S and Sr Isotope Compositions and Trace Element Compositions of the Middle Jurassic Evaporites in Eastern Tibet: Provenance and Palaeogeographic Implications
Abstract
:1. Introduction
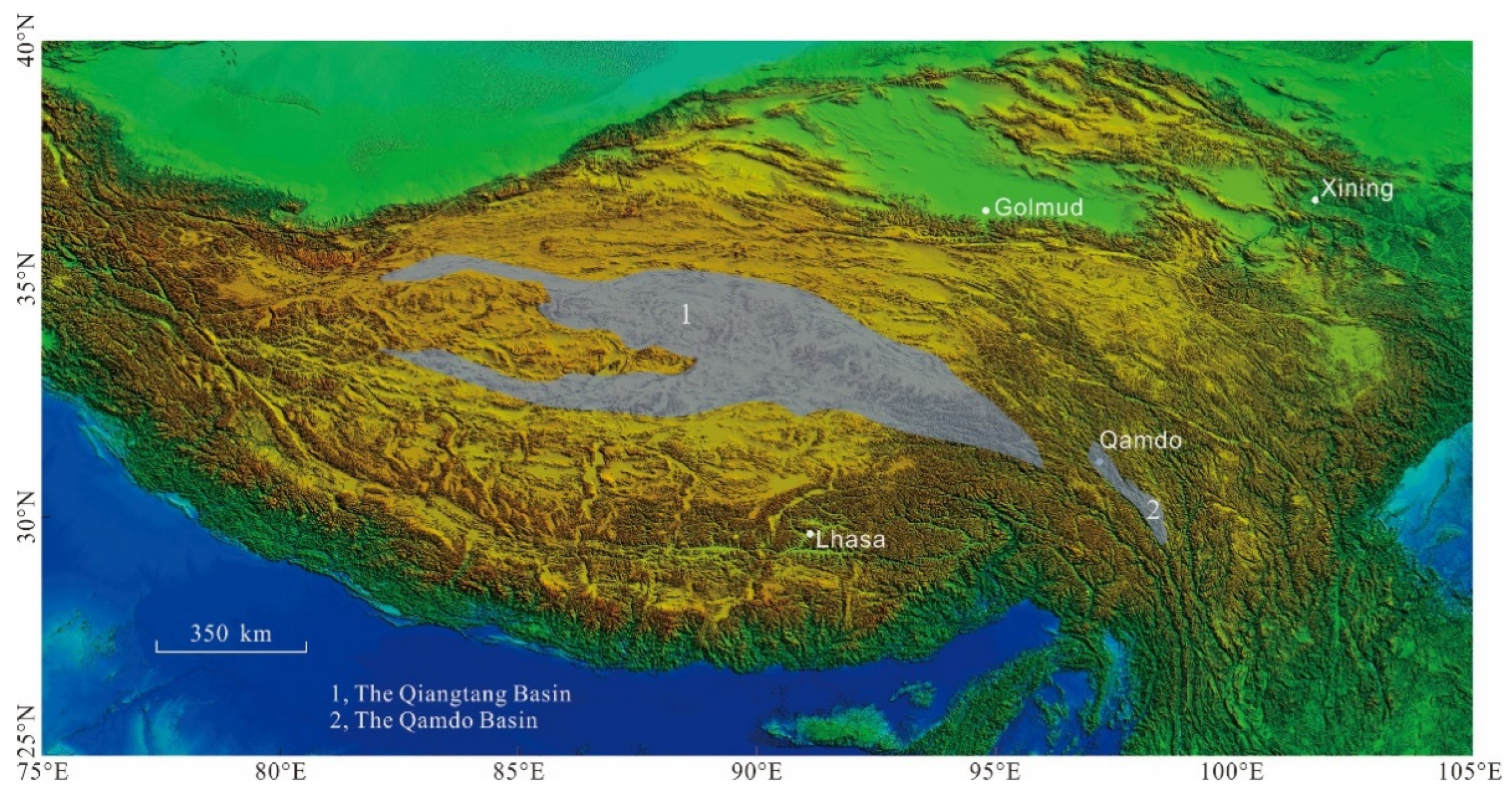
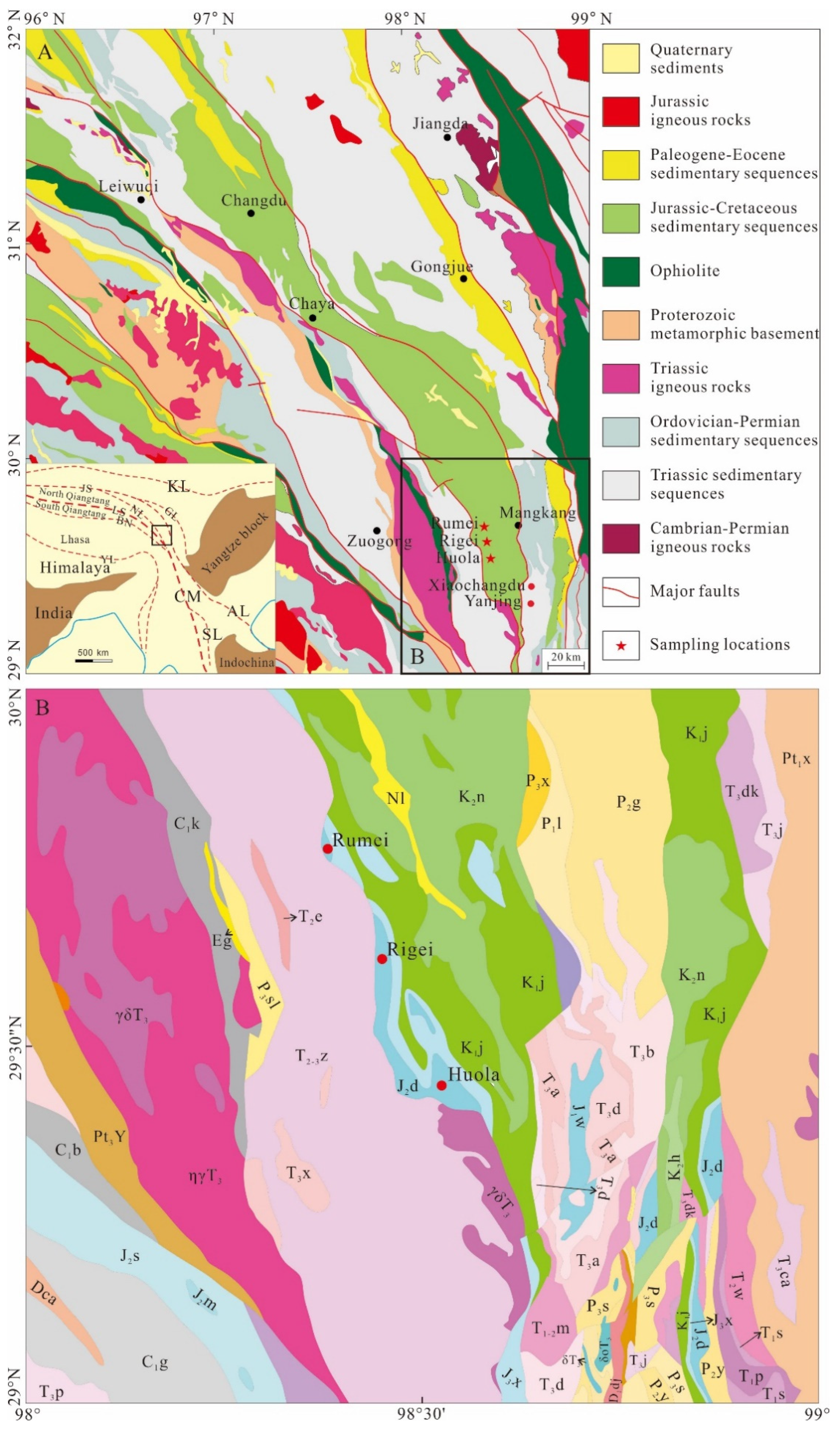
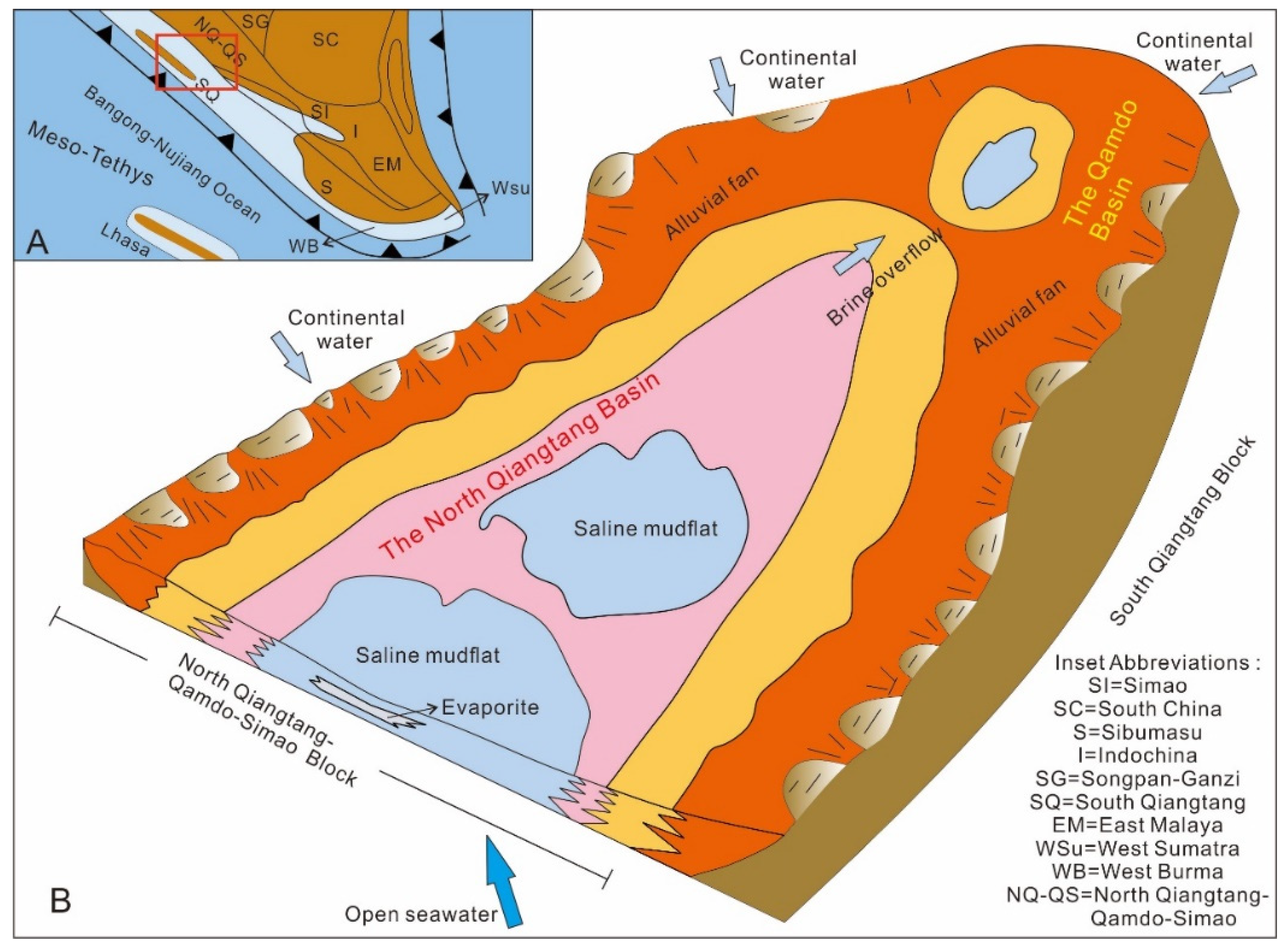
2. Geologic Setting
3. Materials and Methods
4. Results
5. Discussion
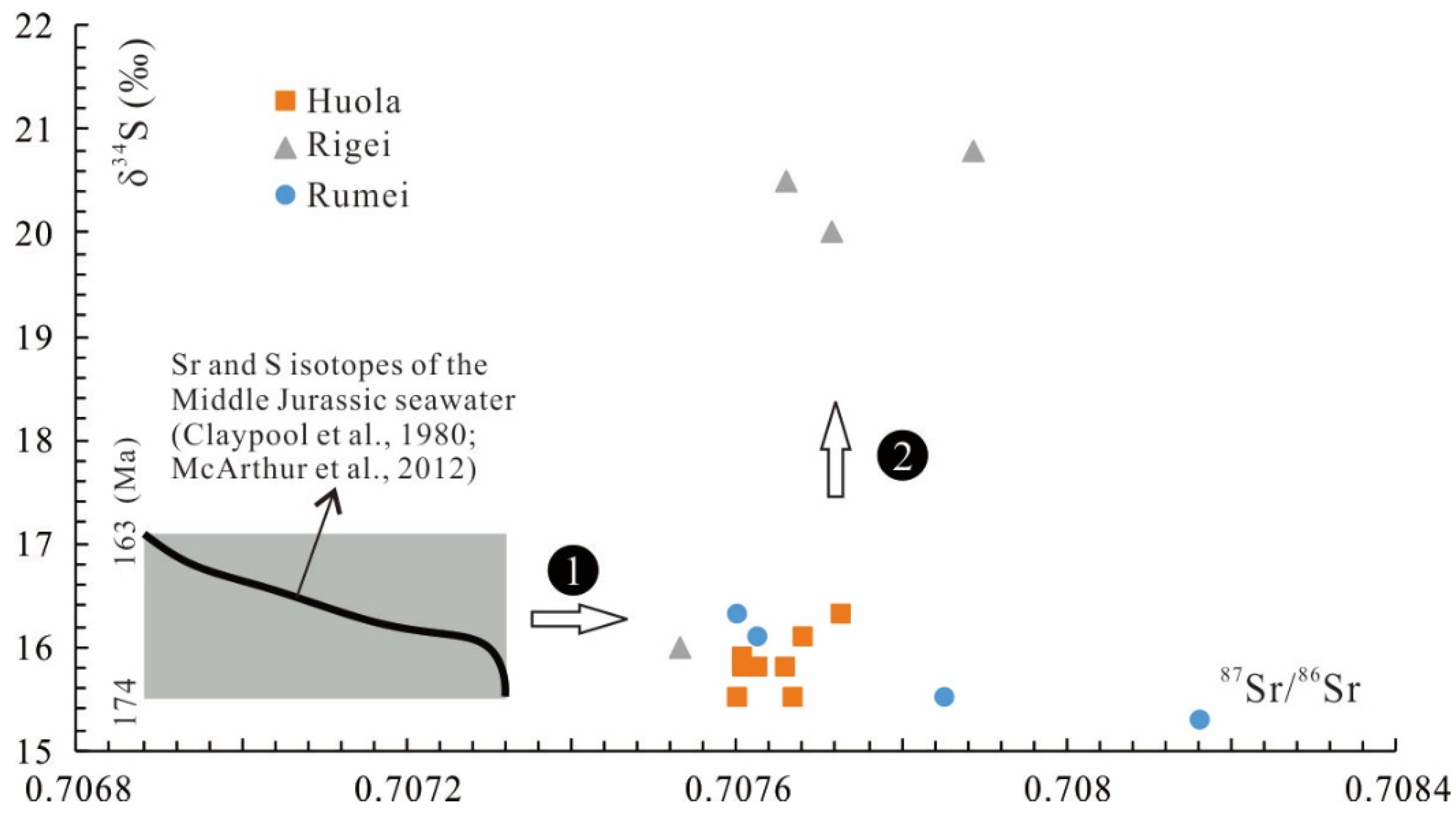
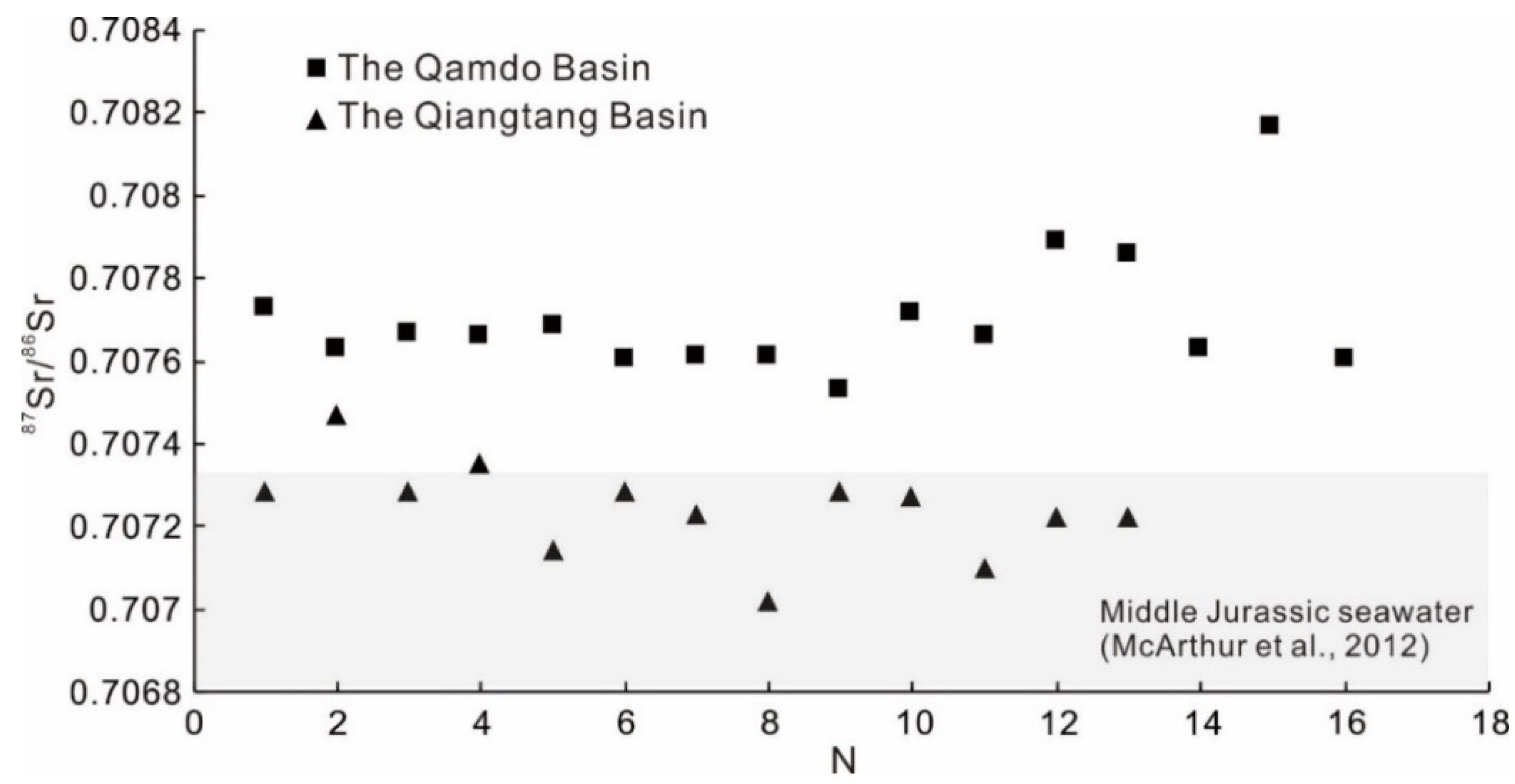
5.1. Sr Isotopes
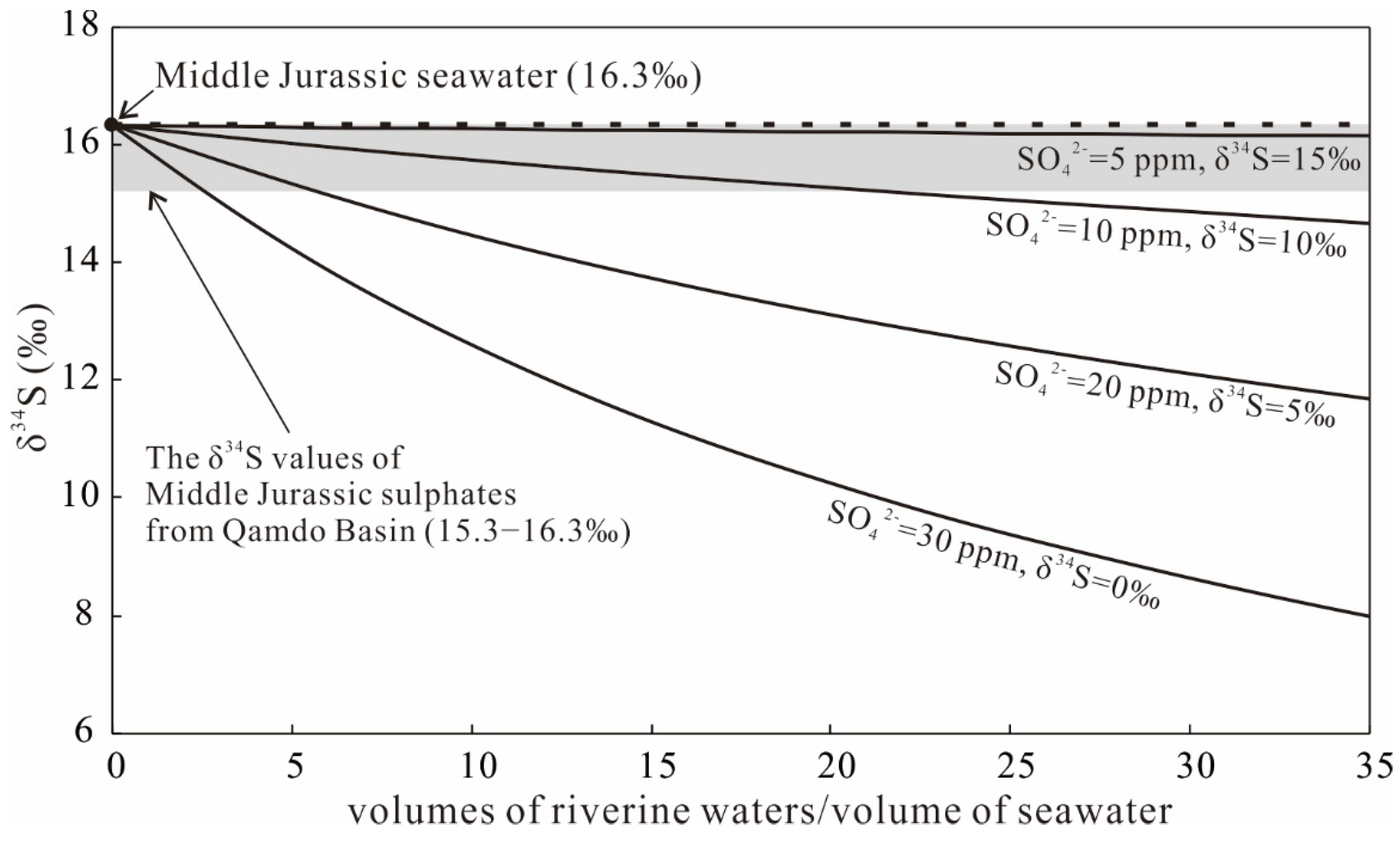
5.2. S Isotopes
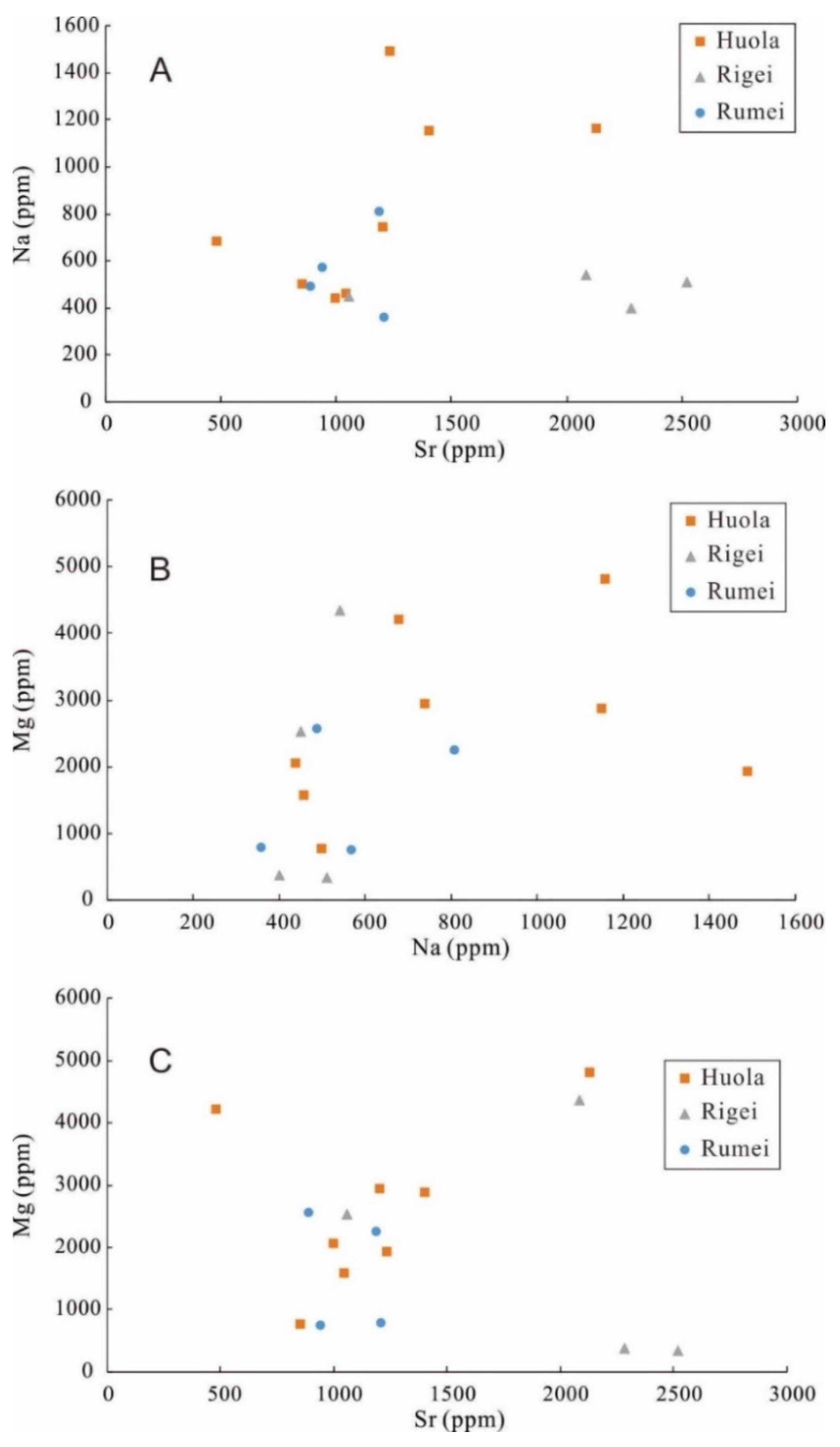
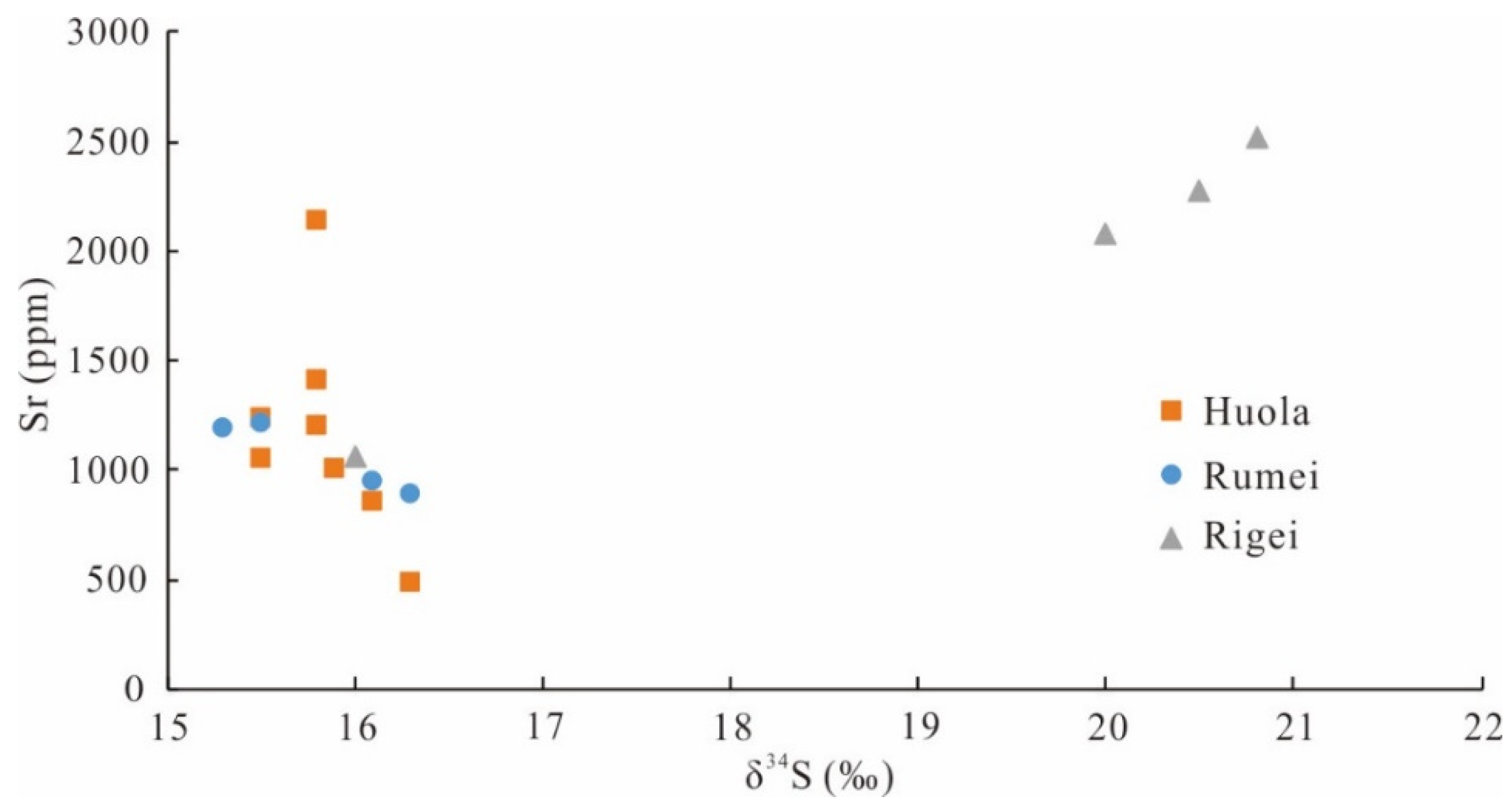
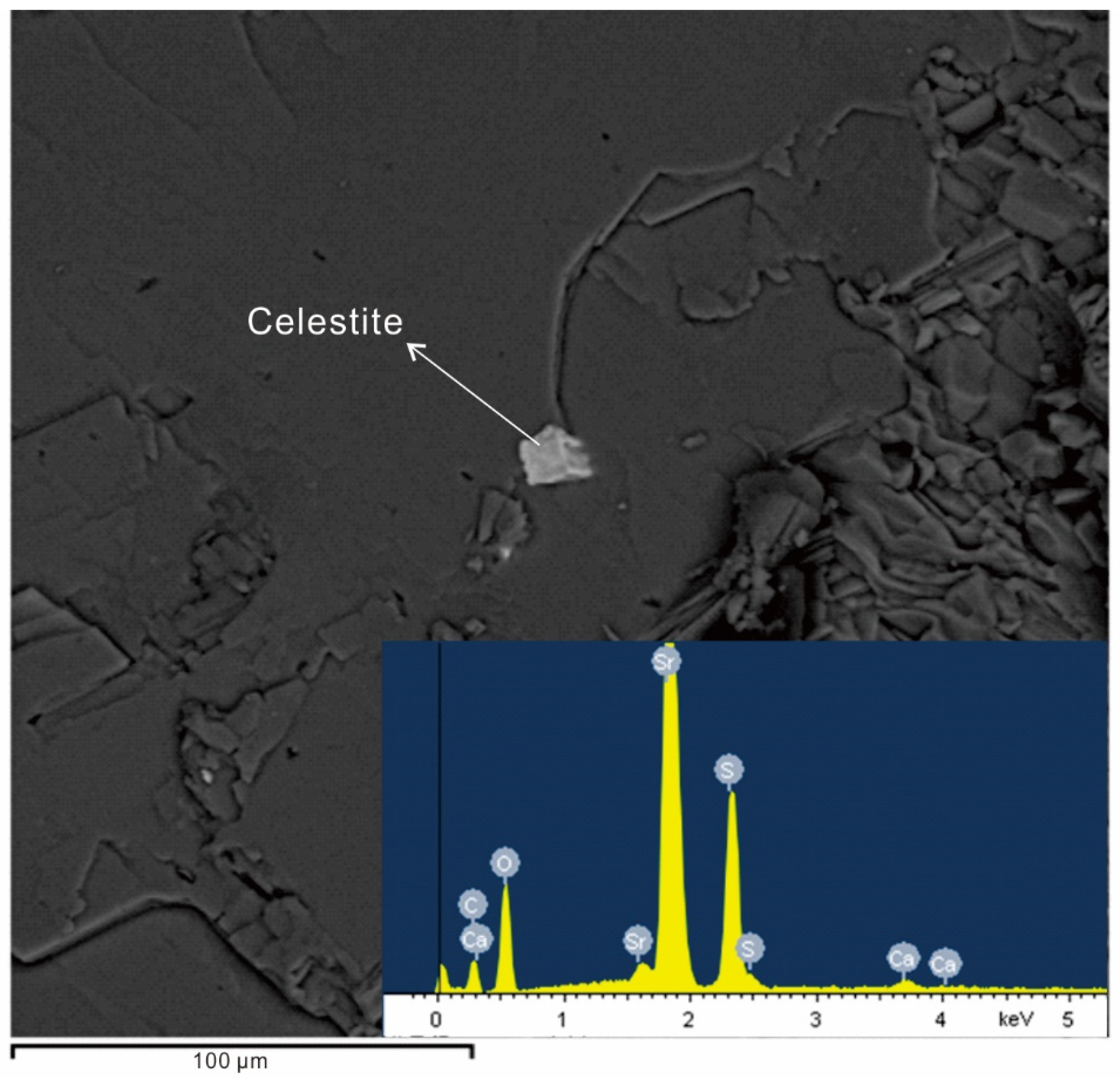
5.3. Trace Elements
5.4. Provenance and Palaeogeographic Implications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Jia, B.; Yang, P.; Chen, Y.; Peng, B.; Li, Z. The application of carbon. Oxygen and strontium isotopes to the study of Middle-Upper Jurassic sequence stratigraphy in Longweicuo area, Qiangtang Basin. Acta Geosci. Sin. 2007, 28, 253–260, (In Chinese with English abstract). [Google Scholar]
- Xue, W.; Hu, X.; Ma, A.; Garzanti, E.; Li, J. Eustatic and tectonic control on the evolution of the Jurassic North Qiangtang Basin, northern Tibet, China: Impact on the petroleum system. Mar. Pet. Geol. 2020, 120, 104558. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, Z.; Xie, Y.; Ye, H.; Tong, Z.; Shen, Q. The gypsolith and its bearings on the oil and gas exploration in the Qiangtang Basin in northern Xizang and southern Qinghai. Sediment. Geol. Tethyan Geol. 2003, 23, 1–8, (In Chinese with English abstract). [Google Scholar]
- Jia, J. Tectonopaleogeographic characteristics and evolution of the Mesozoic in eastern Qiangtang Basin, Tibet. J. Palaeogeogr. 2008, 10, 613–625, (In Chinese with English abstract). [Google Scholar]
