Chlorine-Deficient Analog of Taseqite from Odikhincha Massif (Russia): Genesis and Relation with Other Sr-Rich Eudialyte-Group Minerals
Abstract
:1. Introduction
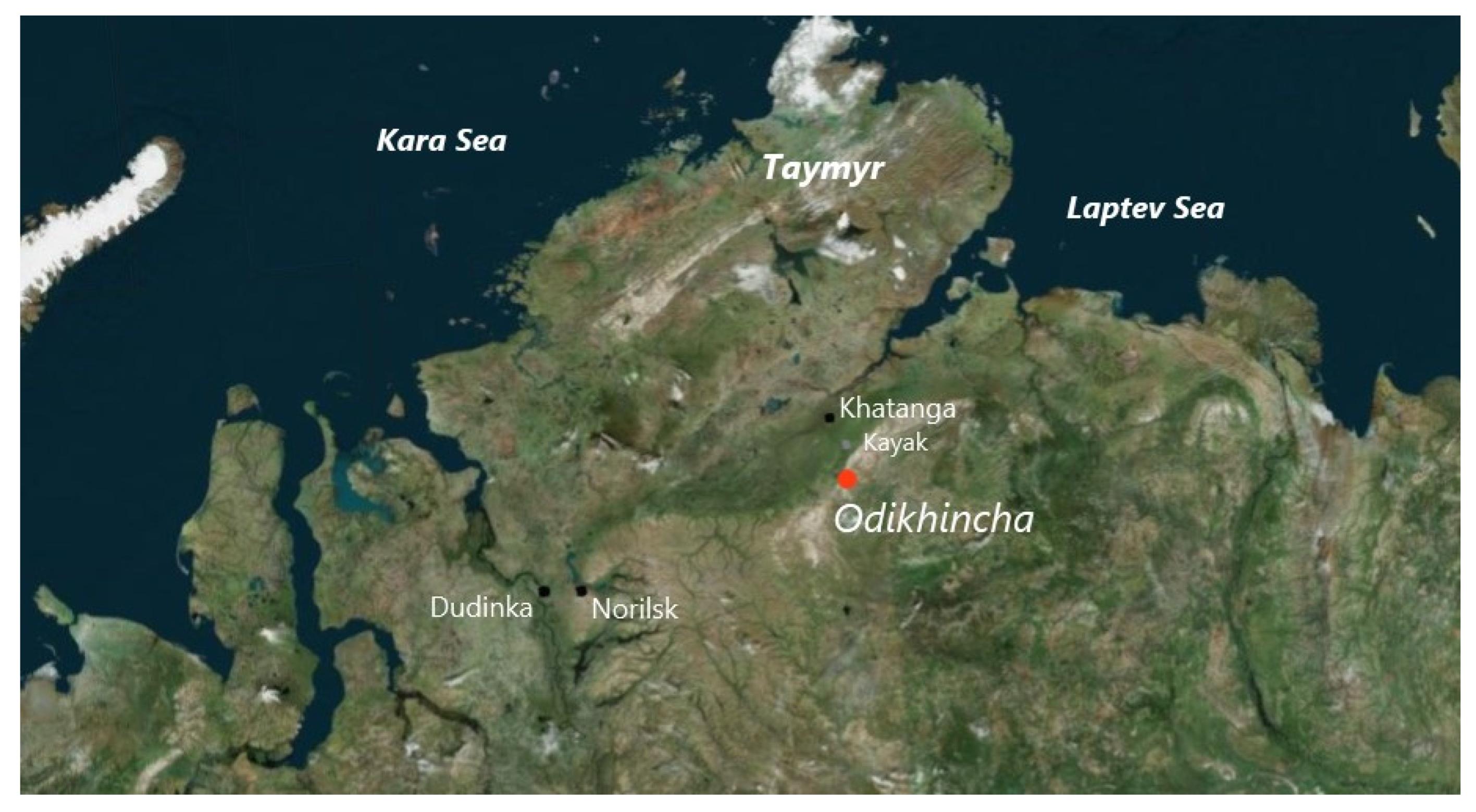
2. Geological Setting
- -
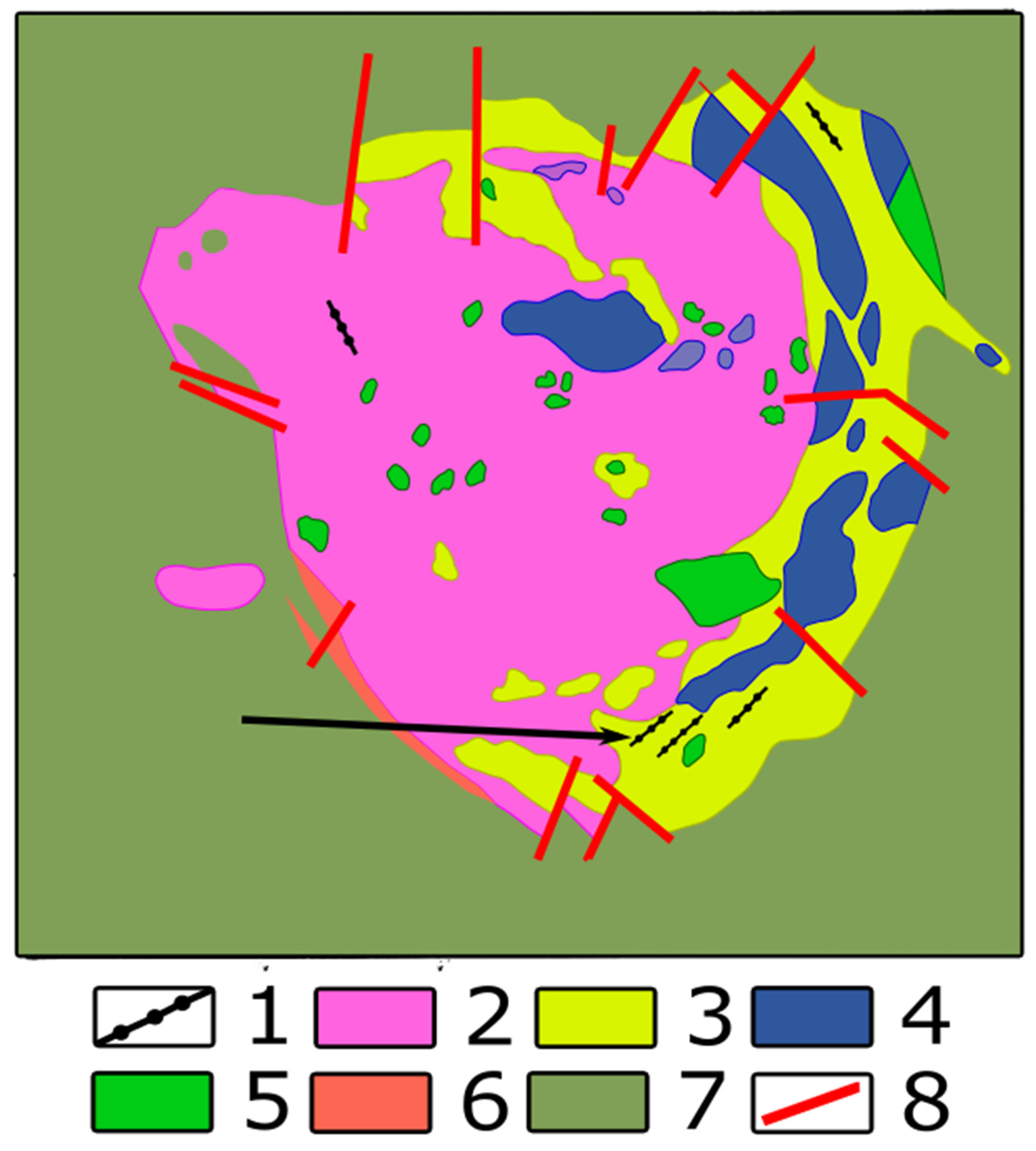
- fine-grained aggregate of nepheline, feldspar, aegirine and wollastonite with poikilitic crystals of lamprophyllite and flakes of rinkite-group minerals;
- -
- coarse-crystalline zone, with tabular crystals of a brown Sr-rich eudialyte-group mineral, and gray plates of the K-Na feldspar surrounded by the aggregate, consist of cancrinite crystals, idiomorphic against zeolite and streaks of interstitial brown mass of rinkite-group minerals. Cancrinite crystals contain irregular inclusions of nepheline. The bronze-color lamprophyllite blades and black needles of pyroxene are disseminated in light mass or gathered in spherules (Figure 3).
3. Methods
3.1. Electron Probe Microanalysis
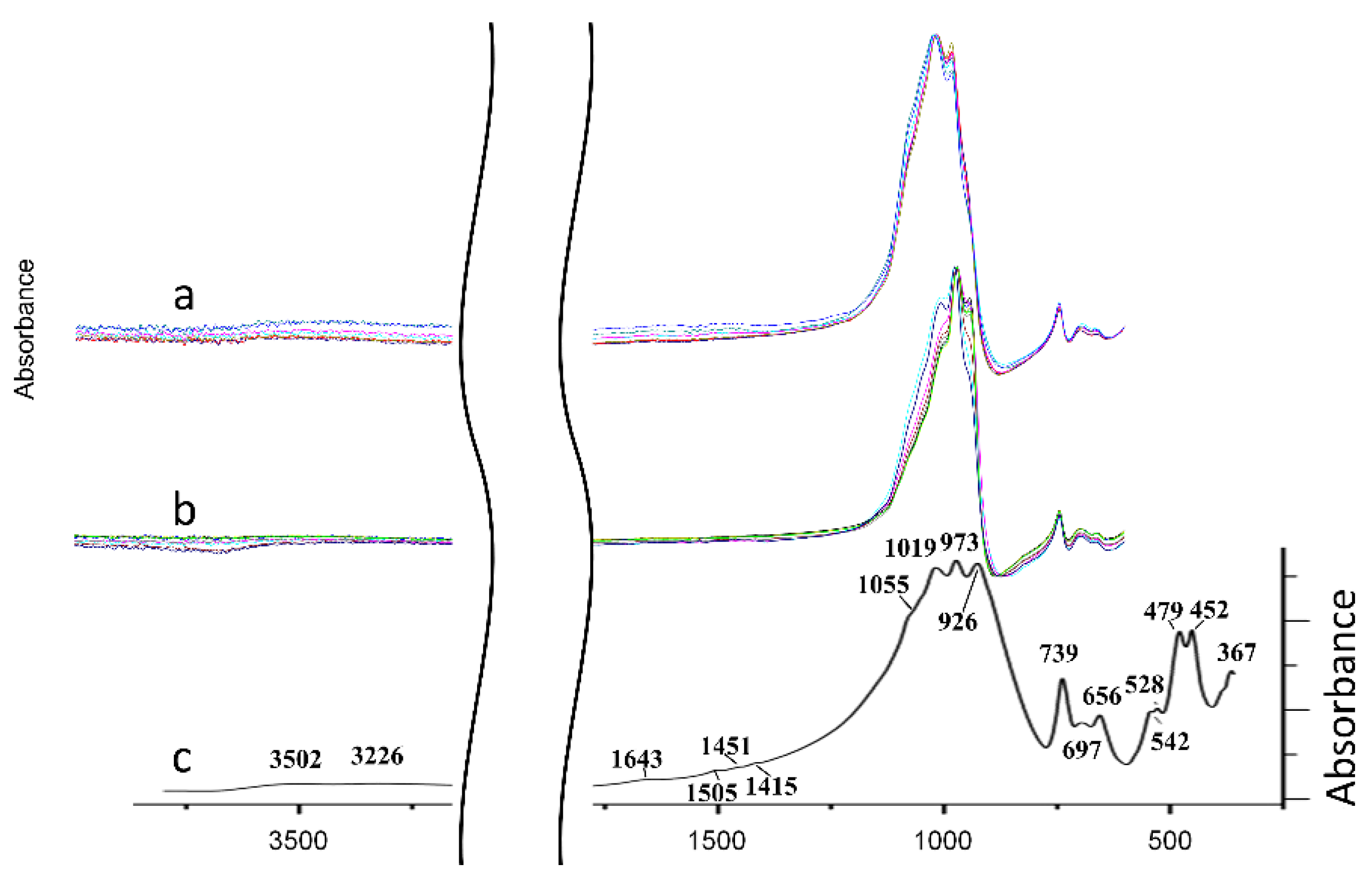
3.2. Infrared Spectroscopy
3.3. X-ray Diffraction
4. Results
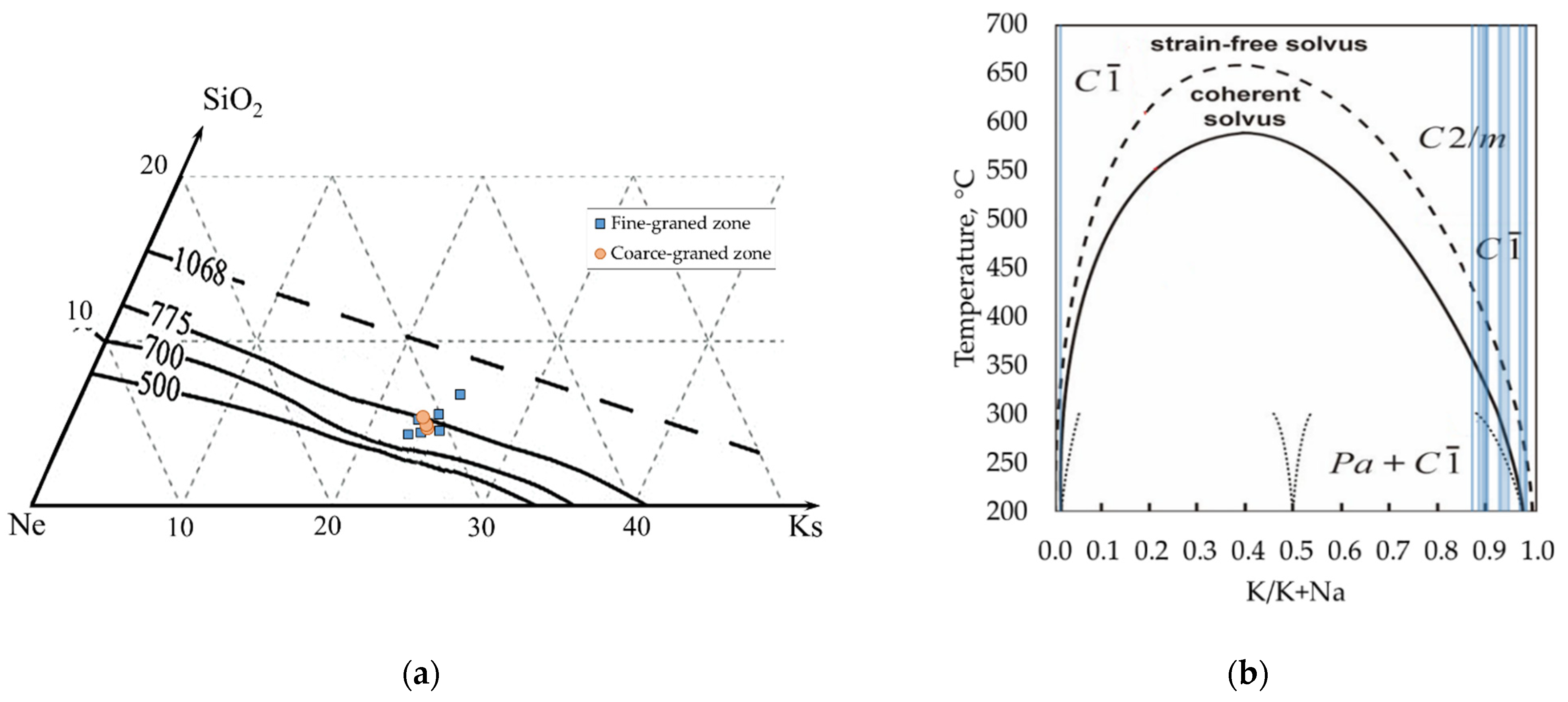
4.1. General Features of Studied Pegmatite
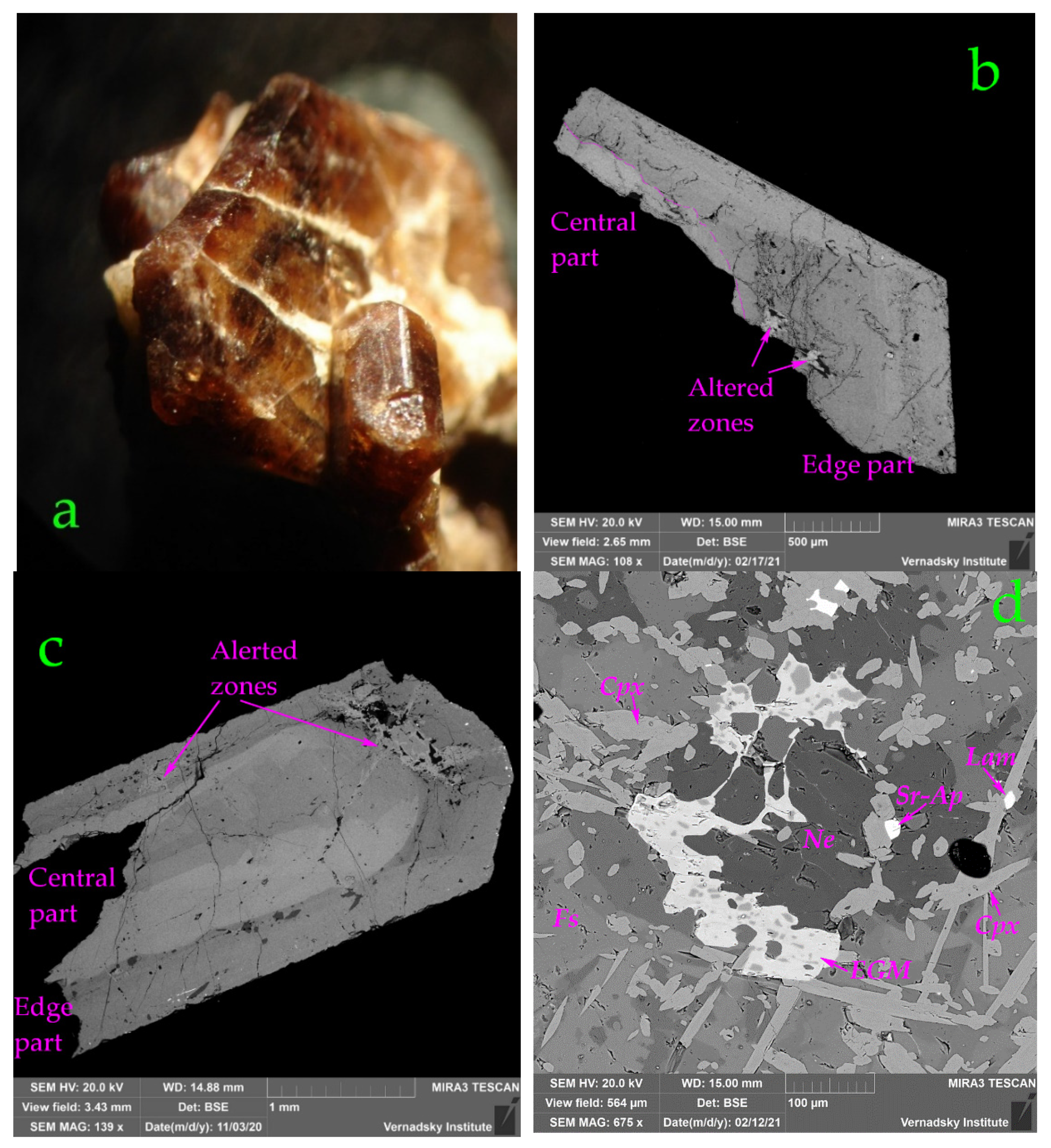
4.2. Morphology, General Physical and Optical Properties of EGMs
4.3. Chemical Composition of EGMs
4.4. Infrared Spectroscopy
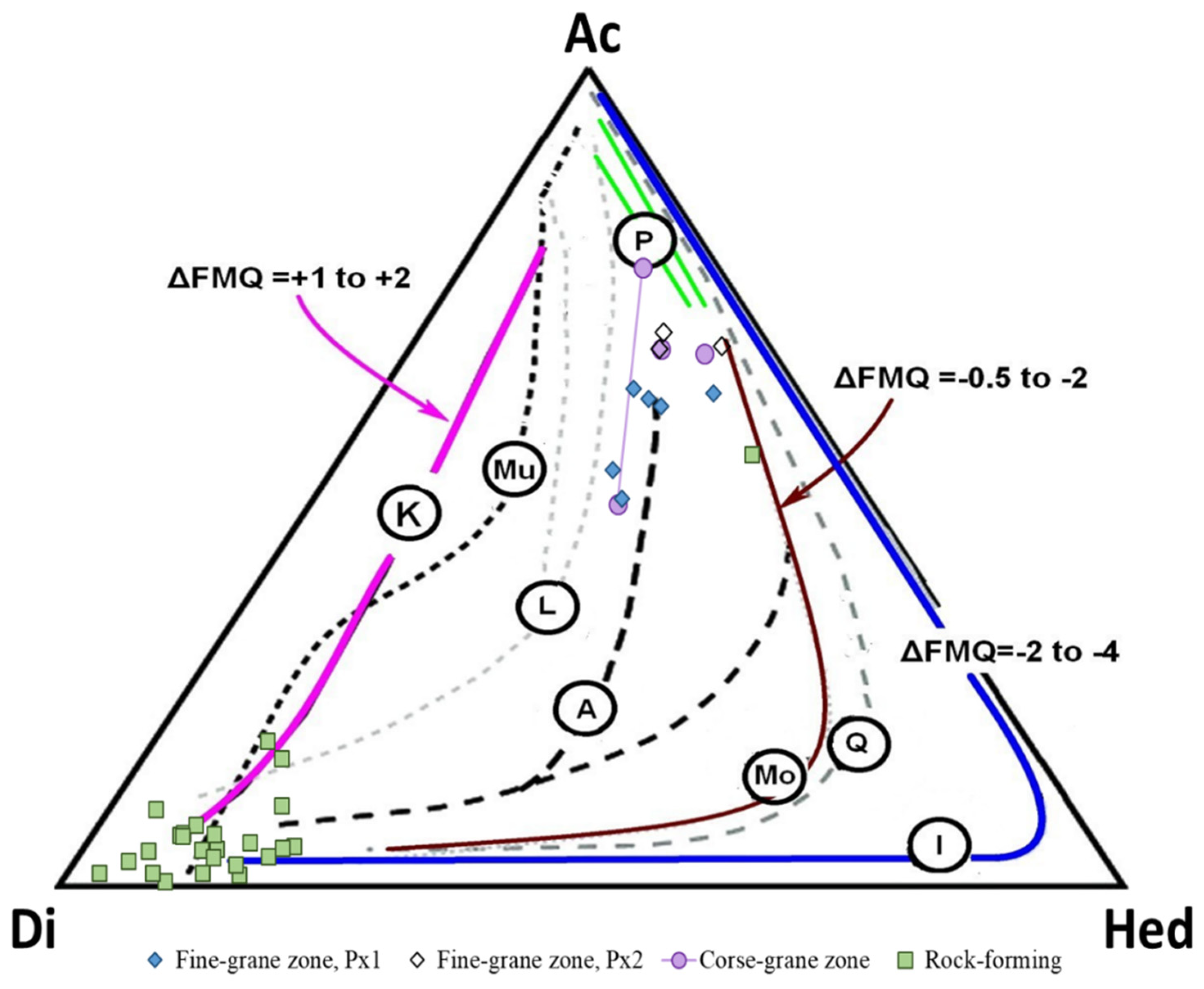
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnsen, O.; Grice, J.D. The Crystal Chemistry of Eudialyte Group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The Nomenclature of Eudialyte-Group Minerals. Can. Mineral. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Aksenov, S.M. Minerals of Eudialyte Group: Crystal Chemistry, Properties, Genesis; University of Nizhni Novgorod: Nizhniy Novgorod, Russia, 2012; ISBN 978-5-91326-207-3. [Google Scholar]
- Aksenov, S.M.; Kabanova, N.A.; Chukanov, N.V.; Panikorovskii, T.L.; Blatov, V.A.; Krivovichev, S.V. The role of local heteropolyhedral substitutions in the stoichiometry, topological characteristics and ion-migration paths in the eudialyte-related structures: A quantitative analysis. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2022, 78, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Rastsvetaeva, R.K.; Chukanov, N.V. Classification of Eudialyte-Group Minerals. Geol. Ore Depos. 2012, 54, 487–497. [Google Scholar] [CrossRef]
