New Geochemical Framework and Geographic Information System Methodologies to Assess Element Occurrence, Persistence, and Mobility in Groundwater and Surface Water
Abstract
1. Introduction
2. Background
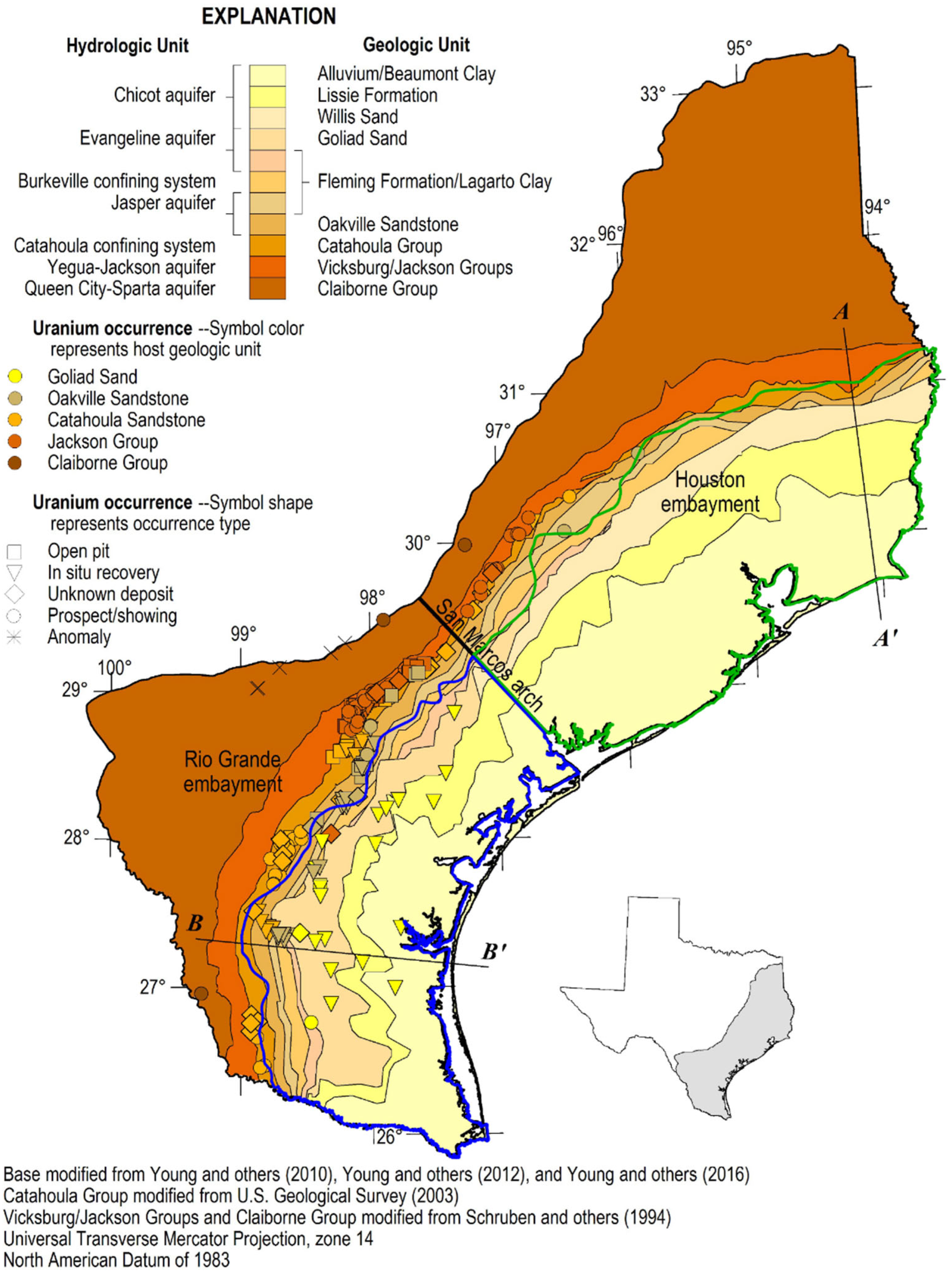
2.1. Overview of the South Texas Coastal Plain Uranium Deposit Formation, Mining, and Constituents of Potential Concern
2.2. Water Quality Vulnerability
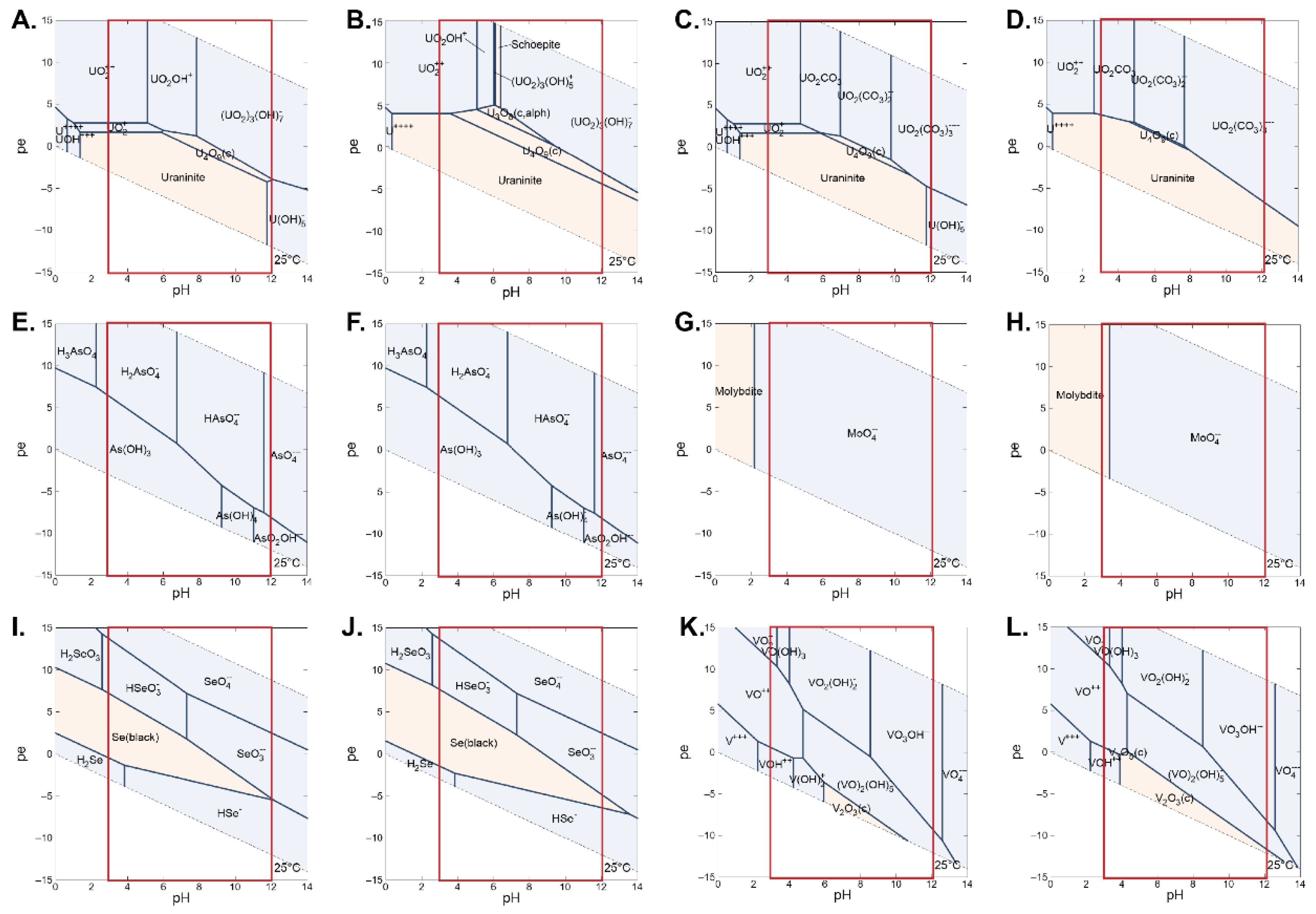
2.3. Geochemical and Physical Conditions Controlling the Mobility of Elements
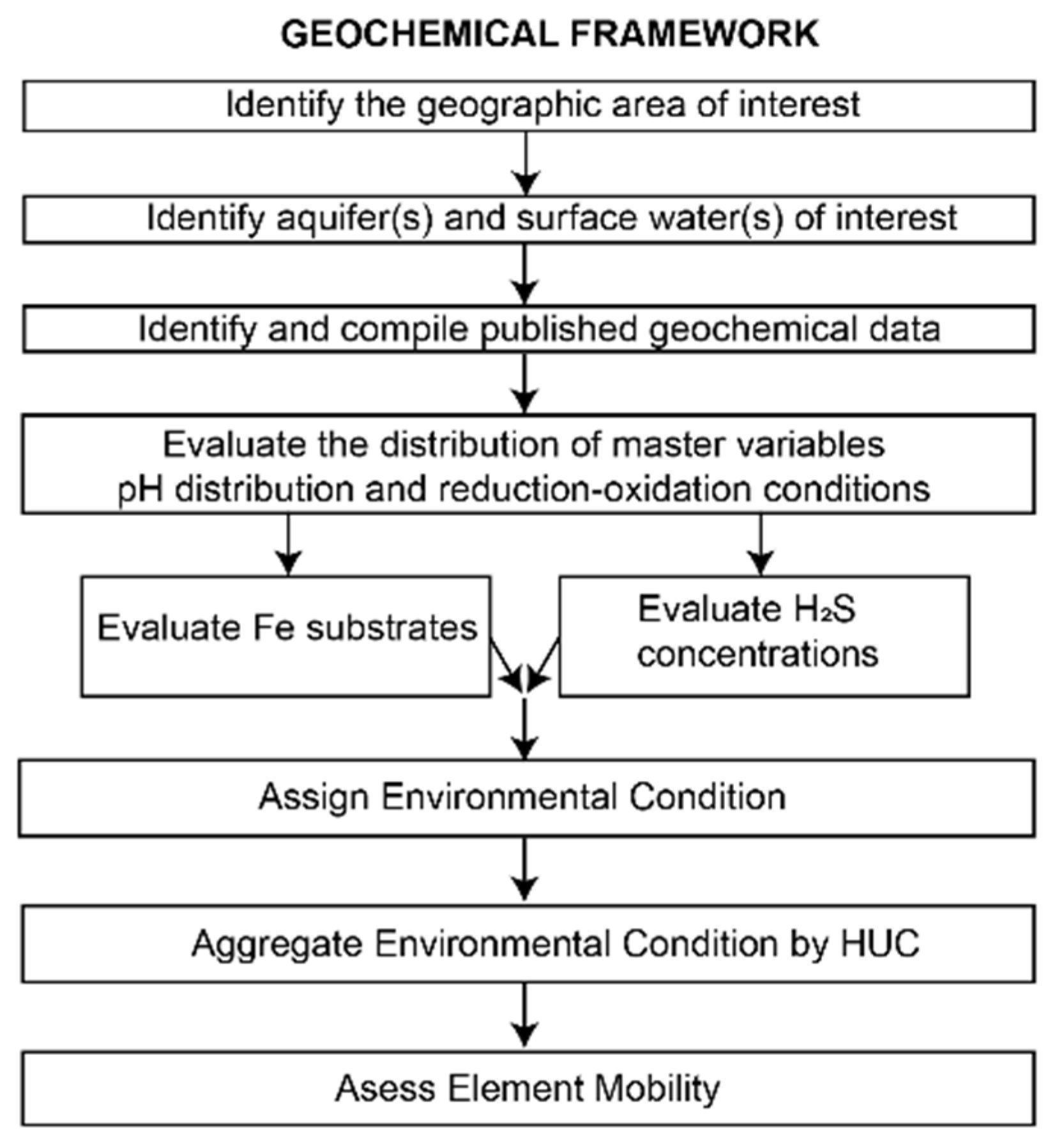
3. Materials and Methods
3.1. Identify the Geographic Area of Interest
