Green and Efficient Utilization of Ferruginous Gibbsite Ore and Ferruginous Manganese Ore by Synergetic Carbothermic Co-Reduction–Magnetic Separation Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Carbothermic Co-Reduction via Magnetic Separation Process
2.3. Magnetic Separation Process
2.4. Separation Indexes
2.5. Analysis and Characterization Methods
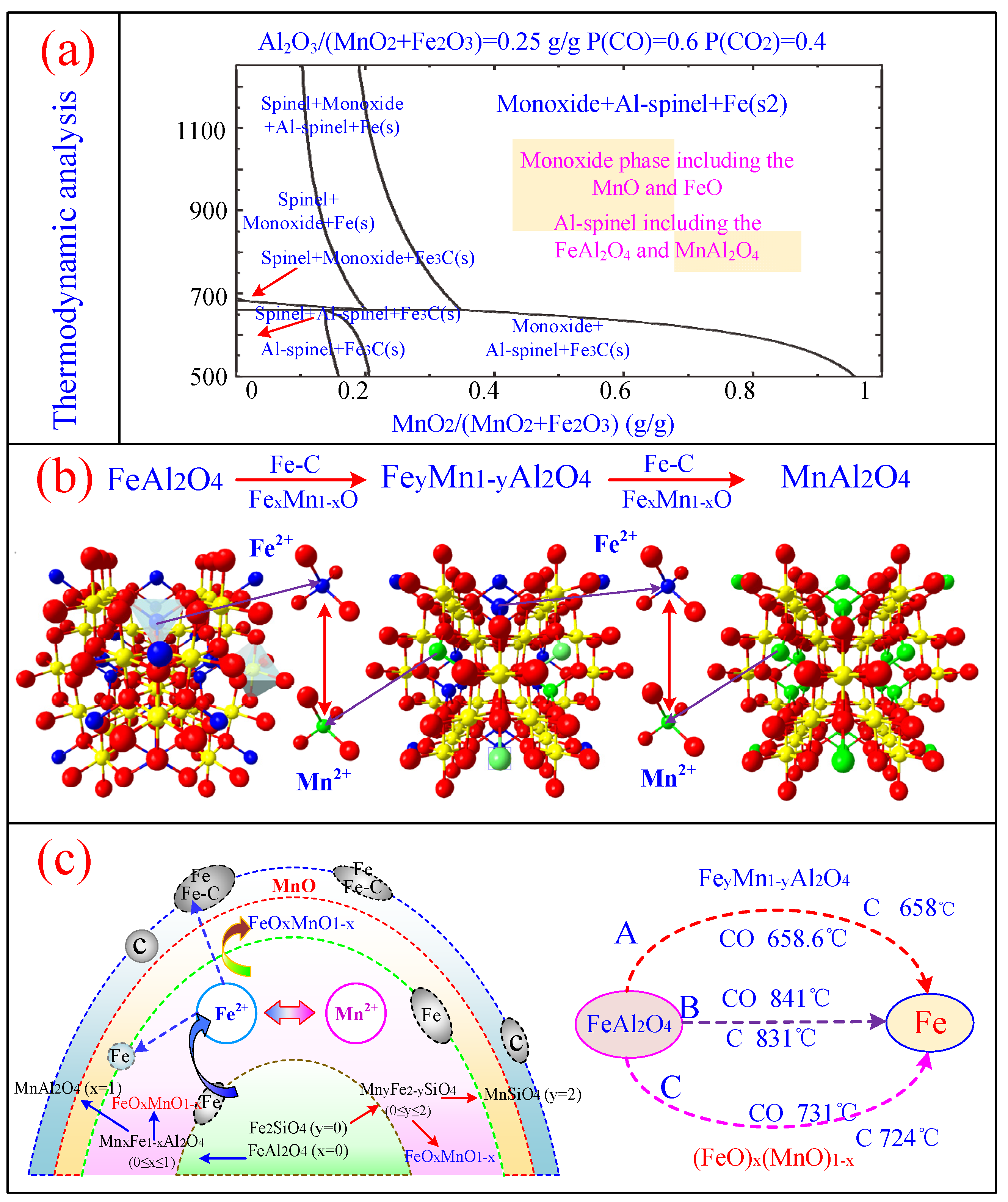
3. Thermodynamic Analysis of the Co-Reduced Al-Fe Ores and Mn-Fe Ores
4. Results and Discussion
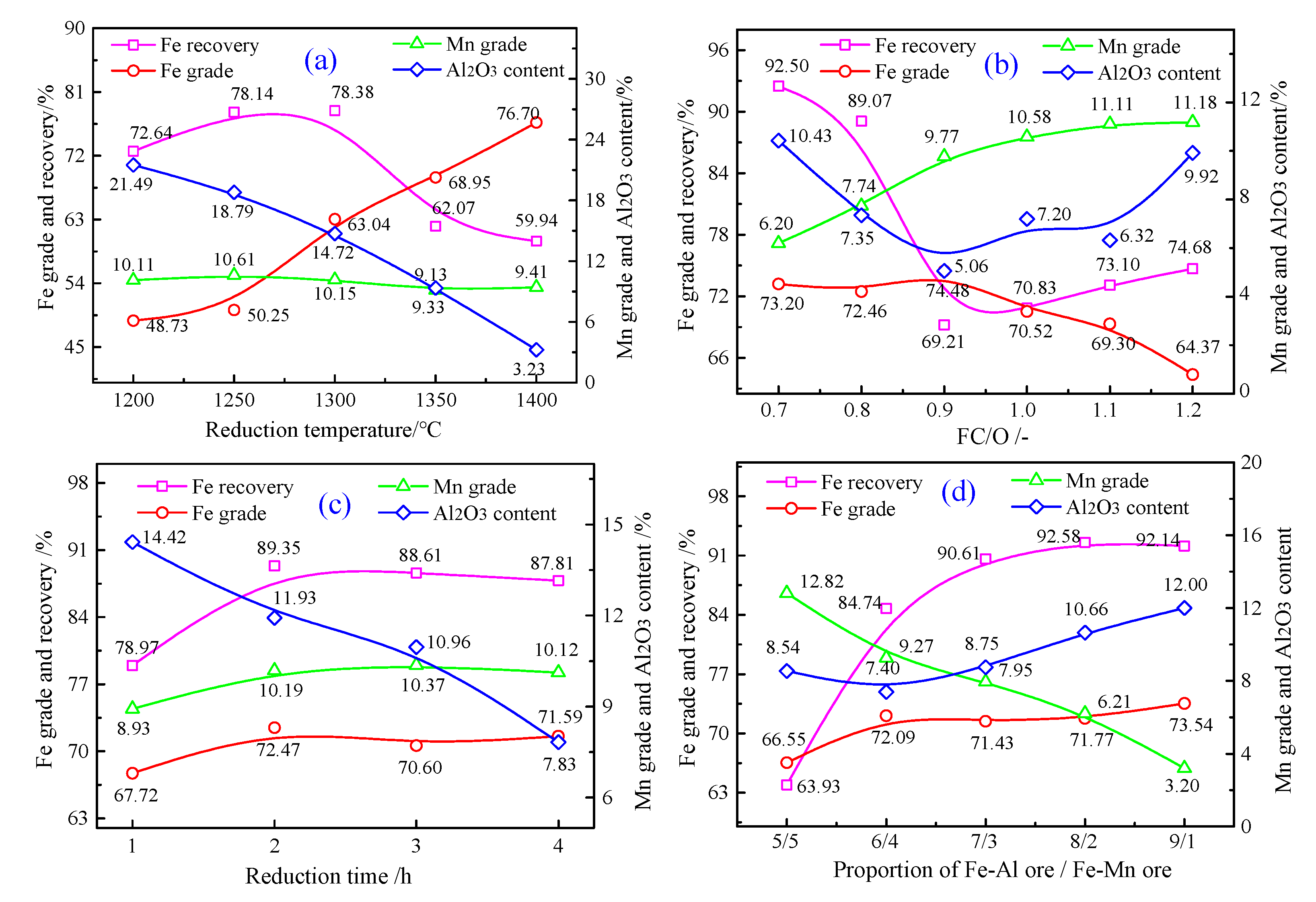
4.1. Results of the Carbothermic Co-Reduction Roasting–Magnetic Separation of Al-Fe Ores/Mn-Fe Ores
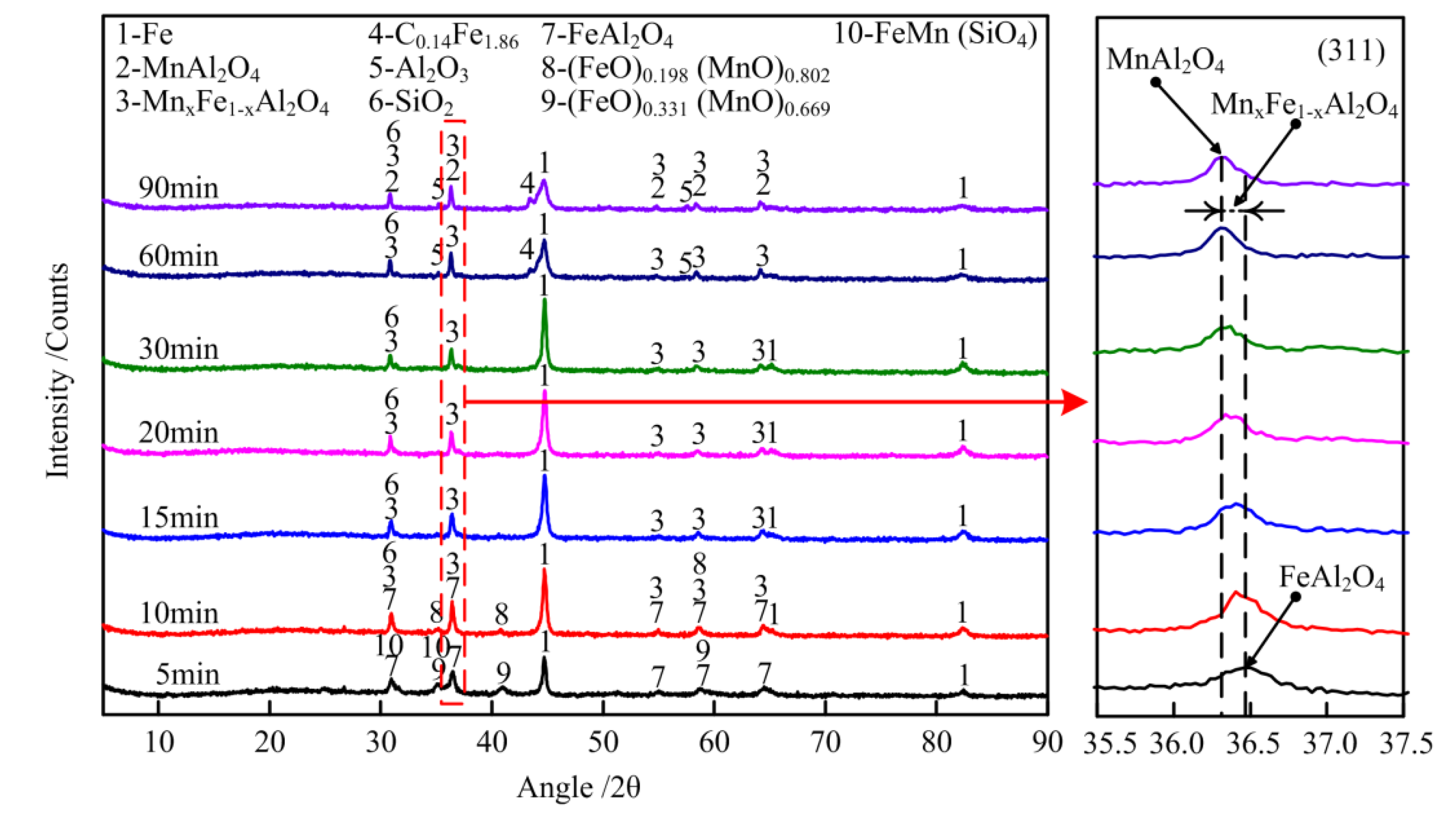
4.2. Phase Transformation of Al-Fe Ores and Mn-Fe Ores during Carbothermic Co-Reduction Roasting
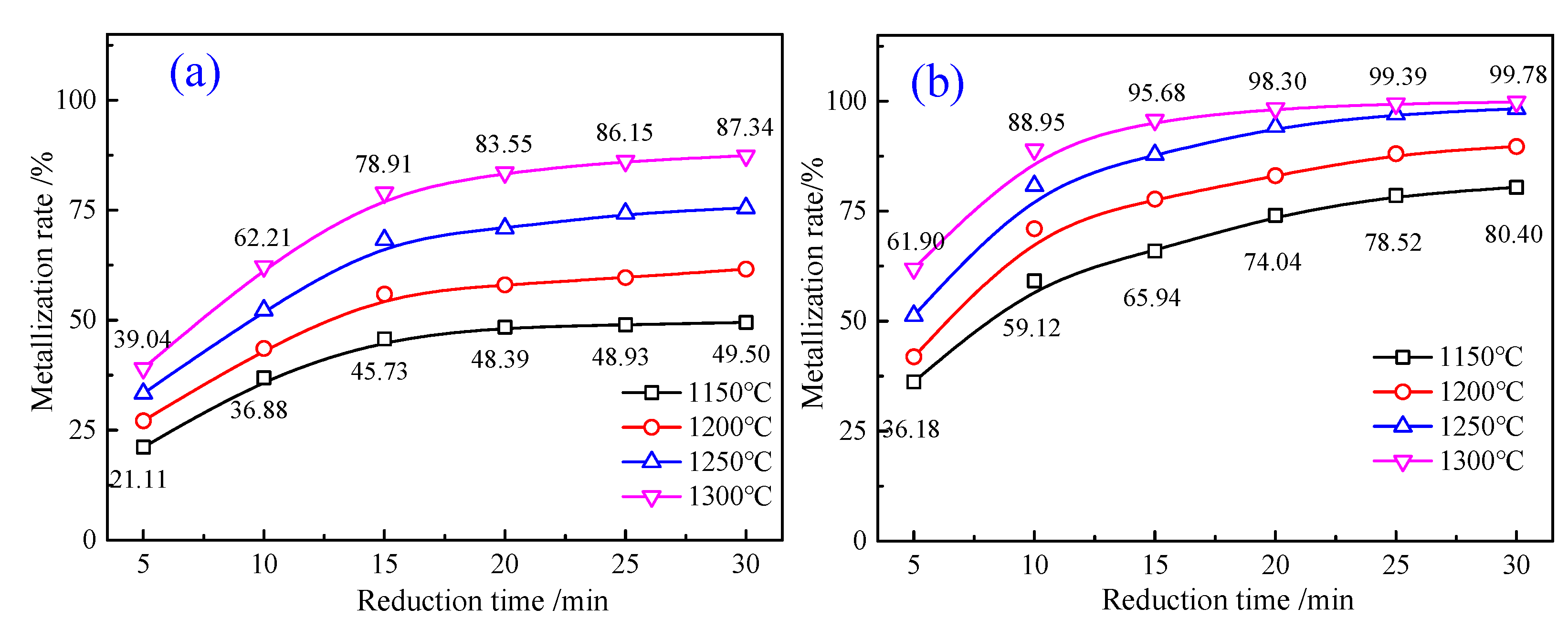
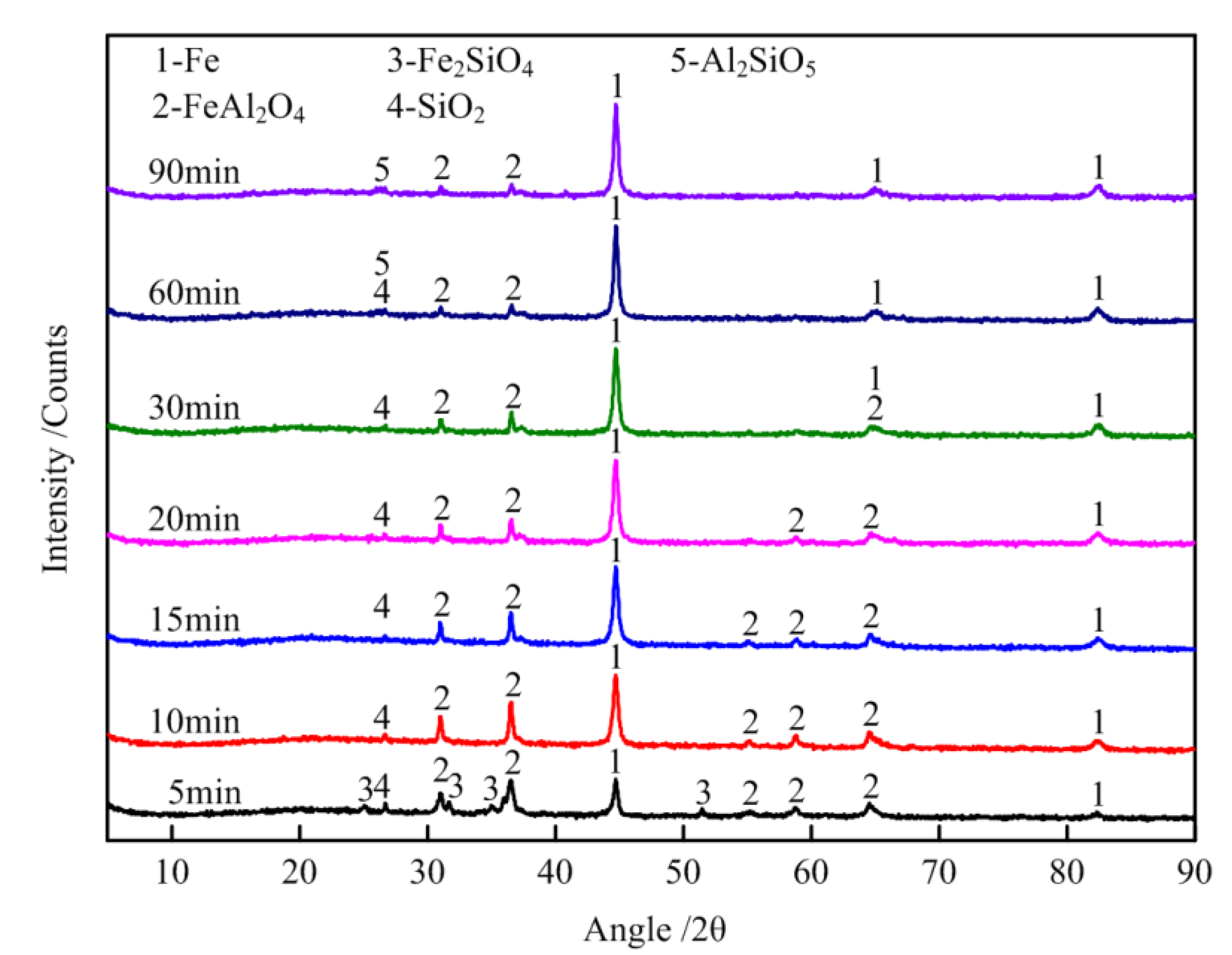
4.2.1. Phase Transformation of Al-Fe Ores in Reduction Roasting Process
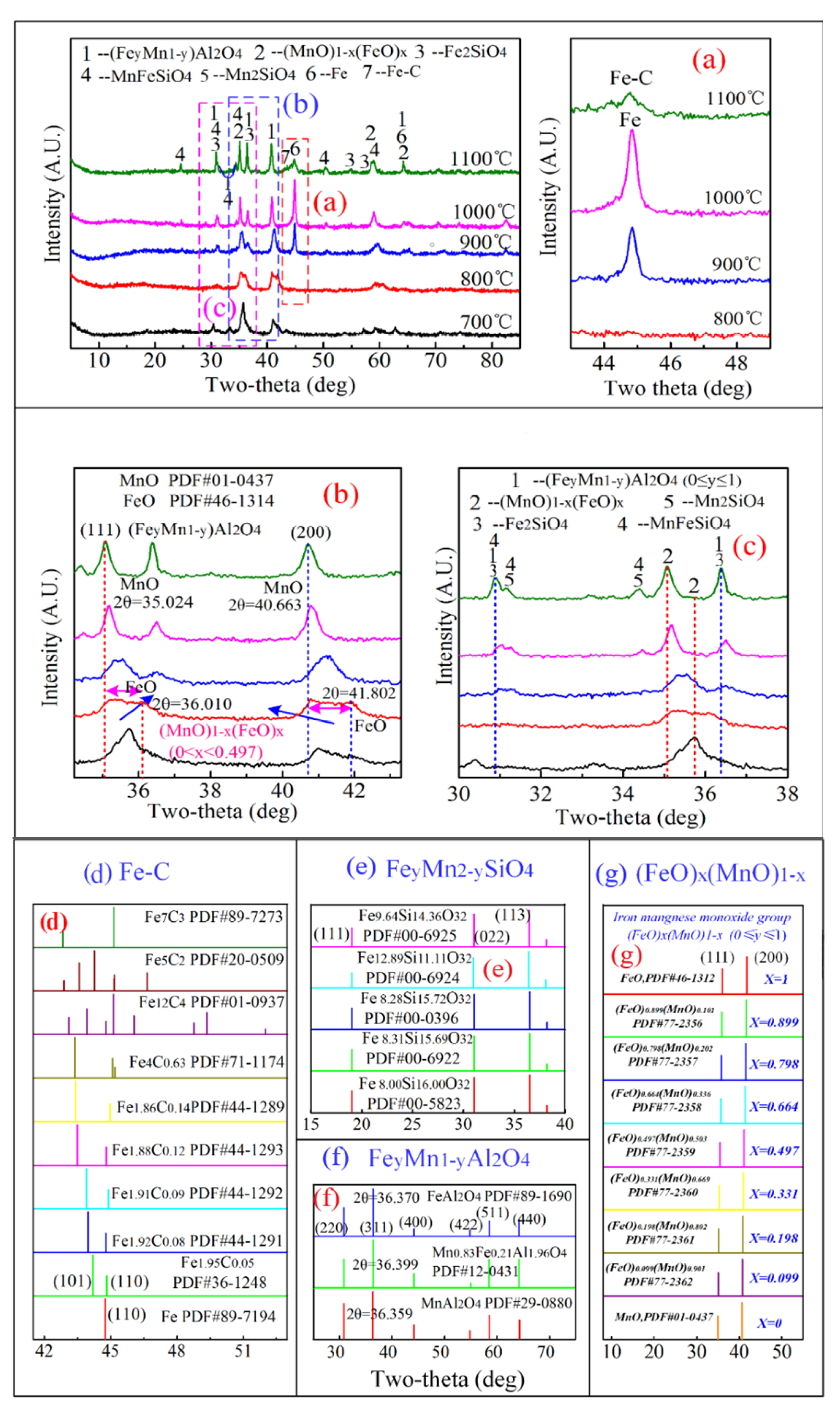
4.2.2. Phase Transformation of Mn-Fe Ores in Reduction Roasting Process
4.2.3. Phase Transformation of Al-Fe Ores/Mn-Fe Ores
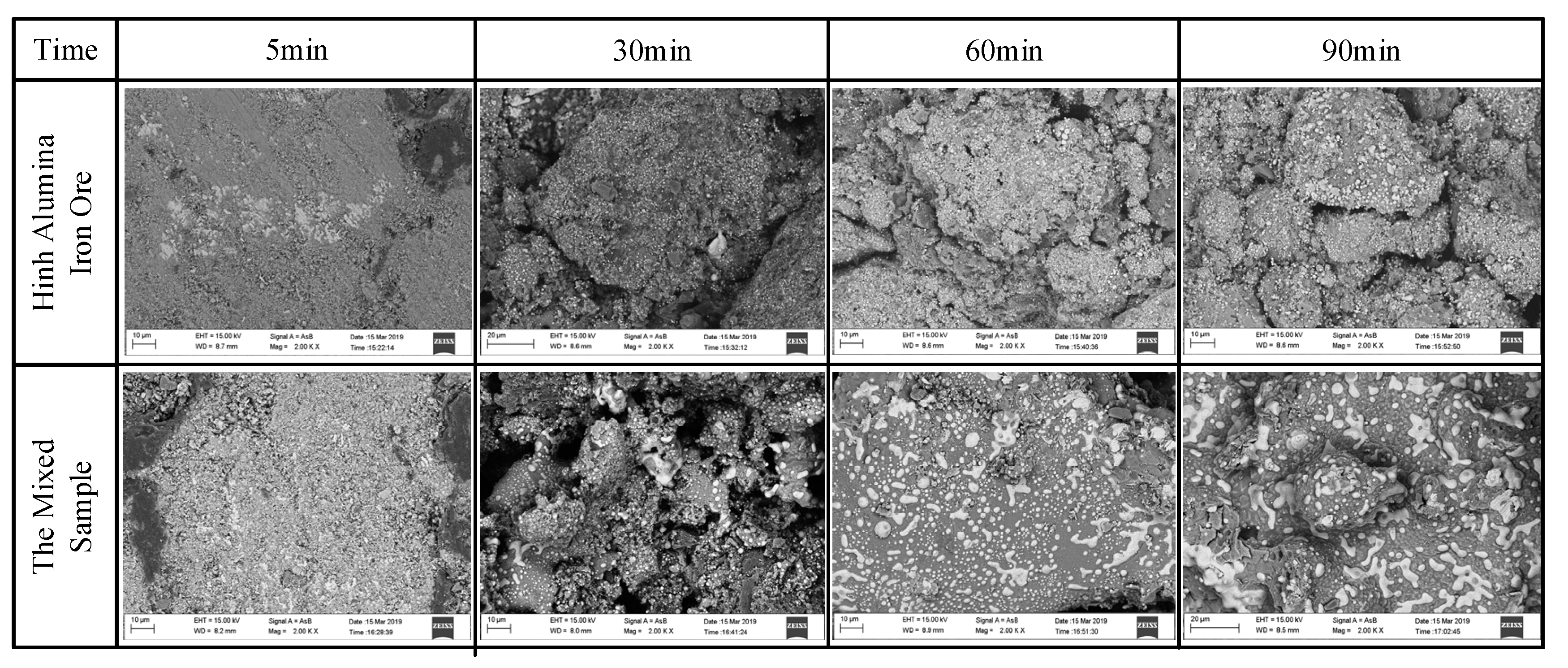
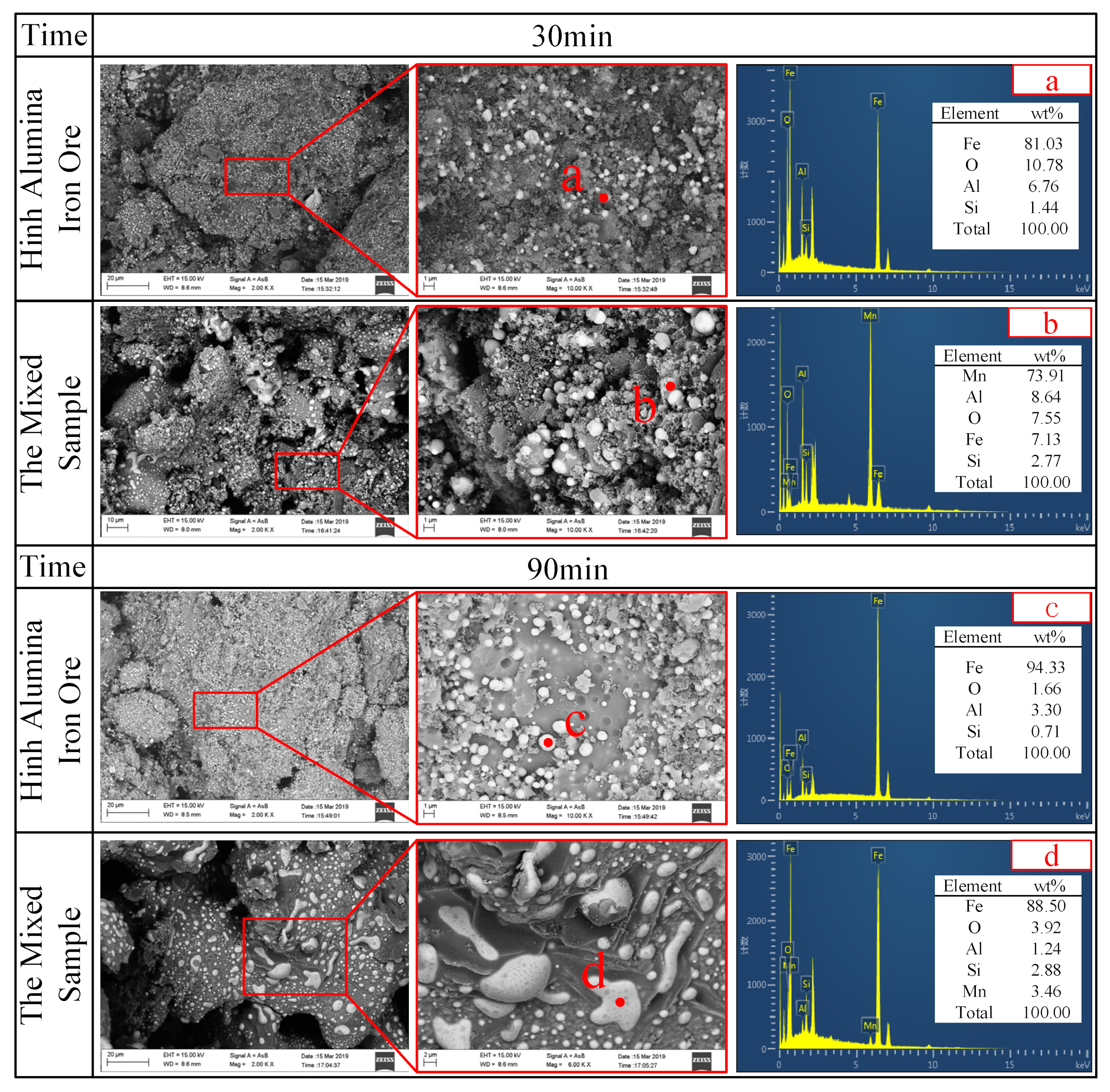
4.3. Morphological Characteristics of Al-Fe Ores and Mn-Fe Ores during Carbothermic Reduction Process
4.4. Carbothermic Co-Reduction Mechanism of Al-Fe Ores and Mn-Fe Ores
[Fe2+]y[Mn2+]1−y[Al3+]2[O2−]4+[Fe2+][O2−]x[Mn2+][O2−]1−x
