Fluid Properties and Genesis of Dolomites in the Devonian Guanwushan Formation of Upper Yangtze Platform, SW China
Abstract
1. Introduction
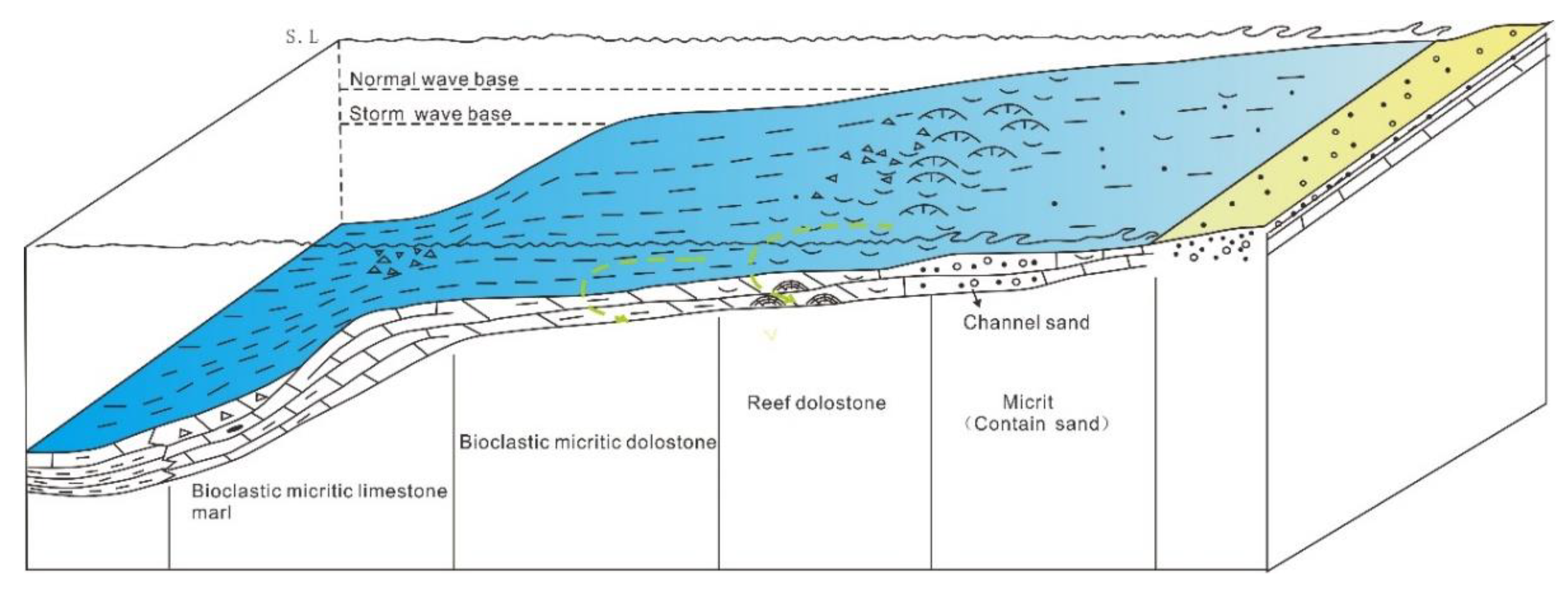
2. Geological Setting
3. Samples and Methods
4. Results
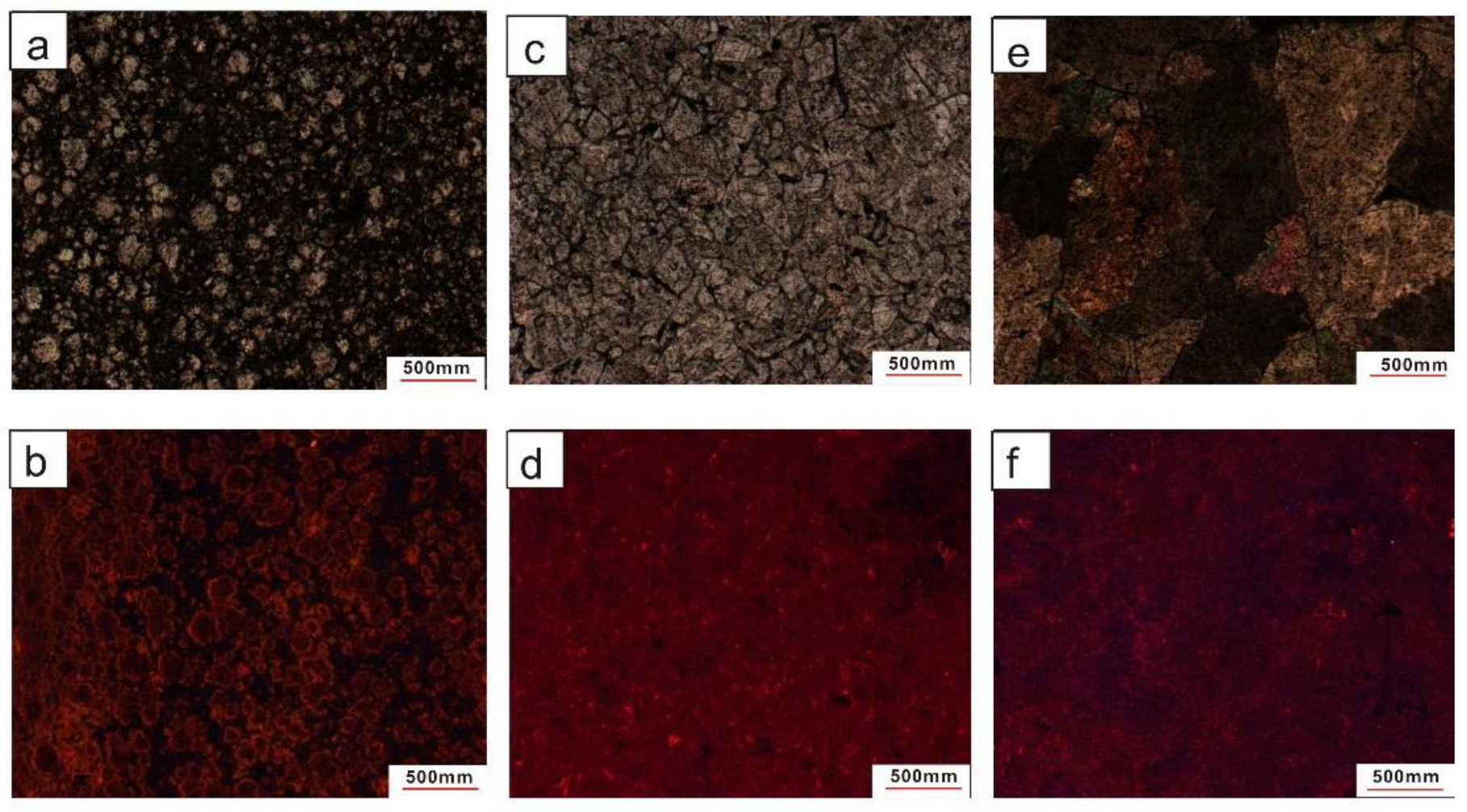
4.1. Petrological and Mineralogical Characteristics of Dolomites
4.1.1. Petrological Characteristic and Classification of Dolomites
Fine Crystalline-Microcrystalline Dolomite (FMD)
Fine Crystalline Dolomite (FCD)
Medium Crystalline Dolomite (MCD)
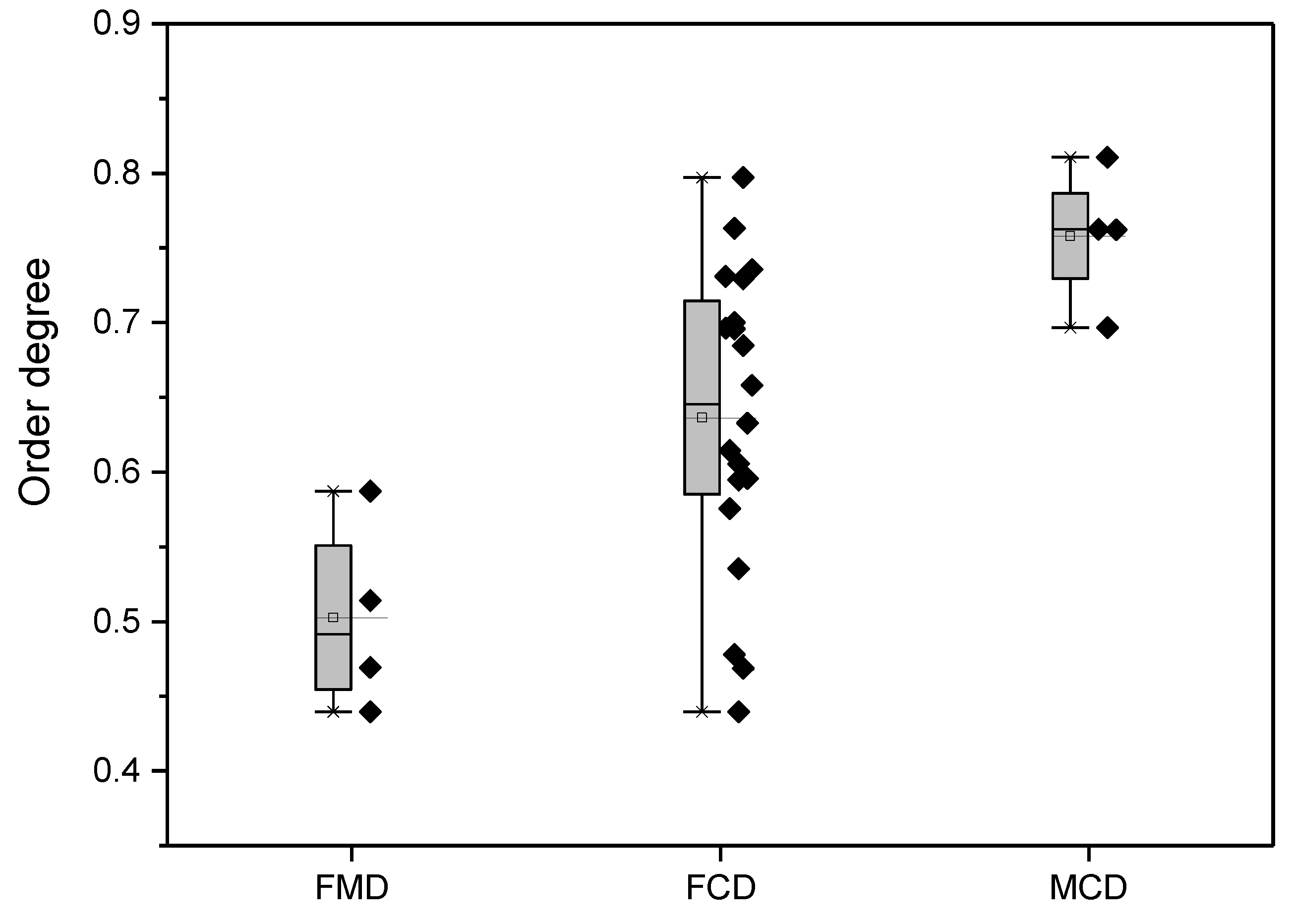
4.1.2. Degree of Order in the Dolomites
4.2. Geochemical Characteristics of Dolomite
4.2.1. Trace Elements
4.2.2. REEs
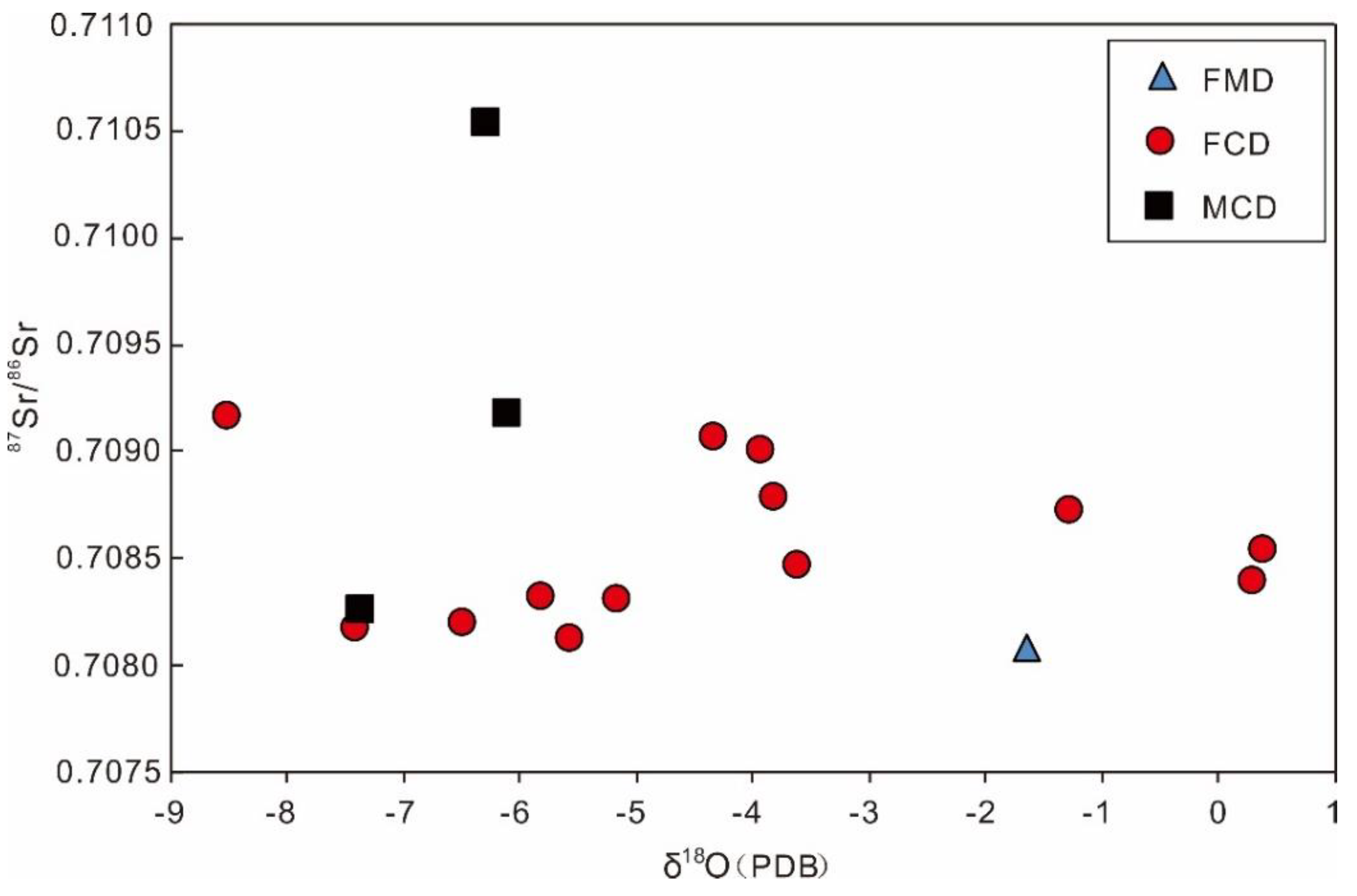
4.2.3. Isotopes of C, O and Sr
5. Discussion
5.1. Dolomitization Fluid’s Properties
5.2. Origin of Dolomite
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, J.K. Dolomite: Occurrence, evolution and economically important associations. Earth Sci. Rev. 2005, 2, 1–81. [Google Scholar] [CrossRef]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Mueller, M.; Igbokwe, O.A.; Walter, B.; Pederson, C.L.; Riechelmann, S.; Richter, D.K.; Albert, R.; Gerdes, A.; Buhl, D.; Neuser, R.D.; et al. Testing the preservation potential of early diagenetic dolomites as geochemical archives. Sedimentology 2020, 67, 849–881. [Google Scholar] [CrossRef]
- He, Y.; Liu, B.; Qin, S. Study on the dolomitization and dolostone genesis. Acta Sci. Nat. Univ. Pekin. 2010, 46, 1010–1020. [Google Scholar]
- Adams, J.E.; Rhodes, M.L. Dolomitization by seepage refluxion. AAPG Bull. 1960, 44, 1912–1920. [Google Scholar]
- Bush, P.R. Some Aspects of the Diagenetic History of the Sabkha in Abu Dhabi, Persian Gulf. In The Persian Gulf; Purser, B.H., Ed.; Springer: New York, NY, USA, 1973; pp. 395–407. [Google Scholar]
- Mckenzie, J.A.; HSü, K.J.; Schneider, J.F. Movement of subsurface waters under the Sabkha, Abu Dhabi, UAE, and its relation to evaporative dolomite genesis. AAPG Bull. 1980, 28, 11–30. [Google Scholar]
