Sulfide and Fluoride Mineralization of the NNE Region of Achemmach (Central Morocco): Paragenetic Sequences and Pyrrhotite-Sphalerite Geothermometry Constraints
Abstract
:1. Introduction

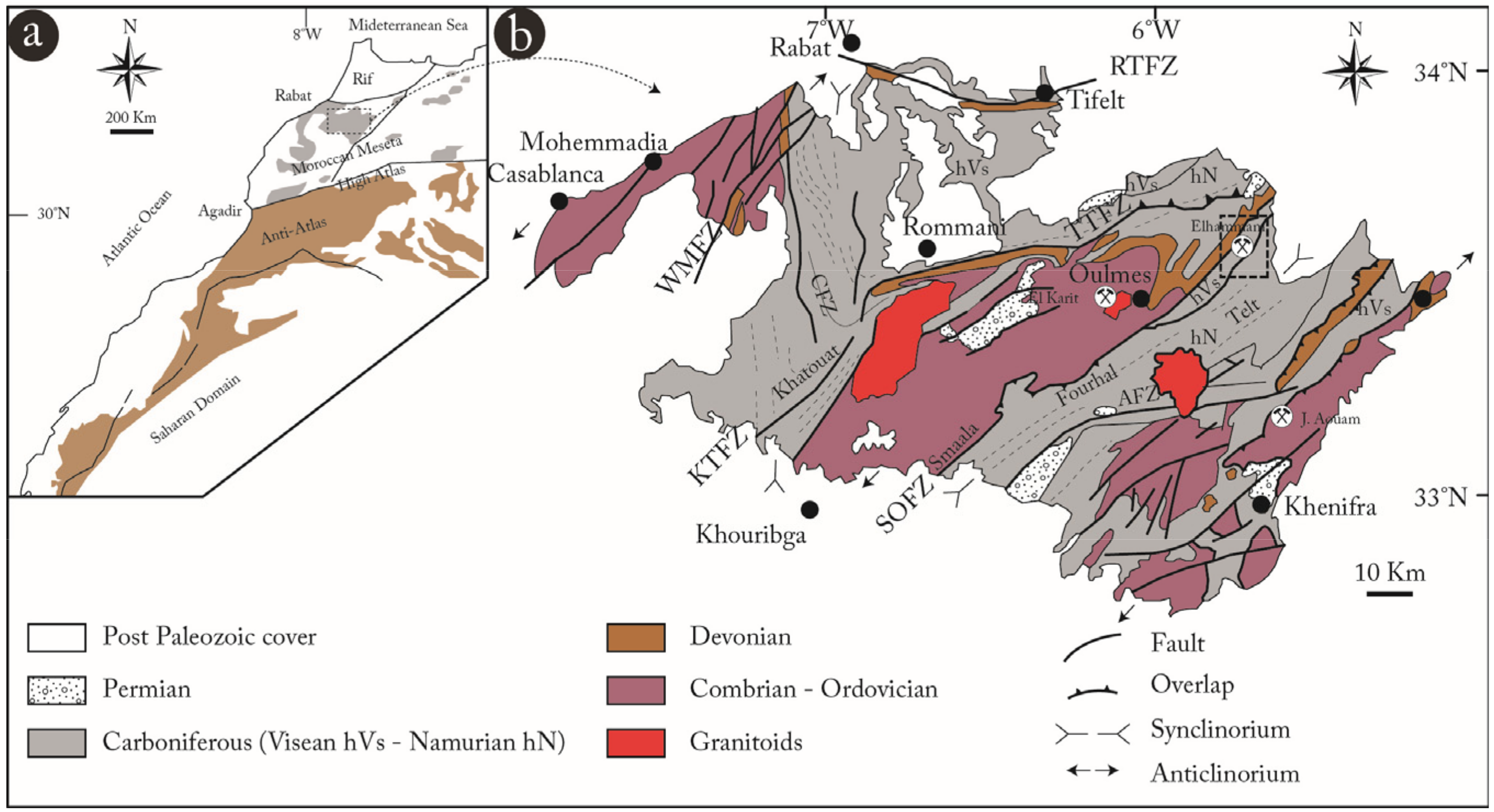
2. Geological Setting
3. Methodology
4. Results
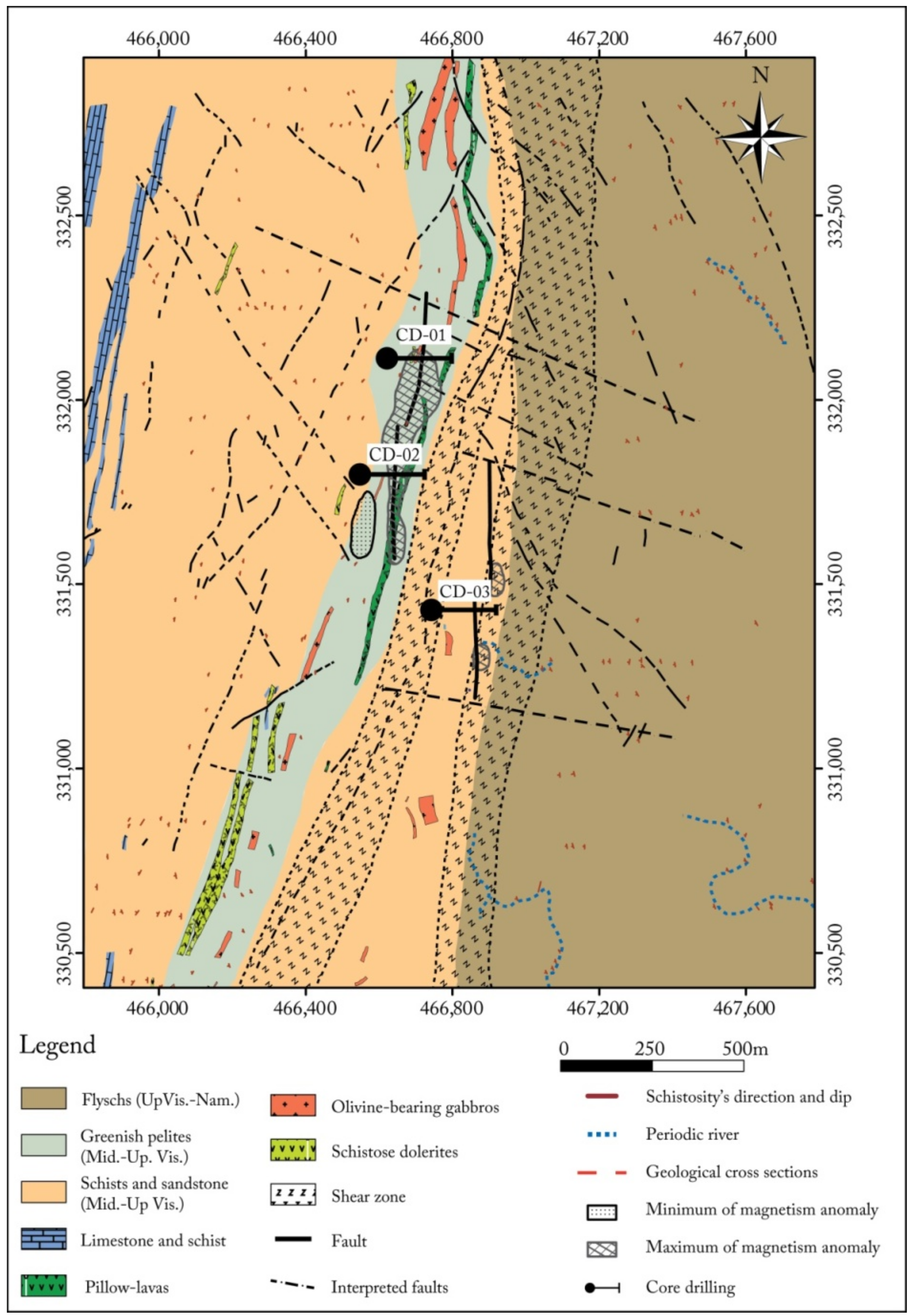
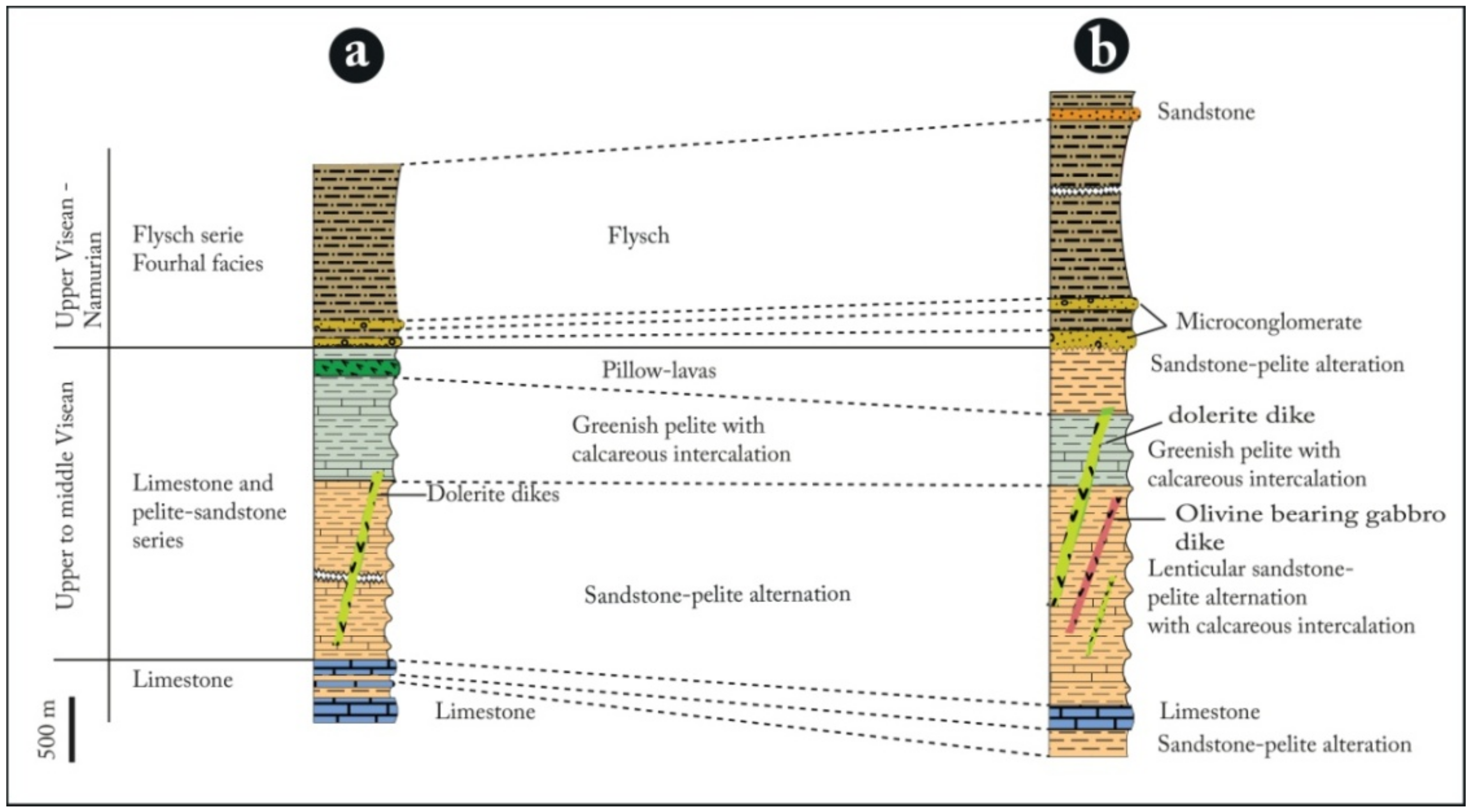
4.1. Local Geology Field Work
- (i)
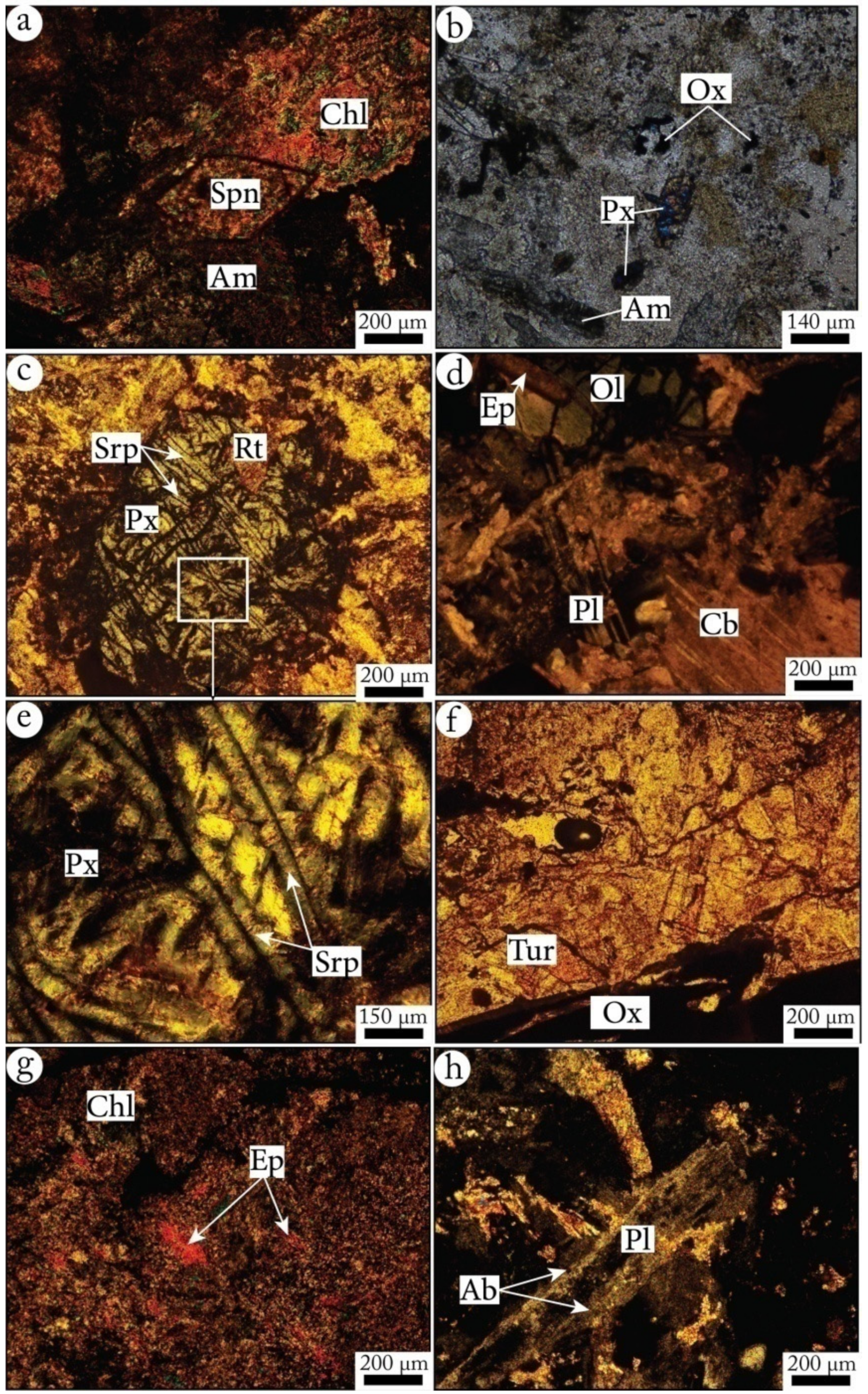
- Decimeter to meter-thick greenish pillow-lavas, with sharp borders and radius fractures underlined by fine greenish pelitic sedimentary intercalations, indicating recurrent volcanic activity in short episodes (Figure 6). Plagioclases and pyroxenes (augite) microlites, and rare phenocrystals are recognizable in a glassy matrix devoid of recognizable olivine (Figure 7);
- (ii)
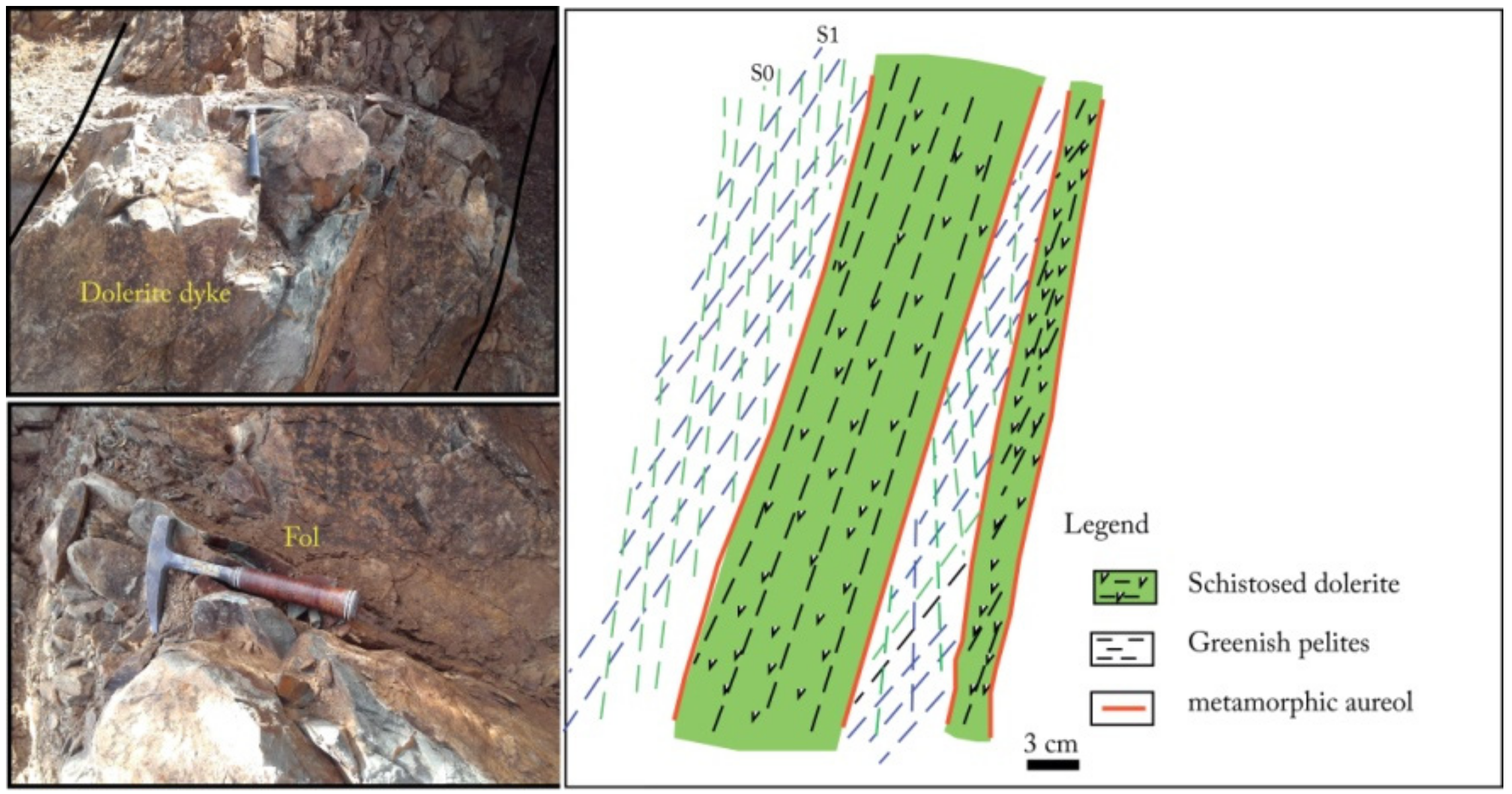
