Basic Evaluation of Phase Relation in a Phosphorus-Containing System Saturated with CaSiO3 at Elevated Temperatures for the Utilization of Steelmaking Slag and Sewage Sludge as Phosphorus Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
3. Results
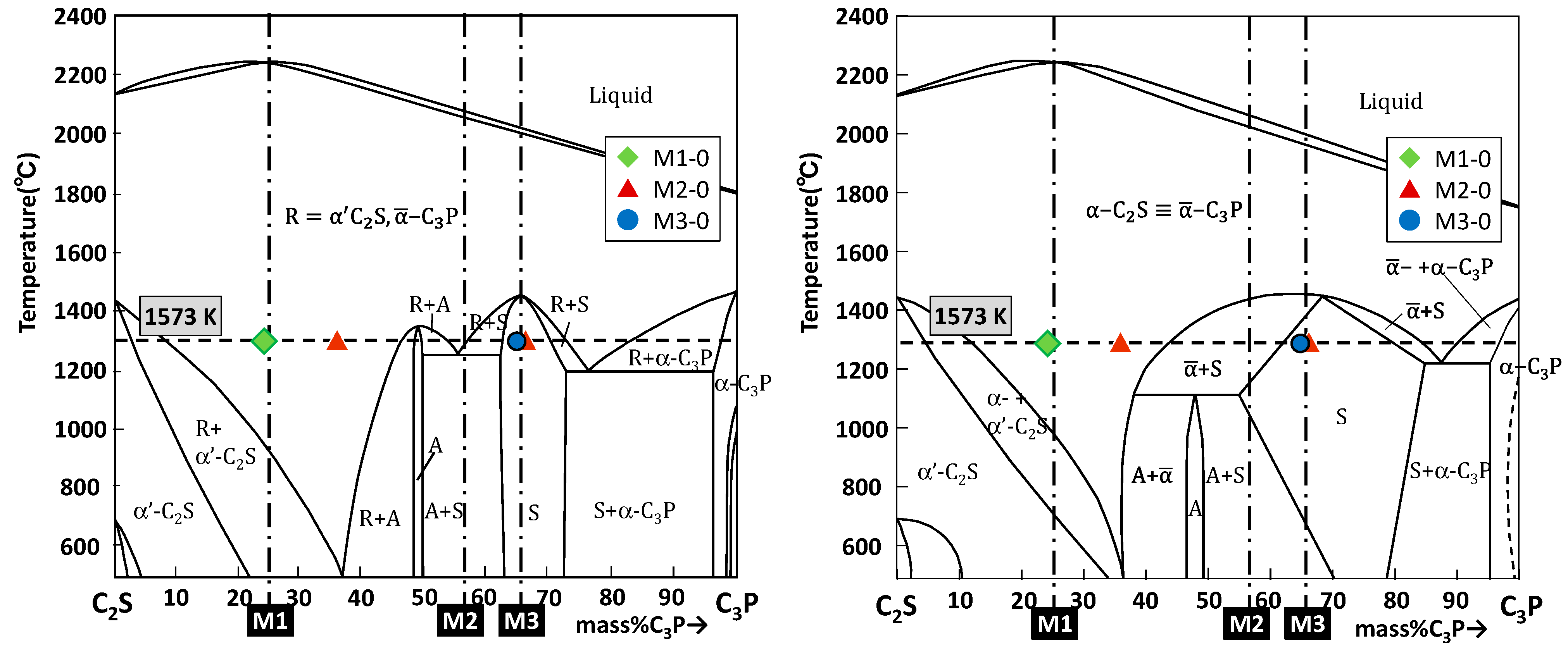
3.1. CaO-SiO2-P2O5 Ternary System
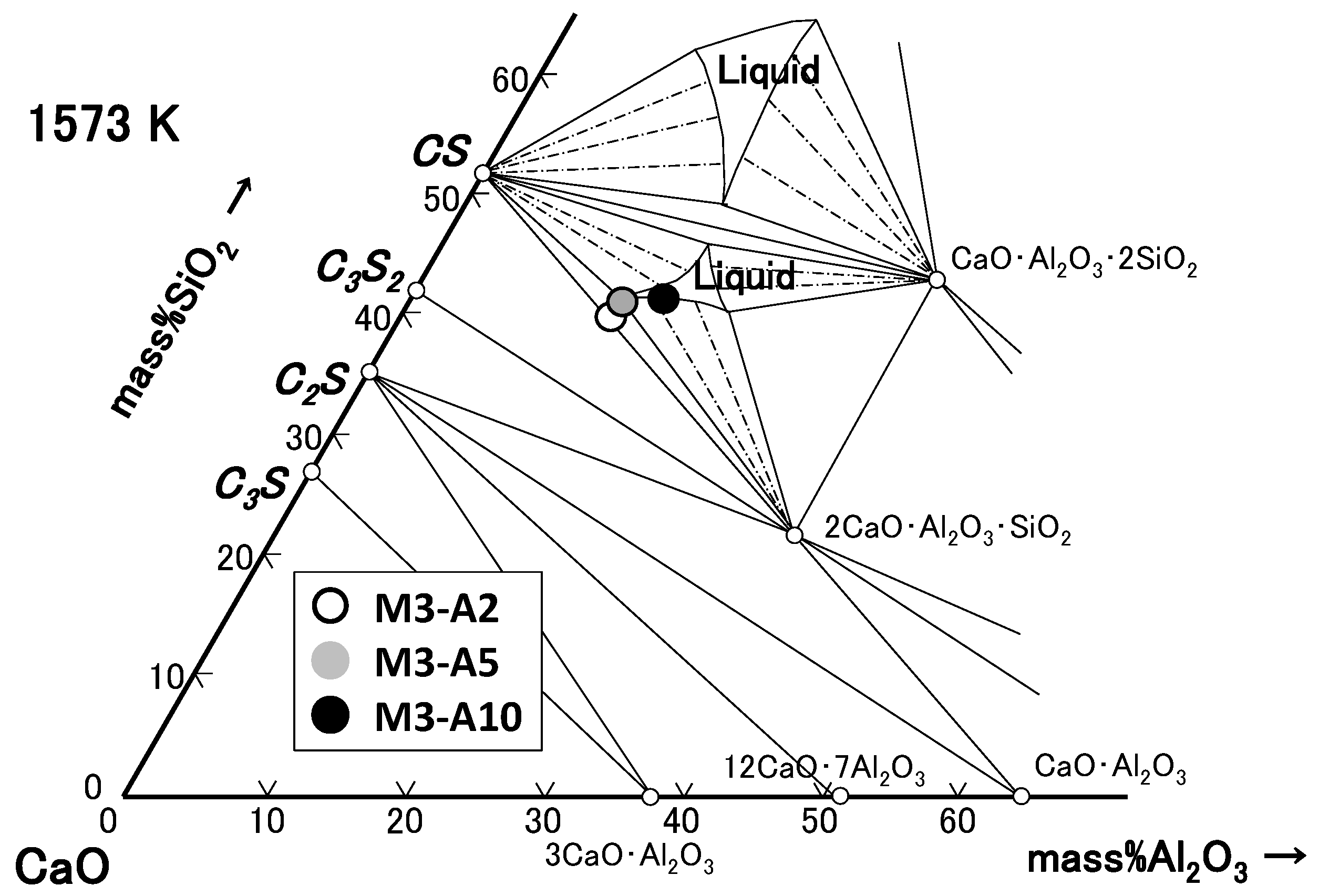
3.2. CaO-SiO2-P2O5–Al2O3/Fe2O3 System
4. Discussion
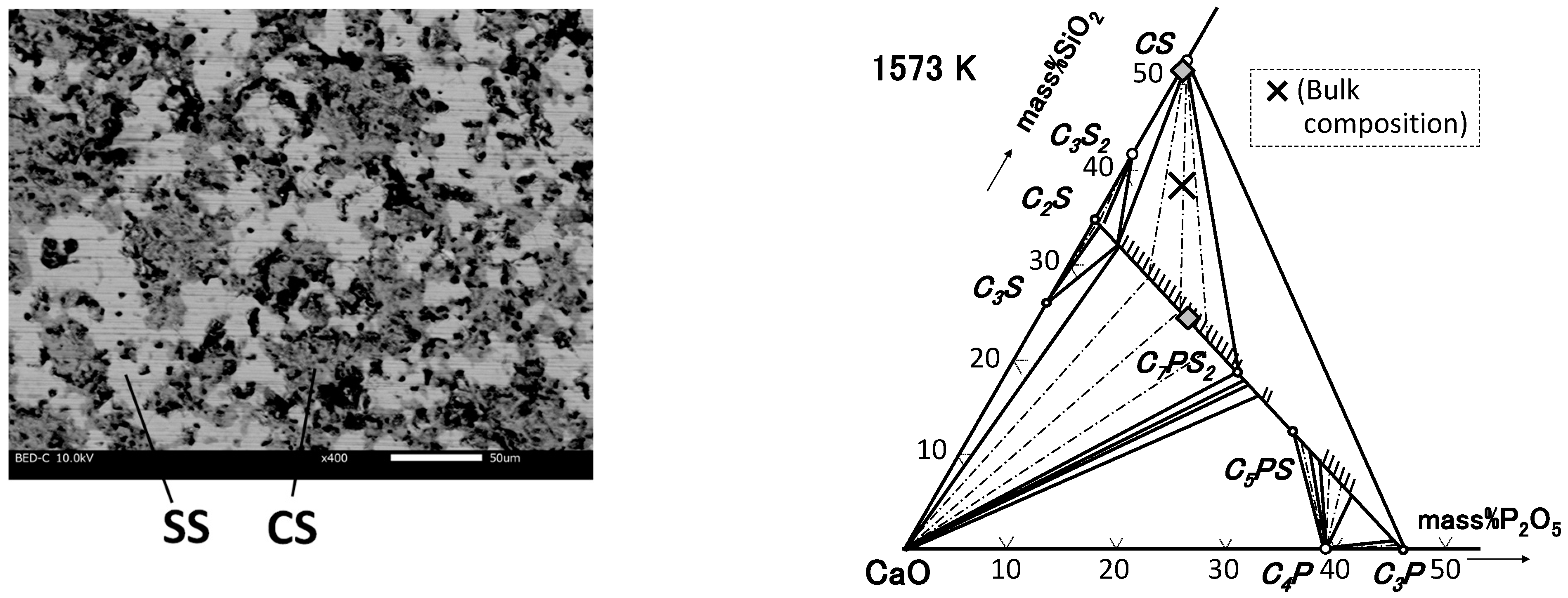
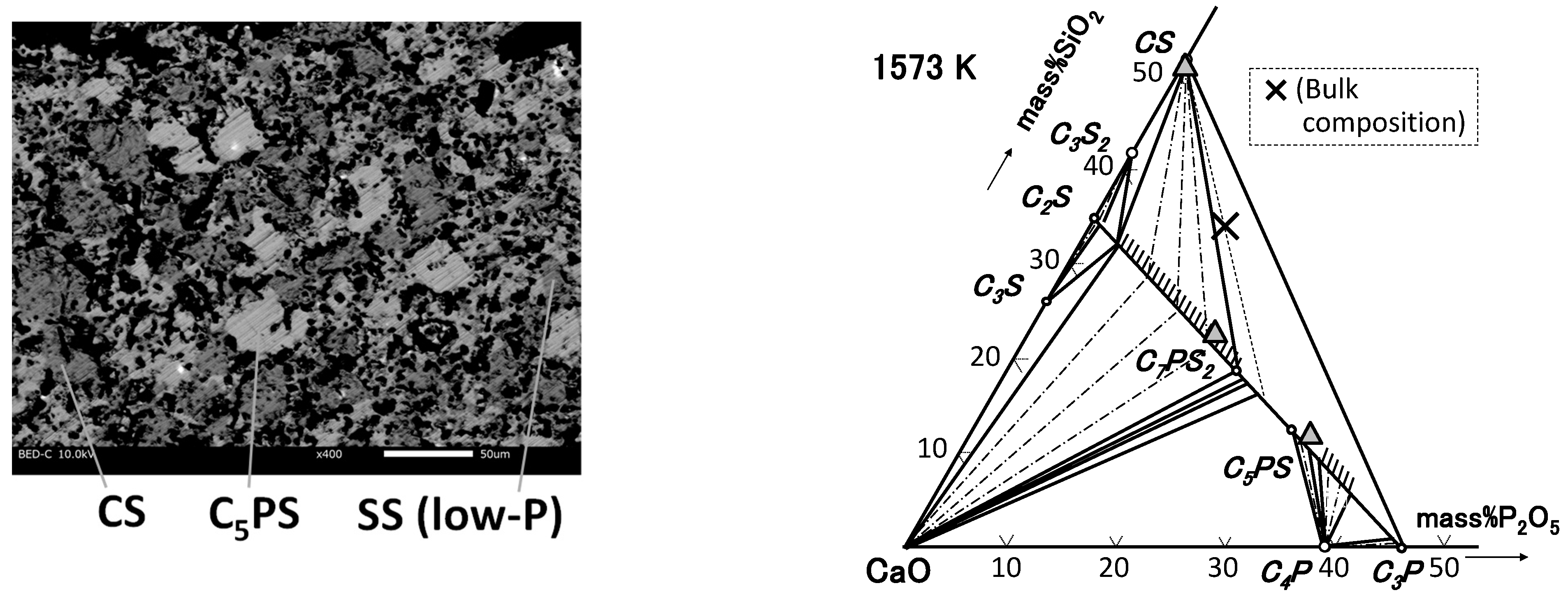
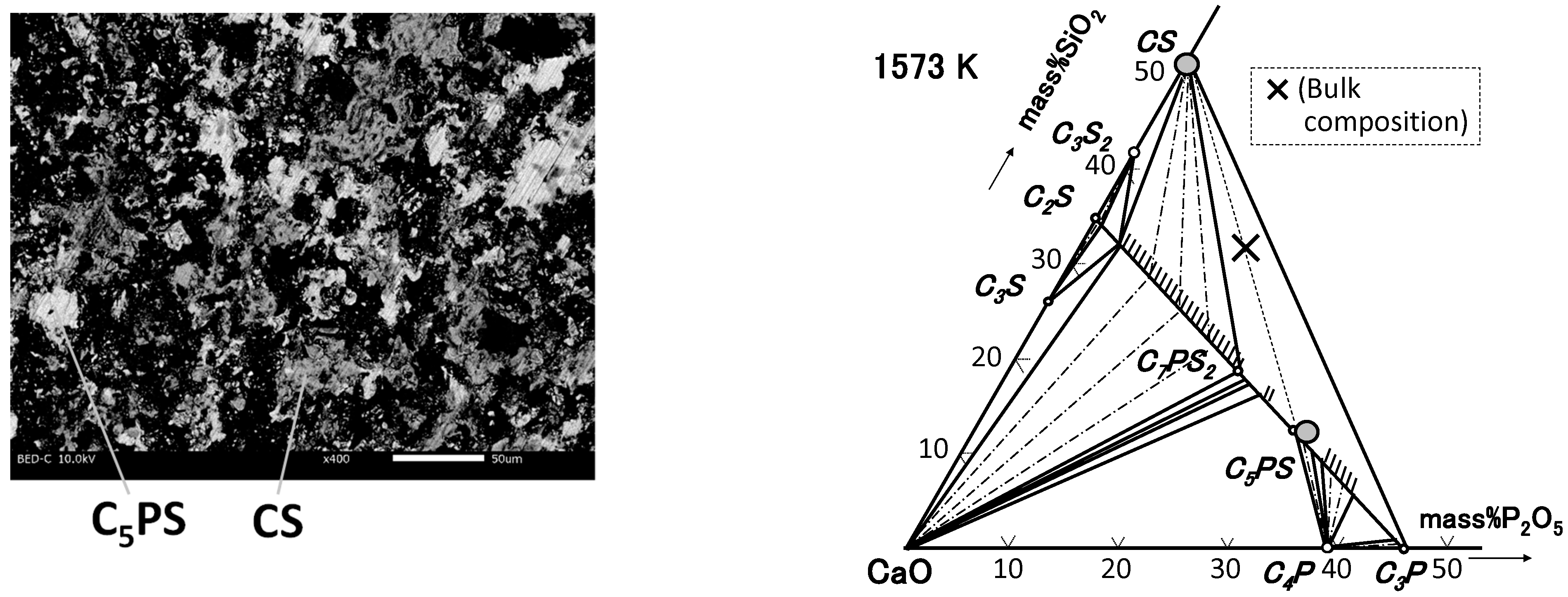
4.1. Phase Relation in Ternary Samples
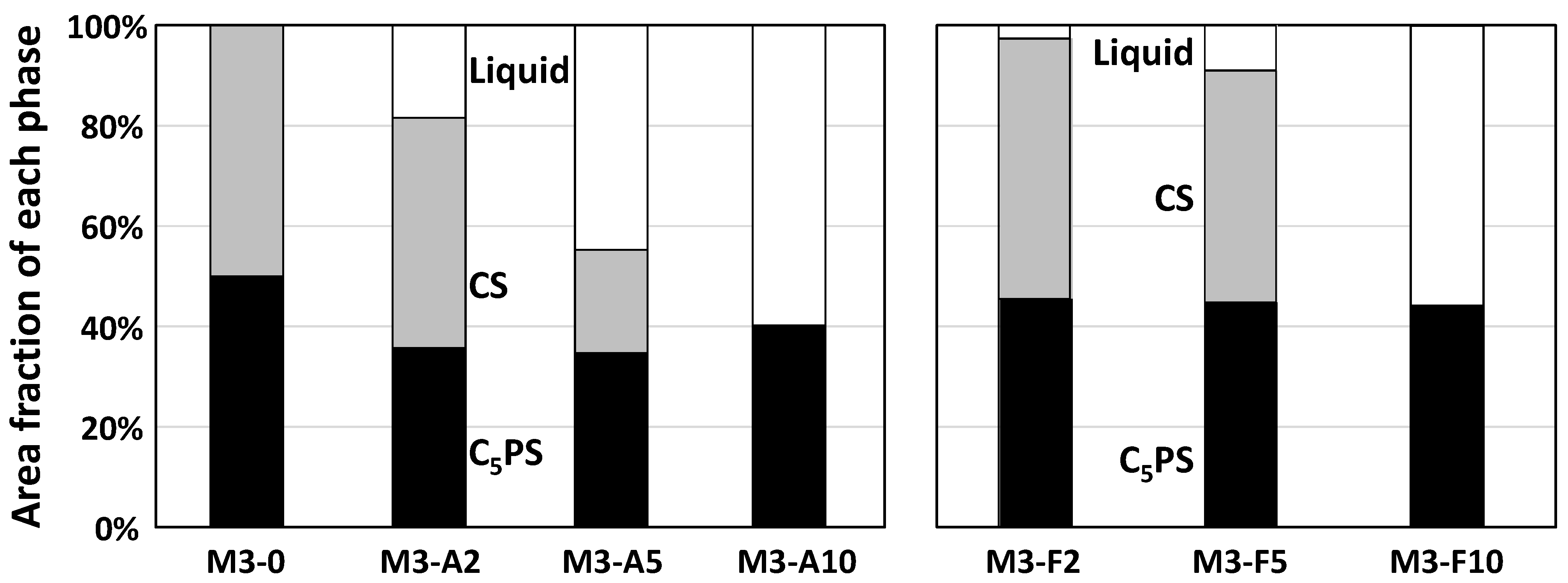
4.2. Phase Relation in Quaternary Samples (Liquid Composition)
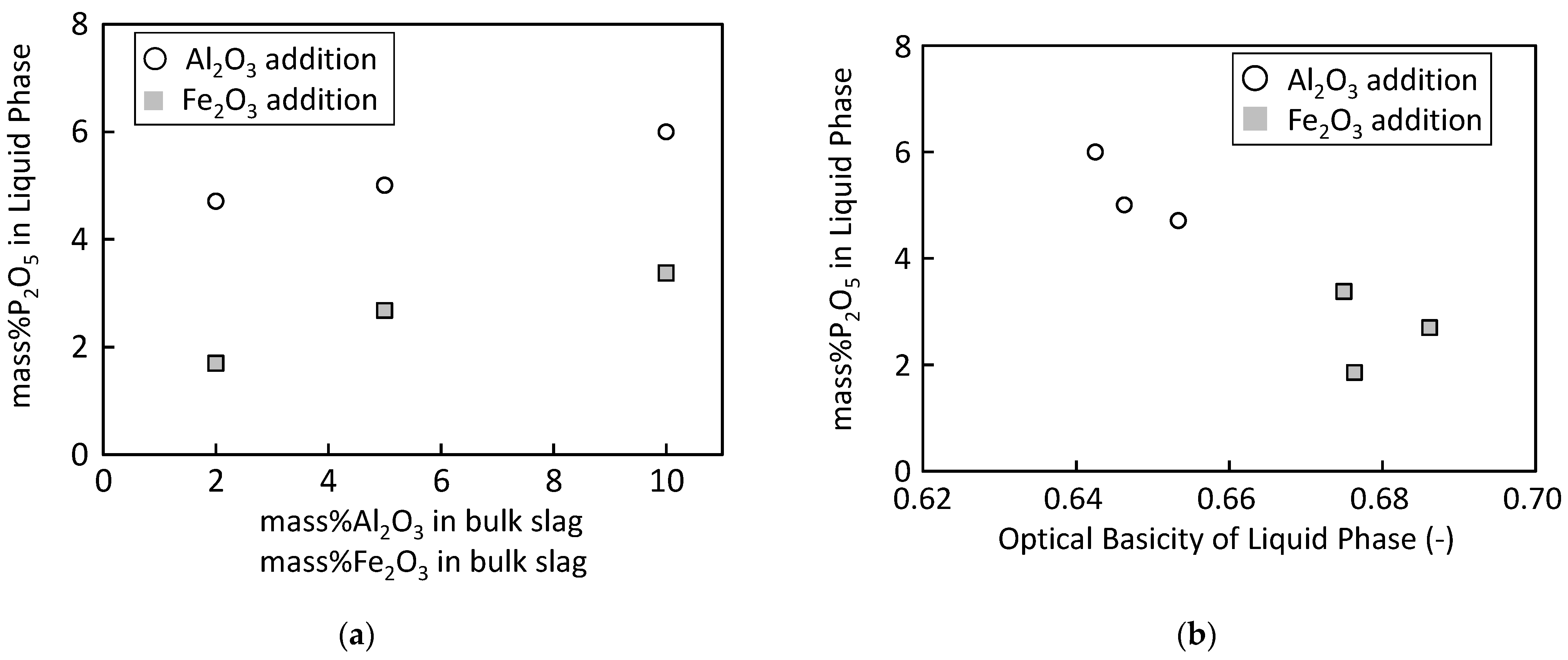
4.3. P2O5 Content in Liquid Phase
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Boer, M.A.; Wolzak, L.; Slootweg, J.C. Phosphorus: Reserves, production and applications. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 75–100. [Google Scholar]
- Miki, T. Phosphorus separation and recovery from steelmaking slag. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 329–337. [Google Scholar]
- Matsui, A.; Nakase, K.; Kikuchi, N.; Kishimoto, Y.; Takahashi, K.; Ishida, K. Phosphorus separation from steelmaking slag by high temperature reduction with mechanical stirring. Tetsu-to-Hagané 2016, 102, 416–422. [Google Scholar] [CrossRef]