- Badiozamani, K. The Dorag dolomitization model-application to the Middle Ordovician of Wiscons. J. Sediment. Petrol. 1973, 43, 965–984. [Google Scholar]
- Meister, P.; Bernasconi, S.M.; Vasconcelos, C.; McKenzie, J.A. Sea-level changes control diagenetic dolomite formation in hemipelagic sediments of the Peru Margin. Mar. Geol. 2008, 252, 166–173. [Google Scholar] [CrossRef]
- Adams, A.; Diamond, L.W.; Aschwanden, L. Dolomitization by hypersaline reflux into dense groundwaters as revealed by vertical trends in strontium and oxygen isotopes: Upper Muschelkalk, Switzerland. Sedimentology 2019, 66, 362–390. [Google Scholar] [CrossRef]
- Matters, B.W.; Mountjoy, E.W. Burial dolomitization of the Upper Devonian Miette buildup, Jasper National Park, Alberta. In Concepts and Models of Dolomitization; Zenger, D.H., Dunham, J.B., Ethington, R.L., Eds.; SEPM Special Publication: Tulsa, OK, USA, 1980; Volume 28, pp. 259–297. [Google Scholar]
- Haedie, L.A. Dolomitization: A critical view of some current views. J. Sediment. Res. 1987, 57, 166–183. [Google Scholar]
- Montaenz, I.P. Late diagenetic dolomitization of Lower Ordovician, Upper Knox Carbonates: A record of the hydrodynamic evolution of the southern Appalachian Basin. AAPG Bull. 1994, 78, 1210–1239. [Google Scholar]
- Vahrenkamp, V.C.; Swart, P.K. Late Cenozoic Dolomites of the Bahamas: Metastable Analogues for the Genesis of Ancient Platform Dolomites; Springer: Berlin/Heidelberg, Germany, 1994; Volume 21, pp. 133–153. [Google Scholar]
- Davies, G.R.; Smith, L.B., Jr. Structurally controlled hydrothermal dolomite reservoir facies: An overview. AAPG Bull. 2006, 90, 1641–1690. [Google Scholar] [CrossRef]
- Zheng, H.F.; Ma, Y.S.; Chi, G.Y.; Qing, H.R.; Liu, B.; Zhang, X.F.; Sheng, Y.C.; Liu, J.Q.; Wang, Y.C. Stratigraphic and Structural Control on Hydrothermal Dolomitization in the Middle Permian Carbonates, Southwestern Sichuan Basin (China). Minerals 2019, 9, 32. [Google Scholar] [CrossRef]
