1. Introduction
Cement is a basic building material, with a global production of 4.1 Gt/year (2020 world production) [
1]. It contributes significantly to the building of our cities and therefore the development of our culture, mainly due to its special characteristics, namely strength, durability, longevity, resilience, etc., in combination with its competitive price. Cement production requires raw materials rich in the oxides CaO, SiO
2, Al
2O
3 and Fe
2O
3. Their common abbreviations, in industry and research of cement, is C, S, A and F respectively [
2,
3,
4]. In order to produce Portland cement, raw materials rich in these oxides are mixed and fired at temperatures usually up to 1450 °C. Therefore, the clinker of Portland cement consists mainly of 4 basic mineralogical phases; tricalcium silicate or alite (C
3S), dicalcium silicate or belite (C
2S), Tetracalcium aluminoferrite or ferrite (C
4AF) and tricalcium aluminate or celite (C
3A) [
3,
4].
During cement production, it is estimated that almost 680 kg total CO
2 emissions per ton of cement are released into the environment in two ways, ranking the cement industry among the largest CO
2 emitters in the world [
5,
6]. This quantity of CO
2 is released in two ways: 60% directly, due to decomposition upon heating of CaCO
3 found in limestone [
3,
7] and 40% indirectly, due to the thermal energy consumption required for the production process [
3,
6]. Moreover, the cement industry is a large energy consumer with a typical energy requirement for Portland cement production, 440–3845 MJ/t of cement, from the quarry to packaging [
8,
9]. The need for reuse, waste recovery, reduced energy consumption and minimization of carbon footprint is imperative and concerns many of the scientific and industrial cement research in the last decades [
8,
10,
11,
12].
Therefore, according to a number of researchers [
8,
13,
14,
15,
16,
17] the reduction of CO
2 emissions in Portland cement production, is possible by applying the circular economy principles, i.e., using alternative fuels in cement kilns and/or using recycled/secondary byproducts as raw materials (wastes) and/or changing the chemistry of cement. These “new types of cement” are often called “low energy cements”. The most common problems these types of cement may face are the low initial strength of the produced mortars [
18,
19] and the “dust effect” due to the formation of γ-belite (γ-C
