Using Top-of-Froth Conductivity to Infer Water Overflow Rate in a Two-Phase Lab-Scale Flotation Column
Abstract
:1. Introduction
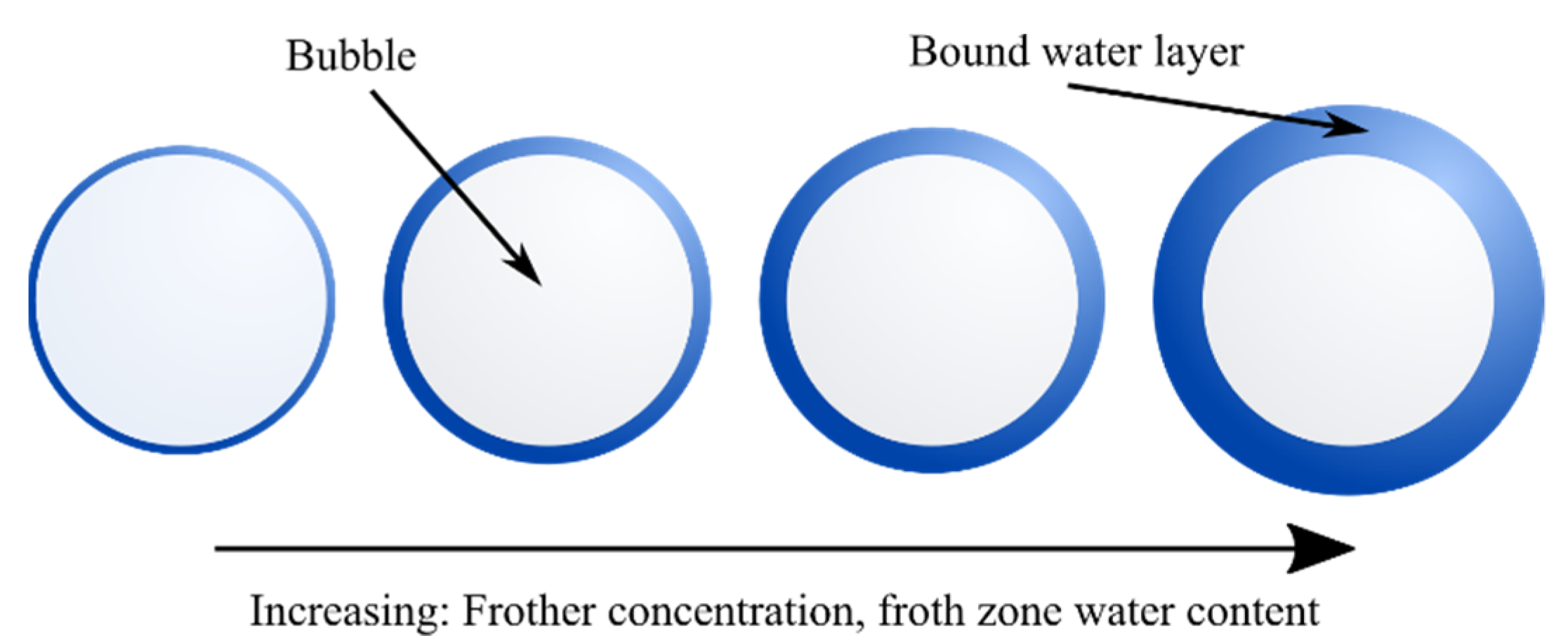
2. Conductivity Measurements of Dispersions
3. Experimental Section
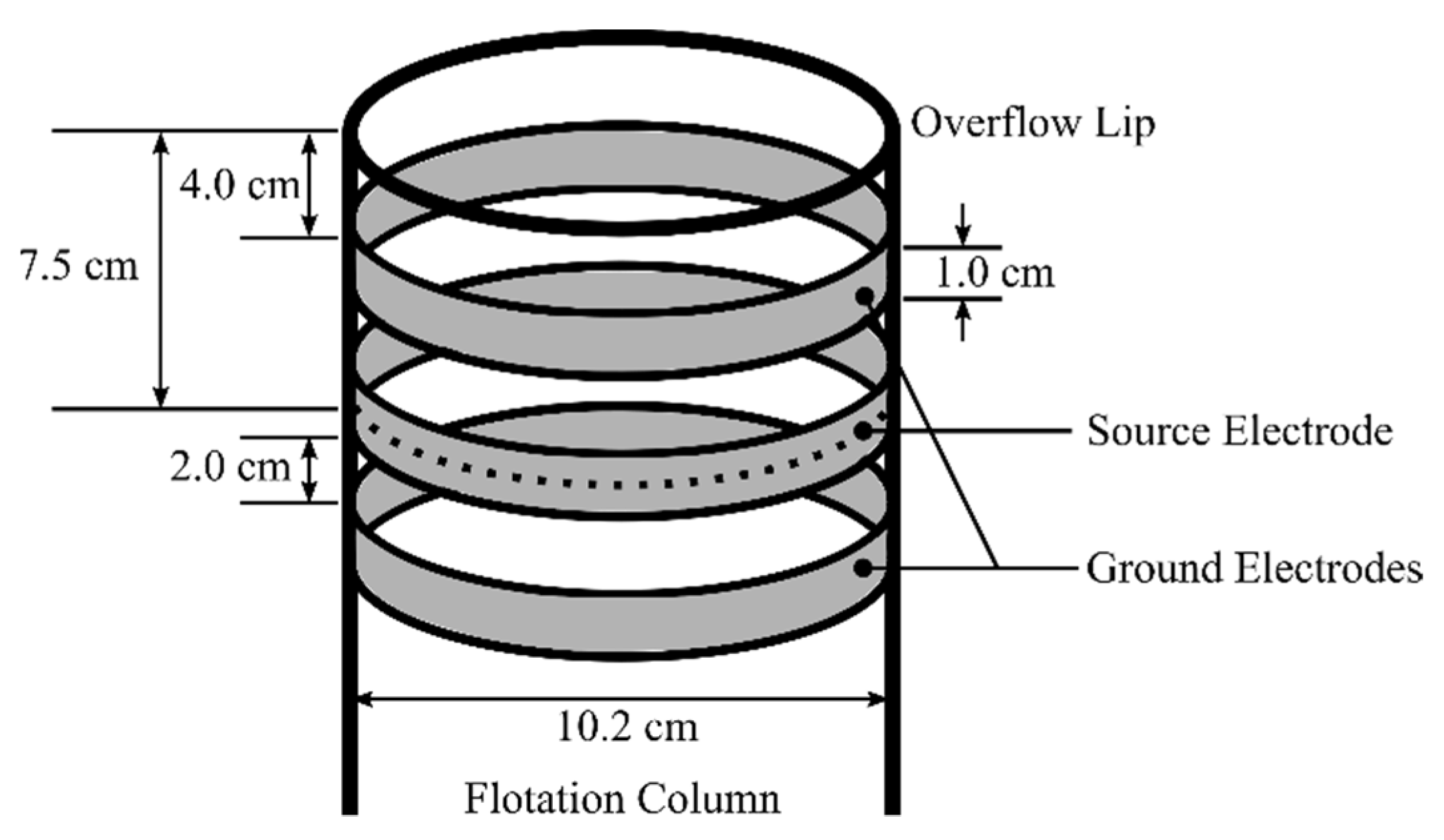
3.1. The Conductivity Sensor
3.2. Emulsion Generation
3.3. Blast Gate Apparatus and Methodology
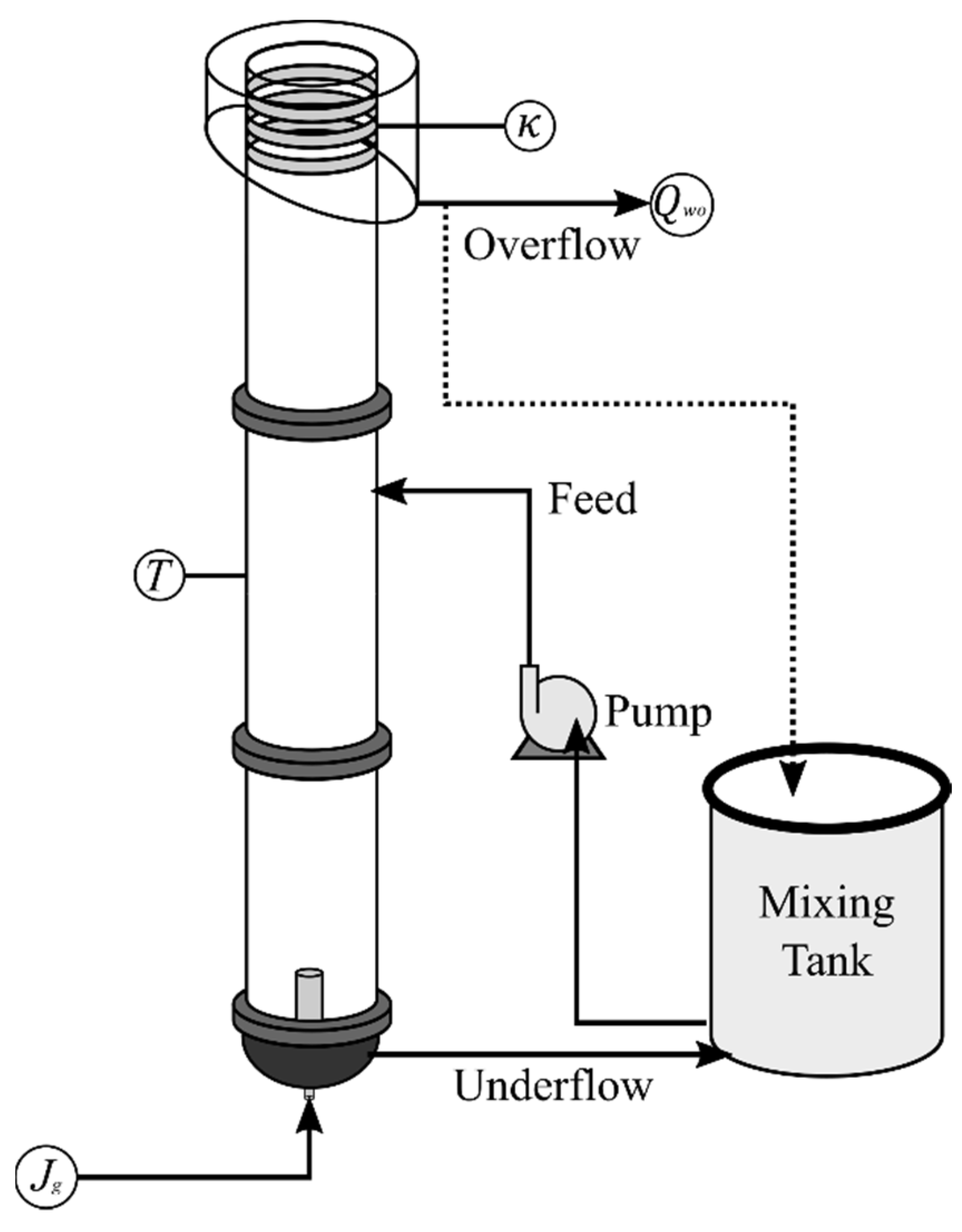
3.4. Laboratory Flotation Column and Methodology
3.5. Materials
3.5.1. Process Water
3.5.2. Frothers
4. Results and Discussion
4.1. Validating the Conductivity Sensor
4.1.1. Determining the Cell Constant
