Mineralogical Evolution and Expansion of Cement Pastes in a Sulfate-Confined Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Exposure Conditions
2.2. Experimental Techniques for Characterization
2.2.1. X-ray Diffraction
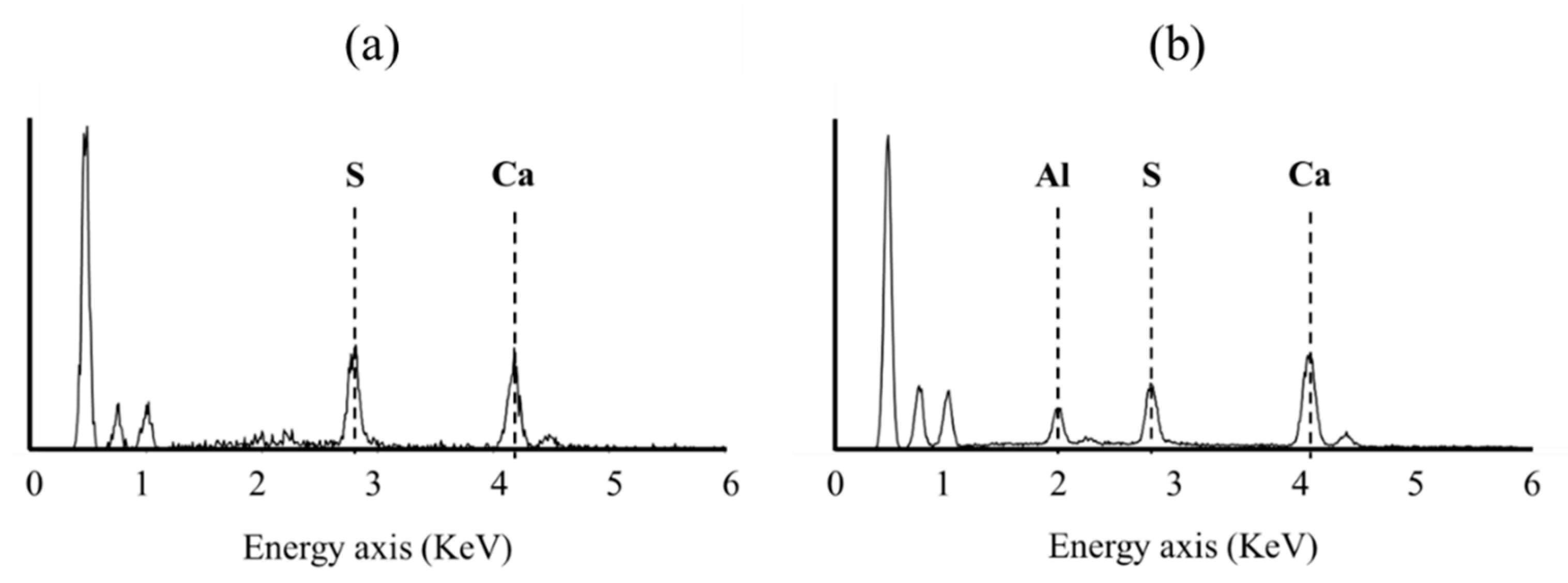
2.2.2. Scanning Electron Microscopy
2.2.3. Micro-Indentation
2.3. Modeling Approach and Data
2.3.1. Reactive Transport Equation
2.3.2. Chemical and Physical Data
2.3.3. Initial State and Configuration of the Cement Pastes
2.3.4. Homogenization Method
3. Results and Discussion
3.1. Evolution of the Mineralogy
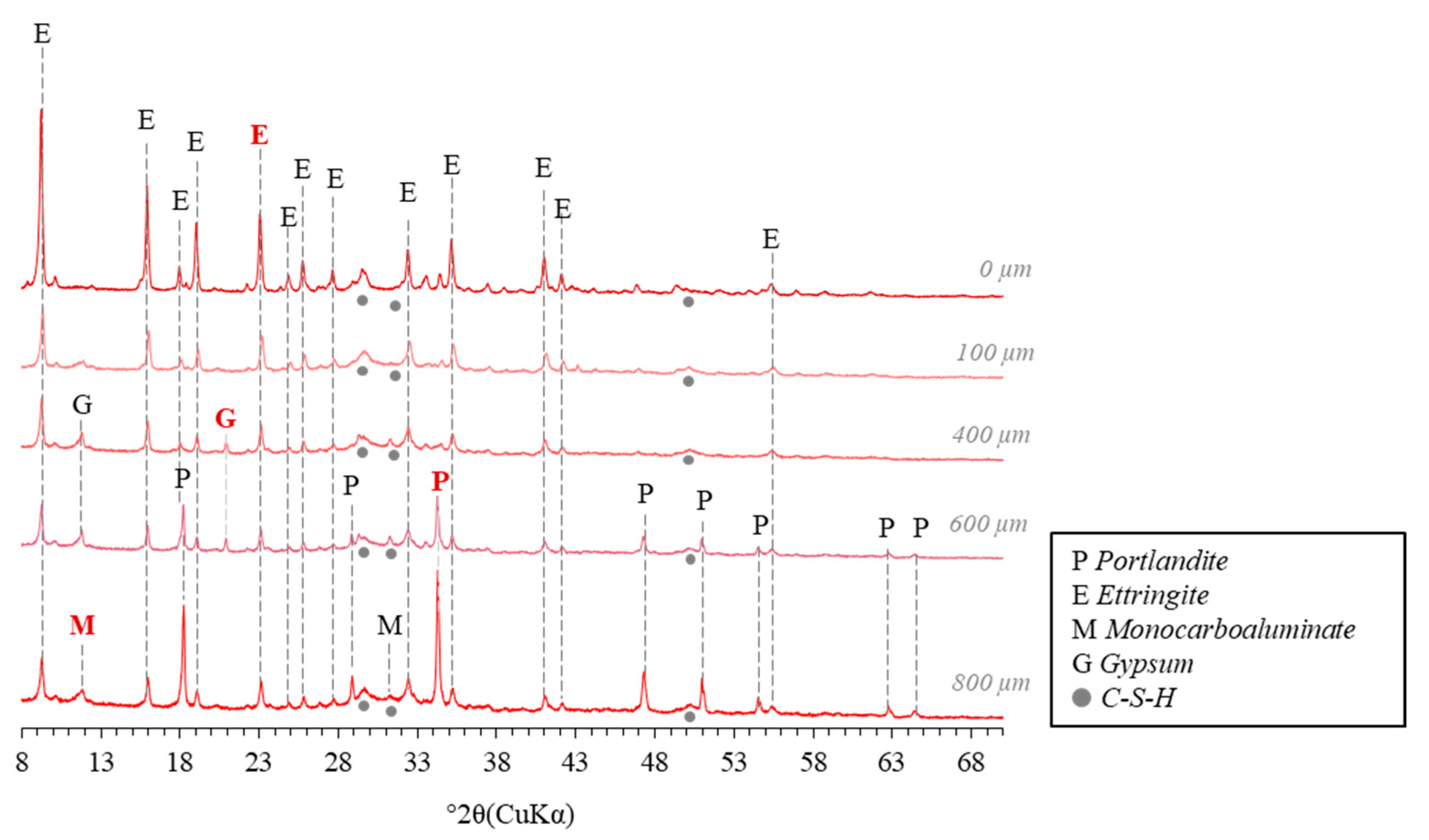
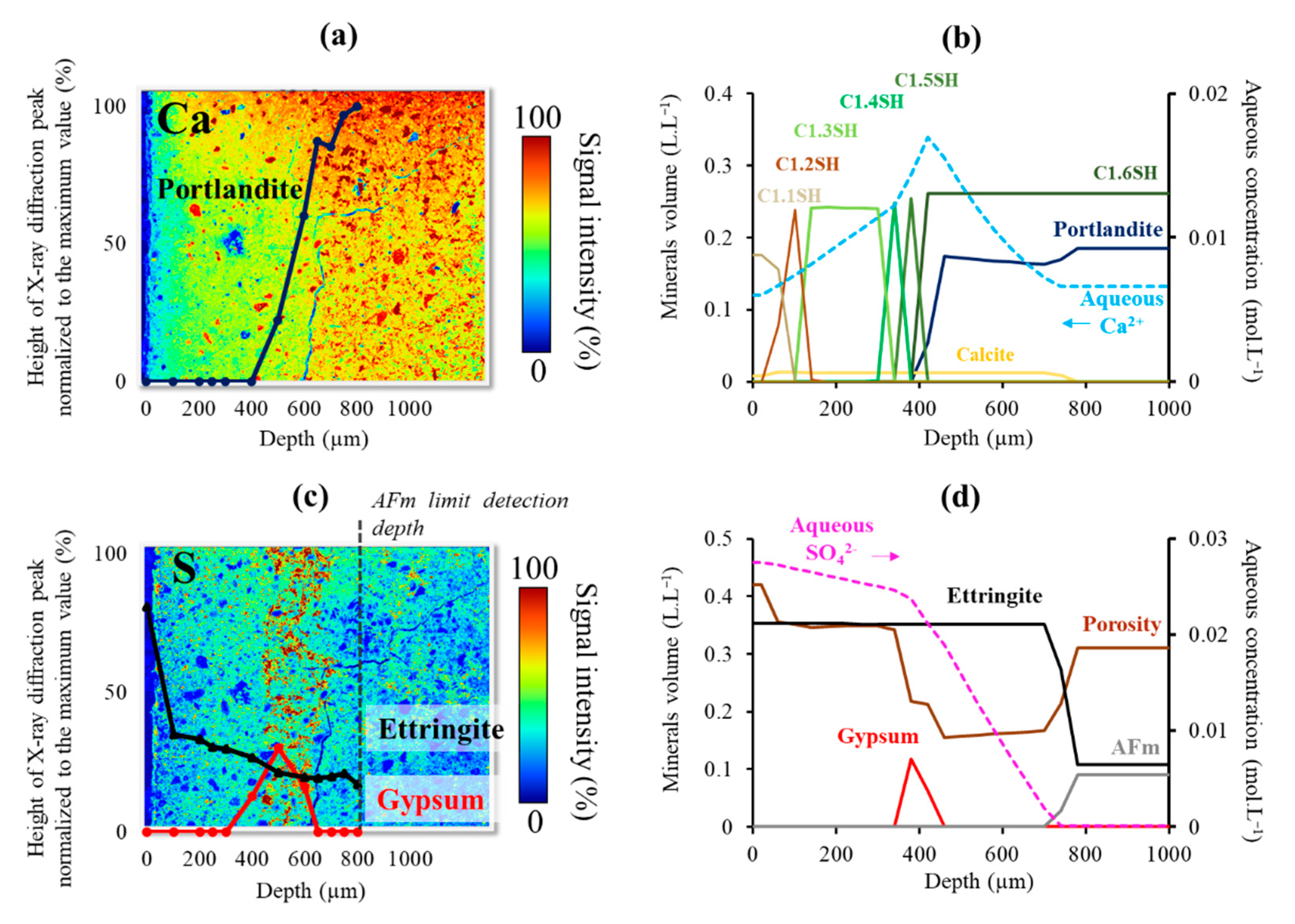
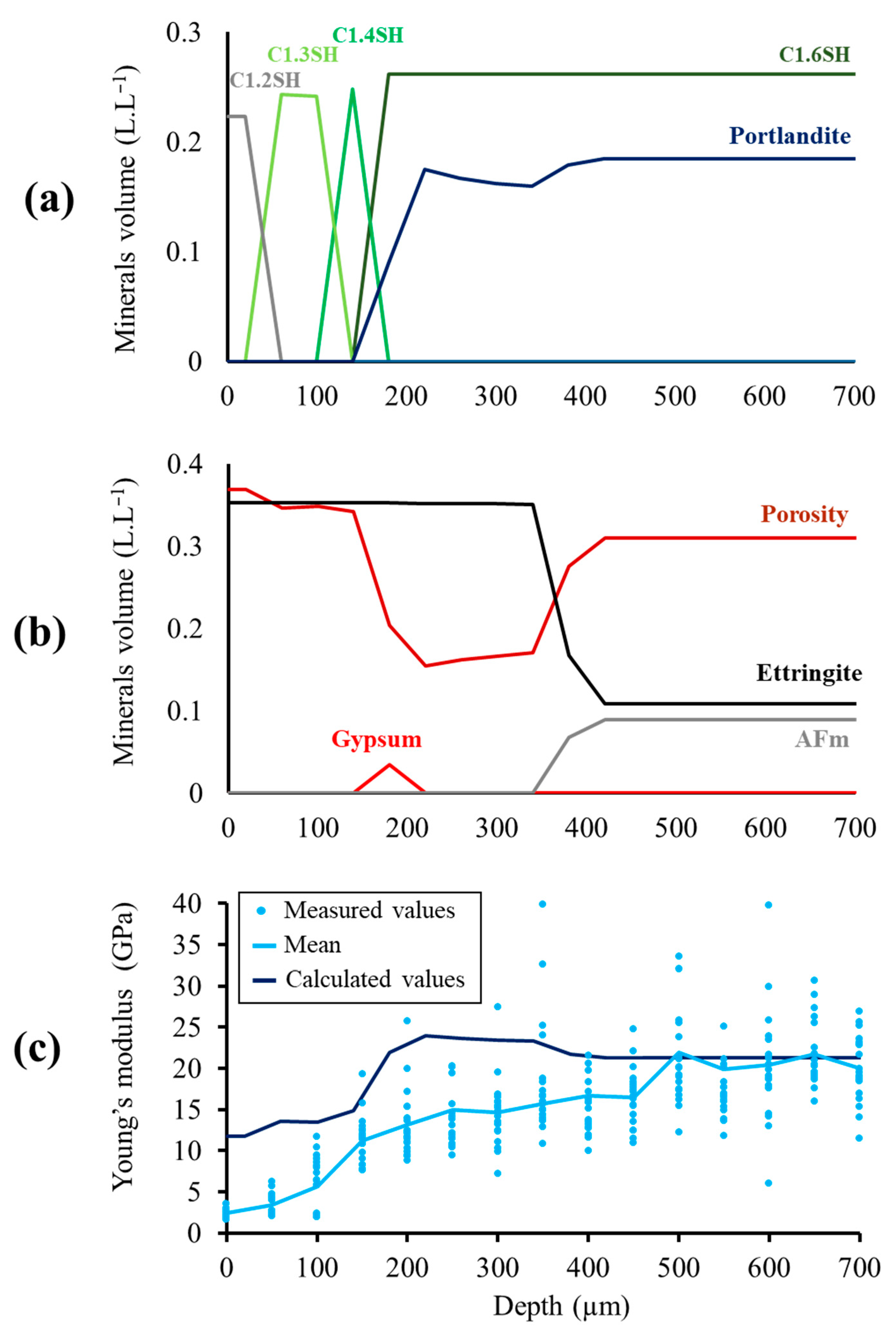
3.1.1. Distribution of the Mineralogical Phases in the Degraded Zone
3.1.2. Ettringite and Gypsum Precipitation
3.2. Evolution of the Degradation over Time
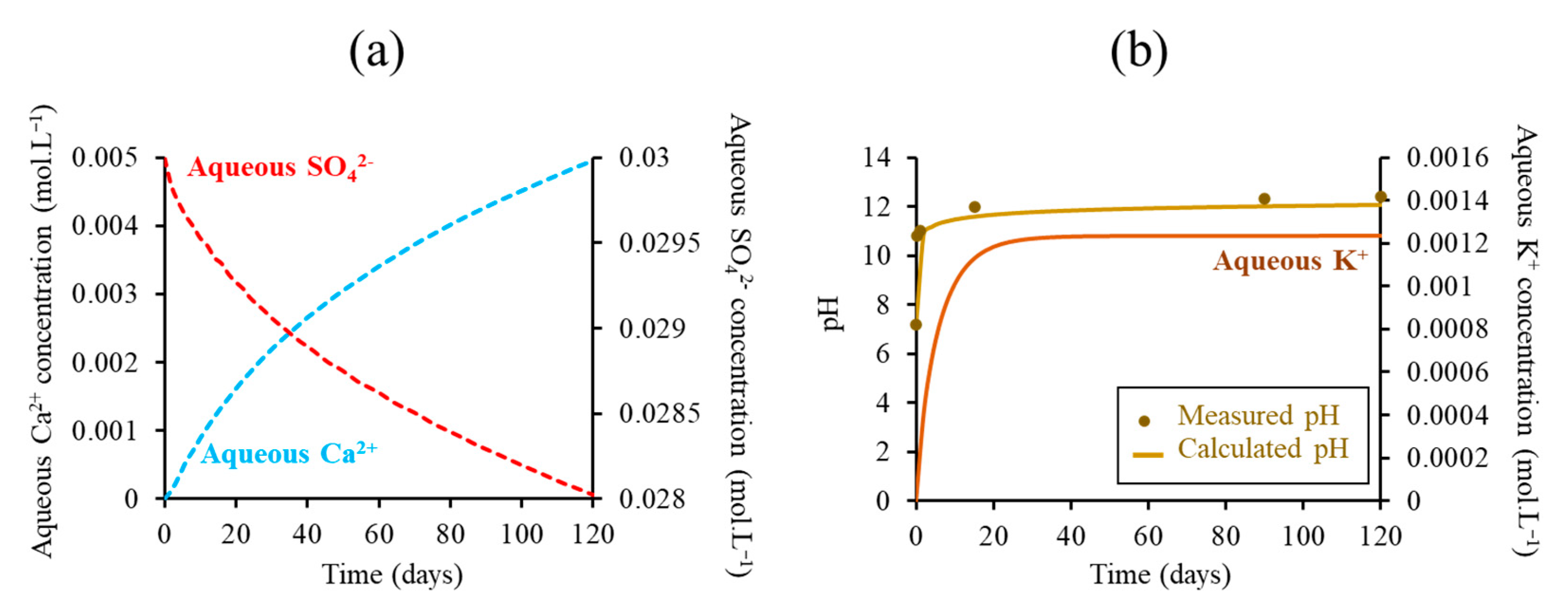
3.2.1. Evolution of the Mineralogical Fronts and the Tank Solution Chemistry
3.2.2. Impact of The Boundary Conditions on The Degradation
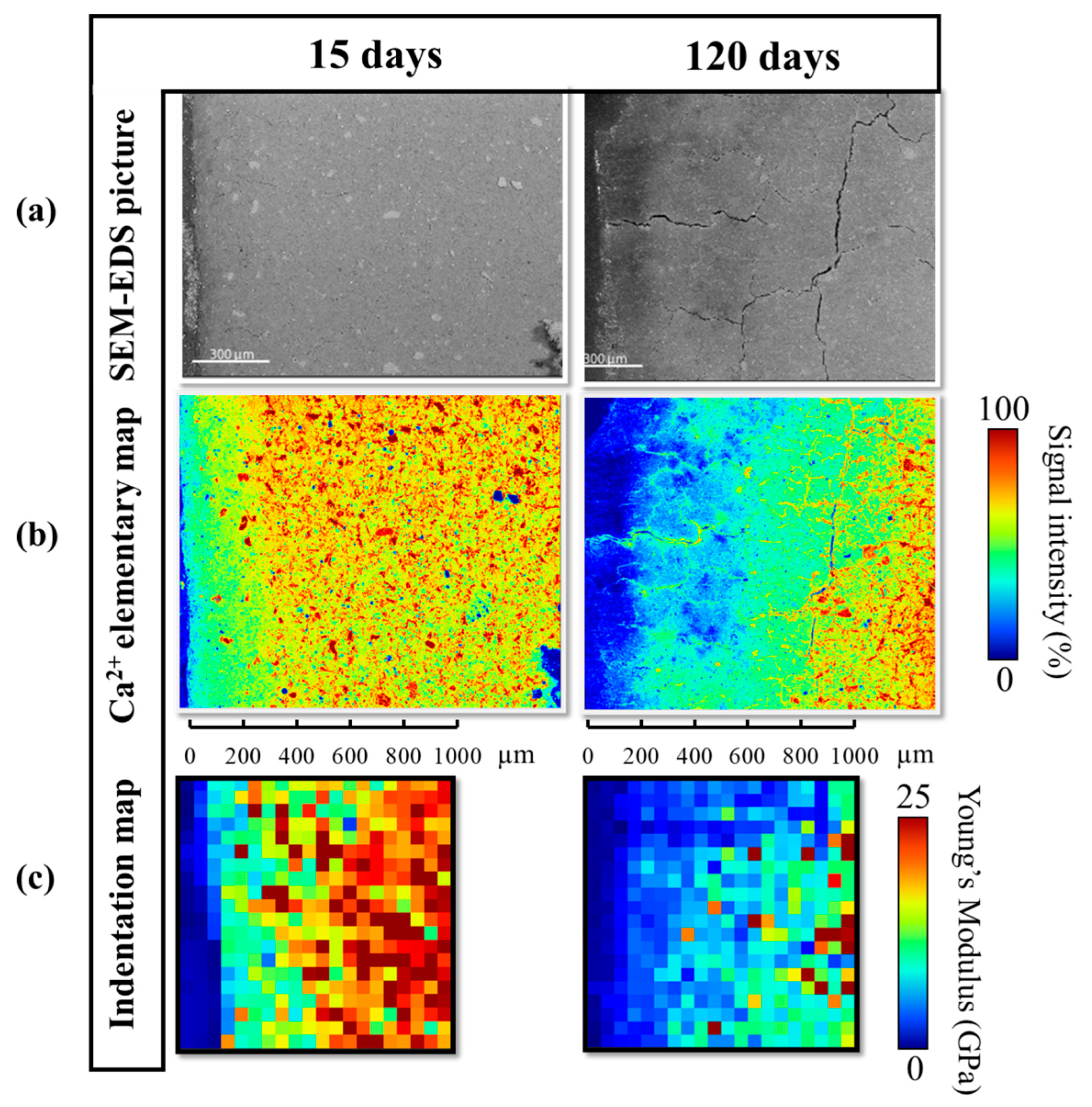
3.3. Impact of the Mineralogy on the Mechanical Properties and Cracking
3.3.1. Effect of the Decalcification
3.3.2. Estimation of the Young’s Modulus by Analytical Homogenization
3.3.3. Macroscopic Cracking and Swelling of the Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Figg, J. Field Studies of Sulfate Attack on Concrete. In Materials Science of Concrete: Sulfate Attack Mechanisms (USA); American Ceramic Society, Inc: Columbus, OH, USA, 1999; pp. 315–323. [Google Scholar]
- Santhanam, M.; Cohen, M.D.; Olek, J. Sulfate Attack Research—Whither Now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Neville, A. The Confused World of Sulfate Attack on Concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of Expansion of Mortars Immersed in Sodium Sulfate Solutions. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.