- Li, Y. Middle and late Jurassic cyclostratigraphy in Qiangtang Basin, northern Qianghai-Tibet Plateau, China. J. Chengdu Univ. Technol. 2004, 31, 623–628, (In Chinese with English abstract). [Google Scholar]
- Xizang Institute of Geological Survey. Regional Geological Survey Reports (Basu, Gongjue, Ranwu, and Mangkang), Scale: 1: 250000; Xizang Institute of Geological Survey: Lhasa, China, 2007. (In Chinese) [Google Scholar]
- Zeng, S. Study on the Sedimentary Sequences and Basin Transformation of the End-Triassic to Early-Middle Jurassic Period in the Northern Qiangtang Basin. Ph.D. Thesis, Chengdu University of Technology, Chengdu, China, 2021. (In Chinese with English abstract). [Google Scholar]
- Palmer, M.R.; Helvací, C.; Fallick, A.E. Sulphur, sulphate oxygen and strontium isotope composition of Cenozoic Turkish evaporites. Chem. Geol. 2004, 209, 341–356. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Veizer, J. Strontium isotopes in seawater through time. Annu. Rev. Earth Planet. Sci. Lett. 1989, 17, 141–167. [Google Scholar] [CrossRef]
- McArthur, J.M.; Howarth, R.J.; Bailey, T.R. Strontium isotope stratigraphy: LOWESS version 3: Best fit to the marine Sr-isotope curve for 0–509 Ma and accompanying look-up table for deriving numerical age. J. Geol. 2001, 109, 155–170. [Google Scholar] [CrossRef]
- Kampschulte, A.; Strauss, H. The sulfur isotopic evolution of Phanerozoic seawater based on the analysis of structurally substituted sulfate in carbonates. Chem. Geol. 2004, 204, 255–286. [Google Scholar] [CrossRef]
- Alonso-Azcárate, J.; Bottrell, S.H.; Mas, J.R. Synsedimentary versus metamorphic control of S, O and Sr isotopic compositions in gypsum evaporites from the Cameros Basin, Spain. Chem. Geol. 2006, 234, 46–57. [Google Scholar] [CrossRef]