4.1.2. Measuring a Known Water Content Dispersion
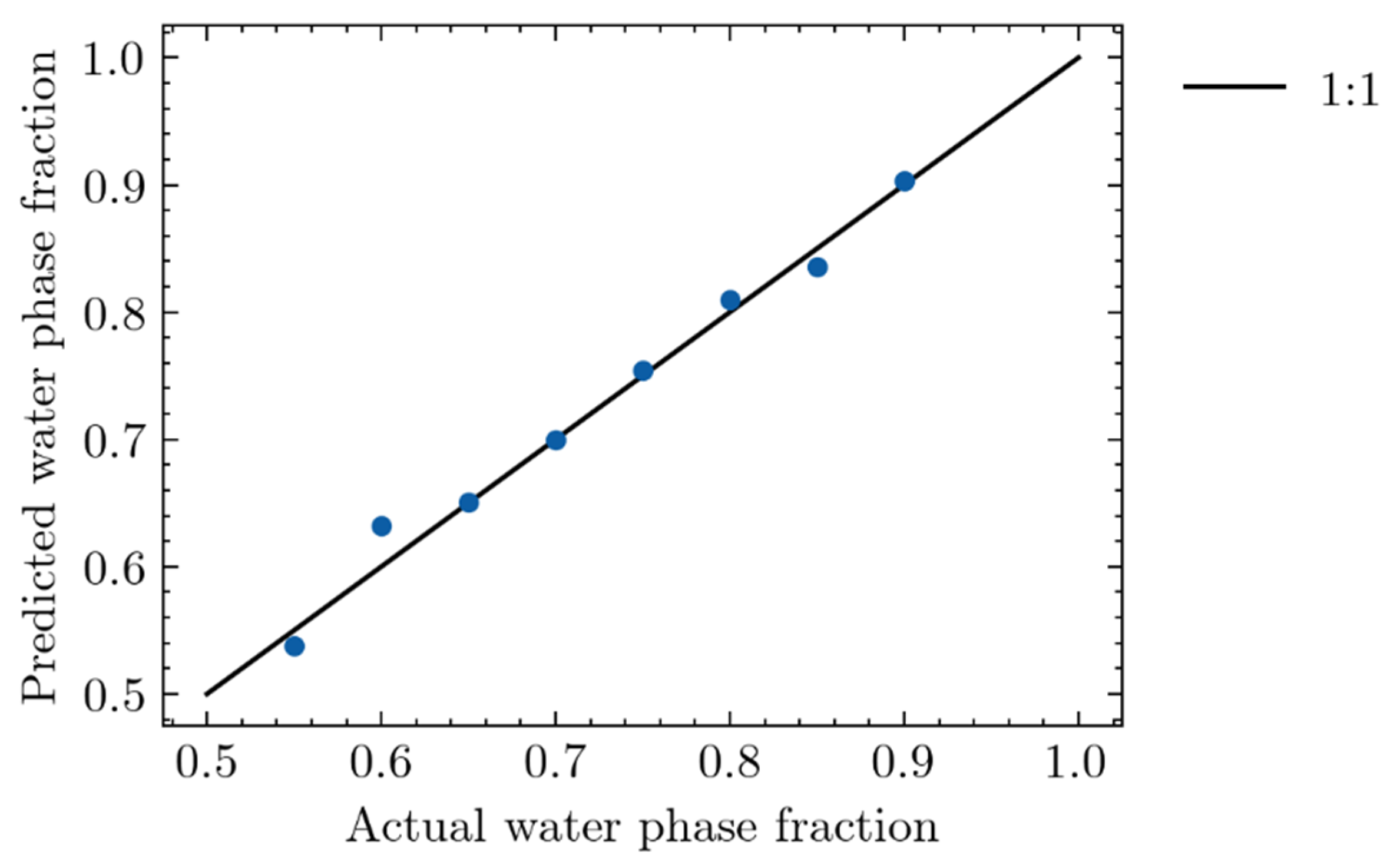
4.1.3. Validating the Relationship of Relative Conductivity to Froth Zone Water Content
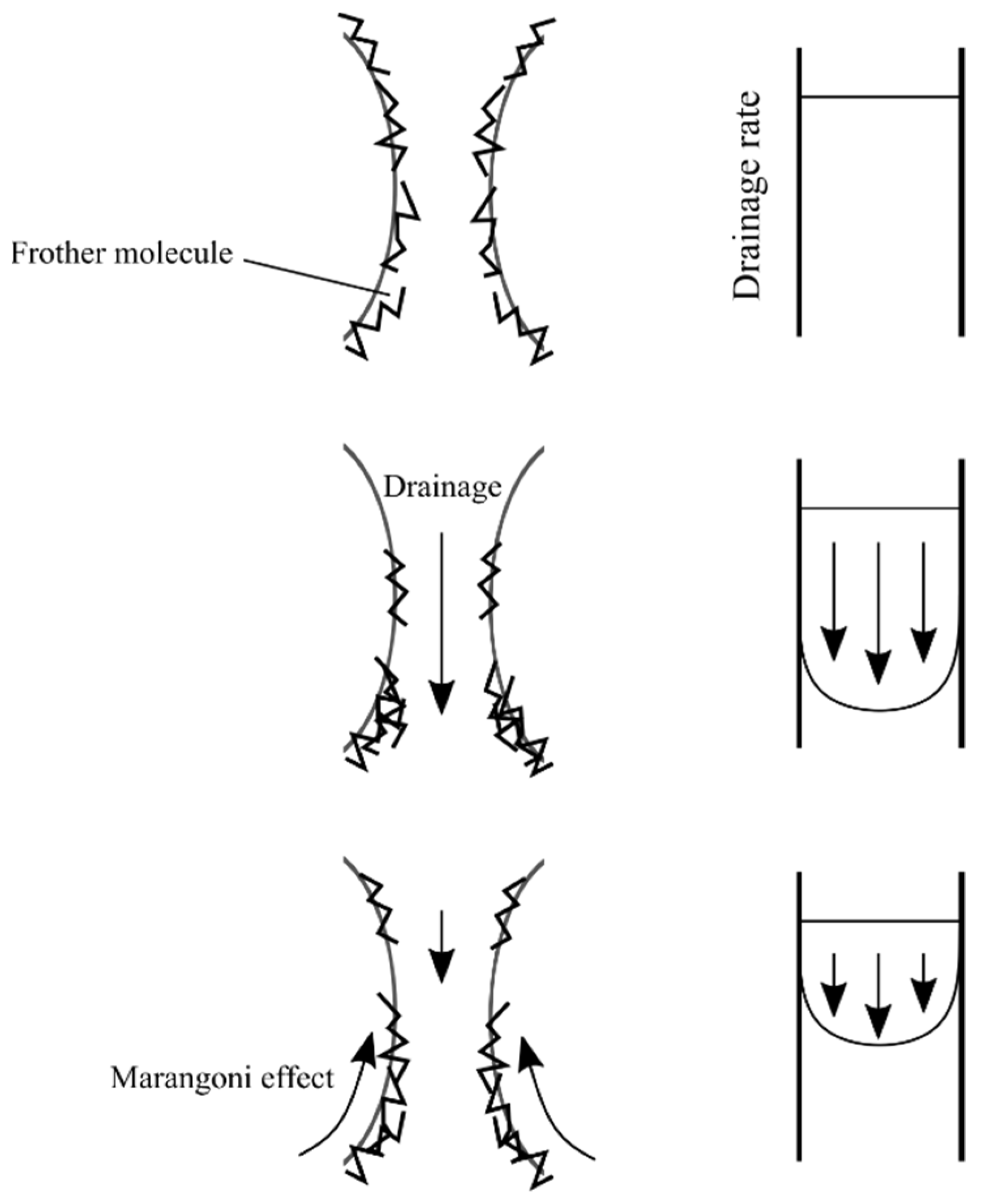
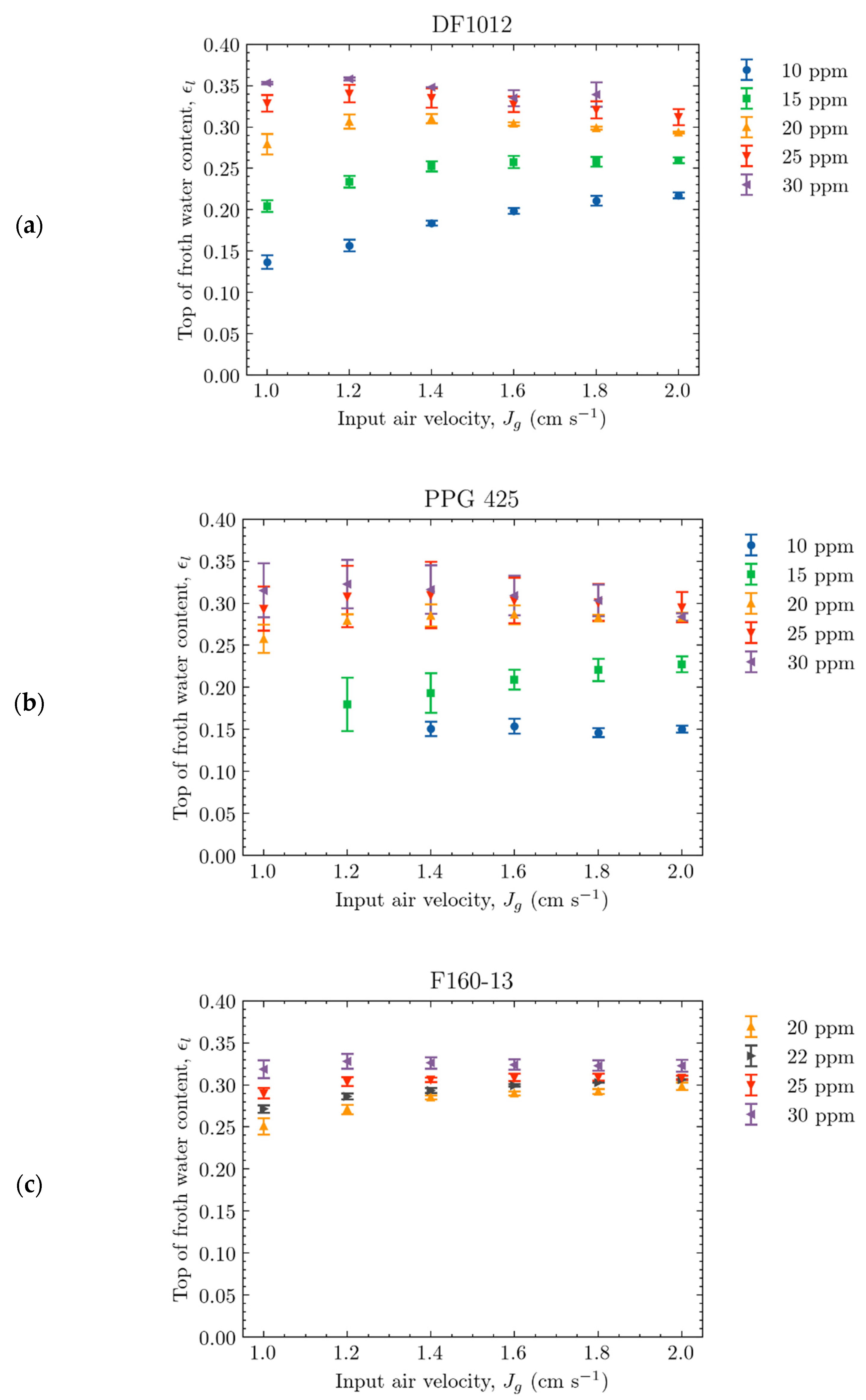
4.2. Effect of Operating Conditions on the Top-of-Froth Zone Water Content
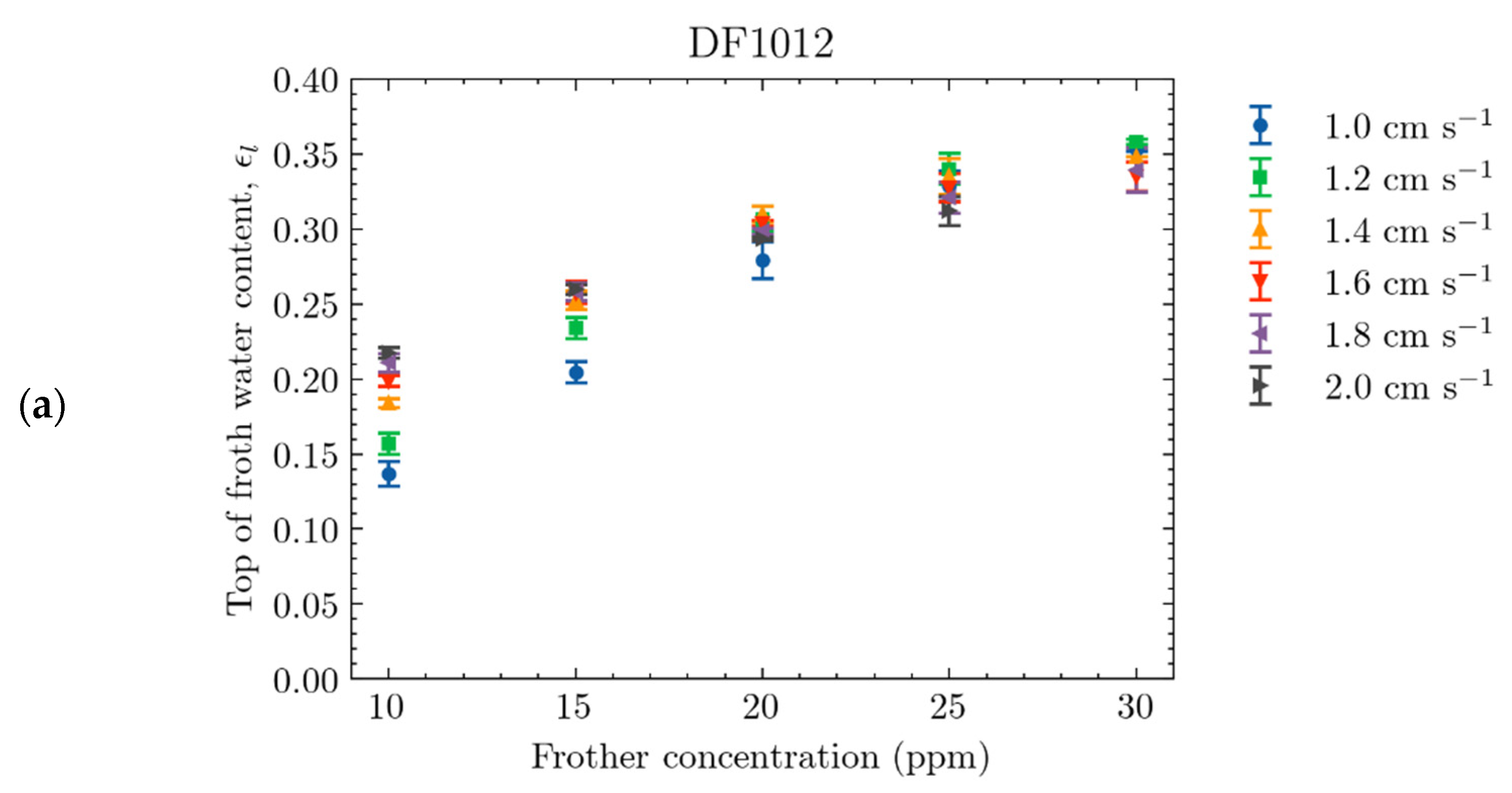
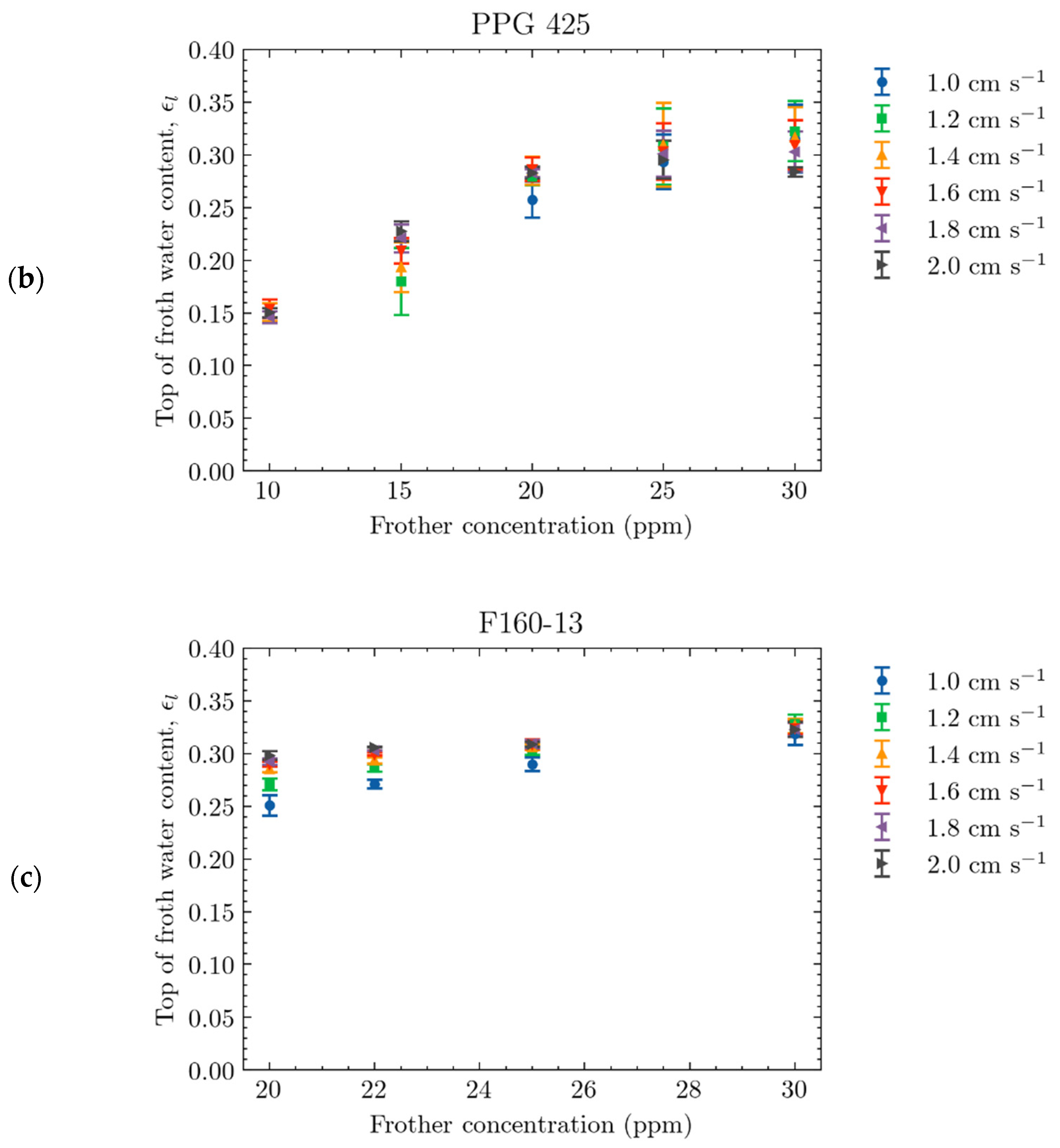
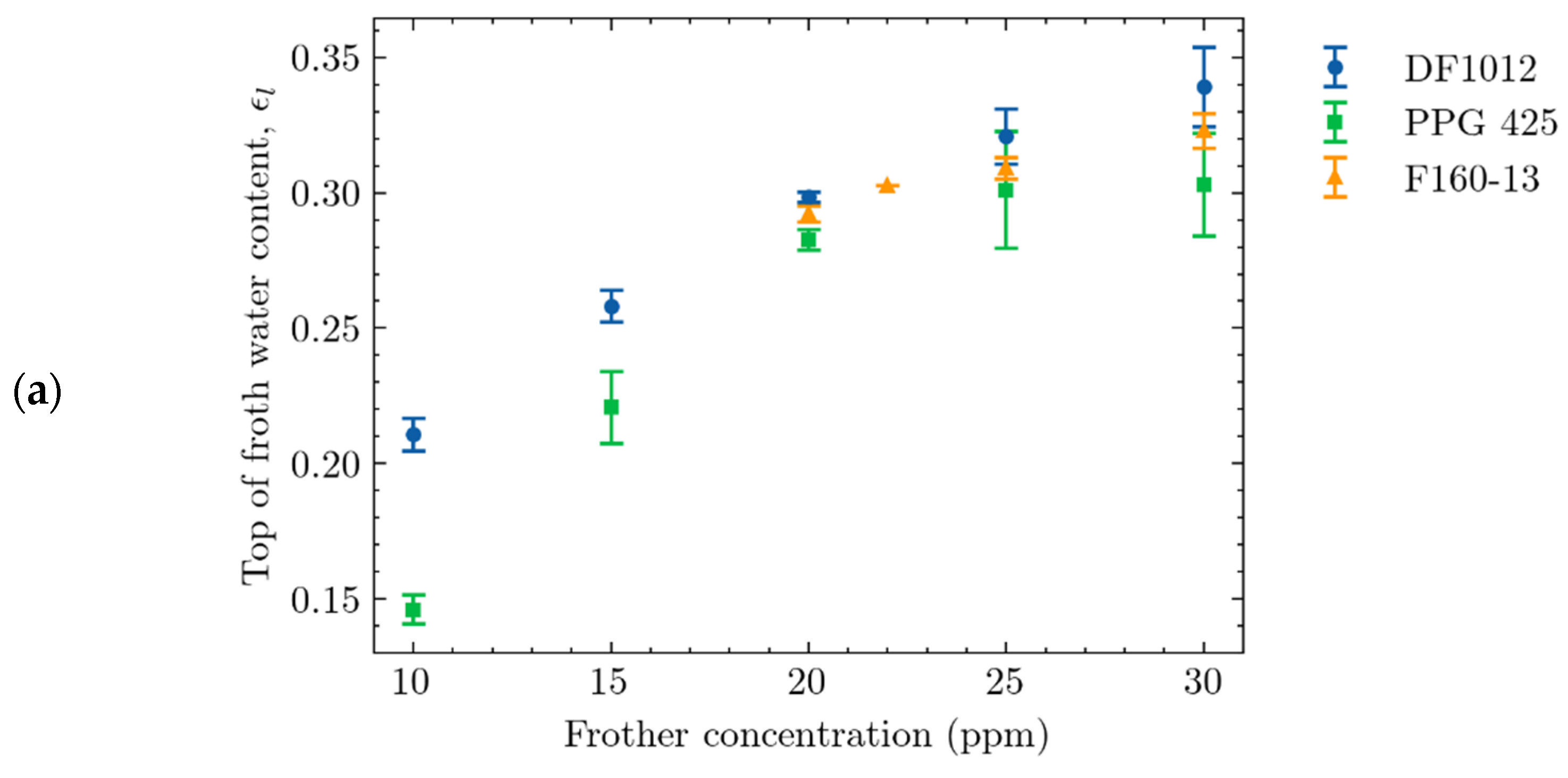
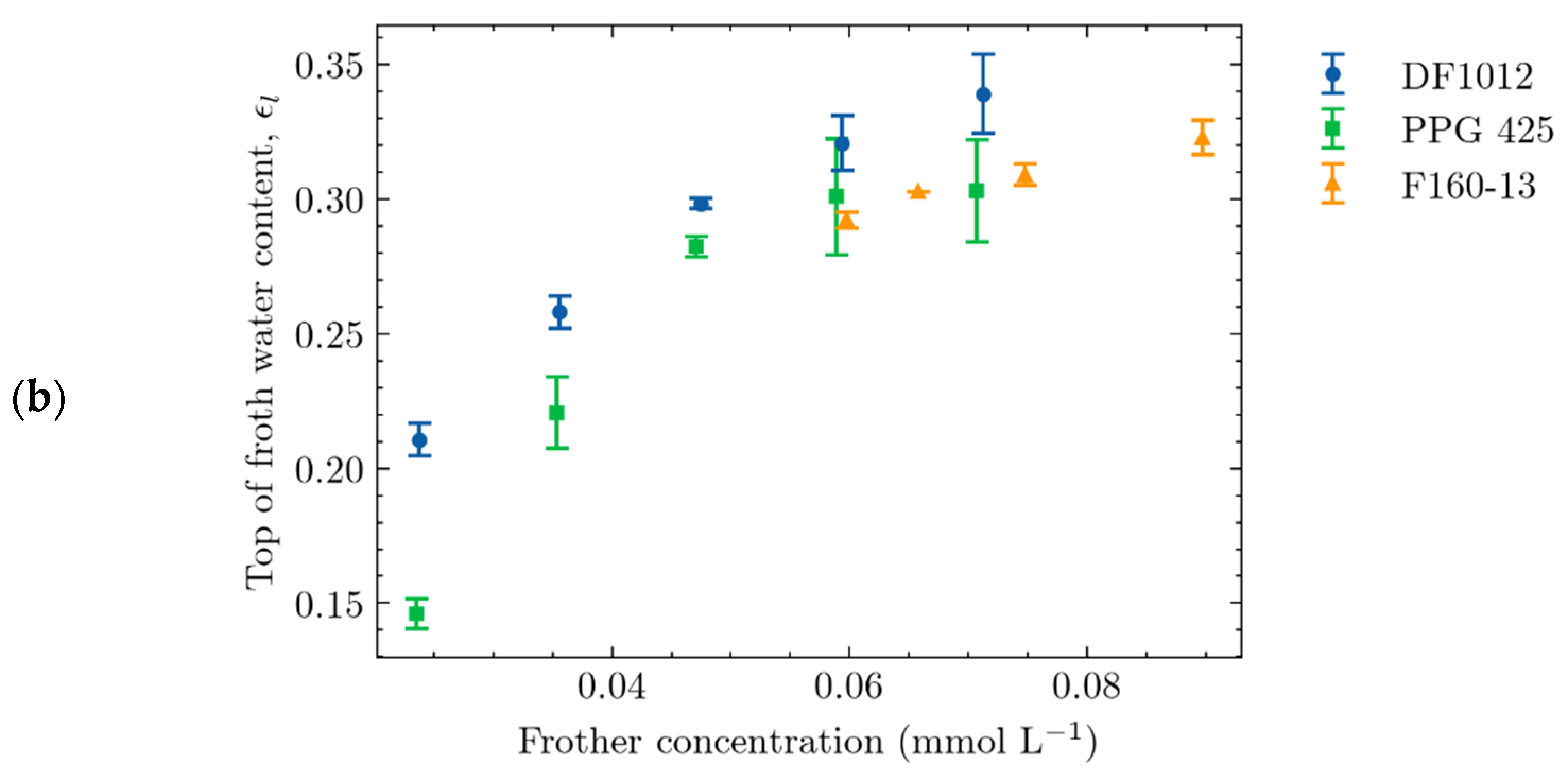
4.2.1. Effect of Frother Concentration
4.2.2. Effect of Input Air Rate
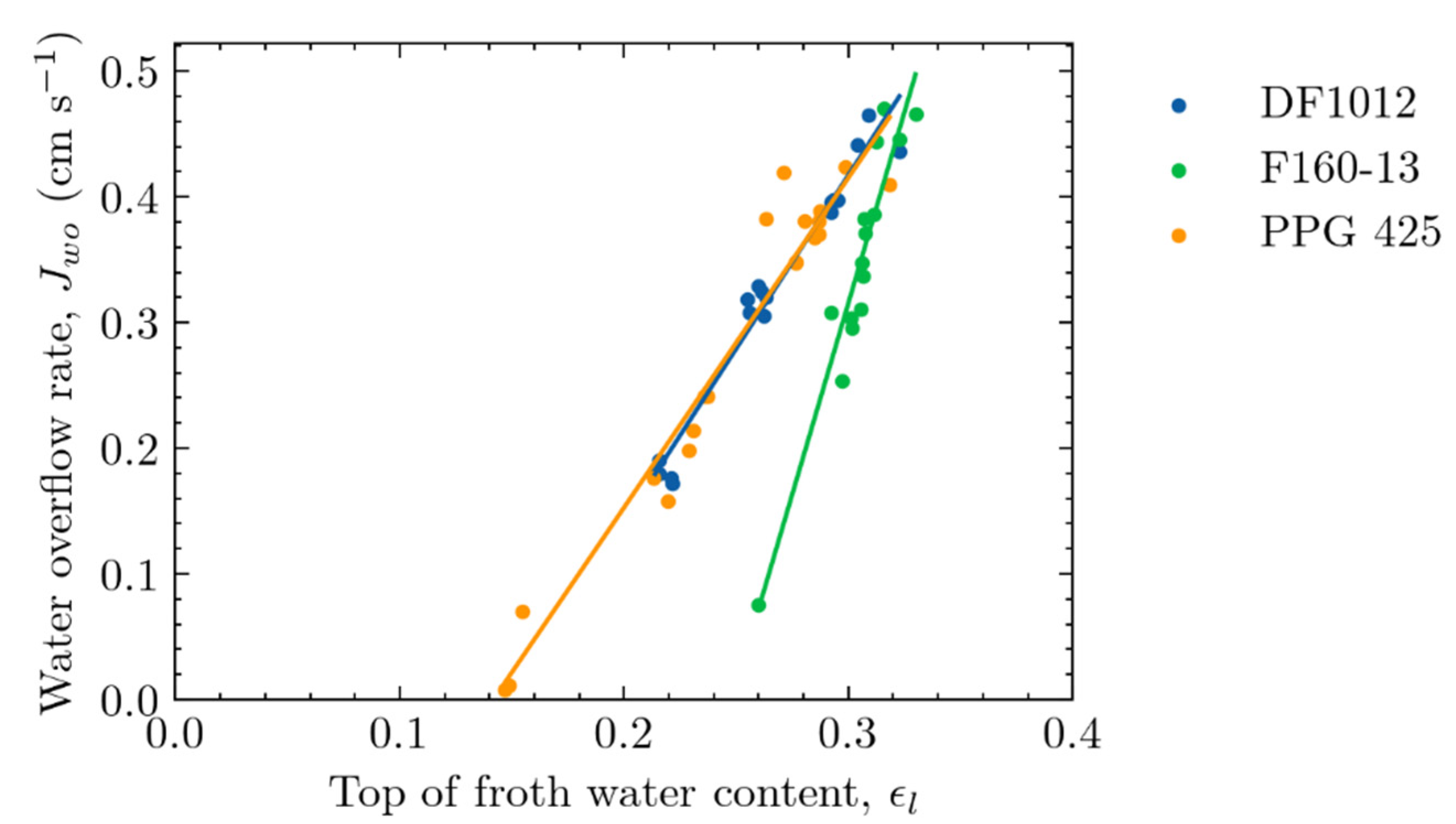