2S) [
18]. The above disadvantages are proposed to be addressed in several ways [
13,
14,
15,
16,
17]. However, to our knowledge, no study exists that utilizes industrial secondary products such as Iron Lamination Scales (ILS), Greek Wet Fly Ash (WFA) and at the same time lower clinkering temperature. The present study attempts to do so.
The cement chemistry implies that alite is formed as a result of the reaction of CaO and C
2S. Under optimal conditions, eutectic melt is formed and alite can be formed at a temperature of 1338 °C [
20], while for all the other main phases, namely belite (C
2S), calcium aluminate (C
3A) and ferrite (C
4AF), the temperature of formation is below 1300 °C [
3]. In addition, C
2S crystallizes in five main structures [
21] (α, α
H′, α
L′, β and γ) depending on the clinkering temperature and all of them, except γ-structure, have very good hydraulic properties. Ono [
22] showed that during rapid cooling a significant proportion of belite remains in the α-form between lamellas of the disordered β-form. However, γ-C
2S is thermodynamically stable at room temperature and is favored by slow cooling [
18]. So, in order to stabilize the desired belite structures, there are two ways: by adding certain oxides that act as stabilizers [
23,
24,
25,
26] or rapid cooling. On one hand, β-C
2S is stabilized by the addition of B
2O
3, Na
2O, K
2O, BaO, MnO
2, Cr
2O
3, SO
3 and combinations of them. Also, α- and α’-C
2S are stabilized by using MgO, A1
2O
3, Fe
2O
3, BaO, K
2O, P
2O
5 and Cr
2O
3 [
25]. On the other hand, rapid cooling could be achieved in various ways, such as using water, compressed air, liquid nitrogen and carbon tetrachloride [
27]. However, the use of water as a refrigerant resulted in a reduction in strength of the produced cement, and carbon tetrachloride led to the collapse of the natural structure of the clinker phases [
27,
28]. The most effective cooling method is achieved with the use of liquid nitrogen, but it presents difficulties in its use. Therefore, the most efficient cooling method is the compressed air blowing and simultaneous breaking of the clinker.
Therefore, the cement compositions of this study were fired over 1300 °C, at 1340 °C, in order to form alite in the clinkers. This was confirmed by the performance of preliminary tests. The produced cement was designed to be Portland cement with more than 65% wt. of C3S, in order to avoid low initial strength.
