Multi-Step Gold Refinement and Collection Using Bi-Minerals in the Laozuoshan Gold Deposit, NE China
Abstract
:1. Introduction
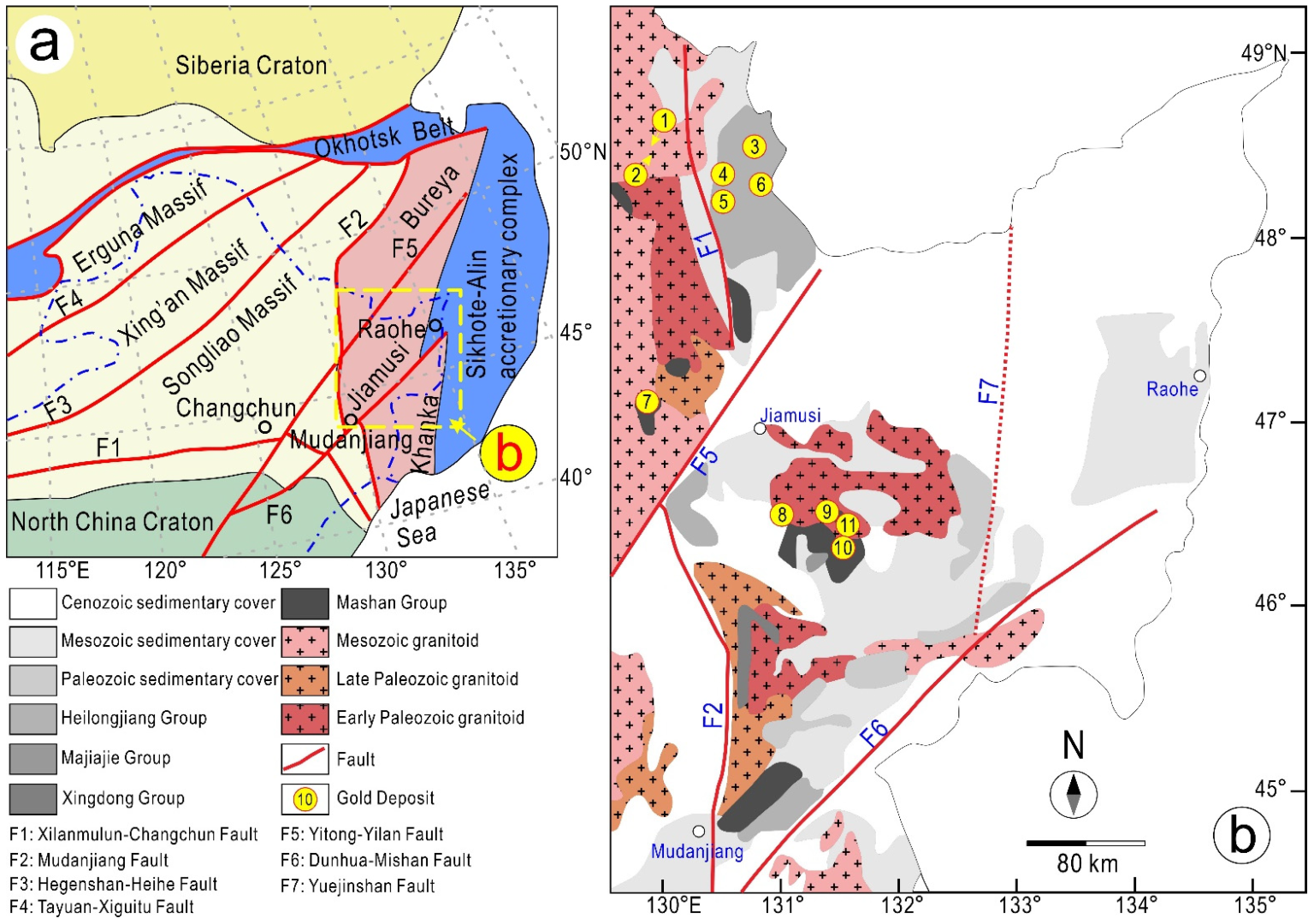
2. Geological Setting
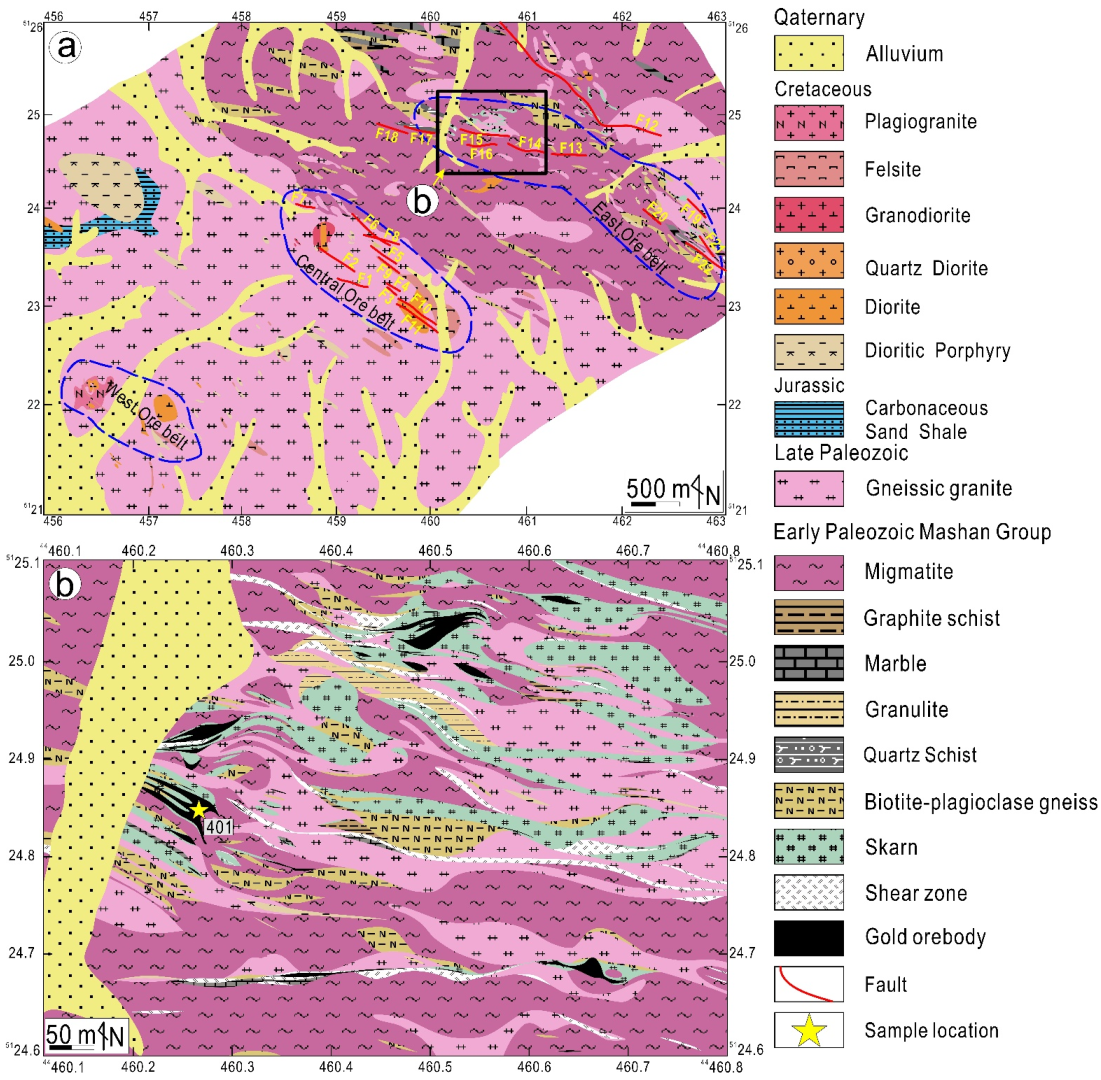
3. Deposit Geology
4. Samples and Analytical Methods
5. Results
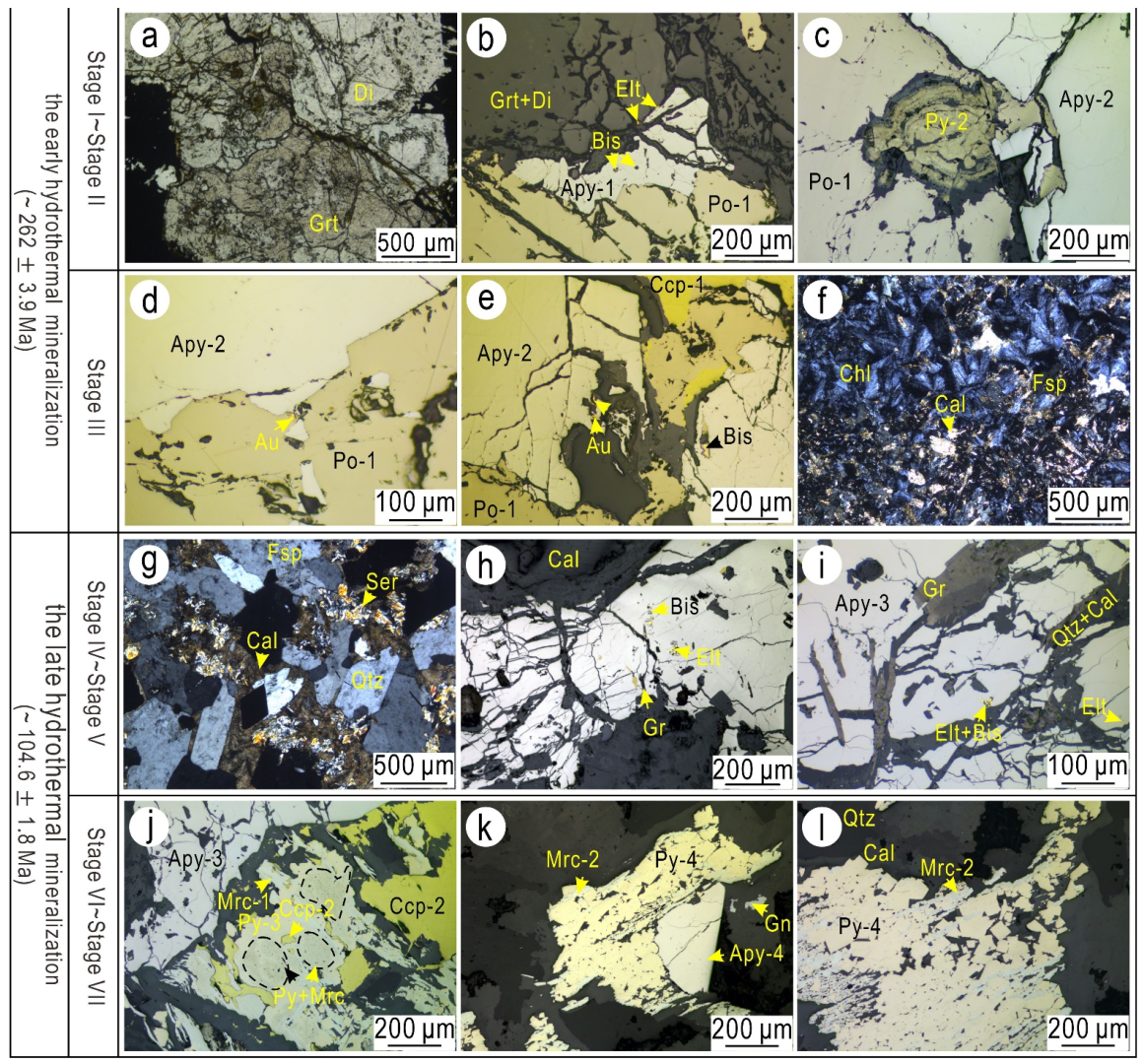
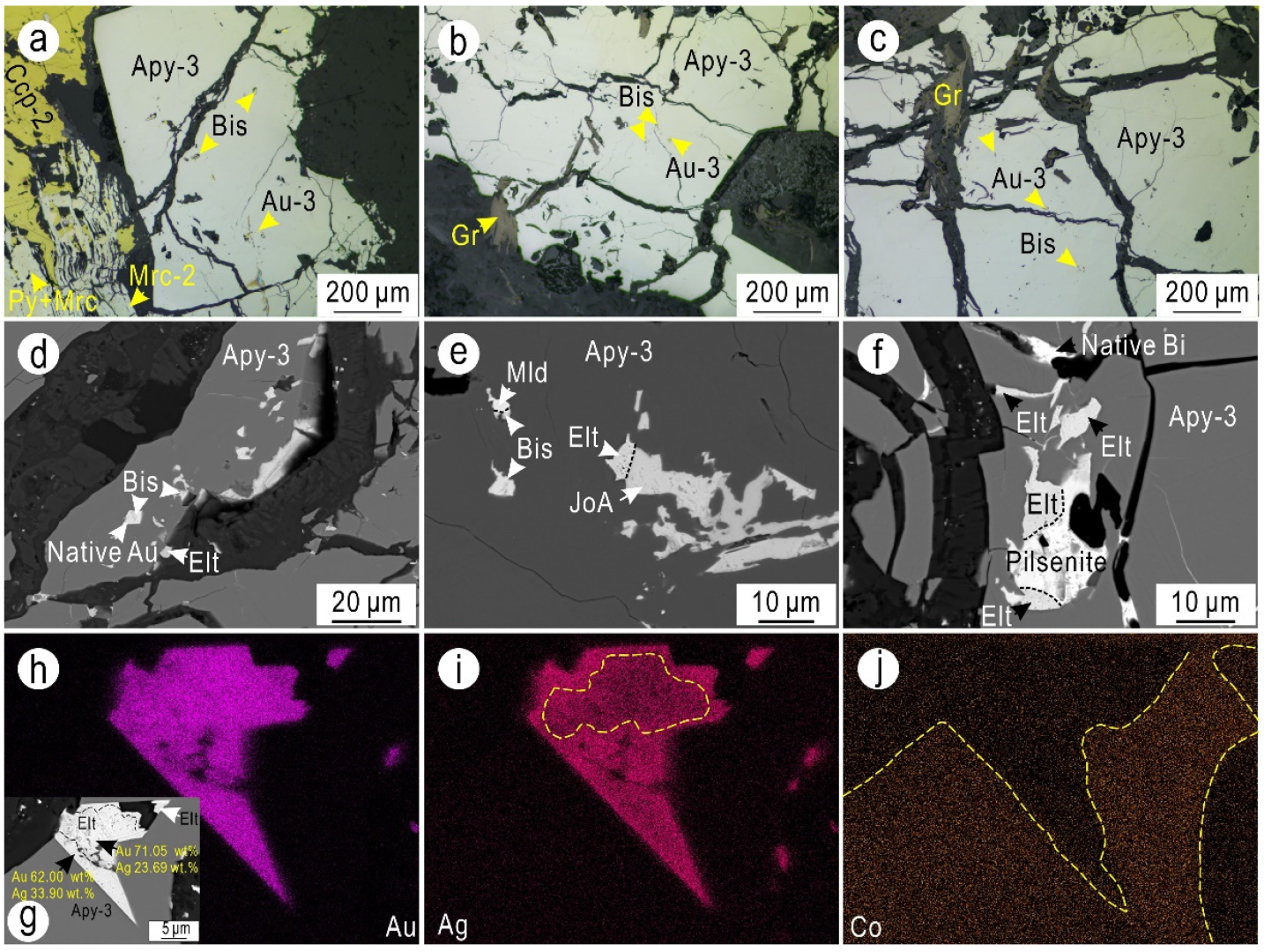
5.1. Texture of the Arsenopyrite, Bi-Minerals, and Gold
5.1.1. Stage I (Pyrrhotite–Arsenopyrite–Calcite Stage)
5.1.2. Stage III (Coarse-Grained Arsenopyrite–Calcite Stage)
5.1.3. Stage V (Coarse-Grained Arsenopyrite-Quartz Stage)
5.2. Compositions of Arsenopyrite, Gold, and Bi-Mineral Assemblages
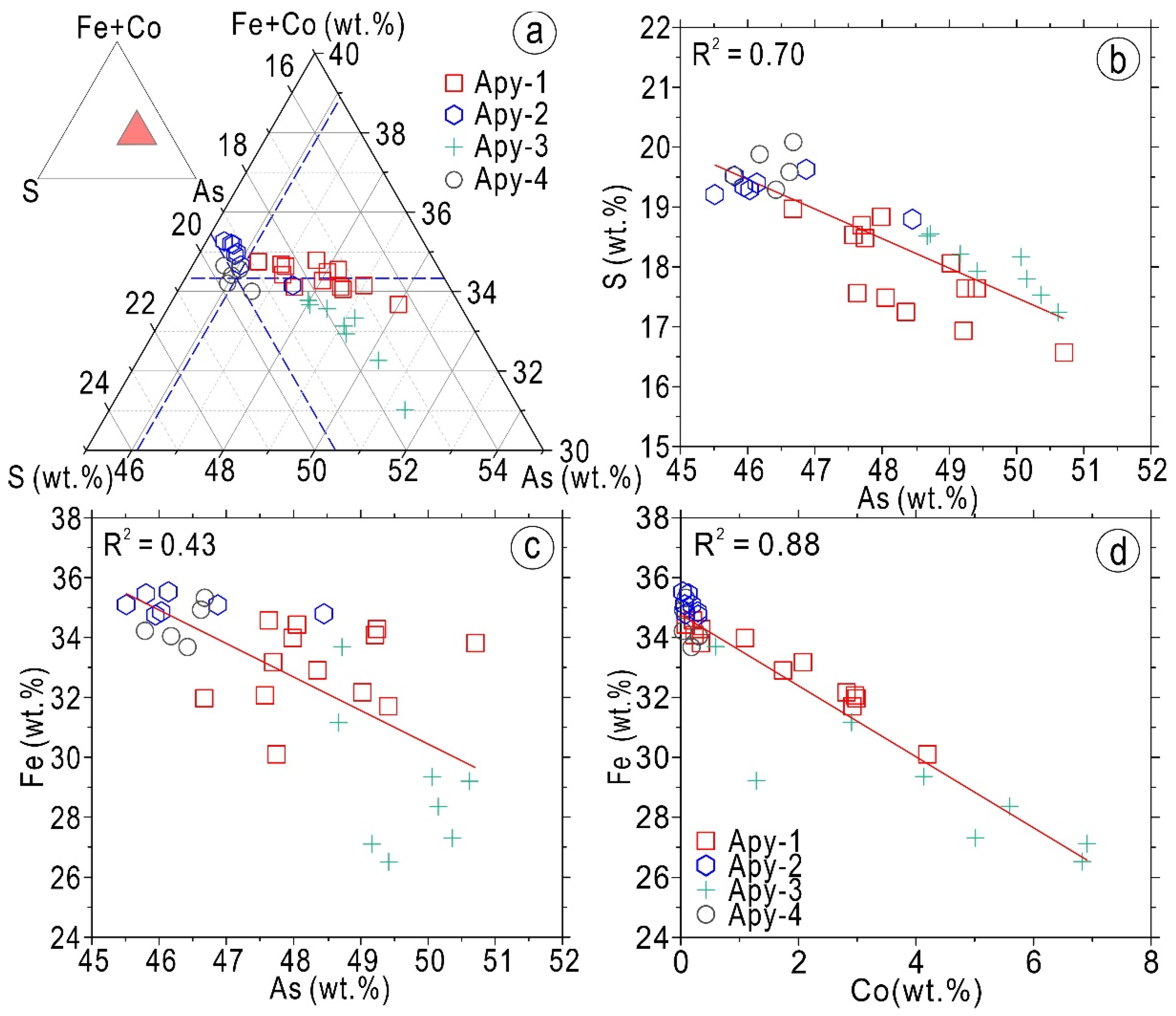
5.2.1. Compositions of Arsenopyrite
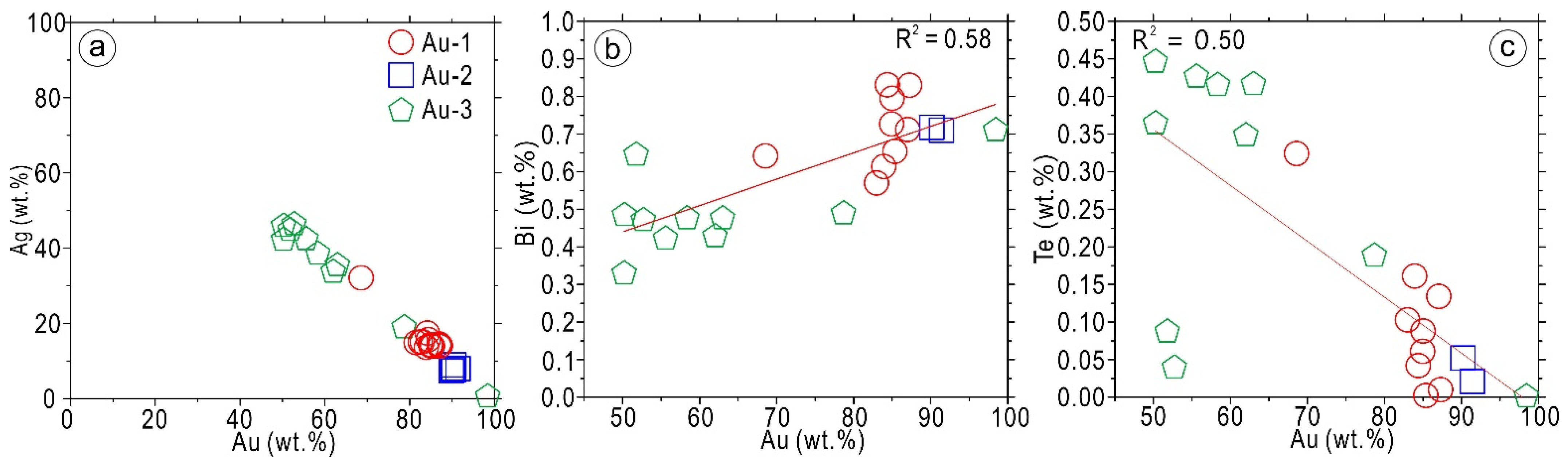
5.2.2. Compositions of Gold
5.2.3. Compositions of Bi-Minerals
6. Discussion
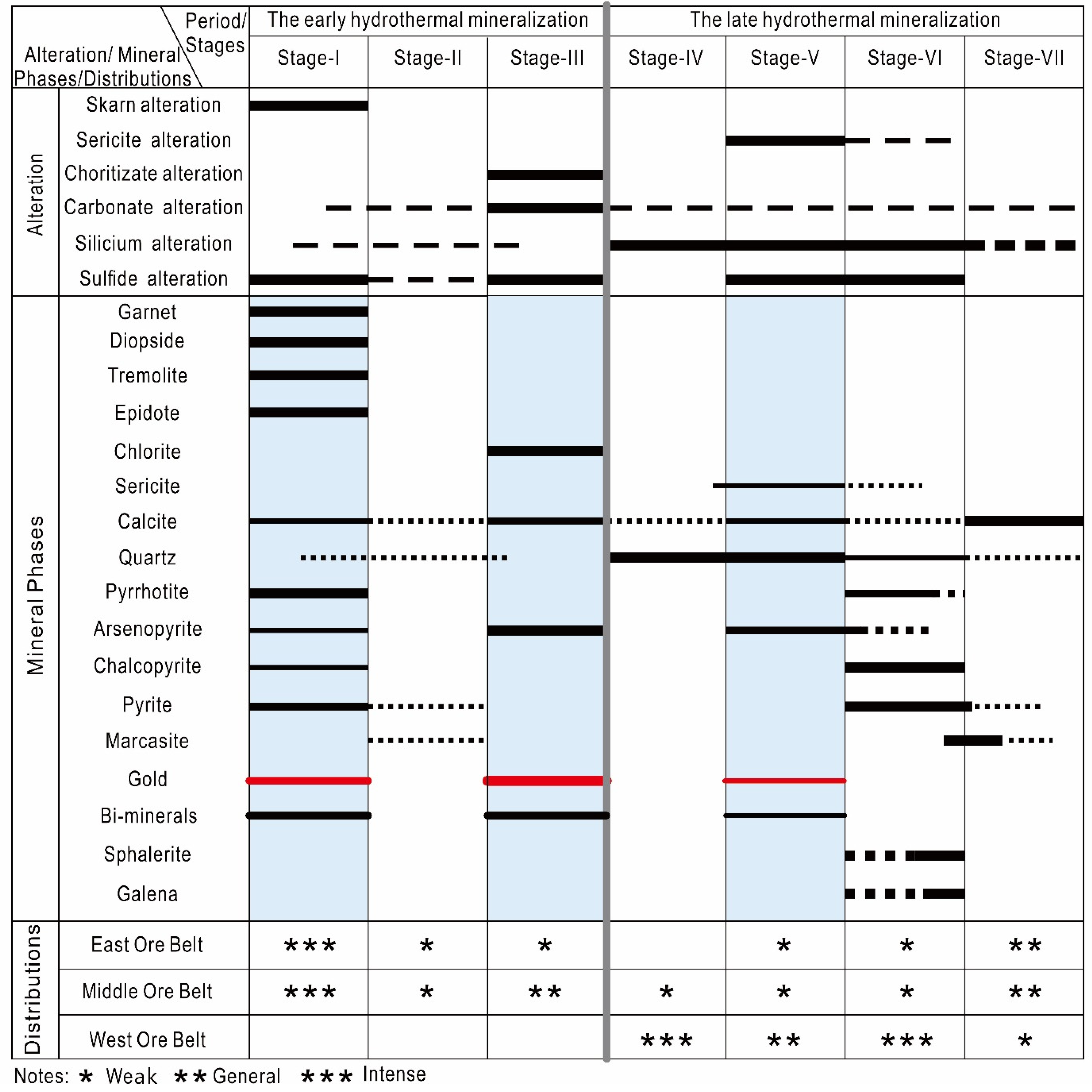
6.1. The Physical–Chemical Conditions of the Ore-Forming Fluids Indicated by Minerals Assemblages
6.2. Gold Scavenging Using Bi-Melts and Refining Processes during the Cooling of the Mineralization System
7. Conclusions
- The Laozuoshan gold deposit has experienced complex mineralization processes (the early hydrothermal mineralization and the late hydrothermal mineralization), with three gold precipitation processes occurring successively. The abundant gold (Au-1 to Au-3) and Bi-mineral (Bis-1 to Bis-3) particles are associated with pyrrhotite (Po-1) + arsenopyrite (Apy-1), chlorite + arsenopyrite (Apy-2), and graphite + arsenopyrite (Apy-3).
- Bi-minerals (such as joséite-B, ingodite, and native bismuth without Bi-sulfosalts) are associated with gold (electrum, native gold, or minor maldonite) and coexist as inclusions or as fracture fillings in arsenopyrite (Apy-1~Apy-3) and pyrrhotite. The mineral assemblages of arsenopyrite, Bi-minerals, and gold exhibit a clear As-Bi-Au mineralogy in the ores, and the ternary diagram of the chemical compositions of the Bi-minerals shows that the Bi-minerals all fall in reducing regions, indicating that Bi-minerals are precipitated under reducing conditions.
- The gold compositions show a positive correlation (R2 = 0.58) between Au and Bi. The gold experienced the concentration of the ore-forming fluids and further Bi-melt scavenging during the mineralization of the Laozuoshan gold deposit. The Bi collector model is essential for interpreting the high-grade gold in the Laozuoshan gold deposit, indicating that the bismuth geochemical anomaly may be a critical potential exploration target for the high-grade gold deposits in the Jiamusi Block.