4.3. Relationship between Water Overflow Rate and Top-of-Froth Zone Water Content
4.4. Practical Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crosbie, R.; Runge, K.; McMaster, J.; Rivett, T.; Peaker, R. The impact of the froth zone on metallurgical performance in a 3m3 RCS flotation cell. In Proceedings of the Flotation 09′, Cape Town, South Africa, 9–12 November 2009. [Google Scholar]
- Massinaei, M.; Kohaldoozan, M.; Noaparast, M.; Oliazadeh, M.; Yianatos, J.; Shamsadini, R.; Yarahmadi, M. Froth zone characterization of an industrial flotation column in rougher circuit. Miner. Eng. 2009, 22, 272–278. [Google Scholar] [CrossRef]
- Laskowski, J.S. Frothers and Frothing. In Frothing in Flotation II; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 1998; pp. 1–50. [Google Scholar]
- Savassi, O.N.; Alexander, D.J.; Franzidis, J.P.; Manlapig, E.V. An empirical model for entrainment in industrial flotation plants. Miner. Eng. 1998, 11, 243–256. [Google Scholar]
- Neethling, S.J.; Cilliers, J.J. The entrainment factor in froth flotation: Model for particle size and other operating parameter effects. Int. J. Miner. Process. 2009, 93, 141–148. [Google Scholar]