3.2. Identify Aquifer(s) and Surface Water(s) of Interest
3.3. Identify and Compile Published Geochemical Data
3.4. Evaluate the Distribution of Master Variables
3.4.1. pH Distribution
3.4.2. Reduction–Oxidation (Redox) Conditions
3.5. Evaluate Fe Substrates
3.6. Evaluate H2S Concentrations
3.7. Assign Environmental Condition
3.8. Aggregate Environmental Condition by HUC
3.9. Assess Element Mobility
3.10. Variance-Qualified Environmental Conditions
4. Results
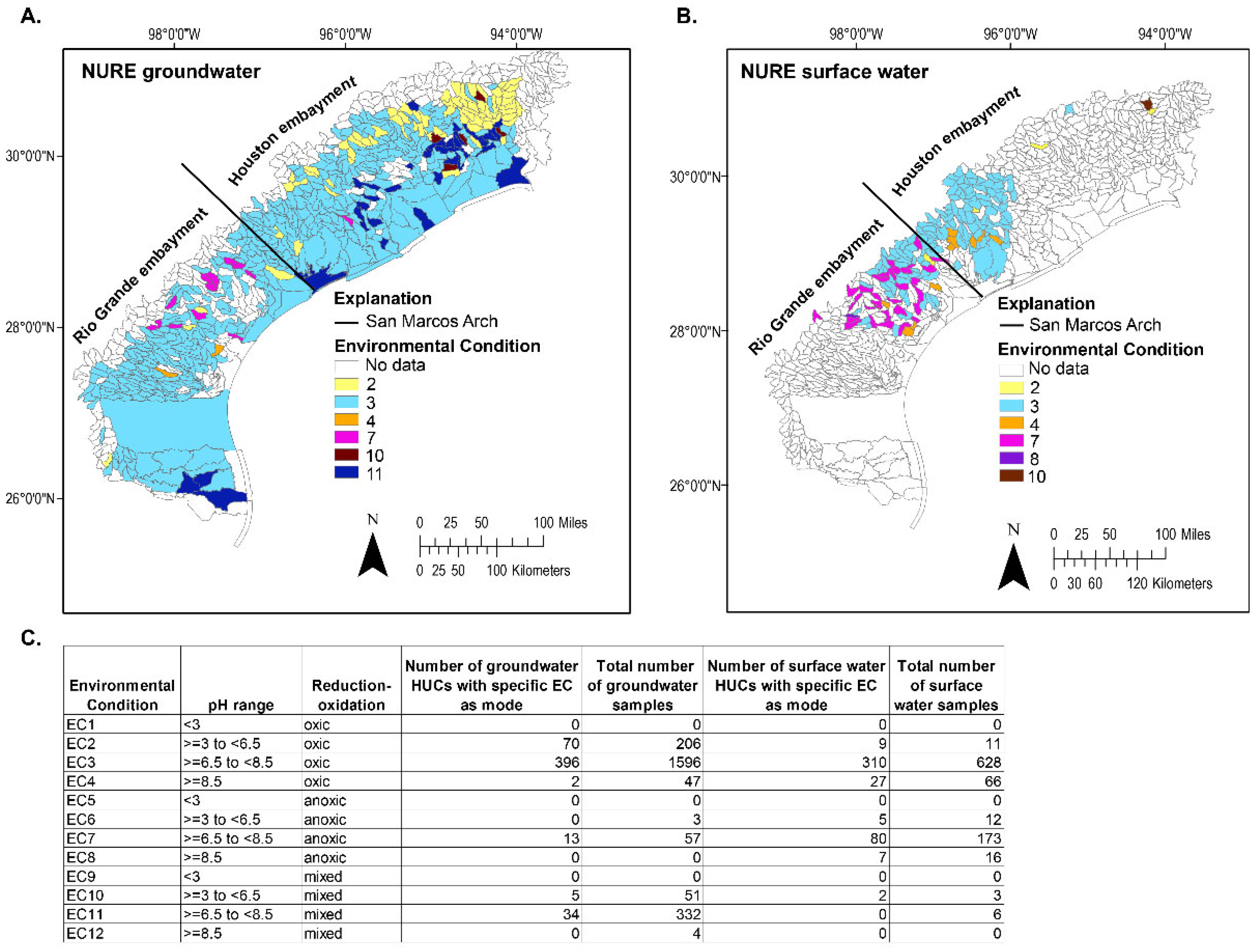
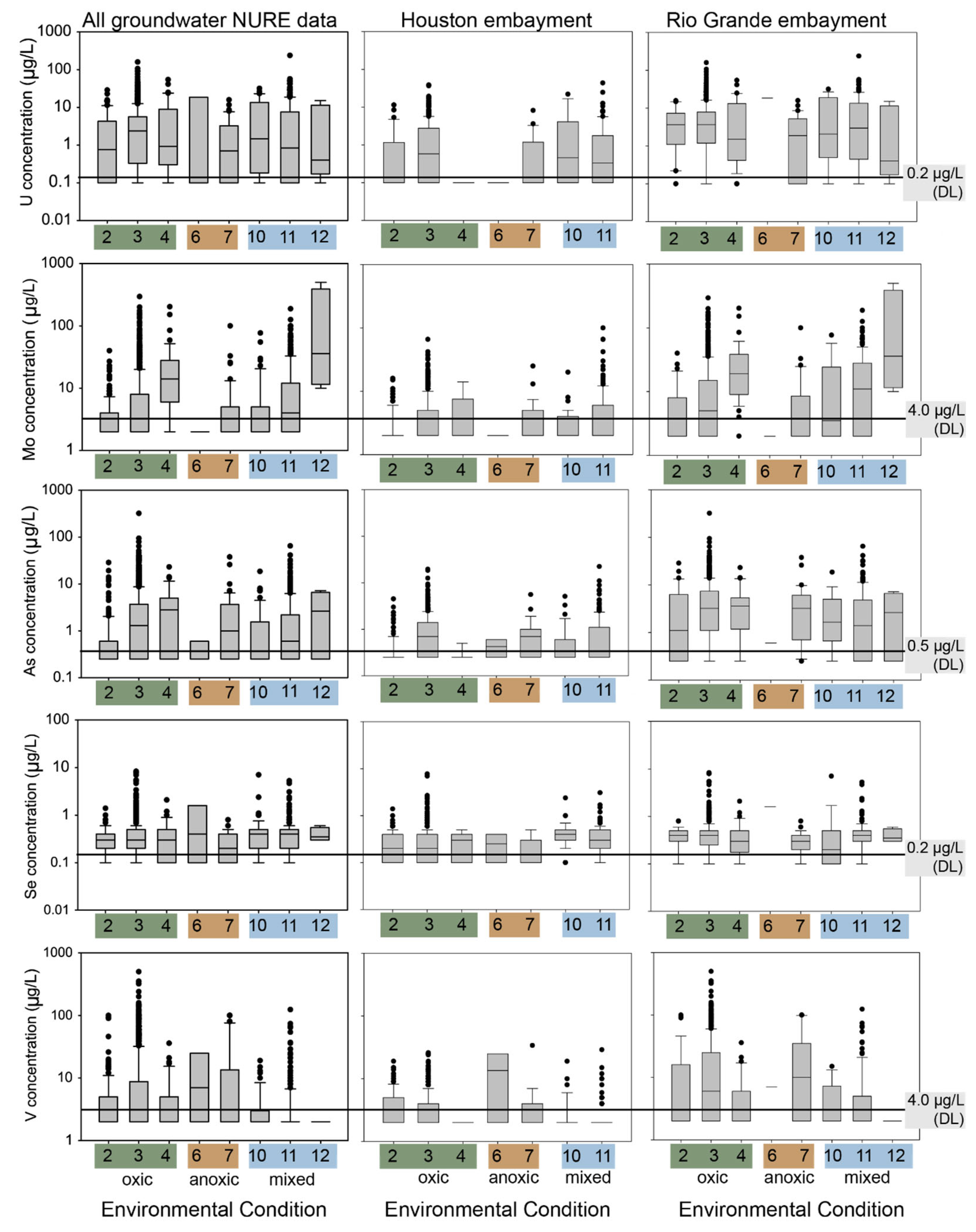
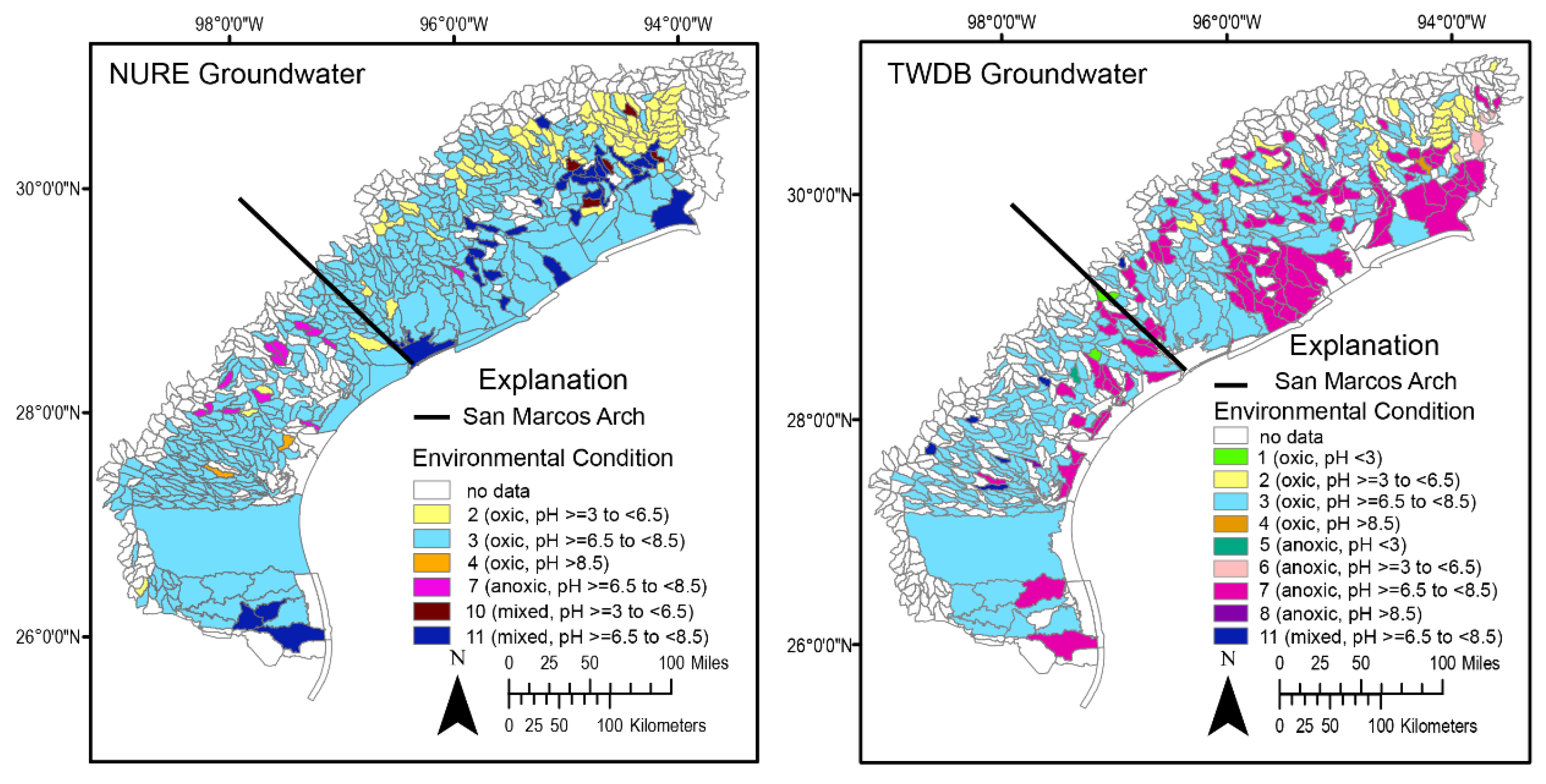
