Effects of Coal Rank and Macerals on the Structure Characteristics of Coal-Based Graphene Materials from Anthracite in Qinshui Coalfield
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.1.1. Samples
2.1.2. Demineralization and Graphitization
2.1.3. Preparation of Coal-Based GS
2.2. Methods
2.2.1. Basic Characteristics
2.2.2. X-ray Diffraction (XRD)
2.2.3. Raman Spectroscopy
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. High-Resolution Transmission Electron Microscope (HRTEM)
3. Results and Discussion
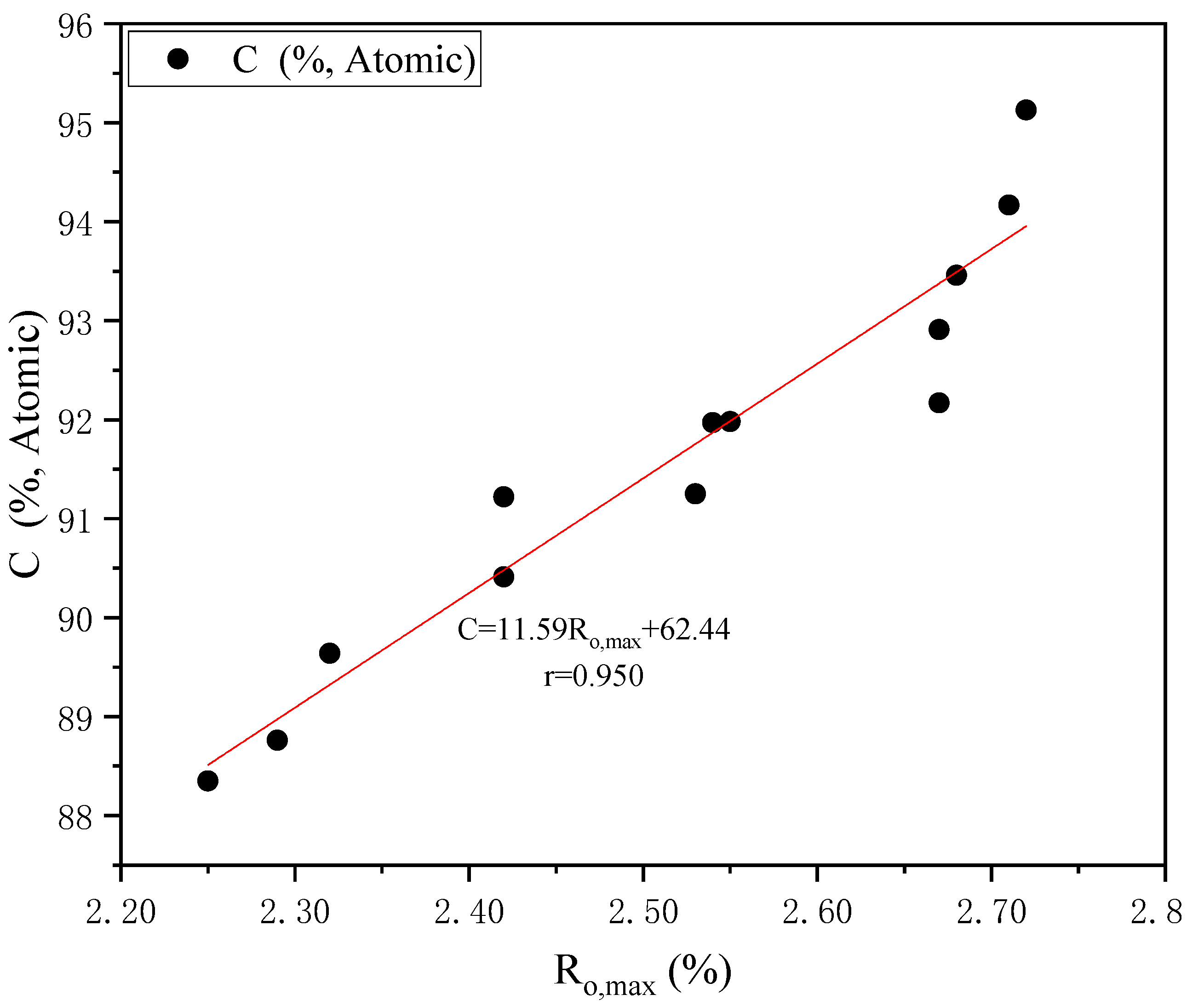
3.1. Basic Sample Properties
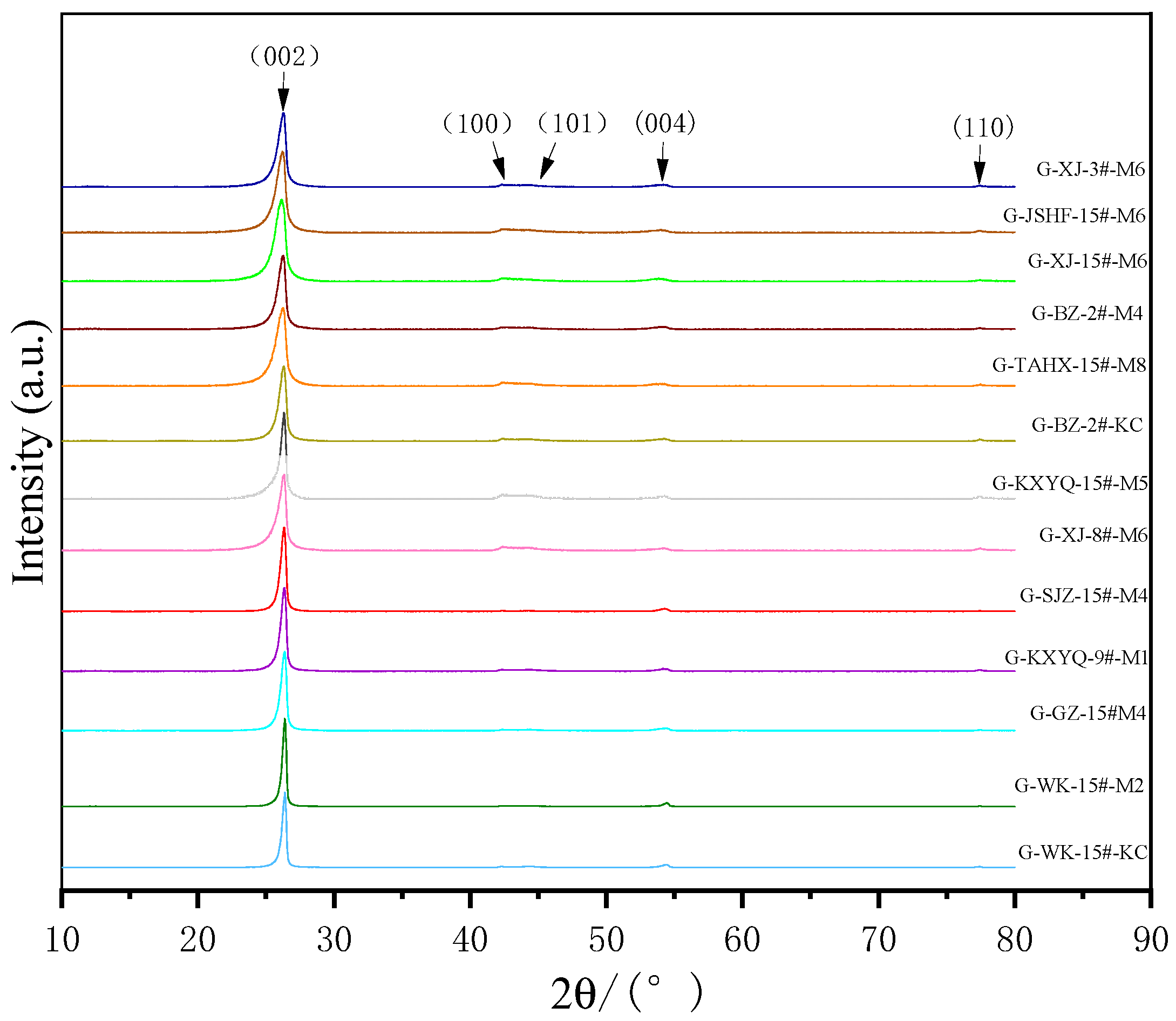
3.2. XRD Characterization of Products
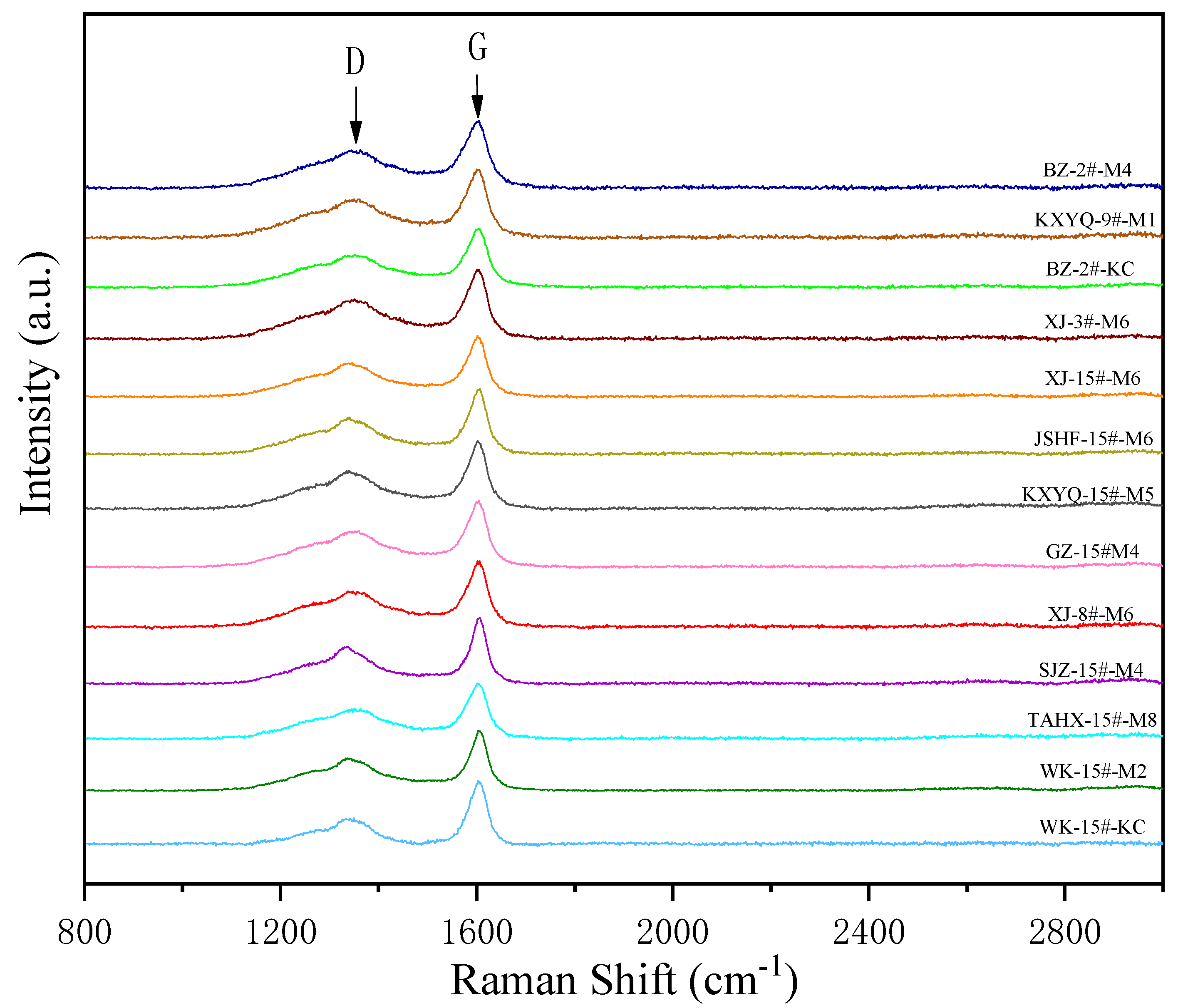
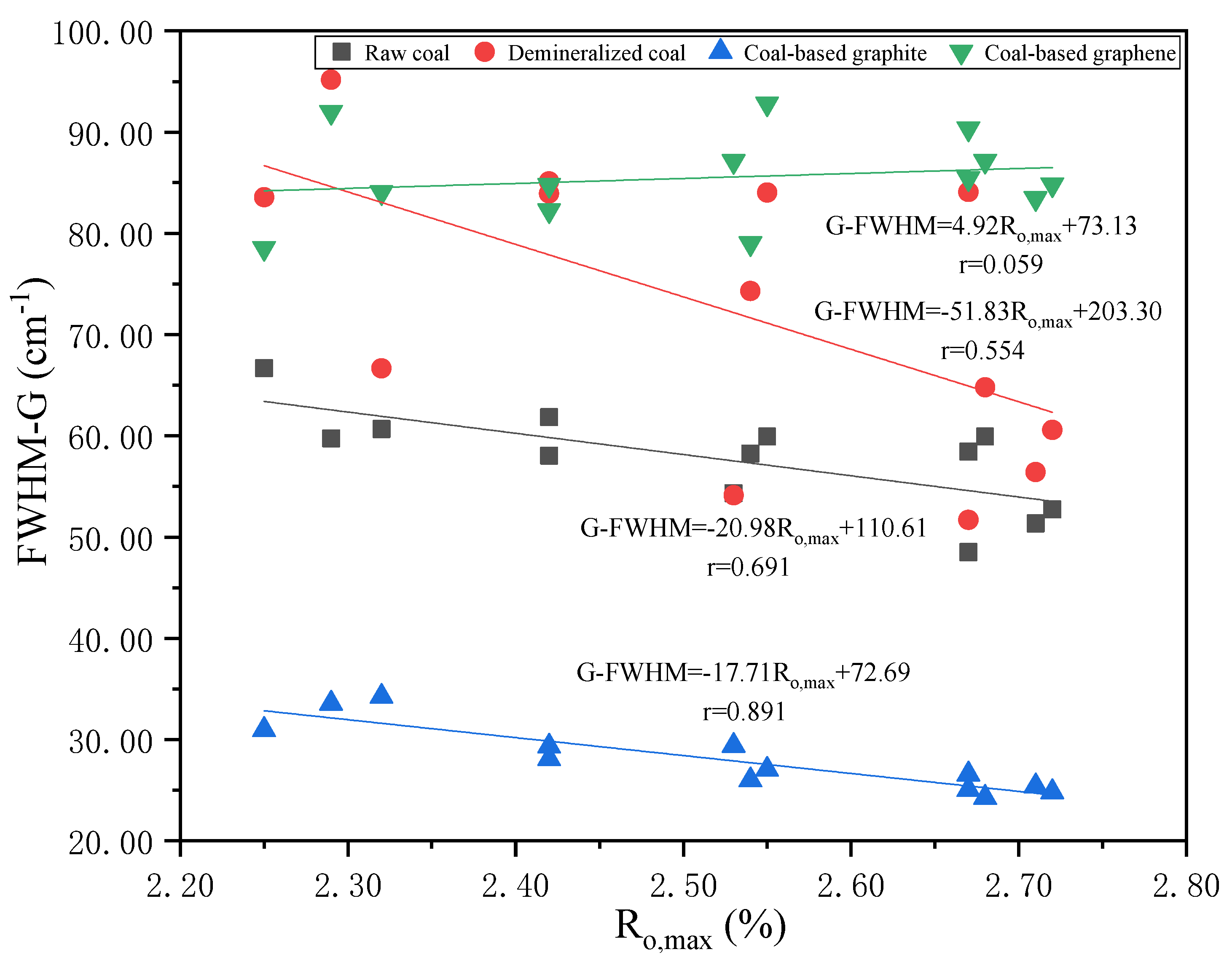
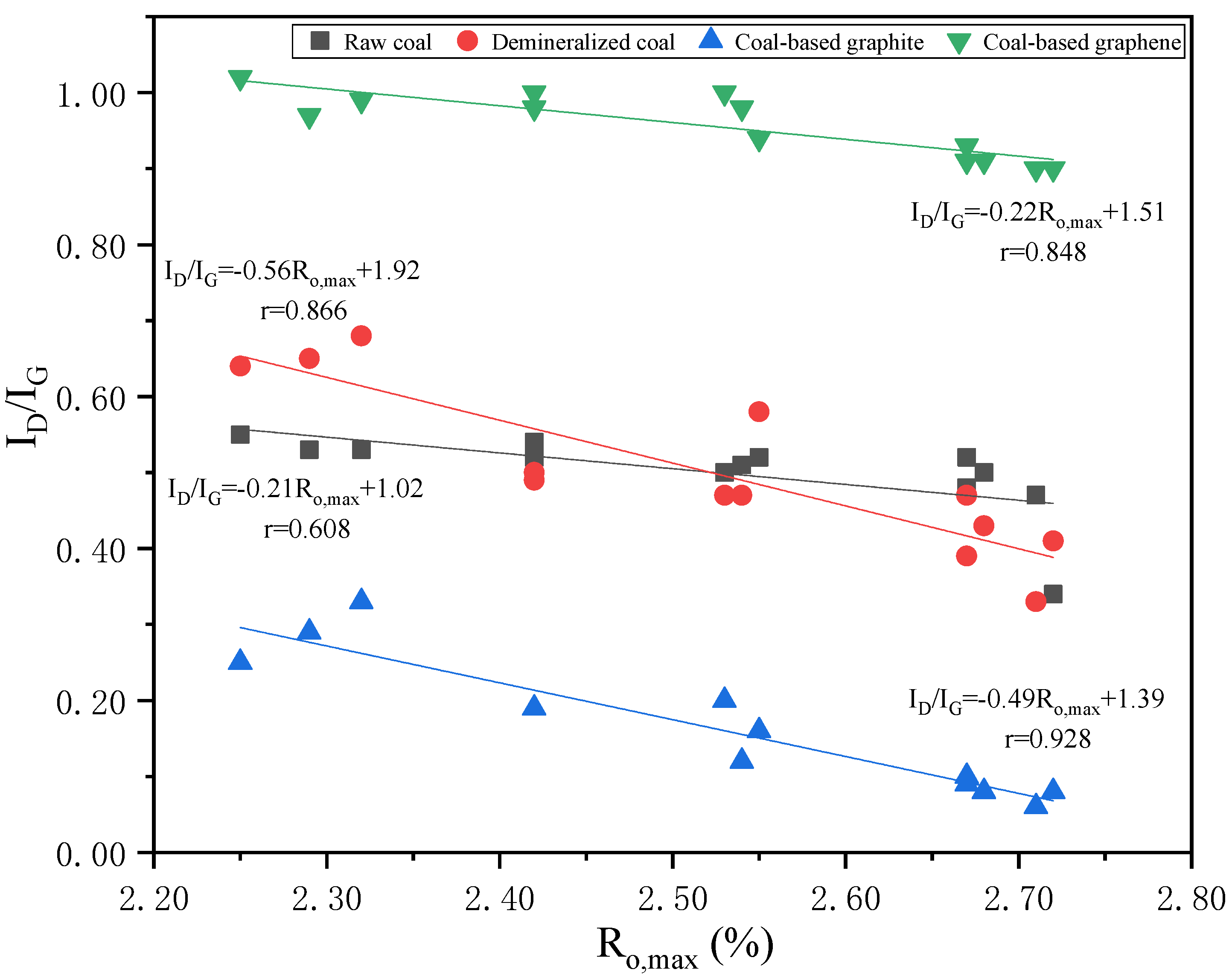
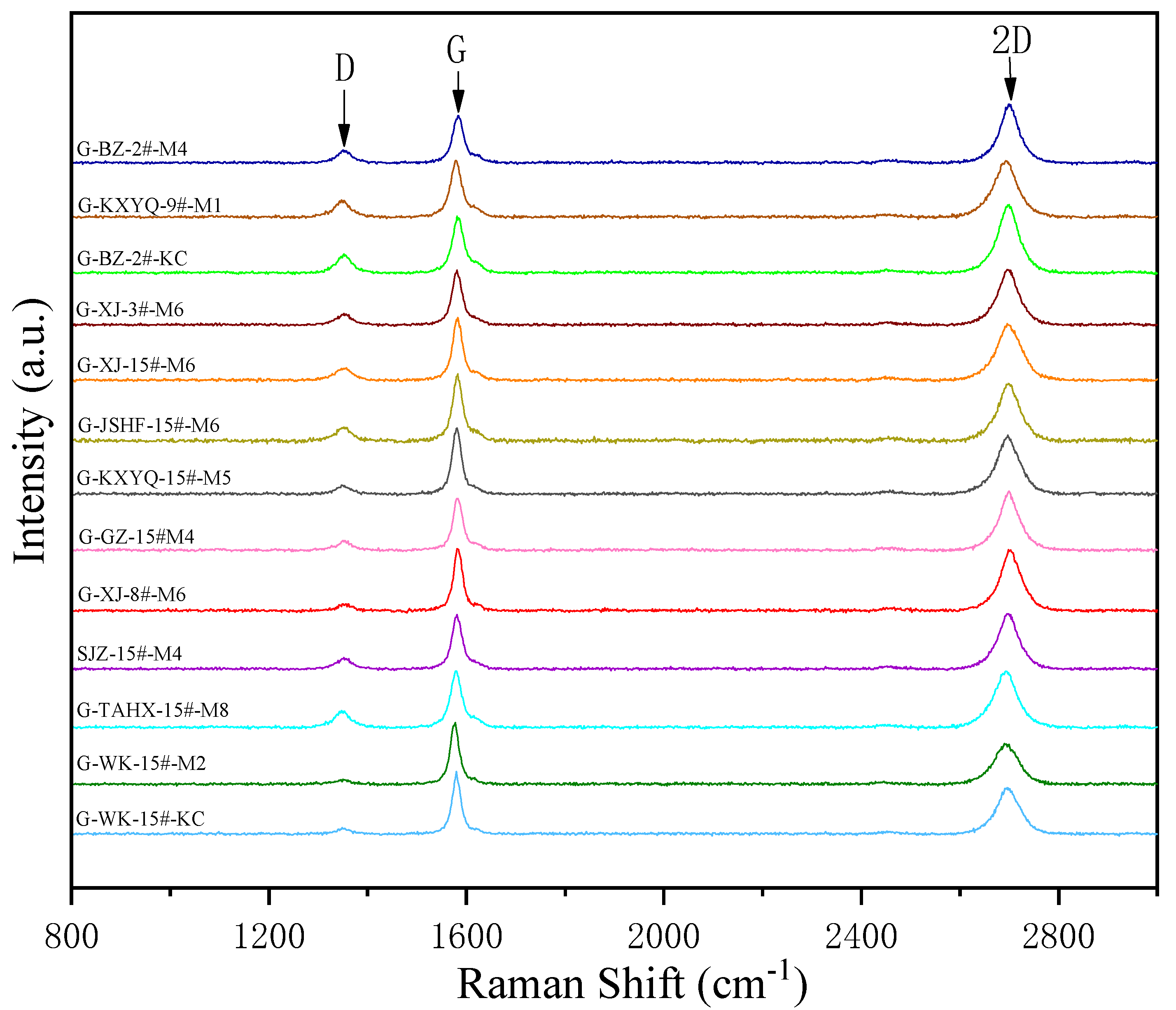
3.3. Raman Characterization of Products
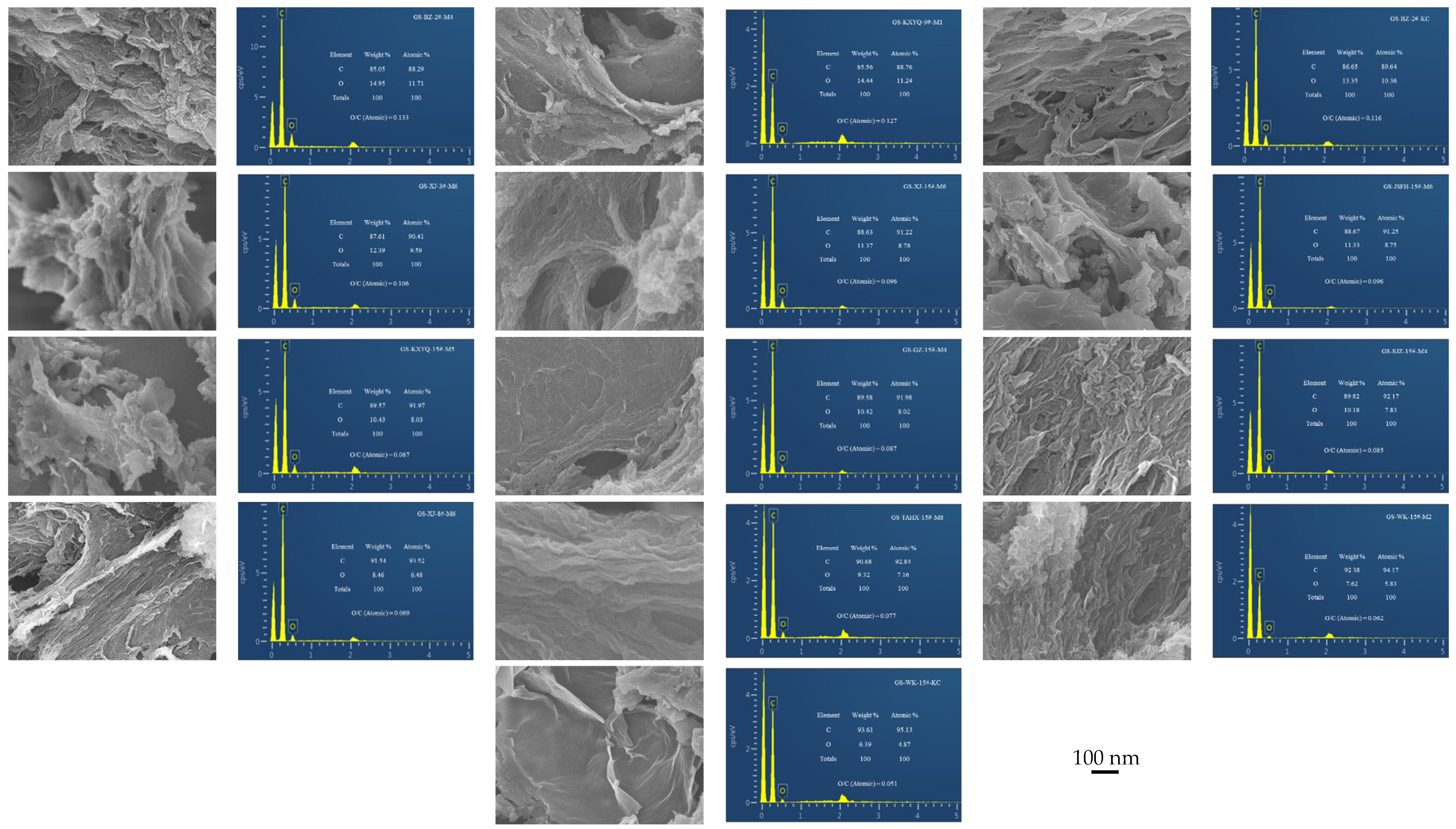
3.4. SEM Characterization of Products
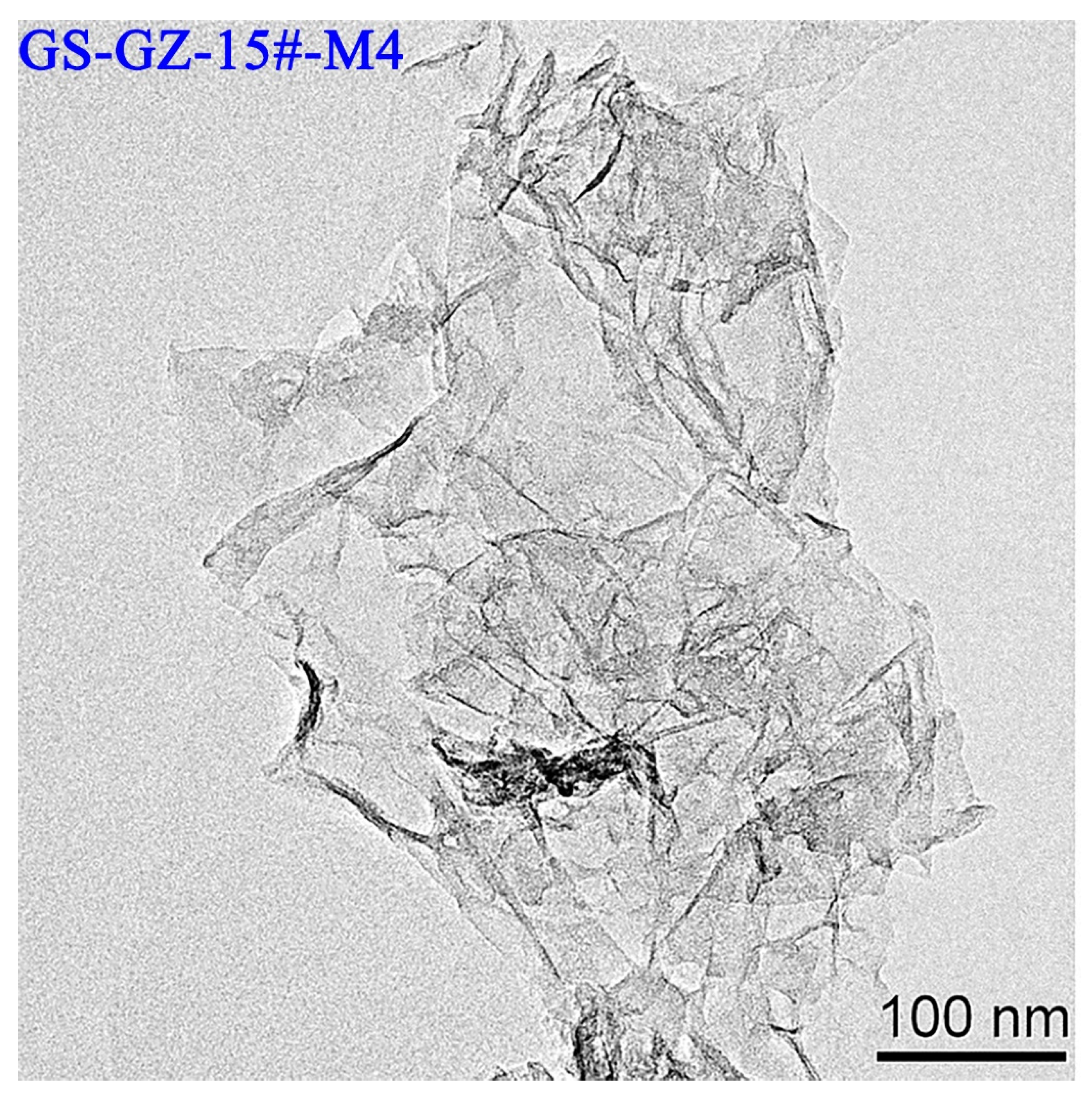
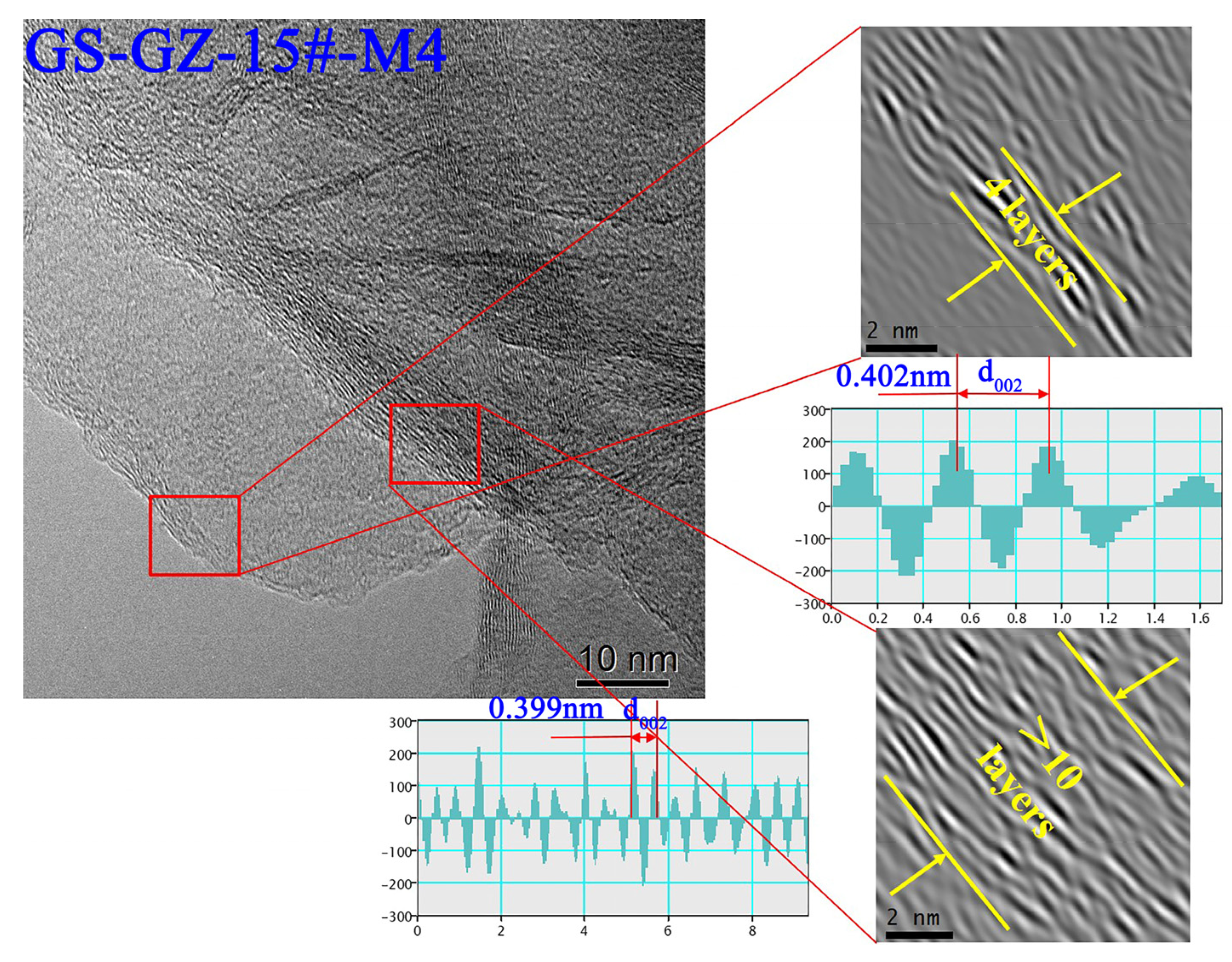
3.5. HRTEM Characterization of Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.G.; Wei, X.D.; Jeffrey, W.K.; James, H. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Dimitrakopoulos, C.; Jenkins, K.A.; Farmer, D.B.; Chiu, H.-Y.; Grill, A.; Avouris, P. 100-GHz transistors from wafer-scale epitaxial graphene. Science 2010, 327, 662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Dan, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Kim, N.H.; Kuila, T.; Lee, J.H. Enhanced mechanical properties of a multiwall carbon nanotube attached pre-stitched graphene oxide filled linear low density polyethylene composite. J. Mater. Chem. A 2014, 2, 2681–2689. [Google Scholar] [CrossRef]
- Editorial Department of Nature. Microscopy: Graphene protects cells for imaging. Nature 2015, 522, 394–395. [Google Scholar] [CrossRef]
- An, X.H.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.K.; Talapatra, S.; Kar, S. Stable aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Harmon, J.B.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef]
- Ramón, M.E.; Gupta, A.; Corbet, C.; Ferrer, D.A.; Movva, H.C.P.; Carpenter, G.; Colombo, L.; Bourianoff, G.; Doczy, M.; Akinwande, D. Cmos-compatible synthesis of large-area, high-mobility graphene by chemical vapor deposition of acetylene on cobalt thin films. ACS Nano 2011, 5, 7198–7204. [Google Scholar] [CrossRef]
- Mathews, J.P.; Chaffee, A.L. The molecular representations of coal—A review. Fuel 2012, 96, 1–14. [Google Scholar] [CrossRef]
- Hoang, V.C.; Hassan, M.; Gomes, V.G. Coal derived carbon nanomaterials-recent advances in synthesis and applications. Appl. Mater. Today 2018, 12, 342–358. [Google Scholar] [CrossRef]
- Achten, C.; Andersson, J.T. Overview of polycyclic aromatic compounds (PAC). Polycycl. Aromat. Compd. 2015, 35, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Huan, X.; Tang, Y.G.; Xu, J.J.; Xu, M.X. Nano-level resolution determination of aromatic nucleus in coal. Fuel 2020, 262, 116532. [Google Scholar] [CrossRef]
- Wang, R.W.; Sun, R.Y.; Liu, G.J.; Yousaf, B.; Wu, D.; Chen, J.; Zhang, H. A review of the biogeochemical controls on the occurrence and distribution of polycyclic aromatic compounds (PACs) in coals. Earth Sci. Rev. 2017, 171, 400–418. [Google Scholar] [CrossRef]
- Hirsch, P.B. X-ray scattering from coals. Proc. R. Soc. A Math. Phys. Eng. Sci. 1954, 226, 143–169. [Google Scholar]
- Cartz, L.; Diamond, R.; Hirsch, P.B. New X-ray data on coals. Nature 1956, 177, 500–502. [Google Scholar] [CrossRef]