- Deformed, metamorphosed and altered dolerite dikes crossingthe Middle to Upper Visean shale-sandstone formations (Figure 8) with an overall NE-SW direction with a NW dip-direction. They have a microlithicmesostasis texture and are composed of sericitized plagioclases in close association with amphibolitized pyroxenes, tourmaline with a variable degree of chlorite substitution, rutile, and opaque minerals (Figure 9);
- (iii)
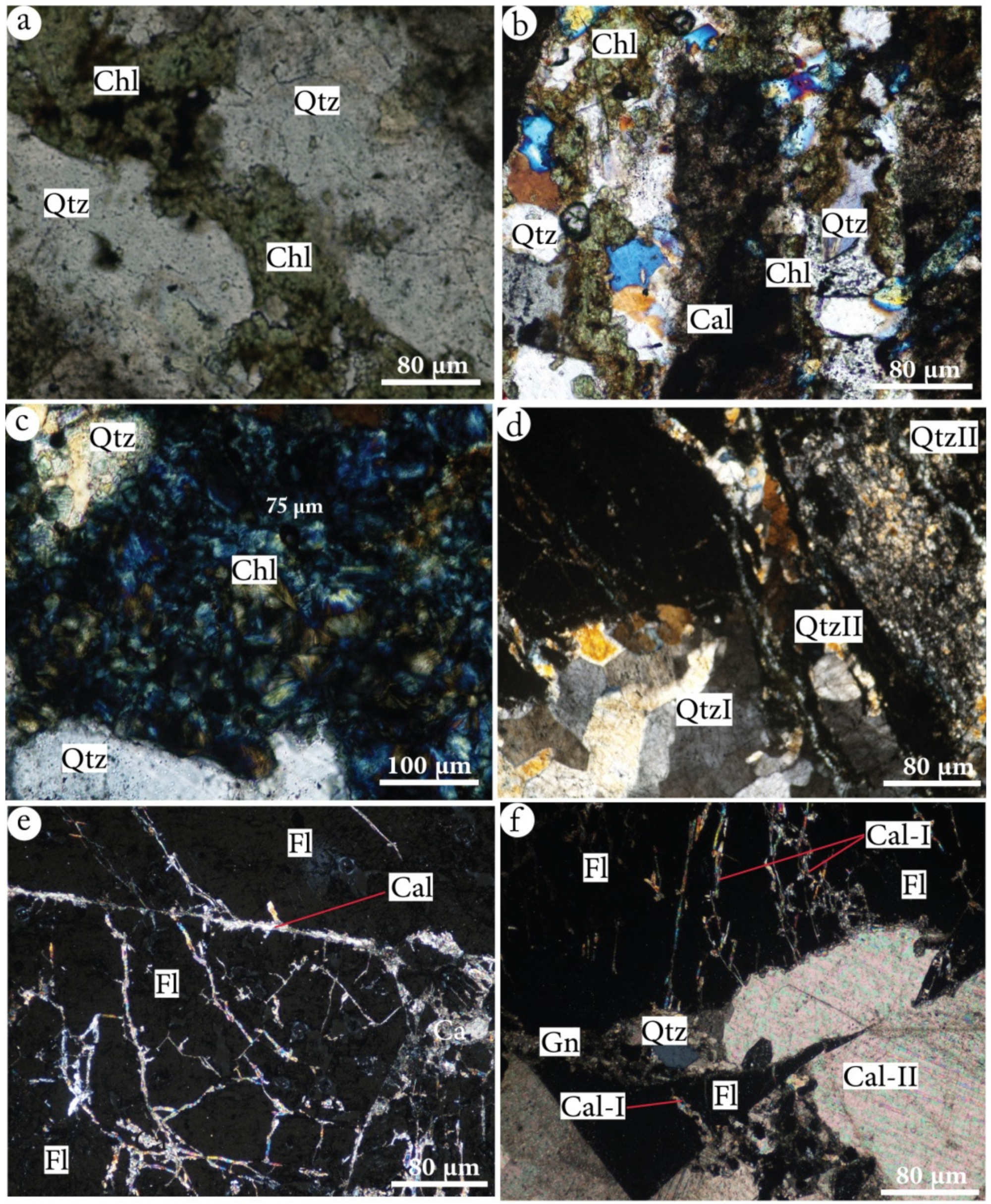
- Olivine-bearing gabbros, outcropped in variable dimensions (few meters to 20 m). These plutonic bodies have a granular texture and are mainly made of plagioclase, pyroxene, olivine, sphene, rutile, apatite and opaque minerals. All their constituting minerals were affected by different degrees of replacement by secondary minerals; the plagioclase was sericitized and albitized, while pyroxene was amphibolitized and epidotized, and olivine was serpentinized and chloritized (Figure 10) [29].
