An Integrated Capture of Red Mud and One-Step Heat-Treatment Process to Recover Platinum Group Metals and Prepare Glass-Ceramics from Spent Auto-Catalysts
Abstract
:1. Introduction
2. Materials and Methods
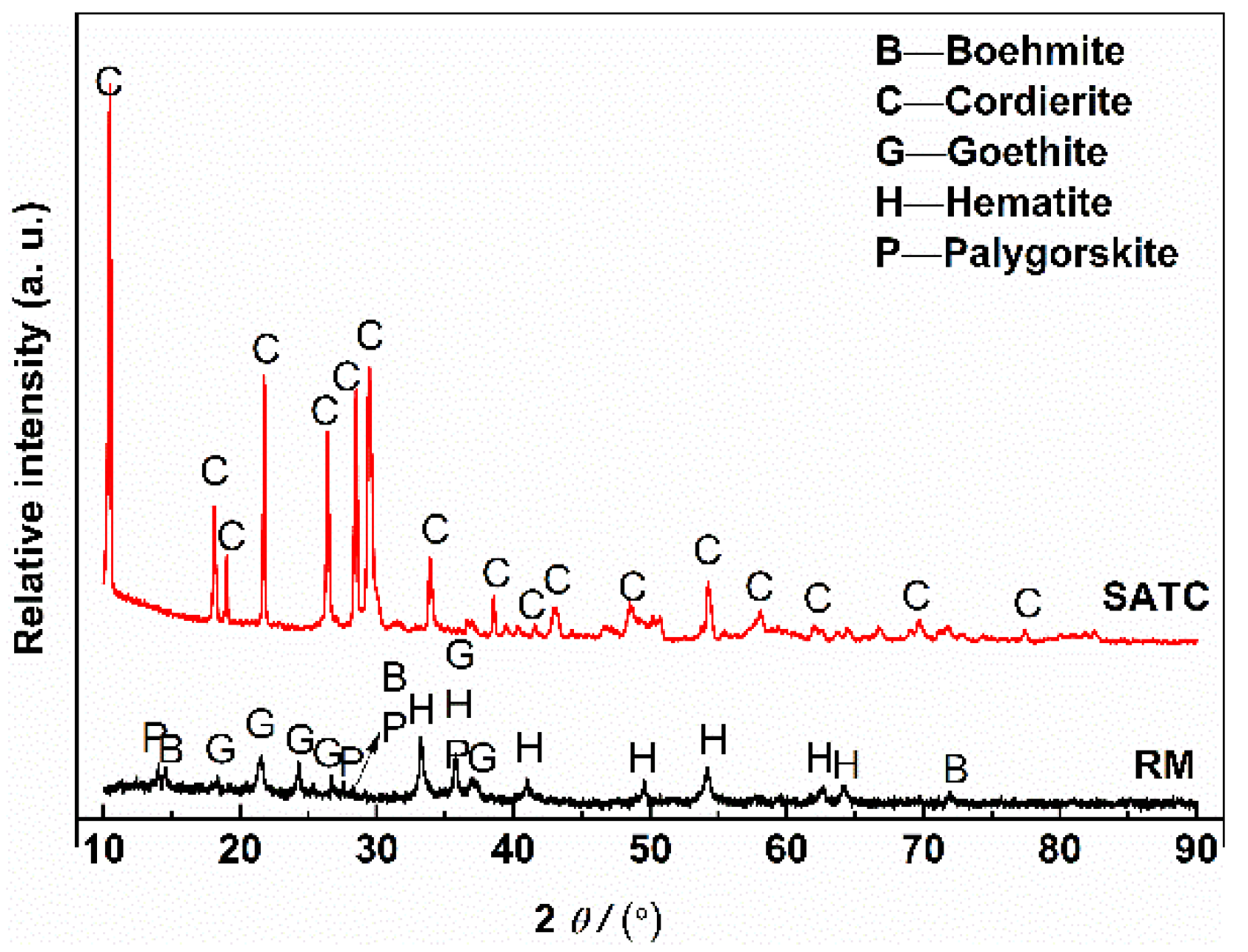
2.1. Starting Materials
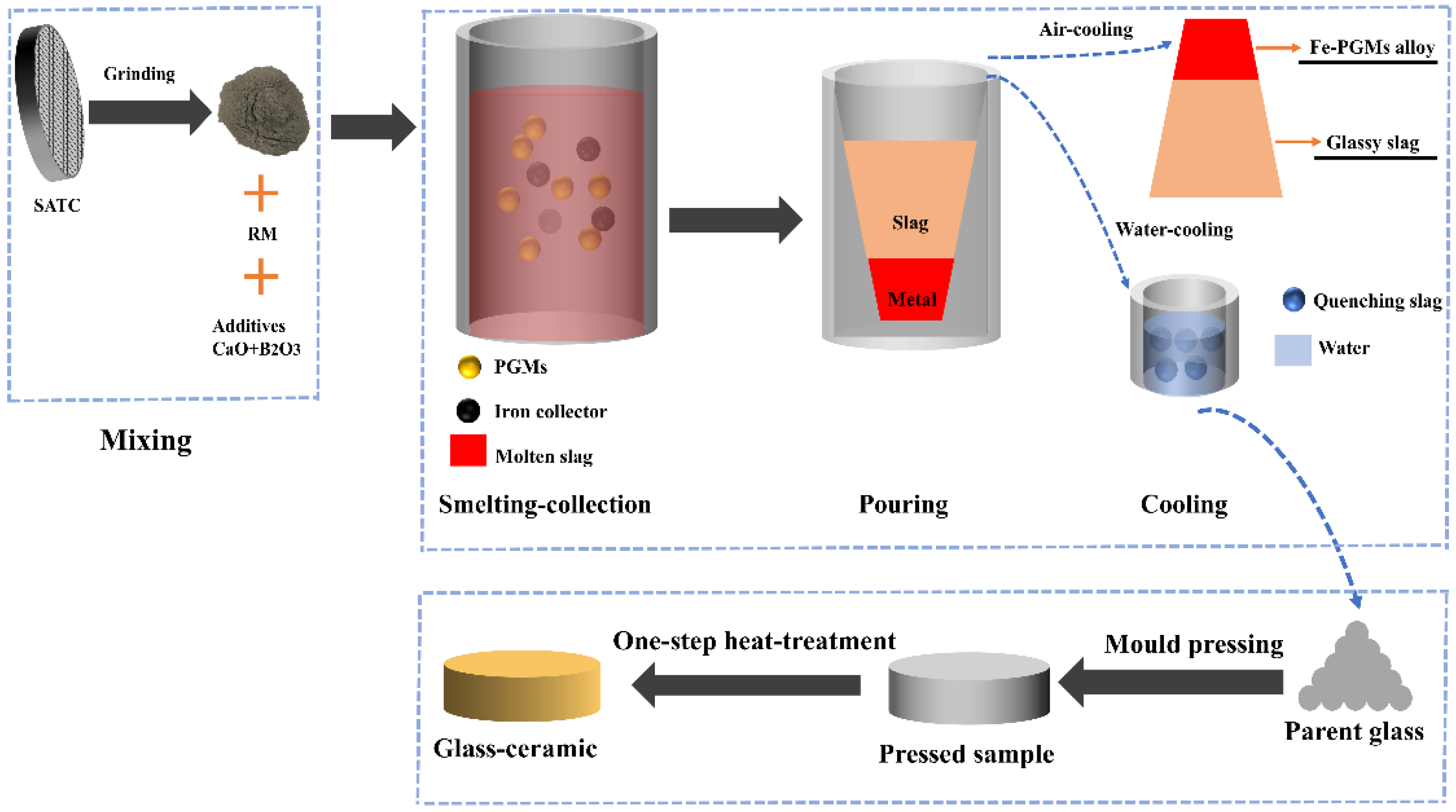