- Chen, P.; Fu, M.Y.; Deng, H.C.; Xu, W.; Wu, D.; He, P.W.; Guo, H.W. The Diagenetic Alteration of the Carbonate Rocks from the Permian Qixia Formation as Response to Two Periods of Hydrothermal Fluids Charging in the Central Uplift of Sichuan Basin, SW China. Minerals 2021, 11, 1212. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Mckenzie, J.A.; Bernasconi, S.; Grujic, D.; Tien, A.J. Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Sánchez-Román, M. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882. [Google Scholar] [CrossRef]
- Petrasha, D.A.; Bialik, O.M.; Bontognali, T.R.R.; Vasconcelos, C.; Roberts, J.A.; McKenzie, J.A.; Konhauser, K.O. Microbially catalyzed dolomite formation: From near-surface to burial. Earth-Sci. Rev. 2017, 171, 558–582. [Google Scholar] [CrossRef]
- Vandeginste, V.; Snell, O.; Hall, M.R.; Steer, E.; Vandeginste, A. Acceleration of dolomitization by zinc in saline waters. Nat. Commun. 2019, 10, 1851. [Google Scholar] [CrossRef]
- Mei, M. Brief introduction of “dolostone problem” in sedimentology according to three scientific ideas. J. Palaeogeogr. 2006, 14, 1–12. [Google Scholar]
- Wang, Z.F.; Huang, K.K.; Zhang, D.J.; You, L.; Liu, X.Y.; Luo, W. Maturation of Neogene dolomite from Xuande Atoll of Xisha archipelago, the South China Sea. Mar. Pet. Geol. 2018, 92, 51–64. [Google Scholar] [CrossRef]
- Ning, M.; Lang, X.G.; Huang, K.J.; Li, C.; Huang, T.Z.; Yuan, H.L.; Xing, C.C.; Yang, R.Y.; Shen, B. Towards understanding the origin of massive dolostones. Earth Planet. Sci. Lett. 2020, 545, 116403. [Google Scholar] [CrossRef]
- Tang, J.; Dietzel, M.; Böhm, F.; Köhler, S.J.; Eisenhauer, A. Sr2+/Ca2+ and 44Ca/40Ca fractionation during inorganic calcite formation: II. Ca isotopes. Geochim. Cosmochim. Acta 2008, 72, 3733–3745. [Google Scholar] [CrossRef]
- Gussone, N.; Schmitt, A.D.; Heuser, A.; Wombacher, F.; Dietzel, M.; Tipper, E.; Schiller, M. Calcium Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Murray, S.T.; Swart, P.K. Evaluating formation fluid models and calibrations using clumped isotope paleothermometry on Bahamian dolomites. Geochim. Cosmochim. Acta 2017, 206, 73–93. [Google Scholar] [CrossRef]
