Abstract
Ulug-Sair Au-Bi-Te-Se mineralization is one prospect for native Au in the Western Tuva, and its origin remains debated. Mineralization consists of gold–sulfide–quartz veins in the host sedimentary rocks (conglomerates, siltstones, shales), quartz–tourmaline, and quartz–carbonate–sericite–altered rocks. To determine its origin, we examined the mineralogical–geochemical features, formation conditions, and fluid sources of the Ulug-Sair ore. A mineralogical–geochemical investigation outlines two substages with Au: an early gold–sulfide–quartz with pyrite, chalcopyrite, galena, gold, and electrum; and a late gold–telluride–sulfide–quartz, characterized by the presence of Bi-bearing minerals (AgBiTe, Bi2Te2Se, Cu3BiS3, Bi), tellurides (Au and Ag), Se-tellurides (Ag and Bi), and selenides (Au, Ag, and Hg). The paragenesis of Au–Ag tellurides, and fluid inclusion study data (microthermometry, Raman spectroscopy, LA-ICP-MS, and crush leach analysis (gas and ion chromatography, ICP-MS) in quartz showed that quartz–tourmaline-altered rocks were formed by an aqueous Mg–Na–K-chloride fluid with a salinity of 8–10 wt % NaCl eq. at 325–370 °C, whereas the host quartz–carbonate–sericite-altered rocks were formed from CO2–H2O fluid containing CH4 and N2, with a salinity of 0.18–6.1 wt % NaCl eq. at 200–400 °C. Gold-bearing mineral assemblages were formed at P ~ 0.75–1.0 kbar (~2.3–3 km) due to CO2–H2O chloride (Na–K ± Fe, Mg) fluid with CH4, Na2SO4, and Na2B2O5, and salinities 1.7–12.5 wt % NaCl eq. at temperatures decreasing from 360 up to 115 °C (gold–sulfide–quartz veins—360–130 °C, and gold–telluride–sulfide–quartz veins—330–115 °C), and variable fO2, fS2, fSe2, and fTe2. Results of the investigation of the isotope composition of S in pyrites indicates the magmatic origin of the fluid (δ18S fluid from −0.4 to +2.5‰). The stable O isotope data in quartz indicates that, at an early substage, the formation of ore involved a fluid of magmatic and metamorphic origin (from +8.2 to +11.6‰), and, in the later substage, multiple sources of hydrothermal fluids (from +3.1 to +10.4‰), including magmatic-derived, metamorphic-derived, and meteoric waters. These data, in conjunction with structurally controlled mineralization, point towards similarities of the Ulug-Sair ore system with orogenic gold deposits.
1. Introduction
Gold–telluride and gold–bismuth (gold–bismuth–telluride) mineralization deposits are often associated with magmatic bodies and are included in epithermal (volcanic), porphyry, intrusion-related, or orogenic types of gold deposits [1,2,3,4,5,6,7,8,9,10], etc.
Gold–bismuth (gold–bismuth–telluride) type deposits [3,4], which are confined to granitoid intrusions, belong to “granitoid related deposits” [7]. Deposits of this type in Alaska and the Yukon [11,12] are large, with reserves of 100 t and more. Gold–bismuth mineralization in Russia include the Pogranichnoye (Eastern Sayany), Ergelyakh, Kurumskoye, Tuguchak, Basaguninskoye, Chuguluk, Nennely, and Galechnoye (northeastern Russia) vein deposits, and Levodybinskoye and Teutedzhak (northeastern Russia) stockwork deposits confined to the apical near-contact zones of granitoid plutons or their marginal near-contact fracture zones [3,4,10,13,14]. It should be noted that deposits of this type are formed mainly from magmatic fluids with the involvement of metamorphic and meteoritic sources [10,15,16,17,18]. They are formed over a wide range of temperatures (437–155 °C, mainly at 400–250 °C) and pressures (1700–90 bar) due to aqueous fluids with Na and K chlorides with wide salinity (46.0–1.1 wt %), at fO2–fS2 variations [10].
Gold–telluride deposits are commonly not distinguished as a separate deposit type, but they are included in epithermal, porphyry, orogenic, or intrusion-related types of gold deposits. Deposits of the gold–telluride mineral type are associated with volcano-plutonic complexes of the calc-alkaline and alkaline series. Typical deposits include the following: Cripple Creek and Golden Sunlight (USA), Golden Mile (Australia), Acupan (Philippines), Yuryang (Korea), Emperor (Fiji), Porgera (Papua New Guinea), Kochbulak (Uzbekistan), Svetlinskoe and Aginsky (Russia), etc. [7,8,19,20,21,22,23,24,25,26,27,28]. The gold–telluride ores were formed by multiple sources of hydrothermal fluids (including magmatic-derived, mantle-derived, metamorphic-derived, and meteoric waters) with variations of fO2, fS2, fSe2, fTe2, pressures (0.05 to 5 kbar) and temperatures (50 to 420 °C). The temperature geochemical barrier is one of the leading ore-forming factors associated with the mixing of magmatic fluids with surface waters and fluid-rock interaction and other processes favorable for gold deposition [8,20,22,23,24,25,26,27,28].
The Ulug-Sair gold mineralization located in the central part of Aldan-Maadyr ore cluster (AMOC) was discovered in 1964 by Victor V. Zaykov during a geological survey at the 1:50,000 map scale [29]. A specific feature of the Ulug-Sair occurrence is the presence of Au–Bi–Te–Se mineral assemblages, namely, petzite, hessite, Se-volynskite, kawazulite, wittichenite, native Bi, fischesserite, naumannite, and tiemannite. However, the topic in question has not been sufficiently studied in terms of genesis, mineralogical and geochemical features, and conditions of ore formation.
The relevance of the research is defined by the need to determine the genesis and formation conditions of gold deposits in the Tuva Republic in order to improve the efficiency of geological prospecting, this increasing the gold mineral resource base of the region.
2. Geological Setting
During the geological survey and prospecting activities during the period 1952–1977 in Western Tuva, gold–quartz ore occurrences were discovered (Ulug-Sair, Aryskan, Khaak-Sair, Dushkunnug, Ak-Dag, etc.). In addition, numerous gold mineralizations are associated with medium-temperature quartz–carbonate–sericite- and quartz–carbonate–fuchite-altered rocks. The largest are Khaak-Sair, in quartz–carbonate–fuchsite rocks [30], and Ulug-Sai, in conglomerates, siltstones, and quartz–carbonate–sericite rocks. They are located in the AMOC, which is considered one of the most promising for native Au (Figure 1) in the region.
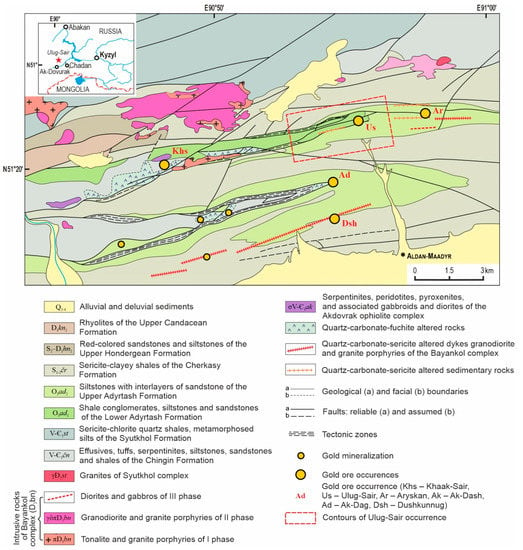
Figure 1.
Geological scheme of the central part of Aldan-Maadyr ore cluster, simplified with permission from [29].
The Aldan-Maadyr ore cluster is located on the left bank of the Khemchik River and is related to the junction zone of: (a) Early Cambrian–Ordovician terrigenous complexes of the Western Sayany; (b) Vendian–Early Cambrian oceanic complexes and Middle Cambrian–Silurian molasses, which, respectively, formed the basement and sedimentary cover of the Khemchik–Systygkhem pre-arc collision; and (c) Devonian magmatic and sedimentary complexes of one of the “branches” of the Tuva riftogenic trough [31].
Gold mineralization is controlled by narrow, linear, near NS anticlines and horst anticlines and the associated fractures of the Sayany-Tuva deep fault of the same orientation cutting them. A similar orientation of the main discontinuous and folded structures led to a linear distribution of igneous rocks and narrow linear quartz–sericite–fuchite–carbonate alteration zones with gold–quartz veins.