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, R.X.; Poon, C.S. Utilization of red mud derived from bauxite in self-compacting concrete. J. Hazard. Mater. 2016, 112, 384–391. [Google Scholar] [CrossRef]
- Huang, X.L.; Badawy, A.E.; Arambewela, M.; Ford, R.; Barlaz, M.; Tolaymat, T. Characterization of salt cake from secondary aluminum production. J. Hazard. Mater. 2014, 273, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Resour. Conserv. Recy. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Tripathy, A.K.; Mahalik, S.; Sarangi, C.K.; Tripathy, B.C.; Sanjay, K.; Bhattacharya, I.N. A pyro-hydrometallurgical process for the recovery of alumina from waste aluminium dross. Miner. Eng. 2019, 137, 181–186. [Google Scholar] [CrossRef]
- Shah, S.S.; Palmieric, M.C.; Sponchiado, S.R.P.; Denise, B. Environmentally sustainable and cost-effective bioleaching of aluminum from low-grade bauxite ore using marine-derived Aspergillus niger. Hydrometallurgy 2020, 195, 105368–105378. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chu, M.S.; Wang, Z. Study on metallized reduction and magnetic separation of iron from fine particles of high iron bauxite ore. High Temp. Mat. Process. 2017, 36, 79–88. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, T.A.; Lyu, G.Z.; Guo, F.F.; Zhang, W.G.; Zhang, Y.H. Recovery of alkali and alumina from bauxite residue (red mud) and complete reuse of the treated residue. Miner. Eng. 2018, 188, 456–465. [Google Scholar] [CrossRef]
