Geochemical Characteristics of the Mineral Assemblages from the Niukutou Pb-Zn Skarn Deposit, East Kunlun Mountains, and Their Metallogenic Implications
Abstract
1. Introduction
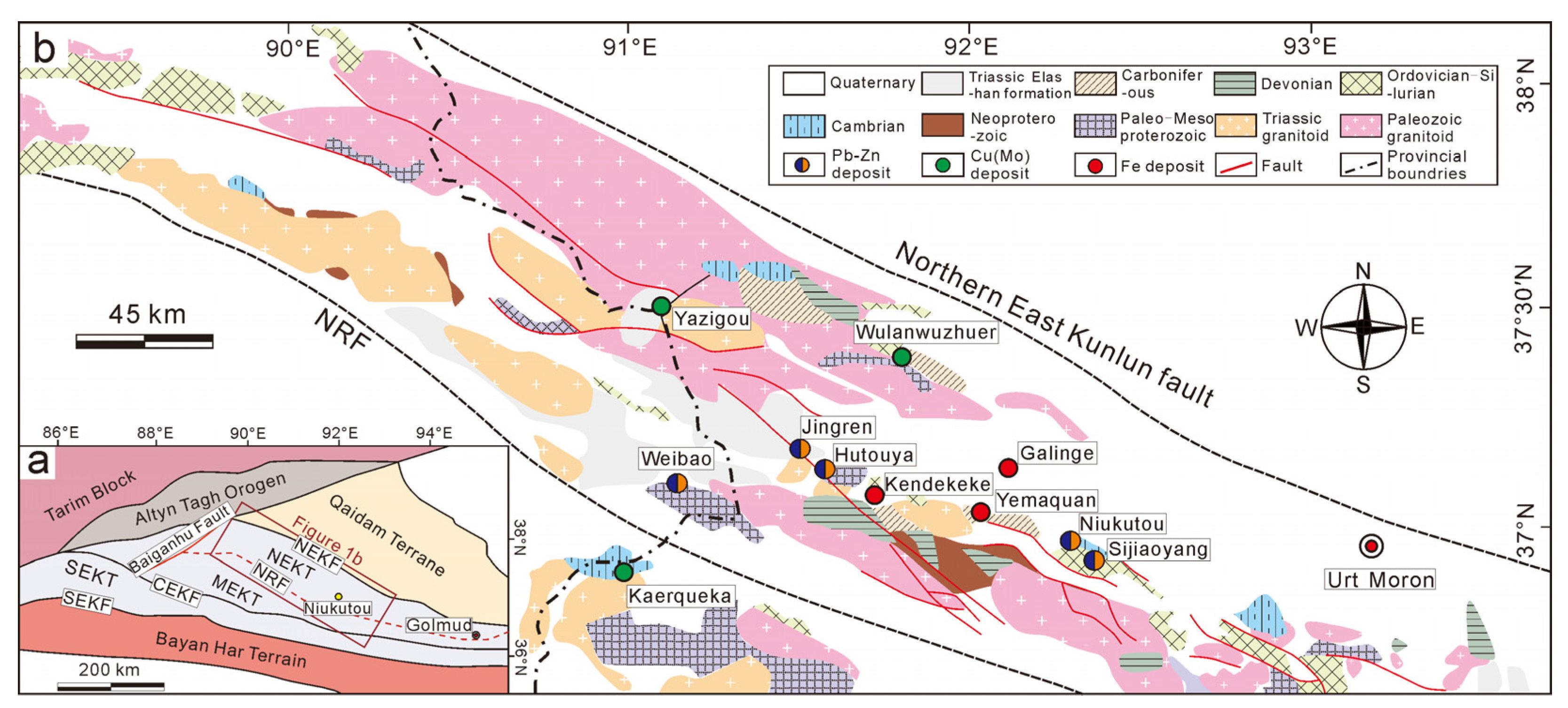
2. Regional Geology
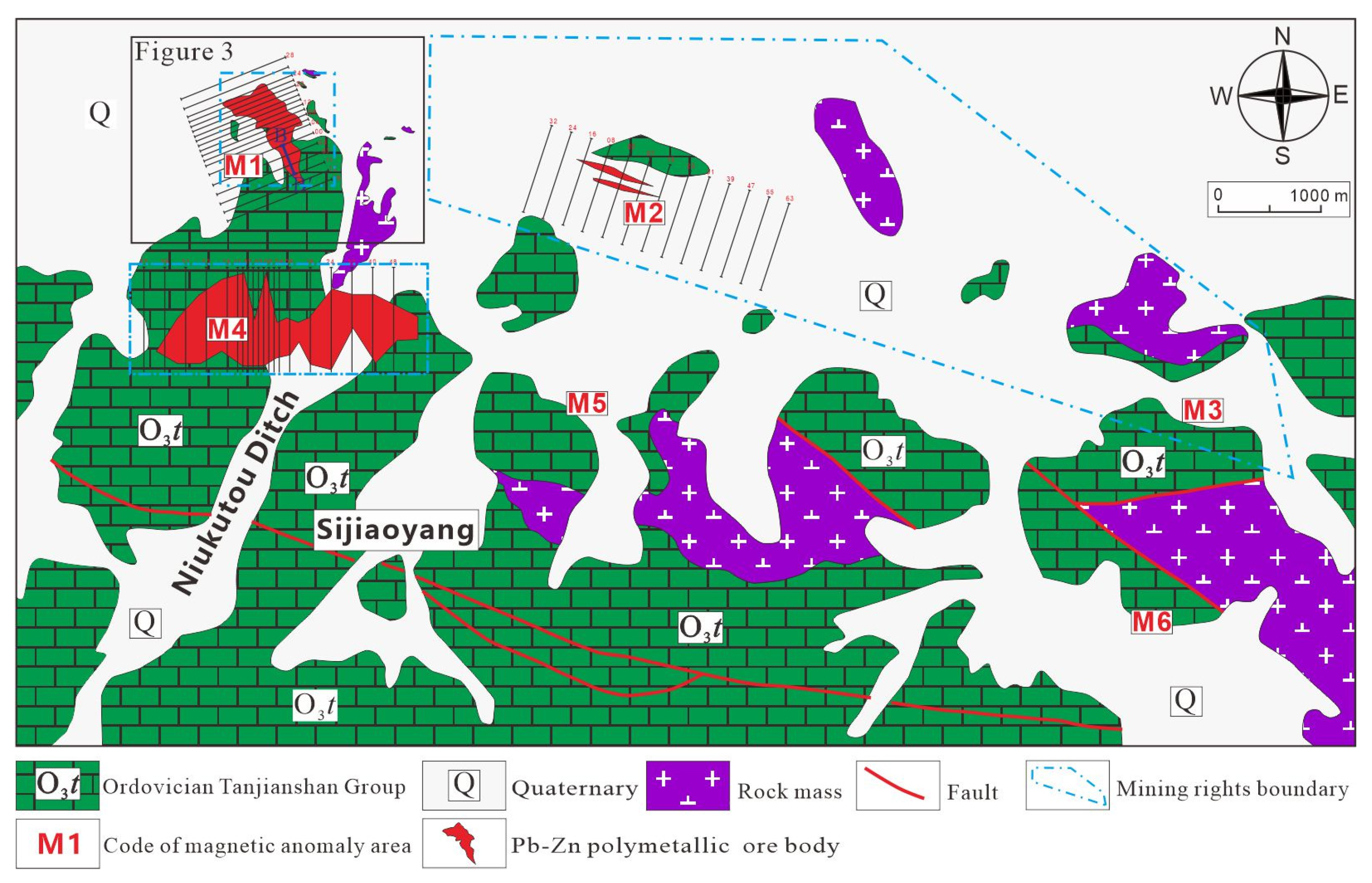
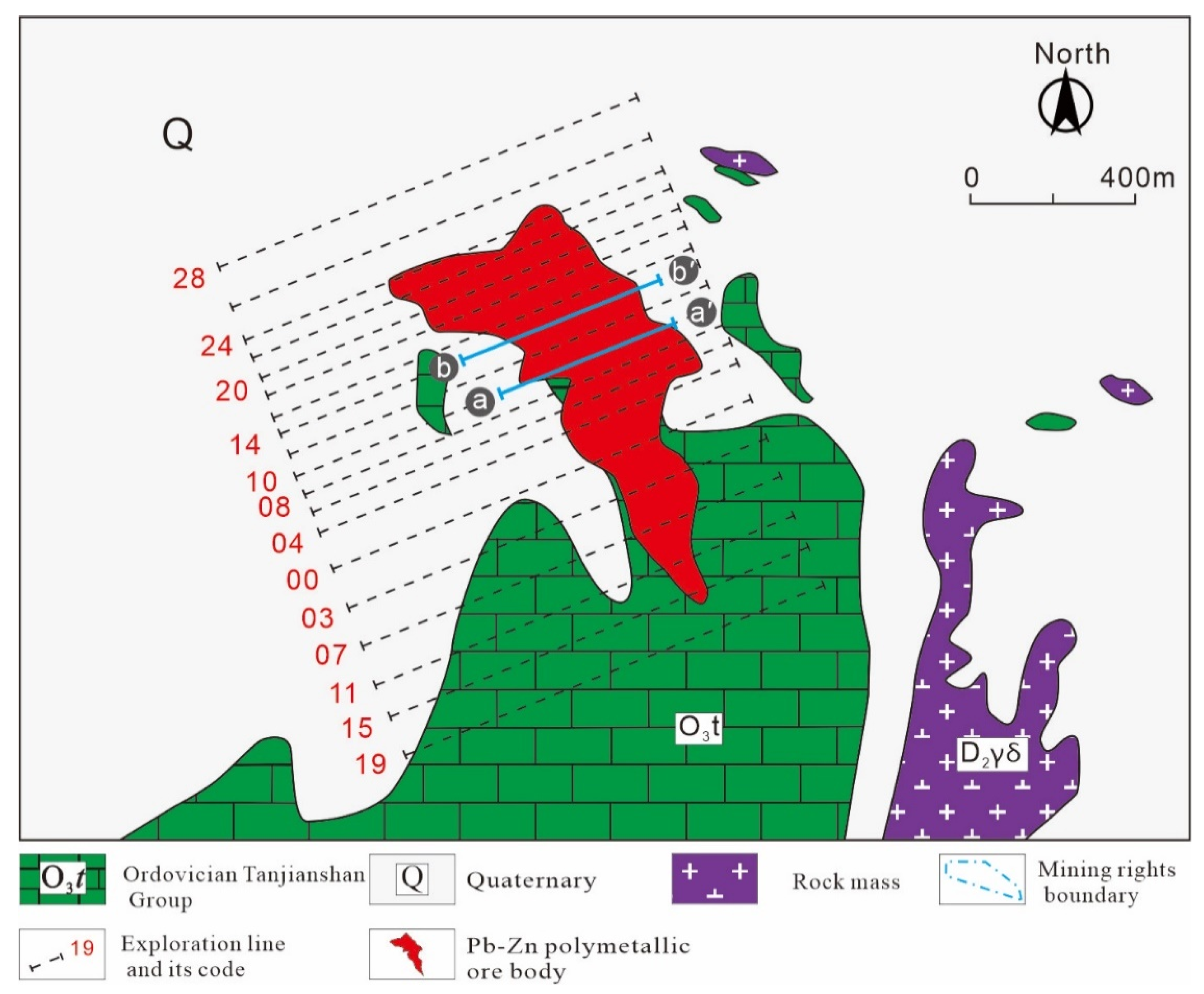
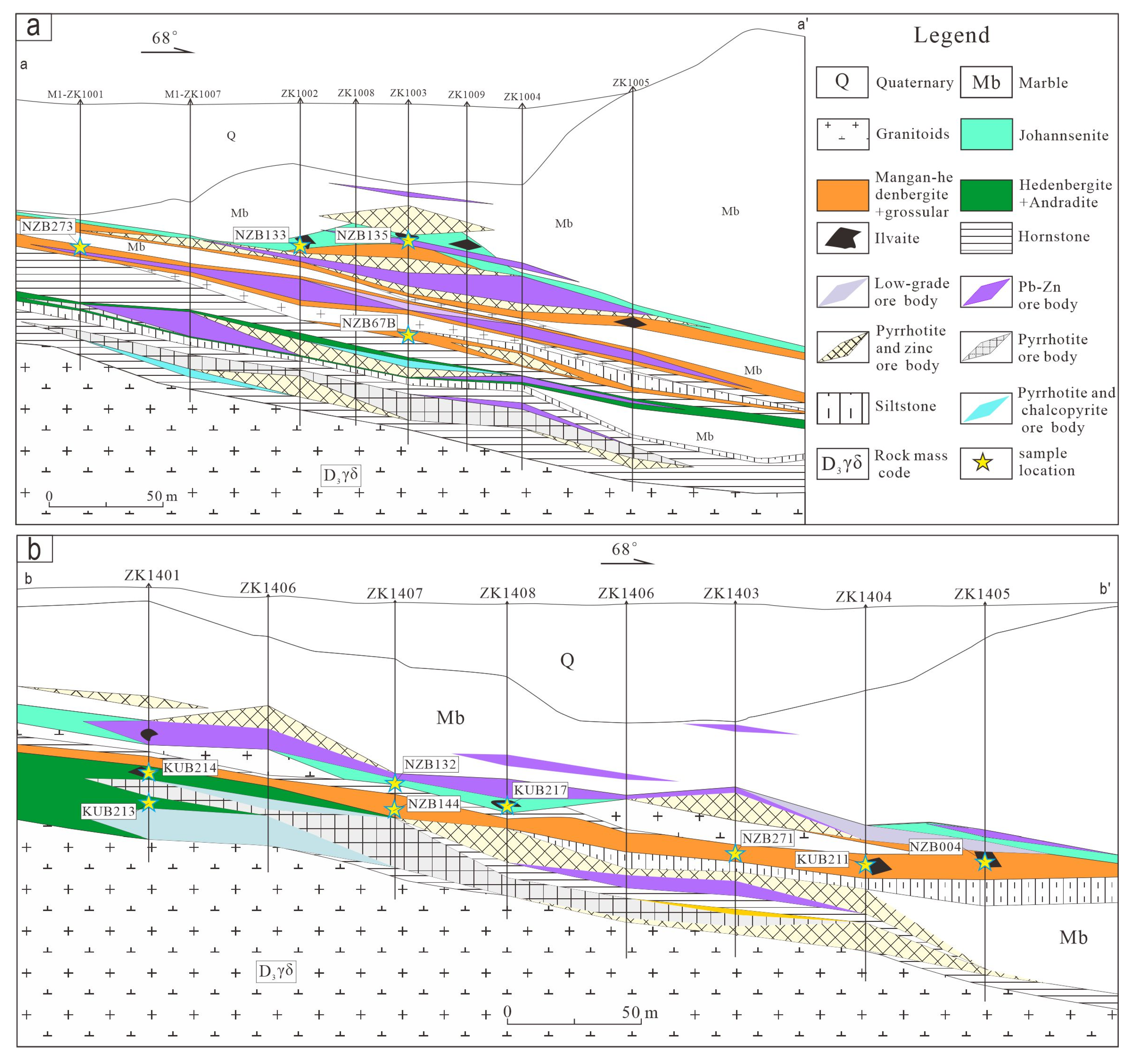
3. Deposit Geology
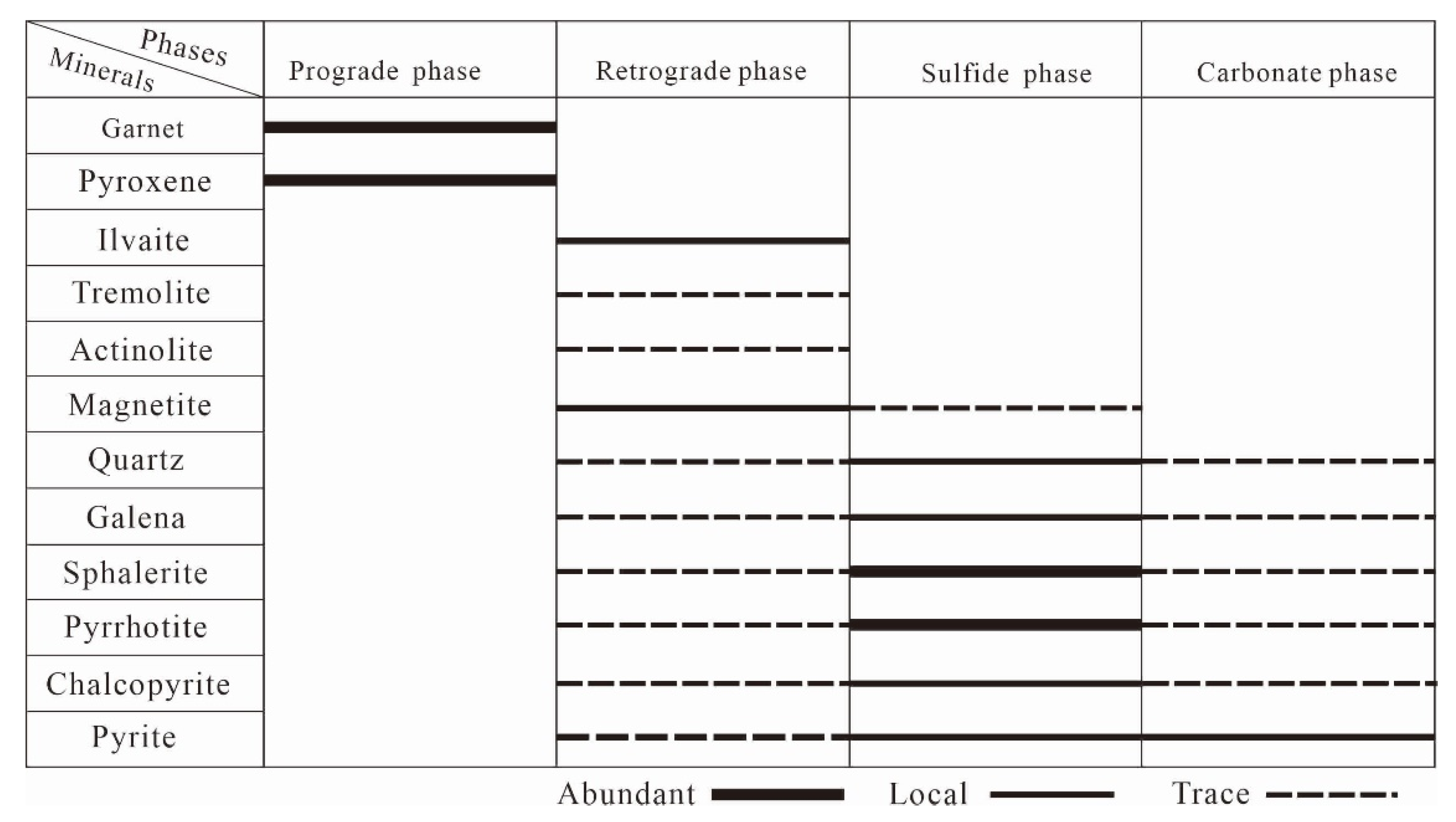
4. Mineralogical Characteristics
4.1. Analytical Methods
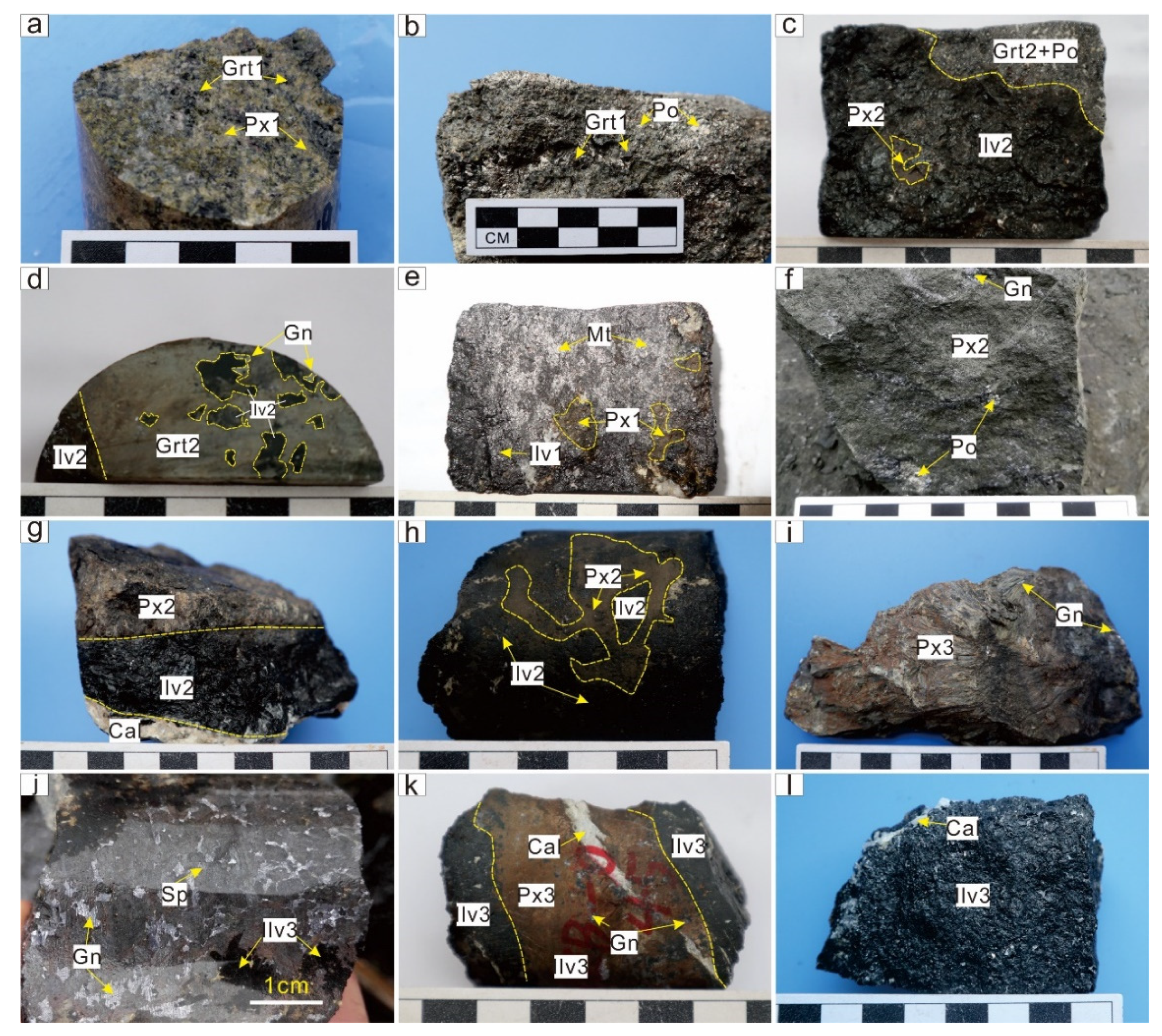
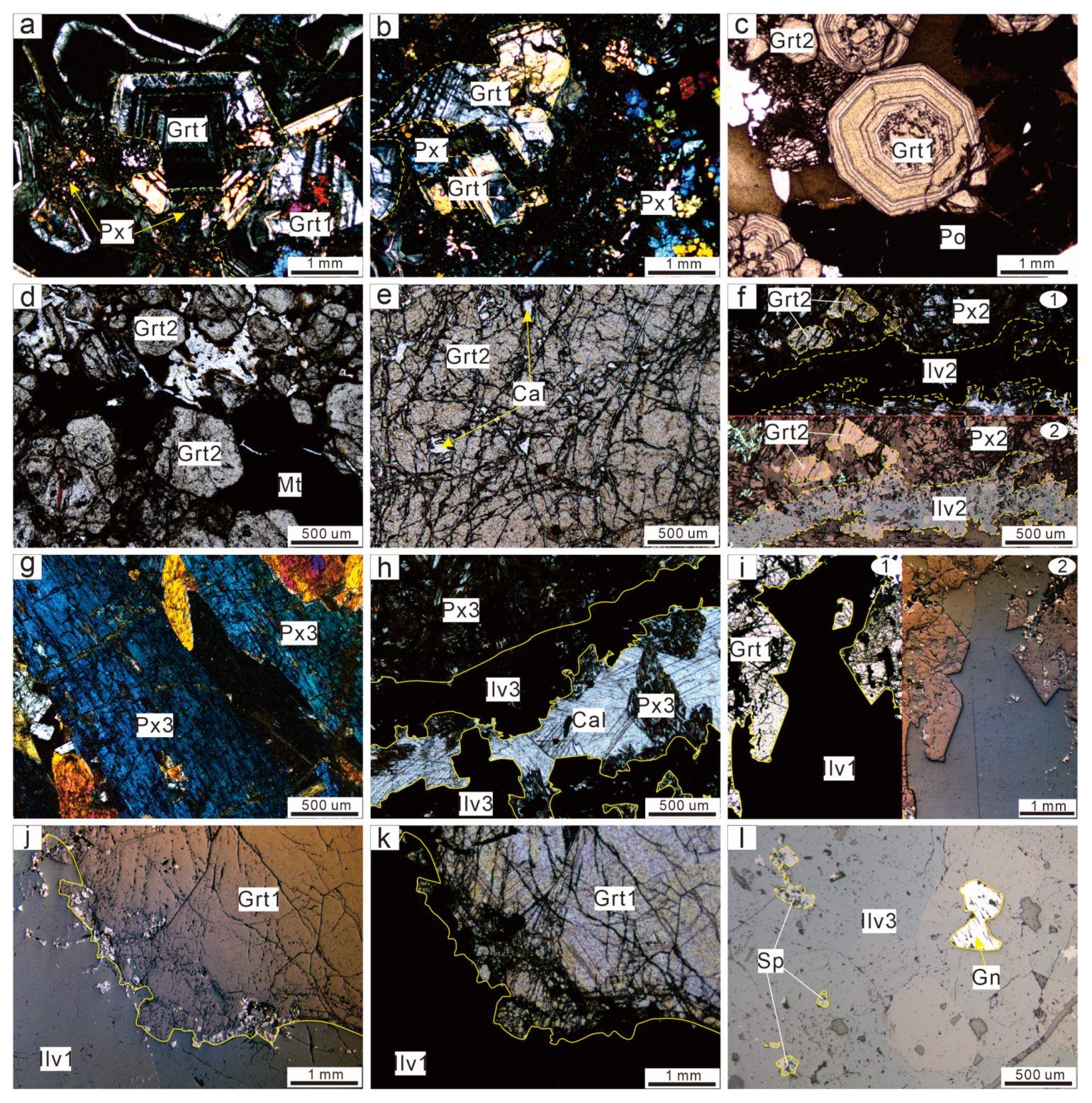
4.2. Mineralogical Characteristics and Petrographic Features
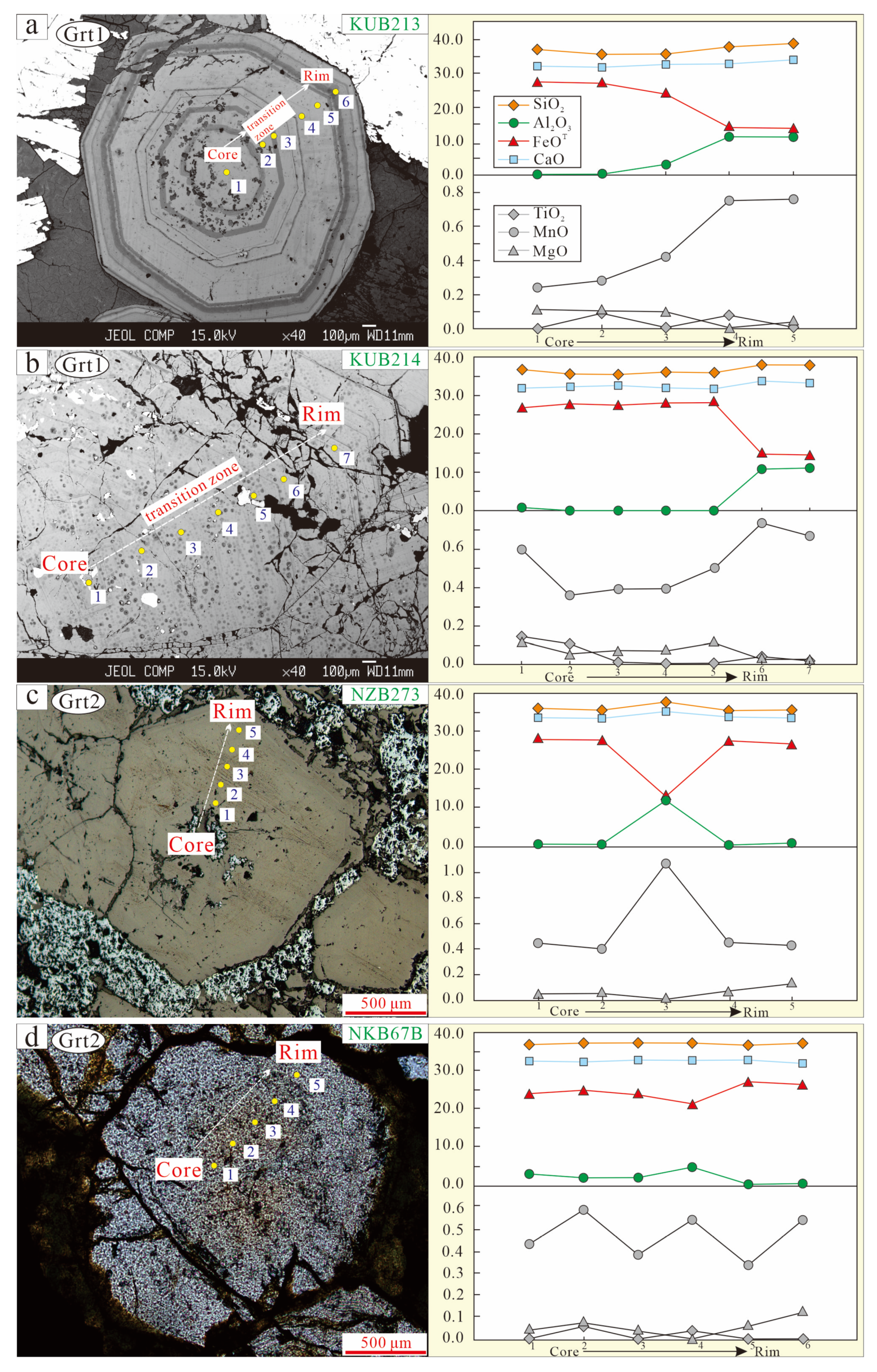
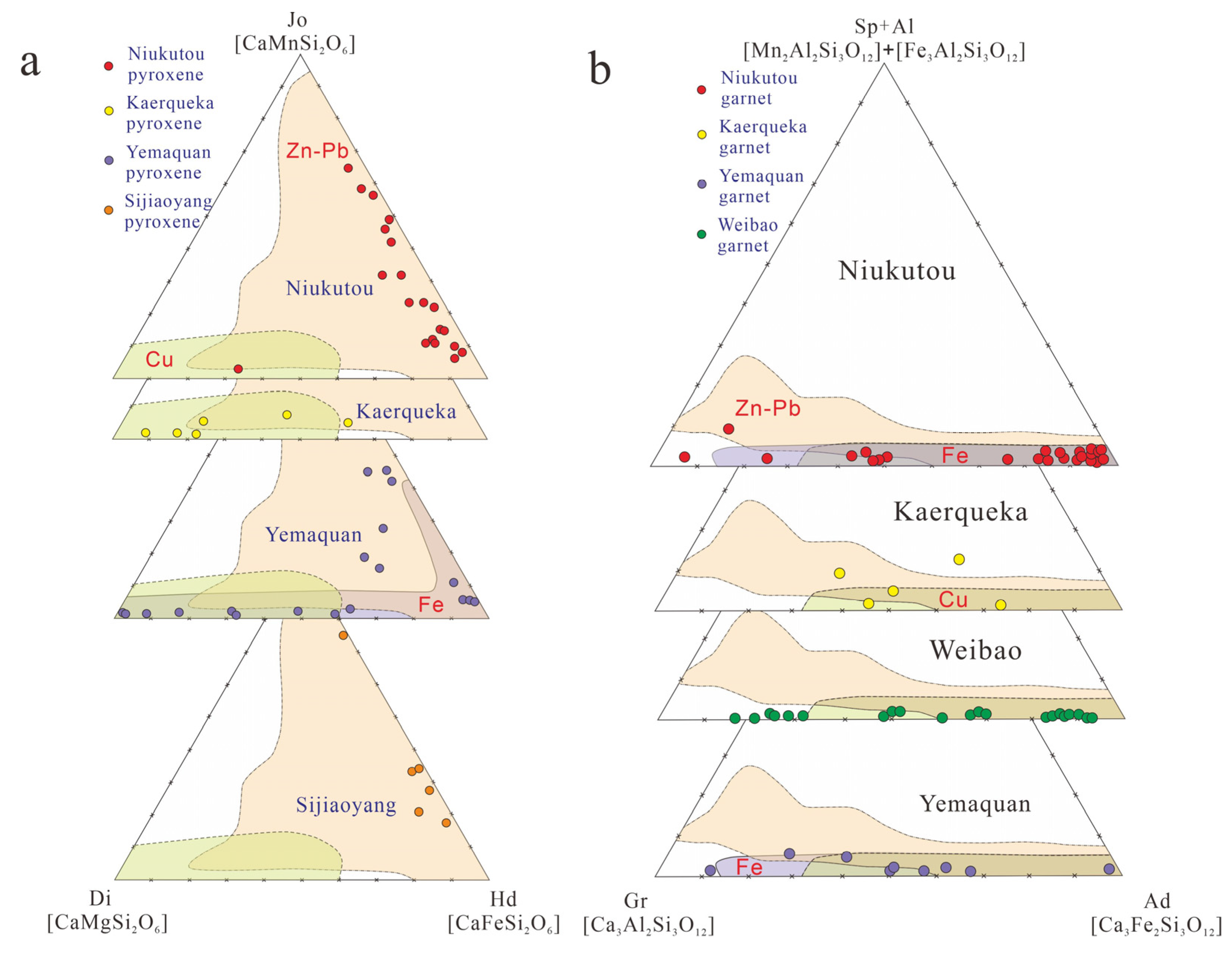
4.2.1. Garnet
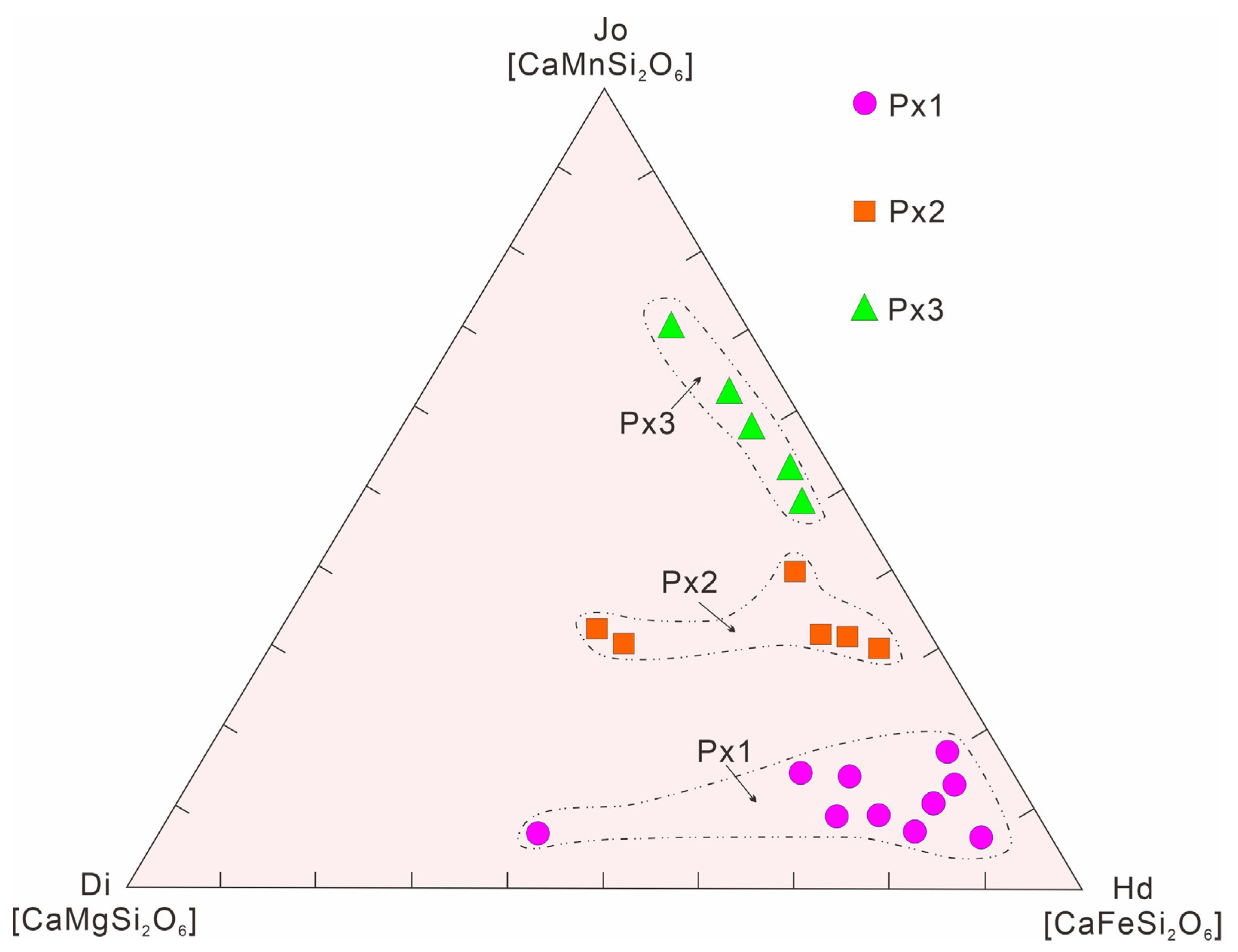
4.2.2. Pyroxene
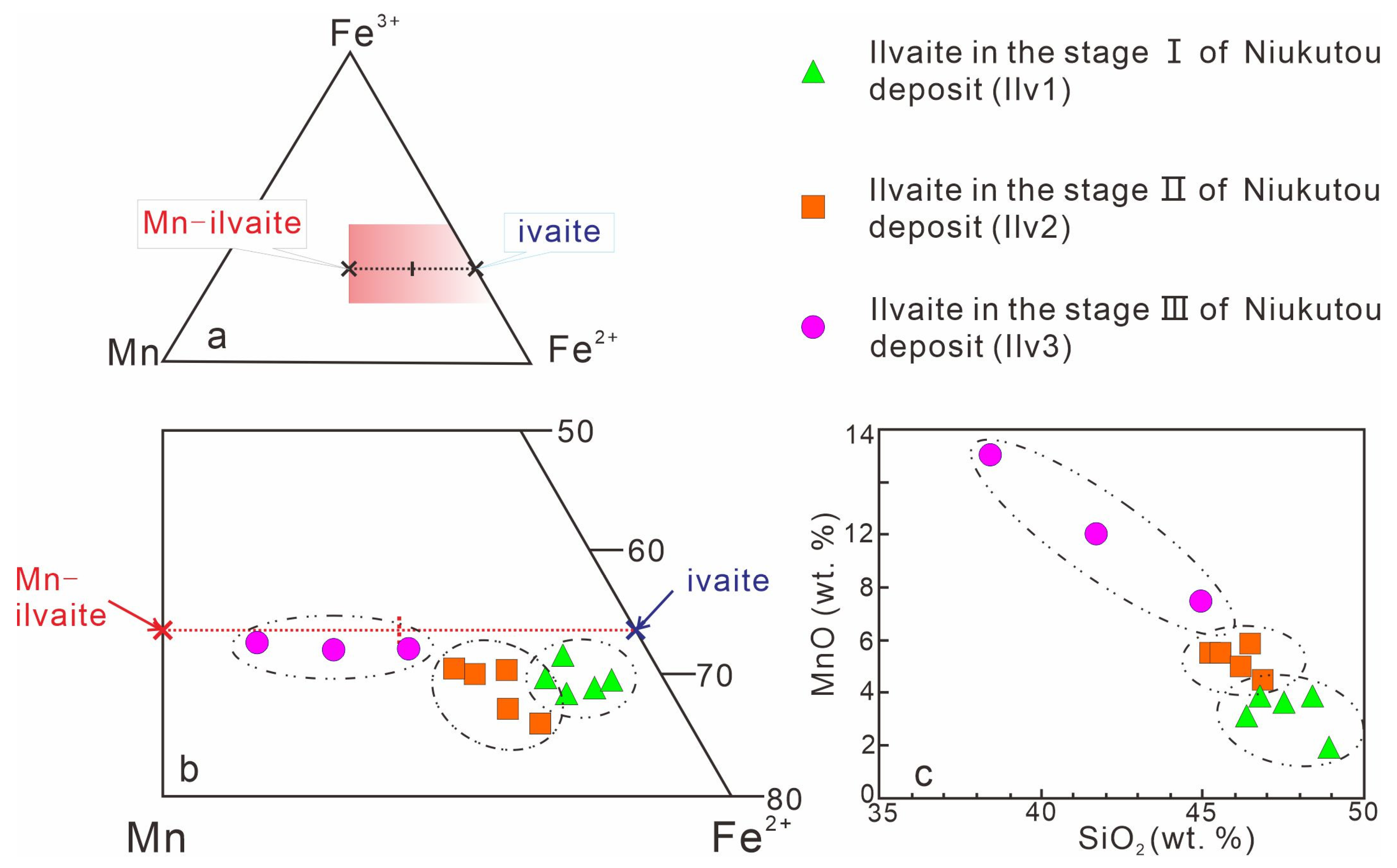
4.2.3. Ilvaite
4.2.4. Epidote
4.2.5. Chlorite
5. Results
5.1. Major Chemical Elements of Minerals
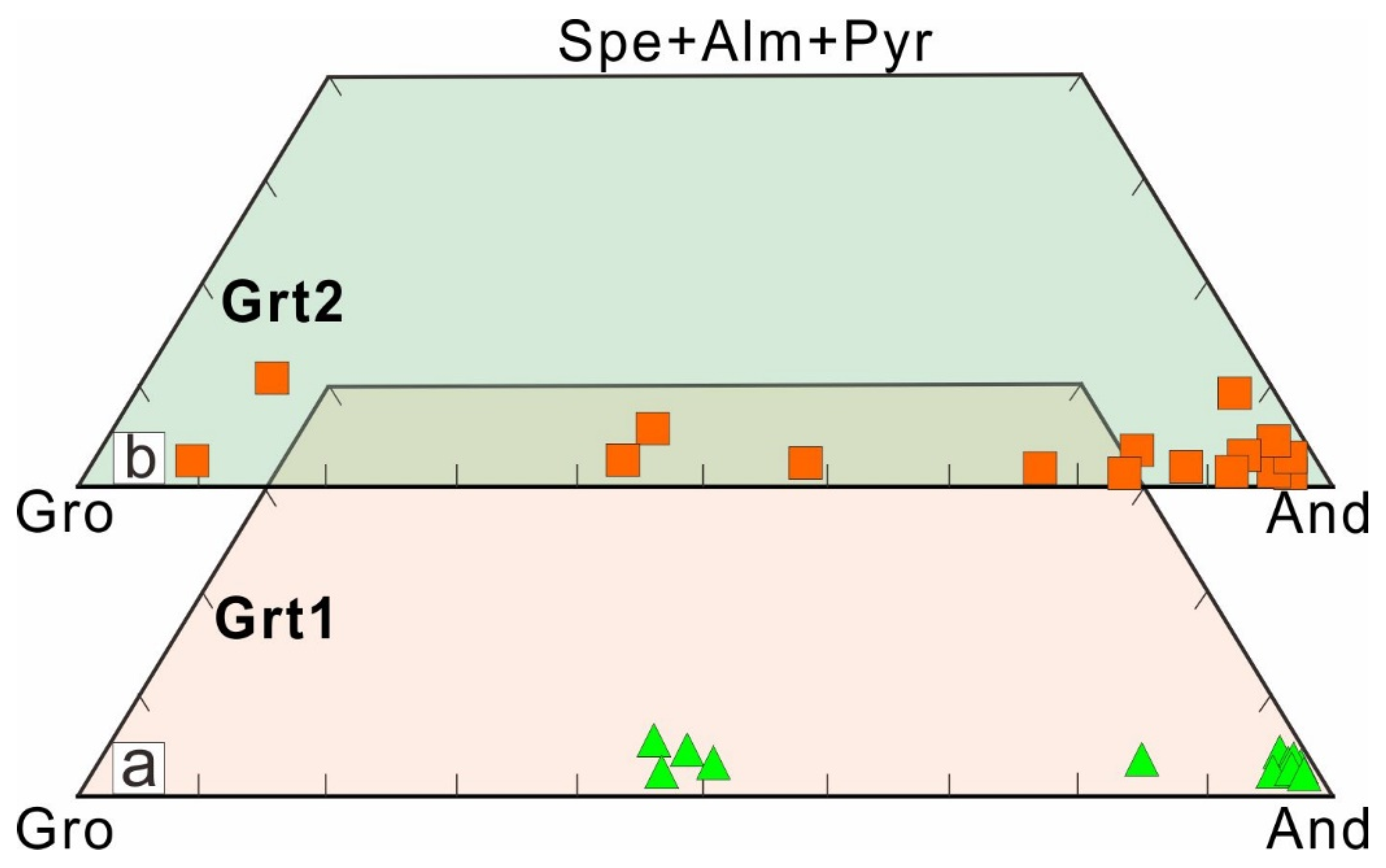
5.1.1. Garnet
5.1.2. Pyroxene
5.1.3. Ilvaite
5.1.4. Epidote
5.1.5. Chlorite
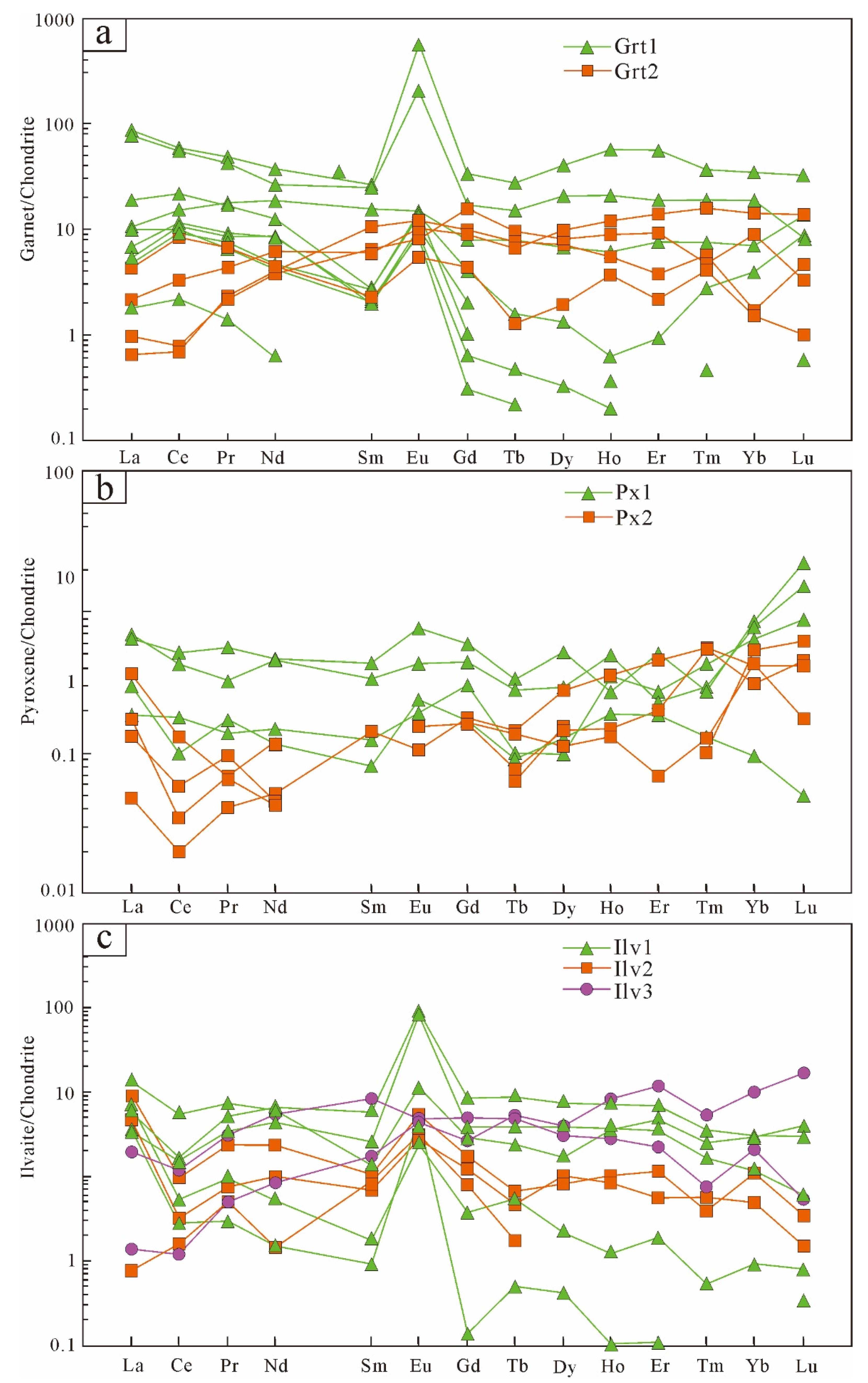
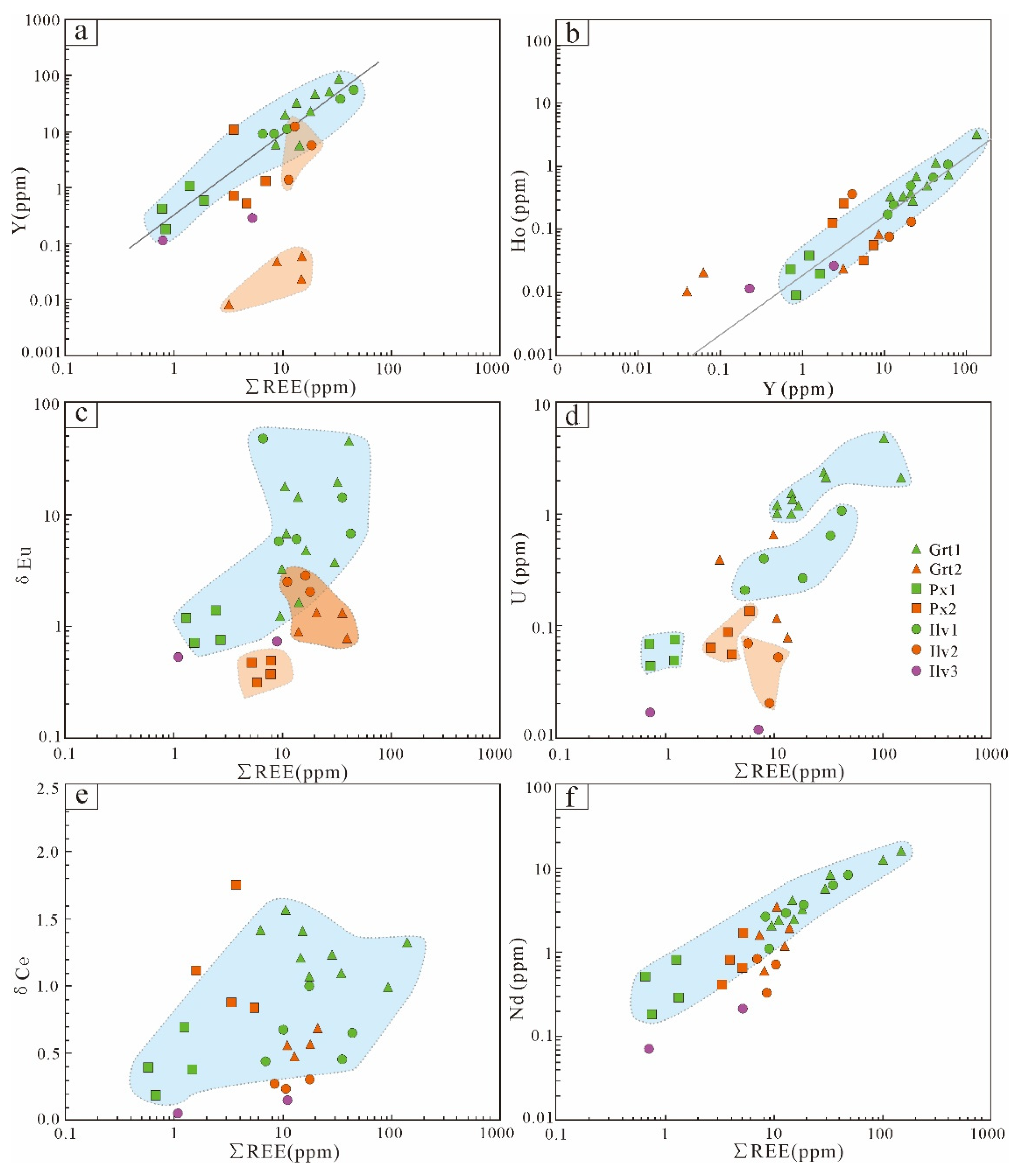
5.2. Trace Elements
6. Discussion
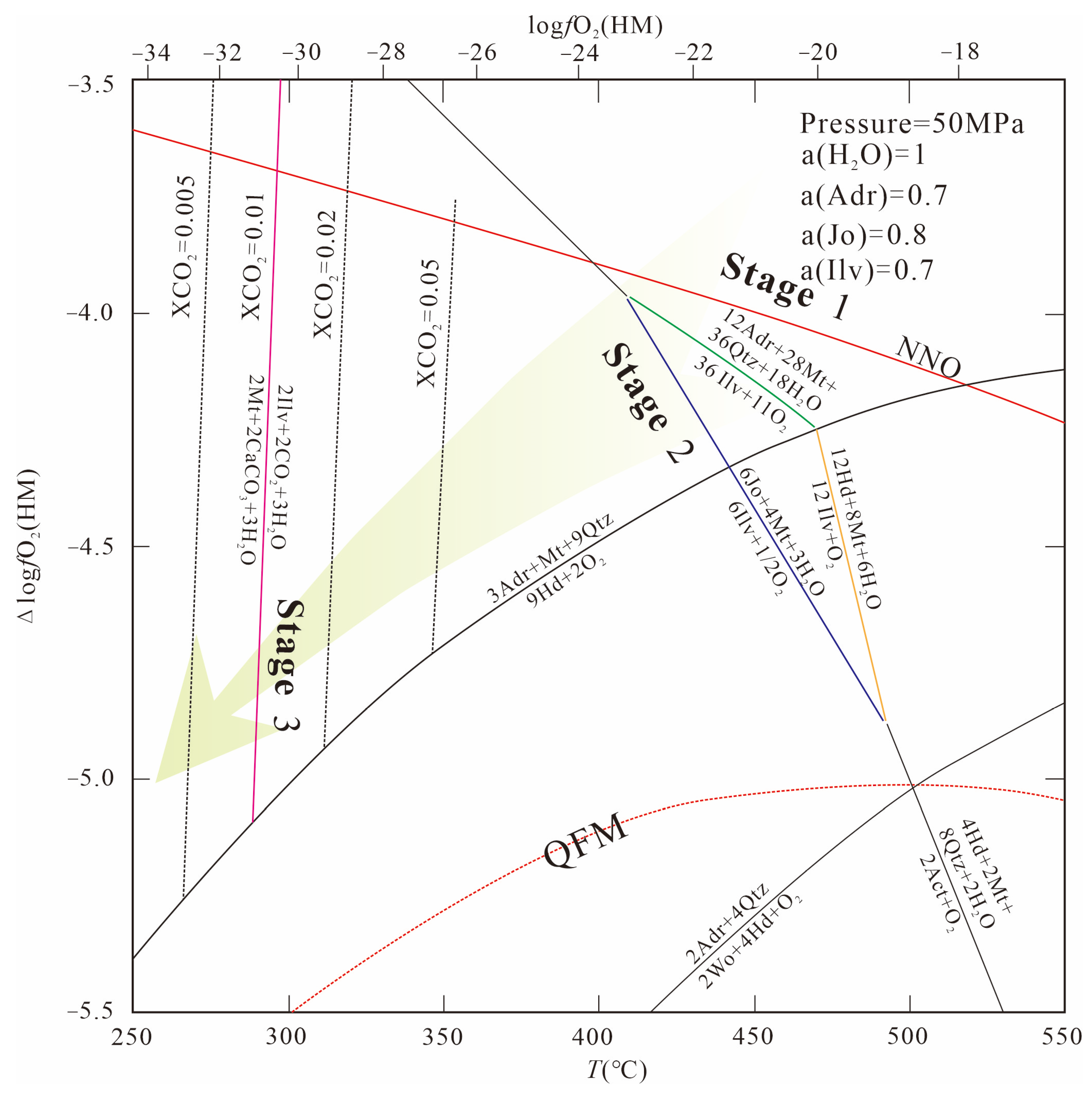
6.1. Physicochemical Conditions
6.1.1. Fluid Properties
6.1.2. Oxygen Fugacity
Prograde Metamorphic Phase
Retrograde Metamorphic Phase
6.1.3. pH
Prograde Metamorphic Phase
Retrograde Metamorphic Phase
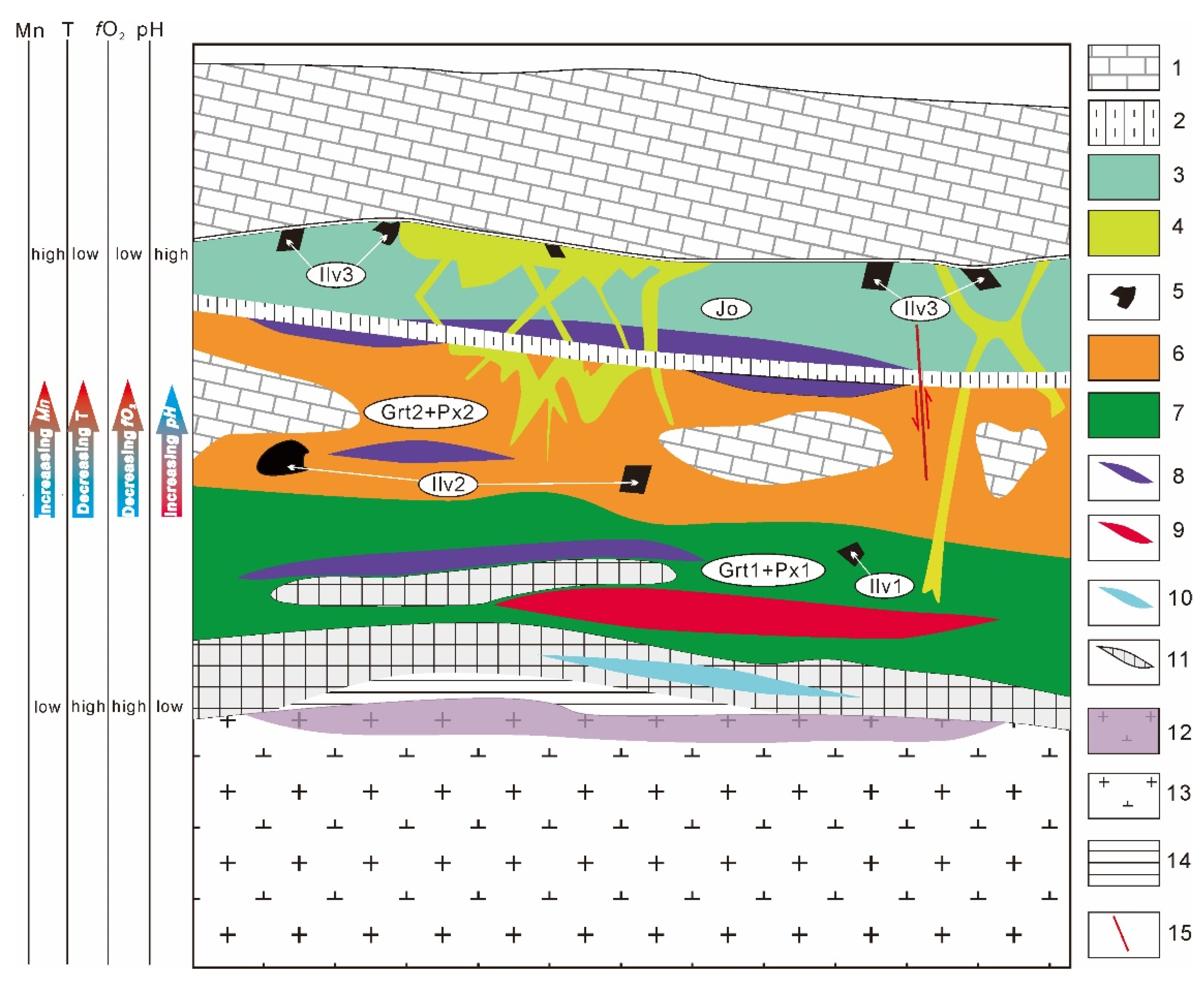
6.2. Ore-Forming Fluid Evolution
6.3. Implications for Mineralization
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, C.Y.; Wang, X.P.; Shu, X.F.; Zhang, A.K.; Xiao, Y.; Liu, J.N.; Ma, S.C.; Li, G.C.; Li, D.X. Isotopic Chronology of the Hutouya Skarn Lead-Zinc Polymetallic Ore District in Qimantage Area of Qinghai Province and Its Geological Significance. J. Jilin Univ. 2011, 41, 1806–1817, (In Chinese with English abstract). [Google Scholar]
- Feng, C.Y.; Wang, S.; Li, G.C.; Ma, S.C.; Li, D.S. Middle to late Triassic granitoids in the Qimantage area, Qinghai Province, China: Chronology, geochemistry and metallogenic significances. Acta Petrol. Sin. 2012, 28, 665–678, (In Chinese with English abstract). [Google Scholar]