Gold mineralization is related to the intrusions and dikes of the Late Devonian Bayankol complex, comprising granodiorites and tonalite, rhyolite, and granodiorite porphyries [29,30]. The 40Ar/39Ar age of ore-bearing quartz–carbonate–fuchsite-altered rocks (379.4 ± 4.4 Ma) of the Khaak-Sair occurrence is 376.5 ± 3.4 Ma for phase III gabbro dikes of the Bayankol complex, thereby confirming the genetic relationship of mineralization with the Late Devonian Bayankol intrusive complex [32].
Ore occurrences of AMOC are sulfide-poor (<3–5%) and contain Au–Bi–Te–Se mineral assemblages [30,32].
The Ulug-Sair occurrence is confined to the axial part of the near EW striking horst-anticline and complex faults: Arzhansky and “Rudny”, which are accompanied by numerous fractures with angles of 75–90°. The core of the horst anticline is composed of Ordovician conglomerates, siltstones, sandstones, and Vendian–Lower Cambrian ophiolites (exposed west of the ore occurrence), the anticline limbs are composed of Ordovician siltstones, Silurian schists, and siltstones (Figure 1).
The Ulug-Sair horst anticline is 6 km long and 2–3 km wide. Lilac-gray fine-pebble foliated conglomerates of the Ordovician Lower Adyrtash sub-formation in the core of the Ulug-Sair horst anticline are folded and host several gold–quartz veins. Pebbles in conglomerates have silty cement and semi-rounded or angular fragments of vein quartz and quartzite from 1 to 5 cm in size. It makes up 50–60% of the rock bulk.
Conglomerates are associated with greenish-grey schistose sandstones of 10 to 25 m thickness and gravels of 0.2–1.5 m thickness. The conglomerates have a gradual transition to and are overlapped with lilac and grey-green siltstones of the Upper Adyrtash sub-formation. Siltstones contain intercalations of greenish-grey, lilac-grey, and red-brown sandstones of 0.01 to 5 m thickness, as well as slices with a thickness of several tens of meters. The total thickness of the Upper Adyrtash formation is 600–700 m.
The rocks of the Silurian Nizhny Chergak formation are concordantly overlapped with the rocks of the Upper Adyrtash formation and are grey and greenish-grey sericite shales with grey-green siltstones and grey sandstones of 68–140 m and 1.5–20 m thick, respectively.
Igneous rocks are represented by dykes, granite-, granodiorite-porphyries (II phase), diorites, and gabbro (III phase) of the Bayankol complex intruding into the Ordovician and Silurian rocks. The strike of dykes is sub-latitudinal and consistent with the directions of the faults. The shape of the dykes is plate-like, slightly curved, and sometimes vein-like; 1–3 m thick and 0.3–0.5 km long, less commonly up to 1 km. Granite-, granodiorite-porphyries have been subjected to propylitization and quartz–carbonate–sericite alteration.
Ulug-Sair comprises quartz–tourmaline and quartz–carbonate–sericite-altered rocks, as well as quartz veins and vein zones with gold mineralization that postdate intrusive and sedimentary rocks (Figure 2). Additional EW striking and steeply dipping 75 gold–quartz veins and several vein zones were identified. The veins are from 15 cm to 2 m thick, and 20–100 m long (less often 200 m); vein zones are 3 to 40 m thick and 20 to 120 m long [29]. The average content of ore minerals in the veins and vein zones does not exceed 3 vol. %.
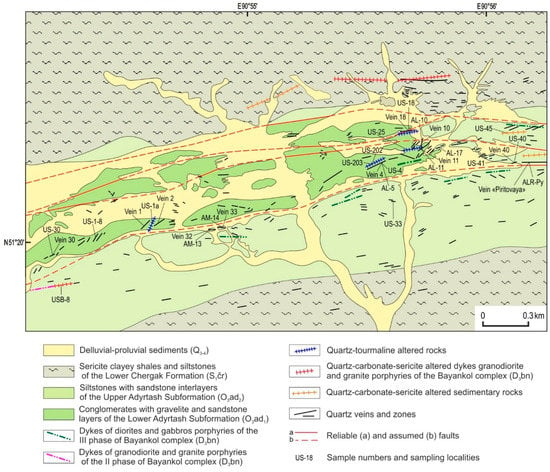
Figure 2.
Geological scheme of the Ulug-Sair occurrence.
The Au in quartz veins ranges from 0.2 to 286 g/t, and Ag ranges up to 300 g/t [28]. The Au resources, according to prospecting work, are 20 t with an average Au grade of 2 ppm [33]. The Cu sulphides in quartz veins are a proxy for gold. A positive correlation between Au and Cu, B, Ag, Sb, As, Te, Bi, Mn, Ba, Sr, Pb, Mg, Mo, Cd, Zn, and W was identified [34].
3. Methods
The samples from hydrothermally-altered rocks and ore veins were collected from various mine workings at a range of depths from 0.6 m to 3 m. More than 100 samples of ores and host rocks were taken, weighing from 500 g to 5 kg. Polished thin sections were prepared in order to determine the paragenetic relationships of ore minerals and to characterise the various stages of alteration. The optical studies were carried out using Olympus BX41, POLAM P-213M, and P-212M microscopes (TuvIENR SB RAS, Kyzyl, Tuva Republic, Russia). The chemical composition of minerals was determined using an MIRA 3 LMU SEM (Tescan Orsay Holding, Brno, Czech Republic) with INCA Energy 450 + XMax 80 and INCA Wave 500 microanalysis systems (Oxford Instruments Nanoanalysis Ltd., Abingdon, UK) (Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia). The compositions of native gold and other minerals were examined at the accelerating voltage of 20 kV, an electron beam current of 1.5 nA, and live acquisition time of spectra of 30 s. The following X-rays were selected: K series for Fe, Cu, and Ni, and L series for Pd, Au, Ag, As, Te, Se, Bi, Sb, and Hg. As the standards, we used FeS2 (on S), PbTe (on Te), PtAs2 (on As), and the pure elements of Fe, Ni, Cu, Se, Ru, Rh, Pd, Ag, Sn, Sb, Pt, Au, and Bi. Phases smaller than 5 µm were analyzed using a point probe, and larger phases were analysed in a small raster mode with the size of the scanned area up to 100 µm2. The formulas of petzite and fischesserite were calculated for 6 at., hessite and naumannite for 3 at., tiemannite for 2 at., Se-volynskite for 4 at., kawazulite for 5 at., and wittichenite for 7 at.
The fluid inclusions were examined in the laboratory of the South Urals State University (Miass) and the CCE “Multielement and Isotope Studies” SB RAS (Novosibirsk) using the Linkam TMS-600 stage equipped with LinkSystem 32 DV-NC and optical Olympus BX51 microscope. The eutectic temperatures of fluid inclusions were interpreted using [35,36]. The salinity of inclusions was determined by the final melting temperatures according to [37]. The homogenization temperatures are the first temperature of the mineral formation [38].
Pressure–temperature conditions for mineral assemblages were also determined using geothermometers, geofugometers, and mineral parageneses. The stability areas of the main ore minerals in the fS2—fTe2, and fS2—fSe2 coordinates were taken from [39,40,41,42].
The gas composition of fluid inclusions was detected by Raman spectroscopy on a Ramanor U-1000 spectrometer with a Horiba DU420E-OE-323 detector (Jobin Yvon) and a Millennia Pro laser from Spectra-Physics (analyst A.A. Redina, Institute of Geology and Mineralogy SB RAS, Novosibirsk). The fluid pressure was calculated using the FLINCOR program using CO2 homogenization temperatures [43].
The bulk analysis of the element composition of fluid was carried out according to [44,45]. The gases from inclusions (H2O, CO2, and CH4) were analyzed using an Agilent 6890 gas chromatograph. The content of anions was determined by ion chromatography (Color-3000) and the cations and trace elements by the ICP-MS analysis (Elan-6100). The NSO3 content was calculated on the balance sheet. To exclude the influence of the matrix, a repeated (“single”) extraction was carried out, the analysis of which was subtracted from the first one.
The oxygen isotopic composition was analyzed on an Isoprime mass spectrometer using the internal AQS standard (Akita Quartz Standard) at the University of Akita, Japan (analysts H. Kawaraya and O. Matsubaya). The powdered quartz samples (~20 mg) were reacted in F2 gas, a technique described by [46], in a nickel tube at 500 °C for 12 h to produce O2 gas. The gas was then converted to CO2 gas in a graphite furnace at 700 °C and collected by a Toeple pump and liquid nitrogen trap. The values of δ18O are in ppm (‰) relative to the SMOW standard.