2.2. Experimental Procedure
2.3. Analytical Methods
3. Result and Discussion
3.1. Optimization of the Cooperative Smelting Process
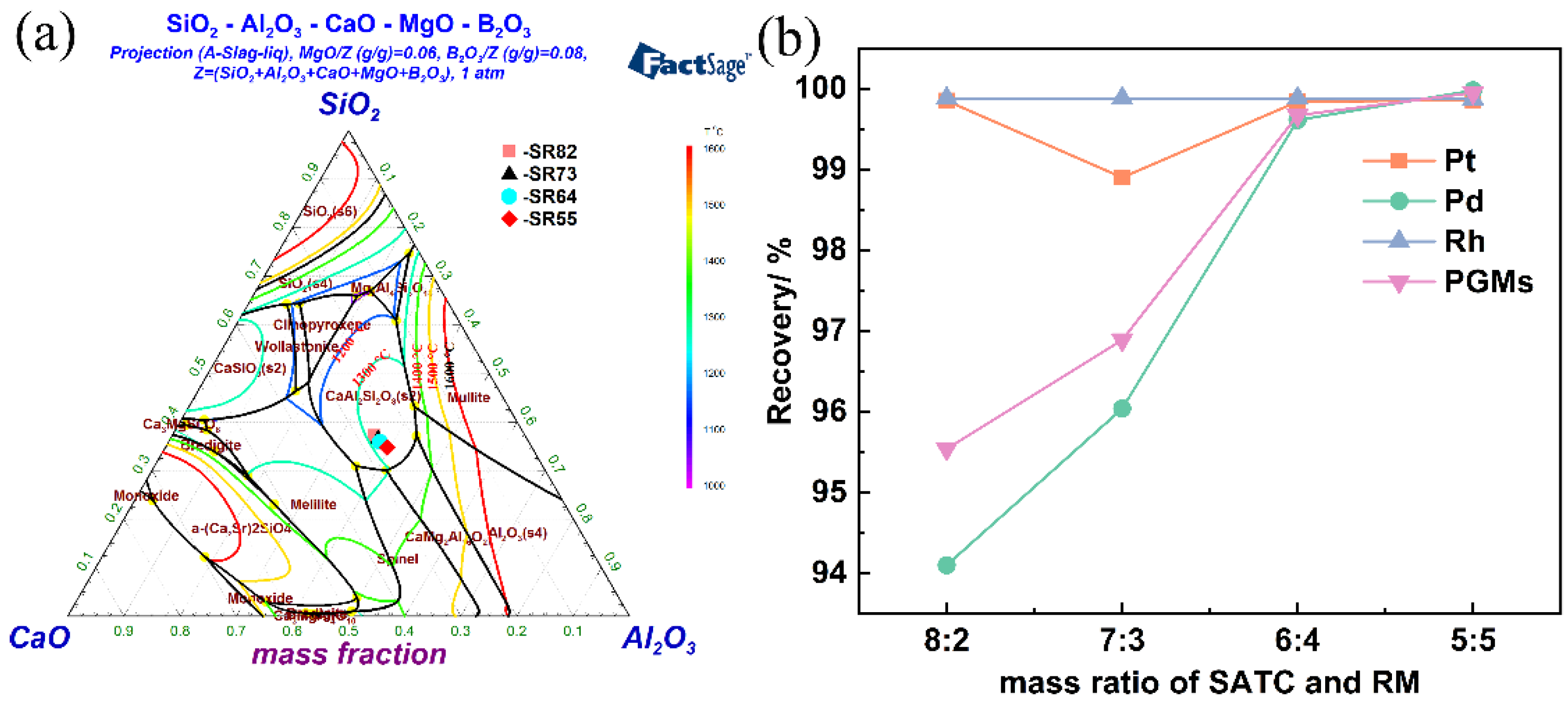
3.1.1. Effect of RM Addition on the PGM Recovery
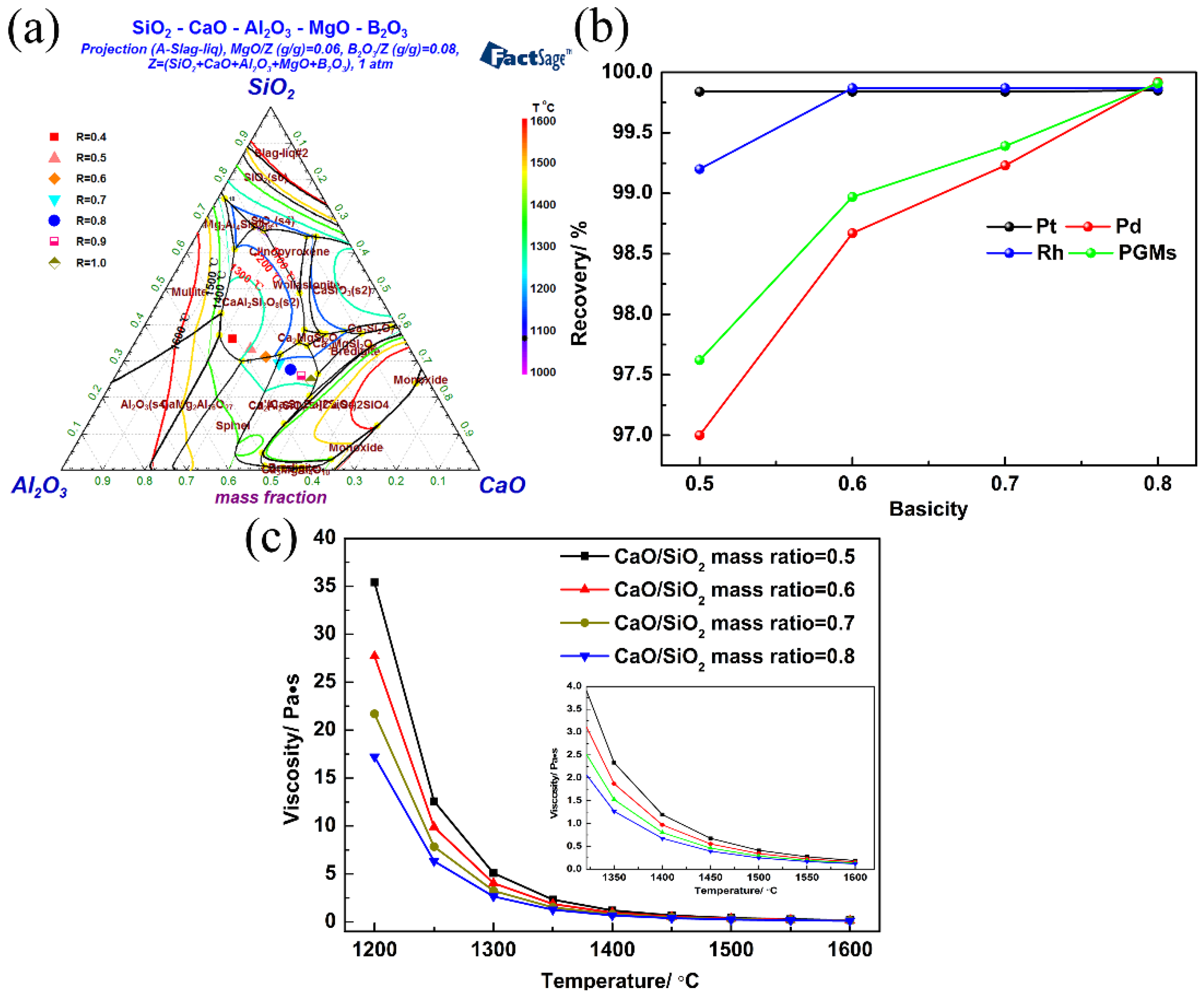
