Abstract
The Italian Northern Apennines contain several Fe-Cu-Zn-bearing, Cyprus-type volcanogenic massive sulfide (VMS) deposits, which significantly contribute to the Cu resources of Italy. The massive sulfide lenses and related stockwork mineralizations are hosted by several levels (from basalt to serpentinite) of the unmetamorphosed ophiolitic series; therefore, this region offers perfect locations to study the ore-forming hydrothermal system in detail. A combination of fluid inclusion microthermometry, Raman spectroscopy, electron probe analyses (chlorite thermometry) and stable and noble gas isotope geochemistry was used to determine the fluid source of the VMS system at Bargone, Boccassuolo, Campegli, Casali–Monte Loreto, Corchia, Reppia and Vigonzano. This question of the fluid source is the focus of modern VMS research worldwide, as it has a direct influence on the metal content of the deposit. The obtained temperature and compositional data are both in the typical range of VMS systems and basically suggest evolved seawater origin for the mineralizing fluid. Modification of seawater was most commonly due to fluid–rock interaction processes, which happened during long-lasting circulation in the crust. The role of a small amount of magmatic fluid input was traced only at the lower block of Boccassuolo, which may be responsible for its higher ore grade. This fluid origin model is evidenced by O, H and C stable isotopic as well as He, Ne and Ar noble gas isotopic values.
1. Introduction
Several studies are dealing with the formation and evolution of volcanogenic massive sulfide (VMS) deposits (see, e.g., [1,2,3,4,5] and the references cited therein). Research projects of the past few decades provided robust genetic and descriptive models of these deposits and significantly improved our knowledge of the source of the hydrothermal fluid, the driving force of the system, the controlling factors of the ore precipitation and the transported metals. However, refinement of the models emerged new questions and challenges; a system-based approach, as well as a modern methodology, is needed to answer them.
Geological evidence generally indicates an evolved seawater origin for the hydrothermal fluid (see, e.g., 1–5 and the references cited therein), though there is a continuous debate regarding the mode of modification, i.e., (1) fluid–rock interaction in the oceanic crust (2) and/or admixture of magmatic fluid (3) and/or phase separation may also play a role. Understanding these processes bears high importance, as they can significantly contribute to the metal content of the deposit (1% magmatic fluid component can bring 85% of the deposit’s metal content [6]). However, studies on VMS deposits have shown that finding reliable evidence of the fluid source can be a challenge (see, e.g., [1,3,4] and the references cited therein). In the present study, several different mineralogical and geochemical research methods are utilized in order to better model the hydrothermal fluid source in VMS deposits through examples from the Northern Apennines, Italy. We not only show the useful combination of fluid inclusion study, Raman spectroscopy, electron probe analyses and stable and noble gas isotope analyses but also highlight those points where data interpretation may be misleading without the combination of the above-mentioned methods. Hence the obtained results can contribute to tracing the hydrothermal fluid source at other similar VMS deposits worldwide.
2. Geological Background
The Ligurian Ophiolites arrived at their present (near) surface position during the orogenesis of the Italian Apennines, beginning from the Oligocene. These ophiolites represent the remnants of the southwestern Alpine Tethys [7]. The ophiolites occurring on the eastern side of the Sestri–Voltaggio Line suffered only very low-grade metamorphism, while the ones in the Western Alps, to the west of this line, experienced high-pressure, low-temperature metamorphism. The non-metamorphosed oceanic crust fragments and the related rocks of the accretion prism form the Liguride Nappe, which is divided into two distinct units. The Internal Ligurides form the upper nappe, and its rocks are formed in a proximal position related to the spreading, whereas the External Ligurides, forming a nappe below, contain rocks representing a more distal position [7,8] (Figure 1).
The Eastern Ligurian ophiolites (where both the Internal and the External Ligurides occur) form an incomplete ophiolitic series, as the sheeted dyke complex and the transitional zone are missing or subordinate. The pillow basalt series is commonly thin (max 200 m), whereas sometimes pelagic sediments can be found on serpentinites due to syntectonic processes [8]. Based on petrological, geochemical and structural characteristics, these ophiolites formed during the early phase of oceanic crust formation, most likely related to marginal basin opening ([9,10] and the references cited therein).
Several Fe-Cu-Zn sulfide mineralizations classified as Cyprus-type volcanogenic massive sulfide deposits are known in the Eastern Ligurian ophiolites (Figure 1) [11]. These mineralizations contain 65% of the ophiolite-hosted Cu in Italy. Based on textural properties and stratigraphical position, they are divided into five groups: stratiform deposits, which occur on the top of (1) serpentinite breccia or (2) pillow basalt; stratabound deposits found (3) within the pillow basalt series; stockwork deposits, which (4) cross-cut the different units of the ophiolitic series (c: basalt, b: gabbro, a: serpentinite); and liquid magmatic massive sulfides, which (5) are hosted by serpentinite [12,13].
Modern studies of the above-mentioned deposits mostly addressed mineralogical, mineral chemical and geochemical questions (see, e.g., [11,12,13,14,15]), but only a little data is available about the characteristics and development of the hydrothermal process, which resulted in the ore formation [16,17]. The latter studies have shown the dynamic evolution of the fluid conditions (e.g., temperature, composition) and characterized the consecutive ore-forming and barren stages of the hydrothermal process; however, the source and development of the hydrothermal fluid remain unclear.
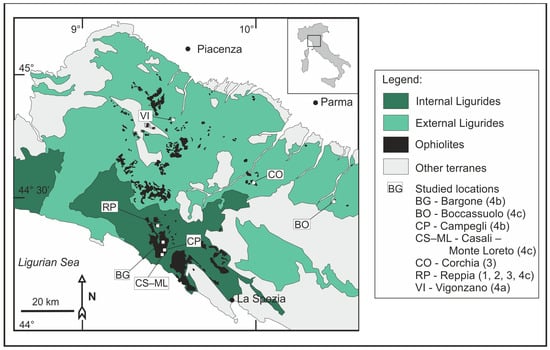
Figure 1.
Simplified geological map (based on [15]) showing the ophiolites of the Northern Apennines and the study locations. Numbers refer to the deposit types introduced in Section 2.
Figure 1.
Simplified geological map (based on [15]) showing the ophiolites of the Northern Apennines and the study locations. Numbers refer to the deposit types introduced in Section 2.
Eight VMS locations (Figure 1) were chosen for further study from the Eastern Ligurian ophiolites, representing all hydrothermal deposit types (types 1–4abc). Vigonzano contains stockwork ore in serpentinite (4a), and Bargone and Campegli are known for their stockwork ore in gabbro (4b), while Boccassuolo, Casali, Monte Loreto and Reppia feature stockwork ore in basalt (4c); Stratabound ore in pillow basalt is known in Corchia and Reppia (3); Above this, Reppia contains stratiform sulfide in serpentinite breccia (1) and pillow basalt (2), too [12]. The most important ore minerals are chalcopyrite, pyrite, sphalerite and rarely pyrrhotite either in the massive sulfide lenses or in the stockwork ore. Local differences are observable in the gangue and accessory minerals (Table 1.).

Table 1.
Mineral assemblage of the studied VMS deposits, based on the research of [13,18] and own observations.
3. Materials and Methods
Careful fieldwork, aiming to collect representative samples, was carried out at each study location. Textural features, classification and observation of the typical features of the hydrothermal infillings were carried out with the help of a hand lens and a stereomicroscope. Further observations on the hydrothermal phenomena, as well as their host rock, were performed with a Zeiss Axioplan polarizing microscope under transmitted and reflected light. Microphotographs were taken with the help of an Olympus Camedia C-5060 camera. These studies were performed on polished 30 µm thin sections as well as on block sections. Fluid inclusion petrography was carried out on doubly polished, 100–150 µm thick sections with an Olympus-BH2 type microscope, while the microthermometric measurements were performed by means of a Linkam FT-IR 600 type heating-freezing stage mounted on an Olympus-BX51-type microscope equipped with up to 100× long working distance objectives, providing optical magnification up to 1000×. For the calibration of the microthermometric stage, quartz-hosted synthetic CO2 and H2O inclusions were used, allowing 0.1 °C precision during the freezing runs and 1 °C precision during the homogenization experiments. Salinity calculations were performed based on the final ice-melting temperatures, using the equation of [19], except in inclusions with the formation of voluminous methane clathrate during the freezing runs. In these latter inclusions, the determination of salinity was based on the Raman spectra of the aqueous phase [20]. In some cases, salinity was not possible to measure due to technical reasons (metastability during freezing, small size of inclusions or strong luminescence of calcite). All the above-mentioned work was carried out at the Eötvös Loránd University, Department of Mineralogy.
Raman spectroscopy analyses of fluid inclusions were performed at the ELTE Research and Industrial Relations Center, Faculty of Science, Eötvös Loránd University, by using a Horiba Jobin Yvon LabRAM HR 800 edge filter (based confocal dispersive Raman spectrometer), with 800 mm focal length and coupled with an Olympus BXFM-type microscope. During the 3 × 20 s-long measurements, frequency-doubled Nd:YAG 532 nm laser, 600 grooves/mm grating, 50 μm confocal aperture and 50× and 100× long working distance objectives were used. The methane content of fluid inclusions was determined according to the method presented in [21]. The high-temperature Raman analyses were performed by mounting the Linkam heating-freezing stage on the microscope of the Raman equipment.
Chlorite thermometry calculations were based on electron microprobe analyses performed at the Eugen F. Stumpfl Microprobe Laboratory (University of Leoben) using a Jeol Superprobe JXA-8200, operated at 15 kV accelerating voltage, 10 nA beam current, ~1 µm beam diameter and counting times of 20 and 10 seconds for peak and backgrounds, respectively. The X-ray Kα lines for Si, Mg, Al, Ti, Cr, Ca, Na, K, Mn and Fe, as well as natural silicates and chromium spinel as standards, were used for the analysis of chlorite. Detection limits for each element are shown in the related tables. After obtaining chlorite EPMA data, we calculated its formula based on 14 O. We checked several different chlorite thermometry methods [22,23,24,25,26,27] and used the one in which criteria (Al (IV) and XFe value ranges, amount of K2O+Na2O+CaO, vacancy) was fulfilled by our measurement results.
