1. Introduction
Borehole mining of minerals, in particular uranium, involves the dissolution of a useful component by a moving solvent stream at the location of the ore body, followed by the removal and lifting of the formed compounds to the surface [
1,
2,
3]. The positive aspects of the use of sulfuric acid solutions at enterprises in Kazakhstan are its low cost, widespread use in the national economy, and the possibility of complete dissolution of uranium mineralization [
4,
5]. However, there are negative aspects, such as the high reactivity of the interaction of sulfuric acid with carbonate-, and clay-minerals of ore-bearing rocks. When sulfuric acid interacts with carbonate minerals, gypsum is formed, and clay minerals swell and increase in size; these factors prevent the leaching process [
6].
At mining enterprises, the number of production wells and technological blocks increases annually; this is caused by a gradual decrease in the productivity of the blocks being opened and a decrease in the utilization rate of wells, from 9.0 to 7.6. This is due to the accompanying difficulties of opening and mining peripheral parts of blocks with a ragged ore structure and high heterogeneity of the productive horizon, and low ore filtration coefficients, ranging from 2.0 to 4.0. At the same time, there are no economically justified, effective tools to improve the filtration characteristics of ores and prevent sedimentation for a long period, in difficult mining and geological conditions of well development.
The development of operational blocks in difficult mining and geological conditions is often accompanied by serious complications and an irreversible decrease in the permeability of the downhole zone of the productive reservoir, which dramatically increases the development time and leads to additional costs.
During uranium leaching, the clay content of rocks and ores, as well as the composition of clay cement, largely determines the primary permeability of rocks and reagent consumption. Due to clay minerals, sulfuric acid is neutralized, as a result of which the pH values of solutions do not reach the required parameters (<2.5). This causes an increase in the consumption of chemical reagents, energy, labor, and other operating costs [
7,
8]. Overcoming the solubility threshold and achieving the required uranium content in PS (Productive Solution) requires an increase in the time period of active leaching, which leads to a decrease in the technical and economic indicators of the process [
9].
When designing the borehole development of uranium deposits and feasibility studies, studies of the mineral composition of ores and host rocks of the productive horizon were provided, as well as conducting experimental work on leaching uranium using sulfuric acid solutions with different acidities. Laboratory experiments on uranium leaching were carried out, to determine the effective parameters of the oxidizer application during the intensification of borehole extraction of uranium from ores [
10,
11]. The experiments included the establishment of mineral and granulometric characteristics of samples, and the leaching of uranium from the core in tubes using solutions with high and standard acidity. A solution with low acidity was prepared separately, but with oxygen feeding into the solution, for conversion from iron (II) to iron (III), for the purpose of effecting subsequent oxidation of uranium (IV) to uranium (VI).
3. Discussion or Results
The process of leaching uranium in natural permeability is a complex process involving several stages, one of which is heterogeneous, controlling at the liquid-solid phase boundary, containing a reagent capable of forming highly soluble compounds when interacting with uranium-containing minerals or rocks [
14]. The calculated data obtained, based on the results of laboratory experiments, made it possible to plot: the change in the solution flow rate, as a function of L:S (
Figure 2); the values of the uranium concentration in productive solutions, as a function of L:S (
Figure 3); andthe extraction of uranium, as a function of L:S (
Figure 4); with the flow rate of the chemical reagent depending on L:S (
Figure 5). The flow rate of the solution in the ore during the processes of interaction with uranium minerals, with further dissolution and transfer to the unloading and lifting zone, is one of the important parameters and is determined by the ore filtration coefficient [
15,
16]. The K
f ore filtration coefficient is determined in (m/day) and is calculated using the formula:
where: Δ
V is the volume of filtered solvent;
L is the length of the tube; Δ
t–is the sample measurement time; Δ
H is the hydrostatic pressure drop;
S is the cross-sectional area of the tube.