3.1.2. Effect of Basicity on PGM Recovery
3.2. Confirmation Experiments
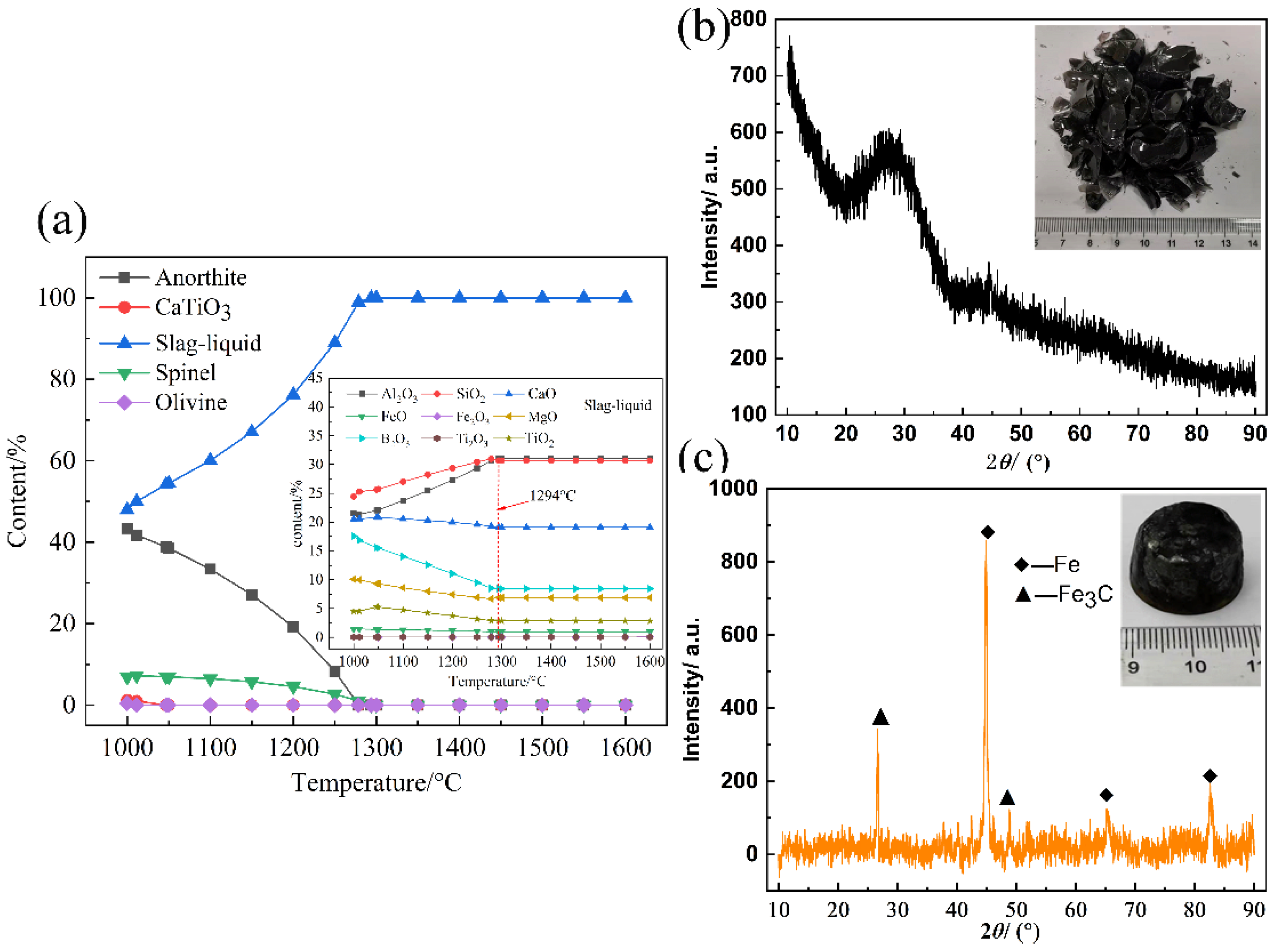
3.2.1. Analysis of the Slag Phase
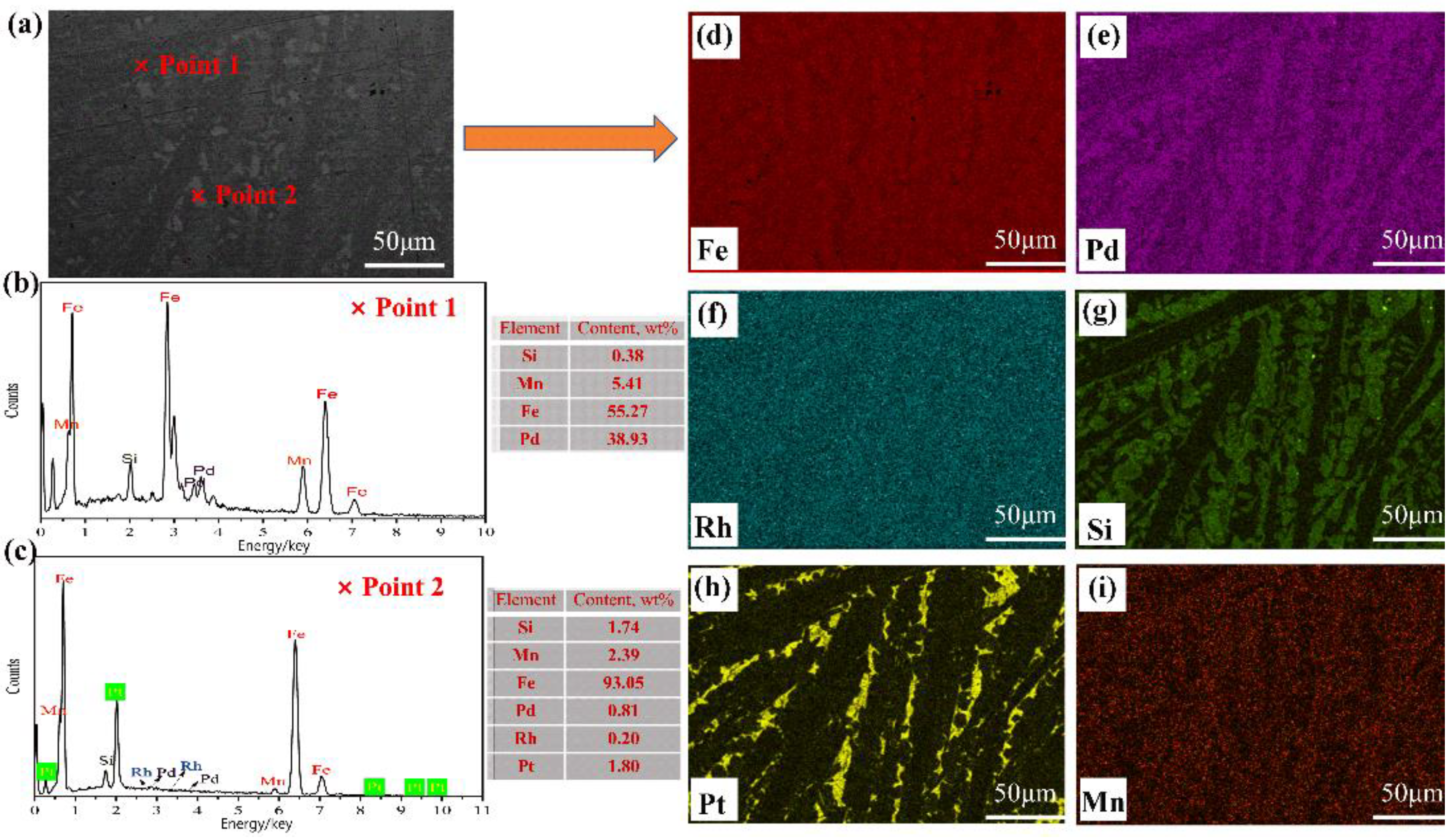
3.2.2. Analysis of the PGM-Enriched Alloy
3.2.3. Economic Evaluation
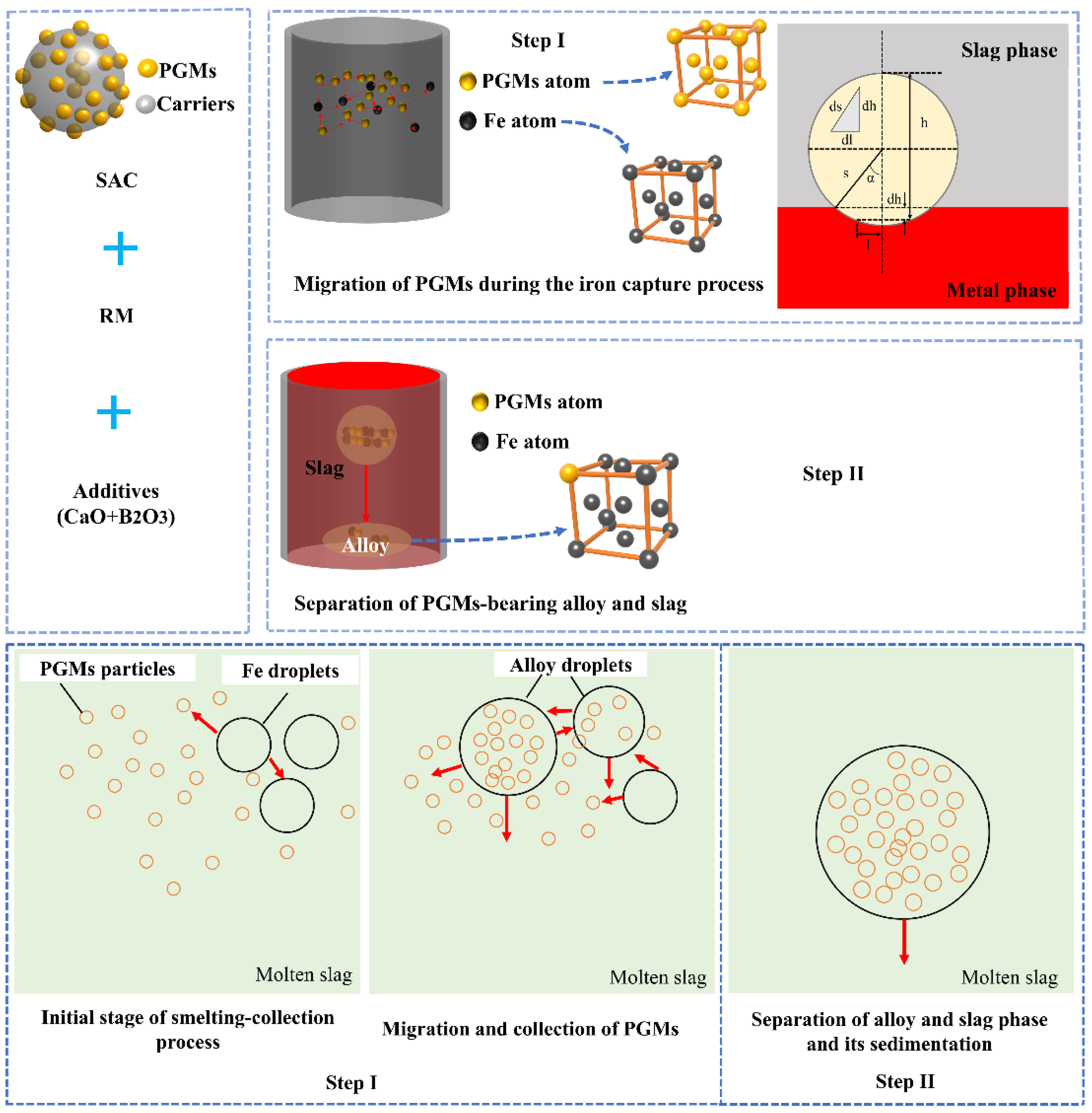