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, M.B.; Alves, F.E.A.; Neto, A.V.C.; Bertolino, L.C.; Araújo, I.M.C.D.P.; Gopon, P.; Mozart, M.S. Gold refinement by the fractionation of Bi-enriched partial melts at the Quadrilatero Ferrifero, Brazil: Implications on the formation of hypozonal deposits. Miner. Depos. 2022, 57, 781–800. [Google Scholar] [CrossRef]
- Hastie, E.C.G.; Schindler, M.; Kontak, D.J.; Lafrance, B. Transport and coarsening of gold nanoparticles in an orogenic deposit by dissolution-reprecipitation and Ostwald ripening. Commun. Earth Environ. 2021, 2, 57. [Google Scholar] [CrossRef]
- Oberthur, T.; Weiser, T.W. Gold-bismuth-telluride-sulphide assemblages at the Viceroy Mine, Harare-Bindura-Shamva greenstone belt, Zimbabwe. Miner. Mag. 2008, 72, 953–970. [Google Scholar] [CrossRef]
- Buzatu, A.; Damian, G.; Dill, H.G.; Buzgar, N.; Apopei, A.I. Mineralogy and geochemistry of sulfosalts from Baia Sprie ore deposit (Romania)—New bismuth minerals occurrence. Ore Geol. Rev. 2015, 65, 132–147. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L. Bismuth tellurides and sulphosalts from the Larga hydrothermal system, Metaliferi Mts., Romania: Paragenesis and genetic significance. Miner. Mag. 2004, 68, 301–321. [Google Scholar] [CrossRef]
- Cepedal, A.; Fuertes-Fuente, M.; Martin-Izard, A.; Gonzalez-Nistal, S.; Barrero, M. Gold-bearing As-rich pyrite and arsenopyrite from the El Valle gold deposit, Asturias, northwestern Spain. Can. Miner. 2008, 46, 233–247. [Google Scholar] [CrossRef]
- Cepedal, A.; Fuertes-Fuente, M.; Martín-Izard, A.; González-Nistal, S.; Rodríguez-Pevida, L. Tellurides, selenides and Bi-mineral assemblages from the Río Narcea Gold Belt, Asturias, Spain: Genetic implications in Cu–Au and Au skarns. Min. Pet. 2006, 87, 277–304. [Google Scholar] [CrossRef]
- Mueller, A.G. The Nevoria gold skarn deposit in Archean iron-formation, Southern Cross greenstone belt, Western Australia; I, Tectonic setting, petrography, and classification. Econ. Geol. 1997, 92, 181–209. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, X.; Wu, Z.; Yang, T.; Li, D.; Ren, Y.; Liu, Q.; Zhu, K.; Yu, H. Mineralogy of Bi-sulfosalts and tellurides from the Yaoan gold deposit, southwest China: Metallogenic implications. Ore Geol. Rev. 2018, 98, 126–140. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, X.; Cook, N.J.; Lin, H.; Fu, Y.; Zhong, R.; Brugger, J. Nano- to Micron-Scale Particulate Gold Hosted by Magnetite: A Product of Gold Scavenging by Bismuth Melts. Econ. Geol. 2017, 112, 993–1010. [Google Scholar] [CrossRef]
