Paste Backfill Corrosion Mechanisms in Chloride and Sulfate Environments
Abstract
:1. Introduction
2. Experimental Materials and Methods
3. Results and Analysis
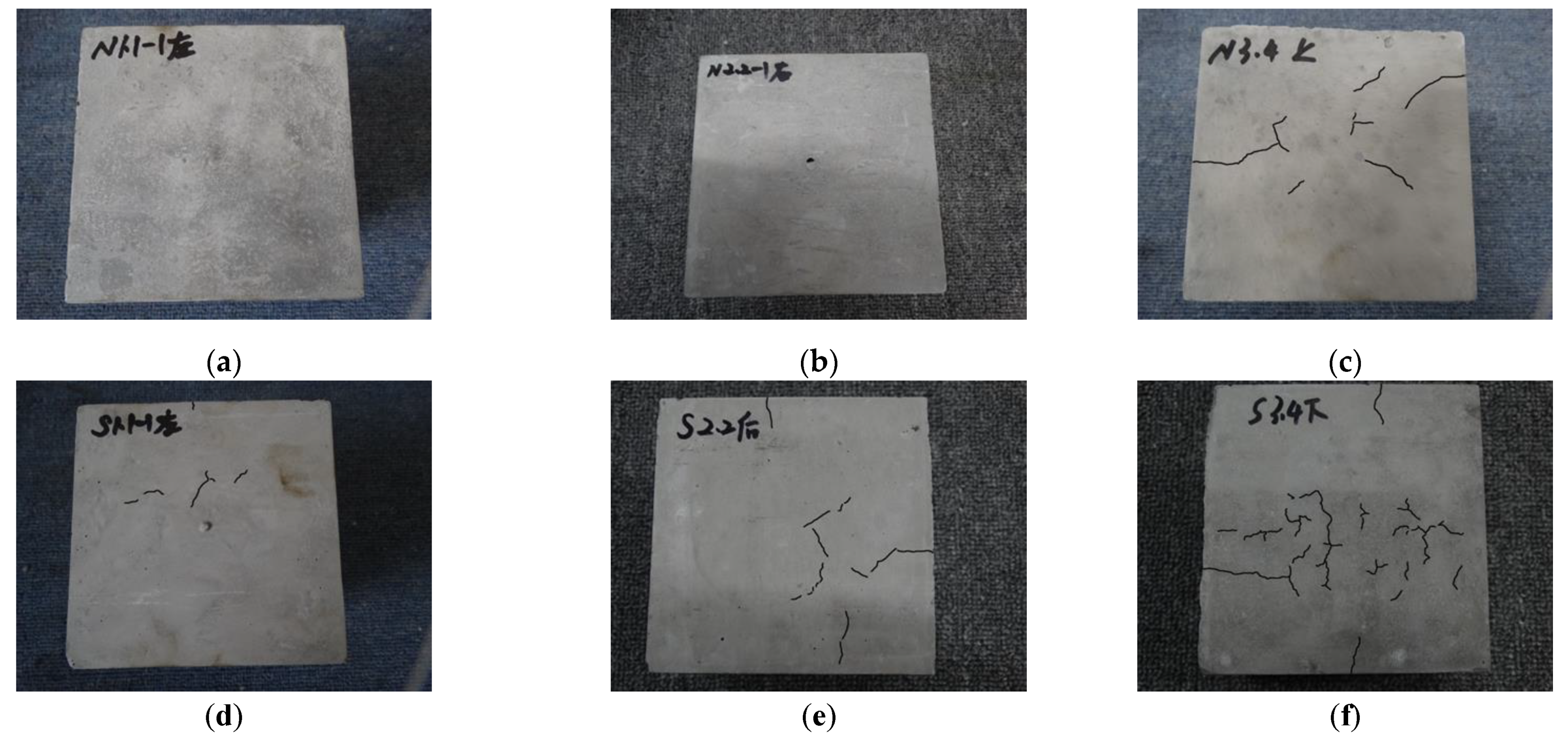
3.1. Effect of Chloride and Sulfate Solutions on the External Surface of the Specimens
3.2. Effect of Chloride and Sulfate Solutions on the Compressive Strength of the Specimens