- Mao, J.W.; Zhou, Z.H.; Feng, C.Y.; Wang, Y.T.; Zhang, C.Q.; Peng, H.J.; Yu, M. A preliminary study of the Triassic large-scale mineralization in China and its geodynamic setting. Geol. China 2012, 39, 1437–1471, (In Chinese with English abstract). [Google Scholar]
- Wang, X.Y.; Zhu, X.Y.; Li, J.D.; Wang, Y.W.; Long, L.L.; Li, S.T.; Wu, J.R.; Cheng, X.Y.; Jiang, B.B. Genesis and geological significance of manganilvaite in the Niukutou deposit, Qinghai Province. Acta Geol. Sin. 2020, 94, 2279–2290, (In Chinese with English abstract). [Google Scholar]
- Wang, X.Y.; Zhu, X.Y.; Li, J.D.; Wang, Y.W.; Jiang, B.B.; Wu, J.R.; Huang, X.K.; Zhao, Z.Y. Two stage magmatisms and their skarn-type mineralization in the Niukutou ore district, Qinghai Provice. Acta Petrol. Sin. 2021, 37, 1567–1586. [Google Scholar]
- Zhong, S.H.; Feng, C.; Seltmann, R.; Li, D. Middle Devonian volcanic rocks in the Weibao Cu–Pb–Zn deposit, East Kunlun Mountains, NW China: Zircon chronology and tectonic implications. Ore Geol. Rev. 2017, 84, 309–327. [Google Scholar] [CrossRef]
- Zhong, S.H.; Feng, C.; Seltmann, R.; Dolgopolova, A.; Andersen, J.C.Ø.; Li, D.; Yu, M. Sources of fluids and metals and evolution models of skarn deposits in the Qimantagh metallogenic belt: A case study from the Weibao deposit, East Kunlun Mountains, northern Tibetan Plateau. Ore Geol. Rev. 2018, 93, 19–37. [Google Scholar] [CrossRef]
- Gao, Y.B.; Li, W.Y.; Qian, B.; Li, K.; Li, D.S.; He, S.Y.; Zhang, Z.W.; Zhang, J.W. Geochronology, geochemistry and Hf isotopic compositions of the granitic rocks related with iron mineralization in Yemaquan deposit, East Kunlun, NW China. Acta. Petrol. Sin. 2014, 30, 1647–1665, (In Chinese with English abstract). [Google Scholar]
- Yu, M.; Feng, C.Y.; Bao, G.Y.; Liu, H.C.; Zhao, Y.M.; Li, D.X.; Xiao, Y.; Liu, J.N. Characteristics and zonation of skarn minerals in Galinge iron deposit, Qinghai Province. Miner. Depos. 2013, 32, 55–76, (In Chinese with English abstract). [Google Scholar]