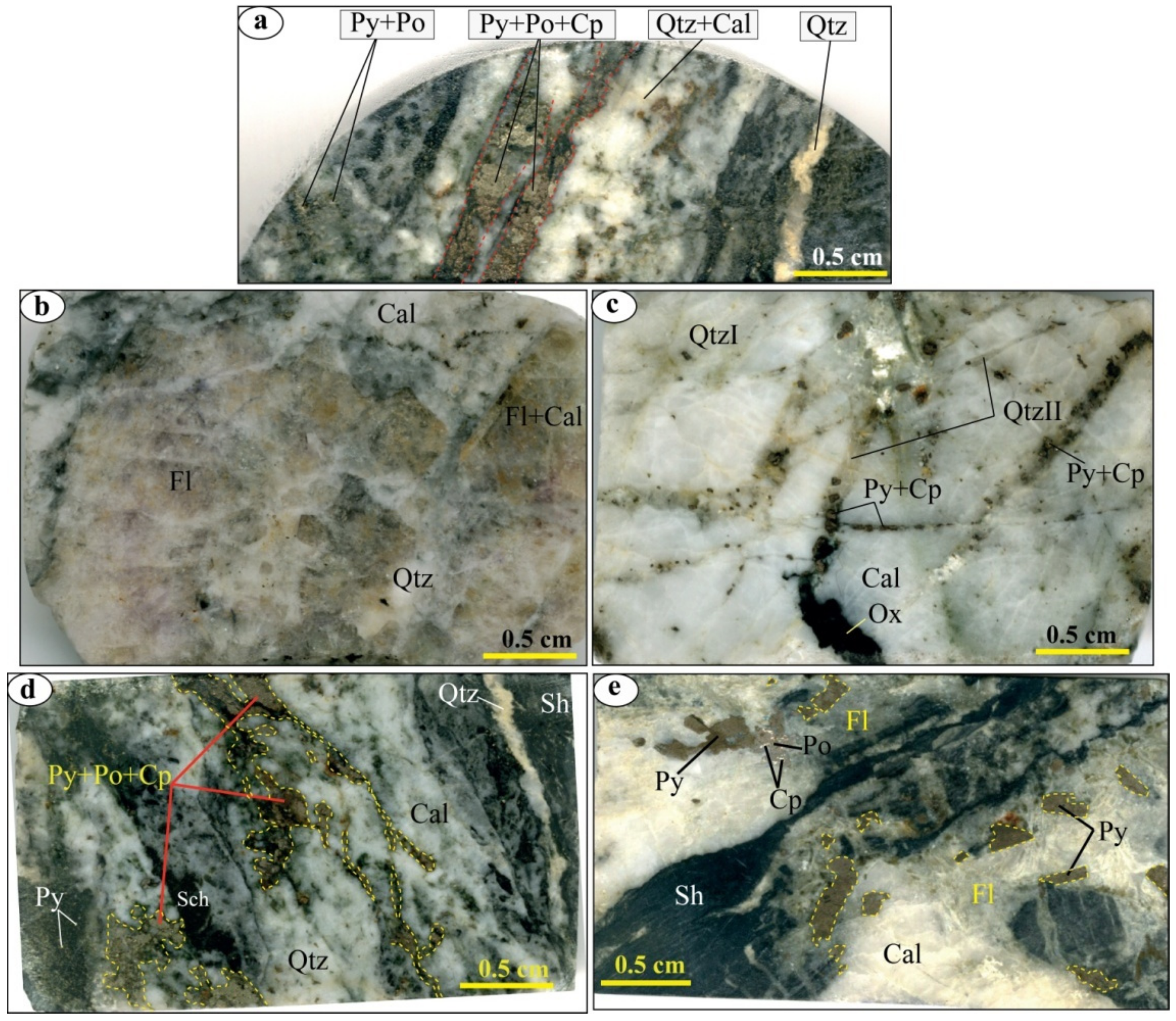
4.2. Mineralization
4.2.1. Morphology
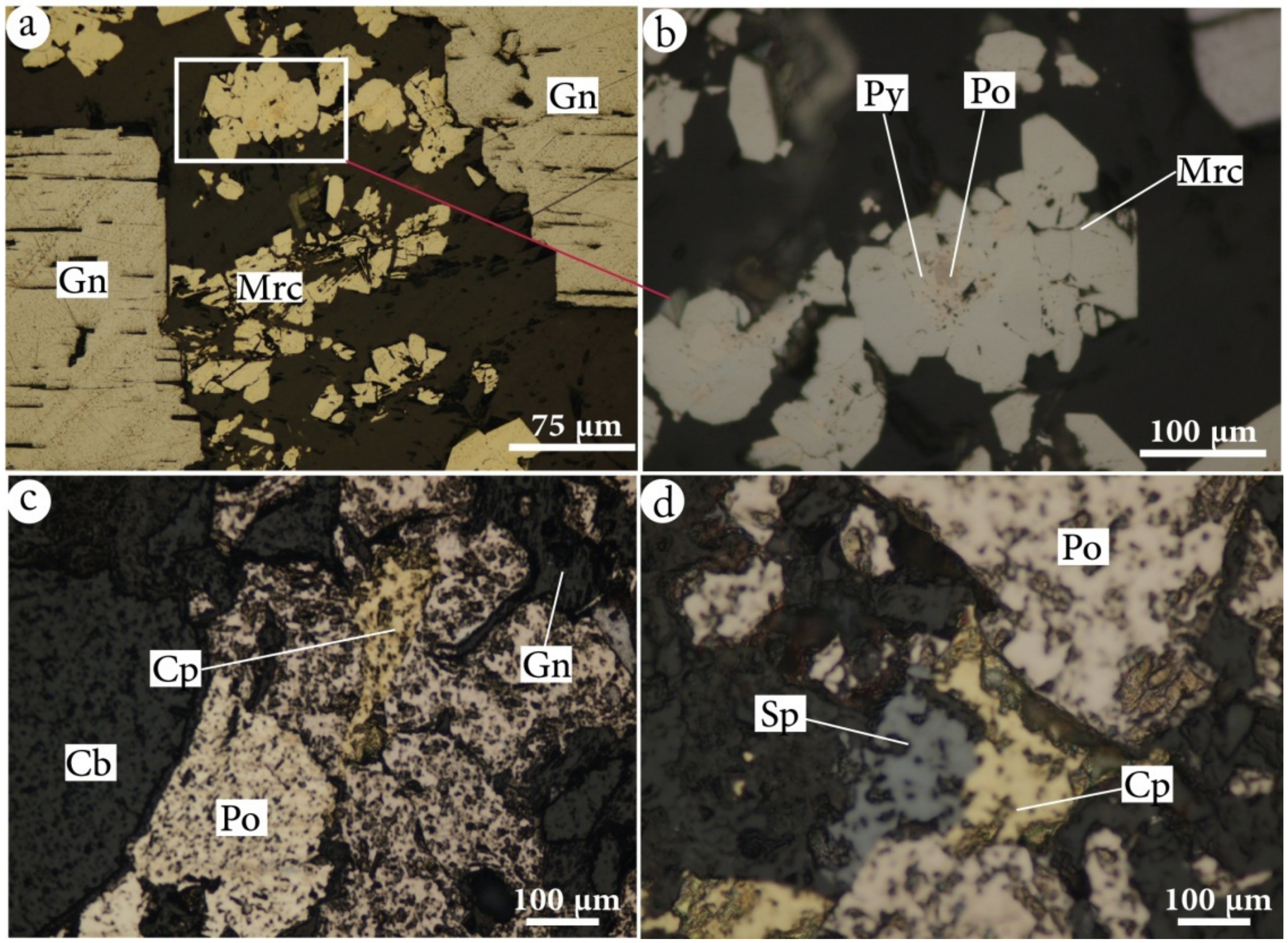
4.2.2. Mineralogy
4.2.3. Sulfide Chemistry
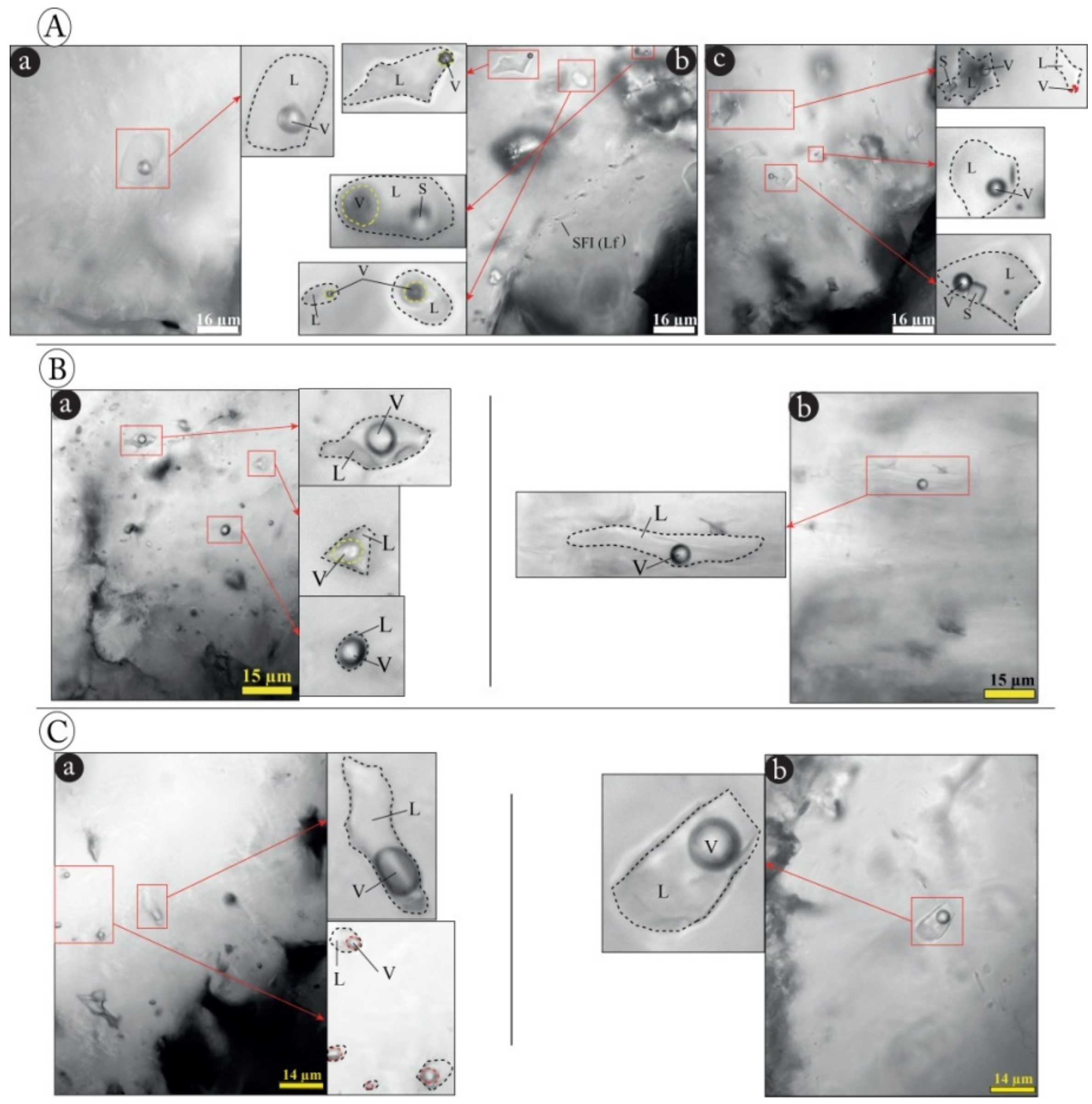
4.3. Fluid Inclusions Results
- (1)
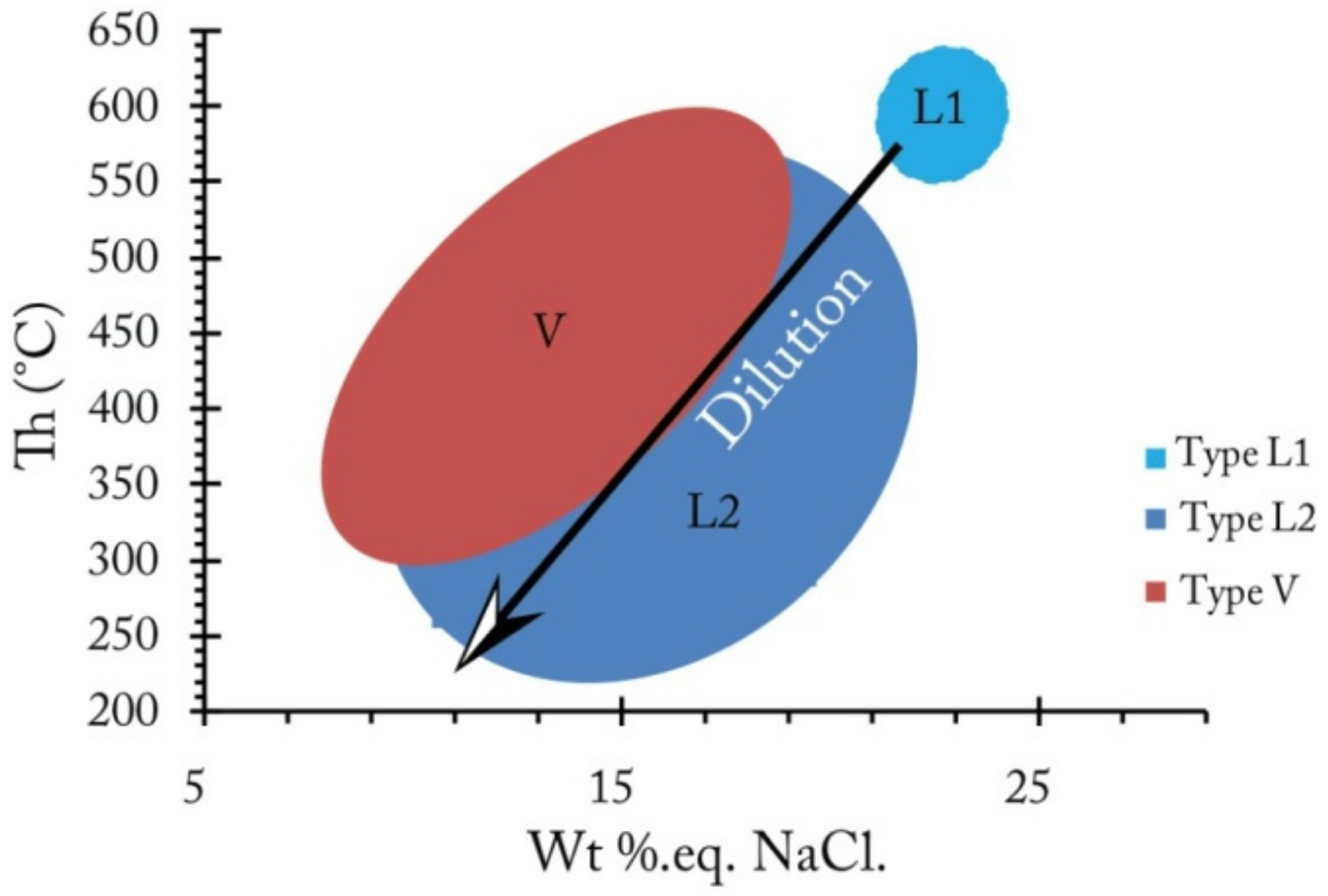
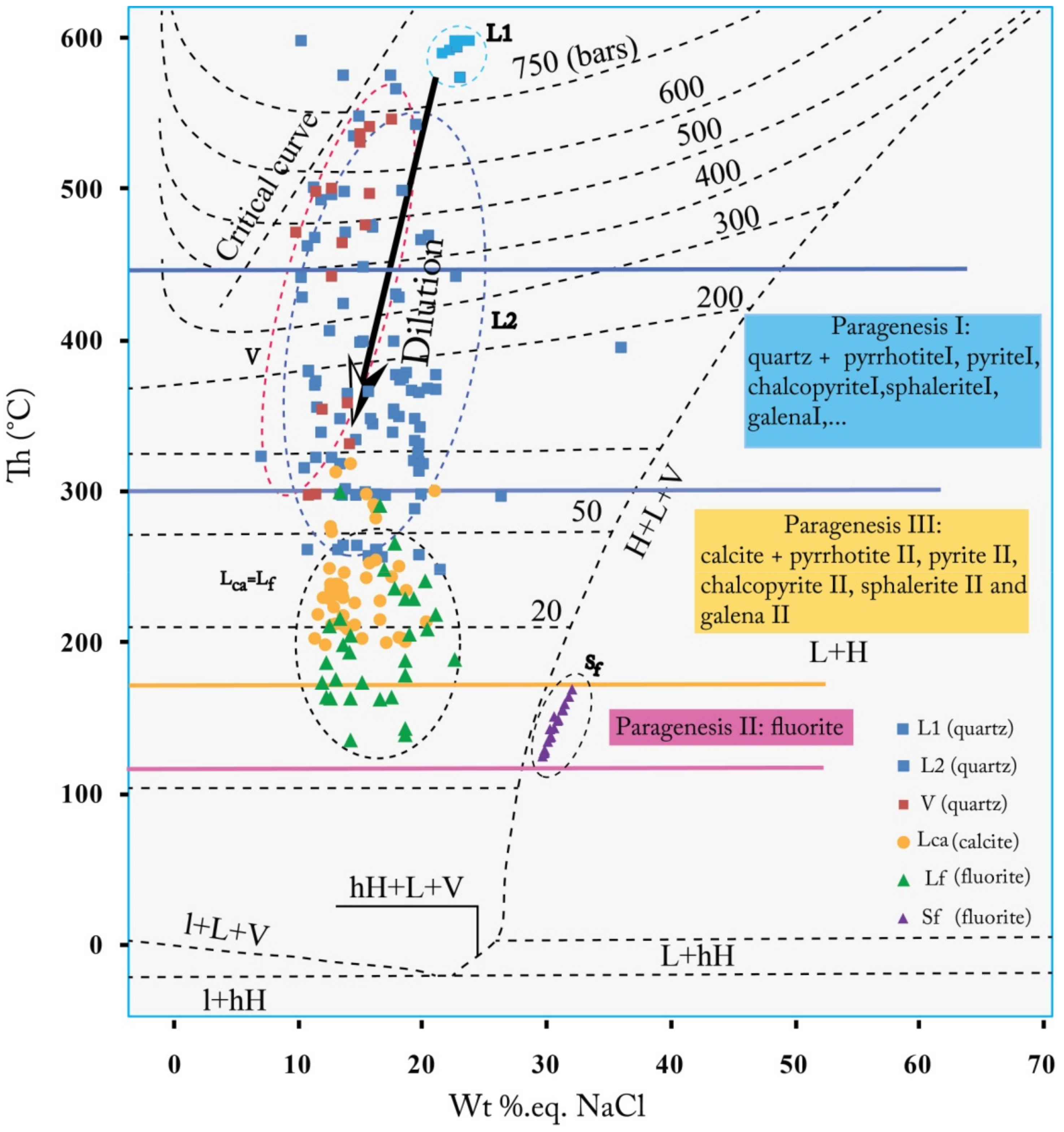
- In quartz, two types of fluid inclusions (L, and V-type) were identified (Figure 20B). They initially corresponded to an early liquid aqueous fluid of high temperature (HT) (Th = 550–600 °C and Wt% 20–25eq.NaCl, type L1), evolving, by mixing with a less salty and colder external fluid, towards two aqueous fluids (L2 and V). These fluids have very similar thermometric characteristics with more moderate homogenization temperatures (Th= 300–450 °C) and low-salinities: 10–20Wt % eq. NaCl (Figure 21B (b)). The eutectic temperatures (Te) measured, show values between −75 and −70 °C (Figure 20B (a)). These temperatures, generally lower than the eutectic temperature of the simple binary system H2O-NaCl (Te =−21.1 °C), suggest the presence of salts other than NaCl in the fluid. The values obtained are close to the eutectic of the H2O-NaCl-Li system (Te = −75 °C). This characteristic supports that the different types of fluids distinguished in the quartz derive from the evolution of the same aqueous fluid of HT and high salinity (L1). This evolution consists of the dilution of the magmatic fluid (type L1), during its ascent to the surface, by a meteoric fluid of low temperature (LT) and low salinity, giving rise to the aqueous fluids L2 and V (Figure 22).