- Acosta-Góngora, P.; Gleeson, S.A.; Samson, I.M.; Ootes, L.; Corriveau, L. Gold Refining by Bismuth Melts in the Iron Oxide-Dominated NICO Au-Co-Bi (±Cu±W) Deposit, NWT, Canada. Econ. Geol. 2015, 110, 291–314. [Google Scholar] [CrossRef]
- Tormanen, T.O.; Koski, R.A. Gold enrichment and the Bi-Au association in pyrrhotite-rich massive sulfide deposits, Escanaba trough, southern Gorda Ridge. Econ. Geol. 2005, 100, 1135–1150. [Google Scholar] [CrossRef]
- Cave, B.J.; Barnes, S.-J.; Pitcairn, I.; Sack, P.J.; Kuikka, H.; Johnson, S.; Duran, C.J. Multi-stage precipitation and redistribution of gold, and its collection by lead-bismuth and lead immiscible liquids in a reduced-intrusion related gold system (RIRGS); Dublin Gulch, western Canada. Ore Geol. Rev. 2019, 106, 28–55. [Google Scholar] [CrossRef]
- Cepedal, A.; Martínez-Abad, I.; Fuertes-Fuente, M.; Lima, A. The presence of plumboan ingodite and a rare Bi-Pb tellurosulfide, Pb3Bi4Te4S5, in the Limarinho gold deposit, Northern Portugal. Can. Miner. 2013, 51, 643–651. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Birch, W.D.; Cook, N.J.; Pring, A.; Grundler, P.V. Petrogenetic significance of Au–Bi–Te–S associations: The example of Maldon, Central Victorian gold province, Australia. Lithos 2010, 116, 1–17. [Google Scholar] [CrossRef]
- McFarlane, C.R.M.; Mavrogenes, J.; Lentz, D.; King, K.; Allibone, A.; Holcombe, R. Geology and Intrusion-Related Affinity of the Morila Gold Mine, Southeast Mali. Econ. Geol. 2011, 106, 727–750. [Google Scholar] [CrossRef]
- Douglas, N.; Mavrogenes, J.; Hack, A.; England, R. The liquid bismuth collector model: An alternative gold deposition mechanism. In Proceedings of the 15th Understanding Planet Earth, Searching for a Sustainable Future, on the Starting Blocks of the Third Millennium: Australian Geological Convention, Sydney, Australia, 3–7 July 2000; p. 135. [Google Scholar]
- Tooth, B.; Brugger, J.; Ciobanu, C.; Liu, W. Modeling of gold scavenging by bismuth melts coexisting with hydrothermal fluids. Geology 2008, 36, 815–818. [Google Scholar] [CrossRef]
- Tooth, B.; Ciobanu, C.L.; Green, L.; O’Neill, B.; Brugger, J. Bi-melt formation and gold scavenging from hydrothermal fluids: An experimental study. Geochim. Cosmochim. Acta 2011, 75, 5423–5443. [Google Scholar] [CrossRef]