- Shiomi, S.; Sano, N.; Matsushita, Y. Removal of phosphorus in BOF slag. Tetsu-to-Hagané 1977, 63, 1520–1528. [Google Scholar]
- Takeuchi, S.; Sano, N.; Matsushita, Y. Separate recovery of iron and phosphorus from BOF slags by using Fe-Si alloy. Tetsu-to-Hagané 1980, 66, 2050–2057. [Google Scholar] [CrossRef][Green Version]
- Morita, K.; Guo, M.; Oka, N.; Sano, N. Resurrection of the iron and phosphorus resource in steel-making slags. J. Mater. Cycl. Waste Manag. 2002, 4, 93–101. [Google Scholar]
- Kubo, H.; Matsubae-Yokoyama, K.; Nagasaka, T. Magnetic separation of phosphorus enriched phase from multiphase dephosphorization slag. ISIJ Int. 2010, 50, 59–64. [Google Scholar] [CrossRef]
- Nakase, K.; Matsui, A.; Kikuchi, N.; Miki, Y. Effect of slag composition on phosphorus separation from steelmaking slag by reduction. ISIJ Int. 2017, 57, 1197–1204. [Google Scholar] [CrossRef]
- Iwama, T.; Du, C.; Gao, X.; Kim, S.J.; Ueda, S.; Kitamura, S. Extraction of phosphorus from steelmaking slag by selective leaching using citric acid. ISIJ Int. 2018, 58, 1351–1360. [Google Scholar] [CrossRef]
- Tromel, G.; Fix, W.; Koch, K. The compositional diagram of the system CaO-FeOn-P2O5-SiO2 at 1600C. Arch. Eisenhuttenwes. 1967, 38, 177–190. [Google Scholar]
- Koch, K.; Fix, W. Examinations in the slag system Fe-CaO-FeOn-P2O5-SiO2 at 1600C. Arch. Eisenhuttenwes. 1970, 41, 111–118. [Google Scholar]