- Polyak, V.; Provencio, P.; Asmerom, Y. U-Pb dating of speleogenetic dolomite: A new sulfuric acid speleogenesis chronometer. Int. J. Speleol. 2016, 45, 103–109. [Google Scholar] [CrossRef]
- Manche, C.J.; Kaczmarek, S.E. Evaluating reflux dolomitization using a novel high-resolution record of dolomite stoichiometry: A case study from the Cretaceous of central Texas, USA. Geology 2019, 47, 586–590. [Google Scholar] [CrossRef]
- Gong, D.M. Conodonts from the top of Guanwushan formation of devonian system in Majiaoba. J. Chengdu Inst. Geol. 1990, 17, 10–17. [Google Scholar]
- Xian, S.Y.; Chen, J.R.; Wan, Z.Q. Devonian ecostratigraphy, sequence stratigraphy and sea-level changes in Ganxi, Longmen Mountain area, Sichuan. Sediment. Facies Palaeogeogr. 1995, 15, 1–47. [Google Scholar]
- Liu, W. Devonian sequence stratigraphy and relative sea level changes in Longmenshan Area, Sichuan. J. China Univ. Geosci. 1996, 7, 60–70. [Google Scholar]
- Zheng, R.C.; Liu, W.J.; Li, X.H.; Chen, Y.R.; Wang, H.F. The genetic types and their sequence stratigraphic significances of Devonian dolostones in Longmen Mountains. J. Miner. Petrol. 1996, 16, 28–37. [Google Scholar]
- Zheng, R.; Liu, W. Depositional systems and cyclic sequences of Pin Yipu Formation Longmenshan area. Acta Sedimentol. Sin. 1997, 15, 1–7. [Google Scholar]
- Hou, H.F.; Wan, Z.Q.; Xian, S.Y. Devonian Stratigraphy, Paleontology and Sedimentary Faceis of Longmenshan, Sichuan; Geological Publishng House: Beijing, China, 1988. (In Chinese) [Google Scholar]
- Chen, H.G.; Li, X.Q.; Yuan, H.L.; Huang, S.J.; Wang, K.Z. Carbon and oxygen isotope variations of Devonian seawater: Isotopic records from brachiopod shell. Acta Geosci. Sin. 2009, 30, 79–88. [Google Scholar]
- Pang, Y.J.; Zhang, B.J.; Feng, R.W.; Wang, Y.R. Evolution of Devonian depositional environment in northern Longmenshan tectonic belt. Glob. Geol. 2010, 29, 561–568. [Google Scholar]