- Shi, K.; Ulrich, T.; Wang, K.Y.; Ma, X.L.; Li, S.D.; Wang, R. Hydrothermal evolution and ore genesis of the Laozuoshan Au skarn deposit, northeast China: Constrains from mineralogy, fluid inclusion, and O-C-S-Pb isotope geochemistry. Ore Geol. Rev. 2020, 127, 103879. [Google Scholar] [CrossRef]
- Bai, C.L.; Sun, J.G.; Zhao, C.T.; Qin, K.Z.; Liu, Y.; Chu, X.L.; Xu, Z.K.; Li, Y.X. The multiple mineralizations and geodynamic settings of the Laozuoshan Cu-Au deposit in the Jiamusi Massif, NE China: Zircon U-Pb geochronological, elemental and Hf isotopic geochemical evidence. Ore Geol. Rev. 2021, 137, 104291. [Google Scholar] [CrossRef]
- Shi, K.; Wang, K.; Ulrich, T.; Ma, X.; Sun, Q.; Wang, R. Two mineralization events in the Laozuoshan Au deposit, north-east China: Evidence from Re–Os geochronology and trace element geochemistry. Geol. J. 2020, 56, 1974–1986. [Google Scholar] [CrossRef]
- Meng, L.; Huang, F.; Gao, W.; Wang, D.; Zhu, J.; Xing, C.; Tan, W.; Tang, X. Multi-stage hydrothermal processes at the Laozuoshan gold deposit in NE China: Insights from textures and compositions of sulfide assemblages. Ore Geol. Rev. 2020, 117, 103275. [Google Scholar] [CrossRef]
- Li, M.F.; Ye, S.Q.; Yang, Y.C.; Zhang, G.B.; Hou, X.G.; Ming, T.X.; Tang, Z. Geological and geochemical characteristics of Laozuoshan gold deposit in Heilongjiang and its metallotectonic setting. Glob. Geol. 2014, 33, 543–555, (In Chinese with English Abstract). [Google Scholar]
- He, B.L. Discussion on the origin of gold ore deposit at the eastern section of Laozuoshan gold ore field. Gold 2002, 23, 7–12, (In Chinese with English abstract). [Google Scholar]
- Wei, C.D.; Li, X.M. The typomorphic characteristic of the main gold-bearing minerals in Laozuoshan gold deposit, Heilongjiang Province. Gold 2001, 22, 5–9, (In Chinese with English Abstract). [Google Scholar]
- HBGMR. Regional Geology of Heilongjiang Province; Geological Publishing House: Beijing, China, 1993. (In Chinese) [Google Scholar]
- Wu, F.Y.; Yang, J.H.; Lo, C.H.; Wilde, S.A.; Sun, D.Y.; Jahn, B.M. The Heilongjiang Group: A Jurassic accretionary complex in the Jiamusi Massif at the western Pacific margin of northeastern China. Isl. Arc 2007, 16, 156–172. [Google Scholar] [CrossRef]
- Li, W.M.; Liu, Y.J.; Zhao, Y.L.; Feng, Z.Q.; Zhou, J.P.; Wen, Q.B.; Liang, C.Y.; Zhang, D. Tectonic evolution of the Jiamusi Block, NE China. Acta Petrol. Sin. 2020, 36, 665–684, (In Chinese with English Abstract). [Google Scholar]