4.1. NURE Groundwater and Surface Water Data
4.2. Groundwater and Surface Water Distribution by EC
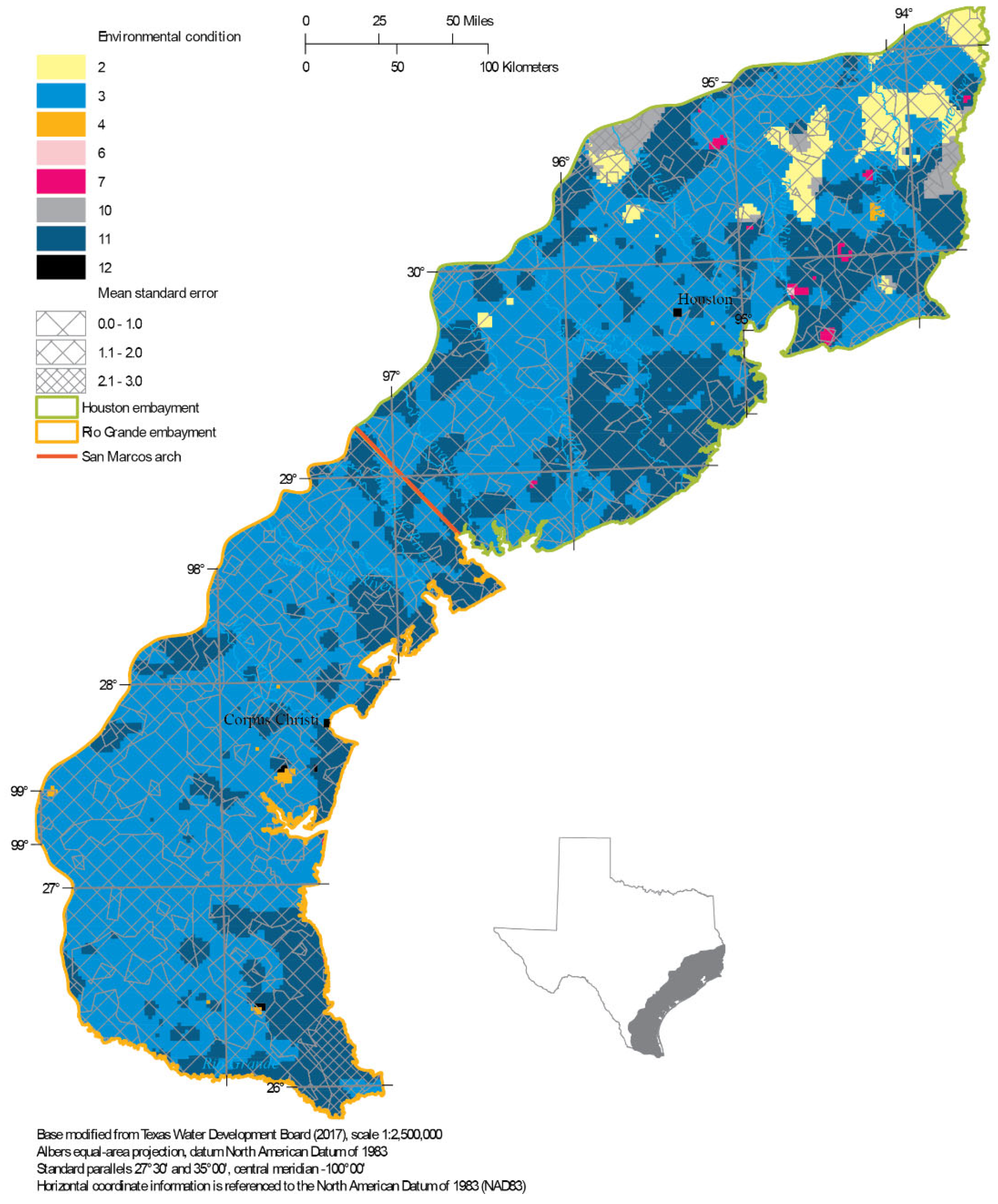
4.3. Variance-Qualified Environmental Condition Maps
4.4. Applying the Geochemical Framework
4.5. Applying Kriging to the Texas Water Development Board Data
5. Discussion
5.1. Implications for Planning
5.2. Limitation on the Model
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haines, S.S.; Diffendorfer, J.E.; Balistrieri, L.; Berger, B.; Cook, T.; DeAngelis, D.; Doremus, H.; Gautier, D.L.; Gallegos, T.; Gerritsen, M.; et al. A framework for quantitative assessment of impacts related to energy and mineral resource development. Nat. Resour. Res. 2013, 23, 3–17. [Google Scholar] [CrossRef][Green Version]
- Jenni, K.E.; Pindilli, E.; Bernknopf, R.; Nieman, T.L.; Shapiro, C. Multi-Resource Analysis—Methodology and Synthesis; U.S. Geological Survey: Reston, VA, USA, 2018; Circular 1442. [CrossRef]
- U.S. Geological Survey. Integrated Uranium Resource and Environmental Assessment. 2019. Available online: https://www.usgs.gov/centers/cersc/science/integrated-uranium-resource-and-environmental-assessment?qt-science_center_objects=0#qt-science_center_objects (accessed on 25 April 2019).