-M.; Roussel, N.; Divet, L.; de Lacaillerie, J.B.D.E. Durability of Cement Pastes Exposed to External Sulfate Attack and Leaching: Physical and Chemical Aspects. Cem. Concr. Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Clifton, J.R.; Pommersheim, J.M. Sulfate Attack of Cementitious Materials: Volumetric Relations and Expansions; National Bureau of Standards: Gaithersburg, MD, USA, 1994; Volume 5390, pp. 4–9. [Google Scholar]
- Sun, D.; Wu, K.; Shi, H.; Miramini, S.; Zhang, L. Deformation Behaviour of Concrete Materials under the Sulfate Attack. Constr. Build. Mater. 2019, 210, 232–241. [Google Scholar] [CrossRef]
- Cefis, N.; Comi, C. Chemo-Mechanical Modelling of the External Sulfate Attack in Concrete. Cem. Concr. Res. 2017, 93, 57–70. [Google Scholar] [CrossRef]
- Jebli, M.; Jamin, F.; Garcia-Diaz, E.; El Omari, M.; El Youssoufi, M.S. Influence of Leaching on the Local Mechanical Properties of an Aggregate-Cement Paste Composite. Cem. Concr. Compos. 2016, 73, 241–250. [Google Scholar] [CrossRef]
- Choi, Y.S.; Yang, E.I. Effect of Calcium Leaching on the Pore Structure, Strength, and Chloride Penetration Resistance in Concrete Specimens. Nucl. Eng. Des. 2013, 259, 126–136. [Google Scholar] [CrossRef]
- Phung, Q.T.; Maes, N.; Jacques, D.; Perko, J.; De Schutter, G.; Ye, G. Modelling the Evolution of Microstructure and Transport Properties of Cement Pastes under Conditions of Accelerated Leaching. Constr. Build. Mater. 2016, 115, 179–192. [Google Scholar] [CrossRef]
- Gaucher, E.C.; Tournassat, C.; Pearson, F.J.; Blanc, P.; Crouzet, C.; Lerouge, C.; Altmann, S. A Robust Model for Pore-Water Chemistry of Clayrock. Geochim. Cosmochim. Acta 2009, 73, 6470–6487. [Google Scholar] [CrossRef]
- Tremosa, J.; Arcos, D.; Matray, J.M.; Bensenouci, F.; Gaucher, E.C.; Tournassat, C.; Hadi, J. Geochemical Characterization and Modelling of the Toarcian/Domerian Porewater at the Tournemire Underground Research Laboratory. Appl. Geochem. 2012, 27, 1417–1431. [Google Scholar] [CrossRef]