- Melo, C.C.A.; Angélica, R.S.; Paz, S.P.A. A proposal for rapid grade control of gibbsitic bauxites using multivariate statistics on XRD data. Miner. Eng. 2020, 157, 106539–106548. [Google Scholar] [CrossRef]
- Liu, Z.G.; Wang, Z.; Tang, J.; Wang, H.T.; Long, H.M. Non-isothermal thermal decomposition kinetics of high iron gibbsite ore based on Popescu method. Trans. Nonferrous Met. Soc. China. 2015, 25, 2415–2421. [Google Scholar] [CrossRef]
- Gibson, B.; Wonyen, D.G.; Chelgani, S.C. A review of pretreatment of diasporic bauxite ores by flotation separation. Miner. Eng. 2017, 114, 64–73. [Google Scholar] [CrossRef]
- Hu, K.T.; Reed, D.; Robshaw, T.J.; Smith, R.M.; Ogden, M.D. Characterisation of aluminium black dross before and after stepwise salt phase dissolution in non-aqueous solvents. J. Hazard. Mater. 2021, 401, 123351–123360. [Google Scholar] [CrossRef] [PubMed]
- He, J.F.; Bai, Q.; Du, T.Y. Beneficiation and upgrading of coarse sized low-grade bauxite using a dry-based fluidized bed separator. Adv. Powder Technol. 2020, 31, 181–189. [Google Scholar] [CrossRef]
- Guan, F.; Zhang, H.; Liu, G.-Y.; Zhao, S.-G.; Xia, L.-Y. Flotation of aluminosilicate minerals using alkylguanidine collectors. Trans. Nonferrous Met. Soc. China. 2009, 19, 228–234. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. The assessment of the recycling process of aluminum hazardous waste and a new route of development. Mater. Today Proc. 2019, 10, 340–347. [Google Scholar] [CrossRef]