- Gallegos, T.J.; Walton-Day, K.; Seal, R.R., II. Conceptual Framework and Approach for Conducting a Geoenvironmental Assessment of Undiscovered Uranium Resources: U.S. Geological Survey Scientific Investigations Report 2018–5104; U.S. Geological Survey: Reston, VA, USA, 2020. [CrossRef]
- Singer, D.A.; Menzie, W.D.; Sutphin, D.M.; Mosier, D.L.; Bliss, J.D. Mineral deposit density—An update, chapter A. In Contributions to Global Mineral Resource Assessment Research; U.S. Geological Survey Professional Paper 1640; Schulz, K.J., Ed.; 2001; pp. A1–A13. Available online: https://pubs.usgs.gov/prof/p1640a/ (accessed on 1 March 2022).
- Ulmer-Scholle, D.S. New Mexico Bureau of Geology & Mineral Resources, Uranium—How Is It Mined? 2019. Available online: https://geoinfo.nmt.edu/resources/uranium/mining.html (accessed on 26 April 2019).
- Mudd, G.M. Critical review of acid in-situ leach uranium mining: 1—USA and Australia. Environ. Geol. 2001, 41, 390–403. [Google Scholar] [CrossRef]
- Saunders, J.A.; Pivetz, B.E.; Voorhies, N.; Wilkin, R.T. Potential aquifer vulnerability in regions down-gradient from uranium in situ recovery (ISR) sites. J. Environ. Manag. 2016, 183, 67–83. [Google Scholar] [CrossRef]
- Hall, S.M.; Mihalasky, M.J.; Tureck, K.R.; Hammarstrom, J.M.; Hannon, M.T. Genetic and grade and tonnage models for sandstone-hosted roll-type uranium deposits, Texas Coastal Plain, USA. Ore Geol. Rev. 2017, 80, 716–753. [Google Scholar] [CrossRef]
- Henry, C.D.; Galloway, W.E.; Smith, G.E. Considerations in the Extraction of Uranium from a Fresh-Water Aquifer-Miocene Oakville Sandstone, South Texas; Report of Investigations No. 126; The University of Texas at Austin, Bureau of Economic Geology: Austin, TX, USA, 1982. [Google Scholar]
- Smith, K.S. Strategies to predict metal mobility in surficial mining environments. In Understanding and Responding to Hazardous Substances at Mine Sites in the Western United States; DeGraff, J.V., Ed.; Geological Society of America: Boulder, CO, USA, 2007; Volume XVII, pp. 25–45. [Google Scholar] [CrossRef]
- Eberts, S.M.; Thomas, M.A.; Jagucki, M.L. The Quality of Our Nation’s Waters—Factors Affecting Public-Supply-Well Vulnerability to Contamination—Understanding Observed Water Quality and Anticipating Future Water Quality; U.S. Geological Survey: Reston, VA, USA, 2013; Circular 1385. [CrossRef]
- Smith, S.M. National Geochemical Database—Reformatted Data from the National Uranium Resource Evaluation (NURE) Hydrogeochemical and Stream Sediment Reconnaissance (HSSR) Program. U.S. Geol. Surv. Open-File Rep. 2006, 97–492. Available online: https://pubs.usgs.gov/of/1997/ofr-97-0492/nurehist.htm (accessed on 1 March 2022).
- Mihalasky, M.J.; Hall, S.M.; Hammarstrom, J.M.; Tureck, K.R.; Hannon, M.T.; Breit, G.N.; Zielinski, R.A.; Elliott, B. Assessment of Undiscovered Sandstone-Hosted Uranium Resources in the Texas Coastal Plain, 2015: U.S. Geological Survey Fact Sheet 2015–3069; U.S. Geological Survey: Denver, CO, USA, 2015. [CrossRef]
- Alam, M.S.; Cheng, T. Uranium release from sediment to groundwater: Influence of water chemistry and insights into release mechanisms. J. Contam. Hydrol. 2017, 164, 72–87. [Google Scholar] [CrossRef]
- Adidas, E. Groundwater quality and availability in and around Bruni, Webb County, Texas. s.l. Tex. Water Dev. Board LP-209 1991, 1–57. [Google Scholar]
- DeSimone, L.A.; McMahon, P.B.; Rosen, M.R. The Quality of Our Nation’s Waters—Water Quality in Principal Aquifers of the United States, 1991–2010; U.S. Geological Survey: Reston, VA, USA, 2014; Circular 1360. [CrossRef]
- U.S. Environmental Protection Agency. Abandoned Mine Site Characterization and Cleanup Handbook. U.S. Environmental Protection Agency [USEPA] 2000, EPA 910-B-00-001. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/2000_08_pdfs_amscch.pdf (accessed on 26 October 2020).
- Reedy, R.C.; Scanlon, B.R.; Walden, S.; Strassberg, G. Naturally Occurring Groundwater Contamination in Texas; The University of Texas at Austin Bureau of Economic Geology: Austin, TX, USA, 2011; Available online: http://www.twdb.texas.gov/publications/reports/contracted_reports/doc/1004831125.pdf (accessed on 26 April 2019).
- Oden, J.H.; Szabo, Z. Arsenic and Radionuclide Occurrence and Relation to Geochemistry in Groundwater of the Gulf Coast Aquifer System in Houston, Texas, 2007–11: U.S. Geological Scientific Investigations Report 2015–5071; U.S. Geological Survey: Austin, TX, USA, 2015. [CrossRef]
- Agency for Toxic Substances & Disease Registry [ATSDR], 2015, Toxic Substances Portal. Available online: https://www.atsdr.cdc.gov/toxguides/ (accessed on 28 January 2020).
- Besser, J.M.; Leib, K.J. Toxicity of metals in water and sediment to aquatic biota. In Integrated Investigations of Environmental Effects of Historical Mining in the Animas River Watershed, San Juan County, Colorado; Church, S.E., von Guerard, P., Finger, S.E., Eds.; U.S. Department of The Interior: Washington, DC, USA, 2007; Professional Paper 1651; pp. 837–850. [Google Scholar]
- U.S. Environmental Agency. Groundwater and Drinking Water—National Primary Drinking Water Regulations. 2019. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 28 January 2020).
- U.S. Environmental Agency. National Recommended Water Quality Criteria—Aquatic Life Criteria Table. 2019. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 28 January 2020).
- National Research Council. Ground Water Vulnerability Assessment: Predicting Relative Contamination Potential Under Conditions of Uncertainty; The National Academies Press: Washington, DC, USA, 1993. [Google Scholar] [CrossRef]
- Focazio, M.J.; Reilly, T.E.; Rupert, M.G.; Helsel, D.R. Assessing Ground-Water Vulnerability to Contamination: Providing Scientifically Defensible Information for Decision Makers; U.S. Geological Survey: Reston, VA, USA, 2002; Circular 1224. Available online: https://pubs.usgs.gov/circ/2002/circ1224/ (accessed on 26 October 2020).