Noble gas isotope analyses were carried out at the Isotope Climatology and Environmental Research Centre, Institute for Nuclear Research, Hungary. The selected rock samples were gently crushed into 0.1–5.0 mm pieces. The pure mineral (sulfide and quartz) separates were cleaned in an acetone ultrasonic bath and dried at 60 °C overnight to remove any adhering contamination. Approximately 1.0–3.5 g mineral separates were loaded into a stainless steel tube attached to the gas purification line. The gas was extracted by crushing (100 strokes) with a piston, which was activated using an external magnet. Different components of the released gas were separated in a cryogenic system consisting of two cold traps and a getter trap. Argon and the chemically active gases (e.g., O2, CH4, CO2, etc.) were adsorbed in an empty trap at 25 K, while He and Ne were adsorbed in a charcoal trap at 10 K. Thereafter, Helium and Ne were desorbed at 42 and 90 K, respectively, whereas Ar was desorbed from the trap at 55 K. The isotope compositions of each noble gas were sequentially measured after purification in a getter trap (SAES) using a HELIX-SFT noble gas mass spectrometer (He) and a VG 5400 noble gas mass spectrometer (Ne, Ar). Signals were collected using a Faraday cup (4He, 40Ar) and an electron multiplier for the other isotopes. The overall measurement procedure was calibrated with known air aliquots and calibration gases. The measured He isotope ratios have been corrected for excess air using the atmospheric 3He/4He and 4He/20Ne ratios, recommended by [28]. The corrections resulted in minor changes (0.01%) in the final data.
Stable carbon and oxygen isotope compositions of approximately 150–200 μg carbonate samples were determined by carrying out the carbonate–orthophosphoric acid reaction at 72 °C [29] and using an automated GASBENCH II sample preparation device attached to a Thermo Finnigan Delta Plus XP mass spectrometer at the Institute for Geological and Geochemical Research (IGGR) in Budapest, Hungary. Precision is better than ±0.1‰ for both δ18O and δ13C.
Stable hydrogen isotope composition of the inclusion-hosted water and the water content of carbonate (dolomite and calcite) samples were determined at the Institute for Geological and Geochemical Research (Budapest, Hungary), using the laser spectrometric method described in detail by [30,31].
4. Results
The studied mineralizations represent each level of the Cyprus-type VMS systems (massive sulfide lens and below the stockwork zone with alteration halo), although not all textural types are known at each location (see Section 2 and Table 1). A special feature of the study locations is the fact that almost every member of the ophiolitic series (basalt, gabbro and peridotite) contained mineralizations due to syntectonic processes; therefore, the studied samples cover a wide range in terms of either host rock or ore texture.
4.1. Massive Sulfide Lenses
4.1.1. Texture and Mineralogy
Massive sulfide lenses occurring in basalt and serpentinite breccia were studied in Reppia and Corchia. This type of mineralization contains more than 80% of ore minerals, including pyrite, chalcopyrite, pyrrhotite, sphalerite and detrital chromite, as well as magnetite, together with their alteration products hematite and limonite. Chlorite, quartz, calcite, clay minerals, barite and anhydrite are common gangue minerals. The gangue minerals most commonly occur among the sulfide grains, forming a massive, granular texture. However, brecciation may also occur (Figure 2).
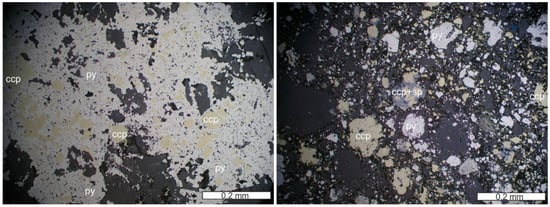
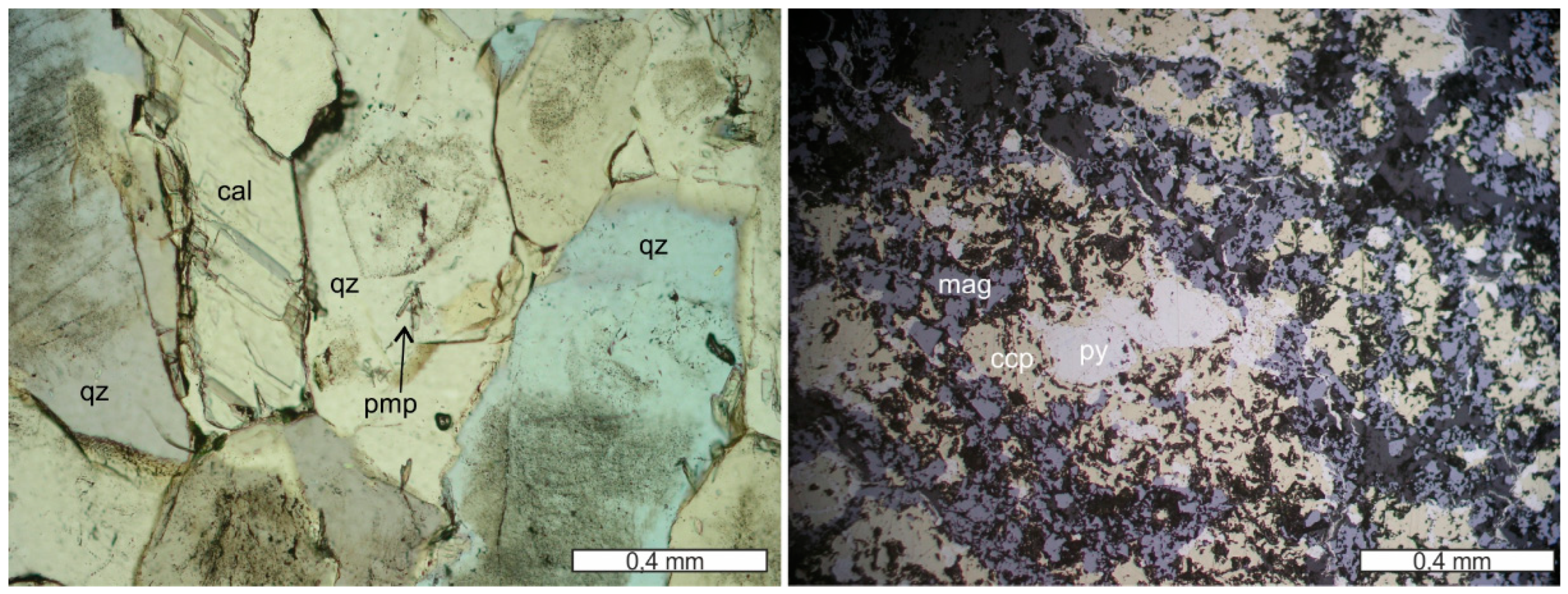
Figure 2.
Massive sulfide (py—pyrite; ccp—chalcopyrite) with gangue minerals (quartz and calcite) from Corchia (reflected light microphotograph, 1N; left) and brecciated ore (pyrite, chalcopyrite; sp—sphalerite) with gangue quartz and calcite from Corchia (reflected light microphotograph, 1N; right).
The most common sulfide mineral is pyrite (50–80%), which is formed in multiple stages during the whole hydrothermal process. Pyrrhotite is formed either earlier or cogenetically with early pyrite, whereas chalcopyrite crystallizes after early pyrite. This was followed by sphalerite crystallization. Late pyrite precipitation lasted longer than chalcopyrite and sphalerite formation.
Chlorite is a common early-formed gangue mineral; the studied grains belong to the type-1 Mg–chlorite group [32] based on their composition (Table 2), and their calculated formation temperature is 198 ± 10 °C [24].

Table 2.
Composition of chlorite based on EPMA data in wt.% (Det. lim.—detection limit; St. dev.—standard deviation; b.d.l.—below detection limit; n.a.—not available). Al (IV) and XFe data are based on 14 O. Formation temperature data are based on [22,23,24].
The basalt in close proximity to the massive sulfide lens shows intensive alteration. Hydrothermal veins are common, and due to the appearance of strongly altered rock-forming minerals, only the relict texture of the original rock is observable. Chlorite, albite and titanite are the most common alteration products in the host rock, whereas quartz, calcite and the above-mentioned sulfides are found in the veins.
4.1.2. Fluid Characteristics
Quartz and calcite are found among the sulfide grains. The coarser-grained quartz could be associated with later sulfides, while fine-grained quartz and calcite are clearly later products. Both may contain common ~5 µm-sized primary fluid inclusions in 3D clouds, far from any secondary plains. The observed constant phase ratio (~10 area% vapor (V) and ~90 area% liquid phase (L), though a rare, accidentally trapped mineral solid phase (S) occurs) suggests trapping from a homogeneous parent liquid (Appendix B, Figure A1). The homogenization temperature was Th(LV-L) = 127–178 °C in coarser-grained quartz (n = 48), Th(LV-L) = 69–91 °C in fine-grained quartz (n = 6) and Th(LV-L) = 49–93 °C in late calcite (n = 26). The salinity was rather stable with values between 2.12–4.18 NaCl equiv. wt.% (Appendix B, Figure A2). Raman spectroscopy identified methane in the vapor phase of each inclusion, and the accidentally trapped mineral was found to be sericite. Where methane content was quantified, 0.3 ± 0.05 mol/kg was calculated. Based on the above-mentioned data, a homogenization pressure of 59 ± 5 MPa was calculated [33] (Appendix A, Table A1).
4.1.3. Stable and Noble Gas Isotopic Composition
The average stable isotopic composition of the fluid inclusions occurring in quartz crystals of Corchia is δD = −42.39‰, while the average composition of calcite is δ13CPDB = −2.376 and δ18OSMOW = 21.238. The calculated [34] average oxygen isotope composition of fluid in equilibrium with calcite is δ18OSMOW = 0.275 (n = 9) (Table 3 and Table 4). To calculate this, we have used the temperature value obtained from the fluid inclusion microthermometry study of the same sample (Corchia: 70 °C). The noble gas isotopic composition of sulfide-hosted fluid inclusions from Reppia is 20Ne/36Ar* (normalized to air) = 0.756 (st. dev. = 0.1), and 4He/36Ar* (normalized to air) = 210.445 (st. dev. = 21.1) (Appendix A, Table A2).

Table 3.
Stable isotope geochemistry of late calcite and the calculated [34] composition of the fluid in equilibrium with calcite.

Table 4.
Results of the H isotope geochemical analyses of quartz-hosted fluid inclusions.
4.2. Stockwork Mineralizations
4.2.1. Texture and Mineralogy
Stockwork mineralizations were studied in basalt (Boccassuolo, Reppia, Casali–Monte Loreto), gabbro (Bargone, Campegli) and peridotite (Vigonzano). In Boccassuolo, the ore veins occur in two major blocks, separated by a subvertical, NW–SE-trending fault. In Reppia, the connection between the stockwork and massive sulfide ores is difficult to observe due to tectonic processes.