- Yu, M.; Feng, C.Y.; Mao, J.W.; Santosh, M.; Zhu, Y.F.; Zhao, Y.M.; Li, D.X. The Qiman Tagh Orogen as a window to the crustal evolution in northern Tibetan Plateau. Earth Sci. Rev. 2017, 167, 103–123. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Tan, H.J.; Xu, Z.N.; Yuan, C.G.; Bi, C.S.; Zheng, R.L.; Li, D.X.; Sun, J.H. The calcic-skarn iron ore deposits of Makeng type in southwestern Fujian. Bull. Inst. Miner. Depos. Chin. Acad. Geol. Sci. (Album) 1983, 1, 1–141, (In Chinese with English abstract). [Google Scholar]
- Zhong, S.H.; Feng, C.; Seltmann, R.; Li, D.; Dai, Z. Geochemical contrasts between Late Triassic ore-bearing and barren intrusions in the Weibao Cu–Pb–Zn deposit, East Kunlun Mountains, NW China: Constraints from accessory minerals (zircon and apatite). Miner. Depos. 2018, 53, 855–870. [Google Scholar] [CrossRef]
- Zhong, S.H.; Feng, C.; Seltmann, R.; Li, D.; Qu, H. Can magmatic zircon be distinguished from hydrothermal zircon by trace element composition? The effect of mineral inclusions on zircon trace element composition. Lithos 2018, 314–315, 646–657. [Google Scholar] [CrossRef]
- Jian, R.T.; Zhao, X.K.; Yang, F.; Li, J.D.; Wu, J.R. New progress in prospecting for Niukutou iron polymetallic deposit, Qinghai. Acta Mineral. Sin. 2017, 34, 821–823, (In Chinese with English abstract). [Google Scholar]
- Li, J.D.; Wang, X.Y.; Zhu, X.Y.; Wu, J.R.; Cai, Y.W.; Li, Y.; Guo, T.J.; Jiang, B.B. Finding and significance of ilvaite in the Qimantag area-with the example of Niukutou lead and zinc deposit. Min. Explor. 2019, 10, 1550–1558, (In Chinese with English abstract). [Google Scholar]
- Li, J.D.; Wang, X.Y.; Zhu, X.Y.; Wu, J.R.; Cai, Y.W.; Li, Y.; Guo, T.J.; Jiang, B.B. The preliminary discussion of the Hercynian metallogenic period in Qimantag area–with the example of Niukutou lead and zinc deposit. Min. Explor. 2019, 10, 1775–1783, (in Chinese with English abstract). [Google Scholar]
- Zhao, X.K.; Jian, R.T.; Yu, C.; Yang, F.; Li, J.D. Geological Characteristics and Prospecting Indicators of Niukutou Fe-Pb-Zn Polymetallic Deposit in Qinghai Province. Mor. Min. 2018, 585, 10–16, (In Chinese with English abstract). [Google Scholar]
- Gaspar, M.; Meinert, L.D.; Moretti, R. REE in skarn systems: A LA-ICP-MS study of garnets from the Crown Jewel gold deposit. Geochim. Cosmochim. Acta 2008, 72, 185–205. [Google Scholar] [CrossRef]