- Gao, L.H.; Liu, Z.G.; Pan, Y.Z.; Feng, C.; Chu, M.S.; Tang, J. Systematic study on separation of Mn and Fe from ferruginous manganese ores by carbothermic reduction roasting process: Phase transformation and morphologies. J. Mater. Res. Technol. 2019, 8, 5591–5609. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Xu, J.W.; Guo, Z.Q.; Pan, J.; Li, S.W.; Pan, L.T.; Yang, C.C. Synergetic utilization of copper slag and ferruginous manganese ore via co-reduction followed by magnetic separation process. J. Clean. Prod. 2020, 250, 119462–119472. [Google Scholar] [CrossRef]
- Coetsee, T. MnO reduction in high carbon ferromanganese production: Practice and theory. Min. Proc. Ext. Met. Rev. 2018, 39, 351–358. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, B.B.; You, Z.X.; Su, Z.J.; Luo, W.; Li, G.H.; Jiang, T. Consolidation behavior of high-Fe manganese ore sinters with natural basicity. Min. Proc. Ext. Met. Rev. 2016, 37, 333–341. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, Y.B.; Wang, J.; Su, Z.J.; Li, G.H.; Jiang, T. New understanding on separation of Mn and Fe from ferruginous manganese ores by the magnetic reduction roasting process. Appl. Surf. Sci. 2018, 444, 133–144. [Google Scholar] [CrossRef]