This stockwork type of mineralization contains less than 20% sulfides; pyrite, pyrrhotite, chalcopyrite, sphalerite and galena commonly occur, but some rare minerals, such as marcasite, millerite, chromite, magnetite, bornite, covellite and Pb-selenides, may also be found. Quartz, calcite and chlorite are common gangue minerals, but clay minerals, prehnite, pumpellyite and epidote also occur (Figure 3). Details on the different steps of gangue mineral formation, as well as their relation to the ore minerals in Bargone, Boccassuolo, Campegli, Reppia and Vigonzano, are found in [16,17].
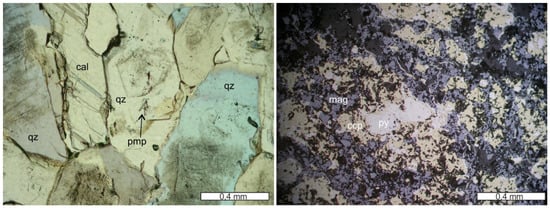
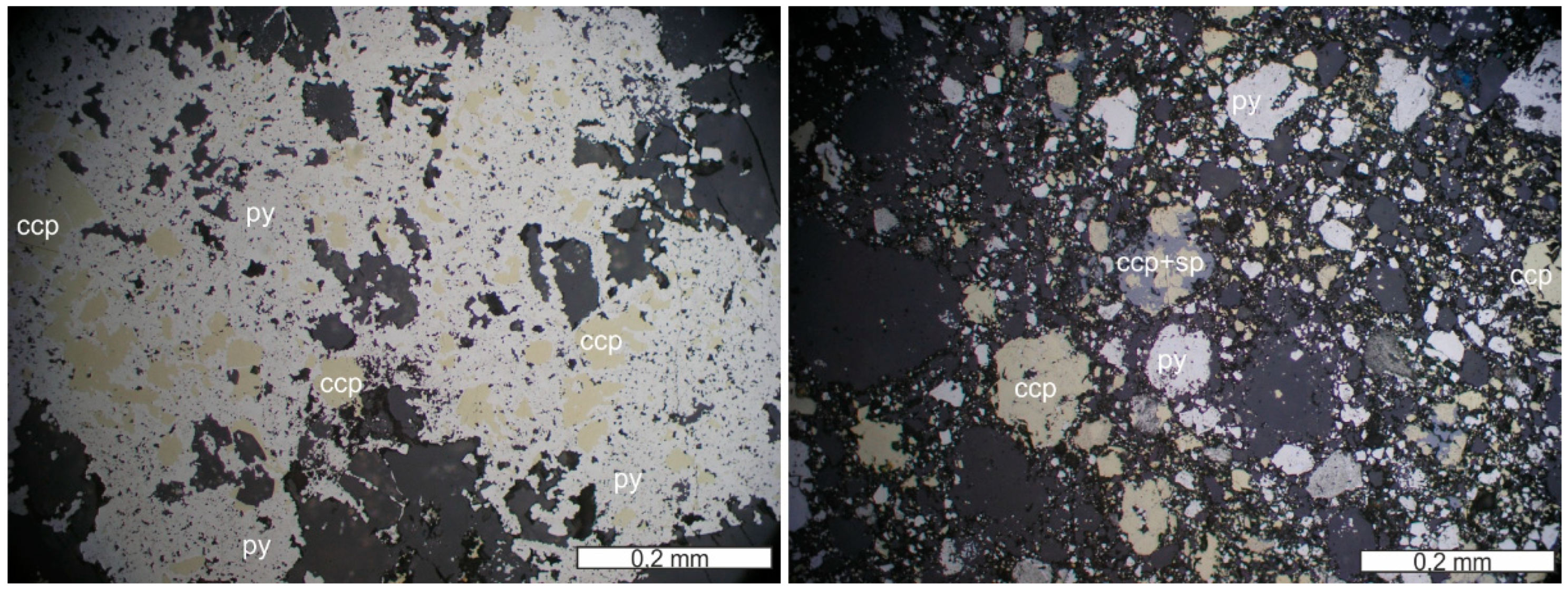
Figure 3.
Coarse-grained early quartz (qz) (at places with growth zones with primary fluid inclusions), later calcite (cal) and pumpellyite (pmp) of a stockwork vein from Campegli (transmitted light microphotograph, 1N; left). Semimassive, brecciated sulfide sample of a thick quartz vein from Vigonzano, showing early pyrite (py) surrounded by later chalcopyrite (ccp), magnetite (mag) and gangue minerals (quartz). Note that pyrite can also occur in later, thin veinlets (reflected light microphotograph, 1N; right).
Chlorite of the stockwork veins is most commonly type 1 Mg–chlorite (nomenclature by [32]) based on their composition (Table 2). This mineral may have formed either before quartz (e.g., in Campegli gabbro, at 220–300 °C, or in Monte Loreto basalt, at 300–340 °C), syngenetically with it (e.g., in Reppia basalt, at 185–215 °C), or as a later product (e.g., at some locations of Boccassuolo, at 120–160 °C; for details on this location, see the corresponding results of [13]) [22,23].
4.2.2. Fluid Characteristics
The fluid inclusion study was performed in different quartz generations and the late calcite, as described in [16,17]. In addition to those results, measurements were also performed in Casali–Monte Loreto. In summary of the fluid inclusion study results, the primary L+V(+S) fluid inclusions are commonly between <1 and 10 µm, occurring along growth zones of the crystals or in 3D inclusion clouds in the core of the crystals (Appendix B, Figure A1). The observed constant phase ratios suggest a homogeneous parent liquid. Based on Raman spectroscopy, sericite is the S phase, and the V phase always contains methane. The homogenization temperatures show a wide range (Th(LV-L) = 50–360 °C) (n = 716), depending on the study location and quartz/calcite generation. Within this range, quartz of Casali–Monte Loreto yielded a Th(LV-L) = 110–190 °C (n = 193) and fluid inclusions of its calcite resulted in a Th(LV-L) = 95–160 °C (n = 62). Slightly changeable salinity and methane content was observed at each location (2.2–9.3 NaCl equiv. wt.% salinity and 0.05–0.33 mol/kg methane), with the newly studied Casali–Monte Loreto showing results in the lower range (2.2–4.6 NaCl equiv. wt.% and 0.05–0.13 mol/kg methane) (Appendix B, Figure A2). Based on these data, the calculated homogenization pressure was between 20–50 MPa [33] (Appendix A, Table A1).
4.2.3. Stable and Noble Gas Isotopic Composition
The stable and noble gas isotopic composition of the hydrothermal minerals, as well as their fluid inclusions, were also studied. The hydrogen isotopic composition of the quartz-hosted fluid inclusions is between δD −65.12 and −30.39‰ (Table 4), while their noble gas isotopic composition is R/Ra = 0.097–0.173 (where R is the measured 3He/4He ratio, and Ra is (1.382 ± 0.006) × 10−6 [35]; (4He/36Ar)air = 17.126–2389.488 and (20Ne/36Ar)air = 0.419–5.579; Appendix A, Table A2). The stable isotopic composition of late calcite is δ18OSMOW = 15.0–22.7‰ and δ18CPDB = 0.71–(−6.96)‰ (n = 30). The calculated [31] oxygen isotope composition of fluid in equilibrium with calcite is δ18OSMOW = (−3.67)–4.28‰ (Table 3). To calculate this, we have used the temperature value obtained from the fluid inclusion microthermometry study of the same sample (Bargone: 117 °C; Boccassuolo—upper block: 80 °C; Boccassuolo—lower block: 90 °C; Monte Loreto: 128 °C).
5. Discussion
The studied Italian VMS deposits represent all levels of a Cyprus-type VMS system; therefore, we can obtain information on the formation of the massive sulfide lens as well as the stockwork ore.
5.1. Formation Conditions, Fluid Characteristics
The massive sulfide lenses form directly on the seafloor due to the rapid interaction between the hot hydrothermal fluid and the cold seawater [4]. The ore and gangue minerals commonly form together or continuously; therefore, we can obtain information on the ore genesis by studying the gangue minerals. Chlorite formed prior to the quartz, calcite and sericite and precipitated at approximately 200 °C. As homogeneous parent fluid was proven by the fluid inclusion petrography, the obtained homogenization temperature and pressure data can be interpreted as minimum formation temperatures and pressures. Thus, the following quartz and calcite precipitation occurred from a continuously cooling and low-salinity (average: 3.3 NaCl equiv. wt.%) fluid at a minimum formation temperature of 50–178 °C. This is caused by dilution and rapid cooling, depicting intensive mixing between hydrothermal fluid and seawater. The calculated minimum formation pressure (59 ± 5 MPa) is higher than the hydrostatic pressure typically affecting the massive sulfide lenses (~25 MPa [3]). This overpressure phenomenon could be caused by the rapid healing of the fluid migration pathways by newly formed hydrothermal minerals. Its result is the brecciation, well-observable in the samples.
Investigation of the relationship between the massive sulfide lenses and the stockwork mineralizations is difficult at several locations due to later tectonic processes, i.e., the obduction of the ophiolitic series. At some locations, certain levels of the system are missing, or parts of obviously different origins are close to each other (see, e.g., the different blocks of Boccassuolo). However, based on the known ore deposit model and analogies (e.g., [3,4,36] and the references cited therein), at least a distant connection between the massive sulfide lens and the stockwork zone can be assumed, and thus, we can model the processes in spite of the missing link. This connection is supported by the observed, similar hydrothermal fluid characteristics, i.e., the observable typical methane content and the similar stable and noble gas isotopic values.
Studying the gangue minerals also contributes to understanding the hydrothermal processes related to stockwork mineralization. This is well illustrated by [17], who studied quartz from Bargone, Boccassuolo, Campegli, Reppia and Vigonzano, demonstrating that combined use of fluid inclusion microthermometry, Raman spectroscopy, SEM-CL imaging and LA-ICP-MS can effectively reconstruct the physico-chemical characteristics of the hydrothermal fluid producing the barren and ore-forming stages of the evolving hydrothermal system. The additional fluid inclusion study results from Casali–Monte Loreto and the new chlorite thermometry data from Campegli, Monte Loreto and Reppia fit well into the above model; both suggest a dynamically changing hydrothermal system comprised of several consecutive steps. The calculated homogenization pressure (which can be interpreted as the minimum formation pressure due to the observed homogeneous parent fluid) is commonly found within the typical 30–60 MPa range of VMS stockwork systems [3,37]. Local variations are the results of the overpressure phenomena caused by the healing of fluid-flow pathways (fractures) by rapid precipitation of hydrothermal minerals. Therefore, pressure correction is not needed on the homogenization temperature (i.e., minimum formation temperature) data; they approximate the formation conditions well. This is also supported by the chlorite thermometry data from Reppia, where chlorite syngenetic with quartz yielded similar formation temperatures as the obtained homogenization temperatures from quartz (Tchl = 120–170 °C vs. TQtz1–2 = 120–200 °C). An important question, however, remained open: the source of the ore-forming hydrothermal fluid.