- Plummer, R.; de Loe, R.; Armitage, D. A systematic review of water vulnerability assessment tools. Water Resour. Manag. 2012, 26, 4327–4346. [Google Scholar] [CrossRef]
- Smith, K.S.; Huyck, H.L.O. An overview of the abundance, relative mobility, bioavailability, and human toxicity of metals. In Reviews in Economic Geology, Volumes 6A and 6B; Society of Economic Geologists: Littleton, CO, USA, 1999; pp. 29–70. [Google Scholar]
- Perel’man, A.I. Geochemical barriers: Theory and practical applications. Appl. Geochem. 1986, 1, 669–680. [Google Scholar] [CrossRef]
- Alekseenko, V.; Maximovich, N.G.; Alekseenko, A.V. Geochemical barriers for soil protection in mining areas. In Assessment, Restoration and Reclamation of Mining Influenced Soils; Bech, J., Bini, C., Pashkevich, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 255–274. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Tang, J. Conservative behavior of arsenic and other oxyanion-forming trace elements in an oxic groundwater flow system. J. Hydrol. 2009, 378, 13–28. [Google Scholar] [CrossRef]
- Blake, J.M.; Avasarala, S.; Artyushkova, K.; Ali, A.S.; Brearley, A.J.; Shuey, C.; Robinson, W.P.; Nez, C.; Bill, S.; Lewis, J.; et al. Elevated concentrations of U and co-occurring metals in abandoned mine wastes in a northeastern Arizona Native American community. Environ. Sci. Technol. 2015, 49, 8506–8514. [Google Scholar] [CrossRef] [PubMed]
- Avasarala, S.; Lichtner, P.C.; Ali, A.S.; Gonzalez-Pinzon, R.; Blake, J.M.; Cerrato, J.M. Reactive transport of U and V from abandoned uranium mine wastes. Environ. Sci. Technol. 2017, 51, 12385–12393. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, R.T.; Lee, T.R.; Beak, D.G.; Anderson, R.; Burns, B. Groundwater co-contaminant behavior of arsenic and selenium at a lead and zinc smelting facility. Appl. Geochem. 2018, 89, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Plant, J.A.; Baldock, J.W.; Smith, B. The role of geochemistry in environmental and epidemiological studies in developing countries: A review. In Environmental Geochemistry and Health; Appleton, J.D., Fuge, R., McCall, G.J.H., Eds.; Geological Society Pub House: Bath, UK, 1996; pp. 7–22. [Google Scholar]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bourg, A.C.M.; Loch, J.P.G. Mobilization of heavy metals as affected by pH and redox conditions. In Biogeodynamics of Pollutants in Soils and Sediments: Risk Assessment of Delayed and Non-Linear Responses; Salomons, W., Stigliani, W.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 87–102. [Google Scholar] [CrossRef]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice-Hall: Hoboken, NJ, USA, 1997. [Google Scholar]
- DeVore, C.L.; Rodriguez-Freire, L.; Ali, A.M.; Ducheneaux, C.; Artyushkova, K.; Zhou, Z.; Latta, D.E.; Lueth, V.W.; Gonzales, M.; Lewis, J.; et al. Effect of bicarbonate and phosphate on arsenic release from mining-impacted sediments in the Cheyenne River watershed, South Dakota, USA. Environ. Sci. Process. Impacts 2019, 21, 456–468. [Google Scholar] [CrossRef]
- Kim, M.; Nriagu, J.; Haack, S. Carbonate ions and arsenic dissolution by groundwater. Environ. Sci. Technol. 2000, 34, 3094–3100. [Google Scholar] [CrossRef]
- Han, M.; Hao, J.; Christodoulatos, C.; Korfiatis, G.P.; Wan, L.; Meng, X. Direct evidence of arsenic (III)-carbonate complexes obtained using electrochemical scanning tunneling microscopy. Anal. Chem. 2007, 79, 3615–3622. [Google Scholar] [CrossRef]
- Jenne, E.A. Adsorption of metals by geomedia: Data analysis, modeling, controlling factors, and related issues. In Adsorption of Metals by Geomedia; Jenne, E.A., Ed.; Academic Press: Cambridge, MA, USA, 1998; pp. 1–73. [Google Scholar]
- Smith, K.S. Metal sorption on mineral surfaces: An overview with examples relating to mineral deposits. In Reviews in Economic Geology; Plumlee, G.S., Logsdon, M.J., Filipek, L.F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volumes 6A–6B, pp. 161–185. [Google Scholar]
- Dong, W.M.; Brooks, S.C. Determination of the formation constants of ternary complexes of uranyl and carbonate with alkaline earth metals (Mg2+, Ca2+, Sr2+, and Ba2+) using anion exchange method. Environ. Sci. Technol. 2006, 40, 4689–4695. [Google Scholar] [CrossRef]
- Texas Water Development Board. Groundwater Database Reports. 2020. Available online: https://www.twdb.texas.gov/groundwater/data/gwdbrpt.asp (accessed on 3 April 2020).
- Jurgens, B.C.; McMahon, P.B.; Chapelle, F.H.; Eberts, S.M. An Excel workbook for identifying redox processes in ground water. U.S. Geol. Surv. Open-File Rep. 2009, 2009, 1004. [Google Scholar]
- U.S. Geological Survey. Mineral Resources On-Line Spatial Data. 2017. Available online: https://mrdata.usgs.gov/geology (accessed on 15 September 2017).
- Solis, R.F. Upper Tertiary and Quaternary Depositional Systems, Central Coastal Plain, Texas—Regional Geology of the Coastal Aquifer and Potential Liquid-Waste Repositories; The University of Texas at Austin, Bureau of Economic Geology: Austin, TX, USA, 1981; Report of Investigations No. 108. [Google Scholar]
- Chowdhury, A.H.; Turco, M.J. Geology of the gulf Coast Aquifer, Texas. In Aquifers of the Gulf Coast of Texas; Mace, R.E., Davidson, S.C., Angle, E.S., Mullican, W.F., III, Eds.; Texas Water Development Board: Austin, TX, USA, 2006; Report 365; pp. 23–50. Available online: https://www.twdb.texas.gov/publications/reports/numbered_reports/doc/R365/R365_Composite.pdf (accessed on 26 September 2020).
- Davis, J.A.; Curtis, G.P.; Randall, J.D. Application of Surface Complexation Modeling to Describe Uranium(VI) Adsorption and Retardation at the Uranium Mill Tailings Site at Naturita, Colorado; U.S. Nuclear Regulatory Commission: Rockville, MD, USA, 2003; NUREG/CR-6820.
- Zheng, Z.; Tokunaga, T.T.; Wan, J. Influence of calcium carbonate on U(VI) sorption to soils. Environ. Sci. Technol. 2003, 37, 5603–5608. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.B.; Chapelle, F.H. Redox processes and water quality of selected principal aquifer systems. Groundwater 2008, 46, 259–271. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.B.; Chapelle, F.H.; Bradley, P.M. Evolution of redox processes in groundwater. In Aquatic Redox Chemistry; Tratnyek, P., Grundl, T.J., Haderlein, S.B., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1071, pp. 581–597. [Google Scholar] [CrossRef]
- Walton-Day, K.; Macaladay, D.L.; Brooks, M.H.; Tate, V.T. Field methods for measurement of ground water redox chemical parameters. Groundw. Monit. Remediat. 1990, 10, 81–89. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparison of Arsenic(V) and Arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef]
- Davis, J.A.; Meece, D.E.; Kohler, M.; Curtis, G.P. Approaches to surface complexation modeling of uranium (VI) adsorption on aquifer sediments. Geochim. Et Cosmochim. Acta 2004, 68, 3621–3641. [Google Scholar] [CrossRef]
- Nicot, J.; Scanlon, B.R.; Yang, C.; Gates, J.B. Geological and Geographical Attributes of the South Texas Uranium Province; The University of Texas at Austin, Bureau of Economic Geology: Austin, TX, USA, 2010; Available online: http://www.beg.utexas.edu/files/publications/cr/CR2010-Nicot-1-QAe5657.pdf (accessed on 18 April 2019).