- Eggenkamp, H.G.M.; Marks, M.A.W.; Atanasova, P.; Wenzel, T.; Markl, G. Changes in Halogen (F, Cl, Br, and I) and S Ratios in Rock-Forming Minerals as Monitors for Magmatic Differentiation, Volatile-Loss, and Hydrothermal Overprint: The Case for Peralkaline Systems. Minerals 2020, 10, 995. [Google Scholar] [CrossRef]
- Schilling, J.; Wu, F.-Y.; McCammon, C.; Wenzel, T.; Marks, M.A.W.; Pfaff, K.; Jacob, D.E.; Markl, G. The Compositional Variability of Eudialyte-Group Minerals. Mineral. Mag. 2011, 75, 87–115. [Google Scholar] [CrossRef]
- Estrade, G.; Salvi, S.; Béziat, D. Crystallization and Destabilization of Eudialyte-Group Minerals in Peralkaline Granite and Pegmatite: A Case Study from the Ambohimirahavavy Complex, Madagascar. Mineral. Mag. 2018, 82, 375–399. [Google Scholar] [CrossRef]
- Sørensen, H. The Ilímaussaq Alkaline Complex, South Greenland an Overview of 200 Years of Research and an Outlook; Meddelelser om Grønland. Geoscience; Museum Tusculanum Press: Copenhagen, Danmark, 2006; ISBN 9788763512718. [Google Scholar]
- Osipov, A.S.; Antonov, A.A.; Panikorovskii, T.L.; Zolotarev, A.A. Hydrated CO3-Bearing Analog of Manganoeudialyte from Alkali Pegmatites of the Konder Pluton, Khabarovsk Krai. Geol. Ore Depos. 2018, 60, 726–735. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K. Aqualite, a New Mineral Species of the Eudialyte Group from the Inagli Alkaline Pluton, Sakha-Yakutia, Russia, and the Problem of Oxonium in Hydrated Eudialytes. Geol. Ore Depos. 2007, 49, 739–751. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Moiseev, M.M.; Rastsvetaeva, R.K.; Rozenberg, K.A.; Zadov, A.E.; Pekov, I.V.; Golyshevite, K.V.V. (Na,Ca)10Ca9Fe2Zr3NbSi25O72(CO3)(OH)3 H2O, and Mogovidite, Na9(Ca,Na)6Ca6Fe2Zr3□Si25O72(CO3)(OH,H2O)4—New Minerals of the Eudialyte Group from Agpaitic Pegmatites of Kovdor Massif, Kola Peninsula. Zap. VMO 2005, 134, 36–47. [Google Scholar]
- Verwoerd, W.J.; Viljoen, E.A.; Chevallier, L. Rare Metal Mineralization at the Salpeterkop Carbonatite Complex, Western Cape Province, South Africa. J. Afr. Earth Sci. 1995, 21, 171–186. [Google Scholar] [CrossRef]
- Egorov, L.S. Ijolite Carbonatite Plutonism (Case History of the Maimecha-Kotui Complexes Northern Siberia); Nedra: Saint Petersburg, Russia, 1991. [Google Scholar]
- Lipenkov, G.V.; Mashak, M.S.; Kirichenko, V.T.; Larichev, A.I.; Nazarov, D.V.; Bigun, I.V.; Kondakova, E.A.; Khabarov, A.N.; Gerasicheva, A.V.; Zavarzin, I.V. State Geological Map of the Russian Federation. Scale 1: 1,000,000 (Third Generation). Anabar-Vilyui Series. Sheet R-48—Khatanga Explan. Lett. 2015, 398. [Google Scholar]