- Tromel, G.; Koch, K.; Fix, W.; Ameling, D. Investigation of the sixcomponent system CaO-FeOn-MgO-MnOn-P2O5-SiO2 at 1600C in equilibrium with iron. Arch. Eisenhuttenwes. 1974, 45, 671–678. [Google Scholar]
- Werme, A.; Lundh, P.A. Distribution of phosphorus between some CaO-FeO-SiO2-P2O5 (10%) slags and C-saturated liquid iron at 1300C. Scand. J. Metall. 1987, 16, 33–41. [Google Scholar]
- Yang, H.; Chang, C. Solid solution of dicalcium silicate and calcium ferrites and phase compatibility in the system CaO-Ca2SiO4-CaFe2O4-Ca3P2O8—Evidence from phosphorus-bearing converter slags. Trans. J. Br. Ceram. Soc. 1990, 89, 159–164. [Google Scholar]
- Matsu-sue, M.; Fushi-tani, K.; Hasegawa, M.; Iwase, M. Electrochemical measurements of oxygen potentials in heterogeneous slags-system CaO-P2O5-SiO2-FexO. Steel Res. Int. 2008, 79, 678–684. [Google Scholar] [CrossRef]
- Uchida, Y.; Kishimoto, Y.; Miki, Y.; Iwase, M. Activities of FexO in industrial slags used for external removal of phosphorous from hot metal. High Temp. Mater. Process. 2012, 31, 479–490. [Google Scholar] [CrossRef]
- Uchida, Y.; Watanabe, C.; Hasegawa, M. Phase equilibria in high phosphate-containing slag without CaO saturation at elevated temperature. Tetsu-to-Hagané 2021, 107, 701–711. [Google Scholar] [CrossRef]
- Arnout, S.; Nagels, E. Modelling thermal phosphorus recovery from sewage sludge ash. CALPHAD 2016, 55, 26–31. [Google Scholar] [CrossRef]