- Zhai, D.G.; Liu, J.J.; Zhang, H.Y.; Wang, J.P.; Su, L.; Yang, X.A.; Wu, S.H. Origin of oscillatory zoned garnetsfrom the Xieertala Fe–Zn skarn deposit, northern China: In situ LA-ICP-MS evidence. Lithos 2014, 190, 279–291. [Google Scholar] [CrossRef]
- Baghban, S.; Hosseinzadeh, M.; Moayyed, M.; Mokhtari, A.A.; Gregory, D.D. Geology, mineral chemistry and formation conditions of calc-silicate minerals of Astamal Fe-LREE distal skarn deposit, Eastern Azarbaijan province, NW Iran. Ore Geol. Rev. 2015, 68, 79–96. [Google Scholar] [CrossRef]
- Feng, C.Y.; Yu, M.; Li, D.X.; Li, G.C.; Zhou, A.S.; Li, X. Fluid inclusion geochemistry of Bashierxi tungsten-tin deposit in Qimantag area, Xinjiang. Miner. Depos. 2013, 32, 20–36, (In Chinese with English abstract). [Google Scholar]
- Gao, Y.B. The Intermediate-Acid Intrusive Magmatism and Mineralization in Qimantag, East Kunlun Mountains. Ph.D. Thesis, Chang’an University, Xi’an, China, 2013. [Google Scholar]
- Lu, Y.F. Geokit: Geochemical software package built by VBA. Geochemistry 2004, 33, 459–464, (In Chinese with English abstract). [Google Scholar]
- Brandelik, A. CALCMIN-an EXCELTM Visual Basic application for calculating mineral structural formulae from electron microprobe analyses. Comput. Geosci. 2009, 35, 1540–1551. [Google Scholar] [CrossRef]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. 1992, 19, 145–162. [Google Scholar]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S. World skarn deposits. Econ. Geol. 100th Anniv. Vol. 2005, 299–336. [Google Scholar] [CrossRef]
- Bonev, I.K.; Vassileva, R.D.; Zotov, N.; Kouzmanov, K. Manganilvaite, CaFe2+Fe3+(Mn, Fe2+)(Si2O7)O(OH), a new mineral of the ilvaite group from Pb-Zn skarn deposits in the Rhodope Moutntains, Bulgaria. Can. Mineral. 2005, 43, 1027–1042. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematic of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Jamtveit, B.; Wogelius, R.A.; Fraser, D.G. Zonation patterns of skarn garnets: Records of hydrothermal system evolution. Geology 1993, 21, 113–116. [Google Scholar] [CrossRef]