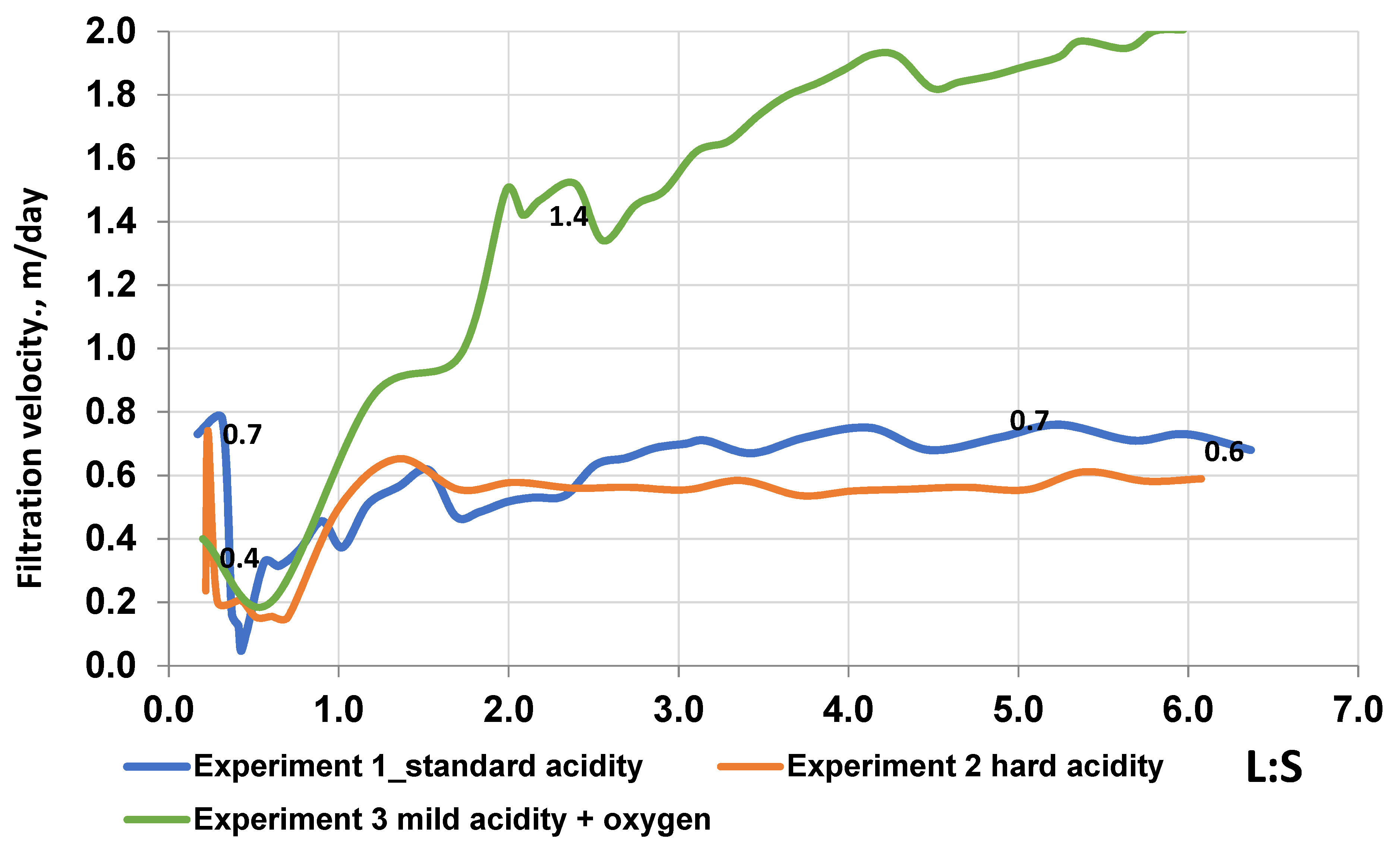
As can be seen from the graph, the changes in the filtration coefficient of Kf depend on the L:S. In experiment 1, when feeding working solutions with a standard mode of acidity, at first the Kf is 0.73 and rises to 0.78, but then it decreases to a minimum value of 0.11 at values of L:S, in the range from 0 to 0.41, with the corresponding acidity parameters of 25–15 g/L. This decrease indicates the formation of colmatation effects, during the interaction of sulfuric acid solutions with ore-containing rocks, that prevent the filtration of solutions. The subsequent gradual increase in the filtration coefficient on the tube from 0.45 to 0.62 at the L:S range, in the range of 0.4–1.4, is due to a decrease in acidity in the working solutions, from 15 to 10 g/L, and a partial reduction in the effect of colmatation. A further decrease in acidity in the solutions did not affect the filtration volume of the solution.