- Arzamastsev, A.A.; Glaznev, V.N.; Arzamastseva, L.V.; Bea, F.; Montero, P. Kola Alkaline Province in the Paleozoic: Evaluation of Primary Mantle Magma Composition and Magma Generation Conditions. Russ. J. Earth Sci. 2001, 3, 1–32. [Google Scholar] [CrossRef]
- Salnikova, E.B.; Chakhmouradian, A.R.; Stifeeva, M.V.; Reguir, E.P.; Kotov, A.B.; Gritsenko, Y.D.; Nikiforov, A.V. Calcic Garnets as a Geochronological and Petrogenetic Tool Applicable to a Wide Variety of Rocks. Lithos 2019, 338–339, 141–154. [Google Scholar] [CrossRef]
- Pokrovsky, B.G. Crustal Contamination of Mantle Magmas on Evidence of Isotope Geochemistry; Transaction of GIN RAS; Nauka: Moscow, Russia, 2000; Volume 535, 228p. [Google Scholar]
- Bagdasaryan, T.E.; Thomson, S.N.; Latyshev, A.V.; Veselovskiy, R.V.; Zaitsev, V.A.; Marfin, A.E.; Zakharov, V.S.; Yudin, D.S. Thermal History of the Siberian Traps Large Igneous Province Revealed by New Thermochronology Data from Intrusions. Tectonophysics 2022, 836, 229385. [Google Scholar] [CrossRef]
- Gritsenko, Y.D.; Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V.; Varlamov, D.A.; Pautov, L.A.; Vozchikova, S.A.; Ksenofontov, D.A.; Britvin, S.N. Odikhinchaite, Na9Sr3[(H2O)2Na]Ca6Mn3Zr3NbSi(Si24O72)O(OH)3(CO3)·H2O, a New Eudialyte-Group Mineral from the Odikhincha Intrusion, Taimyr Peninsula, Russia. Minerals 2020, 10, 1062. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Zaitsev, V.A.; Aksenov, S.M.; Viktorova, K.A. Crystal Structure of Cl-Deficient Analogue of Taseqite from Odikhincha Massif. Crystallogr. Rep. 2018, 63, 349–357. [Google Scholar] [CrossRef]
- Nielsen, T.F.D. The Occurrence and Formation of Ti-Aegirines in Peralkaline Syenites. Contrib. Mineral. Petrol. 1979, 69, 235–244. [Google Scholar] [CrossRef]
- Rass, I.T. Trace Element Fractionation in Coexisting High- and Low-Ca Alkali Ultramafic Series of the Odikhincha Massif (Polar Siberia). Geochem. Int. 2004, 42, 744–754. [Google Scholar]
- Mann, U.; Marks, M.; Markl, G. Influence of Oxygen Fugacity on Mineral Compositions in Peralkaline Melts: The Katzenbuckel Volcano, Southwest Germany. Lithos 2006, 91, 262–285. [Google Scholar] [CrossRef]
- Shubin, I.I.; Filina, M.I.; Kogarko, L.N. Evolution of Pyroxenes of the Lovozero Rare Metal Deposit (Lower Zone). Geochem. Int. 2021, 59, 92–98. [Google Scholar] [CrossRef]
- Andersen, T.; Elburg, M.; Erambert, M. Contrasting Trends of Agpaitic Crystallization in Nepheline Syenite in the Pilanesberg Alkaline Complex, South Africa. Lithos 2018, 312–313, 375–388. [Google Scholar] [CrossRef]