- Lu, F.H.; Meyers, W.J.; Hanson, G.N. Trace elements and environmental significance of Messinian gypsum deposits, the Nijar Basin, southeastern Spain. Chem. Geol. 2002, 192, 149–161. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Li, W.; Wang, B.; Dong, Y.; Yang, F.; Yang, H.; Wu, J. Molybdenite Re-Os dating of the Larong porphyry W-Mo deposit in eastern Tibet and its geological significance. Acta Geosci. Sin. 2019, 93, 1708–1719, (In Chinese with English abstract). [Google Scholar]
- Metcalfe, I. Gondwana dispersion and Asian accretion: Tectonic and palaeogeographic evolution of eastern Tethys. J. Asian Earth Sci. 2013, 66, 1–33. [Google Scholar] [CrossRef]
- Du, D.; Luo, J.; Li, Z. Sedimentary evolution and palaeogeography of the Qamdo Block in Xizang. Lithofacies Paleogeogr. 1997, 17, 1–17, (In Chinese with English abstract). [Google Scholar]
- He, J. Jurassic Litho-Stratigraphic Framework and Sedimentary Basin Evolution in Lancangjiang Region of East Tibet. Master Thesis, Chengdu University of Technology, Chengdu, China, 2018. [Google Scholar]
- Wu, Z.; Gao, R.; Lu, Z.; Ye, P.; Lu, L.; Yin, C. Structures of the Qiangtang Basin and its significance to oil-gas exploration. Acta Geol. Sin. 2014, 88, 1130–1144, (In Chinese with English abstract). [Google Scholar]
- Pan, J.; Song, C.; Bao, J.; Ma, L.; Yan, M.; Fang, X.; Ting, H.; Yang, Y. Geochemical characteristics and salt-forming analysis of Jurassic strata in the Qiangtang Basin. Acta Geol. Sin. 2015, 89, 2152–2160, (In Chinese with English abstract). [Google Scholar]
- Li, Y.; Wang, C.; Li, Y. Characteristics of the Jurassic saline deposits and its significance to hydrocarbon accumulation in Qiangtang Basin of Tibet area. Acta Pet. Sin. 2008, 29, 173–178, (In Chinese with English abstract). [Google Scholar]