3.3. Mechanism of Recovering PGMs via the Iron Capture Process
3.3.1. Migration Process of PGMs
3.3.2. Separation of Slag and Metal Phase
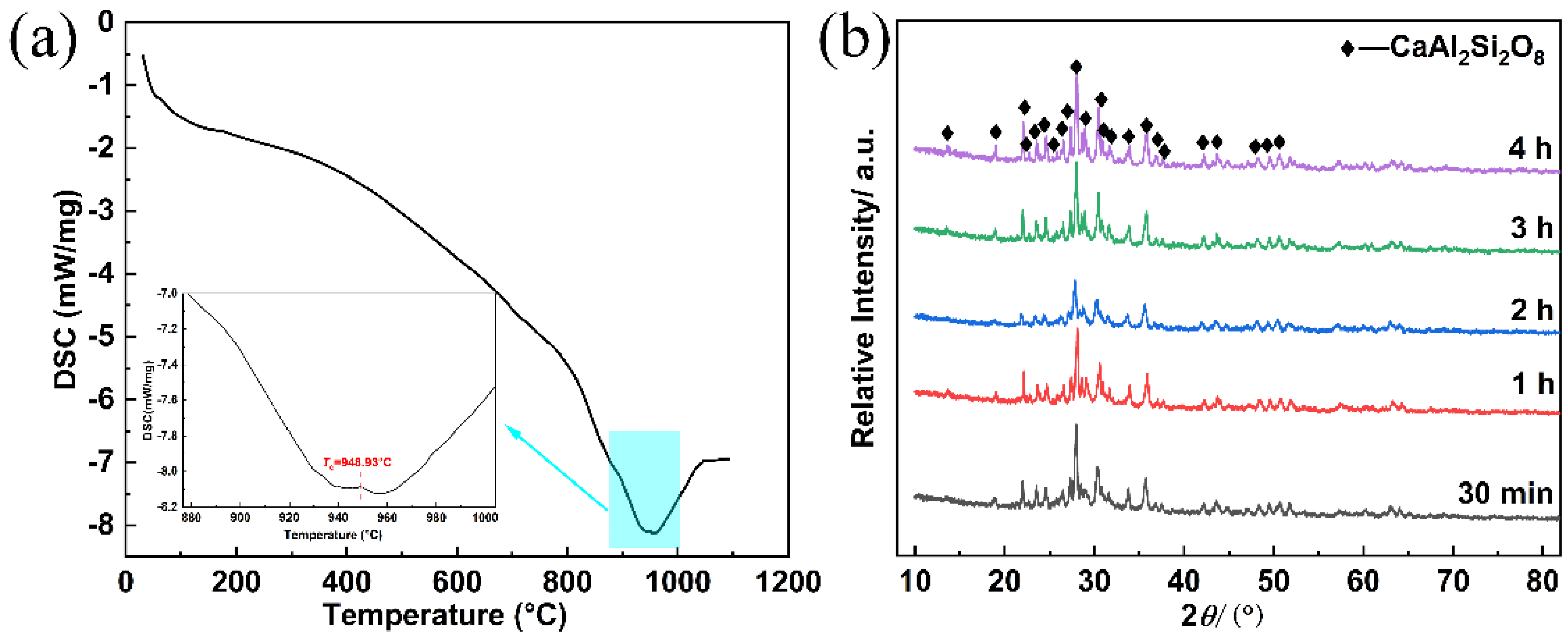
3.4. Synthesis of Glass-Ceramics
4. Conclusions
- PGMs in the SAC were trapped by iron from the reduction process of RM in the form of PGM-bearing iron alloys. The optimal smelting conditions were confirmed to be 40–50 wt% of RM additions, 0.8 of basicity, 1500 °C of smelting temperature, and 40 min of holding time, at which the PGM recovery rates were approximately 100%. The PGMs content in obtained slag was less than 1 g/t.