The sulfur isotopic composition in the sulfides was measured at the Multi-element and Isotope Research Center SB RAS using a Finnigan MAT Delta mass spectrometer in dual-inlet mode (analysts V.N. Reutsky and M.N. Kolbasova, Novosibirsk). The measurement control was provided by the samples with standard isotopic composition in the range δ34S from −15.1 to + 21.8‰, relative to troilite from Canyon Diablo (CDT), including international ones: NBS-123 (δ34S = +17.44) and NBS-127 (δ34S = +21.8). The reproducibility of the values of δ34S, including sample preparation, is not more than 0.1‰ (2σ). The values of δ34S (‰) are given relative to the CDT standard.
4. Results
4.1. Mineralogy
We identified, based on previous works [29] and our data, that the earliest high-temperature quartz–tourmaline stage includes two substages: tourmaline and tourmaline–quartz. Gold–sulfide–quartz mineralization includes substages including: pre-ore-altered rocks and pyrite–quartz; gold–sulfide–quartz (I), and gold–telluride–sulfide–quartz (II), as well as post-ore chlorite–quartz, carbonate–quartz, and chlorite–hematite–quartz substages. Limonite, malachite, azurite, goethite, scorodite, cuprite, cerussite, bismutite, iodargyrite, and chlorargyrite are supergenes (Figure 3).
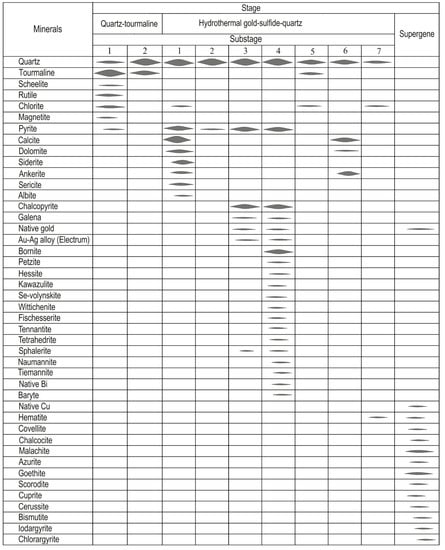
Figure 3.
The para-genetic sequence of mineral formation at the Ulug-Sair ore occurrence.
Quartz–tourmaline rocks (5–7 m thick, and up to 10 m long) and veins (up to 3 mm thick) are widespread throughout the occurrence (Figure 4a,b). They are altered rocks formed from Ordovician siltstones and conglomerates and are composed of light-green needle-prismatic tourmaline. They are brecciated and related to tectonic zones in conglomerates. Quartz–tourmaline-altered rocks contain rutile (including W-containing), F-apatite, scheelite, and pyrite. Veins contain quartz, tourmaline, and pyrite.

Figure 4.
Altered rocks of the Ulug-Sair ore occurrence: (a) quartz–tourmaline rocks (1) with tourmaline–quartz (2) and gold–sulfide–quartz (3) veins of the latest substages; (b) brecciated fragments of quartz–tourmaline rocks (1) in gold–sulfide–quartz mineral aggregates (3); (c,d) ore-bearing conglomerates (1) with tourmaline–quartz (2) and sulfide–quartz (3) veins; (e,f) beresite with gold–sulfide–quartz veins after (e) granite-porphyry (3); (f) quartz sandstone (3).
The chemical composition of tourmaline from quartz–tourmaline-altered rocks and veins shows that they are related to the intermediate members of the dravite–magnesio-foitite series [34].
Quartz–carbonate–sericite-altered intrusive (granite-, granodiorite-porphyries) and sedimentary rocks (Figure 4c,d) consist of horsetail-shaped steep bodies concordant to replaced rocks. These altered rocks contain quartz (30–50%), albite (40–60%), sericite (up to 5–10%), calcite, ankerite (up to 10–3%), and pyrite in the form of well-shaped cubic crystals (1–15%) with an average size of 1–5 mm, reaching a maximum of 1–3 cm.
The altered rocks are from 0.5 to 2 m thick and from 100 to 150 m long. Quartz–carbonate–sericite and tourmaline–quartz-altered rocks contain gold–sulfide–quartz and gold–telluride–sulfide–quartz veins (Figure 5).

Figure 5.
Ore textures of the Ulug-Sair ore: (a,b) vein-disseminated gold–sulfide–quartz ores with chalcopyrite (Csp), pyrite (Py), and early brecciated tourmaline (Tur); (c,d) vein-disseminated gold–telluride–sulfide–quartz mineralization with bornite (Bn) and chalcopyrite (Ccp).
Gold–sulfide–quartz veins (I) are widespread and consist of vein zones composed of quartz, chalcopyrite, pyrite, galena, gold, and Au–Ag alloy (electrum). The major ore mineral—chalcopyrite—is disseminated and forms pockets in the quartz. Pyrite occurs as cubic and pentagon-dodecahedral crystals. Tourmaline occurs as inclusions resembling needles, which are arranged in a chaotic manner or are radially radiant aggregates (Figure 5).
Gold in gold–sulfide–quartz veins (I) are lumpy, interstitial, elongated, crystallomorphic (octahedra, cuboctahedra, crystal intergrowths), and aggregates of poorly-formed crystals and dendritic shapes disseminated in quartz and chalcopyrite, in intergrowth with pyrite and tourmaline (Figure 6). Au–Ag alloy of lumpy-branched and interstitial is detected and overgrows pyrite, tourmaline, and chalcopyrite.
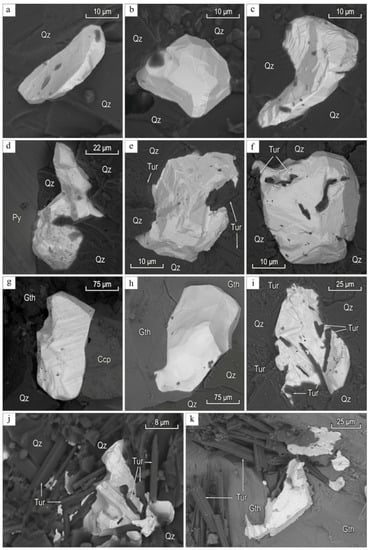
Figure 6.
Gold (light) in gold–sulfide–quartz veins in quartz (a–k) with pyrite (d), chalcopyrite (g), and goethite (Gth) (h,k), and tourmaline (Tur, e,f,i–k). BSE images.
The chemical composition of gold is (wt %) Au 72.12–96.44, Ag 3.36–27.69, Cu 0.00–0.69, Te 0.00–0.04; Au–Ag alloy—Ag 29.80–38.45, Au 61.55–69.71, Cu 0.00–0.46. Gold and electrum grains are weakly zoned with Ag by 3–7 wt %.
Gold–telluride–sulfide–quartz veins (II) with disseminated, nest-like and vein-disseminated mineralization are up to 2 m thick and up to 28 m long. Gold (up to 0.3 mm) of interstitial, lumpy-branched shapes are associated with quartz, chalcocite, hessite, petzite, malachite, and Fe hydroxides. Lumpy and lumpy-branched electrum is found in quartz and Fe hydroxides. Gold and electrum are associated with chalcopyrite, bornite, galena (Se up to 0.64 wt %), tennantite-(Fe), tennantite-(Zn), tennantite-(Cu), tetrahedrite-(Zn), sphalerite, kawazulite, fischesserite, naumannite, tiemannite, Se-volynskite, wittichenite, and native Bi.
The chemical composition of this gold is (wt %) Au 72.56–90.10, Ag 9.47–27.44, Cu 0.00–0.50, and Te 0.00–0.02; Au–Ag alloy—Au 60.37–69.45 and Ag 30.53–40.12. The Au amount is decreased by 2–5 wt % with the increasing of Ag.
Small inclusions (1–50 μm) of hessite and petzite are detected in chalcocite (Figure 7).
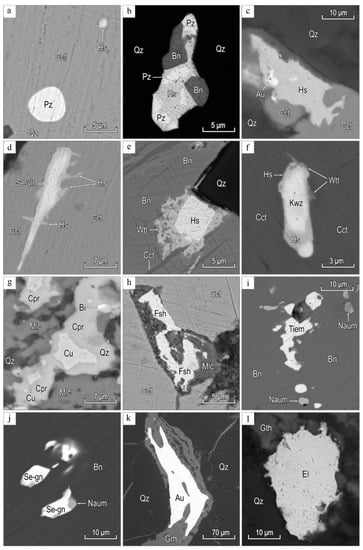
Figure 7.