- Schonenberger, J.; Markl, G. The Magmatic and Fluid Evolution of the Motzfeldt Intrusion in South Greenland: Insights into the Formation of Agpaitic and Miaskitic Rocks. J. Petrol. 2008, 49, 1549–1577. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. The Ilímaussaq Alkaline Complex, South Greenland. In Layered Intrusions (Springer Geology); Charlier, B., Namur, O., Latypov, R., Tegner, C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 649–691. [Google Scholar] [CrossRef]
- Zaitsev, V.A.; Kogarko, L.N. Compositions of Minerals of the Lamprophyllite Group from Alkaline Massifs Worldwide. Geochem. Int. 2002, 40, 313–322. [Google Scholar]
- Borst, A.M.; Finch, A.A.; Friis, H.; Horsburgh, N.J.; Gamaletsos, P.N.; Goettlicher, J.; Steininger, R.; Geraki, K. Structural State of Rare Earth Elements in Eudialyte-Group Minerals. Mineral. Mag. 2020, 84, 19–34. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Stepenshchikov, D.G.; Kalashnikov, A.O.; Aksenov, S.M. Who Is Who in the Eudialyte Group: A New Algorithm for the Express Allocation of a Mineral Name Based on the Chemical Composition. Minerals 2022, 12, 224. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Mineral Species; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7127-7. [Google Scholar]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Lisitsin, D.V.; Voronin, M.V.; Varlamov, D.A. Crystal Structure and Indicatopy Significance of Odikhinchaite from the Khibiny Alkaline Complex. Vestn. Geosci. 2021, 4, 3–9. [Google Scholar] [CrossRef]
- Ekimenkova, I.A.; Rastsvetaeva, R.; Khomyakov, A.P. Refinement of the Crystal Structure of a Fe, Sr-Analogue of Kentbrooksite. Crystallogr. Rep. 2000, 45, 1010–1013. [Google Scholar] [CrossRef]
- Azarova, Y.V. Minerals of the Eudialyte Group and Their Alteration Products as a Geochemical Indicator of Postmagmatic Processes during the Formation of the Lujavrite-Malignite Complex in the Khibiny Massif. Geochem. Int. 2005, 43, 715–720. [Google Scholar]
- Mitchell, R.H.; Liferovich, R.P. Subsolidus Deuteric/Hydrothermal Alteration of Eudialyte in Lujavrite from the Pilansberg Alkaline Complex, South Africa. Lithos 2006, 91, 352–372. [Google Scholar] [CrossRef]
- Petersen, O.V.; Johnsen, O.; Gault, R.A.; Niedermayr, G.; Grice, J.D. Taseqite, a New Member of the Eudialyte Group from the Ilímaussaq Alkaline Complex, South Greenland. Neues Jahrb. Mineral. Mon. 2004, 2004, 83–96. [Google Scholar] [CrossRef]