- A mechanism of the iron collection process, including migration of PGMs during the formation of PGM-bearing alloys and separation of the slag and metal phases, was proposed. The PGMs in molten slag can transfer spontaneously from the slag phase into the metal phase to form the PGM–Fe alloy by thermodynamic analysis, including surface tension and binding energy, and the delocalized electrons theory. Afterwards, the formed PGM–-Fe alloy is more readily separated from the slag phase due to the considerable difference density between the slag and metal phases, approximately 4.5 g/cm3, and the low viscous force during the sedimentation process of the metal phase in molten slag.
- The extracted PGMs from the SAC entered the iron matrix, in which Pd and Rh dispersed in the iron matrix, while Pt was distributed along a certain grain boundary.
- The synthesis of glass-ceramics was successfully achieved by a one-step heat-treatment process at 950 °C for 30 min to 240 min. Consequently, the main phase of CaAl2Si2O8 was determined in the glass-ceramics.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fornalczyk, A.; Saternus, M. Catalytic converters as a source of platinum. Metalurgija 2014, 50, 261–264. [Google Scholar]
- Wei, X.; Liu, C.W.; Cao, H.B.; Ning, P.G.; Jin, W.; Yang, Z.B.; Wang, H.J.; Sun, Z. Understanding the features of PGMs in spent ternary automobile catalysts for development of cleaner recovery technology. J. Clean. Prod. 2019, 239, 118031. [Google Scholar] [CrossRef]
- Ding, Y.J.; Zhang, S.G.; Liu, B.; Zheng, H.D.; Chang, C.C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; McLaren, M.; Laffir, F.; Rooney, D. Characterization of robust combustion catalyst from aluminium foil waste. Chemistry 2018, 3, 1545–1550. [Google Scholar]
- Castellanos, I.; Marie, O. Fe-HFER and Cu-HFER as catalysts for the NOx SCR using acetylene as a reducing agent: Reaction mechanism revealed by FT-IR operando study coupled with (NO)-N-15 isotopic labeling. Appl. Catal. B Environ. 2018, 223, 143–153. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, Y.; Li, S.S.; Xiong, L.; Wang, J.L.; Yuan, S.D.; Chen, Y.Q. Designed synthesis and characterization of nanostructured ceria-zirconia based material with enhanced thermal stability and its application in three-way catalysis. J. Ind. Eng. Chem. 2018, 64, 219–229. [Google Scholar] [CrossRef]
- Shin, D.; Park, J.; Jeong, J.; Kim, B.S. A biological cyanide production and accumulation system and the recovery of platinum-group metals from spent automotive catalysts by biogenic cyanide. Hydrometallurgy 2015, 158, 10–18. [Google Scholar] [CrossRef]
- Dong, H.G.; Zhao, J.V.; Chen, J.L.; Wu, Y.D.; Li, B.J. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, J.; Kim, M.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Peng, Z.W.; Li, Z.Z.; Lin, X.L.; Tang, H.M.; Ye, L.; Ma, Y.T.; Rao, M.J.; Zhang, Y.B.; Li, G.H.; Jiang, T. Pyrometallurgical recovery of platinum group metals from spent catalysts. JOM 2017, 69, 1553–1562. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L.F. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling of electronic scrap at Umicore precious metals refining. Acta. Metall. Slovaca 2006, 12, 111–120. [Google Scholar]
- Zhang, L.G.; Xu, Z.M. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Zhang, L.G.; Song, Q.M.; Liu, Y.; Xu, Z.M. Novel approach for recovery of palladium in spent catalyst from automobile by a capture technology of eutectic copper. J. Clean. Prod. 2019, 239, 118093. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.S.; Polyakov, P.V.; Pavlov, E.A.; Varyukhin, Y.D. Recovery of noble metals from spent catalysts: A review. Metall. Mater. Trans. 2020, 51, 2413–2435. [Google Scholar] [CrossRef]
- Ding, Y.J.; Zheng, H.D.; Zhang, S.G.; Liu, B.; Wu, B.Y.; Jian, Z.M. Highly efficient recovery of platinum, palladium, and rhodium from spent automotive catalysts via iron melting collection. Resour. Conserv. Recycl. 2019, 155, 104644. [Google Scholar] [CrossRef]
- Morcali, M.H. A new approach to recover platinum-group metals from spent catalytic converters via iron matte. Resour. Conserv. Recycl. 2020, 159, 104891. [Google Scholar] [CrossRef]
- Zhu, X.B.; Wang, L.; Guan, X.M. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Liu, S.J.; Yao, Z.Y.; Wu, S.P.; Jiang, H.G.; Liang, M.; Qiao, Y.N. Environmental aspects and pavement properties of red mud waste as the replacement of mineral filler in asphalt mixture. Constr. Build. Mater. 2018, 180, 605–613. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wu, C.S. Stockpiling and comprehensive utilization of red mud research progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef] [Green Version]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilization of Bayer red mud. Resour. Conserv. Recycl. 2018, 141, 483–498. [Google Scholar] [CrossRef]