- Guo, X.Z.; Takasu, A.; Liu, Y.J.; Li, W.M. Zn-Rich Spinel in Association with Quartz in the Al-Rich Metapelites from the Mashan Khondalite Series, NE China. J. Earth Sci. 2014, 25, 207–223. [Google Scholar] [CrossRef]
- Luan, J.P.; Rosenbaum, G.; Fu, J.F. Provenance and depositional history of the Mesozoic Sanjiang Basin (Northeastern China): Implications for the uplift history of the northeastern Asian continental margin. Geol. Mag. 2022, 159, 389–406. [Google Scholar] [CrossRef]
- Luan, J.P.; Wang, F.; Xu, W.L.; Ge, W.C.; Sorokin, A.A.; Wang, Z.W.; Guo, P. Provenance, age, and tectonic implications of Neoproterozoic strata in the Jiamusi Massif: Evidence from U-Pb ages and Hf isotope compositions of detrital and magmatic zircons. Precambr. Res 2017, 297, 19–32. [Google Scholar] [CrossRef]
- Zhou, J.B.; Pu, X.G.; Hou, H.S.; Han, W.; Gao, J.L.; Li, G.Y. The Mesozoic accretionary complex in NE China and its tectonic implications for the subduction of the Paleo-Pacific plate beneath the Eurasia. Acta Petrol. Sin. 2018, 34, 2845–2856, (In Chinese with English Abstract). [Google Scholar]
- Bi, J.H.; Ge, W.C.; Yang, H.; Zhao, G.C.; Yu, J.J.; Zhang, Y.L.; Wang, Z.H.; Tian, D.X. Petrogenesis and tectonic implications of early Paleozoic granitic magmatism in the Jiamusi Massif, NE China: Geochronological, geochemical and Hf isotopic evidence. J. Asian Earth Sci. 2014, 96, 308–331. [Google Scholar] [CrossRef]
- Wu, F.Y.; Sun, D.Y.; Ge, W.C.; Zhang, Y.B.; Grant, M.L.; Wilde, S.A.; Jahn, B.M. Geochronology of the Phanerozoic granitoids in northeastern China. J. Asian Earth Sci. 2011, 41, 1–30. [Google Scholar] [CrossRef]
- Yang, H.; Ge, W.C.; Bi, J.H.; Wang, Z.H.; Tian, D.X.; Dong, Y.; Chen, H.J. The Neoproterozoic-Early Paleozoic evolution of the Jiamusi Block, NE China and its East Gondwana connection: Geochemical and zircon U-Pb-Hf isotopic constrains from the Mashan Complex. Gondwana Res. 2018, 54, 102–121. [Google Scholar] [CrossRef]
- Sun, J.G.; Han, S.J.; Zhang, Y.; Xing, S.W.; Bai, L.A. Diagenesis and metallogenetic mechanisms of the Tuanjiegou gold deposit from the Lesser Xing’an Range, NE China: Zircon U-Pb geochronology and Lu-Hf isotopic constraints. J. Asian Earth Sci. 2013, 62, 373–388. [Google Scholar] [CrossRef]
- Hao, Y.J.; Ren, Y.S.; Zhao, H.L.; Lai, K.; Zhao, X.; Ma, Y.P. Metallogenic Mechanism and Tectonic Setting of Tungsten Mineralization in the Yangbishan Deposit in Northeastern China. Acta Geol. Sin. 2018, 92, 241–267. [Google Scholar] [CrossRef]