5.2. The Source of the Hydrothermal Fluid
The obtained common temperature and compositional data (minimum formation temperature: 50–360 °C, 2.2–9.3 NaCl equiv. wt.% salinity) are both in the typical range of VMS systems and basically suggest the seawater origin of the fluid [1]. Although methane content is not common in this deposit type, it may occur [4, and references therein], mostly related to abiotic processes (e.g., directly emanated from magma, thermal decay of organic material, reduction related to the serpentinization of ultramafic rocks) [38]. The interpretation of the above-shown compositional data and its variability is difficult, as several effects during its circulation could have modified the seawater. Based on the literature data, the most important influencing factors are the fluid–rock interaction during circulation in the crust, mixing with fresh seawater, phase separation (i.e., boiling), passing through evaporites in the host rock series and admixture of magmatic fluid component [1,3,39,40]. In the case of the studied deposits, phase separation and the effect of evaporitic host rock can be excluded based on the geological background as well as our fluid inclusion petrography. However, it is worth discussing the possibility and relative significance of the fluid–rock interaction processes and the magmatic fluid input. This question has both academic and economic significance and is often misinterpreted due to the difficulties of reliable proofing.
The stable O and C isotopic composition of late calcite is similar to the typical ranges of low-temperature alteration zones in VMS systems, mostly depicting fluid–rock interaction processes [4]. Results from Bargone, Boccassuolo (upper block) and Monte Loreto are in the same range (mostly between −3 and 0 δ13CPDB; −16 and −12 δ18OPDB), showing that the different host rock (basalt and gabbro) has a negligible effect on the stable isotopic composition. This is also supported by [41], who have found similarly enriched δ18O isotopic compositions in basalt, gabbro and serpentinite of the Ligurides, indicating an exchange with lower-temperature fluids at a near-seafloor position. However, Boccassuolo (lower block) shows somewhat different characteristics (between 0–1 δ13CPDB; −9 and −7 δ18OPDB), pointing to slightly different fluid characteristics for that location.
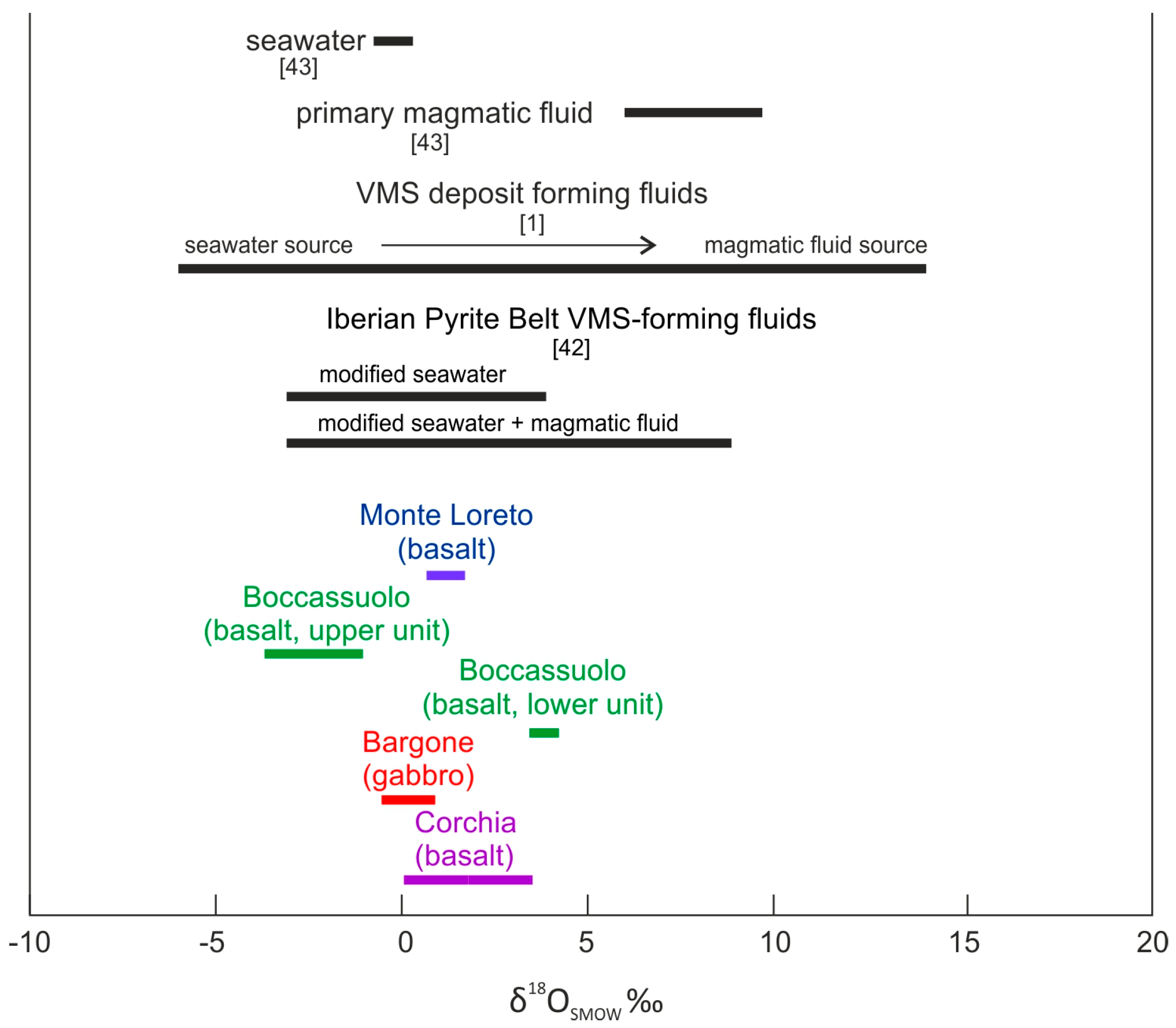
In addition, the calculated oxygen isotopic composition of the fluid in equilibrium with calcite remains in the typical VMS range [1], although the effect, which has modified the original seawater, cannot be unequivocally identified (Figure 4). Similar fluid salinity and O, H stable isotopic values were interpreted to support modified seawater plus magmatic fluid admixture for VMS deposits from the Iberian Pyrite Belt [42], but we would rather beware of such an explicit conclusion. Based on our results, in the case of Boccassuolo (upper block) and Bargone, we can exclude the significant magmatic fluid input, but we cannot reliably decide whether this is the case for Monte Loreto, Corchia and Boccassuolo (lower block) results (Figure 4). The possibility of magmatic fluid involvement is the highest in the case of Boccassuolo (lower block) and the lowest at Boccassuolo (upper block). This is in good correlation with the mineralogical and geochemical observations of [15], who emphasized the effect of crustal fluid circulation and leaching in the case of the anomalously high Pb content of Boccassuolo (upper block).
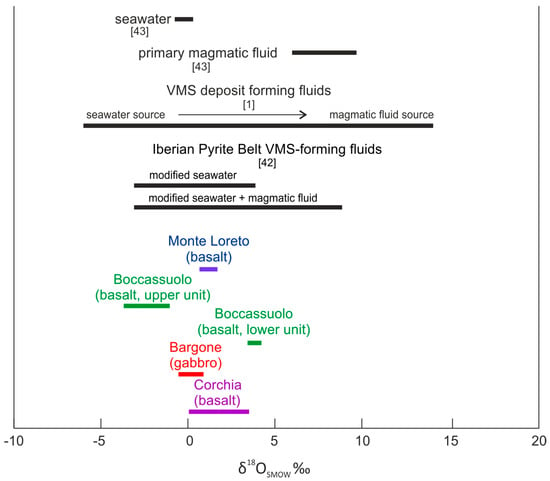
Figure 4.
Stable isotopic composition of the fluid in equilibrium with late calcite from the studied locations. Reference values of [1,42,43] are shown on the top (in black) to assist interpretation.