- Jayasankar, K.; Ray, P.K.; Chaubey, A.K.; Padhi, A.; Satapathy, B.K.; Mukherjee, P.S. Production of pig iron from red mud waste fines using thermal plasma technology. Int. J. Miner. Metall. Mater. 2012, 19, 679–684. [Google Scholar] [CrossRef]
- Liu, C.; Sun, S.C.; Zhu, X.P.; Tu, G.F. Feasibility of platinum recovery from waste automotive catalyst with different carriers via cooperative smelting-collection process. J. Mater. Cycles Waste Manag. 2021, 23, 581–590. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.C.; Jeong, J.; Yang, D.H.; Shin, D.Y.; Lee, K.I. A novel process for extracting precious metals from spent mobile phone PCBs and automobile catalysts. Mater. Trans. 2013, 54, 1045–1048. [Google Scholar] [CrossRef] [Green Version]
- Quan, L.I.; Jianmin, Y.U.; Sha, J.; Wang, H.Y.; Bi, X.G.; Yang, J.F.; Lu, F. Recovery of platinum group metals and valuable metals from spent automotive catalysts by “double hydrometallurgical method”. Precious Met. 2015, 3, 118031. [Google Scholar]
- Erol, M.; Kucukbayrak, S.; Ersoy-Mericboyu, A. Comparison of the properties of glass, glass-ceramic and ceramic materials produced from coal fly ash. J. Hazard. Mater. 2008, 15, 418–425. [Google Scholar] [CrossRef]
- Lv, J.F.; Yang, H.Y.; Jin, Z.N.; Zhao, M.L. Lead extraction and glass-ceramics synthesis from waste cathode ray tube funnel glass through cooperative smelting process with coal fly ash. Waste Manag. 2018, 76, 687–696. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, Y.J. A novel process of extracting precious metals from waste printed circuit boards: Utilization of gold concentrate as a fluxing material. J. Hazard. Mater. 2019, 365, 659–664. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melancon, J.; Pelton, A.D.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Zhang, L.G.; Song, Q.M.; Liu, Y.; Xu, Z.M. An integrated capture of copper scrap and electrodeposition process to enrich and prepare pure palladium for recycling of spent catalyst from automobile. Waste Manag. 2020, 108, 172–182. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.G.; Song, Q.M.; Xu, Z.M. Recovery of palladium and silver from waste multilayer ceramic capacitors by eutectic capture process of copper and mechanism analysis. J. Hazard. Mater. 2019, 388, 122008. [Google Scholar] [CrossRef] [PubMed]
- Fu, J. An investigation of mechanism on the remove of oxide inclusions during ESR process. Acta. Metall. Sin. 1979, 15, 526–539. [Google Scholar]
- Yuan, Z.F.; Ke, J.J.; Li, J. Surface Tension of Metals and Alloys; Science Press: Beijing, China, 2006. [Google Scholar]
- Chen, J. Discussion on the micro-mechanism of precious metals trapped in pyrometallurgical processes by base metals and matte phase. Eng. Sci. 2007, 5, 11–16. [Google Scholar]
- Zheng, H.D.; Ding, Y.J.; Wen, Q.; Zhao, S.Z.; He, X.F.; Zhang, S.G.; Dong, C.F. Slag design and iron capture mechanism for recovering low-grade Pt, Pd, and Rh from leaching residue of spent auto-exhaust catalysts. Sci. Total Environ. 2022, 802, 149830. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.J. Research on the Mechanism and Application of Platinum Group Metals Enrichment from Spent Catalysts; University of Science and Technology of Beijing: Beijing, China, 2019. [Google Scholar]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Takashi, M.; Katsunori, Y. Recovery of palladium and platinum particles suspended in the Al2O3-CaO-SiO2 slag using copper-based extractants at 1723 K. Mater. Trans. 2020, 62, 1495–1501. [Google Scholar]
- Xin, J.J.; Guo, L.; Jiao, L.N. A simple density model for SiO2-Al2O3-CaO-MgOmolten slags. Chin. J. Process. Eng. 2017, 17, 395–399. [Google Scholar]
- Liu, Y.; Song, Q.M.; Zhang, L.G.; Xu, Z.M. Behavior of enrichment and migration path of Cu-Ag-Pd-Bi-Pb in the recovery of waste multilayer ceramic capacitors by eutectic capture of copper. J. Clean. Prod. 2020, 287, 125469. [Google Scholar] [CrossRef]
- Salinigopal, M.S.; Gopakumar, N.; Anjana, P.S.; SureshKumar, B. Structural optical and dielectric properties of aluminoborosilicate glasses. J. Electron. Mater. 2019, 49, 695–704. [Google Scholar] [CrossRef]
- Salinigopal, M.S.; Gopakumar, N.; Anjana, P.S.; Pandey, O.P. Synthesis and characterization of 50BaO-(5-x)Al2O3-xR2O3-30B2O3-15SiO2(R=Nd, Gd) glass-ceramics. J. Non-Cryst. Solids 2020, 535, 119956. [Google Scholar] [CrossRef]