- Wersin, P.; Mazurek, M.; Gimmi, T. Porewater Chemistry of Opalinus Clay Revisited: Findings from 25 Years of Data Collection at the Mont Terri Rock Laboratory. Appl. Geochem. 2022, 138, 105234. [Google Scholar] [CrossRef]
- Planel, D.; Sercombe, J.; Le Bescop, P.; Adenot, F.; Torrenti, J.-M. Long-Term Performance of Cement Paste during Combined Calcium Leaching–Sulfate Attack: Kinetics and Size Effect. Cem. Concr. Res. 2006, 36, 137–143. [Google Scholar] [CrossRef]
- El-Hachem, R.; Rozière, E.; Grondin, F.; Loukili, A. New Procedure to Investigate External Sulphate Attack on Cementitious Materials. Cem. Concr. Compos. 2012, 34, 357–364. [Google Scholar] [CrossRef]
- Cao, H.T.; Bucea, L.; Ray, A.; Yozghatlian, S. The Effect of Cement Composition and pH of Environment on Sulfate Resistance of Portland Cements and Blended Cements. Cem. Concr. Compos. 1997, 19, 161–171. [Google Scholar] [CrossRef]
- Brown, P.W. An Evaluation of the Sulfate Resistance of Cements in a Controlled Environment. Cem. Concr. Res. 1981, 11, 719–727. [Google Scholar] [CrossRef]
- Barbieri-Albert, B. Altération de Matrices Cimentaires Par Des Eaux de Pluie et Des Eaux Sulfatées. Approche Expérimentale et Thermodynamique. Ph.D. Thesis, Ecole Nationale Supérieure des Mines de Saint-Etienne, Saint-Étienne, France, 2002. [Google Scholar]
- Le Saout, G.; Füllmann, T.; Kocaba, V.; Scrivener, K. Quantitative Study of Cementitious Materials by X-Ray Diffraction/Rietveld Analysis Using an External Standard. In Proceedings of the 12th International Congress on the Chemistry of Cement, Montréal, QC, Canada, 8–13 July 2007. [Google Scholar]