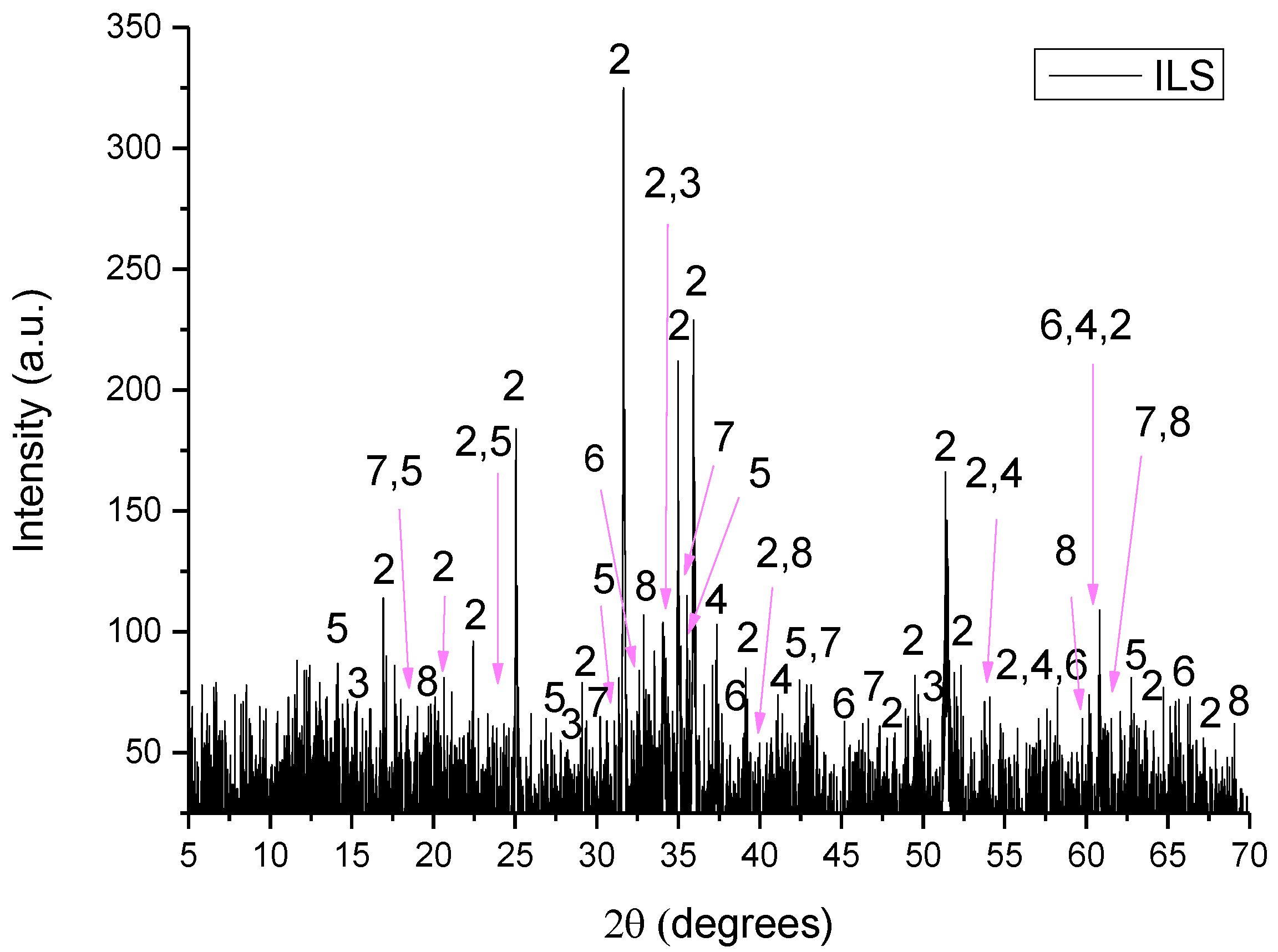
Moreover, this study investigated the reduction in the use of quarry raw materials and their partial replacement with industrial byproducts in the raw meal, along with the reduction of clinkering temperature. The main goal was to produce an alternative Portland cement yielding comparable compressive strength values to commercial ones. Under this prism, two syntheses were designed containing 65% wt. and 71% wt. of alite, and their formation was based on the synergy of conventional raw materials, byproducts and clinkering temperatures. In this case, two different temperatures were studied at 1450 °C and 1340 °C, respectively. Additionally, a commercial Portland clinker (CEM I 42.5N) was also analyzed for comparative reasons. Finally, the used byproducts comprised of Greek Wet Fly Ash (WFA) and Mill Scales, which are rich in SiO
2 and Fe
2O
3 respectively. The type of Mill Scales used in this study are Iron Lamination Scales and are denoted as ILS from this point forward. ILS is a byproduct of the steel production and derives from the process of lamination of iron at high temperatures. The quantity of ILS produced in the steel mills constitutes about 10–15% wt. of the total supply quantity of the kiln, with the result that the metallurgical slags are one of the largest in quantity industrial byproducts [
29]. WFA, on the other hand, is a fine-grained material of spherical glass particles, which is obtained by electrostatic or mechanical precipitation in the exhaust gases of dry combustion furnaces of powdered carbon or lignite, in thermal power plants [
30,
31,
32]. WFA constitutes 85–90% wt. of the total ash produced in the plant and its global production is about 488.5 Mt/y [
33,
34], and in Greece, it amounts to 12 Mt/y [
31].
2. Materials and Methods
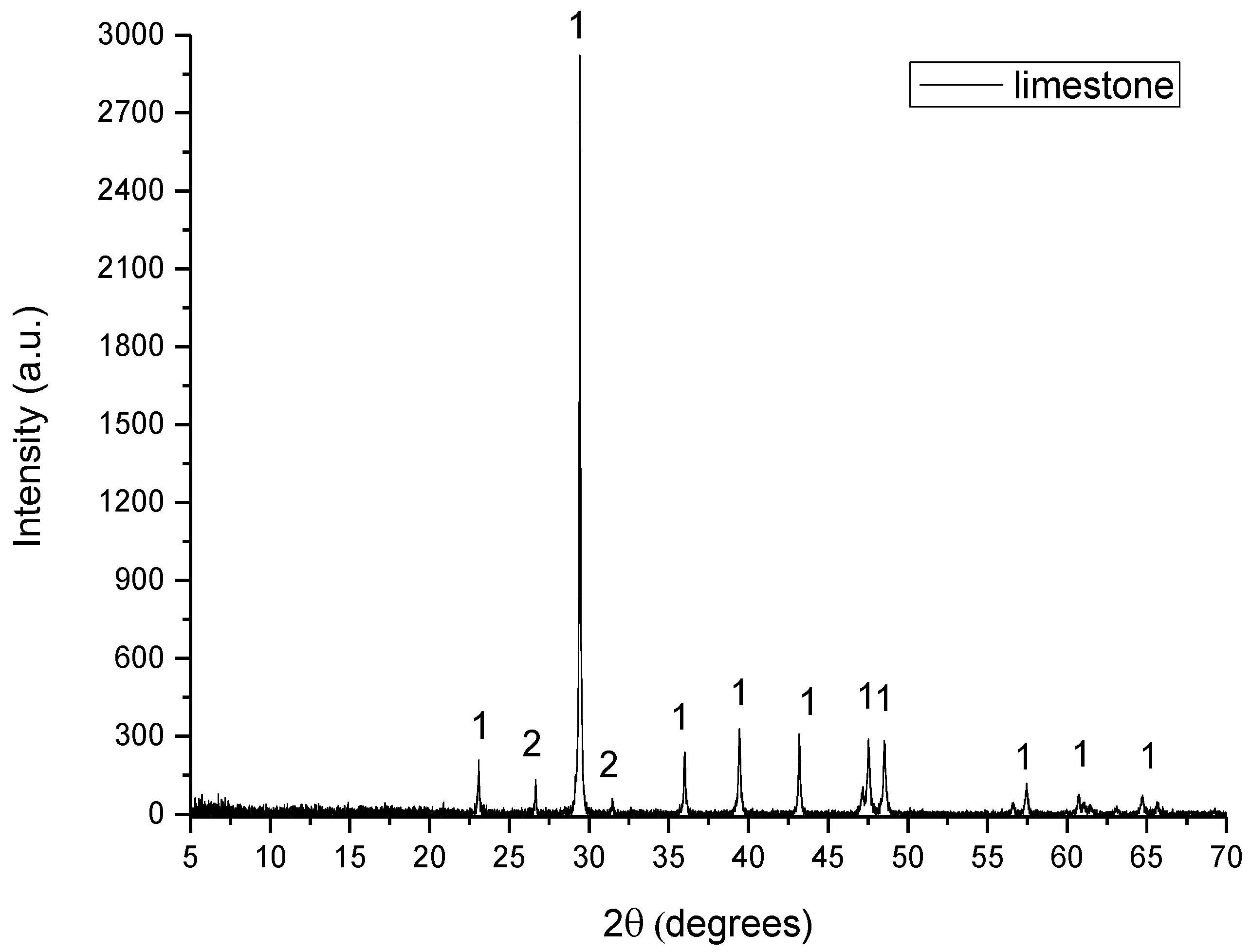
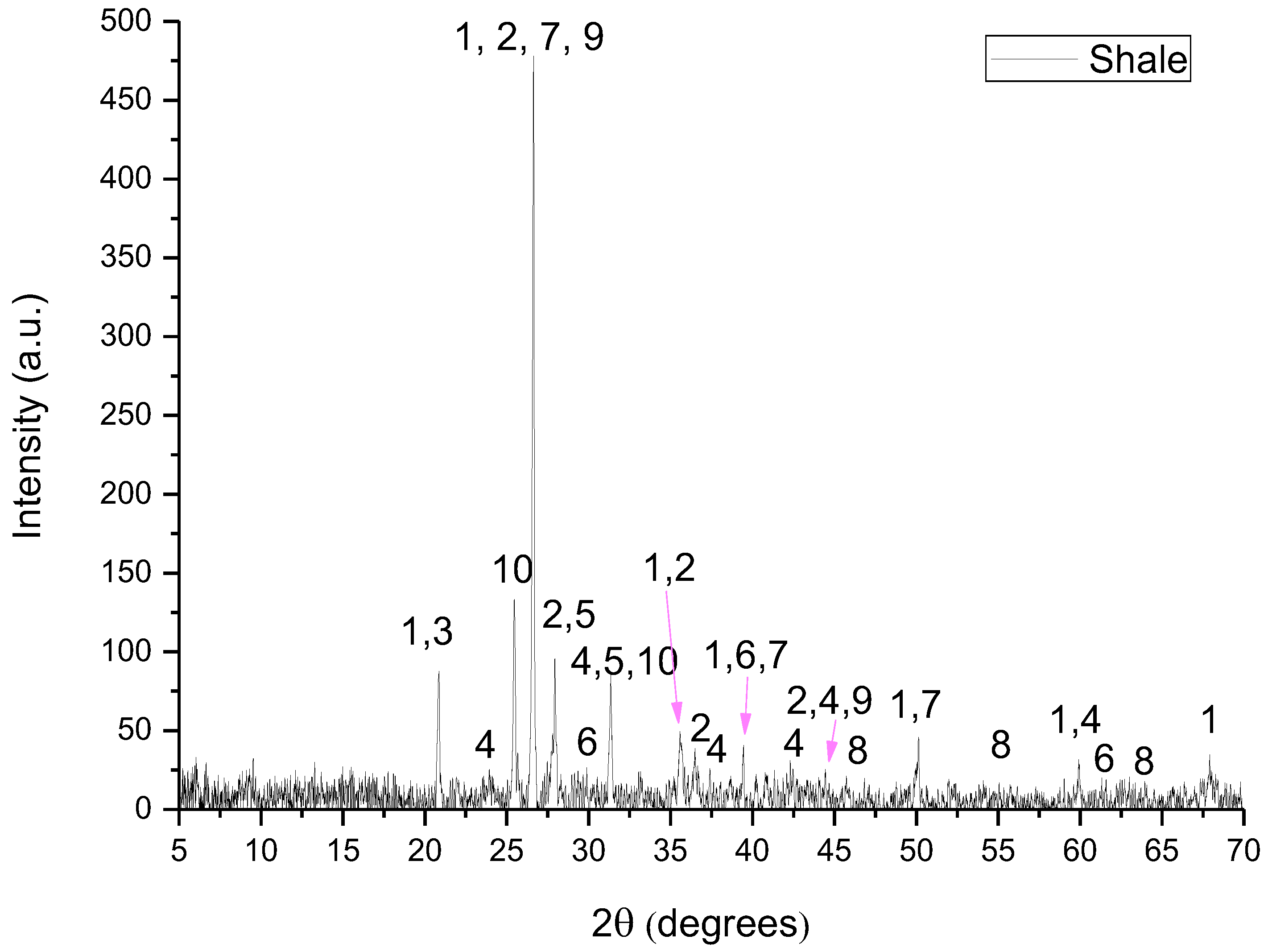
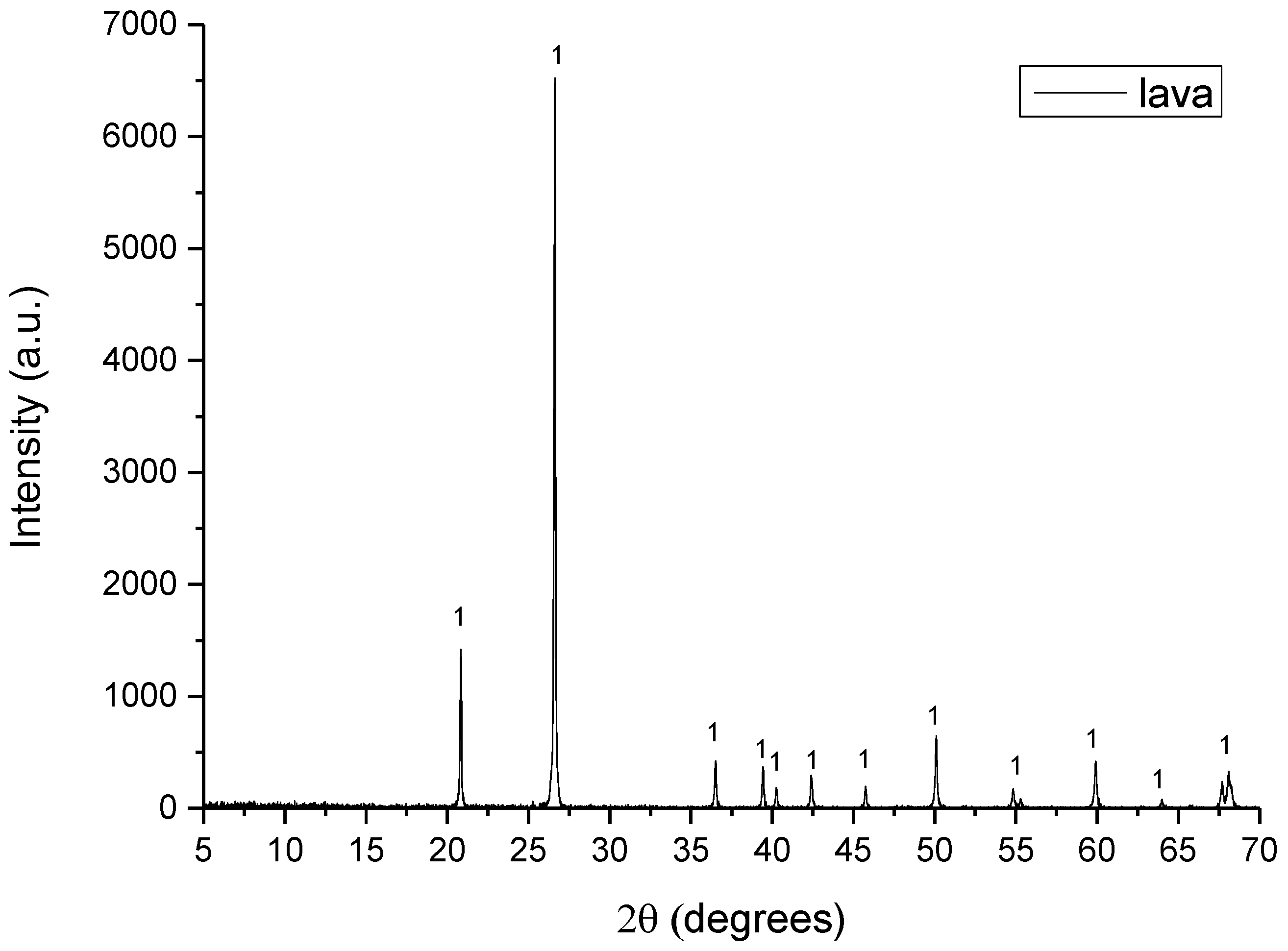
The raw materials used for the preparation of the raw meals were limestone, shale, lava, ILS and WFA. Limestone and shale are quarried raw materials which are typical in cement production due to their oxide content in CaO, SiO2, Al2O3 and Fe2O3. Lava is the solidified form of the magmatic material (Τ = 700–1200 °C). WFA is a byproduct rich in SiO2 and some Al2O3, while ILS is rich in Fe2O3 and some SiO2.