- Cartz, L.; Hirsch, P.B. A contribution to the structure of coals from X-ray diffraction studies. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1960, 252, 557–602. [Google Scholar]
- Radke, M.; Schaefer, R.G.; Leythaeuserv, D.; Teichmüller, M. Composition of soluble organic matter in coals: Relation to rank and liptinite fluorescence. Geochim. Cosmochim. Acta 1980, 44, 1787–1800. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Liu, K.L.; Xie, W.; Pan, W.P.; Riley, J.T. Soluble polycyclic aromatic hydrocarbons in raw coals. J. Hazard. Mater. 2000, 73, 77–85. [Google Scholar] [CrossRef]
- Stach, E.; Mackowsky, M.T.; Teichmüller, M. Stach’s Textbook of Coal Petrology; Gebrüder Borntraeger: Berlin, Germany; Stuttgart, Germany, 1982. [Google Scholar]
- Taylor, G.H. Organic Petrology; Gebrüder Borntraeger: Berlin, Germany, 1998. [Google Scholar]
- Li, J.Q.; Qin, Y.; Chen, Y.L.; Song, Y.; Wang, Z.W. HRTEM observation of morphological and structural evolution of aromatic fringes during the transition from coal to graphite. Carbon 2022, 187, 133–144. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, Q.F.; Mathews, J.P.; Zhang, H.; Wu, Y.K. Quantifying the structural transitions of Chinese coal to coal-derived natural graphite by XRD, Raman spectroscopy, and HRTEM image analyses. Energy Fuels 2021, 35, 2335–2346. [Google Scholar] [CrossRef]
- Diessel, C.F.K.; Brothers, R.N.; Philippa, M.B. Coalification and graphitization in high-pressure schists in New Caledonia. Contrib. Mineral. Petrol. 1978, 68, 63–78. [Google Scholar] [CrossRef]
- Bustin, R.M.; Rouzaud, J.N.; Ross, J.V. Natural graphitization of anthracite: Experimental considerations. Carbon 1995, 33, 679–691. [Google Scholar] [CrossRef]
- Oberlin, A.; Terrier, G. Graphitization studies of anthracites by high resolution electron microscopy. Carbon 1975, 13, 367–376. [Google Scholar] [CrossRef]
- Wertz, D.L.; Bissell, M. Relating the nonideal diffraction from the graphene layer stacking peak to the aliphatic carbon abundance in bituminous coal. Energy Fuels 1994, 8, 613–617. [Google Scholar] [CrossRef]
- Blanche, C.; Rouzaud, J.N.; Dumas, D. New data on anthracite graphitizibility. In Proceedings of the 22nd Biennal Carbon Conference, Extended Abstracts, American Carbon Society, San Diego, CA, USA, 16–21 July 1995; pp. 694–695. [Google Scholar]
- Manoj, B. Synthesis and characterization of porous, mixed phase, wrinkled, few layer graphene like nanocarbon from charcoal. Russ. J. Phys. Chem. A 2015, 89, 2438–2442. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Bratek, K.; Bratek, W.; Gerus-Piasecka, I.; Jasieńko, S.; Wilk, P. Properties and structure of different rank anthracites. Fuel 2002, 81, 97–108. [Google Scholar] [CrossRef]
- Nyathi, M.S.; Clifford, C.B.; Schobert, H.H. Characterization of graphitic materials prepared from different rank Pennsylvania anthracites. Fuel 2013, 114, 244–250. [Google Scholar] [CrossRef]
- Franklin, R.E. Crystallite growth in graphitizing and non-graphitizing carbons. Proc. R. Soc. A Math. Phys. Eng. Sci. 1951, 209, 196–218. [Google Scholar]
- Li, W.; Song, Y.; Yang, W.B.; Mathews, J.P. Structural transformations for a subbituminous coal, impact of temperature on gold-tube pyrolysis chars evaluated using HRTEM. Fuel 2022, 311, 122581. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mahajan, R.L. A facile method for coal to graphene oxide and its application to a biosensor. Carbon 2021, 181, 408–420. [Google Scholar] [CrossRef]