- Vaitkus, A.; Merkys, A.; Gražulis, S. Validation of the Crystallography Open Database Using the Crystallographic Information Framework. J. Appl. Crystallogr. 2021, 54, 661–672. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Constantinides, G.; Chandran, K.R.; Ulm, F.-J.; Van Vliet, K.J. Grid Indentation Analysis of Composite Microstructure and Mechanics: Principles and Validation. Mater. Sci. Eng. A 2006, 430, 189–202. [Google Scholar] [CrossRef]
- Van Der Lee, J.; De Windt, L.; Lagneau, V.; Goblet, P. Module-Oriented Modeling of Reactive Transport with HYTEC. Comput. Geosci. 2003, 29, 265–275. [Google Scholar] [CrossRef]
- de Windt, L.; Devillers, P. Modeling the Degradation of Portland Cement Pastes by Biogenic Organic Acids. Cem. Concr. Res. 2010, 40, 1165–1174. [Google Scholar] [CrossRef]
- Blanc, P.; Lassin, A.; Piantone, P.; Azaroual, M.; Jacquemet, N.; Fabbri, A.; Gaucher, E.C. Thermoddem: A Geochemical Database Focused on Low Temperature Water/Rock Interactions and Waste Materials. Appl. Geochem. 2012, 27, 2107–2116. [Google Scholar] [CrossRef]
- Haecker, C.-J.; Garboczi, E.J.; Bullard, J.W.; Bohn, R.B.; Sun, Z.; Shah, S.P.; Voigt, T. Modeling the Linear Elastic Properties of Portland Cement Paste. Cem. Concr. Res. 2005, 35, 1948–1960. [Google Scholar] [CrossRef]
- Parrot, L.J. Prediction of Cement Hydration. Proc. Br. Ceram. Soc. 1984, 35, 41–53. [Google Scholar]
- Bary, B.; Béjaoui, S. Assessment of diffusive and mechanical properties of hardened cement pastes using a multi-coated sphere assemblage model. Cem. Concr. Res. 2006, 36, 245–258. [Google Scholar] [CrossRef]
- Socié, A.; Monerie, Y.; Péralès, F. Effects of the Microstructural Uncertainties on the Poroelastic and the Diffusive Properties of Mortar. J. Theor. Comput. Overlay Appl. Mech. 2022. [Google Scholar] [CrossRef]
- Lothenbach, B.; Bary, B.; Le Bescop, P.; Schmidt, T.; Leterrier, N. Sulfate Ingress in Portland Cement. Cem. Concr. Res. 2010, 40, 1211–1225. [Google Scholar] [CrossRef]
- Jennings, H.M.; Tennis, P.D. Model for the Developing Microstructure in Portland Cement Pastes. J. Am. Ceram. Soc. 1994, 77, 3161–3172. [Google Scholar] [CrossRef]