- Wilde, S.A.; Zhang, X.; Wu, F. Extension of a newly identified 500Ma metamorphic terrane in North East China: Further U–Pb SHRIMP dating of the Mashan Complex, Heilongjiang Province, China. Tectonophysics 2000, 328, 115–130. [Google Scholar] [CrossRef]
- Wu, M.; Li, L.; Sun, J.G.; Yang, R. Geology, geochemistry, and geochronology of the Laozuoshan gold deposit, Heilongjiang Province, Northeast China: Implications for multiple gold mineralization events and geodynamic setting. Can. J. Earth Sci. 2018, 55, 604–619. [Google Scholar] [CrossRef]
- Meng, L.; Huang, F.; Liu, J.; Xing, M.M.; Wan, Q.; Ling, J.Q.; Gao, S.; Gao, W.Y. The Genesis of Nano-Micron-Sized Spheroidal Aggregates of FeS2 in the Laozuoshan Gold Deposit, Heilongjiang Province, NE China. J. Nanosci. Nanotechnol. 2017, 17, 6539–6548. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Meria, D.; Silcock, D.; Wade, B. Arsenopyrite-Pyrite Association in an Orogenic Gold Ore: Tracing Mineralization History from Textures and Trace Elements. Econ. Geol. 2013, 108, 1273–1283. [Google Scholar] [CrossRef]
- Ciobanu, C.; Cook, N.J. Tellurides, selenides (and Bi-sulphosalts) in gold deposits. In Proceedings of the 11th Quadrennial IAGOD Symposium, Windhoek, Namibia, 22–26 July 2002. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J.; Pring, A. Bismuth tellurides as gold scavengers. In Mineral Deposit Research: Meeting the Global Challenge; Springer: Berlin, Germany, 2005; pp. 1383–1386. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Wagner, T.; Stanley, C.J. Minerals of the system Bi-Te-Se-S related to the tetradymite archetype: Review of classification and compositional variation. Can. Miner. 2007, 45, 665–708. [Google Scholar] [CrossRef]
- Wei, B.; Wang, C.Y.; Wang, Z.C.; Cheng, H.; Xia, X.P.; Tan, W. Mantle-derived gold scavenged by bismuth-(tellurium)-rich melts: Evidence from the mesozoic wulong gold deposit in the North China Craton. Ore Geol. Rev. 2021, 131, 104047. [Google Scholar] [CrossRef]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S. World Skarn Deposits. In Economic Geology: One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 299–336. [Google Scholar]
- Meinert, L.D. Gold in Skarns Related to Epizonal Intrusions. In Gold in 2000; Hagemann, S.G., Brown, P.E., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2000; Volume 13, pp. 347–375. [Google Scholar]
- Zhao, H.; Wang, Q.; Groves, D.I.; Deng, J. A rare Phanerozoic amphibolite-hosted gold deposit at Danba, Yangtze Craton, China: Significance to fluid and metal sources for orogenic gold systems. Miner. Depos. 2019, 54, 133–152. [Google Scholar] [CrossRef]