- (2)
- In fluorite, the following two fluids are distinguished: (i) type Sf corresponding to a hypersaline fluid; characterized by a homogenization temperature between 120 and 160 °C and a salinity ranging from 30 to 35 Wt% eq. NaCl, and (ii) type Lf consisting of a moderately salty aqueous fluid with a homogenization temperature between 150 and 200 °C, and a salinity between 10 and 20 Wt % eq. NaCl, (Figure 21A (a,b)), and (iii). The Th vs. Tfs diagram (Figure 21A (c)), shows that the total homogenization of all the inclusions of this type occurred in the liquid phase due to the disappearance of the vapor after that of the solid (S). This reflects the homogeneity of the initial fluid trapped and the son character of salt crystals.
- (3)
- In calcite, only an aqueous fluid is recognized (type LCa). It is characterized by a relatively low temperature (Th between 200 and 250 °C), a rather moderate salinity (between 10 and 20 Wt % eq. NaCl) (Figure 21C (b,c)) and eutectic temperatures ranging from −60 °C to −95 °C with two modes (−65 to −70 °C and −75 to −80 °C) (Figure 21C (a)).
5. Interpretation and Discussion
6. Conclusions
- The following two Paleozoic groups compose the NNE-A region: (1) ameta-sedimentary group comprising two units; the first one is made of limestone and schisto-sandstone of middle Visean, and the second one is made of flyschoid of the Upper Visean-Namurian, and (2) a magmatic group, described here for the first time, materialized by volcanic (pillow-lavas), hypo-volcanic (dolerites) and plutonic (olivine-bearing gabbros) rocks;
- Pillow basaltic lavas and olivine-bearing gabbros have alkaline affinity, while dolerites have transitional alkaline affinity (alkaline-tholeiitic). They suggest the setting up of this mafic magmatism in intracontinental rifting, beginning with oceanization. These basic rocks have undergone an oceanic hydrothermal alteration, causing their serpentinization, spilitization, chloritization and carbonation;
- Vein or disseminated Fe-Cu-Zn-Pb sulfide and fluorite mineralization, hosted either in Visean sedimentary formations or in the magmatic ones, were established according to the following three paragenetic stages: (i) paragenesis I, with quartz gangue, composed of pyrrhotite I, pyrite I, chalcopyrite I, sphalerite I, galena I, glaucodot, ullmannite, magnetite and rutile, (ii) paragenesis II, mainly formed by fluorite and (iii) paragenesis III, with pyrrhotite II, pyrite II, chalcopyrite II, sphalerite II, galena II and hematite with a carbonate gangue;
- Equilibrium sphalerite-pyrrhotite reveals that the earlier sphalerite was formed at about 420 °C and the late sphalerite at 320 °C. These temperatures were confirmed by the fluid inclusions study;
- The deposition of sulfide and fluorite mineralization is characterized by the circulation of the following three fluids: (i) an aqueous and salty fluid of HT (L1: Th = 550–600 °C and XNaCl = 20–25 Wt% eq. NaCl), resulting from the circulation of magmatic fluids in depth during the rifting stage. The mixture of this fluid, during its rise to the surface, with marine fluids, causes its cooling and evolution into two aqueous fluids; liquid (L2) and vapor (V) at Th = 300–450 °C, and XNaCl = 10–20 Wt % eq. NaCl. This mixture represents the beginning of the deposition of the primary sulfide paragenesis with a quartz gangue, (ii) a hypersaline fluid of a low temperature (brine Sf: Th = 120 to 160 °C and XNaCl = 30 to 35 Wt% eq. NaCl), which would be associated with the deposition of fluorite and (iii) a late aqueous fluid (LCa: XNaCl = 10 to 20 Wt% eq. NaCl, Th = 200 to 250 °C) which would occurs at the deposition of late sulfide ore paragenesis, associated with carbonate gangue, founded as traces in quartz and fluorite (Figure 24);
- The early mineralizing event is linked to the placement of mafic rocks, during the development of continental rifting due to the Devono–Dinantian extension, while those at the origin of fluorite and late sulfides are associated either with the tardi-Hercynian felsic magmatism of El Hammam, or with the effect of the thermal flux of anorogenic Triaso-Jurassic volcanism.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michard, A. Éléments de Géologie Marocaine; Éditions du Service Géologique du Maroc; Service Géologique du Maroc: Rabat, Morocco, 1976. [Google Scholar]
- Fadli, D. Evolution Sédimentaire et Structurale Des Massifs Des Mdakra et Du Khatouat; Deux Segments Hercyniens de La Meseta Marocaine Nord-Occidentale. State Thesis, University of Rabat, Rabat, Morocco, 1990; p. 316. [Google Scholar]
- Kosakevitch, A. Etude Minéralogique Des Minerais d’antimoine Au Maroc; Service Géologique du Maroc: Rabat, Moroco, 1972; p. 145. [Google Scholar]
- Charty, G. Les Gîtes d’antimoine Du Maroc Central Métallotectes et Essai de Classification Typologique. Mines Géol. Énergie 1980, 48, 141–144. [Google Scholar]
- Mezougane, H. Magmatisme et Minéralisations Sulfurées du Secteur NNE d’Achemmach (Maroc central): Pétro-Géochimie, Métallogénie et Caractérisation des Paléocirculations Fluides. Ph.D. Thesis, Faculty of Sciences, Moulay Ismail University, Meknès, Morocco, 2021. [Google Scholar]
- Izart, A.; Tahiri, A.; El Boursoumi, A.; Chèvremont, P. Carte Géologique Du Maroc Au 1/50 000, Feuille de Bouqachmir. Notes Mém. Serv. Géol. Maroc 2001, 60, 1. [Google Scholar]
- Tarrieu, L. Nouvelles Données Minéralogiques, Géochimiques et Géochronologiques Sur Le Gisement Polymétallique de Tighza (Maroc-Central): Contribution à La Métallogénie Des Gisements de Métaux de Base Filoniens En Contexte Post-Collisionnel. Ph.D. Thesis, Université Grenoble Alpes, Savoie, France, 2014. [Google Scholar]
- Tahiri, A.; Montero, P.; El Hadi, H.; Martínez Poyatos, D.; Azor, A.; Bea, F.; Simancas, J.F.; González Lodeiro, F. Geochronological Data on the Rabat–Tiflet Granitoids: Their Bearing on the Tectonics of the Moroccan Variscides. J. Afr. Earth Sci. 2010, 57, 1–13. [Google Scholar] [CrossRef]
- Mahjoubi, E.M.; Chauvet, A.; Badra, L.; Sizaret, S.; Barbanson, L.; El Maz, A.; Chen, Y.; Amann, M. Structural, Mineralogical, and Paleoflow Velocity Constraints on Hercynian Tin Mineralization: The Achmmach Prospect of the Moroccan Central Massif. Miner. Depos. 2016, 51, 431–451. [Google Scholar] [CrossRef]
- Hamoumi, N. La Plateforme Ordovicienne Du Maroc: Dynamique Des Ensembles Sedimentaires. Ph.D. Thesis, Université Louis Pasteur, Strasbourg, Frence, 1988. [Google Scholar]
- Baamar, B.; Badra, L.; Gaouzi, A.; Benyounes, M.; Souiah, M. Contrôle Structural Des Minéralisations Filoniennes à Fluorine Du District d’El Hammam (Maroc Central) et Implication Pour l’exploration Régionale. Bull. L’institut Sci. Rabat 2017, 39, 1–24. [Google Scholar]