- Constantinides, G.; Ulm, F.-J. The Effect of Two Types of CSH on the Elasticity of Cement-Based Materials: Results from Nanoindentation and Micromechanical Modeling. Cem. Concr. Res. 2004, 34, 67–80. [Google Scholar] [CrossRef]
- Stora, E.; Bary, B.; He, Q.-C.; Deville, E.; Montarnal, P. Modelling and Simulations of the Chemo–Mechanical Behaviour of Leached Cement-Based Materials: Leaching Process and Induced Loss of Stiffness. Cem. Concr. Res. 2009, 39, 763–772. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and Microanalytical Studies of Sulfate Attack. I. Ordinary Portland Cement Paste. Cem. Concr. Res. 1992, 22, 1027–1038. [Google Scholar] [CrossRef]
- Wang, J.G. Sulfate Attack on Hardened Cement Paste. Cem. Concr. Res. 1994, 24, 735–742. [Google Scholar] [CrossRef]
- Soive, A.; Roziere, E.; Loukili, A. Parametrical Study of the Cementitious Materials Degradation under External Sulfate Attack through Numerical Modeling. Constr. Build. Mater. 2016, 112, 267–275. [Google Scholar] [CrossRef]
- Maltais, Y.; Samson, E.; Marchand, J. Predicting the Durability of Portland Cement Systems in Aggressive Environments—Laboratory Validation. Cem. Concr. Res. 2004, 34, 1579–1589. [Google Scholar] [CrossRef]
- Hime, W.G.; Mather, B. Sulfate Attack, or Is It? Cem. Concr. Res. 1999, 29, 789–791. [Google Scholar] [CrossRef]
- Matschei, T.; Skapa, R.; Lothenbach, B.; Glasser, F.P. The Distribution of Sulfate in Hydrated Portland Cement Paste. In Proceedings of the 12th International Congress on the Chemistry of Cement, Montréal, QC, Canada, 8–13 July 2007. [Google Scholar]
- Irassar, E.F.; Bonavetti, V.L.; Gonzalez, M. Microstructural Study of Sulfate Attack on Ordinary and Limestone Portland Cements at Ambient Temperature. Cem. Concr. Res. 2003, 33, 31–41. [Google Scholar] [CrossRef]