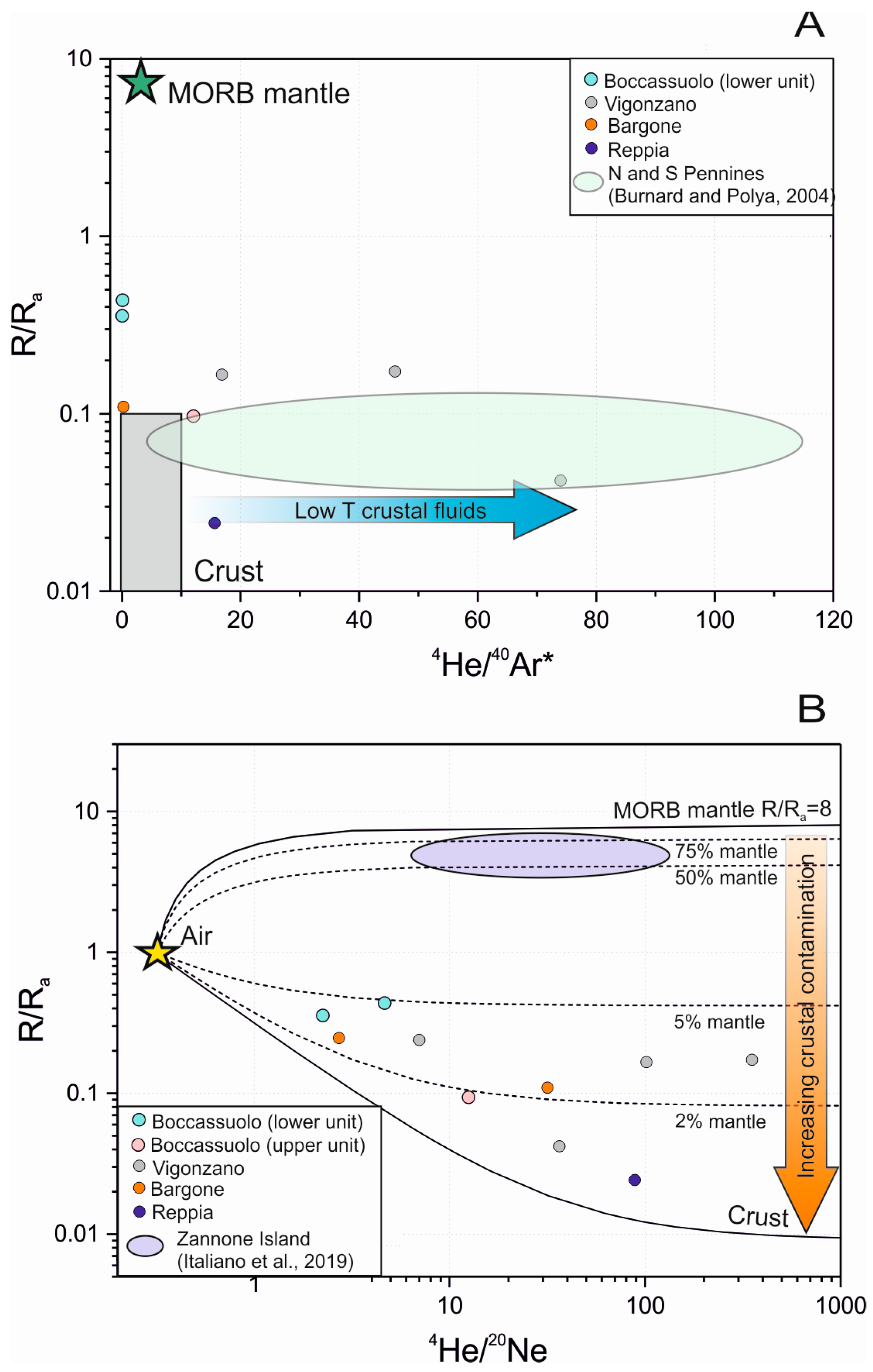
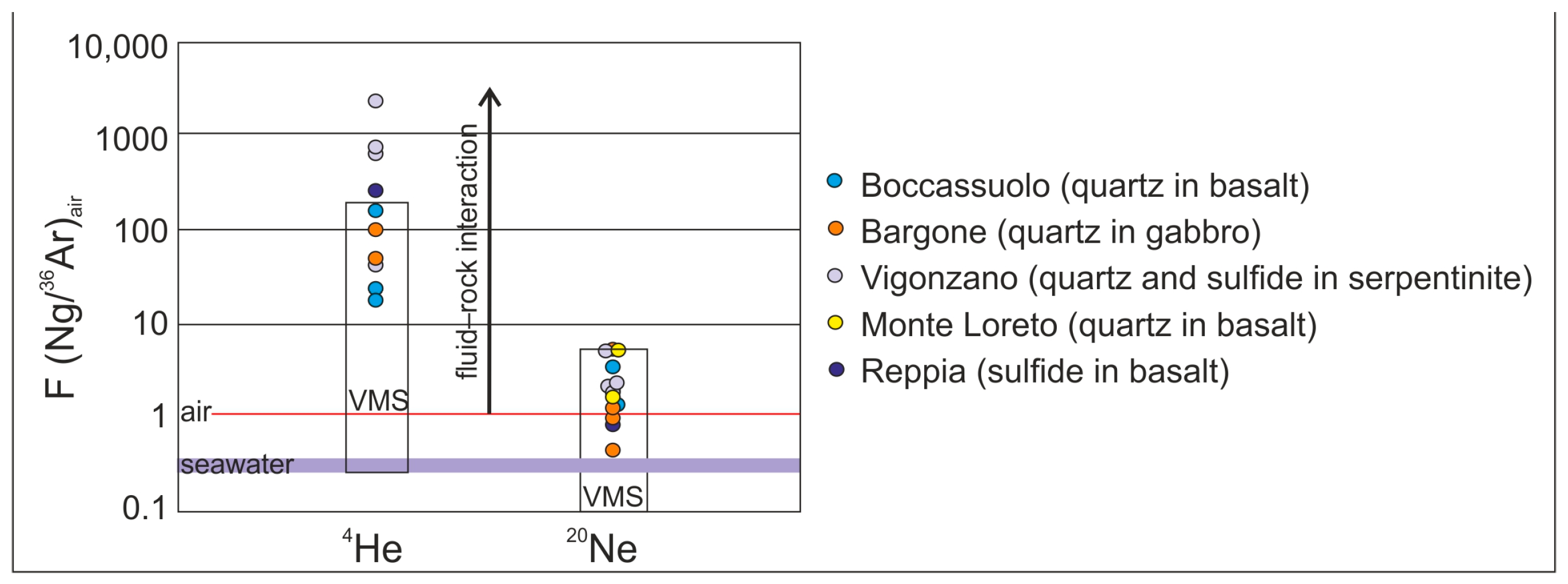
Stable and noble gas isotopic study of quartz and sulfide-hosted fluid inclusions complete the above results. The origin of the hydrothermal fluid can be effectively traced based on the fluid inclusions of hydrothermal minerals, such as quartz and sulfides, as they are less liable to diffusion [44]. This seems to be proven in our case, too, as there is no significant difference between our results obtained from quartz and sulfides. The obtained 40Ar/36Ar data are slightly different from the atmospheric values (40Ar/36Ar = 295 ± 0.5 [45]), showing crustal contribution [44]. Some exceptionally high values (up to 699 ± 29) can be explained by the potassium content of the commonly occurring sericite as an accidentally trapped mineral in the inclusions. The 4He/20Ne, as well as the 4He/40Ar* ratios, also support the deviation from atmospheric characteristics (Figure 5). This variance is most commonly of crustal origin, but in the case of the Boccassuolo (lower block), the magmatic (mantle) origin cannot be ruled out [44]. The ratio of He, Ne and Ar normalized to air also supports the modifying effect of fluid–rock interaction in the crust (Figure 6). However, the low R/Ra values (up to 0.17) are the unambiguous result of this process, and the mantle-derived magmatic component (which would result in higher amounts of 3He) can be clearly excluded for most of the samples [44]. This is supported by the δD values (commonly between −47 and −39‰), which are within the typical VMS range [1] and show lower values than those that are characteristic of the typical field of magmatic fluids (between δD −50 and −85‰ [43]). In the case of the Boccassuolo (lower block) samples, a small amount of mantle-derived (magmatic) fluid input can be assumed based on the combined evaluation of stable isotopic (see Figure 4 and δD between −42.99 and −65.12‰) and noble gas isotopic values (Figure 5).
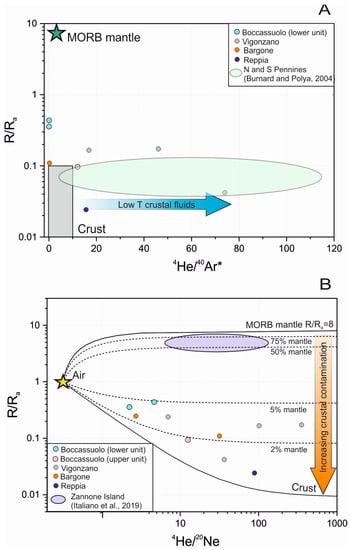
Figure 5.
Interpretation of noble gas isotope composition of quartz and sulfide-hosted fluid inclusions from the studied Italian locations; 4He/40Ar* (A) as well as 4He/20Ne (B) data both support a very slight magmatic fluid input for Boccassuolo (lower unit), while results of a fluid–rock interaction in the crust is clearly seen for the other localities (end-members in the R/Ra vs. 4He/20Ne plot): atmospheric (1 R/Ra, 0.318 4He/20Ne [46]), crustal (0.02 R/Ra, 1000 4He/20Ne [47]) and MORB (8 R/Ra, 1000 4He/20Ne [48]).
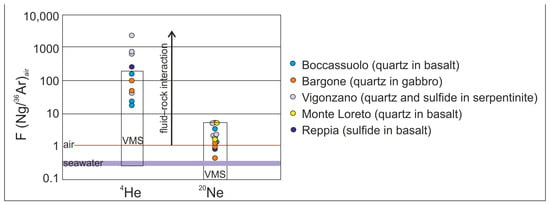
Figure 6.
Noble gas isotopic composition of quartz and sulfide-hosted fluid inclusions show evidence of a fluid–rock interaction. This support the establishment of a large fluid circulation system in the crust (typical VMS fields by [44] are shown for reference).
Summarizing our findings, we conclude that the studied Italian VMS mineralizations were formed by a seawater-originated hydrothermal fluid, in which its composition was modified by the fluid–rock interaction during its crustal circulation. The effect of the different host rocks is negligible, which can be explained by the fact that both basalt, gabbro or serpentinite were at a near-seafloor position at the time of deposit formation due to syntectonic processes [8,41]. Only the Boccassuolo (lower block) shows different stable and noble gas isotopic compositions suggesting a slight magmatic fluid input. This finding explains the difference in the ore grade and mineralogy well; while the lower block contains medium- to high-grade ore with higher Cu and Zn values, the upper block has low- to medium-grade ore with anomalously high Pb content [15]. Thus, the appearance of high-grade ore is in correlation with the possible magmatic fluid input.
6. Conclusions
The present research on VMS deposits of the Italian Northern Apennines has shown that the source of the VMS-forming hydrothermal fluid can be trustworthily traced by the combination of more mineralogical and geochemical study methods (namely, fluid inclusion and stable and noble gas isotopic studies). Our results support that most of the studied Italian VMS locations were formed by hydrothermal fluid of dominantly seawater origin, in which its composition evolved during long-lasting fluid–rock interaction in the crust. The only exception is the lower block of Boccassuolo, where a slight magmatic fluid input is also suggested (aside from the above-mentioned processes). This results in higher ore grades and slightly different mineralogy as well. Though the methods are well-known and often used in VMS case studies separately, a similarly complex approach is hardly available (see, e.g., [49,50]). Therefore, this novel methodology can also help other VMS locations in answering the open questions that remain.
Author Contributions
Conceptualization, G.B.K.; Methodology, G.B.K., K.M., Z.B., L.P. and G.C.; Formal analysis, G.B.K., K.M., Z.B., L.P., G.C., P.S., Z.K. and F.Z.; Investigation, G.B.K., K.M., Z.B., G.C., P.S. and Z.K.; Resources, F.Z., G.G. and G.B.K.; Writing—original draft preparation and editing, G.B.K.; Writing—review, Z.B., G.C., P.S., Z.K, F.Z. and G.G.; Visualization, G.B.K., Z.B.; Supervision, G.B.K.; Project administration, G.B.K.; Funding acquisition, G.B.K. All authors have read and agreed to the published version of the manuscript.