- Morf, I.; Schlumberger, S.; Adam, F.; Diaz Nogueira, G. Urban phosphorus mining in the Canton of Zurich: Phosphorus acid from sewage sludge ash. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 157–177. [Google Scholar]
- Nurse, R.W.; Welch, J.H.; Gutt, W. High-temperature phase equilibria in the system dicalcium silicate—Tricalcium phosphate. J. Chem. Soc. 1959, 1077–1083. [Google Scholar] [CrossRef]
- Fix, W.; Heymann, H.; Heinke, R. Subsolidus relations in the system 2CaO·SiO2–3CaO·P2O5. J. Am. Ceram. Soc. 1969, 52, 346–347. [Google Scholar] [CrossRef]
- Fix, W.; Tromel, G.; Heinke, R. Examinations concerning equilibrium adjustment of the solid phases in the system CaO-2CaO·SiO2-3CaO·P2O5. Arch. Eisenhuttenwes. 1969, 40, 979–984. [Google Scholar]
- Inoue, R.; Suito, H. Phosphorous partition between 2CaO·SiO2 particles and CaO–SiO2–FetO slags. ISIJ Int. 2006, 46, 174–179. [Google Scholar] [CrossRef]
- Matsu-sue, M.; Hasegawa, M.; Fushi-tani, K.; Iwase, M. Phase equilibrium of the system CaO-P2O5-SiO2 between 1473 K and 1673 K. Steel Res. Int. 2007, 78, 465–467. [Google Scholar] [CrossRef]
- Yang, X.; Matsuura, H.; Tsukihashi, F. Condensation of P2O5 at the interface between 2CaO·SiO2 and CaO–SiO2–FeOx–P2O5 slag. ISIJ Int. 2009, 49, 1298–1307. [Google Scholar] [CrossRef]
- Takeshita, H.; Hasegawa, M.; Kashiwaya, Y.; Iwase, M. Formation free energies of solid solution between tri-calcium phosphate and di-calcium silicate. Steel Res. Int. 2010, 81, 100–104. [Google Scholar] [CrossRef]