- Dietvorst, E.J.L. Retrograde garnet zoning at low pressure in metapeletic rocks from kemio, SW Finland. Contrib. Mineral. Petrol. 1982, 79, 37–45. [Google Scholar] [CrossRef]
- Flynn, R.T.; Burnham, C.W. An experimental determination of rare earth partition coe_cients between a chloride containing vapor phase and silicate melts. Geochim. Cosmochim. Acta. 1978, 42, 685–701. [Google Scholar] [CrossRef]
- Park, C.P.; Song, Y.G.; Kang, I.M.; Shim, J.; Chung, D.H.; Park, C.S. Metasomatic changes during periodic fluid flux recorded in grandite garnet from the Weondong W-skarn deposit, South Korea. Chem. Geol. 2017, 451, 135–153. [Google Scholar] [CrossRef]
- Jamtveit, B.; Hervig, R.L. Constraints on transport and kinetics in hydrothermal systems from zoned garnet crystals. Science 1994, 263, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A. The geochemistry of rare earth elements and yttrium in geothermal waters. Spec. Publ. Soc. Econ. Geol. 2003, 10, 133–158. [Google Scholar]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Anders, M.; Grevesse, N. 1989. Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, T.F.; White, N.C.; Zhang, L.J.; Fan, Y.; Wang, F.Y.; Chen, X.F. The formation and trace elements of garnet in the skarn zone from the Xinqiao Cu-S-Fe-Au deposit, Tongling ore district, Anhui province, Eastern China. Lithos 2018, 302, 467–479. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid–rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Sverjensky, D.M. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Van Westernen, W.; Allan, N.L.; Blundy, J.D.; Purton, J.A.; Wood, B.J. Atomistic simulation of trace element incorporation into garnets–comparison with experimental garnet–melt partition data. Geochim. Cosmochim. Acta 2000, 64, 1629–1639. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Jeffries, T.E.R.; Long, J.; Williams, C.T. The rare earth elements and uranium in garnets from the Beinn an Dubhaich Aureole, Skye, Scotland, UK: Constraints on processes in a dynamic hydrothermal system. J. Petrol. 2004, 45, 457–484. [Google Scholar] [CrossRef]