- Mahjoubi, E. Minéralisation Stannifère d’Achmmach (Massif Central Hercynien Marocain): Relations Déformation, Magmatisme et Gîtologie. Ph.D. Thesis, Faculty of Sciences, Moulay Ismail University, Meknès, Morocco, 2017. [Google Scholar]
- Rossi, M.; Gasquet, D.; Cheilletz, A.; Tarrieu, L.; Bounajma, H.; Mantoy, T.; Reisberg, L.; Deloule, E.; Boulvais, P.; Burnard, P. Isotopic and Geochemical Constraints on Lead and Fluid Sources of the PbZnAg Mineralization in the Polymetallic Tighza-Jbel Aouam District (Central Morocco), and Relationships with the Geodynamic Context. J. Afr. Earth Sci. 2017, 127, 194–210. [Google Scholar] [CrossRef] [Green Version]
- Baamar, B. Contribution à l’étude Structurale et Gîtologique Des Minéralisations Fluorifères Du District Polymétallique d’El Hammam (Maroc Central). Ph.D. Thesis, Faculty of Sciences, Moulay Ismail University, Meknès, Morocco, 2018. [Google Scholar]
- Termier, H. Etudes Géologiques Sur Le Maroc Central et Le Moyen-Atlas Septentrional. Ph.D. Thesis, Cadi Ayyad University, Marrakesh, Morocco, 1936. [Google Scholar]
- Piqué, A.; Michard, A. Les zones structurales du Maroc hercynien. Sci. Géol. Bull. Mém. 1981, 34, 135–146. [Google Scholar] [CrossRef]
- Lazraq, N. Contribution à l’étude Micropaléontologique (Principalement Conodontes) Du Dévonien de La Région d’Oulmès (Maroc Central). Ph.D. Thesis, Sorbonne University Pierre and Marie Curie Campus, Paris, Frence, 1983. [Google Scholar]
- Faik, F. Le Paleozoique de La Region de Mrirt (Est Du Maroc Central): Évolution Stratigraphique et Structurale. Ph.D. Thesis, University of Toulouse, Toulouse, Frence, 1988. [Google Scholar]
- Bouabdelli, M. Tectonique et Sédimentation Dans Les Bassins Orogéniques: Le Sillon Viséen d’Azrou-Khénifra (Est Du Massif Hercynien Central). Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 1989; p. 262. [Google Scholar]
- Habibi, M. Le Paleozoique de La Region d’ain Leuh-Souq al Had (NE Du Maroc Central): Recherches Stratigraphiques et Structurales. Master’s Thesis, Université Toulouse 3, Toulouse, France, 1989; p. 186. [Google Scholar]
- El hassani, A. Northern Border of the Moroccan Hercynian Orogeny, Sehoul Caledonian Belt and the Northern Meseta Platform. Ph.D. Thesis, Universite Louis Pasteur, Strasbourg, France, 1990. [Google Scholar]
- Tahiri, A. Le Maroc central septentrional: Stratigraphie, sédimentologie et tectonique du paléozoïque: Un exemple de passage des zones internes aux zones externes de la chaîne hercynienne du Maroc. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, 1991. [Google Scholar]
- Cattanéo, C.; Tahiri, A.; Vachard, D. La Sédimentation Récifale Du Givétien Dans La Meseta Marocaine Nord-Occidentale. Comptes Rendus Acad. Sci. Ser. II V 1993, 317, 73–80. [Google Scholar]
- Zahraoui, M.; El Hassani, A.; Piqué, A.; Tahiri, A. Le Dévonien Inférieur et Moyen. Bull. De L’institut Sci. Rabat 1994, 18, 43–56. [Google Scholar]
- Lakhloufi, A. Etude Structurale de La égion de Brachwa, Parties Centrales et Nord-Orientale Du Bassin Dévono-Dinantien de Sidi Bettache (Maroc Nordoccidental). Ph.D. Thesis, Ecole Nationale Supérieure, Rabat, Morocco, 1988; 281p. [Google Scholar]
- Ben Abbou, M.; Soula, J.-C.; Brusset, S.; Roddaz, M.; N’Tarmouchant, A.; Driouch, Y.; Christophoul, F.; Bouadbelli, M.; Majesté-Menjoulas, C.; Béziat, D.; et al. Contrôle tectonique de la sédimentation dans le système de bassins d’avant-pays de la Meseta marocaine. Comptes Rendus L’académie Des Sci. Ser. II A Earth Planet. Sci. 2001, 332, 703–709. [Google Scholar] [CrossRef]
- Berkhli, M.; Vachard, D. Le Carbonifère du Maroc central: Les formations de Migoumess, de Tirhela et d’Idmarrach. Lithologie, biostratigraphie et conséquences géodynamiques. Comptes Rendus Geosci. 2002, 334, 67–72. [Google Scholar] [CrossRef]
- Hoepffner, C.; Soulaimani, A.; Piqué, A. The Moroccan Hercynides. J. Afr. Earth Sci. 2005, 43, 144–165. [Google Scholar] [CrossRef]
- Mezougane, H.; Aissa, M.; Essalhi, M.; Moussaid, A.; Souiri, M.; Touil, A.; Bilal, E.; Souiah, M. New Petro-Geochemical Data on Carboniferous Mafic Rocks in the Achemmach Area (NW, Fourhal Basin-Moroccan Central Massif). Minerals 2022, 12, 622. [Google Scholar] [CrossRef]
- Allary, A.; Andrieux, J.; Lavenu, A.; Ribeyrolles, M. Présence de Décrochements Dans La Meseta Sud-Orientale Du Maroc Central. Comptes Rendus L’académie Sci. Paris 1972, 274, 653–656. [Google Scholar]
- Ben Abbou, M. Evolution Stratigraphique et Structurale, Au Cours Du Paléozoïque, de La Bordure Nord Du Massif Central (Région d’Agourai, Maroc). Master’s Thesis, University of Fèz, Fès, Morocco, 1990; 214p. [Google Scholar]
- Piqué, A. Géologie du Maroc: Les Domaines Régionaux et Leur Evolution Structurale; Imprimerie el Maarif al Jadida: Rabat, Morocco, 1994. [Google Scholar]
- Sonnet, P. Les Skams à Tungstène, Étain et Bore de La Région d’El Hammam (Maroc Central). Ph.D. Thesis, Université Catholique De Louvain-la-Neuve, Brussel, Belgium, 1981. [Google Scholar]
- Jébrak, M. Contribution à l’histoire Naturelle Des Filons (F, Ba) Du Domaine Varisque. Essai de Caractérisation Structurale et Géochimique Des Filons En Extension et En Décrochement Dans Les Massifs Centraux Français et Marocains; BRGM: Orléans, France, 1985; 473p. [Google Scholar]
- Kharbouch, F. Les Laves Dévono-Dinantiens de La Méséta Marocaine: Étude Petro-Géochimique et Implication Géodynamique. Ph.D. Thesis, Bretagne Occidentale, Brest, Frence, 1994. [Google Scholar]
- Roddaz, M.; Brusset, S.; Soula, J.-C.; Béziat, D.; Abbou, M.B.; Debat, P.; Driouch, Y.; Christophoul, F.; Ntarmouchant, A.; Déramond, J. Foreland Basin Magmatism in the Western Moroccan Meseta and Geodynamic Inferences. Tectonics 2002, 21, 7-1–7-23. [Google Scholar] [CrossRef]
- Ntarmouchant, A. Le Magmatisme Associé à l’orogenèse Du Maroc Varisque: Exemple Du Magmatisme Du Bassin Méridional d’Azrou-Khénifra (Est Du Maroc Hercynien Central). State Thesis, University of Fes, Fez, Morocco, 2003. [Google Scholar]
- Cailleux, Y. Les Écailles Antéviséennes d’Ezzeliga. Leur Importance Dans l’interprétation Structurale Du Maroc Central. C. R. Acad. Sci. Ser. 2 Mec. Phys. Chim. Sci. Univers Sci. Terre 1985, 301, 497–502. [Google Scholar]
- El Wartiti, M. Le Permien Du Maroc Mesetien: Étude Géologique et Implications Paléogéographiques. Ph.D. Thesis, d’Etat, Faculty of Sciences, Mohammed V University, Rabat, Morocco, 1990. [Google Scholar]
- Remmal, T.; Barbarin, B.; Chairaibi, I.; El Hatimi, N. Les Granitoïdes Du District d’EI Hammam (Massif Hercynien Marocain). Mise En Place, Typologie et Relation Avec La Magmatogenèse Acide Tardi-Hercynienne. Les Cah. De La Rech. I 1999, 1, 77–93. [Google Scholar]