All raw materials were dried at 100 °C, for 24 h and ground in a planetary disc mill (Siebtechnik TS 100, Siemens, Mülheim an der Ruhr, Germany) to achieve particle size less than 90 μm. Their chemical composition was analyzed by XRF (ARL9800XP, Thermo Fisher, Waltham, MA, USA). The Loss On Ignition, LOI, was calculated by heating at 950 °C, according to EN 1744-1: 2009 [
35].
The D8–Advance Bruker instrument, using Cu–Kα1 radiation at 40 kV and 40 mA, was used for XRD analysis. The diffractograms were recorded in the 5–70°, 2θ range, with a 0.01° step size and 1°/min scanning speed. The crystalline phases were identified using DIFFRACplus EVA® software Bruker–AXS based on the ICDD Powder Diffraction File.
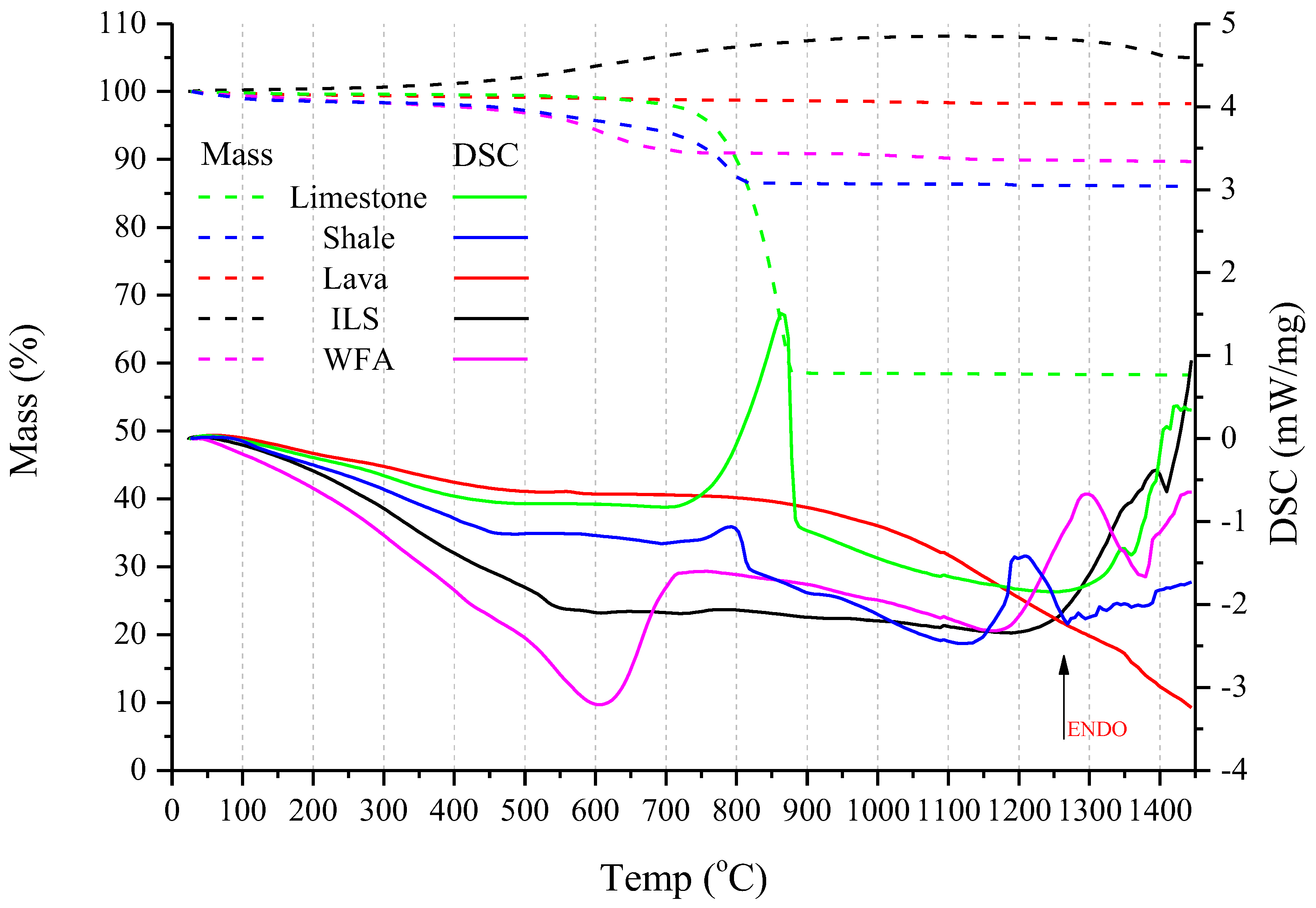
Thermal analysis was performed by Simultaneous TGA/DSC, using a Netzsch (Selb, Deutschland) STA 449F3 with a 10 °C/min heating rate, up to 1450 °C, in a static air, using Al2O3 crucibles.