- Pang, L.S.K.; Vassallo, A.M.; Wilson, M.A. Fullerenes from coal: A self-consistent preparation and purification process. Energy Fuels 1992, 6, 176–179. [Google Scholar] [CrossRef]
- Pang, L.S.K. Fullerenes from brown (lignite) coal. Fuel Process. Technol. 1993, 34, 147–155. [Google Scholar] [CrossRef]
- Taylor, G.H.; Gerald, J.D.F.; Pang, L.S.K.; Wilson, M.A. Cathode deposits in fullerene formation-microstructural evidence for independent pathways of pyrolytic carbon and nanobody formation. J. Cryst. Growth 1994, 135, 157–164. [Google Scholar] [CrossRef]
- Williams, J.H.; Eklund, P.C. Single-wall carbon nanotubes from coal. Chem. Phys. Lett. 1993, 310, 31–37. [Google Scholar] [CrossRef]
- Pang, L.S.K.; Wilson, M.A. Nanotubes from coal. Energy Fuels 1993, 7, 436–437. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Z.B.; Zhang, Y.T.; Meng, B.; Zhou, A.N.; Qiu, J.S. Graphene sheets from graphitized anthracite coal: Preparation, decoration, and application. Energy Fuels 2012, 26, 5186–5192. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Liu, G.Y.; Cai, J.T.; Zhou, A.N.; Zhang, X.Q.; Qiu, J.S. Preparation of graphene from Taixi anthracite and its photocatalyst performance for CO2 conversion. Proc. Inst. Mech. Eng. Part N J. Nanoeng. Nanosyst. 2013, 228, 61–64. [Google Scholar] [CrossRef]
- Powell, C.; Beall, G.W. Graphene oxide and graphene from low grade coal: Synthesis, characterization and applications. Curr. Opin. Colloid Interface Sci. 2015, 20, 362–366. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.L.; Zhang, X.Q.; Lin, Q.L.; Huang, X.L. Preparation of fluffy graphene nanosheets from coal-tar pitch with nano-Al2O3 as filler. J. Anal. Appl. Pyrolysis 2016, 117, 354–356. [Google Scholar] [CrossRef]
- Huan, X.; Tang, Y.G.; Xu, J.J.; Lan, C.Y.; Wang, S.Q. Structural characterization of graphenic material prepared from anthracites of different characteristics: A comparative analysis. Fuel Process. Technol. 2019, 183, 8–18. [Google Scholar] [CrossRef]
- Tang, Y.G.; Xu, J.J.; Huan, X.; Wang, S.Q.; Chen, P.X. Preparation and spectroscopic characterization of coal-based graphene from anthracite in Xiaofalu, Yunnan, China. J. China Coal Soc. 2020, 45, 740–748, (In Chinese with English Abstract). [Google Scholar]
- Ye, R.Q.; Xiang, C.S.; Lin, J.; Peng, Z.W.; Huang, K.W.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.-C.; Ruan, G.D.; et al. Coal as an abundant source of graphene quantum dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef]
- Singamaneni, S.R.; Tol, J.V.; Ye, R.Q.; Tour, J.M. Magnetic defects in graphene quantum dots. In Proceedings of the APS March Meeting 2015, San Antonio, TX, USA, 2–6 March 2015. [Google Scholar]
- Tang, Y.G.; Huan, X.; Lan, C.Y.; Xu, M.X. Effects of coal rank and high organic sulfur on the structure and optical properties of coal-based graphene quantum dots. Acta Geol. Sin. 2018, 92, 1218–1230. [Google Scholar] [CrossRef]
- Aso, H.; Matsuoka, K.; Sharma, A.; Tomita, A. Evaluation of size of graphene sheet in anthracite by a temperature-programmed oxidation method. Energy Fuels 2004, 18, 1309–1314. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.Y.; Zhao, Z.B.; Zhou, Q.; Li, B.B.; Qiu, J.S. A green strategy for the synthesis of graphene supported Mn3O4 nanocomposites from graphitized coal and their supercapacitor application. Carbon 2014, 80, 640–650. [Google Scholar] [CrossRef]
- Vijapur, S.H.; Wang, D.; Botte, G.G. The growth of transparent amorphous carbon thin films from coal. Carbon 2013, 54, 22–28. [Google Scholar] [CrossRef]