- Saidi, A. Etat de Contrainte et Mécanismes d’ouverture et de Fermeture Des Bassins Permiens de La Meseta Marocaine. Apport de La Télédétection à La Reconnaissance Des Faciès et Des Réseaux de Failles. Ph.D. Thesis, Université Mohammed V, Rabat, Morocco, 2005. [Google Scholar]
- Saidi, A.; Tahiri, A.; Ait Brahim, L.; Saidi, M. États de contraintes et mécanismes d’ouverture et de fermeture des bassins permiens du Maroc hercynien. L’exemple des bassins des Jebilet et des Réhamna. Comptes Rendus Geosci. 2002, 334, 221–226. [Google Scholar] [CrossRef]
- Zouine, M. Evolution Structurale Tardi-Hercynienne de La Bordure Septentrionale Du Maroc Central Entre Tiddas et Jbel Triona. Ph.D. Thesis, Ecole Nationale Supérieure, Rabat, Morocco, 1986. [Google Scholar]
- Cogney, G.; Faugeres, J.-C. Précisions Sur La Mise En Place Des Épanchements Basaltiques Des Formations Triasiques de La Bordure Septentrionale Du Maroc Central. Bull. Soc. Géologique Fr. 1975, 7, 721–733. [Google Scholar] [CrossRef]
- Et-Touhami, M.A. Le Trias Évaporitique Du Bassin de Khemisset, Maroc Central: Géométrie Des Dépôts, Évolution Sédimentaire et Géochimie. Ph.D Thesis, Claude Bernard, Lyon, France, 1992; 242p. [Google Scholar]
- Mahmoudi, A.; Bertrand, H. Identification géochimique de la province magmatique de l’Atlantique central en domaine plissé: Exemple du Moyen Atlas marocain. C. R. Geosci. 2007, 339, 545–552. [Google Scholar] [CrossRef]
- Huon, S.; Piqué, A.; Clauer, N. Etude de l’orogenèse Hercynienne Au Maroc Par La Datation K-Ar de l’évolution Métamorphique de Schistes Ardoisiers. Study of the Hercynian Orogeny in Morocco by the K-Ar Isotopic Datation of Metamorphic Evolution in Slates. Sci. Géol. Bull. Mém. 1987, 40, 273–284. [Google Scholar] [CrossRef]
- Tahiri, A.; Hoepffner, C. La Faille d’Oulmès: Cisaillement Ductile et Tectonique Tangentielle, Maroc Central Hercynien. Bull. L’institut Scentifiquei. Rabat Maroc 1987, 11, 59–68. [Google Scholar]
- Oubbih, J. Le Maroc Central Méridional (Région de Moulay Bou Azza): Stratigraphie Du Paléozoïque et Tectonique Hercynienne. Ph.D. Thesis, Mohammed V University, Rabat, Morocco, 1991. [Google Scholar]
- Agard, J. Données Nouvelles Sur Le District Fluorifère d’El Hammam Berkamène (Maroc Central). Rapp. Inédit SEGM 1966, 1–843. [Google Scholar]
- Dahmani, A. Le Métamorphisme Dans L’auréole Du Granite d’Oulmès (Maroc Central): Étude Pétrographique et Relation Avec Les Déformations Hercyniennes. Ph.D. Thesis, Université Mohammed V, Rabat, Morocco, 1985; p. 150. [Google Scholar]
- Diot, H. Mise En Place Des Granitoïdes Hercyniens de La Meseta Marocaine, Étude Structurale Des Massifs de Sebt de Brikine (Rehamna), de Zaër et d’Oulmès (Massif Central) et d’Aouli-Boumia (Haute Moulouya). Implications Géodynamiques. Ph.D. Thesis, Université Paul Sabatier Toulouse, Toulouse, France, 1989. [Google Scholar]
- Gasquet, D.; Stussi, J.-M.; Nachit, H. Les Granitoïdes Hercyniens Du Maroc Dans Le Cadre de l’évolution Géodynamique Régionale. Bull. Soc. Géol. Fr. 1996, 167, 517–528. [Google Scholar]
- Giuliani, G.; Cheilletz, A.; Zimmermann, J.L. The Emplacement, Geochemistry and Petrogenesis of Two Central Morocco Hercynian Granites. Geotectonic Implications. J. Afr. Earth Sci. (Middle East) 1989, 9, 617–629. [Google Scholar] [CrossRef]
- Lagarde, J.-L. Cisaillements Ductiles et Plutons Granitiques Contemporains de La Déformation Hercynienne Post Viséenne de La Méséta Marocaine. Bull. Soc. Géol. Minéral. Bretagne 1985, 1, 29–37. [Google Scholar]
- Boushaba, A.; Cailleux, Y. Les Relations Métamorphisme-Déformation Au Voisinage Des Granitoïdes Hercyniens Du Maroc Central. Bull. Inst. 1992, 16, 15–22. [Google Scholar]
- Mrini, Z.; Rafi, A.; Duthou, J.-L.; Vidal, P. Chronologie Rb-Sr Des Granitoides Hercyniens Du Maroc; Consequences. Bull. Soc. Géol. Fr. 1992, 163, 281–291. [Google Scholar]
- Amenzou, M.; El Mouraouah, A.E. Typologie Du Zircon Des Granitoïdes Hercyniens de La Meseta Marocaine: Zonation Magmatique et Implication Géodynamique. J. Afr. Earth Sci. 1997, 24, 125–139. [Google Scholar] [CrossRef]
- Coogan, L.A.; Wilson, R.N.; Gillis, K.M.; MacLeod, C.J. Near-Solidus Evolution of Oceanic Gabbros: Insights from Amphibole Geochemistry. Geochim. Cosmochim. Acta 2001, 65, 4339–4357. [Google Scholar]
- Gillis, J.J.; Jolliff, B.L.; Elphic, R.C. A Revised Algorithm for Calculating TiO2 from Clementine UVVIS Data: A Synthesis of Rock, Soil, and Remotely Sensed TiO2 Concentrations. J. Geophys. Res. Planets 2003, 108, 1–17. [Google Scholar] [CrossRef]
- Dupuis, C.; Hébert, R.; Dubois-Côté, V.; Wang, C.S.; Li, Y.L.; Li, Z.J. Petrology and Geochemistry of Mafic Rocks from Mélange and Flysch Units Adjacent to the Yarlung Zangbo Suture Zone, Southern Tibet. Chem. Geol. 2005, 214, 287–308. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Craig, J.R. Mineral Chemistry of Natural Sulfides. Am. Mineral. 1985, 70, 1036–1043. [Google Scholar]
- Barton, P.B.; Bethke, P.M. Chalcopyrite Disease in Sphalerite; Pathology and Epidemiology. Am. Mineral. 1987, 72, 451–467. [Google Scholar]
- Sinojmeri, A. Minéralogie et Paragenèse Du Gisement Volcanogène à Cu, Zn, Pb, Au de Munella, Mirdita Central, Albanie. Ph.D. Thesis, Université d’Orléans, Orléans, Frence, 1990. [Google Scholar]
- Yajima, J.; Touray, J.C. Analyse Thermométrique Du Gisement de Fluorite d’el Hammam (Maroc). Miner. Depos. 1970, 5, 23–28. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Zemri, O.; Jébrak, M.; Klügel, A.; Levresse, G.; Maacha, L.; Gaouzi, A.; Souiah, M. Geology and Mineralogy of the El Hammam REE-Rich Fluorite Deposit (Central Morocco): A Product of Transtensional Pangean Rifting and Central Atlantic Opening. In Mineral Deposits of North Africa; Springer: Cham, Switzerland, 2016; pp. 307–324. [Google Scholar]
- Bouabdellah, M.; Banks, D.; Klügel, A. Comments on “A Late Triassic 40Ar/39Ar Age for the El Hammam High-REE Fluorite Deposit (Morocco): Mineralization Related to the Central Atlantic Magmatic Province?” By Cheilletz et al. Miner. Depos. 2010, 45, 729–731. [Google Scholar] [CrossRef]
- Aissa, M. Etude Des Interactions Fluides-Minéraux Des Skarns à Sn, W, B d’El Hammam (Maroc Central). Facteurs Physico-Chimiques Contrôlant Le Développement Du Stade Stannifère. Ph.D. Thesis, University Moulay Ismail, Meknès, Morocco, 1997. [Google Scholar]
- Cheilletz, A.; Gasquet, D.; Filali, F.; Archibald, D.A.; Nespolo, M. A Late Triassic 40 Ar/39 Ar Age for the El Hammam High-REE Fluorite Deposit (Morocco): Mineralization Related to the Central Atlantic Magmatic Province? Miner. Depos. 2010, 45, 323–329. [Google Scholar] [CrossRef]