- Gao, L.H.; Liu, Z.G.; Ge, Y.; Feng, C.; Chu, M.S.; Tang, J. Synthesis and characterization of manganese ferrite MnxFe3-xO4 from ferruginous manganese ores by multi-step roasting and magnetic separation. Powder Technol. 2019, 356, 373–382. [Google Scholar] [CrossRef]
- Gao, L.H.; Liu, Z.G.; Pan, Y.Z.; Feng, C.; Ge, Y.; Chu, M.S. A study on separation of Mn and Fe from high-alumina ferruginous manganese ores by the carbothermal roasting reduction process. Adv. Powder Technol. 2020, 31, 51–60. [Google Scholar] [CrossRef]
- Gao, L.H.; Liu, Z.G.; Chu, M.S.; Wang, R.; Wang, Z.H.; Feng, C. Upgrading of low-grade manganese ore based on reduction roasting and magnetic separation technique. Sep. Sci. Technol. 2019, 54, 195–206. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Du, M.H.; Liu, B.B.; Su, Z.J.; Li, G.H.; Jiang, T. Separation and recovery of iron and manganese from high-iron manganese oxide ores by reduction roasting and magnetic separation technique. Sep. Sci. Technol. 2017, 52, 1321–1332. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, G.C.; Zhao, Y.N. Study in reduction-roast leaching manganese from low-grade manganese dioxide ores using cornstalk as reductant. Hydrometallurgy 2009, 96, 176–179. [Google Scholar] [CrossRef]
- Ye, Q.X.; Chen, J.; Chen, G.; Peng, J.H.; Srinivasakannan, C.; Ruan, R.S. Effect of microwave heating on the microstructures and kinetics of carbothermal reduction of pyrolusite ore. Adv. Powder Technol. 2018, 29, 1871–1878. [Google Scholar] [CrossRef]