Funding
The National Research, Development and Innovation Office (NKFIH) has provided financial support to this research under grant agreement No. OTKA PD 112580 (to G.B.K.). This research was supported by the European Union and the State of Hungary and financed by the European Regional and Development Fund from the GINOP-2.3.2-15-2016-00009 ‘ICER’ project.
Data Availability Statement
All data supporting the reported results can be found in the manuscript and its appendices.
Acknowledgments
The ELTE RIRC is thanked for the access to the Raman spectroscopy laboratory, and the Eugen F. Stumpfl Electron Microprobe Laboratory of UZAG is thanked for the access to the EPMA laboratory.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Summary of primary fluid inclusion data.
Table A1.
Summary of primary fluid inclusion data.
| VMS Massive Sulfide | VMS Stockwork | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Corchia | Reppia | Bargone* | Boccassuolo* | Campegli* | Casali | Monte Loreto | Reppia* | Vigonzano* | ||
| Coarse-grained quartz (Qtz 1) | Th (°C) (core) | 170–270 | 170–225 | 150–205 | 290–330 | |||||
| Th (°C) (rim) | 135–180 | 125–180 | 120–160 | 260–310 | ||||||
| Salinity | 2.6–5.7 | 2.6–4.7 | 3.1–3.9 | 7.2–9.3 | ||||||
| CH4 | x | 0.13–0.22 | x | x | ||||||
| n | 53 | 58 | 70 | 18 | ||||||
| Coarse- and medium-grained quartz (Qtz 1–2) | Th (°C) | 150–360 | 110–180 | 110–190 | ||||||
| salinity | 3.3–8.3 | 3.7–4.6 | 2.2–3.5 | |||||||
| CH4 | 0.19–0.26 | 0.04–0.05 | 0.12–0.13 | |||||||
| n | 98 | 71 | 122 | |||||||
| Medium-grained quartz (Qtz 2) | Th (°C) | 127–178 | 110–158 | 122–171 | ||||||
| Salinity | 2.12–3.26 | 3.1–4.7 | ||||||||
| CH4 | 0.32–0.34 | x | 0.22–0.33 | |||||||
| n | 48 | 9 | 41 | |||||||
| Fine-grained quartz (Qtz 3) | Th (°C) | 69–91 | 107–118 | 80–160 | 50–125 | |||||
| CH4 | ? | ? | x | ? | ||||||
| n | 6 | 3 | 15 | 5 | ||||||
| Calcite | Th (°C) | 49–93 | 110–135 | 60–160 | 132–169 | 95–156 | ||||
| Salinity | 2.41–4.18 | 3.1–6.6 | ||||||||
| CH4 | x | ? | x | x | ? | |||||
| n | 26 | 13 | 35 | 6 | 62 | |||||
Salinity is given in NaCl equiv. wt.%. Methane content is given in mol/kg. x: methane content proven by Raman spectroscopy but not quantified. ?: methane content was not possible to check due to the small size of inclusions or strong luminescence of calcite. *: data from [16,17].

Table A2.
Summary of noble gas isotope measurement data.
Table A2.
Summary of noble gas isotope measurement data.
| Locality | Sample Type | Mass (g) | 3He (ccSTP/g) | St. dev. 3He | 4He (ccSTP/g) | St. dev. 4He | He (ccSTP/g) | R/Ra | St. dev. R/Ra | 20Ne (ccSTP/g) | St. dev. 20Ne | 21Ne (ccSTP/g) | St. dev. 21Ne |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boccassuolo (stockwork basalt) | Basalt, upper block | 1.58 | 2.29E-15 | 1.78E-16 | 1.7042E-08 | 1.92E-10 | 1.7042E-08 | 0.0970 | 0.0076 | 1.41E-09 | 3.75E-11 | 4.00E-12 | 8.37E-14 |
| Basalt, lower block | 1.38 | 2.19E-15 | 2.05E-16 | 4.4485E-09 | 5.02E-11 | 4.4485E-09 | 0.3560 | 0.0336 | 1.98E-09 | 5.25E-11 | 5.93E-12 | 1.17E-13 | |
| 1.50 | 2.56E-15 | 2.07E-16 | 4.2420E-09 | 4.79E-11 | 4.2420E-09 | 0.4364 | 0.0356 | 9.11E-10 | 2.41E-11 | 2.70E-12 | 5.89E-14 | ||
| Corchia (massive sulfide lens) | 1.38 | 3.97E-15 | 2.66E-16 | 1.0698E-07 | 1.21E-09 | 1.0698E-07 | 0.0268 | 0.0018 | 3.33E-12 | 1.48E-13 | |||
| Bargone (stockwork gabbro) | 1.45 | 1.56E-15 | 1.67E-16 | 4.5720E-09 | 5.16E-11 | 4.5720E-09 | 0.2465 | 0.0266 | 1.68E-09 | 4.48E-11 | 5.35E-12 | 1.05E-13 | |
| 1.26 | 2.31E-15 | 2.27E-16 | 1.5216E-08 | 1.72E-10 | 1.5216E-08 | 0.1095 | 0.0109 | 4.80E-10 | 1.28E-11 | 1.41E-12 | 3.98E-14 | ||
| Vigonzano (stockwork serpentinite) | 1.61 | 1.29E-15 | 1.65E-16 | 3.9088E-09 | 4.41E-11 | 3.9088E-09 | 0.2390 | 0.0306 | 5.58E-10 | 1.48E-11 | 1.63E-12 | 4.08E-14 | |
| 1.38 | 3.33E-15 | 2.50E-16 | 5.7313E-08 | 6.47E-10 | 5.7313E-08 | 0.0420 | 0.0032 | 1.57E-09 | 4.17E-11 | 4.75E-12 | 9.69E-14 | ||
| 1.48 | 1.37E-14 | 5.17E-16 | 5.9689E-08 | 6.74E-10 | 5.9689E-08 | 0.1663 | 0.0065 | 5.88E-10 | 1.56E-11 | 1.75E-12 | 4.44E-14 | ||
| 3.41 | 2.11E-14 | 4.64E-16 | 8.8095E-08 | 9.95E-10 | 8.8095E-08 | 0.1728 | 0.0043 | 2.50E-10 | 6.63E-12 | 7.15E-13 | 1.80E-14 | ||
| Reppia (massive sulfide lens) | 2.03 | 7.36E-16 | 1.16E-16 | 2.1960E-08 | 2.48E-10 | 2.1960E-08 | 0.0242 | 0.0038 | 2.48E-10 | 6.57E-12 | 7.96E-13 | 2.21E-14 | |
| 22Ne (ccSTP/g) | St. dev. 22Ne | 36Ar (ccSTP/g) | St. dev. 36Ar | 40Ar (ccSTP/g) | St. dev. 40Ar | Ne (ccSTP/g) | St. dev. Ne | Ar (ccSTP/g) | St. dev. Ar | 20Ne/22Ne | St. dev. 20/22Ne | ||
| Boccassuolo (stockwork basalt) | Basalt, upper block | 1.45E-10 | 4.23E-12 | 8.05E-10 | 8.49E-11 | 1.56E-09 | 6.98E-11 | 9.766 | 0.4 | ||||
| Basalt, lower block | 1.97E-10 | 5.73E-12 | 2.99E-09 | 2.99E-10 | 1.01E-06 | 1.07E-07 | 2.18E-09 | 9.60E-11 | 1.02E-06 | 1.48E-07 | 10.041 | 0.4 | |
| 9.23E-11 | 2.69E-12 | 1.91E-09 | 1.90E-10 | 6.13E-07 | 6.47E-08 | 1.01E-09 | 4.53E-11 | 6.15E-07 | 8.93E-08 | 9.880 | 0.4 | ||
| Corchia (massive sulfide lens) | 1.12E-10 | 3.28E-12 | |||||||||||
| Bargone (stockwork gabbro) | 1.65E-10 | 4.80E-12 | 5.45E-10 | 6.04E-11 | 1.85E-09 | 8.16E-11 | 10.215 | 0.4 | |||||
| 4.69E-11 | 1.38E-12 | 1.10E-09 | 1.11E-10 | 3.85E-07 | 4.09E-08 | 5.28E-10 | 2.57E-11 | 3.86E-07 | 5.64E-08 | 10.245 | 0.4 | ||
| Vigonzano (stockwork serpentinite) | 5.50E-11 | 1.61E-12 | 6.44E-10 | 6.64E-11 | 1.52E-07 | 1.63E-08 | 6.15E-10 | 2.88E-11 | 1.52E-07 | 2.27E-08 | 10.142 | 0.4 | |
| 1.58E-10 | 4.61E-12 | 5.29E-10 | 5.37E-11 | 1.57E-07 | 1.72E-08 | 1.74E-09 | 7.69E-11 | 1.58E-07 | 2.35E-08 | 9.941 | 0.4 | ||
| 6.09E-11 | 1.78E-12 | 4.58E-10 | 4.57E-11 | 1.39E-07 | 1.47E-08 | 6.50E-10 | 3.05E-11 | 1.39E-07 | 2.03E-08 | 9.642 | 0.4 | ||
| 2.44E-11 | 7.13E-13 | 2.20E-10 | 2.19E-11 | 6.69E-08 | 7.05E-09 | 2.75E-10 | 1.29E-11 | 6.71E-08 | 9.74E-09 | 10.235 | 0.4 | ||
| Reppia (massive sulfide lens) | 2.53E-11 | 7.42E-13 | 6.26E-10 | 6.24E-11 | 1.86E-07 | 1.96E-08 | 2.74E-10 | 1.32E-11 | 1.87E-07 | 2.71E-08 | 9.793 | 0.4 | |
| 21Ne/22Ne | St. dev. 21/22Ne | 40Ar/36Ar | St. dev. 40/36Ar | 4He/20Ne | St. dev. 4He/20Ne | 4He/40Ar * | St. dev. 4He/40Ar * | 4He/36Ar * (normalized to air) | St. dev. 4He/36Ar * | 20Ne/36Ar * (normalized to air) | St. dev. 20Ne/36Ar * | ||
| Boccassuolo (stockwork basalt) | Basalt, upper block | 0.028 | 0.001 | 12.1 | 0.3 | 126.9 | 13.5 | 3.4 | 0.4 | ||||
| Basalt, lower block | 0.030 | 0.001 | 338.6 | 49.2 | 2.2 | 0.1 | 0.03 | 0.001 | 8.910 | 0.9 | 1.262 | 0.1 | |
| 0.029 | 0.001 | 321.1 | 46.6 | 4.7 | 0.1 | 0.09 | 0.003 | 13.337 | 1.3 | 0.913 | 0.1 | ||
| Corchia (massive sulfide lens) | 0.030 | 0.002 | 0.0 | ||||||||||
| Bargone (stockwork gabbro) | 0.032 | 0.001 | 2.7 | 0.1 | 50.330 | 5.6 | 5.900 | 0.7 | |||||
| 0.030 | 0.001 | 349.3 | 51.1 | 31.7 | 0.9 | 0.26 | 0.027 | 82.875 | 8.4 | 0.833 | 0.1 | ||
| Vigonzano (stockwork serpentinite) | 0.030 | 0.001 | 235.9 | 35.1 | 7.0 | 0.2 | 36.422 | 3.8 | 1.657 | 0.2 | |||
| 0.030 | 0.001 | 297.0 | 44.3 | 36.4 | 1.0 | 73.99 | 3.323 | 650.163 | 66.5 | 5.683 | 0.6 | ||
| 0.029 | 0.001 | 303.2 | 44.0 | 101.6 | 2.9 | 16.85 | 1.788 | 781.106 | 78.5 | 2.449 | 0.3 | ||
| 0.029 | 0.001 | 304.2 | 44.2 | 352.6 | 10.2 | 46.05 | 1.604 | 2403.878 | 241.5 | 2.172 | 0.2 | ||
| Reppia (massive sulfide lens) | 0.031 | 0.001 | 297.7 | 43.2 | 88.6 | 2.6 | 15.64 | 0.540 | 210.445 | 21.1 | 0.756 | 0.1 | |
St. dev: standard deviation.