- Yagi, K.; Kikuchi, T.; Kakuta, H. Thermal Decomposition of Pectolite and Its Hydrothermal Synthesis. J. Fac. Sci. Hokkaido Univ. Ser. 4 Geol. Mineral. 1968, 14, 123–134. [Google Scholar]
- Edgar, A.D. Studies on Cancrinites; Part 2, Stability Fields and Cell Dimensions of Calcium and Potassium-Rich Cancrinites. Can. Mineral. 1964, 8, 53–67. [Google Scholar]
- Sirbescu, M.; Jenkins, D.M. Experiments on the Stability of Cancrinite in the System Na2O–CaO–Al2O3–SiO2–CO2–H2O. Am. Mineral. 1999, 84, 1850–1860. [Google Scholar] [CrossRef]
- Hamilton, D.L. Nephelines as Crystallization Temperature Indicators. J. Geol. 1961, 69, 321–329. [Google Scholar] [CrossRef]
- Hamilton, D.L.; McKenzie, W.S. Nepheline Solid Solution in the System NaAlSiO4-KAlSiO4-SiO2. J. Petrol. 1960, 1, 56–72. [Google Scholar] [CrossRef]
- Xu, H.; Jin, S.; Lee, S.; Hobbs, F. Nano-Phase KNa(Si6Al2)O16 in Adularia: A New Member in the Alkali Feldspar Series with Ordered K–Na Distribution. Minerals 2019, 9, 649. [Google Scholar] [CrossRef]
- Moiseev, M.M.; Chukanov, N.V. Mineralogy of Alkaline Pegmatites and Hydro-Thermalites of the Kovdor Massif. New Data Miner. 2006, 41, 56–70. [Google Scholar]
- Pekov, I.V.; Chukanov, N.V.; Turchkova, A.G. On the Mineralogy and Behavior of Barium in Differentiates of Alkaline Rocks of the Khibiny Massif, Kola Peninsula. In Proceedings of the XIX Russian Meeting “Geochemistry of Igneous Rocks”, Moscow, Russia, 6–7 April 2000; pp. 111–112. Available online: https://drive.google.com/file/d/101DuzSzT-SBzxOWC4g-fjNhsjxPSEhdv/view?usp=sharing (accessed on 13 May 2022).
- Pekov, I.V.; Lovskaya, E.V.; Turchkova, A.G.; Chukanov, N.V.; Zadov, A.E.; Rastsvetaevs, R.K.; Kononkova, N.N. Thomsonite-Sr, (Sr,Ca)2Na[Al5Si5O20]∙6-7H2O, a New Zeolite from the Khibiny Massif, Kola Peninsula and the Thomsonite-Ca—Thomsonite-Sr Isomorphous Series. Zap. Ross. Mineral. Obs. 2001, 130, 46–55. [Google Scholar]



| Fine-Grained Zone | Coarse-Grained Zone | ||||||
|---|---|---|---|---|---|---|---|
| Nepheline | Orthoclase | Ba-Rich Orthoclase | Albite | Nepheline | Na-Rich Orthoclase | Na-Poor Orthoclase | |
| n | 4 | 6 | 1 | 1 | 3 | 7 | 5 |
| SiO2 | 41.54 | 64.14 | 61.30 | 69.89 | 42.41 | 65.68 | 65.07 |
| Al2O3 | 32.15 | 17.73 | 18.05 | 19.22 | 33.12 | 18.20 | 18.16 |
| Fe2O3 | 0.86 | 0.28 | 0.52 | 0.00 | 0.69 | 0.27 | 0.07 |
| Na2O | 14.79 | 0.91 | 0.94 | 9.78 | 15.91 | 0.99 | 0.20 |