- Perfetti, E.; Pokrovski, G.S.; Ballerat-Busserolles, K.; Majer, V.; Gibert, F. Densities and heat capacities of aqueous arsenious and arsenic acid solutions to 350 °C and 300 bar, and revised thermodynamic properties of As(OH)3°(aq), AsO(OH)3°(aq) and iron sulfarsenide minerals. Geochim. Cosmochim. Acta 2008, 72, 713–731. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kara, S.; Roux, J. Stability and solubility of arsenopyrite, FeAsS, in crustal fluids. Geochim. Cosmochim. Acta 2002, 66, 2361–2378. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Zakirov, I.V.; Roux, J.; Testemale, D.; Hazemann, J.-L.; Bychkov, A.Y.; Golikova, G.V. Experimental study of arsenic speciation in vapor phase to 500 °C: Implications for As transport and fractionation in low-density crustal fluids and volcanic gases. Geochim. Cosmochim. Acta 2002, 66, 3453–3480. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Eadington, P.J. Thermodynamic predictions of the hydrothermal chemistry of arsenic, and their significance for the paragenetic sequence of some cassiterite-arsenopyrite-base metal sulfide deposits. Econ. Geol. 1986, 81, 511–529. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y. Genesis of the Mandongshan gold deposit (Xinjiang, NW China): T-P-ƒS2 and phase equilibria constraints from the Au-As-Fe-S system. Ore Geol. Rev. 2017, 83, 135–151. [Google Scholar] [CrossRef]
- Skirrow, R.G.; Walshe, J.L. Reduced and oxidized Au-Cu-Bi iron oxide deposits of the Tennant Creek inlier, Australia: An integrated geologic and chemical model. Econ. Geol. Bull. Soc. 2002, 97, 1167–1202. [Google Scholar] [CrossRef]
- Voudouris, P.C.; Spry, P.G.; Mavrogonatos, C.; Sakellaris, G.-A.; Bristol, S.K.; Melfos, V.; Fornadel, A.P. Bismuthinite derivatives, lillianite homologues, and bismuth sulfotellurides as indicators of gold mineralization in the Stanos shear-zone related deposit, Chalkidiki, northern Greece. Can. Miner. 2013, 51, 119–142. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in Solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, L.Q.; Li, P.; Xiong, Y.Q. Morphology and chemistry composition of pyrite in the Laowangzhai gold deposit, Ailaoshan orogenic belt, SW China. Acta Petrol. Sin. 2013, 29, 3937–3948, (In Chinese with English Abstract). [Google Scholar]