- Zeng, Y.; Huang, S.; Kulle, H.; Schönfeld, M. The correlation between the dolomites and their forming conditions in the Guanwushan Formation (Middle Devonian) Ganxi, Sichuan, China. Acta Sedimentol. Sin. 1988, 6, 12–23. [Google Scholar]
- Huang, S.J. The Cathodoluminescence and diagenesis of the carbonates of Guanwushan formation, Middle Devonian, Ganxi, Northwestern Sichuan. J. Chendu Univ. Technol. (Sci. Technol. Ed.) 1988, 15, 50–58. [Google Scholar]
- Deng, Y.W.; Hou, M.C.; Chen, A.Q.; Ma, H.L.; Dong, Y.X.; Luo, W.; Huang, S.G. Ree Characteristics of Dolomites and Indication of Dolomitization Fluid in Guanwushan Formation, NW Sichuan Basin, China. J. Chendu Univ. Technol. (Sci. Technol. Ed.) 2018, 45, 282–291. [Google Scholar]
- Huang, S.G.; Hou, M.C.; Xu, S.L.; Chen, A.Q.; Zhang, B.J.; Luo, W.; Chao, H.; Cai, P.C.; Deng, Y.W. Dolomitization Fluid and Genesis of Dolomite in the Devonian Guanwushan Formation in Upper Yangtze. In Advances in Science, Technology & Innovation; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Wen, L.; Ma, H.; Li, Y.; Zhang, B. Natural gas exploration prospect in the Upper Paleozoic strata, NE Sichuan Basin. Nat. Gas Ind. 2016, 36, 11–21. [Google Scholar]
- Sibley, D.F.; Gregg, J.M. Classification of dolomite rock textures. Sediment. Petrol. 1987, 57, 967–975. [Google Scholar]
- Hu, W.X.; Chen, Q.; Wang, X.L.; Cao, J. REE models for the discrimination of fluids in the formation and evolution of dolomite reservoirs. Oil Gas Geol. 2010, 31, 810–818. [Google Scholar]
- Liu, J.Q.; Lin, J.S.; Feng, W.M.; Zhao, Z.; Huang, X.P. The REE geochemical characteristics of Middle–Upper Cambrian dolomite in Southeast Sichuan Basin and its significance: A case study of Changshutian profile in Bijie, Guizhou. Miner. Petrol. 2014, 34, 87–94. [Google Scholar]
- Qing, H.; Mountjoy, E.W. Rare earth element geochemistry of dolomites in the Middle Devonian Presqu’ile barrier Westerm Canada Sedimentary Basin: Implications for fluid-rock ratios during dolomitization. Sedimentology 1994, 41, 787–804. [Google Scholar] [CrossRef]