Appendix B
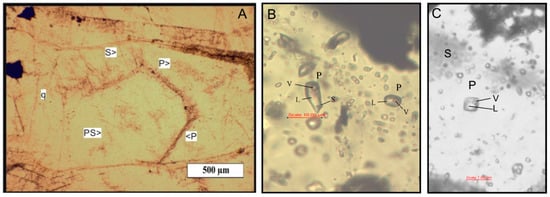
Figure A1.
Microphotographs of fluid inclusions from the stratiform ore of Reppia and Corchia as well as from the stockwork ore of Casali–Monte Loreto. (A): location of primary (P), secondary (S) and pseudosecondary (PS) inclusions in quartz from Casali–Monte Loreto. (B): liquid (L), vapor (V) and accidentally trapped solid (S) containing primary inclusion in quartz from Reppia. (C): negative crystal-shaped two-phase inclusion in calcite from Corchia.
Figure A1.
Microphotographs of fluid inclusions from the stratiform ore of Reppia and Corchia as well as from the stockwork ore of Casali–Monte Loreto. (A): location of primary (P), secondary (S) and pseudosecondary (PS) inclusions in quartz from Casali–Monte Loreto. (B): liquid (L), vapor (V) and accidentally trapped solid (S) containing primary inclusion in quartz from Reppia. (C): negative crystal-shaped two-phase inclusion in calcite from Corchia.
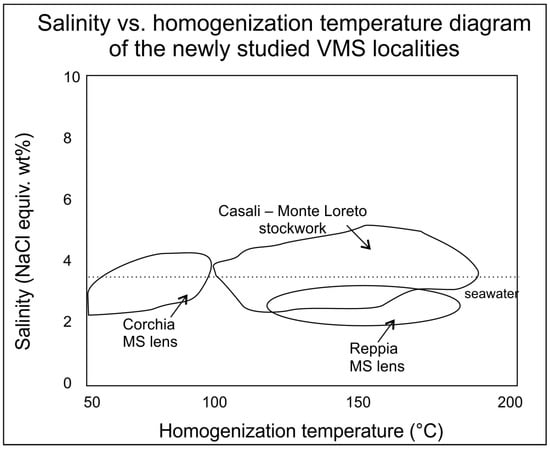
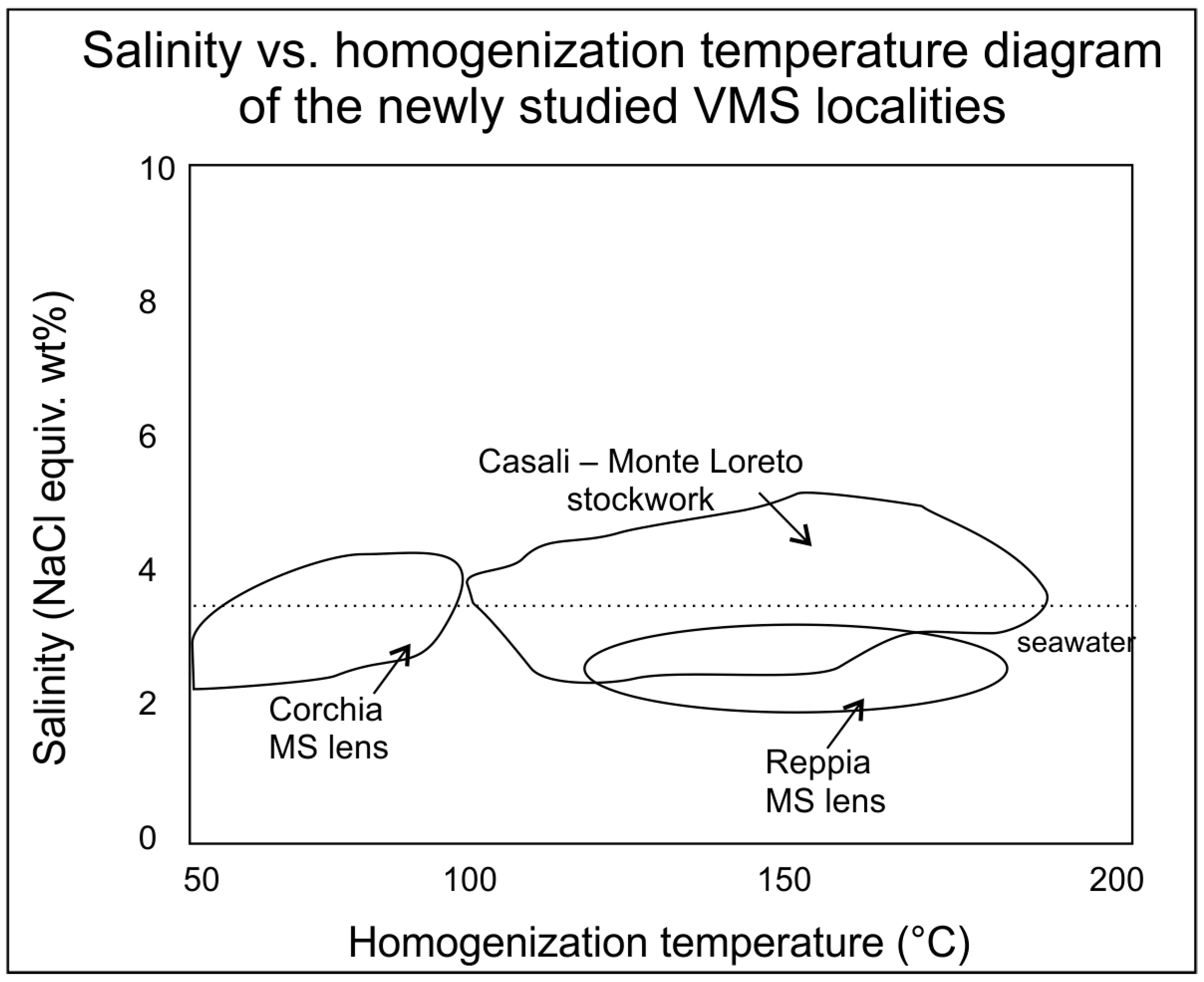
Figure A2.
Salinity vs. homogenization temperature diagram of the newly studied VMS localities (quartz and calcite from the massive sulfide/MS/lenses at Corchia and Reppia and the stockwork veins of Casali–Monte Loreto).
Figure A2.
Salinity vs. homogenization temperature diagram of the newly studied VMS localities (quartz and calcite from the massive sulfide/MS/lenses at Corchia and Reppia and the stockwork veins of Casali–Monte Loreto).
References
- Bodnar, R.J.; Lecumberri-Sanchez, P.; Moncada, D.; Steele-MacInnis, M. Fluid inclusions in hydrothermal ore deposits. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; Volume 13, pp. 119–142. [Google Scholar]
- Herzig, P.M.; Hannington, M.D. Polymetallic massive sulphides at the modern seafloor: A review. Ore Geol. Rev. 1995, 10, 95–115. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer: Dordrecht, The Netherlands, 2009; 1250p. [Google Scholar]
- Shanks, W.C.P.; Thurston, R. (Eds.) Volcanogenic Massive Sulfide Occurrence Model; U.S. Geological Survey Scientific Investigations Report 2010–5070–C; U.S. Geological Survey: Washington, DC, USA, 2012; 345p.