- Niu, Y.; Batiza, R. Trace element evidence from seamounts for recycled oceanic crust in the Eastern Pacific mantle. Earth Planet. Sci. Lett. 1997, 148, 471–483. [Google Scholar] [CrossRef]
- McArthur, J.M.; Howarth, R.J.; Shields, G.A. Strontium isotope stratigraphy. Geol. Time Scale 2012, 1, 127–144. [Google Scholar]
- Long, L.E.; Erwin, M.E.; Fisher, R.S. Rb-Sr ages of diagenesis of Mg-rich clay in Permian sediments, Palo Duro Basin, Texas Panhandle, USA. J. Sediment. Res. 1997, 67, 225–234. [Google Scholar]
- Denison, R.E.; Kirkland, D.W.; Evans, R. Using strontium isotopes to determine the age and origin of gypsum and anhydrite beds. J. Geol. 1998, 106, 1–18. [Google Scholar] [CrossRef]
- Veizer, J.; Compston, W. 87Sr/86Sr composition of seawater during the Phanerozoic. Geochim. Cosmochim. Acta. 1974, 38, 1461–1484. [Google Scholar] [CrossRef]
- Kirkland, D.W.; Denison, R.E.; Evans, R. Middle Jurassic Todilto Formation of northern New Mexico and southwestern Colorado: Marine or nonmarine? New Mex. Bur. Mines Miner. Resour. 1995, 147, 1–39. [Google Scholar]
- Wu, W.; Yang, J.; Xu, S.; Li, G.; Yin, H.; Tao, X. Sr fluxes and isotopic compositions of the eleven rivers originating from the Qinghai-Tibete Plateau and their contributions to 87Sr/86Sr evolution of seawater. Sci. China: Earth Sci. 2009, 39, 655–663, (In Chinese with English abstract). [Google Scholar]
- Taberner, C.; Marshall, J.D.; Hendry, J.P.; Pierre, C.; Thirlwall, M.F. Celestite formation, bacterial sulphate reduction and carbonate cementation of Eocene reefs and basinal sediments (Igualada, NE Spain). Sedimentology 2002, 49, 171–190. [Google Scholar] [CrossRef]
- Holser, W.T.; Kaplan, I.R. Isotope geochemistry of sedimentary sulfates. Chem. Geol. 1966, 1, 93–135. [Google Scholar] [CrossRef]