- Yang, X.; Matsuura, H.; Tsukihashi, F. Reaction behavior of P2O5 at the interface between solid 2CaO·SiO2 and liquid CaO–SiO2–FeOx– P2O5 slags saturated with solid 5CaO·SiO2·P2O5 at 1573 K. ISIJ Int. 2010, 50, 702–711. [Google Scholar] [CrossRef][Green Version]
- Pahlevani, F.; Kitamura, S.; Shibata, H.; Maruoka, N. Distribution of P2O5 between solid solution of 2CaO·SiO2–3CaO·P2O5 and liquid phase. ISIJ Int. 2010, 50, 822–829. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kashiwaya, Y.; Iwase, M. Thermodynamic properties of solid solutions between di-calcium silicate and tri-calcium phosphate. High Temp. Mater. Process 2012, 31, 421–430. [Google Scholar] [CrossRef][Green Version]
- Gao, X.; Matsuura, H.; Sohn, I.; Wang, W.; Min, D.J.; Tsukihashi, F. Phase relationship for the CaO-SiO2-FeO-5 mass%P2O5 system with Oxygen Partial Pressure of 10−8 atm at 1673 and 1623 K. Mater. Trans. 2013, 54, 544–552. [Google Scholar] [CrossRef]
- Matsugi, R.; Miwa, K.; Hasegawa, M. Activities of FeO and P2O5 in dephosphorization slags coexisting with solid solutions between di-calcium silicate and tri-calcium phosphate. ISIJ Int. 2017, 57, 1718–1724. [Google Scholar] [CrossRef]
- Miwa, K.; Matsugi, R.; Hasegawa, M. Activities of FexO in molten slags coexisting with solid CaO and Ca2SiO4–Ca3P2O8 solid solution. ISIJ Int. 2017, 57, 1725–1732. [Google Scholar] [CrossRef]