Minerals gold–telluride–sulfide–quartz veins: (a) inclusions of hessite (Hs), and petzite (Pz) in chalcocite (Cct); (b) inclusions of hessite (Hs), petzite (Pz), and bornite (Bn) in quartz (Qz); (c) inclusions of hessite (Hs), and chalcocite (Cct) in quartz (Qz); (d) intergrowth of Se-volynskite (Vln) with hessite (Hs), in chalcocite (Cct); (f) intergrowth of Se-volynskite (Vln) with hessite (Hs), in chalcocite (Cct); (e) intergrowth of wittichenite (Witt) with hessite (Hs) in bornite (Bn), chalcocite (Cct), and quartz (Qz); (f) intergrowth of kawazulite (Kwz) with wittichenite (Witt), and hessite (Hs) in chalcocite (Cct); (g) intergrowth of native bismuth (Bi), cuprite (Cpr), and native copper (Cu) in malachite (Mlc); (h) inclusions of fischesserite (Fsch) in chalcocite (Cct); (I,j) inclusions of naumannite (Naum), tiemannite (Tiem), and Se-galena (Se-gn) in bornite (Bn); (k) gold (Au), and and goethite (Gth) in quartz (Qz); (l) inclusions of electrum (El) in quartz (Qz), and goethite (Gth). BSE images.
Hessite is intergrown with petzite, wittichenite, and Se-volynskite, locally up to 4 μm thick in the rims kawazulite. Petzite in intergrowths with hessite often has round and oval shapes. The compositions of Au and Ag tellurides are stoichiometric (Table 1).

Table 1.
Chemical composition of petzite, hessite, fischesserite, naumannite, and tiemannite, wt %.
We observed inclusions of fischesserite, naumannite, tiemannite, kawazulite, wittichenite, and Se-volynskite up to 40 μm and their fine intergrowths in quartz, bornite, and chalcocite (see Figure 7).
Fischesserite grains up to 20 μm occur in chalcocite. Fischesserite contains minor Pb (up to 0.98 wt %) and Te (up to 0.62 wt %) (Table 1).
Naumannite and tiemannite up to 25 μm are found in bornite as fine intergrowths.
Kawazulite and Se-volynskite (Se up to 10.45 wt %) form grains 10–40 μm long and 3–7 μm wide. Kawazulite is intergrown with hessite. Less commonly rimmed by Se-wittichenite; wittichenite rim is up to 2 μm around kawazulite (Table 2).

Table 2.
Chemical composition of Se-volynskite, kawazulite, and wittichenite, wt %.
Gold with high and medium fineness prevails; Au–Ag is rare (Figure 8) at the Ulug-Sair ore occurrence. The average gold fineness is 891‰, varied from 602 up to 967‰, and the average Au fineness of gold–sulfide–quartz veins (I) is 885‰ (615–967‰), and gold–telluride–sulfide–quartz veins (II) is 797‰ (601–904‰). Fineness ranges are significantly overlapped.
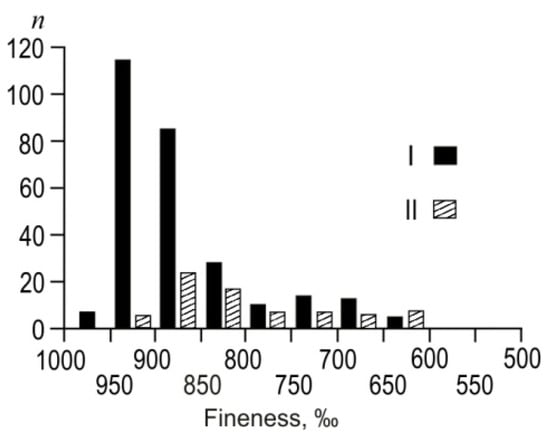
Figure 8.
Frequency of native Au fineness of I and II ore substages of the Ulug-Sair ore occurrence.
4.2. Fluid Inclusions
The fluid inclusions in quartz of mineral assemblages were analyzed to study the formation conditions of gold–quartz veins from the Ulug-Sair ore occurrence (see Figure 2). The fluid inclusion data are in Table 3 and Figure 9.

Table 3.
Fluid inclusion data of the Ulug-Sair ore occurrence.
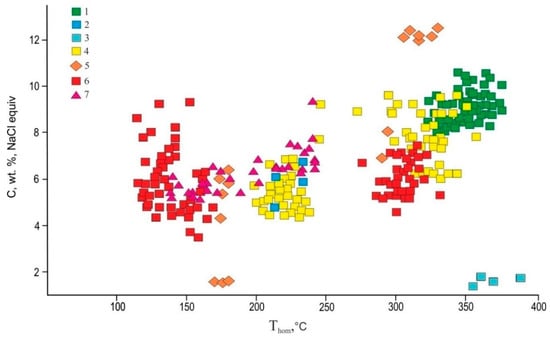
Figure 9.
Homogenization temperatures vs. salinity plot of fluid inclusions in quartz: 1—quartz–tourmaline metasomatites; 2, 3—quartz–carbonate–sericite-altered rocks (2—Ps, 3—P); 4, 5—gold–sulfide–quartz veins (I) (4—P, 5—Ps); 6, 7—gold–telluride–sulfide–quartz veins (II) (6—P, 7—Ps). P—primary, Ps—pseudosecondary fluid inclusions.
In the earliest quartz–tourmaline veins, we analyzed primary and pseudosecondary biphase (vapor + liquid (VL)) fluid inclusions of 10–20 μm, with oval or isometric shapes with smooth boundaries. The eutectic temperatures of the fluid ranged from −33 to −33.9 °C and −23 to −25 °C; therefore, the fluid is NaCl–MgCl2–KCl. Homogenization temperatures (into a liquid phase) range from 325 to 367 °C. The fluid salinity is 8–10 wt % NaCl eq.
Primary and pseudosecondary VL fluid inclusions are analyzed in quartz from quartz–carbonate–sericite-altered rocks. Primary VL inclusions are negative crystal shapes and 8–12 μm. According to Raman spectroscopy, they consist of CO2, CH4, and N2. The fluid contains Na chloride and K bicarbonate according to the eutectic temperatures between −10 and −8 °C. The rare inclusions contain dark-colored mineral phases with elongated shapes that are most likely trapped ore minerals. The salinity is 0.18–0.71 wt % NaCl eq. (temperatures of the final melting are −0.4 to −0.1 °C. Homogenization temperatures (into a liquid phase) vary from 350 to 400 °C. Pseudosecondary fluid inclusions are round shaped and up to 8 μm. The vapor bubbles do not exceed 15 vol. %. Homogenization (into a liquid phase) occurs at 200–240 °C (Table 3).
We analyzed primary and pseudosecondary biphase VL fluid inclusions in the quartz of gold–sulfide–quartz vein No. 18 (I) (see Figure 2) with high-grade gold. They are 8–15 μm with crystallographic outlines and isometric shapes. The eutectic temperatures range between −31.2 and −33.7 °C; therefore, the fluid contains NaCl–MgCl2–H2O. Homogenization temperatures (into a liquid phase) range from 240 to 360 °C. The salinity is 5.5–10 wt % NaCl eq.
Additionally, we analyzed fluid inclusions in quartz from gold–sulfide–quartz vein No. 4 (I) (see Figure 2) with high-grade gold and electrum. Primary VL and VLC inclusions are 12 μm and have elongated shapes. Pseudosecondary VL inclusions are 3–10 μm and are characterized by an isometric shape (Figure 10).
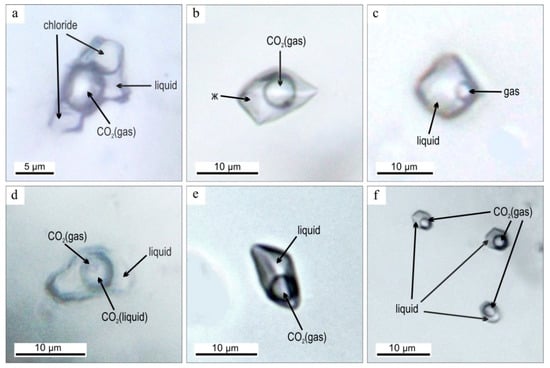
Figure 10.
Fluid inclusions in quartz of the Ulug-Sair ore occurrence: (a) VLS primary (sample US-4); (b) VL pseudosecondary (sample US-4); (c) VL secondary (sample US-4); (d) VLC primary (sample US-40a); (e) VL primary (sample US-40a); (f) VL primary/pseudosecondary (sample US-40a). FIA see in Table 2.