The filtration parameters in experiment 2, with a strict acidity regime of working solutions, approximately correspond to the values of the filtration coefficient in experiment 1, the only dissimilarity being due to a slight difference in acidity (5 g/L). However, the low acidity value in solutions in experiment 3 with the addition of oxygen as an oxidizer positively affected the filtration parameters of the experiment. In experiment 3, with an initial Kf of 0.42 and a further decrease to 0.25 with a change in L:S in the range of 0–0.5, acidity is observed in the working solution of 15 g/L, which indicates a slight formation of the colmatation. A subsequent increase of Kf from 0.25 to 0.9 at values of L:S from 0.5 to 1.5, with an appropriate acidity of 10 g/L, confirms a decrease in the effect of colmatation in the filtrate. The increased values of Kf in experiment 3, compared to experiments 1 and 2, indicate a proportional effect of acidity on the filtration rate and the formation of colmatation effects. The total time spent on conducting experiments, depending on the filtration rate of solutions in core samples, ranged from 320 to 428 h: a total of 320 h were spent on conducting experiment 1; 412 and 428 h were spent on experiments 2 and 3, respectively. A comparative analysis of the results of uranium leaching from core samples using various parameters of solutions allows us to determine the effectiveness of leaching.
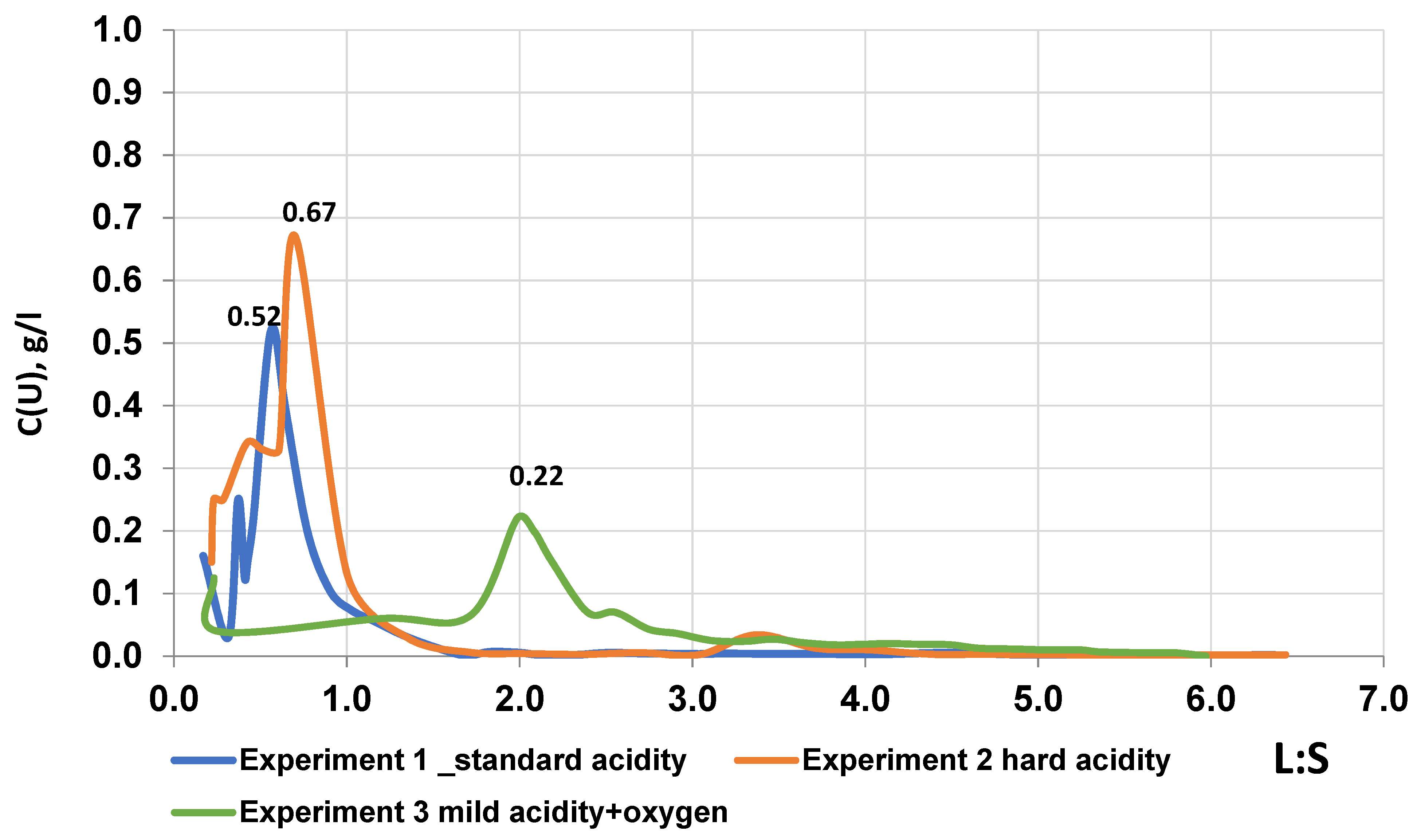
These changes in the concentration of the useful component in the solution depending on the L:S, shows the maximum and minimum concentrations of uranium in the productive solution, with the corresponding volume of the output solution. The concentration of uranium in productive solutions of the
and the ratio of L:S are determined by the formulas:
where
n is the amount of sampling for a given value; Δ
V is the amount of productive solution in the
i-th sample;
—the concentration of uranium in the