For this study, two different compositions of Portland clinkers were designed and prepared on a laboratory scale. The clinkers were designed as P
65A with 65% wt. and P
71A with 71% wt. alite, respectively, and the demand on raw materials were calculated for each experiment by means of an MS Excel, based on XRF-LOI analysis and Bogue equations [
3], assuming thermodynamic equilibrium. Then, the ground and sieved <90 μm raw materials were mixed in the calculated ratios. The meals were homogenized and pellets of 12–15 mm diameter were formed by hand with a minimum addition of deionized water. The pellets were dried at 100 °C for over 20 h, then placed in a tailor-made magnesia–chrome refractory crucible (P201) and covered by a lid of the same material. Each of the two compositions was fired in a Super Kanthal resistance furnace of Nabertherm (Lilienthal, Germany) Mod: HT08/17. In order to find the optimum firing temperature and heating profile of the clinkers (P
65A and P
71A), preliminary firing tests were performed at different temperatures and heating profiles. The optimum heating profile, for each temperature (
Figure 1), was determined based on the best clinkerization process which was indicated by the lowest value of free calcium oxide [
36,
37] and the developed microstructure. The evaluation of these findings led to the conclusion that the major phases of clinker can be formed at temperatures lower than the normal of OPC. The heating profile (
Figure 1) followed a heating rate of 16.25 °C/min up to 1000 °C and soaking time for 40 min. However, to reach the 1450 °C and 1340 °C, lower rates were applied to 7.5 °C/min and 8.5 °C/min, respectively. Moreover, soaking time at 1450 °C was 50 min while at 1340 °C was 40 min. To stabilize the α
H– and β–belite polymorphic forms, the clinkers were cooled rapidly by simultaneous application of air and crushing by a hammer [
22,
28,
37,
38,
39].
The obtained clinkers were studied in two forms: as half pieces of the fired pellets for microscopy observations and as powders for all the other tests. Additionally, a commercial clinker, CEM I 42.5N, with the same raw materials, was studied for comparison reasons. The produced clinkers were ground, according to EN 196-6 [
40], for 150 s per 125 g with the planetary disc mill. Tests were, also, performed for the determination of unreacted free lime content, f(CaO), using the ethylene glycol method [
36] and an indicator mixture [
37]. For the determination of the specific weight, the test method described in ASTM C188-95 [
41] was carried out.
The produced powdered clinkers were analyzed by XRF (ARL9800XP, Thermo Fisher, Waltham, MA, USA) and their mineralogical phases of them were determined by Bruker D8-Advance XRD. Diffraction patterns were measured in the 2θ range of 10–70°, using Cu Ka1 radiation of 40 kV and 30 mA, with a 0.01° step size and a scan speed of 1 deg/min. XRD and quantitative Rietveld analysis were performed using TOPAS 5 software. For the accuracy of the results, adjustments have been performed concerning instrument geometry, background, sample displacement, detector type and mass absorption coefficients of the refined phases.
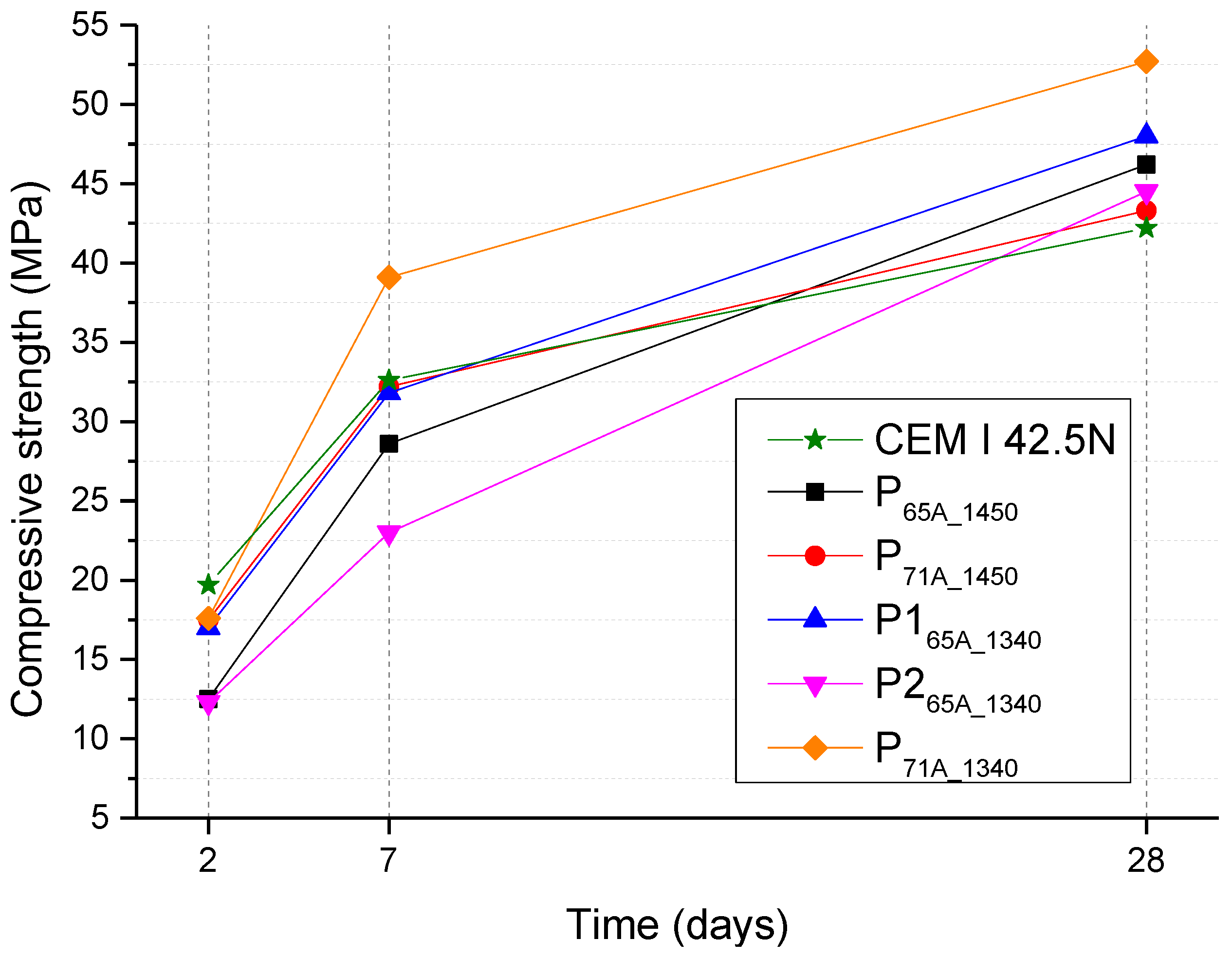
Finally, cement mortar specimens were formed with dimensions of 40 mm × 40 mm × 160 mm, in accordance with the EN 196-1 [
42]. The materials used for their preparation comprised of ground clinkers, 5% wt. gypsum and CEN Standard sand. The water to cement ratio in the concrete mix was 0.5. The curing of the test samples occurred in the molds which were stored in a moist air cabinet at 20 ± 1 °C and 90% humidity for 24 h and after demolding, they were kept apart from each other and submerged in water at 20 ± 1 °C until their test age. Compressive strength tests of the concrete specimens were carried out at 2, 7 and 28 days using a TONICOMP III press (Toni Technik, Berlin, Germany) with a loading rate of 2400 ± 200 N/s.