We detected CO2 in VL primary inclusions using Raman spectroscopy. The final melting temperatures (−8.3 to −4.2 °C) indicate a salinity of 6.7–12.5 wt % NaCl eq. The homogenization temperatures (into a liquid phase) are 290–330 °C. The CO2 primary VLC inclusions are homogenized (into a liquid) at 30.5–31 °C. We used this type of inclusions. The total homogenization of these inclusions was at 170–180 °C. These values correspond to CO2 densities ≈0.51–0.56 g/cm3 and a pressure of 750–900 bars. Pseudosecondary VL inclusions also contain carbon dioxide. Their eutectic temperatures are −21.2 to −21.7 and −23 to −23.9, as shown by Na–K-chloride fluid. The final melting temperatures are −4 to −1 °C, corresponding to the salinity of 1.7–6.8 wt % NaCl eq. The homogenization temperatures are 200–240 °C.
Additionally, we examined the primary and pseudosecondary VL inclusions in quartz from gold–telluride–sulfide–quartz vein No. 30 (II) (see Figure 2) with Au, petzite, hessite, Se-volynskite, kawazulite, and wittichenite. They are 8–15 μm and isometric angular or tabular shape with crystallographic outlines. The eutectic temperatures range between −20.9 and −32.1 °C and determined Na, K, and Mg chlorides in the fluid. Homogenization temperatures (into the liquid) are 270–330 °C. The salinity is 4.5–7.5 wt % NaCl eq.
The primary and pseudosecondary VL inclusions in quartz from Pyritovaya gold–sulfide–quartz vein (II) (sample ALR-Py, see Table 2) (see Figure 2) are up to 15 μm, oval and round shapes, with large vapor bubbles (up to 20–25 vol. %). The eutectic temperatures range between −21.3 and −24.9 °C indicated the presence of Na and K chlorides in the fluid. The salinity varies from 5.0 to 9.5 wt % NaCl eq. The homogenization temperatures (into a liquid phase) are 130–250 °C.
We also analyzed VLC and VL primary and pseudosecondary 8–12 μm fluid inclusions in quartz from gold–telluride–sulfide–quartz veins No. 40 and 41 (II) with Au, electrum, acanthite, and hessite (see Figure 9). Gas CO2 bubbles appear only during the cooling. The CO2 homogenization temperatures (into a liquid) were estimated upon further heating and reached +4 to +16.8 °C. The temperatures of total homogenization have not been detected because, at 230–250 °C, inclusions were decrypted. Primary VL inclusions are 4–10 μm and rounded and elongated. The vapor bubbles of inclusions contain CO2 according to Raman spectroscopy data. The homogenization of inclusions (into a liquid) occurred at 220–235 °C. At a temperature of about 240 °C, we observed the decrepitation of inclusions. The pseudosecondary VL inclusions are 8–12 μm and in the form of negative crystals. The gas phase contains CO2. The homogenization temperatures are 140–160 °C.
The primary VL inclusions in quartz from No. 33 veins (II) are up to 15 μm, acute-angled with crystallographic outlines, with large (up to 20 vol. %) vapor bubbles. Their eutectic temperatures ranged between −22.7 and −23.9 °C, corresponding to Na and K chloride fluid. Large inclusions (more than 20 μm) have lower eutectic temperatures (−29.7 to −37.2 °C) corresponding to MgCl2–H2O ± FeCl2–H2O fluid. The salinity is 3.5–9.5 wt % NaCl eq., and homogenization temperatures (into a liquid phase) are 115–170 °C.
The bulk analysis of water and gas extracts from fluid inclusions in quartz powder provided the data regarding the composition of fluids of the Ulug-Sair mineralization. In the fluid from inclusions in quartz of Au–sulfide–quartz veins (I) (Table 3), Na (5.73–6.48) prevails among cations (g/kg H2O); but K (0.14–0.32), Ca (0.01–0.04), and Mg (0.101–0.005) are subordinate. The significant amounts (g/kg H2O) are detected for CO2 (41.31–56.24), HCO3− (10.04–11.61), Cl− (2.65–4.31), and CH4 (0.027–0.048). The important trace elements are (mg/kg of the fluid): B (74.40–295.5), Ba (53.31–57.85), Sr (17.19–27.67), As (45.42–66.74), Cu (0.03–15.53), Sb (6.27–8.07), Mn (0.40–9.23), Ni (1.26–3.0), and Fe (1.62–2.03) (Table 4).

Table 4.
Element composition of fluid inclusions of the Ulug-Sair ore.
In extracts form fluid inclusions in Au–telluride–sulfide–quartz veins (II) (g/kg H2O) Na (6.7–12.9) prevails, while K (0.16–1.32), Ca (0.00–1.96), and Mg (0.00–0.25) are subordinate. The amounts of volatiles are identified in (g/kg H2O): CO2 (69.77–85.58), HCO3− (13.13–35.74), Cl− (2.81–4.51), and CH4 (0.041–0.196). The significant trace elements are (mg/kg of fluid): B (129.53–696.3), Ba (153–638), Cu (0.00–780), Sr (19.24–82.27), As (101.4–208.8), Sb (7.37–27.87), Ni (0.47–20.38), and Fe (0.00–9.17), Zn (0.00–58.48), Pb (0.00–1.18), W (0.00–9.07), and Mo (0.47–10.89).
The average chemical composition of the fluid is shown in Figure 11.
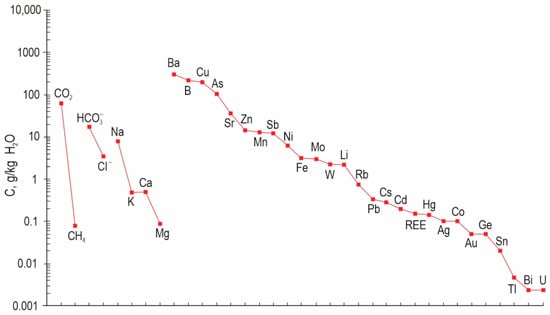
Figure 11.
The average chemical composition of the Ulug-Sair ore occurrence fluid.
4.3. Fluid Isotopic Composition of S and O
The isotopic composition of S in pyrite from the I ore substage is 3.5‰, and in substage II is 1.6‰, and is characterized by stable near-zero values ranging between +1.6 and +3.5‰.
The δ34S values of the fluid coexisting with sulfides, according to the fractionation equation [47,48] of gold–sulfide–quartz veins (I) vary from +2.0 to +2.5‰ (T = 350–250 °C), and in gold–telluride–sulfide–quartz veins (II) from −0.4 to +2.1‰ (T = 280–170 °C), demonstrating the participation of magmatic (0 ± 5‰) or mantle (0 ± 3‰) sulfur [47,48,49,50]. We noted that, while calculating the sulfur isotopic composition of fluids, the estimation of mineral formation values by an electrum-sphalerite geothermometer was 360–280 °C [51], and the parameters of the formation of Ag3AuTe2–Ag2Te–Au parageneses [52] were (280–170 °C).
The δ18O value of quartz from gold–sulfide–quartz veins (I) varies from + 17.2 to +17.5‰, and in gold–telluride–sulfide–quartz veins (II) from +17.3 to +18.5‰.
According to the fractionation equation [53,54], the δ18O of gold–sulfide–quartz veins (I) fluid varies from +8.5 to +11.6‰ (T = 350–260 °C), and in gold–telluride–sulfide–quartz veins (II) from +3.1 to +10.4‰ (T = 280–170 °C).
The points of the oxygen isotopic composition of the fluid of the early mineral association (I) fall in the range of fluids’ magmatic and metamorphic origin. The latest mineral association (I) indicates a mixture of metamorphic or magmatic fluids with meteoric water (Figure 12).
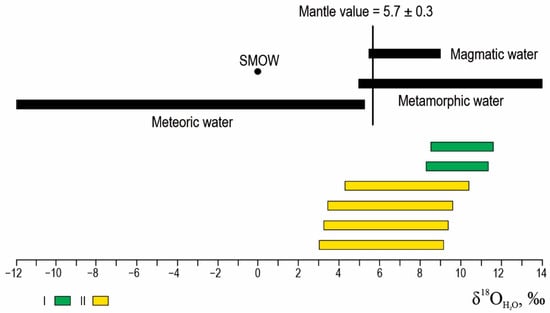
Figure 12.
Oxygen isotopic composition of the fluid of I and II ore substages of the Ulug-Sair occurrence.
Note that the values of δ34S fluid from −0.7 to +2.5‰ suggest the magmatic source of sulfur (0 ± 3‰).
5. Discussion
Gold–sulfide–quartz veins at the Ulug-Sair mineral system are confined to the host sedimentary rocks (conglomerates, siltstones, shales), quartz–tourmaline, and quartz–carbonate–sericite-altered rocks.