i-th specific sample. Counting is performed for all registered samples of productive solution
n.As can be seen from the graph (see
Figure 3), in experiment 1, when leaching using solutions with a standard acidity regime, the maximum uranium content of 520 mg/L is achieved at L:S 0.561, after overcoming the threshold of 250 mg/L and reducing to 125 mg/L. This indicates the gradual overcoming of the solubility threshold and the achievement of active leaching with the required volume of solution and acid. However, there is a decrease in the concentration of uranium in the productive solution, from 520 mg/L to a low value of 30 mg/L, at a range of values of L:S from 0.7 to 1.5. This may be due to a decrease in the acidity of working solutions in 10 g/L. In experiment 2, in the process of leaching under a strict acidity regime, the uranium content in the productive solutions reaches a peak of 670 mg/L within the range of L:S 0.7, with a further sharp decrease in the uranium content in the productive solutions from 670 to 130 mg/L, with an increase in L:S to 1.0, which indicates a decrease in acidity from 25 to 15 g/L. In experiment 3, when leaching uranium with a mild acidity regime of solutions and the addition of oxygen, the uranium content gradually increases to 220 mg/L with a corresponding increase in L:S from 0 to 2.0. This also confirms the gradual overcoming of the solubility threshold by weak sulfuric acid solutions, followed by oxidation and conversion of tetravalent uranium into a soluble form, while creating favorable conditions for stable active leaching and oxidation. The uranium content in the solutions in all experiments showed similar maximum peaks, approximately corresponding to the initial uranium content, depending on the acidity of the leaching solutions. This indicates the neutralization of sulfuric acid during interaction with ore-containing rocks and the influence of an oxidizer during the transfer of uranium mineralization into solution (experiment 3).