| CaO | b.d.l. | 0.06 | b.d.l. | b.d.l. | n.a. | 0.01 | 0.05 |
| K2O | 6.82 | 14.91 | 14.08 | 0.20 | 7.00 | 15.12 | 16.44 |
| BaO | n.a. | 0.71 | 3.11 | 0.00 | n.a. | 0.51 | 0.52 |
| SrO | b.d.l. | b.d.l. | n.a. | 0.60 | n.a. | 0.03 | 0.07 |
| Total | 96.15 | 98.73 | 98.00 | 99.69 | 99.14 | 100.81 | 100.59 |
| Fine-Grained Zone | Coarse-Grained Zone | Inclusions in Eudialyte | ||
|---|---|---|---|---|
| Yellowish-Green to Green | Green to Blueish-Green | |||
| Na2O | 6.73–8.53 | 9.42–9.69 | 7.38–11.31 | 8.52–9.38 |
| MgO | 1.57–4.32 | 0.91–2.03 | 1.35–4.95 | 1.57–1.99 |
| Al2O3 | 0.66–1.02 | 0.68–1.11 | 0.58–0.99 | 0.66–0.85 |
| SiO2 | 50.30–51.79 | 51.15–51.61 | 51.64–54.32 | 50.80–51.64 |
| CaO | 7.13–11.17 | 5.60–6.06 | 4.01–10.54 | 6.11–7.13 |
| TiO2 | 0.92–1.82 | 1.10–1.38 | 1.02–4.05 | 1.00–1.02 |
| MnO | 0.44–0.69 | 0.45–0.61 | 0.52–1.37 | 0.52–0.69 |
| FeO | 20.70–25.43 | 24.24–26.85 | 18.30–26.74 | 24.91–25.43 |
| Total | 94.99–97.79 | 96.21–96.78 | 96.42–101.25 | 95.80–96.42 |
| Lamprophyllite | Rinkite-Group Minerals | Titanite | ||||
|---|---|---|---|---|---|---|
| Rinkite-(Ce) | Mosandrite-(Ce) | Unnamed Mineral | ||||
| n | 4 | 1 | 5 | 3 | 7 | 2 |
| SiO2 | 30.11 | 31.55 | 29.34 | 25.15 | 34.43 | 28.84 |
| TiO2 | 28.67 | 29.68 | 7.66 | 9.62 | 12.88 | 38.33 |
| Al2O3 | 0.09 | 0.35 | 5.19 | 0.21 | ||
| FeO | 2.80 | 2.97 | 0.05 | 0.22 | 0.59 | 1.97 |
| MnO | 1.77 | 1.66 | 0.01 | |||
| MgO | 0.51 | 0.40 | ||||
| CaO | 1.09 | 0.78 | 26.38 | 21.01 | 4.09 | 26.38 |
| SrO | 17.30 | 17.36 | 5.94 | 2.51 | 1.55 | 0.92 |
| BaO | 1.60 | 2.21 | ||||
| K2O | 0.65 | 0.67 | 0.30 | 0.51 | 0.01 | |
| Na2O | 10.13 | 10.87 | 6.34 | 0.70 | 0.16 | 0.76 |
| Nb2O5 | 0.23 | 0.24 | 3.59 | 2.53 | 5.22 | 1.52 |
| ZrO2 | 0.09 | 0.39 | 0.06 | |||
| La2O3 | 4.71 | 4.79 | 7.17 | 0.21 | ||
| Ce2O3 | 5.38 | 9.18 | 9.72 | 0.50 | ||
| Nd2O3 | 0.72 | 0.78 | 1.06 | |||
| Y2O3 | 0.35 | 0.74 | 0.33 | |||
| ThO2 | 1.21 | 1.85 | 2.34 | |||
| UO3 | 0.39 | 0.53 | 0.36 | |||
| F | 1.83 | 2.03 | 6.75 | 1.50 | 0.00 | |
| Total | 94.86 | 98.39 | 92.23 | 80.65 | 85.60 | 99.69 |
| Constituent | Well-Shaped Euhedral Crystal | Xenomorphic | ||||||
|---|---|---|---|---|---|---|---|---|
| Central Part | Edge Part | Alerted Zone | ||||||
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| Na2O | 11.63 | 10.81–12.16 | 11.49 | 9.94–12.53 | 10.48 | 9.27–11.43 | 10.04 | 8.32–11.43 |
| K2O | 0.65 | 0.4–0.79 | 0.62 | 0.42–0.74 | 0.67 | 0.61–0.74 | 0.27 | 0.22–0.37 |