3.2.1. Effect of Cl− Corrosion
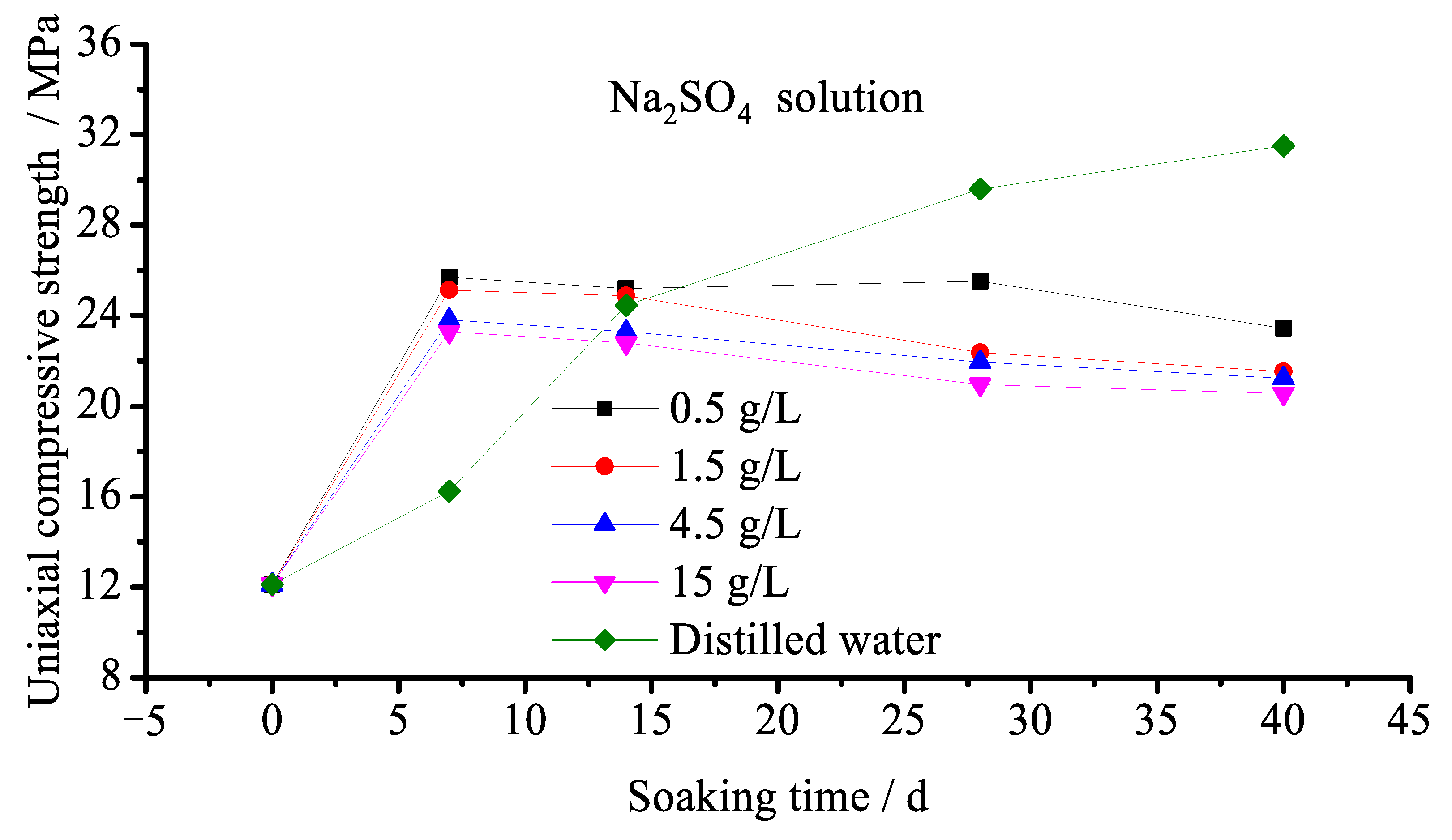
3.2.2. Effect of SO42− Corrosion
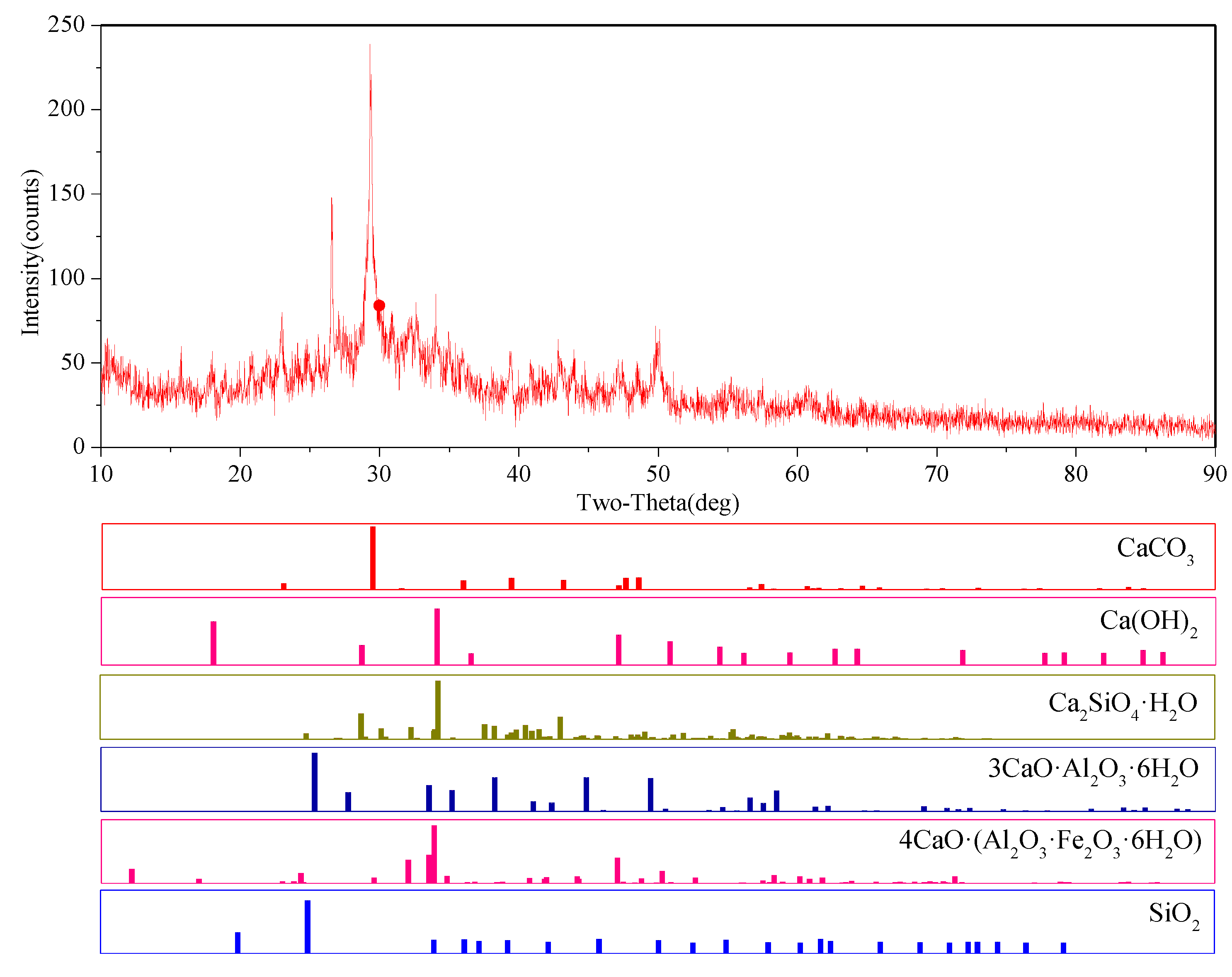
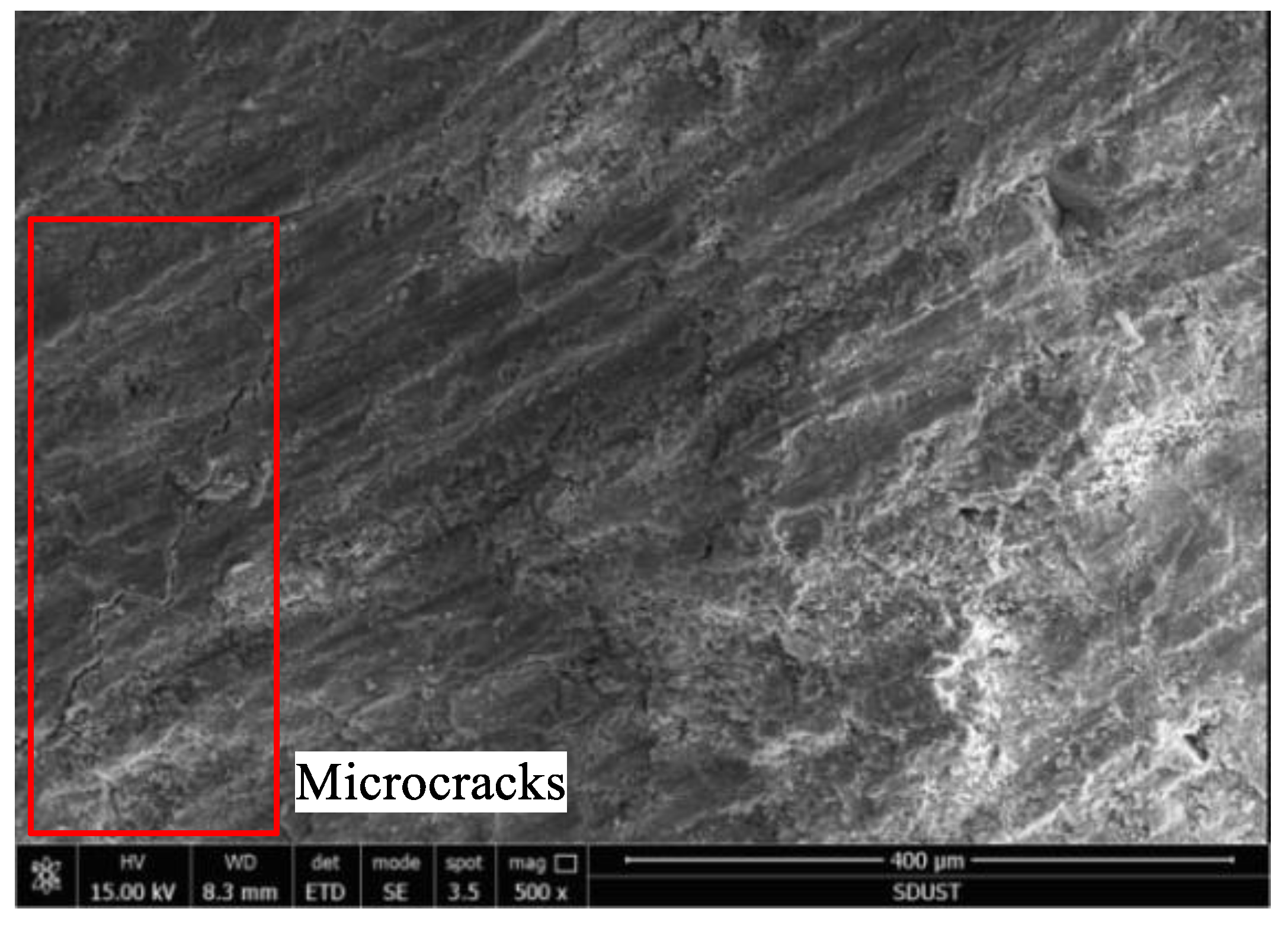
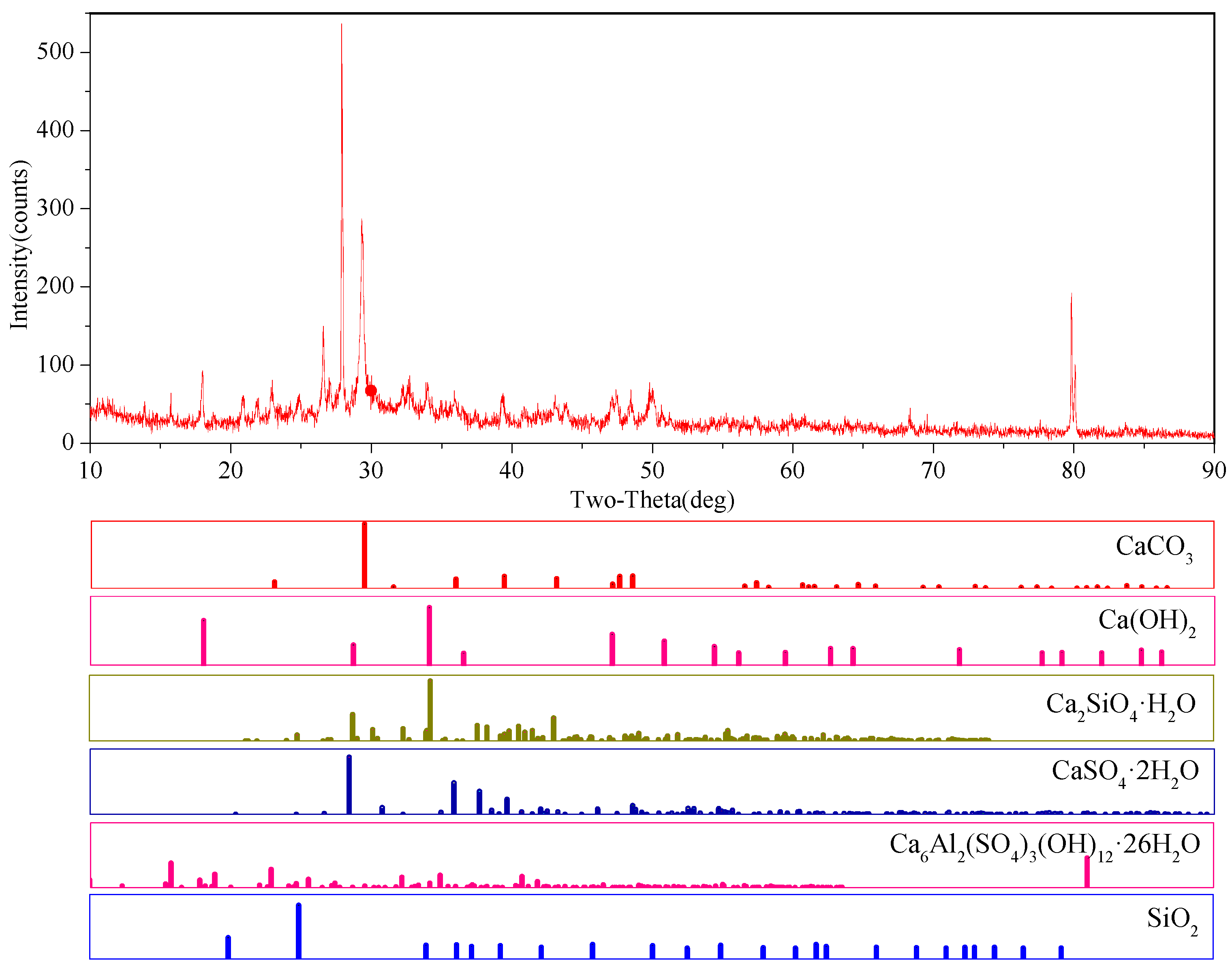
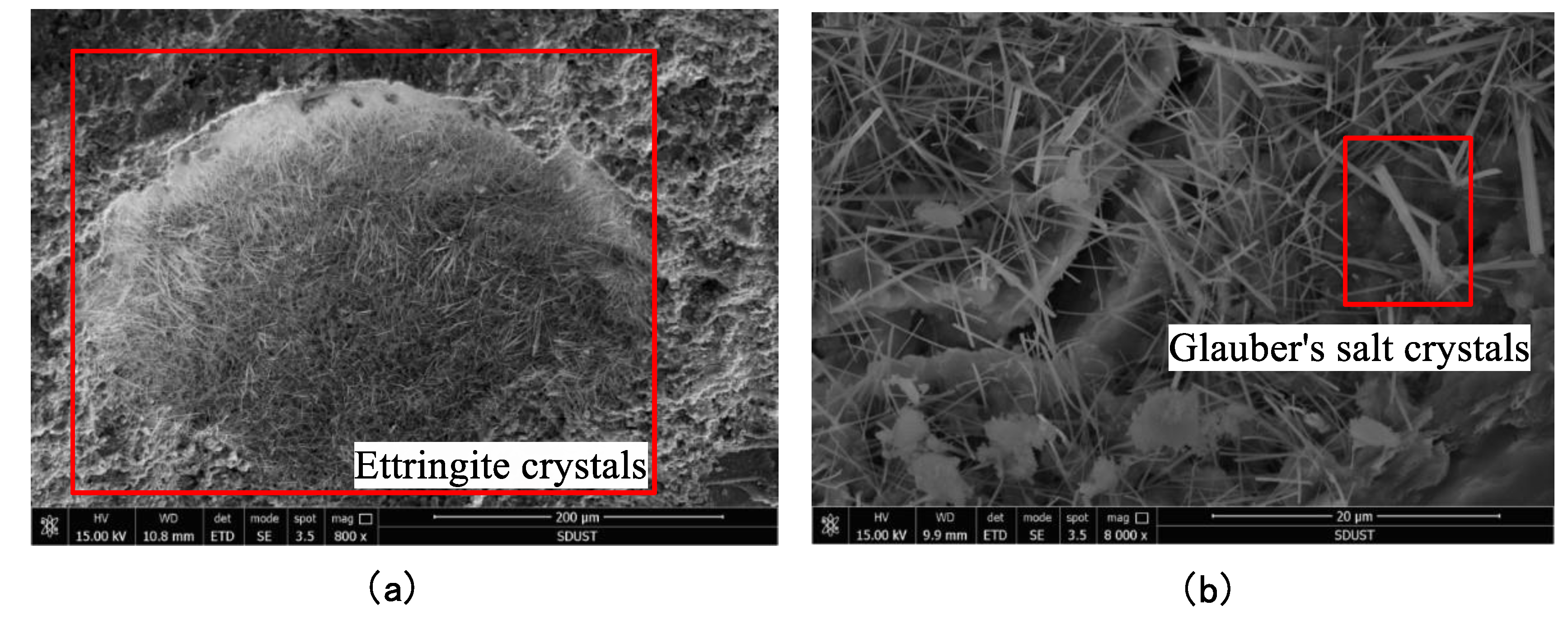
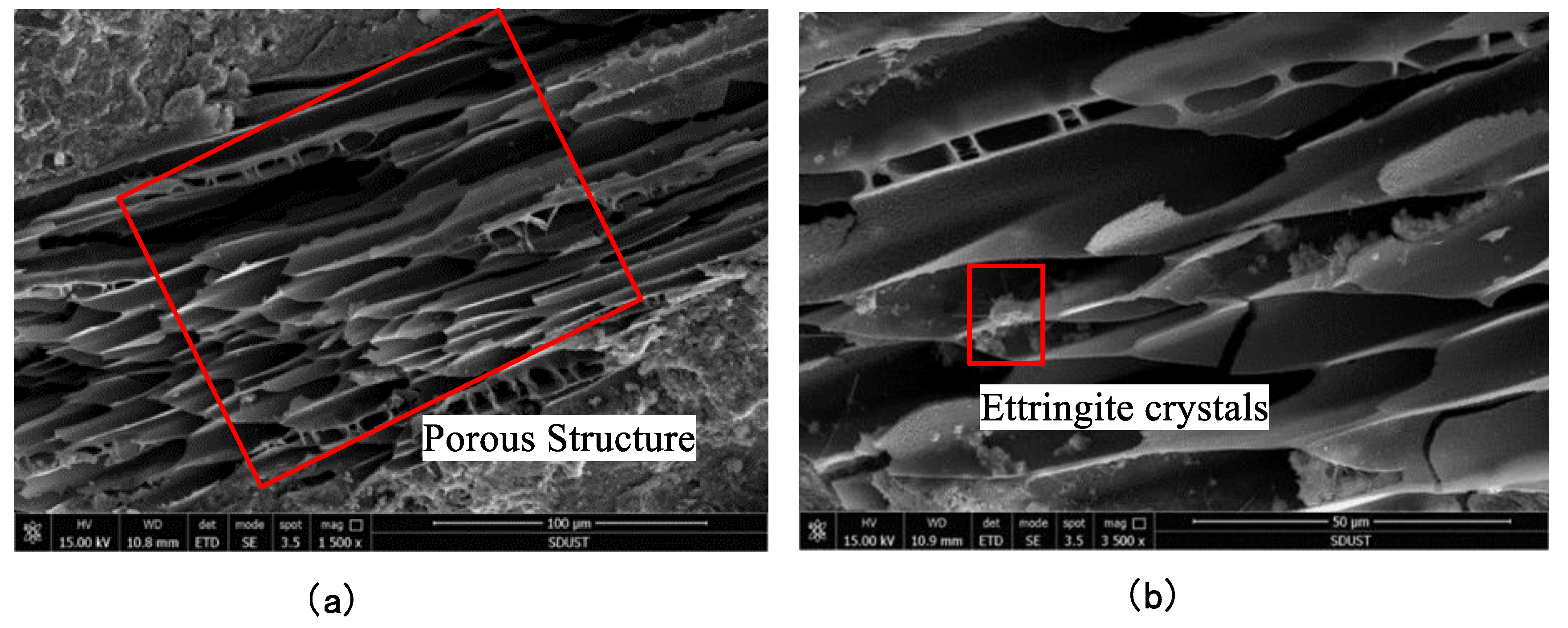
3.3. Microstructural Analysis
4. Conclusions
- The presence of a chloride solution was found to improve the early strength of paste backfill because Cl− participates in the hydration reaction to form calcium chloroaluminate hydrate, which fills the internal pores of the backfill and improves its compactness. After a certain period of time, all internal pores are filled. Because the generated calcium chloroaluminate hydrate is expansive, damage is induced in the backfill material, causing it to gradually crack and ultimately resulting in a reduction in its compressive strength.
- The presence of a sulfate solution was found to, on the one hand, corrode the backfill to produce dihydrate gypsum, ettringite, Glauber’s salt, and other expansive substances, resulting in expansive stress inside the backfill. When this expansive stress exceeds the tensile strength of the backfill, internal damage occurs that gradually destroys the backfill material. This is referred to as crystalline expansion damage. On the other hand, the formation of gypsum, ettringite, etc. was found to consume Ca(OH)2 during the process of filling the pores in the backfill, but Ca(OH)2 serves as the basis for the stable existence of hydration products such as C-S-H. The reduction of Ca(OH)2 will therefore inevitably enhance the decomposition of C-S-H, thereby reducing the internal bond strength of the backfill. This is referred to as reversible reaction damage. The strength and durability of paste backfill subjected to a sulfate solution will decrease under the action of these two types of damage.