- Webb, G.E.; Nothdurft, L.D.; Kamber, B.S.; Kloprogge, J.T.; Zhao, J.X. Rare earth element geochemistry of scleractinian coral skeleton during meteoric diagenesis: A sequence through neomorphism of aragonite to calcite. Sedimentology 2009, 56, 1433–1463. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Kawabe, I. Convex tetrad effect variations in REE abundances of “North American shale composite” and “Post-Archean Australian average shale”. Geochem. J. 1996, 30, 149–153. [Google Scholar] [CrossRef]
- Huang, S.J. A study on carbon and strontium isotopes of late Paleozoic carbonate rocks in the Upper Yangtze platform. Acta Geol. Sin. 1997, 1, 45–53. [Google Scholar]
- Lu, W.C.; Cui, B.Q.; Yang, S.Q.; Zhang, P. Isotope stratigraphic curves of Devonian marine carbonates in Ganqi profile. Acta Sedimentol. Sin. 1994, 3, 12–20. [Google Scholar]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.A.; Diener, A.; Ebneth, S.; Godderis, Y.J. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar] [CrossRef]
- Mazzullo, S.J.; Bischoff, W.D.; Teal, C.S. Holocene shallow subtidal dolomitization by near-normal seawater, northern Belize. Geology 1995, 23, 341–344. [Google Scholar] [CrossRef]
- Sabagh, B.M.; Mahboubi, A.; Al-Aasm, I.; Moussavi-Harami, R.; Nadjafi, M. Multistage dolomitization in the Qal’eh Dokhtar formation (Middle-Upper Jurassic), Central Iran: Petrographic and geochemical evidence. Geol. J. 2018, 53, 22–44. [Google Scholar] [CrossRef]
- Cander, H.S. An example of mixing-zone dolomite, middle Eocene Avon park formation, floridian Aquifer system. J. Sediment. Res. 1994, 64, 615–629. [Google Scholar]
- Harrison, W.J.; Summa, L.L. Paleohydrology of the gulf of Mexico basin. Am. J. Sci. 1991, 291, 109–176. [Google Scholar] [CrossRef]
- Mahboubi, A.; Nowrouzi, Z.; Al-Aasm, I.S.; Moussavi-Harami, R. Dolomitization of the silurian niur formation, tabas block, east central Iran: Fluid flow and dolomite evolution. Mar. Petrol. Geol. 2016, 77, 791–805. [Google Scholar] [CrossRef]