- Mpho, M.; Samson, B.; Ayo, A. Evaluation of reduction roasting and magnetic separation for upgrading Mn/Fe ratio of fine ferromanganese. Int. J. Min. Sci. Technol. 2013, 23, 537–541. [Google Scholar] [CrossRef]
- Yi, L.Y.; Huang, Z.C.; Jiang, T.; Zhao, P.; Zhong, R.H.; Liang, Z.K. Carbothermic reduction of ferruginous manganese ore for Mn/Fe beneficiation: Morphology evolution and separation characteristic. Minerals 2017, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Shi, B.; Ge, W.; Yan, C.J.; Yang, X. Magnetic separation and magnetic properties of low-grade manganese carbonate ore. JOM 2015, 67, 361–368. [Google Scholar] [CrossRef]
- Gao, Y.B.; Olivas-Martinez, M.; Sohn, H.Y.; Han, G.K.; Chan, W.K. Upgrading of low-grade manganese ore by selective reduction of iron oxide and magnetic separation. Metall. Mater. Trans. B 2012, 43, 1465–1475. [Google Scholar] [CrossRef]
- Peng, N.; Pan, Q.L.; Liu, H.; Yang, Z.H.; Wang, G.L. Recovery of iron and manganese from iron-bearing manganese residues by multi-step roasting and magnetic separation. Miner. Eng. 2018, 126, 177–183. [Google Scholar] [CrossRef]
- Singh, V.; Ghosh, T.K.; Ramamurthy, Y.; Tathavadkar, V. Beneficiation and agglomeration process to utilize low-grade ferruginous manganese ore fines. Int. J. Miner. Process 2011, 99, 84–86. [Google Scholar] [CrossRef]