- Galloway, W.E. Epigenetic Zonation and Fluid Flow History of Uranium-Bearing Fluvial Aquifer Systems, South Texas Uranium Province; The University of Texas at Austin Bureau of Economic Geology: Austin, TX, USA, 1982; Available online: http://www.beg.utexas.edu/files/publications/cr/CR1981-Galloway-1_QAe6860.pdf (accessed on 18 April 2019).
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Bethke, C.; Farrell, B. GWB Reference Manual; Aqueous Solutions, LLC: Champaign, IL, USA, 2020; Release 14; Available online: https://www.gwb.com/pdf/GWB14/GWBreference.pdf (accessed on 26 September 2020).
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 2013. Available online: https://pubs.usgs.gov/tm/06/a43/ (accessed on 30 June 2021).
- Plumlee, G.S. The environmental geology of mineral deposits. In The Environmental Geochemistry of Mineral Deposits, Part A. Processes, Techniques, and Health Issues; Plumlee, G.S., Logsdon, M.J., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6A, pp. 71–116. [Google Scholar]
- Miao, Z.; Brusseau, M.L.; Carroll, K.C.; Carreon-Diazconti, C.; Johnson, B. Sulfate reduction in groundwater: Characterization and applications for remediation. Environ. Geochem. Health 2012, 34, 539–550. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 2nd ed.; U.S. Geological Survey: Liston, VA, USA, 1970; Water Supply Paper 1473.
- ArcGIS 10.4.1; Esri: Redlands, CA, USA, 2015.
- U.S. Geological Survey; U.S. Department of Agriculture. Natural Resources Conservation Service. Federal Standards and Procedures for the National Watershed Boundary Dataset (WBD), 4th ed.; U.S. Geological Survey: Liston, VA, USA, 2013; Techniques and Methods 11–A3; p. 63. Available online: http://pubs.usgs.gov/tm/tm11a3/ (accessed on 1 March 2022).
- Mochizuki, A.; Murata, T.; Hosoda, K.; Katano, T.; Tanaka, Y.; Mimura, T.; Mitamura, O.; Nakano, S.; Okazaki, Y.; Sugiyama, Y.; et al. Distributions and geochemical behaviours of oxyanion-forming trace elements and uranium in the Hovsgol-Baikal-Yenisei water system of Mongolia and Russia. J. Geochem. Explor. 2018, 188, 123–136. [Google Scholar] [CrossRef]
- Seequent, 2021, Geosoft Technical Workshop—Topics in Gridding: Broomfield, Calif., Seequent. Available online: https://files.seequent.com/MySeequent/technical-papers/topicsingriddingworkshop.pdf (accessed on 27 October 2020).
- Isaaks, E.H.; Srivastava, R.M. An Introduction to Applied Geostatistics; Oxford University Press: New York, NY, USA, 1989; p. 561. [Google Scholar]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water-Analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Contreras, C. Historical Data Review on Garcitas Creek Tidal. In Performed as Part of the Tidal Stream Use Assessment under TCEQ Contract No. 582-2-48657 (TPWD Contract No. 108287); Resource Protection Division, Texas Parks and Wildlife Department: Austin, TX, USA, 2003. Available online: https://tpwd.texas.gov/landwater/water/conservation/coastal_studies/uaa/media/garcitas.pdf (accessed on 15 September 2020).
- Linam, G.W.; Robertson, S.; Sablan, K.; Burke, R.; Larralde, L.; Donovan, S. Fish Assemblage and Water Quality in the San Antonio River, Texas, between Floresville and Goliad; Inland Fisheries Division, Texas Parks and Wildlife Department: Austin, TX, USA, 2014; River Studies Report No. 22. Available online: https://tpwd.texas.gov/publications/pwdpubs/media/pwd_rp_t3200_1804.pdf (accessed on 15 September 2020).
- Galloway, W.E. Uranium mineralization in a coastal-plain fluvial aquifer system: Catahoula Formation, Texas. Econ. Geol. 1978, 73, 1655–1676. [Google Scholar]
- Reynolds, R.L.; Goldhaber, M.B. Origin of a south Texas roll-type uranium deposit. I. Alteration of iron-titanium oxide minerals. Econ. Geol. 1978, 73, 1677–1689. [Google Scholar] [CrossRef]
- Wilkin, R.T.; McNeil, M.S.; Adair, C.J.; Wilson, J.T. Field measurement of dissolved oxygen: A comparison of methods. Groundw. Monit. Remediat. 2001, 21, 124–132. [Google Scholar] [CrossRef]
- Price, V.; Jones, P.L. Training Manual for Water and Sediment Geochemical Reconnaissance; Du Pont de Nemours (EI) and Co., Aiken, SC (USA). Savannah River Lab.; SRL Internal Doc. DPST-79-219, GJBX-420(81); U.S. Department of Energy: Grand Junction, CO, USA, 1979; 104p.
- Bolivar, S.L. An overview of the National Uranium Resource Evaluation Hydrogeochemical and Stream Sediment Reconnaissance Program, LA-8457-MS; U.S. Department of Energy, Division of Uranium Resources and Enrichment: Washington, DC, USA, 1980.
- Grimes, J.G. NURE HSSR Geochemical Sample Archives Transfer Report Part 3 Geochemical Analysis (K/UR—500-Pt3); Oak Ridge Gaseous Diffusion Plant: Ridge Gaseous, TN, USA, 1984. [Google Scholar]
- Hem, J.D. Study and Interpretation of Chemical Characteristics of Natural Waters, 3rd ed.; US Geological Survey Water Supply Paper 2254; U.S. Geological Survey: Reston, VA, USA, 1989; 363p. [Google Scholar]
- Schwertmann, U.; Fitzpatrick, R.W. Iron minerals in surface environments. Catena Suppl. 1992, 21, 7–30. [Google Scholar]
- Kennedy, V.C.; Zellweger, G.W.; Jones, B.F. Filter pore-size effects on the analysis of Al, Fe, Mn, and Ti in Water. Water Resour. Res. 1974, 10, 4. [Google Scholar] [CrossRef]
- Grundl, T.J.; Haderlein, S.; Nurmi, J.T.; Tratnyek, P.B. Chapter 1: Introduction to Aquatic Redox Chemistry in Tratneyk et al., Aquatic Redox chemistry. ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 2nd ed.; Wiley Interscience: Hoboken, NJ, USA, 1981. [Google Scholar]
- Eargle, D.H.; Dickinson, K.A.; Davis, B.O. South Texas Uranium Deposits. AAPG Bull. 1975, 59, 766–779. [Google Scholar]
- Hamlin, H.S. Salt domes in the Gulf coast Aquifer. In Texas Water Development Board Report 365: Aquifers of the Gulf Coast of Texas; Mace, R.E., Davidson, S.C., Angle, E.S., Mullican, W.F., III, Eds.; Available online: https://www.twdb.texas.gov/publications/reports/numbered_reports/doc/R365/R365_Composite.pdf (accessed on 1 January 2022).