- Machel, H.G.; Mountjoy, E.W.; Jones, G.D.; Rostron, B.J. Toward a sequence stratigraphic framework for the Frasnian of the Western Canada Basin. Bull. Can. Pet. Geol. 2002, 50, 332–338. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, M.; Yuan, Y.; Zhao, Y.; Shan, J.; He, Z.; Tian, Y.; Hu, S. Palaeogeothermal response and record of the effusing of Emeishan basalts in the Sichuan basin. Chin. Sci. Bull. 2010, 55, 949–956. [Google Scholar] [CrossRef]
- Al-Aasm, I.S.; Packard, J.J. Stabilization of early-formed dolomite: A tale of divergence from two Mississippian dolomites. Sediment. Geol. 2000, 131, 97–108. [Google Scholar] [CrossRef]





| Sample | Lithology | Section/Well | Mn(ppm) | Fe(ppm) | Sr(ppm) | Mn/Sr | δ13C (‰ VPDB) | δ18O (‰ VPDB) | 87Sr/86 Sr (±2σ) | Order Degree |
|---|---|---|---|---|---|---|---|---|---|---|
| HJL8-4 | Lime | HJL | 85.53 | 6766.13 | 254.98 | 0.335 | −0.49 | −6.33 | 0.708572 ± 19 | |
| MJB-12 | Lime | MJB | 49.87 | 2993.39 | 133.78 | 0.373 | −2.18 | −5.65 | / | |
| HJL9-3 | FMD | HJL | 83.13 | 3791.38 | 98.24 | 0.85 | 3.36 | −2.12 | / | 0.514 |
| MJB-05 | FMD | MJB | 95.16 | 2165.04 | 62.06 | 1.53 | 1.51 | −5.22 | / | / |
| GX-13 | FMD | GX | 102.76 | 3313.75 | 99.70 | 1.03 | 2.40 | −1.04 | / | 0.587 |
| GX-14 | FMD | GX | 55.70 | 2003.07 | 171.41 | 0.32 | 2.45 | −0.59 | / | 0.469 |
| GX-15 | FMD | GX | 73.54 | 2511.23 | 172.28 | 0.43 | 2.31 | −1.65 | 0.708086 ± 17 | 0.440 |
| 7574.8 | FMD | ST1 | / | / | / | 0.96 | −3.77 | / | / | |
| HJL9-1 | FCD | HJL | 316.72 | 5957.34 | 162.47 | 1.95 | 0.95 | −5.82 | 0.708335 ± 16 | 0.575 |
| HJL10-2 | FCD | HJL | 139.34 | 2913.00 | 136.35 | 1.02 | 0.08 | −1.29 | 0.708734 ± 18 | 0.595 |
| HJL11-3 | FCD | HJL | 779.16 | 3576.71 | 91.15 | 8.55 | −0.34 | −5.58 | 0.708143 ± 13 | 0.685 |
| HJL13-2 | FCD | HJL | 97.23 | 1871.05 | 53.42 | 1.82 | 2.62 | −7.44 | 0.708187 ± 14 | 0.797 |
| HJL13-7 | FCD | HJL | 79.41 | 6328.27 | 115.64 | 0.69 | 1.55 | −4.34 | 0.709076 ± 19 | 0.606 |
| HJL15-1 | FCD | HJL | 87.20 | 5543.94 | 127.42 | 0.68 | 1.83 | 0.28 | 0.708408 ± 16 | 0.535 |