- Scanning the backfill specimens using SEM revealed that there were fine carbon particles in the backfill, introduced by the fly ash. Though the porous structure of carbon particles provides space for hydration products, helping to densify the backfill as it cures, it also provides space for the growth of expansive substances, reducing the strength of the backfill.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Li, M.; Taheri, A.; Zhang, W.; Wu, Z.; Song, W. Properties and application of backfill materials in coal mines in China. Minerals 2019, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Sivakugan, N.; Rankine, R.; Rankine, K.; Rankine, K. Geotechnical considerations in mine backfilling in Australia. J. Clean. Prod. 2006, 14, 1168–1175. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, C.; Sun, X. Solid waste paste filling for none-village-relocation coal mining. J. China Univ. Min. Technol. 2004, 33, 154–158. [Google Scholar]
- Liu, J.; Li, X.; He, T. Application status and prospect of backfill mining in Chinese coal mines. J. China Coal Soc. 2020, 45, 141–150. [Google Scholar]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Aseniero, J.P.J.; Opiso, E.M.; Banda, M.H.T.; Tabelin, C.B. Potential utilization of artisanal gold-mine tailings as geopolymeric source material: Preliminary investigation. SN Appl. Sci. 2019, 1, 35. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Beltran, A.B.; Orbecido, A.H.; Bernardo-Arugay, I.; Resabal, V.J.; Villacorte-Tabelin, M.; Dalona, I.M.; Opiso, E.; Alloro, R.; Alonzo, D. Systems Approach toward a Greener Eco-efficient Mineral Extraction and Sustainable Land Use Management in the Philippines. Chem. Eng. Trans. 2021, 88, 1171–1176. [Google Scholar]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Feng, M.; Chen, Z.; Mao, X.; Han, G.; Wang, Y. Particle size distribution effects on the strength characteristic of cemented paste backfill. Minerals 2018, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Rong, H.; Zhou, M.; Hou, H. Pore structure evolution and its effect on strength development of sulfate-containing cemented paste backfill. Minerals 2017, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zheng, J.; Guo, L.; Zhao, Y. Effect of gypsum addition on the mechanical and microstructural performance of sulphide-rich cemented paste backfill. Minerals 2021, 11, 283. [Google Scholar] [CrossRef]
- Jiang, H.; Fall, M. Yield stress and strength of saline cemented tailings materials in sub-zero environments: Slag-paste backfill. J. Sustain. Cem. Based Mater. 2017, 6, 314–331. [Google Scholar] [CrossRef]
- Chen, M.; Zang, C.; Ding, Z.; Zhou, G.; Jiang, B.; Zhang, G.; Zhang, C. Effects of confining pressure on deformation failure behavior of jointed rock. J. Cent. South Univ. 2022, 29, 1–15. [Google Scholar]
- Ying, J.; Jiang, Z.; Xiao, J. Synergistic effects of three-dimensional graphene and silica fume on mechanical and chloride diffusion properties of hardened cement paste. Constr. Build. Mater. 2022, 316, 125756. [Google Scholar] [CrossRef]
- Deng, H.; Liu, Y.; Zhang, W.; Yu, S.; Tian, G. Study on the Strength Evolution Characteristics of Cemented Tailings Backfill from the Perspective of Porosity. Minerals 2021, 11, 82. [Google Scholar] [CrossRef]
- Jiang, G.; Wu Ax, L. Long-term strength performance of sulfur tailings filling and its affecting factors. J. Cent. South Univ. Sci. Technol. 2018, 49, 1504–1510. [Google Scholar]
- Wu, D.; Sun, G.; Huang, G. Experimental and simulation study on seepage characteristics of cemented tailings backfill. J. Cent. South Univ. Sci. Technol. 2015, 46, 1050–1057. [Google Scholar]
- Kitazume, M.; Nakamura, T.; Terashi, M.; Ohishi, K. Laboratory Tests on Long-Term Strength of Cement Treated Soil. In Proceedings of the International Conference on Grouting and Ground Treatment, New Orleans, LA, USA, 10–12 February 2003; pp. 586–597. [Google Scholar]
- Bai, X.H.; Zhao, Y.Q.; Han, P.J.; Qiao, J.Y.; Zhi-An, W.U. Experimental study on mechanical property of cemented soil under environmental contaminations. Chin. J. Geotech. Eng. 2007, 29, 1260–1263. [Google Scholar]
- Chew, S.H.; Kamruzzaman, A.; Lee, F.H. Physicochemical and Engineering Behavior of Cement Treated Clays. J. Geotech. Geoenviron. Eng. 2004, 130, 696–706. [Google Scholar] [CrossRef]
- Chen, S.; Du, Z.; Zhang, Z.; Zhang, H.; Xia, Z.; Feng, F. Effects of chloride on the early mechanical properties and microstructure of gangue-cemented paste backfill. Constr. Build. Mater. 2020, 235, 117504. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Acid mine drainage sources and hydrogeochemistry at the Yatani mine, Yamagata, Japan: A geochemical and isotopic study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef]