- Zhong, S.H.; Seltmann, R.; Shen, P. Two different types of granitoids in the Suyunhe large porphyry Mo deposit, NW China and their genetic relationships with molybdenum mineralization. Ore Geol. Rev. 2017, 88, 116–139. [Google Scholar] [CrossRef]
- Zhong, S.H.; Seltmann, R.; Qu, H.; Song, Y. Characterization of the zircon Ce anomaly for estimation of oxidation state of magmas: A revised Ce/Ce* method. Mineral. Charact. Petrol. 2019, 113, 755–763. [Google Scholar] [CrossRef]
- Delgado, J.; Soler, A. Ilvaite stability in skarns from the northern contact of the Maladeta batholith, Central Pyrenees (Spain). Eur. J. Mineral. 2010, 22, 363–380. [Google Scholar]
- Yu, M. Metallogenic Mechanism of the Galinge Polymetallic Iron Skarn Deposit, QimanTagh Mountains, Qinghai Province. Ph.D. Thesis, Peking University, Beijing, China, 2017. [Google Scholar]
- Dziggel, A.; Wul, K.; Kolb, J.; Meyer, F.M.; Lahaye, Y. Significance of oscillatory and bell-shaped growth zoning in hydrothermal garnet: Evidence from the Navachab gold deposit, Namibia. Chem. Geol. 2009, 262, 262–276. [Google Scholar] [CrossRef]
- Inguaggiato, C.; Censi, P.; Zuddas, P.; Londoño, J.M.; Chacón, Z.; Alzate, D.; Brusca, L.; D’Alessandro, W. Geochemistry of REE, Zr and Hf in a wide range of pH and water composition: The Nevado del Ruiz volcano-hydrothermal system (Colombia). Chem. Geol. 2015, 417, 125–133. [Google Scholar] [CrossRef]
- Michard, A. Rare earth element systematics in hydrothermal fluids. Geochim. Cosmochim. Acta. 1989, 53, 745–750. [Google Scholar] [CrossRef]
- Peng, H.J.; Zhang, C.Q.; Mao, J.W.; Santosh, M.; Zhou, Y.M.; Houe, L. Garnets in porphyry–skarn systems: A LA-ICP-MS, fluid inclusion, and stable isotope study of garnets from the Hongniu–Hongshan copper deposit, Zhongdian area, NW Yunnan Province, China. J. Asian Earth Sci. 2015, 103, 229–251. [Google Scholar] [CrossRef]
- Feng, C.Y.; Zhao, Y.M.; Li, D.X.; Liu, J.N.; Xiao, Y.; Li, G.C.; Ma, S.C. Skarn typesand mineralogical characteristics of the Fe-Cu-polymetallic Skarn deposits in the Qimantage area, western Qinghai Province. Acta. Geol. Sin. 2011, 85, 1108–1115, (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.M.; Lin, W.W.; Bi, C.S.; Li, D.X.; Jiang, C.J. Skarn