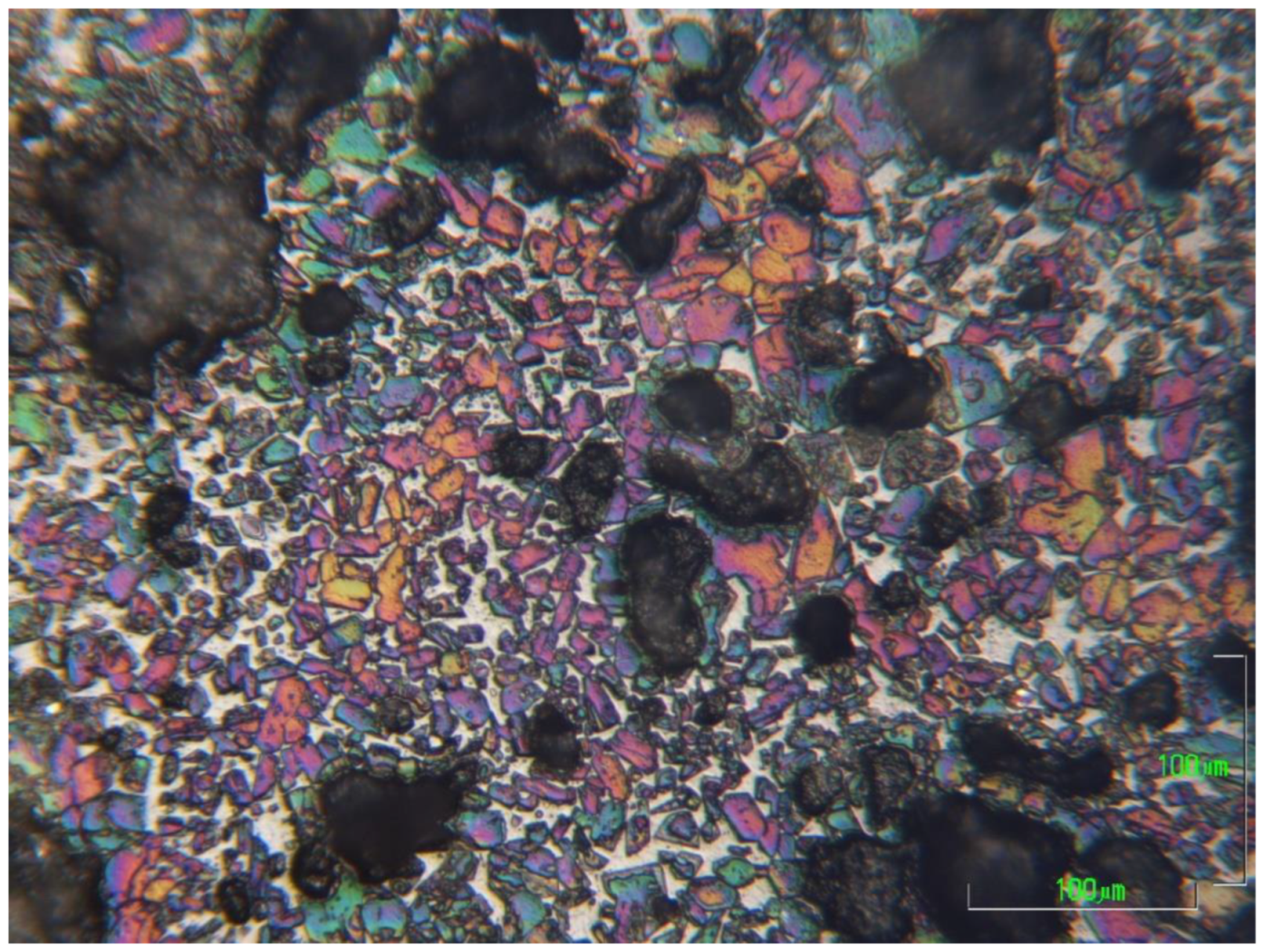
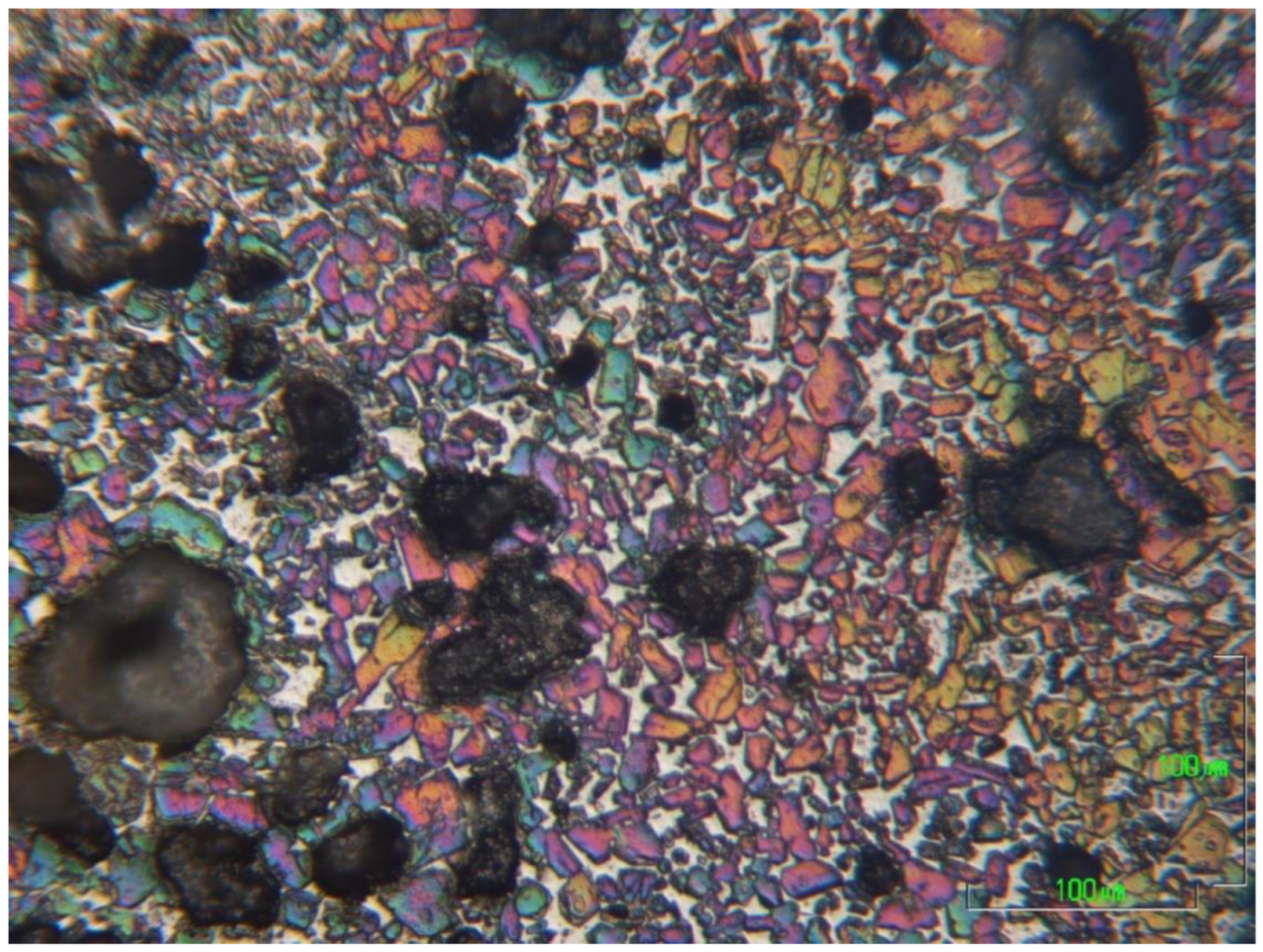
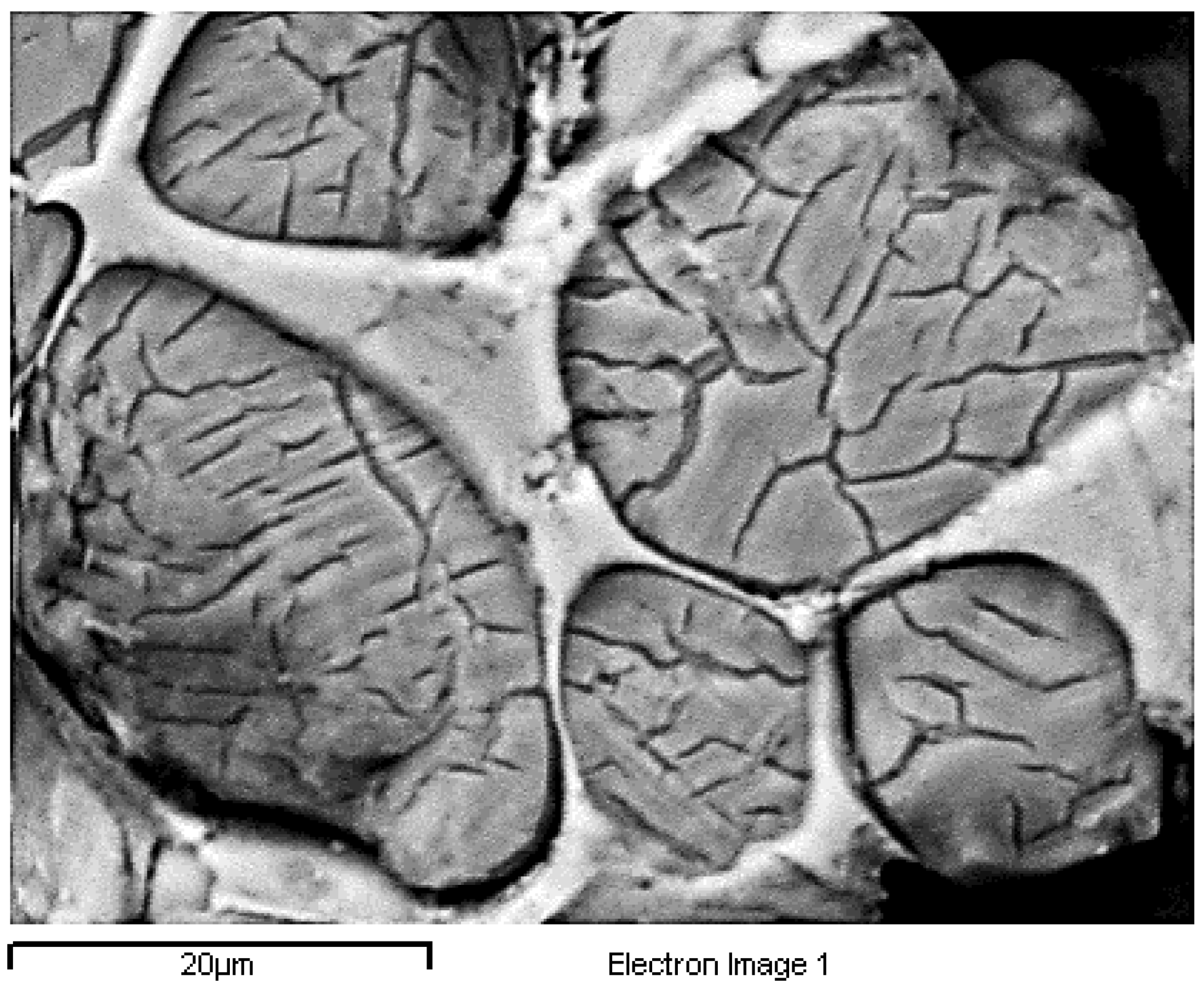
For the optical microscopy, half pieces of the clinkers were mounted in nonconductive resin, by PRONTOPRESS-2 (Struers, CLE, USA). The sample processing included gradual grinding on a rotating wheel (Buehler Metaserv, Motopol 12, Coventry, UK) using SiC abrasive papers from P500 to P4000 and ethylene alcohol (>97%) as a lubricant. Finally, the sample surfaces were etched with 1% Nital [
43,
44] for 5–7 s, depending on the sample, dried by air and immediately observed by optical microscopy (Metalloplan Leitz with attached DS camera head DS–5M Nikon and Camera control unit DS–L1).
