrov, A.Y. Efficiency of Photoelectrochemical Methods of Gold and Molybdenum Leaching from Technogenic Mineral Formations in Zabaikalye. Vestn. Zabaykal’skogo Gorn. kolledzha Im. M.I. Agoshkova Agoshkovskiye chteniya 2013, 5, 11–18. [Google Scholar]
- Lavrov, A.Y. Development of Physicochemical Geotechnologies Based on Complex Activation of Mineral Media and Working Solutions. Vestn. ZabGU 2015, 25–36. [Google Scholar]
- Lodeishchikov, V.V. Some Possibilities for Processing of Refractory Gold Ores. Zolotodobyvayushchaya promyshlennost’ 2008, 64. [Google Scholar]
- Sedelnikova, G.V.; Romanchuk, A.I. Effective Technologies for Extracting Gold from Ores and Concentrates. Gorn. Zhurnal 2007, 45–50. [Google Scholar]
- Tsarkov, V.A.; Dobroskokin, V.B. Extracting Gold from Hardrock Ores in Nevada. Gorn. Zhurnal 2000, 99–101. [Google Scholar]
- Potapov, S.A.; Chanturiya, V.A.; Polyakov, V.A.; Rostovtsev, V.I. Influence of the Accelerated Electron Beam on the Technological Properties of Ferruginous Quartzite from the Mikhailovskoe Deposit. J. Min. Sci. 1992, 10–14. [Google Scholar]
- Gzogyan, T.N. Intensification of Ore Dressing and Enrichment of Ferruginous Quartzite Based on Energy Impacts. Min. Inf. Anal. Bull. 2001, 41–53. [Google Scholar]
- Ismagilov, R.I.; Kozub, A.V.; Gridasov, I.N.; Shelepov, E.V. Modern Directions of Improving the Efficiency of Processing of Ferruginous Quartzites on the Example of JSC “Mikhailovsky GOK Named after AV Varichev”. Russ. Min. Ind. 2020, 98–103. [Google Scholar]
- Vaisberg, L.A.; Safronov, A.N. Vibratory disintegration application in processing of different materials. Obogashchenie Rud 2018, 1, 3–11. [Google Scholar] [CrossRef]
- Dong, H.; Liu, C.; Zhao, Y.; Zhao, L. Influence of Vibration Mode on the Screening Process. Int. J. Min. Sci. Technol. 2013, 23, 95–98. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, Y.; Duan, C.; Zhang, C.; Diao, H.; Wang, Z.; Fan, X. Properties of Technological Factors on Screening Performance of Coal in an Equal-Thickness Screen with Variable Amplitude. Fuel 2017, 188, 511–521. [Google Scholar] [CrossRef]
- Bortnikov, A.V.; Samukov, A.D. Vibrating Disintegration in Ore Grinding Processes of Concentrators. Obogashchenie Rud 2018, 3–10. [Google Scholar] [CrossRef]
- Liao, Y.; Ma, Z.; Cao, Y. Improving Reverse Flotation of Magnetite Ore Using Pulse Magnetic Field. Miner. Eng. 2019, 138, 108–111. [Google Scholar] [CrossRef]
- Pelevin, A.E. Improving Magnetite Concentrate Quality in an Alternating Magnetic Field. Obogashchenie Rud 2019, 2019, 19–24. [Google Scholar] [CrossRef]
- Andrianandraina, S.H.; Dionne, J.; Darvishi-Alamdari, H.; Blais, J.F. Effect of Grain Size on the Bacterial Oxidation of a Refractory Gold Sulfide Concentrate and Its Dissolution by Cyanidation. Miner. Eng. 2022, 176, 107360. [Google Scholar] [CrossRef]
- Yaghobi Moghaddam, M.; Ranjbar, M.; Manafi, Z.; Schaffie, M.; Jahani, M. Modeling and Optimizing Bacterial Leaching Process Parameters to Increase Copper Extraction from a Low-Grade Ore. Miner. Eng. 2012, 32, 5–7. [Google Scholar] [CrossRef]
- Lopez, L.Y.; Merma, A.G.; Torem, M.L.; Pino, G.H. Fundamental Aspects of Hematite Flotation Using the Bacterial Strain Rhodococcus Ruber as Bioreagent. Miner. Eng. 2015, 75, 63–69. [Google Scholar] [CrossRef]
- May, F.; Hamann, S.; Quade, A.; Brüser, V. Froth Flotation Improvement by Plasma Pretreatment of Sulfide Minerals. Miner. Eng. 2017, 113, 95–101. [Google Scholar] [CrossRef]
- May, F.; Hamann, S.; Quade, A.; Brüser, V. Study on Cu<inf>2</Inf>S Mineral Surface Modification by Low Temperature Ar/O<inf>2</Inf> Plasmas. Miner. Eng. 2013, 50–51, 48–56. [Google Scholar] [CrossRef]