- Raab, M.; Spiro, B. Sulfur isotopic variations during seawater evaporation with fractional crystallization. Chem. Geol. Isot. Geosci. 1991, 86, 323–333. [Google Scholar] [CrossRef]
- Horita, J.; Zimmermann, H.; Holland, H.D. Chemical evolution of seawater during the Phanerozoic: Implications from the record of marine evaporites. Geochim. Cosmochim. Acta 2002, 66, 3733–3756. [Google Scholar] [CrossRef]
- Kurtz, A.C.; Kump, L.R.; Arthur, M.A.; Zachos, J.C.; Paytan, A. Early Cenozoic decoupling of the global carbon and sulfur cycles. Paleoceanography 2003, 18, 14.1–14.14. [Google Scholar] [CrossRef]
- Canfield, D.E. The evolution of the Earth surface sulfur reservoir. Am. J. Sci. 2004, 304, 839–861. [Google Scholar] [CrossRef]
- Canfield, D.E. Sulfur isotopes in coal constrain the evolution of the Phanerozoic sulfur cycle. Proc. Natl. Acad. Sci. 2013, 110, 8443–8446. [Google Scholar] [CrossRef]
- Burke, A.; Present, T.M.; Paris, G.; Rae, E.C.; Sandilands, B.H.; Gaillardet, J.; Peucker-Ehrenbrink, B.; Fischer, W.W.; McClelland, J.W.; Spencer, R.G.; et al. Sulfur isotopes in rivers: Insights into global weathering budgets, pyrite oxidation, and the modern sulfur cycle. Earth Planet. Sci. Lett. 2018, 496, 168–177. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Xia, X.; Zhang, L. Major element chemistry of the Changjiang (Yangtze River). Chem. Geol. 2002, 187, 231–255. [Google Scholar] [CrossRef]
- Bernasconi, S.M.; Meier, I.; Wohlwend, S.; Brack, P.; Hochuli, P.A.; Bläsi, H.; Wortmann, U.G.; Ramseyer, K. An evaporite-based high-resolution sulfur isotope record of Late Permian and Triassic seawater sulfate. Geochim. Cosmochim. Acta 2017, 204, 331–349. [Google Scholar] [CrossRef]
- Lu, F.H.; Meyers, W.J.; Schoonen, M.A. Minor and trace element analyses on gypsum: An experimental study. Chem. Geol. 1997, 142, 1–10. [Google Scholar] [CrossRef]