| CaO | 11.13 | 10.56–12.07 | 11.38 | 10.47–11.88 | 11.45 | 11.14–12.08 | 11.25 | 11.08–11.42 |
| SrO | 5.20 | 3.82–6.37 | 4.96 | 3.94–5.7 | 5.91 | 5.02–6.84 | 4.75 | 3.82–5.39 |
| BaO | 0.10 | 0.1–0.1 | 0.04 | 0.04–0.04 | 0.35 | 0.35–0.35 | ||
| Y2O3 | 0.01 | 0–0.05 | 0.01 | 0–0.03 | 0.02 | 0–0.11 | ||
| La2O3 | 0.27 | 0.1–0.55 | 0.20 | 0.04–0.47 | 0.47 | 0–0.87 | 0.14 | 0.09–0.19 |
| Ce2O3 | 0.26 | 0–0.54 | 0.24 | 0.06–0.48 | 0.43 | 0.09–0.83 | 0.27 | 0.24–0.29 |
| Nd2O3 | 0.04 | 0.04–0.06 | 0.06 | 0–0.11 | 0.08 | 0.08–0.08 | 0.04 | 0–0.12 |
| MnO | 1.46 | 1.06–2.17 | 1.61 | 0.37–2.07 | 1.77 | 0.45–2.47 | 1.86 | 1.73–2.04 |
| FeO | 4.99 | 4.71–5.36 | 4.80 | 4.22–5.35 | 4.16 | 3.74–4.61 | 5.12 | 4.91–5.49 |
| TiO2 | 0.76 | 0.4–1.63 | 0.98 | 0.43–1.78 | 0.40 | 0.22–0.57 | 0.89 | 0.68–1.27 |
| Nb2O5 | 2.37 | 1.56–3.61 | 2.34 | 1.81–3.13 | 3.01 | 2.21–3.68 | 2.22 | 2.03–2.36 |
| ZrO2 | 11.14 | 9.36–11.94 | 10.56 | 9.53–11.73 | 11.50 | 10.46–12.42 | 10.14 | 9.66–10.97 |
| HfO2 | 0.13 | 0.09–0.17 | 0.08 | 0–0.13 | 0.19 | 0.15–0.25 | 0.12 | 0.08–0.17 |
| SiO2 | 48.49 | 45.3–51.09 | 49.36 | 47.39–52.03 | 47.58 | 44.96–49.62 | 49.02 | 48.45–49.48 |
| SO3 | 0.17 | 0.08–0.24 | 0.18 | 0–0.27 | 0.14 | 0.06–0.23 | ||
| Cl | 0.96 | 0.8–1.08 | 0.95 | 0.81–1.05 | 0.67 | 0.49–0.93 | 0.77 | 0.74–0.8 |
| Total | 99.53 | 97.27–101.93 | 99.71 | 95.71–102.66 | 98.71 | 94.98–102.82 | 96.89 | 95.86–97.64 |
| Central Part | Edge Part | Alerted Zone | Xenomorphic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N sites | Na | 11.60 | 10.85–12.21 | 11.23 | 9.78–12.32 | 10.74 | 9.43–11.87 | 10.13 | 8.44–11.50 |
| Ca | 0.32 | 0.00–0.60 | 0.36 | 0.00–0.58 | 0.66 | 0.40–1.00 | 0.36 | 0.24–0.51 | |
| Sr | 1.57 | 1.10–2.03 | 1.48 | 1.21–1.71 | 1.81 | 1.50–2.09 | 1.43 | 1.15–1.64 | |
| K | 0.43 | 0.26–0.53 | 0.41 | 0.28–0.48 | 0.45 | 0.39–0.51 | 0.18 | 0.15–0.24 | |
| M(1) site | Ca | 5.90 | 5.79–5.96 | 5.92 | 5.82–5.97 | 5.83 | 5.68–5.97 | 5.92 | 5.90–5.94 |
| REE | 0.10 | 0.04–0.21 | 0.08 | 0.03–0.18 | 0.17 | 0.03–0.32 | 0.08 | 0.06–0.10 | |
| M(2) site | Fe | 2.17 | 2.01–2.37 | 2.07 | 1.86–2.32 | 1.84 | 1.65–2.03 | 2.23 | 2.15–2.39 |
| Mn | 0.66 | 0.46–0.95 | 0.69 | 0.00–0.90 | 0.73 | 0.00–1.09 | 0.82 | 0.77–0.90 | |
| Na | 0.17 | 0.00–0.36 | 0.24 | 0.01–0.83 | 0.43 | 0.14–1.17 | 0.03 | 0.00–0.08 | |
| M(3,4)sites | Nb | 0.57 | 0.36–0.90 | 0.55 | 0.42–0.75 | 0.72 | 0.51–0.88 | 0.52 | 0.48–0.55 |
| Si | 0.31 | 0.00–0.69 | 0.42 | 0.12–0.79 | 0.17 | 0.00–0.44 | 0.54 | 0.36–0.76 | |