The effectiveness of the parameter of the proposed solution, with reduced acidity and with the addition of an oxidizer, was determined on the basis of a comparative analysis with the parameters of uranium extraction under standard and rigid modes of acidity of solutions. For this purpose, experimental data were collected and processed, calculations were made and schedules for uranium extraction were constructed. Uranium extraction depends on the uranium content in the productive solution and the volume of the most productive uranium; these data are the most informative and fundamental in assessing the solubility and stability of uranium minerals. Uranium extraction is calculated as the ratio of the mass of uranium in the output solutions to its initial calculated mass in the sample:
where
—the concentration of uranium in the initial sample;
—is the concentration of uranium in the productive solution of the
i-th sample;
Mp is the mass of ore in the initial sample; Δ
Vi is the volume of the productive solution in the
i-th sample.
The graph in
Figure 4 shows that the maximum extraction values in Experiment 1 reached 49% of the mass of uranium contained in the sample; this indicates insufficient solvent capacity of working solutions with standard acidity and low filtration values. A sharp increase in extraction occurs in the range of L:S from 0.5 to 0.9 to 38.6%, followed by a slowdown in extraction at L:S > 1; the acidity of working solutions in this range was maximum: 25–15 g/L. The extraction data correlate with the graph of the uranium content in the solution, where the peak of the maximum uranium content in the PS falls in the range of L:S from 0.5 to 0.8. A further slowdown in the extraction of the useful component is due to a decrease in the uranium content in the solution, due to a decrease in the acidity of working solutions from 15 to 10 g/L. In experiment 2, the increase in uranium extraction occurs at L:S parameters from 0 to 1.0 with the achievement of 56% extraction, followed by a slowdown of 63%, which indicates intensive extraction of uranium in solutions with a strict acidity regime. In the range of L:S from 0 to 1.0, the acidity of the solutions supplied to tube 2 was maximum and amounted to: 25–20–15 g/L. Intensive extraction is due to the high uranium content in the solution: 320–670 mg/L and the corresponding filtration coefficient of the solution is 0.34–0.44 in the range of L:S from 0 to 0.9. The extraction of the useful component in experiment 3, when feeding working solutions with a mild acidity regime with the addition of oxygen, reached a maximum value of 68% with a change in L:S to 3.0. However, the active extraction of uranium in Experiment 3 also occurred in the range of L:S from 1.7 to 3.0, from 27.6 to 60.3%, respectively. The intensive extraction of uranium in experiment 3 in the range L:S 1.7–3.0 is due to the achievement of the solubility threshold and the creation of conditions for active leaching, with the oxidation of iron (II) and the interaction of iron (III) for the oxidation of uranium (IV) [
17,
18]:
To determine the economic efficiency of using an oxidizer in the intensification of uranium leaching, and a comparative analysis of solvent consumption, graphs were constructed based on the experiments performed. The consumption of sulfuric acid as a solvent per kilogram of extracted uranium
PK is calculated as the ratio of the total mass of the reagent consumed during the experiment to the calculated mass of extracted uranium:
where
is the initial concentration of the reagent in the leaching solution (determined when using a mother liquor with the initial acidity);
—the actual concentration of the reagent in the
i-th sample of the leaching solution (actual);
the actual concentration of uranium in the productive solution of the
i-th sample;
Mp the total mass of ore used in the experiment; Δ
Vi the total volume of leaching solution in the
i-th sample.
Reagent costs per unit of processed ore mass (calculated to determine the acid capacity of ore) are determined by the formula:
where
is the initial concentration of the solvent reagent in the leaching solution (determined when using sorption curves with residual acidity);
—is the concentration of the solvent reagent in the
i-th sample of the leaching solution (actual);
Mp is the mass of ore used in the experiment; Δ
Vi is the volume of the leaching solution in the
i-th sample.