- Feng, P.; Garboczi, E.J.; Miao, C.; Bullard, J.W. Microstructural Origins of Cement Paste Degradation by External Sulfate Attack. Constr. Build. Mater. 2015, 96, 391–403. [Google Scholar] [CrossRef]
- Le Bescop, P.; Solet, C. External Sulphate Attack by Ground Water: Experimental Study on CEM I Cement Pastes. Rev. Eur. Génie Civ. 2006, 10, 1127–1145. [Google Scholar] [CrossRef]
- El-Hachem, R.; Rozière, E.; Grondin, F.; Loukili, A. Multi-Criteria Analysis of the Mechanism of Degradation of Portland Cement Based Mortars Exposed to External Sulphate Attack. Cem. Concr. Res. 2012, 42, 1327–1335. [Google Scholar] [CrossRef]
- Adenot, F.; Richet, C.; Faucon, P. Long-Term Prediction of Concrete Durability in Radioactive Waste Management: Influence of the PH of the Aggressive Solution. In Proceedings of the International Conference on Engineering Materials, Ottawa, ON, Canada, 8 June 1997; Volume 2. [Google Scholar]
- Pouya, J.; Neji, M.; De Windt, L.; Péralès, F.; Socié, A.; Corvisier, J. Multi-Technical Study of Chemical and Cracking Processes of Cement Paste Degradation Subjected to a Low Concentration External Sulfate Attack. Cem. Concr. Compos. 2022. submitted. [Google Scholar]
- Bary, B. Simplified Coupled Chemo-Mechanical Modeling of Cement Pastes Behavior Subjected to Combined Leaching and External Sulfate Attack. Int. J. Numer. Anal. Methods Geomech. 2008, 32, 1791–1816. [Google Scholar] [CrossRef]
- Rougelot, T.; Burlion, N.; Bernard, D.; Skoczylas, F. About Microcracking Due to Leaching in Cementitious Composites: X-Ray Microtomography Description and Numerical Approach. Cem. Concr. Res. 2010, 40, 271–283. [Google Scholar] [CrossRef]



| Chemical Analysis (g/100g) | |
| SiO2 | 19.3 |
| Al2O3 | 5.3 |
| Fe2O3 | 2.6 |
| CaO | 63.2 |
| SO3 | 3.5 |
| Na2O | 0.08 |
| K2O | 0.94 |
| Normative phase composition (g/100 g) | |
| C3A | 11 |
| C3S | 66 |
| C2S | 10 |
| C4AF | 8 |
| Water | |
| W/C (mass) | 0.5 |