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Yang, K.; Scott, S.D. Magmatic sources of volatiles and metals for volcanogenic massive sulfide deposits on modern and ancient seafloors: Evidence from melt inclusions. In Mineral Deposit Research: Meeting the Global Challenge; Mao, J., Bierlein, F.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 715–718. [Google Scholar]
- Molli, G.; Crispini, L.; Mosca, P.; Piana, P.; Federico, L. Geology of the Western Alps-Northern Apennine junction area: A regional review. J. Virtual Explor. 2010, 36, 10. [Google Scholar] [CrossRef]
- Barrett, T.J. Review of stratigraphic aspects of the ophiolitic rocks and pelagic sediments of the Vara complex, North Apennines, Italy. Ofioliti 1982, 7, 3–46. [Google Scholar]
- Abbate, E.; Bortolotti, V.; Principi, G. Apennine ophiolites: A peculiar oceanic crust. In Tethyan Ophiolites; Ofioliti Special Issue; Rocci, G., Ed.; Pitagora Editrici: Bologna, Italy, 1980; Volume 1, pp. 59–96. [Google Scholar]
- Piccardo, G.B.; Rampone, E.; Romairone, A. Formation and composition of the oceanic lithosphere of the Ligurian Tethys: Inferences from the Ligurian ophiolites. Ofioliti 2002, 27, 145–161. [Google Scholar]
- Garuti, G.; Zaccarini, F. Assemblage and composition of Au, Ag and U minerals in Volcanic-Associated Massive Sulfide deposits of the Northern Apennine Ophiolites (Italy). Can. Mineral. 2005, 43, 935–950. [Google Scholar] [CrossRef]
- Garuti, G.; Bartoli, O.; Scacchetti, M.; Zaccarini, F. Geological setting and structural styles of Volcanic Massive Sulphide deposits in the Northern Apennines (Italy): Evidence for seafloor and sub-seafloor hydrothermal activity in unconventional ophiolites of the Mesozoic Tethys. Boletín Soc. Geológica Mex. 2008, 60, 121–145. [Google Scholar] [CrossRef]
- Zaccarini, F.; Garuti, G. Mineralogy and chemical composition of VMS deposits of northern Apennine ophiolites, Italy: Evidence for the influence of country rock type on ore composition. Mineral. Petrol. 2008, 94, 61–83. [Google Scholar] [CrossRef]
- Garuti, G.; Alfonso, P.; Zaccarini, F.; Proenza, J.A. Sulfur-isotope variations in sulphide minerals from massive sulphide deposits of the Northern Apennine ophiolites: Inorganic and biogenic constraints. Ofioliti 2009, 34, 43–62. [Google Scholar]
- Garuti, G.; Zaccarini, F.; Scacchetti, M.; Bartoli, O. The Pb-rich sulphide veins in the Boccassuolo ophiolite: Implications for the evolution of hydrothermal activity across the ocean-continent transition in the Ligurian Tethys (Northern-Apennine, Italy). Lithos 2011, 124, 243–254. [Google Scholar] [CrossRef]
- Kiss, G.B. Fluid inclusion study of the Boccassuolo VMS-related stockwork deposit (Northern Apennine ophiolites, Italy). Geol. Croat. 2015, 68, 285–302. [Google Scholar] [CrossRef]
- Kiss, G.B.; Bendő, Z.; Garuti, G.; Zaccarini, F.; Király, E.; Molnár, F. Reconstruction of hydrothermal processes in the Cyprus type Fe-Cu-Zn deposits of the Italian Northern Apennines: Results of combined fluid inclusion microthermometry, SEM-CL im-aging and trace element analyses by LA-ICP-MS. Minerals 2021, 11, 165. [Google Scholar] [CrossRef]
- Bertolani, M. I giacimenti cupriferi nelle ofioliti di Sestri Levante (Liguria). Period. Mineral. 1952, 21, 149–170. [Google Scholar]
- Hall, D.L.; Sterner, S.M.; Bodnar, R.J. Freezing point depression of NaCl-KCl-H2O solutions. Econ. Geol. 1988, 83, 197–202. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, L.; Li, N.; Liu, J. Raman spectroscopic study for the determination of Cl-concentration (molarity scale) in aqueous solutions: Application to fluid inclusions. Chem. Geol. 2010, 272, 55–61. [Google Scholar] [CrossRef]
- Guillaume, D.; Teinturier, S.; Dubessy, J.; Pironon, J. Calibration of methane analysis by Raman spectroscopy in H2O–NaCl–CH4 fluid inclusions. Chem. Geol. 2003, 194, 41–49. [Google Scholar] [CrossRef]
- Kranidiotis, P.; MacLean, W.H. Systematics of chlorite alteration at the Phelps Dodge massive sulfide deposit, Matagami, Quebec. Econ. Geol. 1987, 82, 1898–1911. [Google Scholar] [CrossRef]
- Cathelineau, M.; Izquierdo, G. Temperature-Composition relationships of authigenic micaceous minerals in the Los Azufres geothermal system. Contrib. Mineral. Petrol. 1988, 100, 418–428. [Google Scholar] [CrossRef]
- Zang, W.; Fyfe, W.S. Chloritization of the hydrothermally altered bedrock at the Igarapé Bahia gold deposit, Carajás, Brazil. Miner. Depos. 1995, 30, 30–38. [Google Scholar] [CrossRef]
- Inoue, A.; Meunier, A.; Patrier-Mas, P.; Rigault, C.; Beaufort, D.; Vieillard, P. Application of chemical geothermometry to low-temperature trioctahedral chlorites. Clays Clay Mineral. 2009, 57, 371–382. [Google Scholar] [CrossRef]
- Bourdelle, F.; Parra, T.; Chopin, C.; Beyssac, O. A new chlorite geothermometer for diagenetic to low-grade metamorphic conditions. Contrib. Mineral. Petrol. 2013, 165, 723–735. [Google Scholar] [CrossRef]
- Lanari, P.; Wagner, T.; Vidal, O. A thermodynamic model for di-trioctahedral chlorite from experimental and natural data in the system MgO–FeO–Al2O3–SiO2–H2O: Applications to P–T sections and geothermometry. Contrib. Mineral. Petrol. 2014, 167, 968. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Burgess, R.; Marty, B. Tracing fluid origin, transport and interaction in the crust. Rev. Mineral. Geochem. 2002, 47, 539–614. [Google Scholar] [CrossRef]
- Spötl, C.; Vennemann, T.W. Continuous–flow isotope ratio mass spectrometric analysis of carbonate minerals. Rapid Commun. Mass Spectrom. 2003, 19, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Czuppon, G.; Ramsay, R.R.; Özgenc, I.; Demény, A.; Gwalani, L.G.; Rogers, K.; Eves, A.; Papp, L.; Palcsu, L.; Berkesi, M.; et al. Stable (H, O, C) and noble-gas (He and Ar) isotopic compositions from calcite and fluorite in the Speewah dome, Kimberley region, western Australia: Implications for the conditions of crystallization and evidence for the influence of crustal-fluid mixing. Mineral. Petrol. 2014, 108, 759–775. [Google Scholar] [CrossRef]
- Demény, A.; Czuppon, G.; Kern, Z.; Leél-Őssy, S.; Németh, A.; Szabó, M.; Tóth, M.; Wu, C.-C.; Shen, C.-C.; Molnár, M.; et al. Recrystallization-induced oxygen isotope changes in inclusion-hosted water of speleothems—Paleoclimatological implications. Quatern. Int. 2016, 415, 25–32. [Google Scholar] [CrossRef]
- Zane, A.; Weiss, Z. A procedure for classifying rock-forming chlorites based on microprobe data. Rend. Lincei Sci. Fis. Nat. 1998, 9, 51–56. [Google Scholar] [CrossRef]
- Duan, Z.; Mao, S. A thermodynamic model for calculating methane solubility, density and gas phase composition of methane-bearing aqueous fluids from 273 to 523 K and from 1 to 2000 bar. Geochim. Cosmochim. Acta 2006, 70, 3369–3386. [Google Scholar] [CrossRef]
- Friedman, I.; O’Neil, J.R. Compilation of stable isotope fractionation factors of geochemical interest. In Data of Geochemistry; USGS Numbered Series, Professional Paper 440-KK; US Government Printing Office: Washington, DC, USA, 1977. [Google Scholar]
- Sano, Y.; Marty, B.; Burnard, P. Noble gases in the atmosphere. In The Noble Gases as Geochemical Tracers; Burnard, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 17–31. [Google Scholar]
- Barrie, C.T.; Hannington, M.D. Introduction: Classification of VMS deposits based on host rock composition. Rev. Econ. Geol. 1999, 8, 2–10. [Google Scholar]
- Steele-Macinnis, M.; Han, L.; Lowell, R.P.; Rimstidt, J.D.; Bodnar, R.J. Quartz precipitation and fluid inclusion characteristics in sub-seafloor hydrothermal systems associated with volcanogenic massive sulphide deposits. Centr. Eur. J. Geosci. 2012, 4, 275–286. [Google Scholar]
- Etiope, G.; Schoell, M. Abiotic gas: Atypical but not rare. Elements 2014, 10, 291–296. [Google Scholar] [CrossRef]
- Foustoukos, D.I.; Seyfried, W.E. Fluid phase separation processes in submarine hydrothermal systems. Rev. Mineral. Geochem. 2007, 65, 213–239. [Google Scholar] [CrossRef]
- Nehlig, P. Salinity of oceanic hydrothermal fluids: A fluid inclusion study. Earth Planet. Sci. Lett. 1991, 102, 310–325. [Google Scholar] [CrossRef]
- Barrett, T.J.; Friedrichsen, H. Stable isotopic composition of atypical ophiolitic rocks from east Liguria, Italy. Chem. Geol. 1989, 80, 71–84. [Google Scholar] [CrossRef]
- Tornos, F. Environment of formation and styles of volcanogenic massive sulfides: The Iberian Pyrite Belt. Ore Geol. Rev. 2006, 28, 259–307. [Google Scholar] [CrossRef]
- Taylor, H.P., Jr. Oxygen isotope studies of hydrothermal mineral deposits. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Holt, Rinehart and Wiston: New York, NY, USA, 1967; pp. 109–142. [Google Scholar]
- Kendrick, M.A.; Burnard, P. Noble gases and halogens in fluid inclusions: A journey through the earth’s crust. In The Noble Gases as Geochemical Tracers—Advances in Isotope Geochemistry; Burnard, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 319–370. [Google Scholar]
- Nier, A.O. A redetermination of the relative abundances of the isotopes of carbon, nitrogen, oxygen, argon, and potassium. Phys. Rev. 1950, 77, 789–793. [Google Scholar] [CrossRef]
- Sano, Y.; Wakita, H. Geographical distribution of 3He/4He ratios in Japan: Implications for arc tectonics and incipient magmatism. J. Geophys. Res. Solid Earth 1985, 90, 8729–8741. [Google Scholar] [CrossRef]
- Sano, Y.; Marty, B. Origin of carbon in fumarolic gas from island arcs. Chem. Geol. 1995, 119, 265–274. [Google Scholar] [CrossRef]
- Graham, D.W. Noble Gas Isotope Geochemistry of Mid-Ocean Ridge and Ocean Island Basalts: Characterization of Mantle Source Reservoirs. Rev. Mineral. Geochem. 2002, 47, 247–317. [Google Scholar] [CrossRef]
- Yang, F.; Li, Q.; Yang, C.; Zhang, Z. A combined fluid inclusion and S–H–O–He–Ar isotope study of the Devonian Ashele VMS-type copper–zinc deposit in the Altay orogenic belt, northwest China. J. Asian Earth Sci. 2018, 161, 139–163. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, B.; Yang, F.; Li, Q.; Wang, Y. Zircon U–Pb age, fluid inclusion, and H–C–O–He–Ar–S isotopic compositions as an index to the VMS-type mineralization: A case study from the Wulasigou polymetallic deposit, Altay Orogenic Belt, Northwest China. J. Geochem. Expl. 2021, 222, 106720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).