- Saito, T.; Nishimura, T.; Saito, K.; Hasegawa, M. Activities of P2O5 in solid solutions between di-calcium silicate and tri-calcium phosphate at 1573 K. ISIJ Int. 2020, 60, 2780–2786. [Google Scholar] [CrossRef]
- Yu, H.; Miki, T.; Sasaki, Y.; Nagasaka, T. Crystallography of the high-temperature Ca2SiO4–Ca3P2O8 solid solutions. Metall. Mater. Trans. B 2020, 51B, 3007–3015. [Google Scholar] [CrossRef]
- St. Pierre, P.D.S. Constitution of bone china III. High-temperature phase equilibrium studies in the system tricalciumphosphate-anorthite-silica. J. Am. Ceram. Soc. 1956, 39, 147–150. [Google Scholar] [CrossRef]
- Osborn, E.F.; Muan, A. Phase Equilibrium Diagram of Oxide Systems; American Ceramic Society: Columbus, OH, USA, 1960; Plate 1 and Plate 10. [Google Scholar]
- Mills, K.C. Basicity and optical basicities of slags. In Slag Atlas, 2nd ed.; Eisenhuttenleute, V.D., Ed.; Verlag Stahleisen: Dusseldorf, Germany, 1995; pp. 9–19. [Google Scholar]
- Nakamura, T.; Ueda, Y.; Toguri, J.M. A new development of the optical basicity. J. Japan Inst. Metals 1986, 50, 456–461. [Google Scholar] [CrossRef]
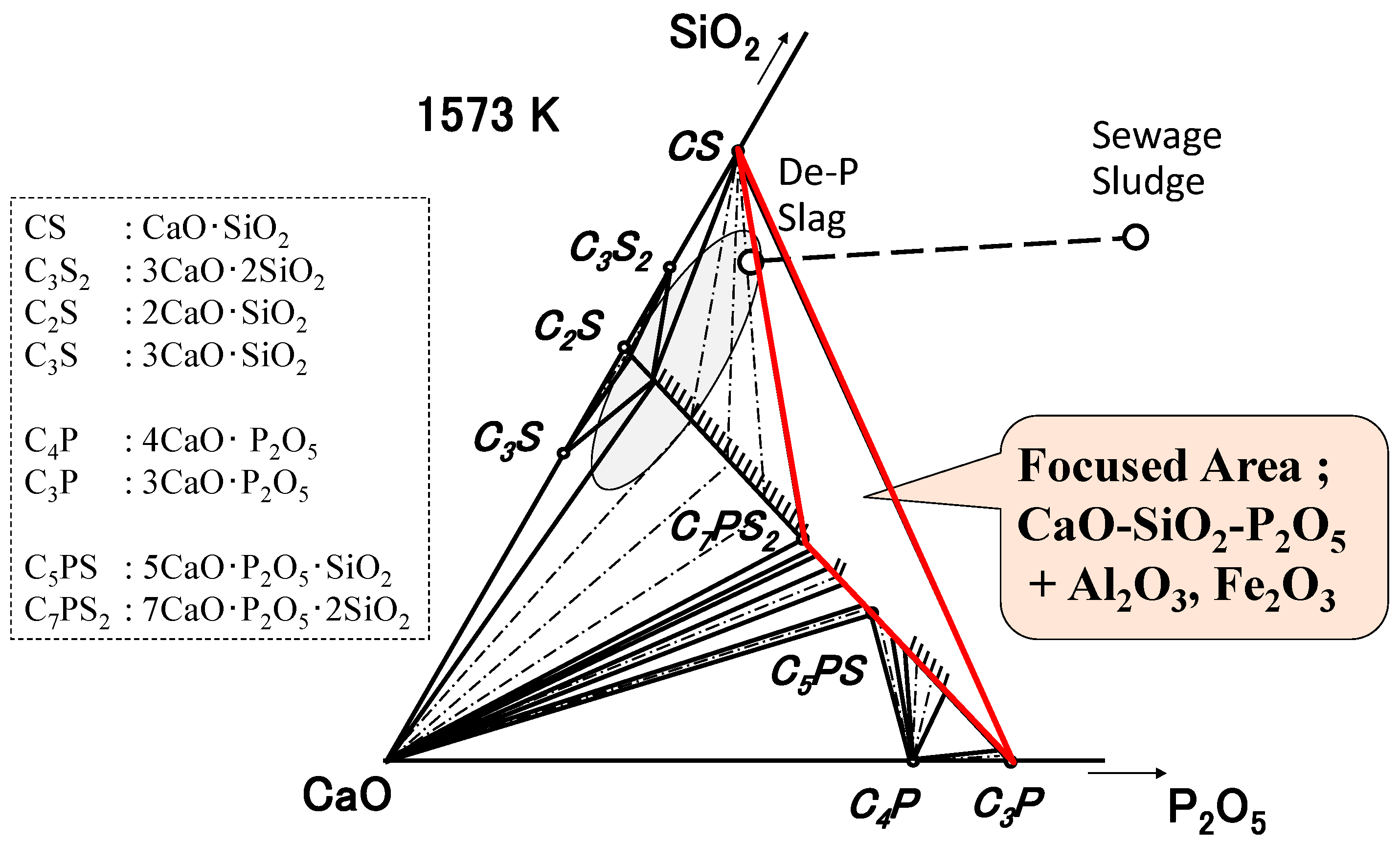


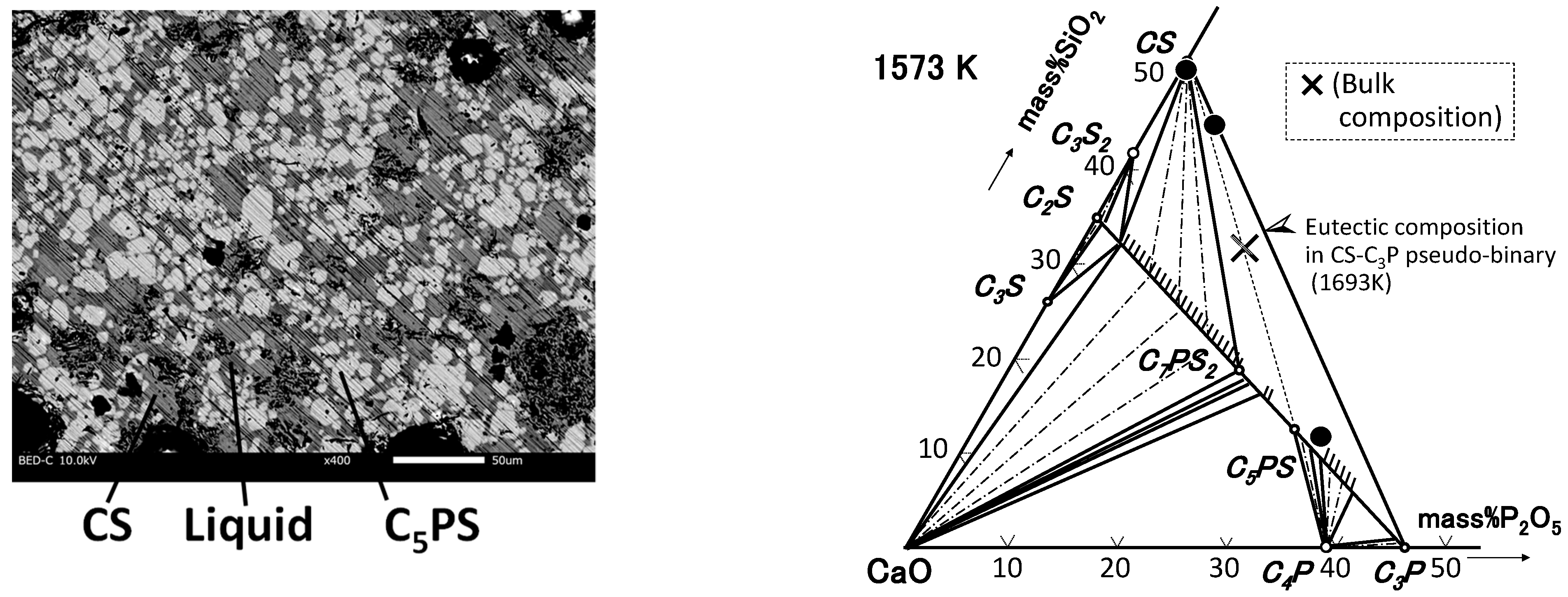
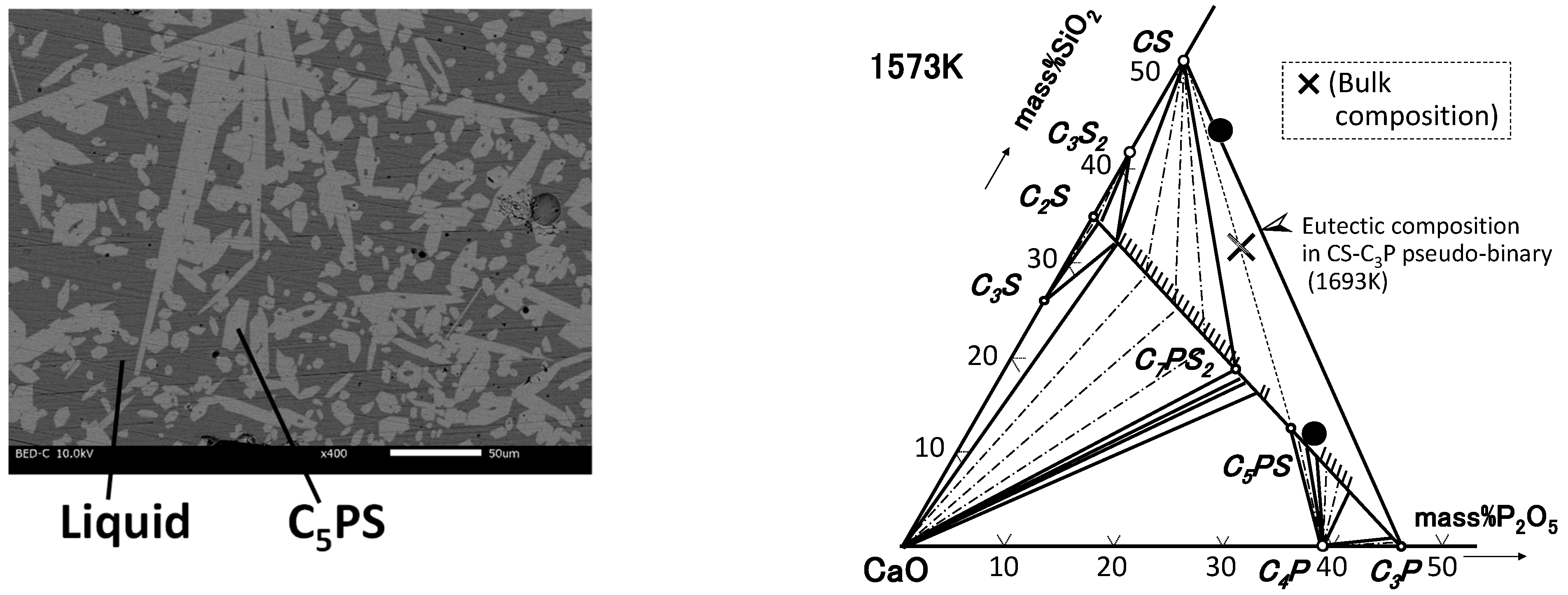
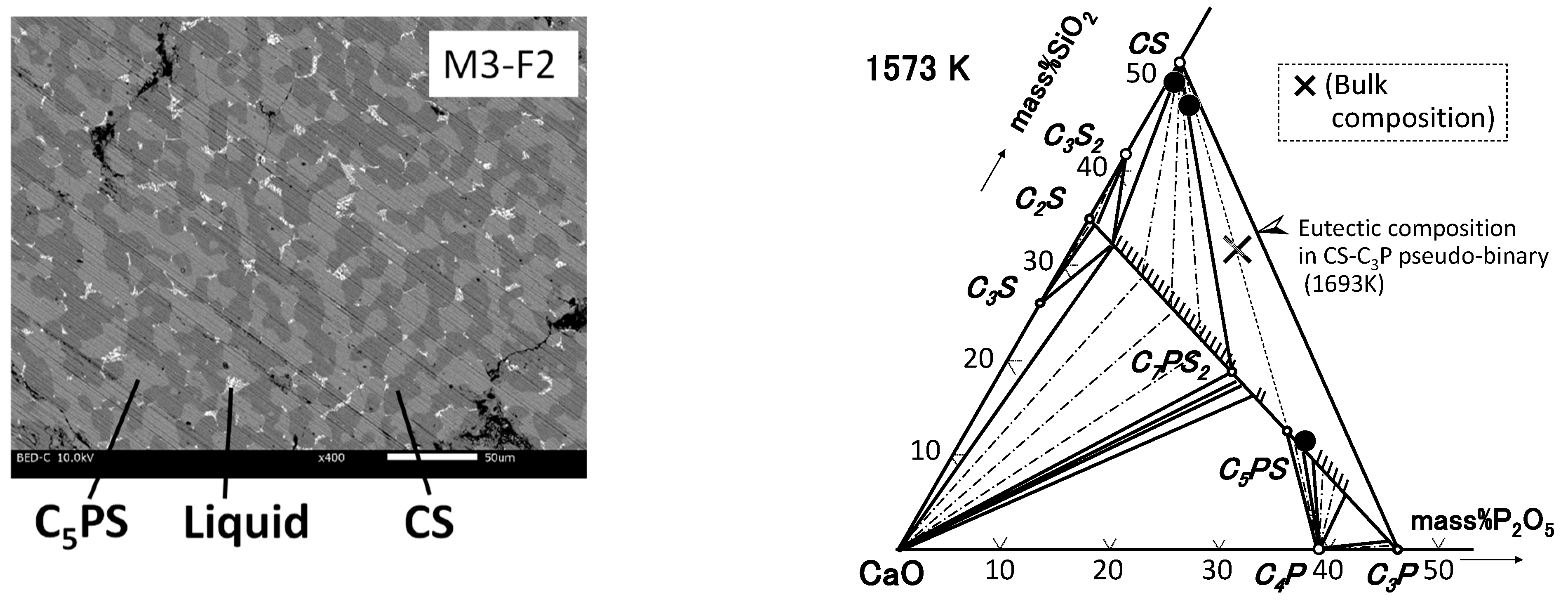

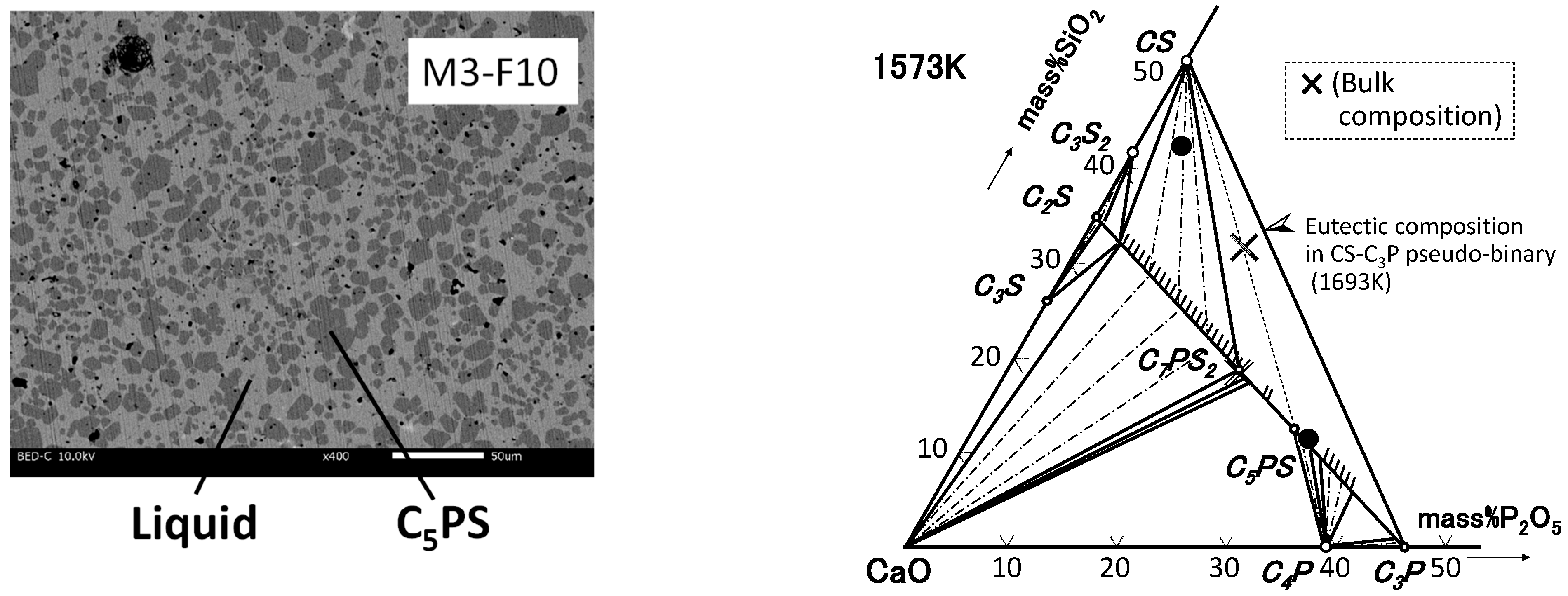

| By-Products | CaO | SiO2 | P2O5 | FeO | Fe2O3 | Al2O3 | MgO | MnO | C/S * |
|---|---|---|---|---|---|---|---|---|---|
| De-P Slag | 26.3 | 22.2 | 3.0 | 24.7 | 7.6 | 5.1 | 6.1 | 4.9 | 1.2 |