Early high-temperature quartz–tourmaline-altered rocks are widespread at Ulug-Sair. For the nearby Khaak-Sair occurrence, they are confined to intrusions of tonalite–porphyry of the Bayankol complex [29,30]. The widespread tourmaline mineralization suggests the presence of a deep-seated granitoid intrusion. The presence of boron is indirectly indicated by data on the salt composition of the fluid according to the thermometric results and ICP-MS. We note that the scheelite–quartz–tourmaline veins and quartz–tourmaline-altered rocks of the Berezovsky (Urals, Russia) intrusion-related gold deposit are confined to the Shartash intrusion [55].
Native gold was formed during two substages of mineralization formation. The grains of gold in both ore substages are similar in composition, but the II substage is characterized by an exceptional mineral diversity and the presence of tellurides (petzite Ag3AuTe2, Ag2Te), selenides (fischesserite Ag3AuSe2, naumannite Ag2Se, and tiemannite HgSe), and diverse Bi-minerals (kawazulite Bi2Te2Se, Se-volynskite AgBiTe2, native Bi, and wittichenite Cu3BiS3. In terms of mineralogical and geochemical features, the Ulug-Sair ore is close to gold–telluride and gold–bismuth (gold–bismuth–telluride) mineralization-type deposits.
Raman spectroscopy and fluid inclusions data demonstrated that quartz–tourmaline and quartz–carbonate–sericite-altered rocks, and Au-bearing veins of the Ulug-Sair ore occurrence were formed by carbon dioxide–water–chloride (Na + K ± Mg) fluid. The fluid oxidation ratio (CO2/CO2 + CH4 + N) during mineral formation is stable, ranging between 1.04 and 1.12. The presence of selenides and Se-bearing minerals indicates the oxidized environment of mineral formation and the participation of acidic fluids [56].
Fluid inclusions data indicated that the quartz–carbonate–sericite-altered rocks were formed due to methane–carbon dioxide–water-chloride low–medium-salt fluid with a concentration of 0.18–6.1 wt % NaCl eq. at temperatures of at least 200–400 °C, corresponding to the temperatures of the formation of mesothermal gold deposits, including the large deposits of the Urals associated with quartz–carbonate–sericite-altered rocks [55,56,57].
Fluid salinity in the early gold–sulfide–quartz veins range from 12.5 to 1.7 wt % NaCl eq at temperatures of 200–360 °C. These data are consistent with previous studies of fluid inclusions in the quartz of such veins [58], showing that they were deposited at temperatures of 250–370 °C from fluid with salinities of 4–10 wt % NaC1 eq. and pressures of 0.9–1 kbar. The pressure during the formation of early gold–sulfide–quartz veins was 0.75–1.0 kbar (~2.3–3 km) according to our results on three-phase inclusions.
Mineral paragenesis of gold–sulfide–quartz veins show that ore formation occurred with high fugacity (f) sulfide sulfur lg f(S2) = 10–14.3–10–8.6 (T = 250 °C) [39,40].
The latest gold–telluride–sulfide–quartz veins (II) were formed by a fluid with a salinity of 9.5–3.5 wt % NaCl eq., with wide variations of temperatures—110–330 °C.
We note that the parameters of the formation of Ag3AuTe2–Ag2Te–Au parageneses range between 170 and 280 °C, at f(Te2) = 10−18–10−10 [52]. The paragenesis of tellurides, sulfides, and selenides in gold–telluride–sulfide–quartz veins was produced at fTe2 from 10−21 to 10−9, fS2—10−25–10−9, fSe2—10−21–10−12 at 200 °C [39,40,41,42].
ICP-MS data show that hydrocarbonate with a concentration significantly higher than chlorine prevailed among the anions in the fluid. The fluid within the cations is most enriched in Na with impurities of Ca, K, and Mg, and it can be attributed to the hydrocarbonate–chloride–sodium type, which does not contradict the fluid inclusion data. The elevated amounts of Ca and Mg (Mn) and the bicarbonate ion are shown in the formation of carbonates in the host quartz–carbonate–sericite-altered rocks. The fluid composition with ore elements (B, As, Sb, Cu, Fe Ag, Ba) reflects the components of the gold-bearing mineral assemblages.
The isotopic composition of δ34Sfluid (from −0.7 to +2.5‰) indicates the juvenile or magmatic origin of ore elements [47,48,50].
The isotopic composition of δ34Ofluid implies that, during the early substages, the formation of ore involved a fluid of metamorphic origin (from +8.2 to +11.6‰). During the latest substages, multiple sources of the hydrothermal fluids (from +3.1 to +10.4‰), including magmatic-derived, metamorphic-derived, and meteoric waters, with predominance the metamorphic-derived fluid typical for orogenic gold deposits [59] are shown.
The fluid salinity up to 12.51 wt % NaCl eq. and a complex composition with Na, K, and Mg chlorides, Na hydrocarbons, and borates indicate the involvement of fluid of magmatic origin. The elevated amounts of boron in fluid and the presence of “magmaphile” elements (W, Sb, Mo, Sb) also confirm involvement of the magmatic genesis of the fluid [60,61].
The salinity reduction during the mineral formation from 12.5 to 1.74 wt % NaCl eq. may be caused by a dilution of igneous fluid with increased salinity and heated meteoric waters [62]. Note that the Se-containing minerals in ores indicate a high oxidation rate of the ore environment that could be caused by mixing ore-bearing fluid with meteoric waters.
The involvement of magmatic, metamorphic, or meteoric fluids in ore development is typical for several gold deposits in Russia (Beresovskoye, Kochkar) with a dominant role of magmatic fluid [56].
The determined conditions of ore genesis allow us to classify the Ulug-Sair gold mineral system as a mesozonal to epizonal orogenic gold. It should be noted that orogenic gold deposits are subdivided into hypozonal, mesozonal, and epizonal subtypes, distinguished by their differences in depth of formation [63]. Moreover, they may differ in sources of ore-forming fluids and metals [59,64]. The origin of orogenic deposits is most commonly explained by the metamorphism or slab devolatilization model [65,66,67]. Nevertheless, some orogenic gold deposits may have been generated from magmatic fluids [64,68,69].
6. Conclusions
The results herein presented support the hypothesis that mineralization is associated with tectonic-magmatic activity in which sub-latitudinal tectonic disruption becomes a favorable environment for the circulation of hydrothermal fluids. This resulted in the formation of linear zones with gold–quartz veins in quartz–tourmaline and quartz–carbonate–sericite-altered rocks, conglomerates, and siltstones. The present study proves a multiple source for the ore-forming fluids in the Ulug-Sair hydrothermal system. It is assumed that the magmatic fluids were generated by dykes and small granitoid intrusions of the Late Devonian Bayankol complex, which are assumed to be deep-seated. The Ulug-Sair ore can be referred to as an orogenic gold deposit on the basis of geological, mineralogical, and fluid inclusion, and on isotopic data.
Author Contributions
Conceptualization, R.V.K.; methodology, R.V.K. and N.N.A.; expeditionary work, R.V.K., A.A.M. and Y.V.B.; investigation, R.V.K., N.N.A. and visualization, R.V.K. and N.V.S.-M.; writing—original draft preparation, R.V.K., N.N.A. and F.P.; data interpretation, review, and editing, F.P.; writing—review and editing, R.V.K., N.N.A., N.V.S.-M. and A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of science and higher education of the Russian Federation (project TuvIENR SB RAS No. 2021-0002 and project IM SU FRS MG UB RAS No. 075-00880-22ΠP).