- Singh, V.; Biswas, A. Physicochemical processing of low grade ferruginous manganese ores. Int. J. Miner. Process 2017, 158, 35–44. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Banerjee, P.K.; Suresh, N. Effect of desliming on the magnetic separation of low-grade ferruginous manganese ore. Int. J. Min. Met. Mater. 2015, 22, 661–673. [Google Scholar] [CrossRef]
- Gao, L.H.; Liu, Z.G.; Yang, Z.C.; Cao, L.G.; Feng, C.; Chu, M.S.; Tang, J. Synthesis and magnetism property of manganese ferrite MnFe2O4 by selective reduction and oxidization roasting process. Appl. Surf. Sci. 2020, 508, 145292–1452099. [Google Scholar] [CrossRef]
- Zhu, L.; He, J.N.; Yan, D.R.; Dong, Y.C.; Zhang, J.X.; Lia, X.Z.; Liao, H.L. Atmospheric reactive plasma sprayed Al-Fe2O3-FeAl2O4 composite coating and its property evaluation. Appl. Surf. Sci. 2011, 257, 10282–10288. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Pan, L.Q.; Guo, Z.Q.; Pan, J. Utilization of limonitic nickel laterite to produce ferronickel concentrate by the selective reduction-magnetic separation process. Adv. Powder Technol. 2019, 30, 451–460. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, Y.B.; Lu, M.M.; Su, Z.J.; Li, G.H.; Jiang, T. A study on the carbonization and alloying process of MnO2 by methane-hydrogen gas mixture in the presence of Fe2O3. Powder Technol. 