- Smonton, S.; Spears, M. Human health effects from exposure to low-level concentrations of hydrogen sulfide. Occup. Health Saf. 2007, 76, 102–104. [Google Scholar]

| Environmental Condition | pH Range | Reduction–Oxidation | Number of Samples |
|---|---|---|---|
| EC1 | <3 | oxic | 0 |
| EC2 | ≥3 to <6.5 | oxic | 11 |
| EC3 | ≥6.5 to <8.5 | oxic | 628 |
| EC4 | ≥8.5 | oxic | 66 |
| EC5 | <3 | anoxic | 0 |
| EC6 | ≥3 to <6.5 | anoxic | 12 |
| EC7 | ≥6.5 to <8.5 | anoxic | 173 |
| EC8 | ≥8.5 | anoxic | 16 |
| EC9 | <3 | mixed | 0 |
| EC10 | ≥3 to <6.5 | mixed | 3 |
| EC11 | ≥6.5 to <8.5 | mixed | 6 |
| EC12 | ≥8.5 | mixed | 0 |
| Element | Detection Limit (NURE) (Smith, 2006) | Detection Limit (TWDB, 2020) | Drinking-Water Maximum Contaminant Level (USEPA, 2019b) | Aquatic Life Freshwater Acute Criteria Maximum Concentration (USEPA, 2019a) | Aquatic Life Freshwater Chronic Criteria Continuous Concentration (USEPA, 2019a) |
|---|---|---|---|---|---|
| All values in μg/L | |||||
| U | 0.2 | 1.0 | 30 | none identified | none identified |
| Mo | 4.0 | 1.0 | none identified (70 By WHO (Smedley and Kinniburgh 2017)) | none identified | none identified |
| As | 0.5 | 1.0 | 10 | 340 | 150 |
| Se | 0.2 | 2.0 | 50 | none identified | none identified |
| V | 4.00 | 1.0 | none identified | none identified | none identified |
| Smith, 2007 | Perelman, 1986 | Blake et al., This Study | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environmental Condition | Mobile | Scarcely Mobile to Immobile | Environmental Condition | Oxidized Water | Reduced Water (no H2S) | Reduced Water with H2S | Environmental Condition | Mobile | Scarcely Mobile to Immobile | Fe Substrates Present | H2S Present | ||||
| Adsorption to Fe Substrate | Contact with Reduced Water without H2S | H2S Introduced | Adsorption to Fe Substrate | Contact with Reduced Water without H2S | H2S Introduced | Adsorption to Fe Substrate | |||||||||
| Oxidizing with pH < 3 | As, Mo, Se, U, V | -- | pH < 3 | As immobile | U, Mo immobile | As, Mo, U immobile | V, As immobile | U, Mo immobile | -- | As, V immobile | (1) pH < 3, oxic | no data but would expect As, Mo, Se, U, V | -- | As immobile | -- |
| Oxidizing with pH > 5 to circumneutral, no iron substrates | As, Mo, Se, U, V | -- | pH 3 to 6.5 | As, Mo, U, V immobile | U, Mo immobile | Mo, U immobile | U immobile | U, Mo immobile | U immobile | -- | (2) pH ≥ 3 to <6.5, oxic | As, Mo, Se, U, V | -- | As, Mo, U, V immobile | -- |
| Oxidizing with pH > 5 to circumneutral, abundant iron substrates | Se | As, Mo, U, V | pH 6.5 to 8.5 | As, Mo, V potentially immobile | U, Mo, Se, V immobile | Mo, U, Se, V immobile | -- | U, Mo immobile | Mo, U potentially immobile | -- | (3) pH ≥ 6.5 to <8.5, oxic | As, Mo, Se, U, V | -- | As, Mo, V scarcely mobile | -- |
| Reducing with pH > 5 to circumneutral, no hydrogen sulfide | As | Mo, Se, U, V | pH > 8.5 | As, Mo, V potentially immobile | U, Mo, Se, V, As immobile | As, Mo, U, V immobile | -- | U, Mo immobile | U potentially immobile | -- | (4) pH ≥ 8.5, oxic | As, Mo, Se, U, V | -- | As immobile Mo, V scarcely mobile | -- |
| Reducing with pH > 5 to circumneutral, with hydrogen sulfide | -- | As, Mo, Se, U, V | (5) pH < 3, anoxic | no data but would expect As, Mo, V | no data but would expect Se, U | As, V immobile | As, Mo, Se can form solids with sulfide | ||||||||
| (6) pH ≥ 3 to <6.5, anoxic | As, Mo, V | Se, U | As, Se immboile | As, Mo, Se can form solids with sulfide | |||||||||||
| (7) pH ≥ 6.5 to <8.5, anoxic | As, Mo, Se, V | Se, U, V | As immobile, Se scarcely mobile | As, Mo, Se can form solids with sulfide | |||||||||||
| (8) pH ≥ 8.5, anoxic | no data but would expect As, Mo, Se, U, V | -- | -- | -- | |||||||||||
| (9) pH < 3, mixed | no data | no data | Se potentially immobile | As, Mo, Se can form solids with sulfide | |||||||||||
| (10) pH ≥ 3 to <6.5, mixed | U | As, Mo, Se, V | Se potentially immobile | As, Mo, Se can form solids with sulfide | |||||||||||
| (11) pH ≥ 6.5 to <8.5, mixed | U, As | Mo, Se, V | Mo, Se immobile | As, Mo, Se can form solids with sulfide | |||||||||||
| (12) pH ≥ 8.5, mixed | U, As, Mo | Se, V | Mo mobile | -- | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blake, J.M.; Walton-Day, K.; Gallegos, T.J.; Yager, D.B.; Teeple, A.; Humberson, D.; Stengel, V.; Becher, K. New Geochemical Framework and Geographic Information System Methodologies to Assess Element Occurrence, Persistence, and Mobility in Groundwater and Surface Water. Minerals 2022, 12, 411. https://doi.org/10.3390/min12040411
Blake JM, Walton-Day K, Gallegos TJ, Yager DB, Teeple A, Humberson D, Stengel V, Becher K. New Geochemical Framework and Geographic Information System Methodologies to Assess Element Occurrence, Persistence, and Mobility in Groundwater and Surface Water. Minerals. 2022; 12(4):411. https://doi.org/10.3390/min12040411
Chicago/Turabian StyleBlake, Johanna M., Katherine Walton-Day, Tanya J. Gallegos, Douglas B. Yager, Andrew Teeple, Delbert Humberson, Victoria Stengel, and Kent Becher. 2022. "New Geochemical Framework and Geographic Information System Methodologies to Assess Element Occurrence, Persistence, and Mobility in Groundwater and Surface Water" Minerals 12, no. 4: 411. https://doi.org/10.3390/min12040411
APA StyleBlake, J. M., Walton-Day, K., Gallegos, T. J., Yager, D. B., Teeple, A., Humberson, D., Stengel, V., & Becher, K. (2022). New Geochemical Framework and Geographic Information System Methodologies to Assess Element Occurrence, Persistence, and Mobility in Groundwater and Surface Water. Minerals, 12(4), 411. https://doi.org/10.3390/min12040411