- Vijapur, S.H.; Wang, D.; Ingram, D.C.; Botte, G.G. An investigation of growth mechanism of coal derived graphene films. Mater. Today Commun. 2017, 11, 147–155. [Google Scholar] [CrossRef]
- Savitskii, D.P. Preparation and characterization of colloidal dispersions of graphene-like structures from different ranks of coals. J. Fuel Chem. Technol. 2017, 45, 897–907. [Google Scholar] [CrossRef]
- Evans, E.L.; Jenkins, J.L.; Thomas, J.M. Direct electron microscopic studies of graphitic regions in heat-treated coals and coal extracts. Carbon 1972, 10, 637–642. [Google Scholar] [CrossRef]
- Lan, C.Y.; Tang, Y.G.; Huan, X.; Che, Q.L. Effects of minerals in anthracite on the formation of coal-based graphene. Chem. Select 2019, 19, 5937–5944. [Google Scholar] [CrossRef]
- Liu, L.; Du, M.L.; Li, G.; Fan, J.W.; Cai, Y.C. Pyrolysis behavior and product distribution of exinite submacerals. J. Anal. Appl. Pyrolysis 2020, 152, 104957. [Google Scholar] [CrossRef]
- Du, M.L.; Liu, L.; Fan, J.W.; Li, G.; Yang, J.L. Prediction and characterization of macromolecular structure of cutinite from Luquan cutinitic liptobiolith with molecular simulation. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Li, Y. On the difference of characterization and supercapacitive performance of graphene nanosheets from precursors of inertinite- and vitrinite-rich coal. J. Alloy. Compd. 2019, 815, 152502. [Google Scholar] [CrossRef]
- Awasthi, S.; Awasthi, K.; Ghosh, A.K.; Srivastava, S.K.; Srivastava, O.N. Formation of single and multi-walled carbon nanotubes and graphene from Indian bituminous coal. Fuel 2015, 147, 35–42. [Google Scholar] [CrossRef]
- Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaría, R.; Menéndez, R. Cokes of different origin as precursors of graphene oxide. Fuel 2016, 166, 400–403. [Google Scholar] [CrossRef]
- Ge, B.; Yin, G.; Li, C. A preliminary study on sedimentary environments and law of coal-bearing formation in Yangquan, Shanxi. Acta Sedimentol. Sin. 1985, 3, 33–44, (In Chinese with English Abstract). [Google Scholar]
- Shao, L.; Xiao, Z.; Wang, H.; Lu, J.; Zhou, J. Permo-Carboniferous coal measures in the Qinshui Basin: High-resolution sequence stratigraphy and coal accumulating models. Chin. J. Geol. 2008, 43, 777–791, (In Chinese with English Abstract). [Google Scholar]
- Bishop, M.; Ward, D.L. The direct determination of mineral matter in coal. Fuel 1958, 37, 191–200. [Google Scholar]
- Rodrigues, S.I.; Suárez-Ruiz, M.M.; Flores, D.; Camean, I.; García, A.B. Development of graphite-like particles from the high temperature treatment of carbonized anthracites. Int. J. Coal Geol. 2011, 85, 219–226. [Google Scholar] [CrossRef]
- Botas, C.; Pérez-Mas, A.M.; Álvarez, P.; Santamaría, R.; Menéndez, R. Optimization of the size and yield of graphene oxide sheets in the exfoliation step. Carbon 2013, 63, 576–578. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie method. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- ASTM Standard D2798-11a; Standard Test Method for Microscopical Determination of the Vitrinite Reflectance of Coal. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3173-11; Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3174-11; Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3175-11; Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3177-02; Test Method for Total Sulfur in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM Standard D3177-02; Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal. ASTM International: West Conshohocken, PA, USA, 2011.