- Ciobanu, C.L.; Cook, N.J.; Damian, F.; Damian, G. Gold scavenged by bismuth melts: An example from Alpine shear-remobilizates in the Highiş Massif, Romania. Miner. Petrol. 2006, 87, 351–384. [Google Scholar] [CrossRef]
- An, R.; Wang, K.Y.; Ma, X.L.; Liang, Y.M.; Wang, Y.C.; Wang, Z.G. Evolution mode for the ore-forming fluid system of the Laozuoshan skarn-magmatic hydrothermal vein gold deposit, Heilongjiang Province, China. Northwest. Geol. 2017, 50, 122–135, (In Chinese with English Abstract). [Google Scholar]
- Cockerton, A.B.D.; Tomkins, A.G. Insights into the Liquid Bismuth Collector Model through Analysis of the Bi-Au Stormont Skarn Prospect, Northwest Tasmania. Econ. Geol. 2012, 107, 667–682. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Mao, J.W. Textural control on gold distribution in As-free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China Craton (Hebei Province, China). Chem. Geol. 2009, 264, 101–121. [Google Scholar] [CrossRef]
- Fougerouse, D.; Micklethwaite, S.; Tomkins, A.G.; Mei, Y.; Kilburn, M.; Guagliardo, P.; Fisher, L.A.; Halfpenny, A.; Gee, M.; Paterson, D.; et al. Gold remobilisation and formation of high grade ore shoots driven by dissolution-reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochim. Cosmochim. Acta 2016, 178, 143–159. [Google Scholar] [CrossRef]
- Morey, A.A.; Tomkins, A.G.; Bierlein, F.P.; Weinberg, R.F.; Davidson, G.J. Bimodal Distribution of Gold in Pyrite and Arsenopyrite: Examples from the Archean Boorara and Bardoc Shear Systems, Yilgarn Craton, Western Australia. Econ. Geol. 2008, 103, 599–614. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Huang, F.; Gao, W.; Gao, R.; Zhao, F.; Zhou, Y.; Li, Y. Multi-Step Gold Refinement and Collection Using Bi-Minerals in the Laozuoshan Gold Deposit, NE China. Minerals 2022, 12, 1137. https://doi.org/10.3390/min12091137
Meng L, Huang F, Gao W, Gao R, Zhao F, Zhou Y, Li Y. Multi-Step Gold Refinement and Collection Using Bi-Minerals in the Laozuoshan Gold Deposit, NE China. Minerals. 2022; 12(9):1137. https://doi.org/10.3390/min12091137
Chicago/Turabian StyleMeng, Lin, Fei Huang, Wenyuan Gao, Rongzhen Gao, Fude Zhao, Yiran Zhou, and Yongli Li. 2022. "Multi-Step Gold Refinement and Collection Using Bi-Minerals in the Laozuoshan Gold Deposit, NE China" Minerals 12, no. 9: 1137. https://doi.org/10.3390/min12091137
APA StyleMeng, L., Huang, F., Gao, W., Gao, R., Zhao, F., Zhou, Y., & Li, Y. (2022). Multi-Step Gold Refinement and Collection Using Bi-Minerals in the Laozuoshan Gold Deposit, NE China. Minerals, 12(9), 1137. https://doi.org/10.3390/min12091137