2018, 325, 271–279. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Pan, J.; Zhu, D.Q.; Zhang, F. Green and efficient utilization of waste ferric-oxide desulfurizer to clean waste copper slag by the smelting reduction-sulfurizing process. J. Clean. Prod. 2018, 199, 891–899. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Gao, L.; Zhan, W.; He, Z. Green and Efficient Utilization of Ferruginous Gibbsite Ore and Ferruginous Manganese Ore by Synergetic Carbothermic Co-Reduction–Magnetic Separation Process. Minerals 2022, 12, 671. https://doi.org/10.3390/min12060671
Chen H, Gao L, Zhan W, He Z. Green and Efficient Utilization of Ferruginous Gibbsite Ore and Ferruginous Manganese Ore by Synergetic Carbothermic Co-Reduction–Magnetic Separation Process. Minerals. 2022; 12(6):671. https://doi.org/10.3390/min12060671
Chicago/Turabian StyleChen, Haipeng, Lihua Gao, Wenlong Zhan, and Zhijun He. 2022. "Green and Efficient Utilization of Ferruginous Gibbsite Ore and Ferruginous Manganese Ore by Synergetic Carbothermic Co-Reduction–Magnetic Separation Process" Minerals 12, no. 6: 671. https://doi.org/10.3390/min12060671
APA StyleChen, H., Gao, L., Zhan, W., & He, Z. (2022). Green and Efficient Utilization of Ferruginous Gibbsite Ore and Ferruginous Manganese Ore by Synergetic Carbothermic Co-Reduction–Magnetic Separation Process. Minerals, 12(6), 671. https://doi.org/10.3390/min12060671