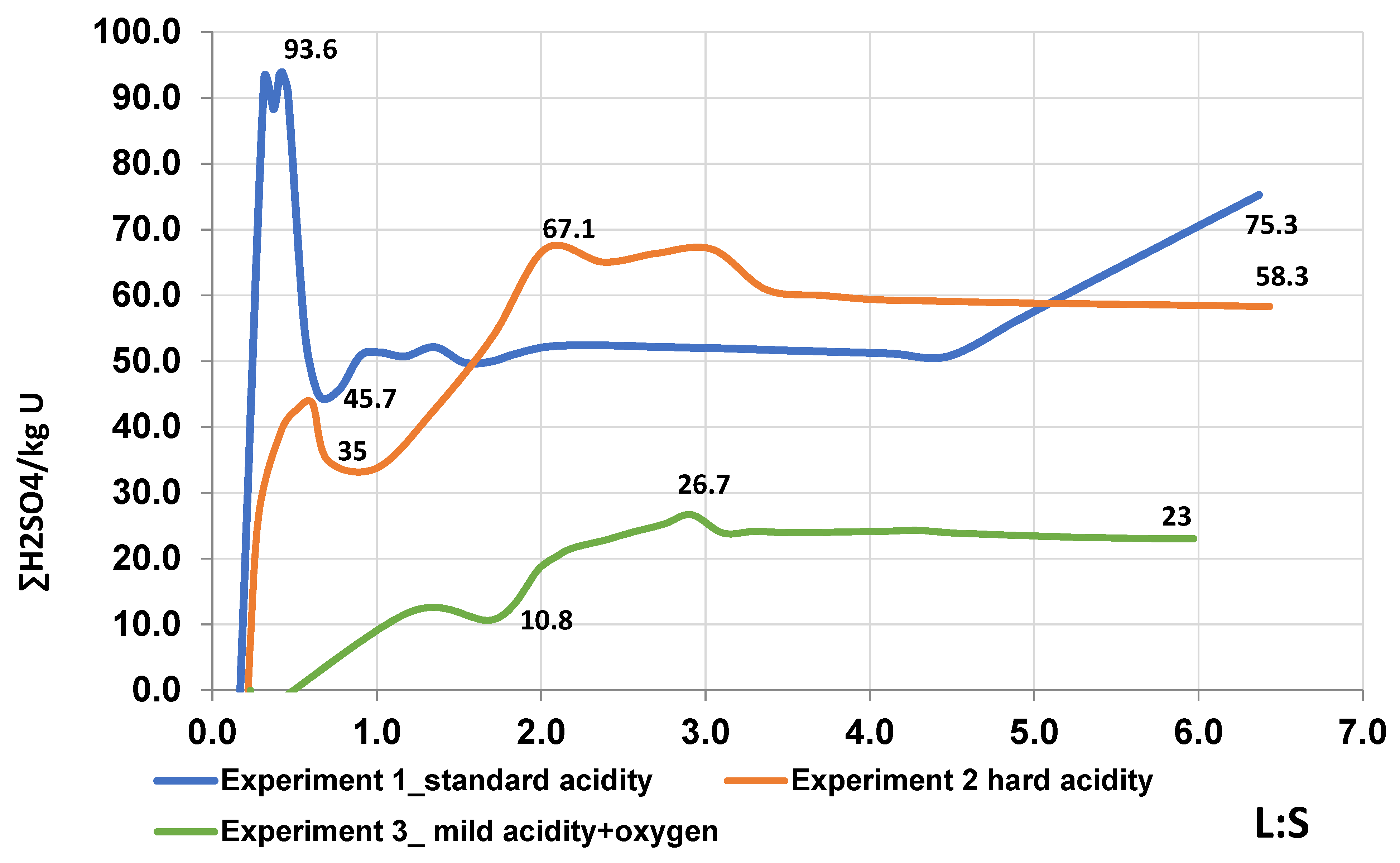
The graph in
Figure 5 shows that the consumption of sulfuric acid in Experiment 1 reaches maximum values of 90–93 kg/kg U in the L:S range: 0.2–0.7, after which it sharply decreases to values of 48–50 kg/kg U. A sharp increase in the consumption of the solvent reagent is associated with the acidity in the leaching solution and low parameters of uranium extraction in the range of L:S from 0 to 0.8. After increasing the extraction of uranium from 25.8 to 47% in the L:S range from 0.647 to 1.689, there is a noticeable decrease in the consumption of the solvent reagent. The maximum values of the reagent consumption in experiment 2, when feeding working solutions with a hard acidity regime, reach 67 kg/kg U at L:S 2.0. A gradual increase in the specific consumption in experiment 2, with a hard acidity regime of working solutions, is due to a slowdown in the extraction of uranium in the corresponding range L:S. The maximum values of the specific consumption of sulfuric acid in experiment 3 are not able to reach the corresponding indicators in experiment 2 and amount to 26.7 kg/kg U with a change in L:S from 2.0 to 3.0, after which they decrease to the minimum values of 23 kg/kg U. Reduction of reagent consumption in the L:S range >3.0 is due to intensive uranium extraction in this interval. In a comparative analysis of the solvent reagent consumption, the lowest values are observed in Experiment 3, with a mild acidity regime of working solutions and with the addition of oxygen as an oxidizer. The decrease in reagent consumption is caused by intensive uranium extraction, due to the oxidizing effect of iron (III) on uranium (IV) in its conversion to uranium (VI), during its further