| Solid Phase | Chemical Equation | Log K (a) 25 °C | Density (a) (kg·m−3) | Young Mod. (b) (GPa) | Poisson Coeff. (b) |
|---|---|---|---|---|---|
| Cement hydrates | |||||
| C1.6SH | 3.2Ca2+ + 2H4SiO4 + 2.6128H2O − 6.4H+ → (CaO)3.2(SiO2)2(H2O)3.4128 | −55.99 | 2506 | 23.8 (1) | 0.24 |
| C1.5SH | 3Ca2+ + 2H4SiO4 + 2.2631H2O − 6H+ → (CaO)3(SiO2)2(H2O)3.2631 | −51.44 | 2478 | 21.4 (1) | 0.24 |
| C1.4SH | 2.8Ca2+ + 2H4SiO4 + 1.9144H2O − 5.6H+ → (CaO)2.8(SiO2)2(H2O)3.1144 | −46.93 | 2447 | 18.9 (1) | 0.24 |
| C1.3SH | 2.6Ca2+ + 2H4SiO4 + 1.5659H2O − 5.2H+ → (CaO)2.6(SiO2)2(H2O)2.9659 | −42.47 | 2415 | 16.5 (1) | 0.24 |
| C1.2SH | 2.4Ca2+ + 2H4SiO4 + 1.1895H2O − 4.8H+ → (CaO)2.4(SiO2)2(H2O)2.7895 | −38.09 | 2389 | 14.1 (1) | 0.24 |
| C1.1SH | 2.2Ca2+ + 2H4SiO4 + 0.7491H2O − 4.4H+ → (CaO)2.2(SiO2)2(H2O)2.5491 | −33.76 | 2380 | 11.7 (1) | 0.24 |
| C1SH | 2Ca2+ + 2H4SiO4 + 0.3978H2O − 4H+ → (CaO)2(SiO2)2(H2O)2.3978 | −29.47 | 2358 | 9.2 (1) | 0.24 |
| C0.9SH | 1.8Ca2+ + 2H4SiO4 + 0.1062 H2O − 3.6H+ → (CaO)1.8(SiO2)2(H2O)2.3062 | −25.25 | 2327 | 6.7 (1) | 0.24 |
| C0.8SH | 1.6 Ca2+ +2H4SiO4 − 0.218H2O − 3.2H+ → (CaO)1.6(SiO2)2(H2O)2.182 | −21.18 | 2299 | 4.3 (1) | 0.24 |
| C0.7SH | 1.4Ca2+ +2H4SiO4 − 0.6724 H2O − 2.8H+ → (CaO)1.4(SiO2)2(H2O)1.9276 | −17.80 | 2292 | 4.3 (1) | 0.24 |
| Ettringite | 2Al3+ + 6Ca2+ + 3SO42− + 38H2O → Ca6Al2(SO4)3(OH)12·26H2O + 12H+ | −57.00 | 1770 | 22.4 | 0.25 |
| Portlandite | Ca2+ + 2H2O → Ca(OH)2 + 2H+ | −22.81 | 2241 | 42.3 | 0.324 |
| Monocarboaluminate | 2Al3+ + 4Ca2+ + HCO3− + 16.7 H2O → Ca4Al2(CO3)(OH)12·5H2O + 13H+ | −80.55 | 2148 | 42.3 | 0.324 |
| Hemicarboaluminate | 4Al3+ + 8Ca2+ + HCO3− + 35 H2O → Ca8Al4(CO3)(OH)26·18H2O + 27H+ | −183.65 | 1921 | - | - |
| Monosulfoaluminate | 2Al3+ + 4Ca2+ + SO42− + 18H2O → Ca4Al2(SO42−)(OH)12·6H2O + 12H+ | −73.06 | 2000 | 42.3 | 0.324 |
| Other phases | |||||
| Gypsum | Ca2+ + SO42− + 2H2O → CaSO4(H2O)2 | 4.61 | 2305 | 45.7 | 0.33 |