| Ti | 0.14 | 0.00–0.28 | 0.07 | 0.00–0.44 | 0.10 | 0.00–0.21 | 0.03 | 0.00–0.10 | |
| Z site | Zr | 2.80 | 2.37–3.00 | 2.65 | 2.40–2.88 | 2.96 | 2.69–3.15 | 2.58 | 2.44–2.80 |
| Ti | 0.19 | 0.00–0.62 | 0.35 | 0.12–0.59 | 0.06 | 0.00–0.23 | 0.31 | 0.17–0.50 | |
| Hf | 0.00 | 0.00–0.03 | 0.00 | 0.00–0.02 | 0.00 | 0.00–0.04 | 0.02 | 0.01–0.03 | |
| X site | Cl | 0.85 | 0.72–0.94 | 0.83 | 0.70–0.90 | 0.60 | 0.44–0.81 | 0.68 | 0.66–0.70 |
| Site | «Taseqite», Odikhincha [21] | Taseqite, Ilímaussaq [37] | «Fe,Sr-Analog of Kentbrooksite», Lovozero [34] | Odikhinchaite, Odikhincha [20] |
|---|---|---|---|---|
| M(1) | 5.85 Ca + 0.15 REE | 4.98 Ca + 0.58 Mn + + 0.3 Sr + 0.14Y | 4.85 Ca + 0.85 Mn + 0.30 REE | 6 Ca |
| M(2a) | 2.43 FeV | 1.25 Fe2+,V + 0.92 MnV | 0.87 Fe2+,IV | 2.49 Mn + 0.51 Fe |
| M(2b) | 0.45 MnV | 0.68 Fe2+,V | 1.11 Fe2+,V + 0.67 MnV + 0.19 TiV + 0.1ZrV + 0.04 HfV | |
| M(3a) | 0.64 Nb | 0.97 Nb | 0.7 Nb + 0.3 Si | Nb |
| M(3b) | 0.33 Si | |||
| M(4a) | 0.67 Si | 0.3 Nb | Si | 0.82 Si |
| M(4b) | 0.27 Ti | 0.45 Si | 0.18 Si | |
| N(3) | 2.5 Na + 0.5 K | 1.9 Na + 1.1 Sr | 3 Na | 2Sr + 0.45 K + 0.2 REE Ln |
| 0.35 Na | ||||
| N(4) | 1.71 Sr + 1.29 Na | 2.47 Sr + 0.53 Na | 1.8 Sr + 0.96 Na + 0.24 K | 3 Na |
| N(5) | 3 Na | 2.56 Na | 3 Na | 1.8 H2O + 1.2 Na |
| X(1) | 0.74 Cl | 0.91Cl | 0.53 CO3 + 0.35 Cl | |
| 0.12 Cl | ||||
| X(2) | 0.18 H2O | 0.83 Cl | 0.6 H2O | |
| 0.4 (OH,F) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, V.A.; Chukanov, N.V.; Aksenov, S.M. Chlorine-Deficient Analog of Taseqite from Odikhincha Massif (Russia): Genesis and Relation with Other Sr-Rich Eudialyte-Group Minerals. Minerals 2022, 12, 1015. https://doi.org/10.3390/min12081015
Zaitsev VA, Chukanov NV, Aksenov SM. Chlorine-Deficient Analog of Taseqite from Odikhincha Massif (Russia): Genesis and Relation with Other Sr-Rich Eudialyte-Group Minerals. Minerals. 2022; 12(8):1015. https://doi.org/10.3390/min12081015
Chicago/Turabian StyleZaitsev, Victor A., Nikita V. Chukanov, and Sergey M. Aksenov. 2022. "Chlorine-Deficient Analog of Taseqite from Odikhincha Massif (Russia): Genesis and Relation with Other Sr-Rich Eudialyte-Group Minerals" Minerals 12, no. 8: 1015. https://doi.org/10.3390/min12081015
APA StyleZaitsev, V. A., Chukanov, N. V., & Aksenov, S. M. (2022). Chlorine-Deficient Analog of Taseqite from Odikhincha Massif (Russia): Genesis and Relation with Other Sr-Rich Eudialyte-Group Minerals. Minerals, 12(8), 1015. https://doi.org/10.3390/min12081015