- Kushnir, J. The coprecipitation of strontium, magnesium, sodium, potassium and chloride ions with gypsum. An experimental study. Geochim. Cosmochim. Acta 1980, 44, 1471–1482. [Google Scholar] [CrossRef]
- Shen, L.; Wang, L.; Liu, C.; Zhao, Y. Sr, S, and O Isotope Compositions of Evaporites in the Lanping–Simao Basin, China. Miner 2021, 11, 96. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Liu, J.; Luo, J.; Zhang, S.; Yang, B.; Li, M. Carbon, oxygen and strontium isotopic responses of carbonate rocks and the Middle Jurassic sequence stratigraphy in the Nadigangri area, Qiangtang Basin. Acta Sedimentol. Sin. 2002, 20, 188–196, (In Chinese with English abstract). [Google Scholar]
- Ortí, F.; Pérez-López, A.; Salvany, J.M. Triassic evaporites of Iberia: Sedimentological and palaeogeographical implications for the western Neotethys evolution during the Middle Triassic–Earliest Jurassic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 471, 157–180. [Google Scholar] [CrossRef]

| Location | Sample ID | Age | Formation | Lithology |
|---|---|---|---|---|
| Huola | MKHL-20 | Middle Jurassic | Dongdaqiao | Outcropped gypsum laminae occurred within clastic rocks of Dongdaqiao Formation with corroded surface. Gypsum crystals are subhedral to euhedral and aligned linearly. |
| MKHL-21 | Middle Jurassic | Dongdaqiao | ||
| MKHL-22 | Middle Jurassic | Dongdaqiao | ||
| MKHL-23 | Middle Jurassic | Dongdaqiao | ||
| MKHL-24 | Middle Jurassic | Dongdaqiao | ||
| MKHL-25 | Middle Jurassic | Dongdaqiao | ||
| MKHL-26 | Middle Jurassic | Dongdaqiao | ||
| MKHL-27 | Middle Jurassic | Dongdaqiao | ||
| Rigei | MKRG-28 | Middle Jurassic | Dongdaqiao | Alternating white and brown gypsum layers, which are mainly composed of subhedral-to-euhedral relatively coarse grains. |
| MKRG-29 | Middle Jurassic | Dongdaqiao | ||