- International Committee for Coal and Organic Petrology. The new vitrinite classification (ICCP system 1994). Fuel 1998, 77, 349–358. [Google Scholar] [CrossRef]
- International Committee for Coal and Organic Petrology. The new inertinite classification (ICCP system 1994). Fuel 2001, 80, 459–471. [Google Scholar] [CrossRef]
- Duber, S.; Pusz, S.; Kwiecińska, B.K.; Rouzaud, J.N. On the optically biaxial character and heterogeneity of anthracites. Int. J. Coal Geol. 2000, 44, 227–250. [Google Scholar] [CrossRef]
- Pusz, S.; Borrego, A.G.; Alvarez, D.; Camean, I.; Cann, V.D.; Duber, S.; Kalkreuth, W.; Komorek, J.; Kus, J.; Kwiecińska, B.K.; et al. Application of reflectance parameters in the estimation of the structural order of coals and carbonaceous materials. Precision and bias of measurements derived from the ICCP structural working group. Int. J. Coal Geol. 2014, 131, 147–161. [Google Scholar] [CrossRef][Green Version]
- Sheng, C.D. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 11760, Classification of Coals; ISO: Geneva, Switzerland, 2018; p. 9. [Google Scholar]
- Chou, C.L. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401–187404. [Google Scholar] [CrossRef] [PubMed]
- Lyon, L.A. Raman spectroscopy. Anal. Chem. 1998, 70, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Pachfule, P.; Shinde, D.; Majumder, M.; Xu, Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework. Nat. Chem. 2016, 8, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorioa, A.; Saitoe, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Hou, Y.G.; Zhang, K.P.; Wang, F.R.; He, S.; Dong, T.; Wang, C.; Qin, W.; Xiao, Y.; Tang, B.; Yu, R.; et al. Structural evolution of organic matter and implications for graphitization in over-mature marine shales. South China. Mar. Pet. Geol. 2019, 109, 304–316. [Google Scholar] [CrossRef]
- Robertson, A.W.; Bachmatiuk, A.; Wu, Y.A.; Schaffel, F.; Buchner, B.; Rummeli, M.H.; Warner, J.H. Structural distortions in few-layer graphene creases. ACS Nano 2012, 5, 9984–9991. [Google Scholar] [CrossRef]









| Sample | Description | Age | Location |
|---|---|---|---|
| WK-15#-M2 | 30 cm, 2nd bench sample, semi-bright | Taiyuan Formation, Carboniferous | No.15 coal of Wukuang mine |
| WK-15#-KC | channel sample | Taiyuan Formation, Carboniferous | No.15 coal of Wukuang mine |
| BZ-2#-M4 | 25 cm, 4th bench sample, semi-bright | Shanxi Formation, Permian | No.2 coal of Buzi mine |
| BZ-2#-KC | channel sample | Shanxi Formation, Permian, | No.2 coal of Buzi mine |
| JSFH-15#-M6 | 26 cm, 6th bench sample, semi-bright | Taiyuan Formation, Carboniferous | No.15 coal of Fenhong mine |
| KXYQ-9#-M1 | 30 cm, 1st bench sample, semi-dull | Taiyuan Formation, Carboniferous | No.9 coal of Yunquan mine |
| KXYQ-15#-M5 | 23 cm, 5th bench sample, semi-bright | Taiyuan Formation, Carboniferous | No.15 coal of Yunquan mine |
| TAHX-15#-M8 | 20 cm, 8th bench sample, bright | Taiyuan Formation, Carboniferous | No.15 coal of Hongxiang mine, |
| GZ-15#-M4 | 25 cm, 4th bench sample, semi-bright | Taiyuan Formation, Carboniferous | No.15 coal of Gaizhou mine |
| SJZ-15#-M4 | 30 cm, 4th bench sample, bright | Taiyuan Formation, Carboniferous | No.15 coal of Shijiahzuang mine, |
| XJ-3#-M6 | 25 cm, 6th bench sample, bright | Shanxi Formation, Permian | No.3 coal of Xingjing mine |
| XJ-8#-M6 | 25 cm, 6th bench sample, semi-bright | Taiyuan Formation, Carboniferous | No.8 coal of Xingjing mine |
| XJ-15#-M6 | 25 cm, 6th bench sample, semi-bright | Taiyuan Formation, Permian | No.15 coal of Xingjing mine |