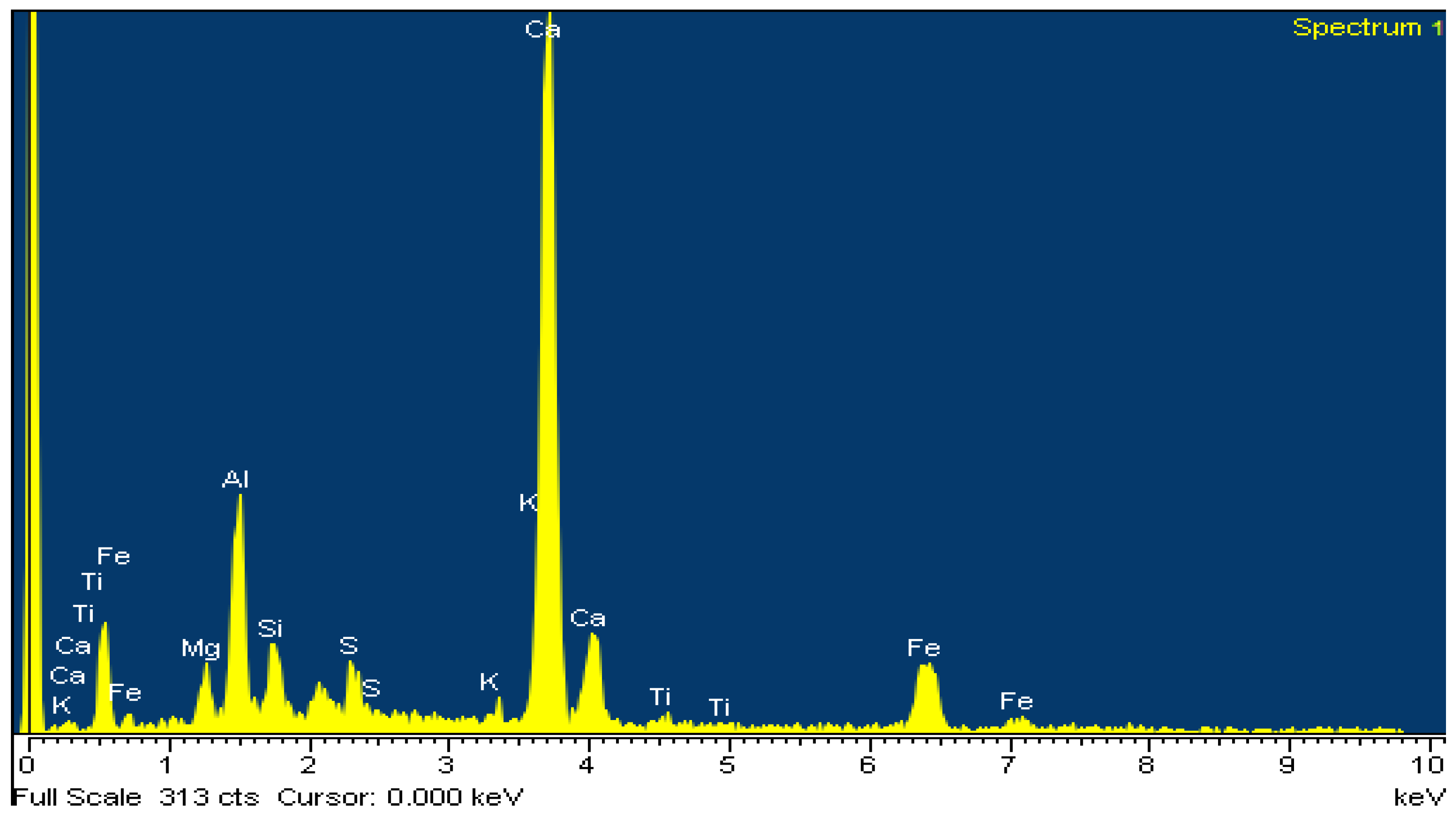
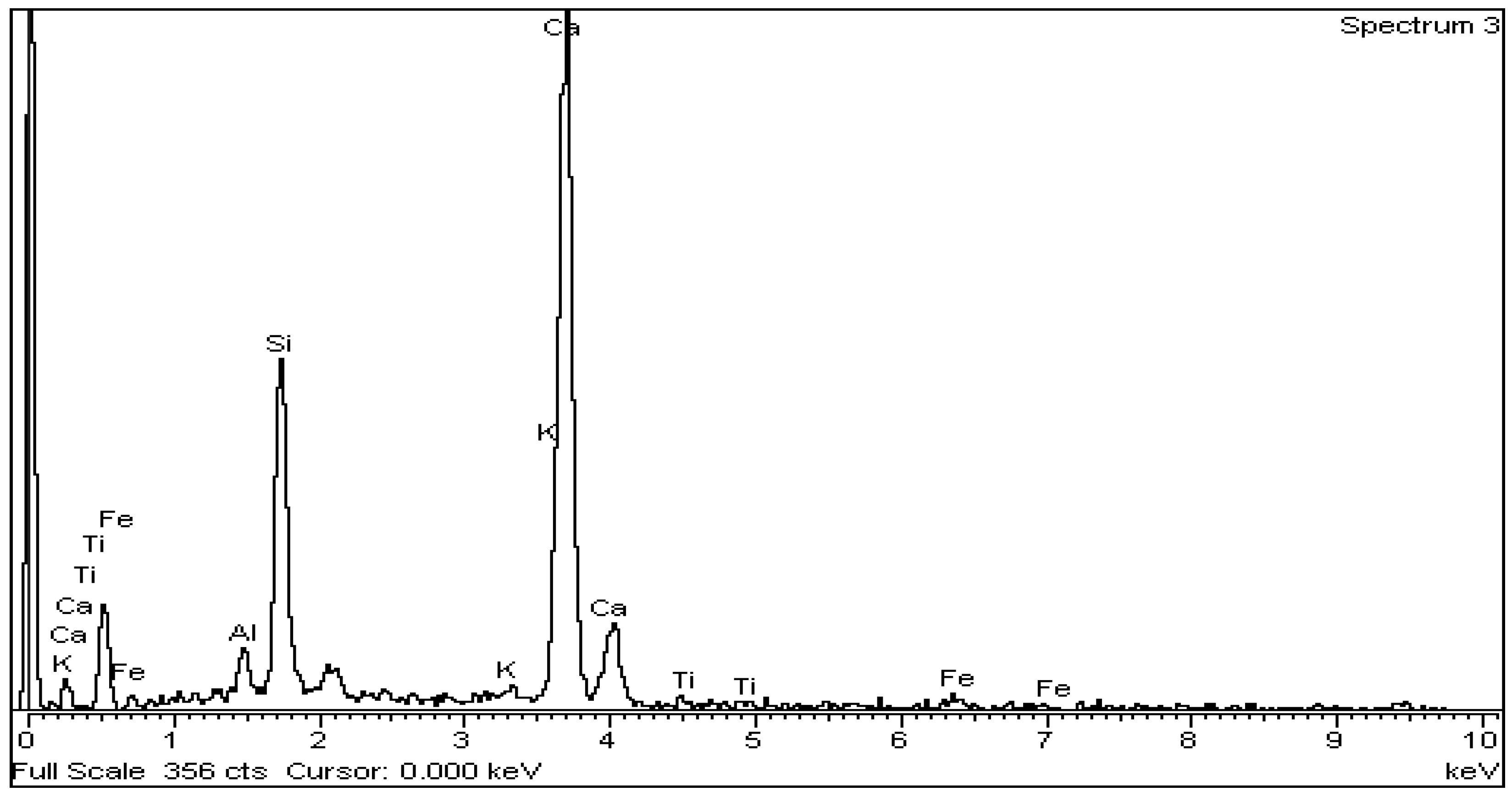
For the microstructural observation, the surfaces were polished again using a diamond paste of 1 μm (DP-Paste M, Struers, CLE, USA) on a synthetic cloth (MD-Nap, Struers, CLE, USA) and DP Lubricant Red (Struers, CLE, USA). The morphology of the microstructure and X-ray microanalysis of carbon-coated surfaces was performed by SEM/EDS (JSM 5600 of Jeol, Tokyo, Japan).