- Yang, J.; Liu, B.; Zhang, S.G.; Volinsky, A.A. Glass-Ceramics one-step crystallization accomplished by building Ca2+ and Mg2+ fast diffusion layer around diopside crystal. J. Alloys Compd. 2016, 688, 709–714. [Google Scholar] [CrossRef]
- Ozabaci, M.; Aksan, M.A.; Kirat, G.; Kizilaslan, O.; Yakinci, M.E. Preparation and characterization of CaO-Al2O3-SiO2 (CAS) glass-ceramics. J. Non-Cryst. Solids 2016, 454, 8–12. [Google Scholar] [CrossRef]
- Allu, A.R.; Balaji, S.; Tulyaganov, D.U.; Mather, G.C.; Margit, F.; Pascual, M.J.; Siegel, R.J.; Milius, W.; Senker, J.; Agarkov, D.A.; et al. Understanding the formation of CaAl2Si2O8 in melilite-based glass-ceramics: Combined diffraction and spectroscopic studies. ACS Omega 2017, 2, 6233–6243. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.G. Thermodynamic evaluation of the surface tension of molten CaO-SiO2-Al2O3 ternary slag. ISIJ Int. 2002, 42, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lv, X.; Bai, C. Prediction for the Surface Tension of FeO-TiO2-Ti2O3-X (SiO2, CaO, MgO) Slag Systems; John Wiley & Sons, Ltd.: Chichester, UK, 2015. [Google Scholar]
- Nakamoto, M.; Tanaka, T.; Holappa, L.; Haemaelaeinen, M. Surface tension evaluation of molten silicates contain-ing surface-active components (B2O3, CaF2 or Na2O). ISIJ Int. 2007, 47, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, M.; Kiyose, A.; Tanaka, T. Evaluation of the surface tension of ternary silicate melts containing Al2O3, CaO, FeO, MgO or MnO. ISIJ Int. 2007, 47, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Heikkinen, E.P.; Riipi, J.; Fabritius, T. Computational modelling of oxide surface tensions in secondary metallurgy. Steel Res. Int. 2010, 81, 959–964. [Google Scholar] [CrossRef]
| Components | Concentration (wt%) | |
|---|---|---|
| SAC | RM | |
| SiO2 | 34.6 | 7.18 |
| Al2O3 | 31.0 | 16.36 |
| CaO | 1.7 | 1.05 |
| MgO | 8.9 | 0.14 |
| TFe | 2.5 | 38.74 |
| K2O | 0.3 | 0.18 |
| Na2O | 2.88 | |
| ZrO2 | 4.1 | 0.21 |
| MnO | 0.6 | |
| S | 0.004 | |
| P | 0.11 | |
| V | 0.15 | |
| ZnO | 0.2 | |
| Cr2O3 | 0.15 | |
| TiO2 | 0.7 | 6.22 |
| Pt/Pd/Rh a | 97.0/642.0/118.0 | |
| LOI b | 12.6 | 9.4 |
| Products | Compositions (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slag phase | SiO2 | CaO | Al2O3 | MgO | B2O3 | TFe | TiO2 | Pt * | Pd * | Rh * |
| 30.26 | 18.84 | 30.68 | 6.78 | 8.32 | 0.75 | 2.82 | BDL | 0.86 | BDL | |
| Metal phase | TFe | Si | C | Mn | Pt * | Pd * | Rh * | |||
| 88.2 | 1.79 | 3.9 | 0.62 | 392.6 | 2945.3 | 459.2 | ||||
| Samples | TCLP Results (mg/L) | |||
|---|---|---|---|---|
| Zn | Cr | Mn | Pb | |
| Glassy slag | 1 | 0.072 | 4.7 | BDL |
| GC0.5 | 0.34 | BDL | 3.22 | BDL |
| GC1 | 0.33 | BDL | 3.69 | BDL |
| GC2 | 0.29 | BDL | 3.77 | BDL |
| GC3 | 0.28 | BDL | 2.44 | BDL |
| GC4 | 0.28 | BDL | 2.48 | BDL |
| US-EPA limit | 500 | 5 | 5 | 5 |
| Reference Method [16] | This Work | ||||
|---|---|---|---|---|---|
| Chemicals | Amount/kg | Cost/USD | Chemicals | Amount/kg | Cost/USD |
| SiO2 | 0 | 0 | SiO2 | 0 | 0 |
| CaO | 0.3605 | 0.0649 | CaO | 0.2598 | 0.0468 |
| Iron powder | 0.1500 | 0.0825 | Iron powder | 0 | 0 |
| C | 0.0500 | 0.0080 | C | 0 | 0 |
| Na2B4O7 | 0.0850 | 0.0374 | H3BO3 | 0.1400 | 0.1526 |
| Na2CO3 | 0.3498 | 0.0826 | RM | 0.5000 | 0 |
| CaF2 | 0.0500 | 0.0204 | / | / | / |
| Sum | 1.0453 | 0.2958 | Sum | 0.8998 | 0.1994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Sun, S.; Tu, G.; Xiao, F. An Integrated Capture of Red Mud and One-Step Heat-Treatment Process to Recover Platinum Group Metals and Prepare Glass-Ceramics from Spent Auto-Catalysts. Minerals 2022, 12, 360. https://doi.org/10.3390/min12030360
Liu C, Sun S, Tu G, Xiao F. An Integrated Capture of Red Mud and One-Step Heat-Treatment Process to Recover Platinum Group Metals and Prepare Glass-Ceramics from Spent Auto-Catalysts. Minerals. 2022; 12(3):360. https://doi.org/10.3390/min12030360
Chicago/Turabian StyleLiu, Chuan, Shuchen Sun, Ganfeng Tu, and Faxin Xiao. 2022. "An Integrated Capture of Red Mud and One-Step Heat-Treatment Process to Recover Platinum Group Metals and Prepare Glass-Ceramics from Spent Auto-Catalysts" Minerals 12, no. 3: 360. https://doi.org/10.3390/min12030360
APA StyleLiu, C., Sun, S., Tu, G., & Xiao, F. (2022). An Integrated Capture of Red Mud and One-Step Heat-Treatment Process to Recover Platinum Group Metals and Prepare Glass-Ceramics from Spent Auto-Catalysts. Minerals, 12(3), 360. https://doi.org/10.3390/min12030360