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are grateful to Anna A. Redina and Ilya R. Prokopyev for their analytical works. The authors are deeply grateful to the reviewers for allowing us to make this paper better.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cook, N.J.; Ciobanu, C.L.; Spry, P.G.; Voudouris, P.; the participants of IGCP-486. Understanding gold-(silver)-telluride-(selenide) mineral deposits. Episodes 2009, 32, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Cooke, D.R.; Deyell, C.L.; Waters, P.J.; Gonzales, R.I.; Zaw, K. Evidence for magmatic-hydrothermal fluids and ore-forming processes in epithermal and porphyry deposits of the Baguio District, Philippines. Econ. Geol. 2011, 106, 1399–1424. [Google Scholar] [CrossRef]
- Gamyanin, G.N.; Goncharov, V.I.; Goryachev, N.A. Gold-Raremetal deposits of northeastern Russia. Tikhookeanskaya 1998, 17, 94–103. [Google Scholar]
- Goryachev, N.A.; Gamyanin, G.N. Gold-Bismuth (Gold-Raremetal) Deposits of North East Russia: Types, and Exploration Perspectives. Gold ore Deposits of East Russia; NESC FEB RAS: Magadan, Russian, 2006; pp. 50–62. [Google Scholar]
- Hedenquist, J.W.; Arribas, A., Jr.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system: Far Southeast-Lepanto porphyry-epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef] [Green Version]
- Sillitoe, R.H.; Thompson, F.H. Intrusion-related vein gold deposits: Types, tectono-magmatic settings, and difficulties of distinction from orogenic gold deposits. Resour. Geol. 1998, 48, 237–250. [Google Scholar] [CrossRef]
- Lang, J.R.; Baker, T. Intrusion related gold systems: The present level of understanding. Miner. Depos. 2001, 36, 477–489. [Google Scholar] [CrossRef]
- Pak, S.J.; Choi, S.-G.; Oh, C.-W.; Heo, C.-H.; Choi, S.-H.; Kim, S.-W. Genetic environment of the intrusion-related Yuryang Au-Te deposit in the Cheonan metallogenic province, Korea. Resour. Geol. 2006, 56, 117–132. [Google Scholar] [CrossRef]
- Rowins, S.M. Reduced porphyry copper-gold deposits: A new variation on an old theme. Geology 2000, 28, 491–494. [Google Scholar] [CrossRef]
- Vikent’eva, O.V.; Prokofiev, V.Y.; Gamyanin, G.N.; Goryachev, N.A.; Bortnikov, N.S. Intrusion-related gold–bismuth deposits of North-East Russia: PTX parameters and sources of hydrothermal fluids. Ore Geol. Rev. 2018, 100, 240–259. [Google Scholar] [CrossRef]
- Smith, M.T.; Thompson, J.F.H.; Bressler, J.; Layer, P.; Mortensen, J.K.; Abe, I.; Takaoka, H. Geology of the Liese Zone, Pogo property, east-central Alaska. SEG Discov. 1999, 38, 1–21. [Google Scholar] [CrossRef]
- Hart, C.J.R.; McCoy, D.; Goldfarb, R.J.; Smith, M.; Roberts, P.; Hulstein, R.; Blake, A.A.; Bundtzen, T.K. Geology, exploration and discovery in the Tintina gold province, Alaska and Yukon. Soc. Econ. Geol. Spec. Publ. 2002, 9, 241–274. [Google Scholar]
- Damdinov, B.B.; Garmaev, B.L.; Mironov, A.G.; Dashinmaev, Z.B. Gold-bismuth mineralization in the southeastern part of the Eastern Sayan. Dokl. Earth Sci. 2009, 425, 256–259. [Google Scholar] [CrossRef]
- Garmaev, B.L.; Damdinov, B.B.; Mironov, A.G. Pogranichnoe Au–Bi occurrence, Eastern Sayan: Composition and link to magmatism. Geol. Ore Deposits. 2013, 55, 455–466. [Google Scholar] [CrossRef]
- Moravek, P. The Mokrsko gold deposit. In Gold Deposits of the Central and SW Part of the Bohemian Massif. Third Biennial Society for Geology Applied to Mineral Deposits Meeting; Moravek, P., Ed.; Excursion Guide: Prague, Czech Republic, 1995; pp. 33–61. [Google Scholar]
- Nie, F.-J.; Jiang, S.-H.; Liu, Y. Intrusion-Related Gold Deposits of North China Craton, People’s Republic of China. Resour. Geol. 2004, 54, 299–324. [Google Scholar] [CrossRef]
- Baker, T.; Pollard, P.J.; Mustard, R.; Mark, G.; Graham, J.L. A comparison of granite-related tin, tungsten, and gold-bismuth deposits: Implications for exploration. SEG Newsl. 2005, 61, 5–17. [Google Scholar] [CrossRef]
- Goryachev, N.A.; Pirajno, F. Gold deposits and gold metallogeny of Far East Russia. Ore Geol. Rev. 2014, 59, 123–151. [Google Scholar] [CrossRef]
- Lindgren, W. Mineral Deposits, 4th ed.; McGraw–Hill Book Company: New York, NY, USA, 1933; p. 930. [Google Scholar]
- Hedenquist, J.W. The ascent of magmatic fluid: Discharge versus mineralization. Mineral. Assoc. Can. Short Course Ser. 1995, 23, 263–289. [Google Scholar]
- Richards, J.P. Alcalic-type epithermal gold deposits—A review. Mineral. Assoc. Can. Short Course Ser. 1995, 23, 367–400. [Google Scholar]
- Shackleton, J.M.; Spry, P.G.; Bateman, R. Telluride mineralogy of the Golden Mile deposit, Kalgoorlie, Western Australia. Can. Mineral. 2003, 41, 1503–1524. [Google Scholar] [CrossRef] [Green Version]
- Spry, P.; Foster, F.; Truckle, J.; Chadwick, T. The mineralogy of the Golden Sunlight gold–silver telluride deposit, Whitehall, Montana, USA. Mineral. Petrol. 1997, 59, 143–164. [Google Scholar] [CrossRef]
- Kelley, K.D.; Romberger, S.B.; Beaty, D.W.; Pontius, J.A.; Snee, L.W.; Stein, H.J.; Thompson, T.B. Geochemical and geochronological constraints on the genesis of Au-Te Deposits at Cripple Creek, Colorado. Econ. Geol. 1998, 93, 981–1012. [Google Scholar] [CrossRef]
- Ahmad, H.; Solomon, M.; Walshe, J.L. Mineralogical and geochemical studies of the Emperor gold telluride deposit, Fiji. Econ. Geol. 1987, 82, 345–370. [Google Scholar] [CrossRef]
- Pals, D.; Spry, P. Telluride mineralogy of the low-sulfidation epithermal Emperor gold deposit, Vatukoula, Fiji. Mineral. Petrol. 2003, 79, 285–307. [Google Scholar] [CrossRef]
- Cooke, D.R.; McPhail, D. Epithermal Au–Ag–Te mineralization, Acupan, Baguio district, Philippines: Numerical simulations of mineral deposition. Econ. Geol. 2001, 96, 109–131. [Google Scholar]
- Vikent’eva, O.; Prokofiev, V.; Borovikov, A.; Kryazhev, S.; Groznova, E.; Pritchin, M.; Vikentyev, I.; Bortnikov, N. Contrasting Fluids in the Svetlinsk Gold-Telluride Hydrothermal System, South Urals. Minerals 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Zaykov, V.V.; Melekestseva, I.Y.; Ankusheva, N.N.; Mongush, A.A.; Kuzhuget, R.V. The Aldan-Maadyr gold-bearing zone in Hg-listvenites and tourmaline altered rocks, Republic of Tuva: Mineralogy, forming conditions and resources. In Large Igneous Provinces of Asia, Mantle Plumes and Metallogeny: Abs. of the International Symposium; Sibprint: Novosibirsk, Russia, 2009; pp. 414–417. [Google Scholar]
- Kuzhuget, R.V.; Zaykov, V.V.; Lebedev, V.I.; Mongush, A.A. Gold mineralization of the Khaak-Sair gold-quartz ore occurrence in listwanites (western Tuva). Russ. Geol. Geophys. 2015, 56, 1332–1348. [Google Scholar] [CrossRef]
- Berzin, N.A.; Kungurtsev, L.V. Geodynamic interpretation of Altai–Sayan Geological complexes. Russ. Geol. Geophys. 1996, 37, 56–73. [Google Scholar]
- Kuzhuget, R.V.; Ankusheva, N.N.; Redina, A.A.; Prokopyev, I.R.; Ponomarchuk, A.V. Khaak-Sair gold-sulfide-quartz ore occurrence (Western Tuva): Dating, PT parameters, fluid composition, and isotopes of S, O and C. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2021, 332, 148–163. [Google Scholar] [CrossRef]
- Kuzhuget, R.V.; Ankusheva, N.N.; Redina, A.A.; Prokopevd, I.R.; Ondar, E.V. Aryskan gold-sulphide-quartz ore occurrence (Western Tuva): Conditions of formation and geochemical peculiarities of fluid. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2020, 331, 224–237. [Google Scholar] [CrossRef]
- Kuzhuget, R.V. Gold-Telluride Mineralization of the Aldan-Maadyr Ore Cluster (Western Tuva): Mineralogical and Geochemical Features of Ores and Conditions for Their Formation. Ph.D. Thesis, TuvIENR SB RAS, Novosibirsk, Russia, 2014; p. 20. [Google Scholar]