dissolution. In the hard mode of acidity (experiment 2), the specific acid consumption is higher than in the soft mode of acidity, with the addition of an oxidizer, but lower than in the standard mode of acidity (experiment 1), due to the intensive extraction of uranium from the tube.
4. Conclusions
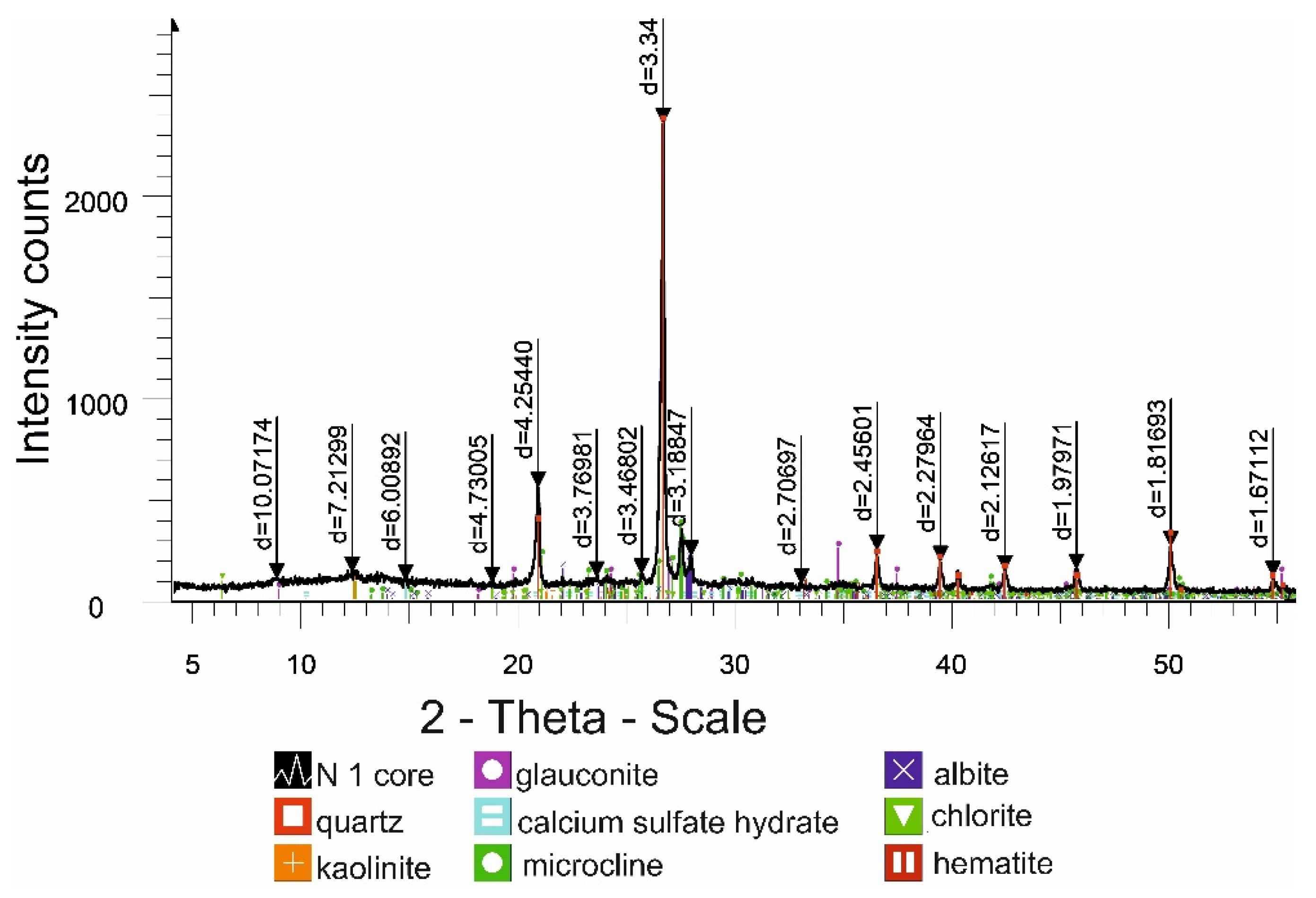
X-ray phase studies of ore-bearing rocks indicate their diverse mineral composition, and the presence of calcium sulfate will contribute to chemical colmatation in wells. The predominance of a fine fraction of fine-grained sands (57% of the total mass) prevents the filtration of solutions in the productive horizon. The data indicate a complex structure of the productive horizon with low filtration parameters. As practice shows, in such conditions, the leaching processes are hindered by the low uranium content in the PS and low parameters of the turnover of solutions, which requires continuous intensification of the process by hydrodynamic methods.
The data indicate the direct influence of the concentration of sulfuric acid on the dissolution of uranium minerals and the content of uranium in productive solutions. However, the influence of filtration characteristics on the overall indicators of the process (extraction, specific acid consumption) is no less important when solving issues of production intensification. The addition of oxygen as an oxidizer to sulfuric acid solutions, with reduced acidity, positively affects the intensification of uranium leaching. Reducing the acidity of working solutions significantly increases the filtration characteristics of samples, due to the oxidizing ability of iron (III) to uranium (IV); the uranium content in the solutions reach the design values of 300 mg/L. A decrease in the consumption of the solvent reagent (37 kg H2SO4/kg U) indicates the economic feasibility of using an oxidizer at the first stage of well production, in areas with a predominance of ores containing uranium (IV). In addition, the low acidity of the leaching solution will not create sedimentation, will lead to intensive development of blocks and reduce operating costs, electricity costs and labor costs.
These studies allowed us to establish that the uranium content in the productive solution directly depends on the concentration of acid in the leaching solution. However, an increase in the concentration of the solvent reagent does not always lead to an increase in its consumption. The analysis of the values of uranium extraction against time allows us to determine the optimal concentration and consumption of reagents during borehole production. The optimal concentration is one in which an excess leads to a sharp consumption of acid and a slight increase in the intensity of uranium extraction. The value of the optimal acid concentration depends on the properties of the specific ore minerals in the test samples.
The use of air at the enterprises’ uranium mining, in areas with low filtration characteristics of ores, has become possible thanks to special devices, the design of which allows for dosing of air into the LS pipeline. This technology does not need capital investments and allows for reductions in the consumption of chemical reagents, as well as increases in the extraction of uranium, by improving the filtration characteristics of ores and host rocks. Scientists and specialists at Satbayev University have already developed this area and have achieved positive results [
19].