Deposits in China; Beijing; Geological Publishing House: Beijing, China, 1990; pp. 1–354, (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.M.; Feng, C.Y.; Li, D.X.; Liu, J.N.; Xiao, Y.; Yu, M.; Ma, S.C. Metallogenic setting and mineralization-alteration characteristics of major skarn Fe-polymetallic deposits in Qimantag area, western Qinghai Province. Miner. Depos. 2013, 32, 1–19, (In Chinese with English abstract). [Google Scholar]
- Li, D.X.; Feng, C.Y.; Zhao, Y.M.; Li, Z.F.; Liu, J.N.; Xiao, Y. Mineralization and alteration types and skarn minerology of Kaerqueka copper polymetallic deposit in Qinghai Province. J. Jilin Univ. 2011, 41, 1818–1830, (In Chinese with English abstract). [Google Scholar]
- Liu, J.N.; Feng, C.Y.; Zhao, Y.M.; Li, D.X.; Xiao, Y.; Zhou, J.H.; Ma, Y.S. Characteristics of intrusiverock, metasomatites, mineralization and alteration in Yemaquan skarn Fe-Zn-polymetallic deposit, Qinghai Province. Miner. Depos. 2013, 32, 77–93, (In Chinese with English abstract). [Google Scholar]
- Ma, S.C.; Feng, C.Y.; Zhang, D.J.; Shu, X.F.; Liu, J.N.; Du, S.J. Alteration and mineralization zoning of Hutouya polymetallic deposit in Qimantage area, Qinghai Province. Miner. Depos. 2013, 32, 109–121, (In Chinese with English abstract). [Google Scholar]
- Xiao, Y.; Feng, C.Y.; Liu, J.N.; Yu, M.; Zhou, J.H.; Li, D.X.; Zhao, Y.M. LA-MC-ICPMS zircon U-Pb dating and sulfur isotope characteristics of Kendekeke Fe-polymetallic deposit, Qinghai Province. Miner. Depos. 2013, 32, 177–186, (In Chinese with English abstract). [Google Scholar]
- Wang, S.; Feng, C.Y.; Li, S.J.; Jiang, J.H.; Li, D.S.; Su, B.S. Zircon SHRIMP U-Pb dating of granodiorite in the Kaerqueka polymetallic ore deposit, Qimantage Mountain, Qinghai Province, and its geological implications. Geol. China 2009, 36, 74–84, (In Chinese with English abstract). [Google Scholar]