- Spenser, R.J.; Moller, N.; Weare, J.N. The prediction of mineral solubilities in mineral waters: A chemical equilibrium model for the Na-K-Ca-Mg-Cl-SO4 system at temperatures below 25 °C. Geochim. Et Cosmochim. Acta 1990, 54, 575–590. [Google Scholar] [CrossRef]
- Davis, D.W.; Lowenstein, T.K.; Spenser, R.J. Melting behavior of fluid inclusions in laboratory-grown halite crystals in the systems NaCl-H2O, NaCl-KCl-H2O, NaCl-MgCl2-H2O, and CaCl2-NaCl-H2O. Geochim. Cosmochim. Acta 1990, 54, 591–601. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O–NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications; De Vivo, B., Frezzotti, M.L., Eds.; Fluids Research Laboratory, Department of Geological Sciences, Virginia Tech: Blacksburg, VA, USA, 1994; pp. 117–130. [Google Scholar]
- Roedder, E. Fluid inclusions. In Reviews in Mineralogy and Geochemistry; De Gruyter: Berlin, Germany, 1984; Volume 12, p. 646. [Google Scholar]
- Barton, P.B.; Skinner, B.J. Sulfide mineral stabilities. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Willey & Sons: New York, NY, USA, 1979; pp. 278–403. [Google Scholar]
- Afifi, A.M.; Kelly, W.C.; Essene, E.J. Phase relations among tellurides, sulphides and oxides: I. Thermochemical data and calculated equilibria. Econ. Geol. 1988, 83, 377–404. [Google Scholar] [CrossRef]
- Zhuravkova, T.V.; Palyanova, G.A.; Kalinin, Y.A.; Goryachev, N.A.; Zinina, V.Y.; Zhitova, L.M. Physicochemical conditions of formation of gold and silver parageneses at the valunistoe deposit (Chukchi peninsula). Russ. Geol. Geophys. 2019, 60, 1247–1256. [Google Scholar] [CrossRef]
- Palyanova, G.A.; Savva, N.E.; Zhuravkova, T.V.; Kolova, E.E. Minerals of gold and silver in pyrites of low-sulfide ores of the Juliet deposit (North-East of Russia). Russ. Geol. Geophys. 2016, 57, 1488–1510. [Google Scholar] [CrossRef]
- Brown, P.E. Flincor: A microcomputer program for the reduction and investigation of fluid inclusion data. Am. Miner. 1989, 74, 1390–1393. [Google Scholar]
- Kryazhev, S.G.; Prokof’ev, V.Y.; Vasyuta, Y.V. Use of method ICP MS at the analysis of composition of ore-forming fluids. Vestn. MSU Geol. 2006, 4, 30–36. [Google Scholar]
- Prokofiev, V.Y.; Kiseleva, G.D.; Dolomanova-Topol, A.A.; Borisovsky, S.E.; Trubkin, N.V.; Magazina, L.V.; Kryazhev, S.G.; Krasnov, A.N.; Zorina, L.D. Mineralogy and Formation Conditions of Novoshirokinsky Base Metal–Gold Deposit, Eastern Transbaikal Region, Russia. Geol. Ore Deposits. 2017, 59, 521–550. [Google Scholar] [CrossRef]
- Kita, I.; Matsubaya, O. F2-technique for the oxygen isotopic analysis of silica minerals. Rep. Res. Inst. Nat. Resour. Min. Coll. Akita Univ. 1983, 48, 25–33. [Google Scholar]
- Ohmoto, H.; Rye, R.O. Isotopes of Sulfur and Carbon. Geochemistry of Hydrothermal Ore Deposits; Wiley: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Li, Y.; Liu, J. Calculation of sulfur isotope fractionation in sulfides. Geochim. Cosmochim. Acta 2006, 70, 1789–1795. [Google Scholar] [CrossRef]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. In Stable Isotopes in High Temperature Geological Processes. Rev. Mineral. Geochem. 1986, 16, 491–560. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2009; p. 281. [Google Scholar]
- Shikazono, N. A comparison of temperatures estimated from the electrum-sphalerite-pyrite-argentite assemblage and filling temperatures of fluid implications from epitermal Au-Ag vein-type deposits in Japan. Econ. Geol. 1985, 80, 1415–1424. [Google Scholar] [CrossRef]
- Bortnikov, N.S.; Kramer, H.; Genkin, A.D. Paragenesis of tellurides of gold and silver in the gold ore deposit Florencia (Republic of Cuba). Geol. Ore Deposits. 1988, 2, 49–61. [Google Scholar] [CrossRef]
- Zhang, L.-G.; Liu, J.-X.; Zhou, H.B.; Chen, Z.-S. Oxygen isotope fractionation in the quartz-water-salt system. Econ. Geol. 1989, 89, 1643–1650. [Google Scholar] [CrossRef]
- Zheng, Y.F. Oxygen isotope fractionation in carbonate and sulfate minerals. Geochem. J. 1999, 33, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Vikent’eva, O.V.; Bortnikov, N.S.; Vikentyev, I.V.; Groznova, E.O.; Lyubimtseva, N.G.; Murzin, V.V. The Berezovsk Giant Intrusion-Related Gold Quartz deposit, Urals, Russia: Evidence for Multiple Magmatic and Metamorphic Fluid Reservoirs. Ore Geol. Rev. 2017, 91, 837–863. [Google Scholar] [CrossRef]
- Bortnikov, N.S. Geochemistry and the origin of ore-forming fluids in hydrothermal-magmatic systems in tectonically active zones. Geol. Ore Depos. 2006, 48, 3–28. [Google Scholar] [CrossRef]
- Belogub, E.V.; Melekestseva, I.Y.; Novoselov, K.A.; Zabotina, M.V.; Tretyakov, G.A.; Zaykov, V.V.; Yuminov, A.M. Listvenite-related gold deposits of the South Urals (Russia): A review. Ore Geol. Rev. 2017, 85, 247–270. [Google Scholar] [CrossRef]
- Borisenko, A.S.; Lebedev, V.I.; Obolensky, A.S.; Zaykov, V.V.; Tyulkin, V.G. Physical and chemical conditions for the formation of hydrothermal deposits in Western Tuva. In Basic Parameters of Natural Processes of Endogenous Ore Formation; Nauka: Novosibirsk, Russia, 1979; pp. 226–235. [Google Scholar]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common or evolving fluid and metal sources through time. Lithos 2015, 233, 2–26. [Google Scholar] [CrossRef]
- Prokof’ev, V.; Zorina, L.; Reyf, F.; Ishkov, Y.; Kudryavtseva, O.; Baksheev, I. Conditions of Gold Mineralization by Boron-Rich Fluids. In Proceedings of the 17th International Conference European Current Research on Fluid Inclusions, Budapest, Hungary, 5–7 June 2003; Acta Mineralogica-Petrographica Abstract Series 2. pp. 165–166. [Google Scholar]
- Kun, L.; Ruidong, Y.; Wenyong, C.; Rui, L.; Ping, T. Trace element and REE geochemistry of the Zhewang gold deposit, southeastern Guizhou Province. Chin. J. Geochem. 2014, 3, 109–118. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Gebre-Mariam, M.; Hagemann, S.G.; Robert, F. Orogenic gold deposits: A proposed classification in the context of their crustal distribution and relationship to other gold deposit types. Ore Geol. Rev. 1998, 13, 7–27. [Google Scholar] [CrossRef]
- Ridley, J.R.; Diamond, L.W. Fluid chemistry of orogenic lode gold deposits and implications for genetic models. SEG Rev. 2000, 13, 141–162. [Google Scholar] [CrossRef]
- Phillips, G.N.; Powell, R. Formation of gold deposits: A metamorphic devolatilization model. J. Metamorph. Geol. 2010, 28, 689–718. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Deng, J.; Wang, Q.; Yang, L.; Zhang, L. A holistic model for the origin of orogenic gold deposits and its implications for exploration. Miner. Depos. 2020, 55, 275–292. [Google Scholar] [CrossRef]
- Kouhestani, H.; Pashidnejad-Omran, N.; Rastad, E.; Mohajjol, M.; Goldfarb, R.; Ghaderi, M. Orogenic gold mineralization at the Chah Bagh deposit, Muteh gold district, Iran. J. Asian Earth Sci. 2014, 91, 89–106. [Google Scholar] [CrossRef]
- Wang, C.; Shao, Y.-J.; Evans, N.J.; Li, H.; Zhou, H.-D.; Huang, K.-X.; Liu, Z.-F.; Chen, Y.; Lai Ch Liu, Q.-Q. Genesis of Zixi gold deposit in Xuefengshan, Jiangnan Orogen (South China): Age, geology and isotopic constraints. Ore Geol. Rev. 2020, 117, 103301. [Google Scholar] [CrossRef]
- Izvekova, A.D.; Damdinov, B.B.; Damdinova, L.B.; Moskvitina, M.L. Gold-telluride mineralization in ore of Pionerskoe goldquartz deposit (Eastern Sayan, Russia). Geol. Ore Depos. 2021, 63, 579–598. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).