| Sewage Sludge | 15.3 | 30.8 | 22.2 | 0.9 | 16.5 | 14.1 | 0.1 | 0.5 |
| Sample No. | CaO | SiO2 | P2O5 | Al2O3 | Fe2O3 |
|---|---|---|---|---|---|
| M1-0 | 55.3 | 38.6 | 6.1 | ||
| M2-0 | 53.6 | 33.6 | 12.8 | ||
| M3-0 | 53.2 | 32.1 | 14.7 | ||
| M3-A2 | 52.1 | 31.5 | 14.4 | 2.0 | |
| M3-A5 | 50.5 | 30.5 | 14.0 | 5.0 | |
| M3-A10 | 47.9 | 28.9 | 13.2 | 10.0 | |
| M3-F2 | 52.1 | 31.5 | 14.4 | 2.0 | |
| M3-F5 | 50.5 | 30.5 | 14.0 | 5.0 | |
| M3-F10 | 47.9 | 28.9 | 13.2 | 10.0 |
| Sample No. | CaO | SiO2 | P2O5 | Note 1 |
|---|---|---|---|---|
| M1-0 | 49.3 | 50.6 | 0.1 | CS |
| 61.7 | 24.2 | 14.1 | SS | |
| M2-0 | 48.7 | 51.1 | 0.2 | CS |
| 55.5 | 12.1 | 32.4 | C5PS | |
| 59.7 | 23.4 | 16.9 | SS | |
| M3-0 | 48.4 | 51.4 | 0.2 | CS |
| 56.8 | 11.9 | 31.3 | C5PS |
| Sample No. | CaO | SiO2 | P2O5 | Al2O3 | Note 1 |
|---|---|---|---|---|---|
| M3-A2 | 48.6 | 51.2 | 0.2 | 0.0 | CS |
| 56.0 | 11.7 | 32.3 | 0.0 | C5PS | |
| 43.8 | 37.3 | 4.7 | 14.2 | Liq | |
| M3-A5 | 49.0 | 50.6 | 0.3 | 0.0 | CS |
| 56.1 | 11.8 | 32.0 | 0.0 | C5PS | |
| 42.3 | 38.4 | 5.0 | 14.3 | Liq | |
| M3-A10 | 55.0 | 11.6 | 31.6 | 1.8 | C5PS |
| 40.4 | 36.8 | 6.0 | 16.8 | Liq |
| Sample No. | CaO | SiO2 | P2O5 | Fe2O3 | Note 1 |
|---|---|---|---|---|---|
| M3-F2 | 49.9 | 49.1 | 0.8 | 0.1 | CS |
| 56.5 | 11.4 | 31.9 | 0.3 | C5PS | |
| 25.9 | 23.7 | 1.7 | 48.7 | Liq | |
| M3-F5 | 49.7 | 50.1 | 0.1 | 0.1 | CS |
| 56.6 | 11.7 | 31.2 | 0.5 | C5PS | |