- Hu, H.; Li, J.W.; Lentz, D.; Ren, Z.; Zhao, X.F.; Deng, X.D.; Hall, D. Dissolution -reprecipitation process of magnetite from the Chengchao iron deposit: Insights into ore genesis and implication for in-situ chemical analysis of magnetite. Ore Geol. Rev. 2014, 57, 393–405. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Wang, J.B.; Wang, Y.W.; Long, L.L. Geochemical Characteristics of Mineral Assemblages from the Yamansu Iron Deposit, NW China, and Their Metallogenic Implications. Minerals 2020, 39, 39. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.J.; Zhang, R.Q.; Li, D.F.; Liu, Z.F.; Chen, H.Y. Dating Ore Deposit Using Garnet U–Pb Geochronology: Example from the Xinqiao Cu–S–Fe–Au Deposit, Eastern China. Minerals 2018, 8, 31. [Google Scholar] [CrossRef]
- Kolb, J. The role of fluids in partitioning brittle deformation and ductile creep in auriferous shear zones between 500 and 700 °C. Tectonophysics 2008, 446, 1–15. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Wang, J.B.; Wang, Y.W.; Long, L.L.; Hu, Q.T.; Wang, M.L.; Li, D.D.; Xie, H.J. Two generations of garnets and their relevance for the hydrothermal fluid evolution of the Hongyuntan deposit, NW China. Ore Geol. Rev. 2022, 122, 103513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, S.; Zhang, H.; Wang, Y.; Zhu, X.; Yang, X. Geochemical Characteristics of the Mineral Assemblages from the Niukutou Pb-Zn Skarn Deposit, East Kunlun Mountains, and Their Metallogenic Implications. Minerals 2023, 13, 18. https://doi.org/10.3390/min13010018
Wang X, Wang S, Zhang H, Wang Y, Zhu X, Yang X. Geochemical Characteristics of the Mineral Assemblages from the Niukutou Pb-Zn Skarn Deposit, East Kunlun Mountains, and Their Metallogenic Implications. Minerals. 2023; 13(1):18. https://doi.org/10.3390/min13010018
Chicago/Turabian StyleWang, Xinyu, Shulai Wang, Huiqiong Zhang, Yuwang Wang, Xinyou Zhu, and Xing Yang. 2023. "Geochemical Characteristics of the Mineral Assemblages from the Niukutou Pb-Zn Skarn Deposit, East Kunlun Mountains, and Their Metallogenic Implications" Minerals 13, no. 1: 18. https://doi.org/10.3390/min13010018
APA StyleWang, X., Wang, S., Zhang, H., Wang, Y., Zhu, X., & Yang, X. (2023). Geochemical Characteristics of the Mineral Assemblages from the Niukutou Pb-Zn Skarn Deposit, East Kunlun Mountains, and Their Metallogenic Implications. Minerals, 13(1), 18. https://doi.org/10.3390/min13010018