- Ross, V.E. Mechanisms Operating Flotation Froths. In Froth in Flotation II; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 1998; pp. 109–144. [Google Scholar]
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise of Electricity and Magnetism; Oxford University Press: London, UK, 1892. [Google Scholar]
- Cortes-Lopez, F. Design of a Gas Holdup Sensor for Flotation Diagnosis. Masters’s Thesis, McGill University, Montreal, QC, Canada, 1998. [Google Scholar]
- Gomez, C.O.; Finch, J.A. Gas dispersion measurements in flotation cells. Int. J. Miner. Process. 2007, 84, 51–58. [Google Scholar] [CrossRef]
- Maldonado, M.; Pinto, A.; Magne, L.; Gomez, C.O.; Finch, J.A. Gas holdup estimation using Maxwell equation in flotation systems: Revisited. Miner. Eng. 2016, 98, 9–13. [Google Scholar] [CrossRef]
- Moyo, P.; Gomez, C.O.; Finch, J.A. Characterizing Frothers using Water Carrying Rate. Can. Metall. Q. 2007, 46, 215–220. [Google Scholar] [CrossRef]
- Banisi, S.; Finch, J.A.; Laplante, A.R. Electrical Conductivity of Dispersions: A Review. Miner. Eng. 1993, 6, 369–385. [Google Scholar] [CrossRef]
- Feitosa, K.; Marze, S.; Saint-Jalmes, A.; Durian, D.J. Electrical conductivity of dispersions: From dry foams to dilute suspensions. J. Phys. Condens. Matter 2005, 17, 6301. [Google Scholar] [CrossRef]
- Tavera, F.J. Flow Cells to Measure Electrical Conductivity: Use in Estimating Gas Holdup in Flotation Systems. Ph.D. Thesis, Department of Mining and Metallurgical Engineering, McGill University, Montreal, QC, Canada, 1996. [Google Scholar]
- Zhang, W.; Nesset, J.; Rao, R.; Finch, J. Characterizing Frothers through Critical Coalescence Concentration (CCC)95-Hydrophile-Lipophile Balance (HLB) Relationship. Minerals 2012, 2, 208–227. [Google Scholar] [CrossRef] [Green Version]
- CAN/CGSB-3.3-2019; Kerosene. Government of Canada: Gatineau, QC, Canada, 2019.