| MKRG-30 | Middle Jurassic | Dongdaqiao | ||
| MKRG-31 | Middle Jurassic | Dongdaqiao | ||
| Rumei | MKRM-33 | Middle Jurassic | Dongdaqiao | Lenticular gypsum interbedded with purplish sandstone, and massive unconsolidated gypsum mixed with carbonates breccias. Amoeboid gypsum crystals show subhedral-to-euhedral characteristics. |
| MKRM-34 | Middle Jurassic | Dongdaqiao | ||
| MKRM-35 | Middle Jurassic | Dongdaqiao | ||
| MKRM-36 | Middle Jurassic | Dongdaqiao |
| Sample ID | Location | 87Sr/86Sr | Δ34SV-CDT | Rb | Sr | Mg | Na | Si | Ca | Rb/Sr |
|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | (ppm) | (ppm) | (ppm) | (%) | (%) | |||||
| MKHL-20 | Huola | 0.707729 ± 0.000017 | 16.3 | 1.71 | 483 | 4206 | 680 | 1.01 | 32.06 | 0.0035 |
| MKHL-21 | 0.707628 ± 0.000011 | 15.8 | 2.61 | 1405 | 2874 | 1150 | 1.67 | 31.16 | 0.0019 | |
| MKHL-22 | 0.707668 ± 0.000018 | 15.5 | 1.4 | 1048 | 1572 | 460 | 0.993 | 31.97 | 0.0013 | |
| MKHL-23 | 0.707659 ± 0.000016 | 15.8 | 2.7 | 2132 | 4806 | 1160 | 2.08 | 31.12 | 0.0013 | |
| MKHL-24 | 0.707683 ± 0.000011 | 16.1 | 0.585 | 855 | 768 | 500 | 0.378 | 32.17 | 0.0007 | |
| MKHL-25 | 0.707602 ± 0.000013 | 15.5 | 0.867 | 1236 | 1920 | 1490 | 0.746 | 31.8 | 0.0007 | |
| MKHL-26 | 0.707608 ± 0.000014 | 15.8 | 1.6 | 1206 | 2940 | 740 | 1.1 | 31.65 | 0.0013 | |
| MKHL-27 | 0.707608 ± 0.000012 | 15.9 | 1.48 | 1002 | 2052 | 440 | 0.603 | 31.84 | 0.0015 | |
| MKRG-28 | Rigei | 0.707531 ± 0.000013 | 16 | 0.267 | 1057 | 2532 | 450 | 0.184 | 32.14 | 0.0003 |
| MKRG-29 | 0.707715 ± 0.000018 | 20.0 | 2.78 | 2084 | 4356 | 540 | 0.897 | 31.66 | 0.0013 | |
| MKRG-30 | 0.707661 ± 0.000017 | 20.5 | 0.11 | 2281 | 378 | 400 | <0.010 | 32.32 | 0.0000 | |
| MKRG-31 | 0.707886 ± 0.000020 | 20.8 | 0.069 | 2521 | 342 | 510 | <0.010 | 32.48 | 0.0000 | |