| Sample | Ro,max (%) | Proximate Analysis (wt%) | Ultimate Analyses (wt%) | H/C (Atom) | O/C (Atom) | Maceral Composition (vol%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | St, d | Cdaf | Hdaf | Ndaf | St, daf | Odaf * | V | I | M | ||||
| BZ-2#-M4 | 2.25 | 1.56 | 7.84 | 11.13 | 0.45 | 91.26 | 3.68 | 1.53 | 0.49 | 3.04 | 0.484 | 0.025 | 72.8 | 25.6 | 1.6 |
| KXYQ-9#-M1 | 2.29 | 0.86 | 13.34 | 9.66 | 1.99 | 90.47 | 3.74 | 2.24 | 2.30 | 1.25 | 0.496 | 0.010 | 44.4 | 54.0 | 1.6 |
| BZ-2#-KC | 2.32 | 1.77 | 10.16 | 10.41 | 0.43 | 90.80 | 3.96 | 1.56 | 0.48 | 3.20 | 0.523 | 0.026 | 74.8 | 22.8 | 2.4 |
| XJ-3#-M6 | 2.42 | 1.10 | 2.09 | 8.45 | 0.57 | 91.95 | 4.00 | 1.38 | 0.58 | 2.09 | 0.522 | 0.017 | 79.2 | 15.6 | 5.2 |
| XJ-15#-M6 | 2.42 | 1.10 | 4.28 | 7.30 | 1.98 | 91.43 | 3.59 | 1.25 | 2.07 | 1.66 | 0.471 | 0.014 | 74.8 | 24.4 | 0.8 |
| JSFH-15#-M6 | 2.53 | 1.44 | 7.61 | 5.08 | 1.72 | 91.76 | 2.70 | 0.82 | 1.86 | 2.86 | 0.353 | 0.023 | 80.4 | 18.0 | 1.6 |
| KXYQ-15#-M5 | 2.54 | 1.09 | 6.94 | 9.55 | 4.02 | 89.06 | 3.54 | 2.08 | 4.32 | 1.00 | 0.477 | 0.008 | 78.0 | 20.8 | 1.2 |
| GZ-15#-M4 | 2.55 | 1.95 | 13.24 | 9.69 | 2.84 | 87.55 | 3.53 | 0.99 | 3.27 | 4.66 | 0.484 | 0.040 | 52.8 | 34.8 | 12.4 |
| XJ-8#-M6 | 2.67 | 2.04 | 10.73 | 7.85 | 0.76 | 91.34 | 3.53 | 1.35 | 0.85 | 2.93 | 0.464 | 0.024 | 59.8 | 35.4 | 4.8 |
| SJZ-15#-M4 | 2.67 | 1.11 | 7.24 | 10.09 | 0.79 | 91.31 | 3.73 | 1.35 | 0.85 | 2.76 | 0.490 | 0.023 | 82.0 | 15.6 | 2.4 |
| TAHX-15#-M8 | 2.68 | 0.87 | 10.09 | 7.46 | 3.89 | 89.96 | 3.26 | 1.56 | 4.33 | 0.89 | 0.435 | 0.007 | 77.6 | 21.2 | 1.2 |
| WK-15#-M2 | 2.71 | 2.50 | 4.90 | 6.99 | 0.87 | 92.21 | 3.08 | 1.34 | 0.91 | 2.46 | 0.401 | 0.020 | 54.8 | 44.0 | 1.2 |
| WK-15#-KC | 2.72 | 2.10 | 13.52 | 7.23 | 0.90 | 91.71 | 3.39 | 1.39 | 1.04 | 2.47 | 0.444 | 0.020 | 62.8 | 34.4 | 2.8 |
| G-LT | - | 2.40 | 3.62 | 1.71 | - | 98.25 | 0.58 | 0.21 | - | - | - | - | - | - | - |
| Sample | 2θ002/(°) | FWHM002 | 2θ100/(°) | FWHM100 | d002/nm | La/nm | Lc/nm | Nave | G |
|---|---|---|---|---|---|---|---|---|---|
| G-BZ-2#-M4 | 26.28 | 0.63 | 42.37 | 0.99 | 0.3388 | 17.591 | 13.522 | 40.907 | 60.05% |
| G-KXYQ-9#-M1 | 26.30 | 0.59 | 42.33 | 0.70 | 0.3386 | 24.876 | 14.439 | 43.644 | 62.99% |
| G-BZ-2#-KC | 26.29 | 0.75 | 42.39 | 0.63 | 0.3387 | 24.645 | 11.359 | 34.545 | 61.52% |
| G-XJ-3#-M6 | 26.25 | 0.65 | 42.36 | 0.76 | 0.3392 | 22.914 | 13.105 | 39.633 | 55.62% |
| G-XJ-15#-M6 | 26.28 | 0.43 | 42.36 | 0.55 | 0.3388 | 31.663 | 19.811 | 59.468 | 60.05% |
| G-JSFH-15#-M6 | 26.26 | 0.73 | 42.31 | 0.93 | 0.3391 | 18.722 | 11.669 | 35.413 | 57.10% |
| G-KXYQ-15#-M5 | 26.32 | 0.52 | 42.39 | 0.77 | 0.3383 | 22.619 | 16.384 | 49.425 | 65.93% |
| G-GZ-15#-M4 | 26.38 | 0.49 | 42.37 | 0.63 | 0.3376 | 27.643 | 17.389 | 52.511 | 74.72% |
| G-XJ-8#-M6 | 26.33 | 0.62 | 42.35 | 0.82 | 0.3382 | 21.237 | 13.7413 | 41.630 | 67.40% |
| G-SJZ-15#-M4 | 26.34 | 0.47 | 42.36 | 0.48 | 0.3381 | 36.281 | 18.1272 | 54.619 | 68.86% |
| G-TAHX-15#-M8 | 26.34 | 0.50 | 42.39 | 0.63 | 0.3381 | 27.645 | 17.0396 | 51.401 | 68.86% |
| G-WK-15#-M2 | 26.38 | 0.37 | 42.36 | 0.36 | 0.3376 | 48.374 | 23.0283 | 69.217 | 74.72% |
| G-WK-15#-KC | 26.39 | 0.38 | 42.33 | 0.44 | 0.3374 | 39.575 | 22.4228 | 67.448 | 76.18% |
| G-LT | 26.24 | 0.47 | 43.98 | 2.52 | 0.3393 | 6.949 | 18.1235 | 54.408 | 54.15% |
| Sample | 2θ002/(°) | FWHM002 | 2θ100/(°) | FWHM100 | d002/nm | La/nm | Lc/nm | Nave |
|---|---|---|---|---|---|---|---|---|
| GS-BZ-2#-M4 | 24.53 | 10.40 | 43.440 | 3.51 | 0.3626 | 4.980 | 0.816 | 3.251 |