| 39.5 | 29.2 | 2.7 | 28.6 | Liq | |
| M3-F10 | 56.5 | 11.3 | 31.7 | 0.5 | C5PS |
| 40.6 | 32.3 | 3.4 | 23.8 | Liq |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, Y.-i.; Watanabe, C.; Tsuruoka, H. Basic Evaluation of Phase Relation in a Phosphorus-Containing System Saturated with CaSiO3 at Elevated Temperatures for the Utilization of Steelmaking Slag and Sewage Sludge as Phosphorus Resources. Minerals 2022, 12, 266. https://doi.org/10.3390/min12020266
Uchida Y-i, Watanabe C, Tsuruoka H. Basic Evaluation of Phase Relation in a Phosphorus-Containing System Saturated with CaSiO3 at Elevated Temperatures for the Utilization of Steelmaking Slag and Sewage Sludge as Phosphorus Resources. Minerals. 2022; 12(2):266. https://doi.org/10.3390/min12020266
Chicago/Turabian StyleUchida, Yu-ichi, Chiho Watanabe, and Hideki Tsuruoka. 2022. "Basic Evaluation of Phase Relation in a Phosphorus-Containing System Saturated with CaSiO3 at Elevated Temperatures for the Utilization of Steelmaking Slag and Sewage Sludge as Phosphorus Resources" Minerals 12, no. 2: 266. https://doi.org/10.3390/min12020266
APA StyleUchida, Y.-i., Watanabe, C., & Tsuruoka, H. (2022). Basic Evaluation of Phase Relation in a Phosphorus-Containing System Saturated with CaSiO3 at Elevated Temperatures for the Utilization of Steelmaking Slag and Sewage Sludge as Phosphorus Resources. Minerals, 12(2), 266. https://doi.org/10.3390/min12020266