| MKRM-33 | Rumei | 0.707854 ± 0.000020 | 15.5 | 0.258 | 1211 | 774 | 360 | 1.18 | 31.8 | 0.0002 |
| MKRM-34 | 0.707627 ± 0.000012 | 16.1 | 0.508 | 943 | 738 | 570 | 0.385 | 32.31 | 0.0005 | |
| MKRM-35 | 0.708163 ± 0.000011 | 15.3 | 1.29 | 1190 | 2250 | 810 | 2.27 | 33.51 | 0.0011 | |
| MKRM-36 | 0.707602 ± 0.000008 | 16.3 | 0.71 | 890 | 2556 | 490 | 0.472 | 32.05 | 0.0008 |
| Sr Contribution of River Water (%) | Parameters | ||
|---|---|---|---|
| 87Sr/86Sr of River Water | 87Sr/86Sr of Sea Water | 87Sr/86Sr of Evaporites | |
| 45.6% | 0.70974 (minimum) | 0.70684 (minimum) | 0.708163 (maximum) |
| 11.6% | 0.7102 (maximum) | 0.70732 (maximum) | 0.707602 (minimum) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, J.; Shen, L.; Guan, X.; Sun, Z. S and Sr Isotope Compositions and Trace Element Compositions of the Middle Jurassic Evaporites in Eastern Tibet: Provenance and Palaeogeographic Implications. Minerals 2022, 12, 1039. https://doi.org/10.3390/min12081039
Fei J, Shen L, Guan X, Sun Z. S and Sr Isotope Compositions and Trace Element Compositions of the Middle Jurassic Evaporites in Eastern Tibet: Provenance and Palaeogeographic Implications. Minerals. 2022; 12(8):1039. https://doi.org/10.3390/min12081039
Chicago/Turabian StyleFei, Jinna, Lijian Shen, Xin Guan, and Zhicheng Sun. 2022. "S and Sr Isotope Compositions and Trace Element Compositions of the Middle Jurassic Evaporites in Eastern Tibet: Provenance and Palaeogeographic Implications" Minerals 12, no. 8: 1039. https://doi.org/10.3390/min12081039
APA StyleFei, J., Shen, L., Guan, X., & Sun, Z. (2022). S and Sr Isotope Compositions and Trace Element Compositions of the Middle Jurassic Evaporites in Eastern Tibet: Provenance and Palaeogeographic Implications. Minerals, 12(8), 1039. https://doi.org/10.3390/min12081039