| GS-KXYQ-9#-M1 | 24.20 | 9.15 | 43.520 | 3.13 | 0.3675 | 5.586 | 0.927 | 3.523 |
| GS-BZ-2#-KC | 24.59 | 9.90 | 43.560 | 3.82 | 0.3617 | 4.578 | 0.858 | 3.371 |
| GS-XJ-3#-M6 | 24.72 | 10.57 | 43.590 | 3.97 | 0.3599 | 4.405 | 0.804 | 3.233 |
| GS-XJ-15#-M6 | 24.72 | 10.38 | 43.490 | 3.07 | 0.3599 | 5.695 | 0.818 | 3.274 |
| GS-JSFH-15#-M6 | 24.48 | 10.50 | 43.400 | 3.14 | 0.3633 | 5.566 | 0.808 | 3.225 |
| GS-KXYQ-15#-M5 | 24.42 | 9.27 | 43.440 | 3.14 | 0.3642 | 5.567 | 0.916 | 3.514 |
| GS-GZ-15#-M4 | 24.49 | 8.45 | 43.450 | 3.02 | 0.3632 | 5.788 | 1.005 | 3.766 |
| GS-XJ-8#-M6 | 24.75 | 9.73 | 42.470 | 3.15 | 0.3594 | 5.531 | 0.873 | 3.429 |
| GS-SJZ-15#-M4 | 23.71 | 8.13 | 43.410 | 3.18 | 0.3749 | 5.496 | 1.043 | 3.781 |
| GS-TAHX-15#-M8 | 24.31 | 8.51 | 43.480 | 2.95 | 0.3658 | 5.926 | 0.997 | 3.726 |
| GS-WK-15#-M2 | 24.66 | 8.32 | 43.460 | 2.80 | 0.3607 | 6.243 | 1.021 | 3.829 |
| GS-WK-15#-KC | 24.64 | 7.42 | 43.430 | 2.80 | 0.3610 | 6.242 | 1.144 | 4.170 |
| GS-LT | 25.30 | 3.45 | 43.16 | 3.07 | 0.3520 | 5.680 | 2.360 | 7.700 |
| Sample | D/cm−1 | G/cm−1 | G-D (cm−1) | ID/IG | ||
|---|---|---|---|---|---|---|
| Frequency (cm−1) | FWHM | Frequency (cm−1) | FWHM | |||
| BZ-2#-M4 | 1349.17 | 256.39 | 1597.97 | 66.66 | 248.80 | 0.55 |
| KXYQ-9#-M1 | 1345.69 | 248.57 | 1599.24 | 59.73 | 253.55 | 0.53 |
| BZ-2#-KC | 1347.40 | 262.60 | 1600.52 | 60.67 | 253.12 | 0.53 |
| XJ-3#-M6 | 1347.02 | 257.07 | 1598.86 | 61.85 | 251.84 | 0.54 |
| XJ-15#-M6 | 1341.25 | 240.43 | 1598.99 | 58.04 | 257.74 | 0.52 |
| JSFH-15#-M6 | 1338.98 | 233.18 | 1601.35 | 54.32 | 262.37 | 0.50 |
| KXYQ-15#-M5 | 1341.50 | 240.13 | 1600.36 | 58.25 | 258.86 | 0.51 |
| GZ-15#-M4 | 1346.69 | 255.02 | 1599.75 | 59.92 | 253.06 | 0.52 |
| XJ-8#-M6 | 1346.31 | 254.36 | 1600.80 | 58.42 | 254.49 | 0.52 |
| SJZ-15#-M4 | 1331.53 | 210.29 | 1603.38 | 48.50 | 271.85 | 0.48 |
| TAHX-15#-M8 | 1345.85 | 233.18 | 1600.26 | 59.91 | 254.41 | 0.50 |
| WK-15#-M2 | 1339.78 | 226.38 | 1603.47 | 51.34 | 263.69 | 0.47 |
| WK-15#-KC | 1333.46 | 159.01 | 1601.52 | 52.73 | 268.06 | 0.34 |
| Sample | D/cm−1 | G/cm−1 | G-D (cm−1) | ID/IG | ||
|---|---|---|---|---|---|---|
| Frequency (cm−1) | FWHM | Frequency (cm-1) | FWHM | |||
| d-BZ-2#-M4 | 1360.88 | 200.56 | 1581.88 | 83.57 | 221.00 | 0.64 |
| d-KXYQ-9#-M1 | 1355.99 | 194.40 | 1586.42 | 95.18 | 230.43 | 0.65 |
| d-BZ-2#-KC | 1354.23 | 279.78 | 1591.52 | 66.66 | 237.29 | 0.68 |
| d-XJ-3#-M6 | 1356.57 | 202.42 | 1588.30 | 85.12 | 231.73 | 0.49 |
| d-XJ-15#-M6 | 1355.13 | 215.17 | 1589.00 | 83.94 | 233.87 | 0.50 |
| d-JSFH-15#-M6 | 1324.87 | 158.24 | 1597.32 | 54.13 | 272.45 | 0.47 |
| d-KXYQ-15#-M5 | 1350.27 | 221.02 | 1593.00 | 74.30 | 242.73 | 0.47 |
| d-GZ-15#-M4 | 1353.48 | 203.03 | 1591.21 | 84.03 | 237.73 | 0.58 |
| d-XJ-8#-M6 | 1351.49 | 192.86 | 1589.12 | 84.09 | 237.63 | 0.47 |
| d-SJZ-15#-M4 | 1334.65 | 186.87 | 1601.67 | 51.70 | 267.02 | 0.39 |
| d-TAHX-15#-M8 | 1335.81 | 201.14 | 1592.93 | 64.78 | 257.12 | 0.43 |
| d-WK-15#-M2 | 1327.86 | 164.95 | 1597.22 | 56.41 | 269.36 | 0.33 |
| d-WK-15#-KC | 1337.05 | 204.88 | 1595.56 | 60.58 | 258.51 | 0.41 |
| Sample | D/cm−1 | G/cm−1 | G-D (cm−1) | ID/IG | ||
|---|---|---|---|---|---|---|
| Frequency (cm−1) | FWHM | Frequency (cm−1) | FWHM | |||
| G-BZ-2#-M4 | 1352.58 | 37.82 | 1583.40 | 30.95 | 230.82 | 0.25 |
| G-KXYQ-9#-M1 | 1348.21 | 42.18 | 1579.35 | 33.57 | 231.14 | 0.29 |
| G-BZ-2#-KC | 1351.77 | 39.30 | 1583.56 | 34.24 | 231.79 | 0.33 |
| G-XJ-3#-M6 | 1351.71 | 39.89 | 1581.19 | 29.32 | 229.48 | 0.19 |