- Cameron, N.R.; Sherrington, D.C. High internal phase emulsions (HIPEs)—Structure, properties and use in polymer preparation. In Biopolymers Liquid Crystalline Polymers Phase Emulsion; Springer: Berlin/Heidelberg, Germany, 1996; pp. 163–214. [Google Scholar]
- Lemlich, R. Semitheoretical Equation To Relate Conductivity to Volumetric Foam Density. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 686–687. [Google Scholar] [CrossRef]
- Yianatos, J.B.; Laplante, A.R.; Finch, J.A. Estimation of local holdup in the bubbling and froth zones of a gas—Liquid column. Chem. Eng. Sci. 1985, 40, 1965–1968. [Google Scholar] [CrossRef]
- Hollenbach, R.; Oeppling, S.; Delavault, A.; Völp, A.R.; Willenbacher, N.; Rudat, J.; Ochsenreither, K.; Syldatk, C. Comparative study on interfacial and foaming properties of glycolipids in relation to the gas applied for foam generation. RSC Adv. 2021, 11, 34235–34244. [Google Scholar] [CrossRef]
- Völp, A.R.; Kagerbauer, L.; Engmann, J.; Gunes, D.Z.; Gehin-Delval, C.; Willenbacher, N. In-situ rheological and structural characterization of milk foams in a commercial foaming device. J. Food Eng. 2021, 290, 110150. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Effect of floation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Elmahdy, A.M.; Finch, J.A. Effect of frother blends on hydrodynamic properties. Int. J. Miner. Process. 2013, 123, 60–63. [Google Scholar] [CrossRef]
- Subrahmanyam, T.V.; Forssberg, E. Froth stability, particle entrainment and drainage in flotation—A review. Int. J. Miner. Process. 1988, 23, 33–53. [Google Scholar] [CrossRef]
- Tan, S.N.; Fornasiero, D.; Sedev, R.; Ralston, J. The interfacial conformation of polypropylene glycols and foam behaviour. Colloids Surf. A: Physicochem. Eng. Asp. 2004, 250, 307–315. [Google Scholar] [CrossRef]
- Harvey, P.A.; Nguyen, A.V.; Jameson, G.J.; Evans, G.M. Influence of sodium dodecyl sulphate and Dowfroth frothers on froth stability. Miner. Eng. 2005, 18, 311–315. [Google Scholar] [CrossRef]
- Drzymala, J.; Kowalczuk, P.B. Classification of Flotation Frothers. Minerals 2018, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, J. Testing flotation frothers. 2004 SME Annu. Meet. Prepr. 2004, 38, 13–22. [Google Scholar]
- Finch, J.A.; Nesset, J.E.; Acuña, C. Role of frother on bubble production and behaviour in flotation. Miner. Eng. 2008, 21, 949–957. [Google Scholar] [CrossRef]
- Tan, Y.H.; Zhang, W.; Finch, J.A. Frother structure-property relationship: Effect of polypropylene glycol alkyl ethers on bubble rise velocity. In Proceedings of the XXVIII International Mineral Processing Congress Proceedings, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Tan, Y.H.; Finch, J.A. Frother structure-property relationship: Effect of alkyl chain length in alcohols and polyglycol ethers on bubble rise velocity. Miner. Eng. 2016, 95, 14–20. [Google Scholar] [CrossRef]
- Tan, Y.H.; Zhang, W.; Finch, J.A. Frother structure-property relationship: Effect of polyethylene glycols on bubble rise velocity. Miner. Eng. 2018, 116, 56–61. [Google Scholar] [CrossRef]
- Tan, Y. Structure Effect of Alchohols and Polyglycols on Bubble Rise Velocity. Ph.D. Thesis, McGill University, Montréal, QC, Canda, 2013. [Google Scholar]
- Finch, J.A.; Gelinas, S.; Moyo, P. Frother-related research at McGill University. Miner. Eng. 2006, 19, 726–733. [Google Scholar] [CrossRef]
- Gelinas, S.; Finch, J.A.; Gouet-Kaplan, M. Comparative real-time characterization of frother bubble thin films. J. Colloid Interface Sci. 2005, 291, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A. Effect of Frothers on Foamability, Foam Stability, and Bubble Size. Coal Prep. 2007, 27, 107–125. [Google Scholar] [CrossRef]
- Hadler, K.; Cilliers, J.J. The Effect of Particles on Surface Tension and Flotation Froth Stability. Min. Metall. Explor. 2019, 36, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Hutzler, S.; Cox, S.J.; Wang, G. Foam drainage in two dimensions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 263, 178–183. [Google Scholar] [CrossRef]
- Lepage, M.R.; Visconti, L.; Liboiron-Ladouceur, O.; Waters, K.E. Vertical water content profiles of two-phase flotation froths measured through conductivity. Miner. Eng. 2020, 154, 106399. [Google Scholar] [CrossRef]
- Malysa, K. Water contents in froths obtained from solutions of α-terpineol, n-pentatnol and n-octanol. Int. J. Miner. Process. 1993, 40, 69–81. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Chapter 12—Froth Flotation. In Wills’ Mineral Processing Technology, 8th ed.; Wills, B.A., Finch, J.A., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2016; pp. 265–380. [Google Scholar] [CrossRef]
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid Interface Sci. 2011, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cappuccitti, F.; Nesset, J.E. Frother and collector effects on flotation cell hydrodynamics and their implications on circuit performance. In Proceedings of the 48th Conference of Metallurgists, Sudbury, ON, Canada, 23–26 August 2009. [Google Scholar]
- Tan, Y.; Finch, J. Frothers and gas dispersion: A review of the structure-property-function relationship. Physicochem. Probl. Miner. Process. 2018, 54, 40–53. [Google Scholar] [CrossRef]
- Zhang, W. The effects of frothers and particles on the characteristics of pulp and froth properties in flotation—A critical review. J. Miner. Mater. Charact. Eng. 2016, 4, 251–269. [Google Scholar] [CrossRef] [Green Version]
- Uribe-Salas, A.; Gomez, C.O.; Finch, J.A. A conductivity technique for gas and solids holdup determination in three-phase reactors. Chem. Eng. Sci. 1994, 49, 1–10. [Google Scholar] [CrossRef]
- Banisi, S.; Finch, J.A.; Laplante, A.R. On-line gas and solids holdup estimation in solid-liquid-gas systems. Miner. Eng. 1994, 7, 1099–1113. [Google Scholar] [CrossRef]




| Symbol | Measurement | Units | Method |
|---|---|---|---|
| Conductivity | mS cm−1 | Conductivity sensor | |
| Water overflow rate | cm s−1 | Bucket and stopwatch (60 s collection) | |
| Temperature | C | Temperature transmitter | |
| Input air velocity | cm s−1 | Gas flow meter |
| Frother | Structure |
|---|---|
| DF1012 | |
| F160-13 | * |
| PPG 425 |
| Model | Source |
|---|---|
| [8] | |
| [19] | |
| [14] | |
| [20] |
| Model | R2 |
|---|---|
| Maxwell [8] | 0.556 |
| Lemlich [19] | 0.947 |
| Feitosa et al. [14] | 0.949 |
| Yianatos et al. [20] | 0.949 |
| Jg (cm s−1) | PPG 425 | DF1012 | F160-13 |
|---|---|---|---|
| 1.0 | 0.688 | 0.855 | 0.879 |
| 1.2 | 0.861 | 0.903 | 0.869 |
| 1.4 | 0.898 | 0.948 | 0.914 |
| 1.6 | 0.917 | 0.956 | 0.908 |
| 1.8 | 0.932 | 0.966 | 0.908 |
| 2.0 | 0.946 | 0.966 | 0.896 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepage, M.R.; Gomez, C.O.; Waters, K.E. Using Top-of-Froth Conductivity to Infer Water Overflow Rate in a Two-Phase Lab-Scale Flotation Column. Minerals 2022, 12, 454. https://doi.org/10.3390/min12040454
Lepage MR, Gomez CO, Waters KE. Using Top-of-Froth Conductivity to Infer Water Overflow Rate in a Two-Phase Lab-Scale Flotation Column. Minerals. 2022; 12(4):454. https://doi.org/10.3390/min12040454
Chicago/Turabian StyleLepage, Mark R., Cesar O. Gomez, and Kristian E. Waters. 2022. "Using Top-of-Froth Conductivity to Infer Water Overflow Rate in a Two-Phase Lab-Scale Flotation Column" Minerals 12, no. 4: 454. https://doi.org/10.3390/min12040454
APA StyleLepage, M. R., Gomez, C. O., & Waters, K. E. (2022). Using Top-of-Froth Conductivity to Infer Water Overflow Rate in a Two-Phase Lab-Scale Flotation Column. Minerals, 12(4), 454. https://doi.org/10.3390/min12040454