| Calcite | Ca2+ + HCO3− → CaCO3 + H+ | −1.85 | 2710 | 79.6 | 0.31 |
| Gibbsite | Al3+ + 3H2O → Al(OH)3 + 3H+ | −7.73 | 2441 | - | - |
| Amorphous silica | SiO2(aq) → SiO2(am) | 2.69 | 2072 | - | - |
| Volume Fraction | |
|---|---|
| Minerals | |
| Portlandite | 0.18 |
| C1.6SH | 0.26 |
| Ettringite | 0.11 |
| Monocarboaluminate | 0.09 |
| Unhydrated clinker | |
| C3S | 0.03 |
| C2S | 0.01 |
| C3A | 0.006 |
| C4AF | 0.006 |
| Porosity | 0.31 |
| Num. Data (HYTEC) Secondary Ettringite Precipitation Depth (µm) | Exp. Data (XRD) Monocarboaluminate Detection Depth (µm) | |
|---|---|---|
| 15 days | 480 | 550 |
| 60 days | 900 | 800 |
| 120 days | 1300 | 950 |
| Decalcification Limit Depth (µm) | Gypsum Precipitation Depth (µm) | Gypsum Thickness (µm) | |
|---|---|---|---|
| Small vol. of solution with non-regulated pH | 900 | 500–800 | 300 |
| Large vol. of solution with pH control around 7 | 1400 | 800–1300 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouya, J.; Neji, M.; De Windt, L.; Péralès, F.; Socié, A.; Corvisier, J. Mineralogical Evolution and Expansion of Cement Pastes in a Sulfate-Confined Environment. Minerals 2023, 13, 1. https://doi.org/10.3390/min13010001
Pouya J, Neji M, De Windt L, Péralès F, Socié A, Corvisier J. Mineralogical Evolution and Expansion of Cement Pastes in a Sulfate-Confined Environment. Minerals. 2023; 13(1):1. https://doi.org/10.3390/min13010001
Chicago/Turabian StylePouya, Julie, Mejdi Neji, Laurent De Windt, Frédéric Péralès, Adrien Socié, and Jérôme Corvisier. 2023. "Mineralogical Evolution and Expansion of Cement Pastes in a Sulfate-Confined Environment" Minerals 13, no. 1: 1. https://doi.org/10.3390/min13010001
APA StylePouya, J., Neji, M., De Windt, L., Péralès, F., Socié, A., & Corvisier, J. (2023). Mineralogical Evolution and Expansion of Cement Pastes in a Sulfate-Confined Environment. Minerals, 13(1), 1. https://doi.org/10.3390/min13010001