- Guo, Y.; Ran, H.; Feng, G.; Wang, P.; Wang, Z. Strength and creep characteristics of cemented gangue backfill in acid environment. J. Min. Saf. Eng. 2021, 38, 361–369. [Google Scholar]
- Chen, X.B.; Tang, M.X.; Ma, K.L. Underground concrete structure exposure to sulfate and chloride invading environment. J. Cent. South Univ. 2012, 43, 2803–2812. [Google Scholar]
- Luo, T.; Fan, G.; Guo, B.; Zhang, S. Experimental study on the influence of hydro-chemical erosion on morphology parameters and shear properties of limestone fractures. Acta Geotech. 2021, 16, 3867–3880. [Google Scholar] [CrossRef]
- Cui, B.; Liu, Y.; Feng, G.; Bai, J.; Du, X.; Wang, C.; Wang, H. Experimental study on the effect of fly ash content in cemented paste backfill on its anti-sulfate erosion. Int. J. Green Energy 2020, 17, 730–741. [Google Scholar] [CrossRef]
- He, X.; Hu, D.; Hu, Z. Research on technology for high mineralized mine water treatment. Coal Sci. Technol. 2002, 30, 38–41. [Google Scholar]
- Feng, Q.; Wang, H.; Li, X.; Hao, L. Characteristics and utilization of mine water in east China. J. China Univ. Min. Technol. 2004, 33, 193–196. [Google Scholar]
- Hao, L.I.; Liu, Y.; Yao, L.U.; Cui, B.; Guo, H. Experimental Study on the Effect of Sulfate Erosion on the Strength of Coal Filling Paste. Min. Res. Dev. 2018, 3, 139–144. [Google Scholar]
- Dong, Q.; Liang, B.; Jia, L.; Jiang, L. Effect of sulfide on the long-term strength of lead-zinc tailings cemented paste backfill. Constr. Build. Mater. 2019, 200, 436–446. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, A.-X.; Wang, H.; Wang, Y. Experimental study on the strength deterioration of sulfidic paste backfill. Chin. J. Eng. 2017, 39, 1493–1497. [Google Scholar]
- Chen, W.; Chen, H.; Qiao, D. Regulation of mechanical damage of filling body with sulfur based on coagulation and retarding technology. J. China Coal Soc. 2019, 44, 553–559. [Google Scholar]
- Liu, J.; Wu, R.; Wu, A.; Wang, S. Bleeding characteristics and improving mechanism of self-flowing tailings filling slurry with low concentration. Minerals 2017, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Ouellet, S.; Bussiere, B.; Aubertin, M.; Benzaazoua, M. Characterization of cemented paste backfill pore structure using SEM and IA analysis. Bull. Eng. Geol. Environ. 2008, 67, 139–152. [Google Scholar] [CrossRef]
- Mouret, M.; Bascoul, A.; Escadeillas, G. Microstructural features of concrete in relation to initial temperature—SEM and ESEM characterization. Cem. Concr. Res. 1999, 29, 369–375. [Google Scholar] [CrossRef]
- Du, Z.; Chen, S.; Yin, D.; Yao, D.; Zhang, Z. Experimental study of stability of paste backfill under chloride erosion environment. J. China Univ. Min. Technol. 2021, 50, 532–538+547. [Google Scholar]
- Luo, Q.; Jones, A. High-precision determination of residual stress of polycrystalline coatings using optimised XRD-sin2ψ technique. Surf. Coat. Technol. 2010, 205, 1403–1408. [Google Scholar] [CrossRef] [Green Version]
- Fry, A.T.; Kandil, F. A study of parameters affecting the quality of residual stress measurements using XRD. Mater. Sci. Forum 2002, 404, 579–586. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, C.; Chen, G.; Xin, J.; Fang, Z. Erosion mechanism of sulfur-bearing tailings in micro-scale. J. Xi’An Univ. Sci. Technol. 2018, 38, 553. [Google Scholar]
- Gao, Y.; Jing, H.; Yu, Z.; Li, L.; Wu, J.; Chen, W. Particle size distribution of aggregate effects on the reinforcing roles of carbon nanotubes in enhancing concrete ITZ. Constr. Build. Mater. 2022, 327, 126964. [Google Scholar] [CrossRef]



| Name of Coal Mine | Chloride (mg/L) | Sulfate (mg/L) |
|---|---|---|
| Xinzhuangzi | 39.99 | 327.93 |
| Kongji | 115.16 | 43.16 |
| Panyi | 889.72 | 198.03 |
| Wusu | 412.7 | 1026.3 |
| Zhangji | 259.4 | 472 |
| Shuanggou | 182 | 882.01 |
| Linhuan | 213 | 1580.6 |
| Quangou | 107.7 | 1094.5 |
| Lingxin | 1193.6 | 884.3 |
| Haizi | 146.0 | 1028.0 |
| Bulianta | 235.19 | 227.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Fan, K.; Wang, K.; Ning, J. Paste Backfill Corrosion Mechanisms in Chloride and Sulfate Environments. Minerals 2022, 12, 551. https://doi.org/10.3390/min12050551
Xu G, Fan K, Wang K, Ning J. Paste Backfill Corrosion Mechanisms in Chloride and Sulfate Environments. Minerals. 2022; 12(5):551. https://doi.org/10.3390/min12050551
Chicago/Turabian StyleXu, Guangzheng, Kegong Fan, Kun Wang, and Jianguo Ning. 2022. "Paste Backfill Corrosion Mechanisms in Chloride and Sulfate Environments" Minerals 12, no. 5: 551. https://doi.org/10.3390/min12050551
APA StyleXu, G., Fan, K., Wang, K., & Ning, J. (2022). Paste Backfill Corrosion Mechanisms in Chloride and Sulfate Environments. Minerals, 12(5), 551. https://doi.org/10.3390/min12050551