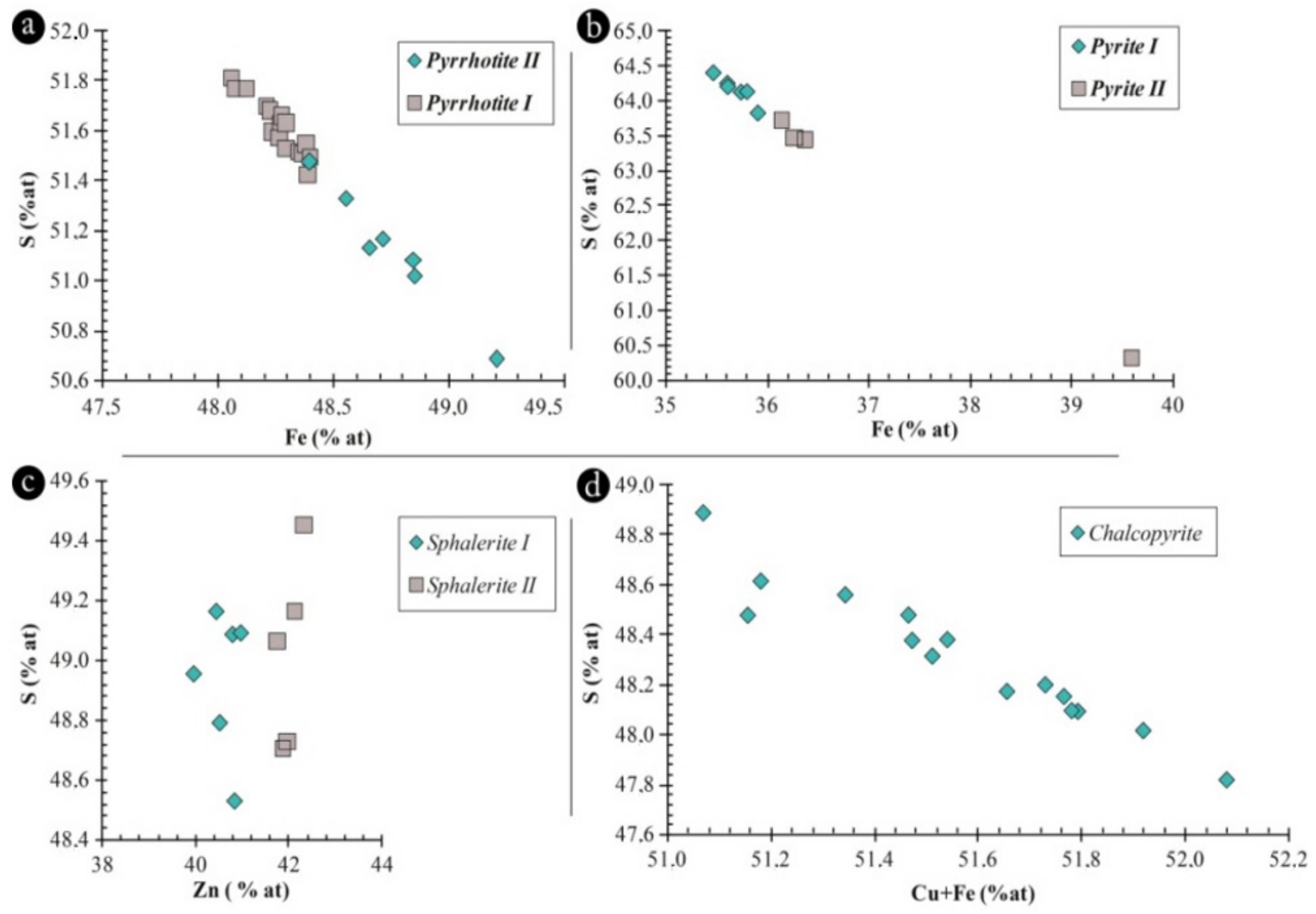




| % Wt | Pyrite I | Pyrite II | Chalcopyrite | Pyrrhotite | Sphalerite I | Sphalerite II | Galena |
|---|---|---|---|---|---|---|---|
| Fe | (49.17) | (49.23) | (31.22) | (61.82) | (12.09) | (9.99) | (0.25) |
| [48.83_ 49.54] | [49.02_ 49.37] | [30.68_ 31.73] | [60.89_ 63.12] | [11.00_ 14.42] | [9.23_ 10.61] | [0.04_ 0.46] | |
| Zn | (0.02) | (0.10) | (0.08) | (0.04) | (53.69) | (56.18) | (0.10) |
| [0.00_ 0.11] | [0.03_ 0.14] | [0.00_ 0.33] | [0.00_ 0.19] | [48.11_ 55.50] | [55.70_ 56.54] | [0.00_ 0.20] | |
| S | (50.67) | (49.66) | (33.16) | (37.74) | (32.35) | (32.15) | (12.54) |
| [49.70_ 50.98] | [49.37_ 50.02] | [32.50_ 33.55] | [37.08_ 38.12] | [32.12_ 32.61] | [31.82_ 32.40] | [12.41_ 12.67] | |
| Pb | (0.20) | (0.22) | (0.14) | (0.14) | (0.08) | (0.07) | (86.57) |
| [0.12_ 0.29] | [0.20_ 0.24] | [0.03_ 0.25] | [0.04_ 0.27] | [0.03_ 0.14] | [0.03_ 0.11] | [86.20_ 86.93] | |
| Ag | (0.03) | (0.03) | (0.01) | (0.02) | (0.02) | (0.01) | (0.12) |
| [0.00_ 0.06] | [0.02_ 0.05] | [0.00_ 0.04] | [0.00_ 0.06] | [0.00_ 0.04] | [0.00_ 0.03] | [0.05_ 0.19] | |
| Cd | (0.03) | (0.01) | (0.01) | (0.02) | (0.30) | (0.14) | (0.01) |
| [0.00_ 0.04] | [0.00_ 0.01] | [0.00_ 0.03] | [0.00_ 0.04] | [0.28_ 0.36] | [0.05_ 0.38] | [0.00_ 0.02] | |
| In | (0.01) | (0.05) | (0.02) | (0.01) | (0.04) | (0.03) | (0.01) |
| [0.01_ 0.03] | [0.04_ 0.06] | [0.00_ 0.05] | [0.00_ 0.04] | [0.00_ 0.05] | [0.00_ 0.06] | [0.00_ 0.02] | |
| Sb | (0.03) | (0.08) | (0.01) | (0.01) | (0.01) | (0.05) | (0.02) |
| [0.00_ 0.08] | [0.04_ 0.11] | [0.00_ 0.04] | [0.00_ 0.04] | [0.00_ 0.02] | [0.00_ 0.10] | [0.01_ 0.03] | |
| Cu | (0.01) | (0.03) | (34.62) | (0.01) | (0.66) | (0.10) | (0.01) |
| [0.00_ 0.03] | [0.00_ 0.05] | [34.05_ 34.93] | [0.00_ 0.04] | [0.03_ 3.83] | [0.02_ 0.32] | [0.00_ 0.02] | |
| Ni | (0.01) | (0.02) | (0.00) | (0.02) | (0.01) | (0.00) | 0.00 |
| [0.00_ 0.03] | [0.02_ 0.03] | [0.00_ 0.03] | [0.00_ 0.07] | [0.00_ 0.02] | [0.00_ 0.01] | [0.00_ 0.00] | |
| Co | (0.01) | (0.03) | (0.00) | (0.01) | (0.01) | (0.03) | (0.02) |
| [0.00_ 0.03] | [0.03_ 0.04] | [0.00_ 0.03] | [0.00_ 0.06] | [0.00_ 0.03] | [0.00_ 0.05] | [0.01_ 0.03] | |
| As | (0.04) | (0.01) | (0.02) | (0.02) | (0.04) | (0.02) | (0.02) |
| [0.00_ 0.10] | [0.00_ 0.03] | [0.00_ 0.07] | [0.00_ 0.06] | [0.00_ 0.06] | [0.00_ 0.04] | [0.01_ 0.02] | |
| Total | (100.21) | (99.47) | (99.29) | (99.86) | (99.28) | (98.77) | (99.66) |
| [99.17_ 100.78] | [99.15_ 99.69] | [98.67_ 99.97] | [98.60_ 101.20] | [98.37_ 100.49] | [98.43_ 99.77] | [99.14_ 100.18] | |
| % Atomic | |||||||
| Fe | (35.69) | (37.33) | (26.11) | (48.41) | (10.50) | (8.75) | (0.54) |
| [35.47_ 35.91] | [36.15_ 39.58] | [25.84_ 26.44] | [48.06_ 49.21] | [9.65_ 12.44] | [8.09_ 9.32] | [0.08_ 1.00] | |
| Zn | (0.03) | (0.02) | (0.06) | (0.03) | (39.84) | (42.01) | (0.19) |
| [0.00_ 0.09] | [0.00_ 0.07] | [0.00_ 0.24] | [0.00_ 0.12] | [35.45_ 40.96] | [41.75_ 42.32] | [0.00_ 0.38] | |
| S | (64.16) | (62.51) | (48.31) | (51.47) | (48.94) | (49.02) | (47.87) |
| [63.82_ 64.41] | [60.32_ 63.73] | [47.82_ 48.89] | [50.68_ 51.82] | [48.53_ 49.16] | [48.70_ 49.45] | [47.80_ 47.94] | |
| Pb | (0.04) | (0.03) | (0.03) | (0.03) | (0.02) | (0.02) | (51.13) |
| [0.03_ 0.06] | [0.02_ 0.04] | [0.01_ 0.06] | [0.01_ 0.06] | [0.01_ 0.03] | [0.01_ 0.03] | [50.88_ 51.39] | |
| Ag | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.00) | (0.14) |
| [0.00_ 0.02] | [0.007_ 0.01] | [0.00_ 0.02] | [0.00_ 0.02] | [0.00_ 0.02] | [0.00_ 0.01] | [0.05_ 0.22] | |
| Cd | (0.01) | (0.01) | (0.01) | (0.01) | (0.13) | (0.06) | (0.01) |
| [0.00_ 0.01] | [0.00_ 0.01] | [0.00_ 0.01] | [0.00_ 0.02] | [0.12_ 0.15] | [0.02_ 0.16] | [0.00_ 0.02] | |
| In | (0.01) | (0.01) | (0.01) | (0.01) | (0.02) | (0.01) | (0.01) |
| [0.00_ 0.02] | [0.01_ 0.02] | [0.00_ 0.02] | [0.00_ 0.01] | [0.00_ 0.02] | [0.00_ 0.02] | [0.00_ 0.02] | |
| Sb | (0.01) | (0.03) | (0.00) | (0.00) | (0.00) | (0.02) | (0.02) |
| [0.00_ 0.03] | [0.02_ 0.04] | [0.00_ 0.01] | [0.00_ 0.01] | [0.00_ 0.01] | [0.00_ 0.04] | [0.01_ 0.03] | |
| Cu | (0.01) | (0.01) | (25.45) | (0.01) | (0.50) | (0.08) | (0.02) |
| [0.00_ 0.02] | [0.00_ 0.03] | [25.04_ 25.76] | [0.00_ 0.03] | [0.02_ 2.90] | [0.01_ 0.25] | [0.00_ 0.05] | |
| Ni | (0.01) | (0.01) | (0.00) | (0.02) | (0.00) | (0.00) | 0.00 |
| [0.00_ 0.02] | [0.00_ 0.02] | [0.00_ 0.02] | [0.00_ 0.05] | [0.00_ 0.02] | [0.00_ 0.01] | [0.00_ 0.00] | |
| Co | (0.01) | (0.01) | (0.00) | (0.01) | (0.01) | (0.02) | (0.05) |
| [0.00_ 0.02] | [0.00_ 0.02] | [0.00_ 0.02] | [0.00_ 0.04] | [0.00_ 0.03] | [0.00_ 0.04] | [0.03_ 0.07] | |
| As | (0.02) | (0.01) | (0.01) | (0.01) | (0.02) | (0.01) | (0.03) |
| [0.00_ 0.05] | [0.00_ 0.01] | [0.00_ 0.04] | [0.00_ 0.03] | [0.00_ 0.04] | [0.00_ 0.03] | [0.02_ 0.03] | |
| Total | (100.00) | (99.99) | (100.00) | (100.00) | (100.00) | (100.00) | (100.00) |
| [99.95_ 100.03] | [99.97_ 100.01] | [99.999_ 100.00] | [99.999_ 100.002] | [99.999_ 100.001] | [100.00_ 100.001] | [100.00_ 100.001] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezougane, H.; Aissa, M.; Muhammad, S.; Moussaid, A.; El Basbas, A.; Essalhi, M.; Kharis, A.-a.; El Azmi, M.; Touil, A.; Bilal, E. Sulfide and Fluoride Mineralization of the NNE Region of Achemmach (Central Morocco): Paragenetic Sequences and Pyrrhotite-Sphalerite Geothermometry Constraints. Minerals 2022, 12, 790. https://doi.org/10.3390/min12070790
Mezougane H, Aissa M, Muhammad S, Moussaid A, El Basbas A, Essalhi M, Kharis A-a, El Azmi M, Touil A, Bilal E. Sulfide and Fluoride Mineralization of the NNE Region of Achemmach (Central Morocco): Paragenetic Sequences and Pyrrhotite-Sphalerite Geothermometry Constraints. Minerals. 2022; 12(7):790. https://doi.org/10.3390/min12070790
Chicago/Turabian StyleMezougane, Hafid, Mohamed Aissa, Souiri Muhammad, Azizi Moussaid, Abdelaziz El Basbas, Mourad Essalhi, Abdel-ali Kharis, Mohammed El Azmi, Ahmed Touil, and Essaid Bilal. 2022. "Sulfide and Fluoride Mineralization of the NNE Region of Achemmach (Central Morocco): Paragenetic Sequences and Pyrrhotite-Sphalerite Geothermometry Constraints" Minerals 12, no. 7: 790. https://doi.org/10.3390/min12070790
APA StyleMezougane, H., Aissa, M., Muhammad, S., Moussaid, A., El Basbas, A., Essalhi, M., Kharis, A.-a., El Azmi, M., Touil, A., & Bilal, E. (2022). Sulfide and Fluoride Mineralization of the NNE Region of Achemmach (Central Morocco): Paragenetic Sequences and Pyrrhotite-Sphalerite Geothermometry Constraints. Minerals, 12(7), 790. https://doi.org/10.3390/min12070790