- May, F.; Gock, E.; Vogt, V.; Brüser, V. Plasma-Modification of Sulfides for Optimizing Froth-Flotation Properties. Miner. Eng. 2012, 35, 67–74. [Google Scholar] [CrossRef]
- Litvintsev, V.S.; Melnikova, T.N.; Yatlukova, N.G.; Litvinova, N.M. Mechanical Activation in the Ore Pretreatment Processes. Min. Inf. Anal. Bull. 2005, 306–311. [Google Scholar]
- Boronenko, M.P.; Lavrikov, V.V.; Seregin, A.E.; Yurukin, P.A.; Yuhimyk, R.F. Energy Control and Grinding Mechanoactivation Planetary Mill AGO-3. Yugra State Univ. Bull. 2016, 12, 7–16. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO<inf>2</Inf> and Graphite from Spent Lithium-Ion Batteries by Cryogenic Grinding and Froth Flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Jaiswal, S.; Singh, R.; Singh, V.; Mukherjee, A.K. A Study of the Effects of Thermal Shocks on Liberation Characteristics of High Coal Ash Particles. Fuel 2018, 233, 215–223. [Google Scholar] [CrossRef]
- De Moraes Tamura, H.; Vurobi, S.; Maeda, M.Y.; Capocchi, J.D.T.; Cintho, O.M. Syntesis of Niobium Nitride Using Cryogenic Milling. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 802, pp. 46–50. [Google Scholar] [CrossRef]
- Kolay, E. Modeling the Effect of Freezing and Thawing for Sedimentary Rocks. Environ. Earth Sci. 2016, 75, 210. [Google Scholar] [CrossRef]
- Binal, A. A New Laboratory Rock Test Based on Freeze-Thaw Using a Steel Chamber. Q. J. Eng. Geol. Hydrogeol. 2009, 42, 179–198. [Google Scholar] [CrossRef]
- Chatterji, S.; Christensen, P. A Mechanism of Breakdown of Limestone Nodules in a Freeze-Thaw Environment. Cem. Concr. Res. 1979, 9, 741–746. [Google Scholar] [CrossRef]
- Mariano, R.A.; Evans, C.L. The Effect of Breakage Energies on the Mineral Liberation Properties of Ores. Miner. Eng. 2018, 126, 184–193. [Google Scholar] [CrossRef]
- Mariano, R.A.; Evans, C.L.; Manlapig, E. Definition of Random and Non-Random Breakage in Mineral Liberation—A Review. Miner. Eng. 2016, 94, 51–60. [Google Scholar] [CrossRef]
- Tungpalan, K.; Wightman, E.; Keeney, L.; Manlapig, E. A Geometallurgical Approach for Predicting Separation Performance. Miner. Eng. 2021, 171, 107065. [Google Scholar] [CrossRef]
- Tungpalan, K.; Wightman, E.; Manlapig, E. The Role of Vein-Type Mineralisation in Mineral Liberation. Miner. Eng. 2018, 116, 209–212. [Google Scholar] [CrossRef]
- Carrasco, C.; Keeney, L.; Walters, S.G. Development of a Novel Methodology to Characterise Preferential Grade by Size Deportment and Its Operational Significance. Miner. Eng. 2016, 91, 100–107. [Google Scholar] [CrossRef]
- Owens, D.; Wendt, R. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, Z.; Li, H.; Gao, X.; Fan, X. Microwave-Assisted Pyrolysis of Plastics with Iron-Based Catalysts for Hydrogen and Carbon Nanotubes Production. Mater. Today Chem. 2022, 26, 101166. [Google Scholar] [CrossRef]
- Noble, J.P.P.; Bending, S.J.; Sartbaeva, A.; Muxworthy, A.R.; Hill, A.K. A Novel In Situ High-Temperature Magnetometry Method for Radiofrequency Heating Applications. Adv. Energy Mater. 2022, 12, 2102515. [Google Scholar] [CrossRef]
- Altarawneh, S.; Al-Harahsheh, M.; Buttress, A.; Dodds, C.; Rodriguez, J.; Kingman, S. Microwave Selective Heating of Electric Arc Furnace Dust Constituents toward Sustainable Recycling: Contribution of Electric and Magnetic Fields. J. Ind. Eng. Chem. 2021, 104, 521–528. [Google Scholar] [CrossRef]
- Aleksandrova, T.N.; Afanasova, A.V.; Aleksandrova, A.V. Microwave Treatment to Reduce Refractoriness of Carbonic Concentrates. J. Min. Sci. 2020, 56, 136–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).