| G-XJ-15#-M6 | 1350.88 | 43.27 | 1582.11 | 28.08 | 231.23 | 0.19 |
| G-JSFH-15#-M6 | 1350.67 | 39.93 | 1582.17 | 29.39 | 231.50 | 0.20 |
| G-KXYQ-15#-M5 | 1350.47 | 36.56 | 1580.41 | 26.01 | 229.94 | 0.12 |
| G-GZ-15#-M4 | 1352.27 | 38.48 | 1582.29 | 27.05 | 230.02 | 0.16 |
| G-XJ-8#-M6 | 1353.73 | 33.39 | 1583.05 | 25.04 | 229.32 | 0.10 |
| G-SJZ-15#-M4 | 1351.07 | 36.18 | 1580.39 | 26.56 | 229.32 | 0.09 |
| G-TAHX-15#-M8 | 1350.35 | 38.47 | 1578.83 | 24.25 | 228.48 | 0.08 |
| G-WK-15#-M2 | 1352.43 | 40.55 | 1576.23 | 25.39 | 223.80 | 0.06 |
| G-WK-15#-KC | 1350.50 | 34.53 | 1579.88 | 24.82 | 229.38 | 0.08 |
| Sample | D/cm−1 | G/cm−1 | G-D (cm−1) | ID/IG | ||
|---|---|---|---|---|---|---|
| Frequency (cm−1) | FWHM | Frequency (cm−1) | FWHM | |||
| GS-BZ-2#-M4 | 1346.31 | 116.44 | 1594.26 | 78.54 | 247.95 | 1.02 |
| GS-KXYQ-9#-M1 | 1342.21 | 143.89 | 1581.97 | 91.98 | 239.76 | 0.97 |
| GS-BZ-2#-KC | 1342.21 | 138.58 | 1594.26 | 84.06 | 252.05 | 0.99 |
| GS-XJ-3#-M6 | 1346.31 | 140.76 | 1602.46 | 82.26 | 256.15 | 0.98 |
| GS-XJ-15#-M6 | 1338.11 | 141.49 | 1598.36 | 84.78 | 260.25 | 1.00 |
| GS-JSFH-15#-M6 | 1346.31 | 153.07 | 1598.36 | 87.14 | 252.05 | 1.00 |
| GS-KXYQ-15#-M5 | 1338.11 | 123.16 | 1598.36 | 79.01 | 260.25 | 0.98 |
| GS-GZ-15#-M4 | 1346.31 | 149.51 | 1586.07 | 92.83 | 247.95 | 0.94 |
| GS-XJ-8#-M6 | 1346.31 | 166.67 | 1594.26 | 90.36 | 247.95 | 0.93 |
| GS-SJZ-15#-M4 | 1342.21 | 165.46 | 1594.26 | 85.43 | 252.05 | 0.91 |
| GS-TAHX-15#-M8 | 1346.31 | 154.62 | 1598.36 | 87.15 | 252.05 | 0.91 |
| GS-WK-15#-KC | 1350.41 | 158.96 | 1598.36 | 83.48 | 247.95 | 0.90 |
| GS-WK-15#-M2 | 1354.51 | 156.69 | 1598.36 | 84.82 | 243.85 | 0.90 |
| Sample | d002 (nm) | Nave | ||
|---|---|---|---|---|
| XRD | HRTEM | XRD | HRTEM | |
| GS-BZ-2#-M4 | 0.363 | 0.433 | 3.25 | 3.00 |
| GS-KXYQ-9#-M1 | 0.368 | 0.457 | 3.52 | 4.00 |
| GS-BZ-2#-KC | 0.362 | 0.413 | 3.37 | 4.00 |
| GS-XJ-3#-M6 | 0.360 | 0.514 | 3.23 | 5.00 |
| GS-XJ-15#-M6 | 0.360 | 0.422 | 3.27 | 5.00 |
| GS-JSFH-15#-M6 | 0.363 | 0.414 | 3.23 | 5.00 |
| GS-KXYQ-15#-M5 | 0.364 | 0.470 | 3.51 | 6.00 |
| GS-GZ-15#-M4 | 0.363 | 0.425 | 3.77 | 6.00 |
| GS-XJ-8#-M6 | 0.359 | 0.414 | 3.43 | 6.00 |
| GS-SJZ-15#-M4 | 0.375 | 0.512 | 3.78 | 7.00 |
| GS-TAHX-15#-M8 | 0.366 | 0.438 | 3.73 | 7.00 |
| GS-WK-15#-M2 | 0.361 | 0.392 | 3.83 | 7.00 |
| GS-WK-15#-KC | 0.361 | 0.394 | 4.17 | 8.00 |
| Average | 0.363 | 0.438 | 3.55 | 5.62 |
| 0.401 | 5.00 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Tang, Y.; Che, Q.; Ma, P.; Luo, P.; Lu, X.; Dong, M. Effects of Coal Rank and Macerals on the Structure Characteristics of Coal-Based Graphene Materials from Anthracite in Qinshui Coalfield. Minerals 2022, 12, 588. https://doi.org/10.3390/min12050588
Li R, Tang Y, Che Q, Ma P, Luo P, Lu X, Dong M. Effects of Coal Rank and Macerals on the Structure Characteristics of Coal-Based Graphene Materials from Anthracite in Qinshui Coalfield. Minerals. 2022; 12(5):588. https://doi.org/10.3390/min12050588
Chicago/Turabian StyleLi, Ruiqing, Yuegang Tang, Qili Che, Pengliang Ma, Peng Luo, Xin Lu, and Min Dong. 2022. "Effects of Coal Rank and Macerals on the Structure Characteristics of Coal-Based Graphene Materials from Anthracite in Qinshui Coalfield" Minerals 12, no. 5: 588. https://doi.org/10.3390/min12050588
APA StyleLi, R., Tang, Y., Che, Q., Ma, P., Luo, P., Lu, X., & Dong, M. (2022). Effects of Coal Rank and Macerals on the Structure Characteristics of Coal-Based Graphene Materials from Anthracite in Qinshui Coalfield. Minerals, 12(5), 588. https://doi.org/10.3390/min12050588