| HJL16-1 | FCD | HJL | 599.45 | 14495.13 | 137.37 | 4.36 | −1.34 | 1.58 | / | 0.440 |
| HJL17-1 | FCD | HJL | 70.71 | 9136.83 | 122.30 | 0.58 | 2.24 | 0.37 | 0.708557 ± 18 | 0.469 |
| HJL18-1 | FCD | HJL | 383.05 | 4942.74 | 91.81 | 4.17 | 0.71 | −5.51 | / | 0.730 |
| HJL18-2B | FCD | HJL | 207.84 | 13497.81 | 102.43 | 2.03 | 0.29 | −5.98 | / | 0.696 |
| HJL18-4 | FCD | HJL | 174.69 | 7556.98 | 115.37 | 1.51 | 1.55 | 0.19 | / | 0.478 |
| HJL19-2 | FCD | HJL | 252.65 | 8208.77 | 127.39 | 1.98 | 0.29 | 0.11 | / | 0.700 |
| HJL21-1 | FCD | HJL | / | / | / | / | / | / | 0.70838 ± 14 | |
| MJB-02 | FCD | MJB | 119.02 | 2035.54 | 69.36 | 1.72 | 1.71 | −4.41 | / | 0.763 |
| MJB-04 | FCD | MJB | 147.87 | 2378.02 | 87.93 | 1.68 | 2.23 | −5.18 | 0.708320 ± 15 | 0.596 |
| MJB-09 | FCD | MJB | 131.56 | 3170.95 | 91.93 | 1.43 | 0.39 | −3.63 | 0.708478 ± 17 | 0.736 |
| MJB-11 | FCD | MJB | 110.94 | 2027.57 | 57.34 | 1.93 | 0.26 | −6.51 | 0.708217 ± 18 | 0.614 |
| GX-06 | FCD | GX | 98.66 | 2010.18 | 54.23 | 1.82 | 1.64 | −9.09 | / | 0.633 |
| GX-08 | FCD | GX | 80.45 | 1568.42 | 45.94 | 1.75 | 2.29 | −9.48 | / | 0.731 |
| GX-10 | FCD | GX | 101.10 | 2583.03 | 63.69 | 1.59 | 3.98 | −8.53 | 0.709172 ± 19 | 0.658 |
| 7572.04 | FCD | ST1 | / | / | / | / | 2.45 | −4.15 | / | / |
| 7578.13 | FCD | ST1 | / | / | / | / | 0.97 | −3.97 | / | / |
| 7579.73 | FCD | ST1 | / | / | / | / | −1.20 | −3.94 | 0.709012 ± 17 | / |
| 7582.68 | FCD | ST1 | / | / | / | / | −2.56 | −3.78 | / | 0.696 |
| 7588.5 | FCD | ST1 | / | / | / | / | −1.65 | −3.84 | 0.708798 ± 15 | / |
| HJL12-2 | MCD | HJL | 221.46 | 4286.53 | 82.79 | 2.67 | 1.66 | −6.21 | / | 0.811 |
| HJL13-3A | MCD | HJL | 96.90 | 5064.68 | 80.90 | 1.20 | 2.42 | −6.12 | 0.709193 ± 16 | 0.762 |
| HJL13-5 | MCD | HJL | 124.95 | 2162.66 | 68.66 | 1.82 | 2.24 | −7.39 | 0.708267 ± 19 | 0.697 |
| HJL14-1 | MCD | HJL | 473.72 | 2690.19 | 89.44 | 5.30 | 1.96 | −6.30 | 0.710546 ± 13 | 0.763 |
| Sample | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HJL8-4 | 4.433 | 9.415 | 1.170 | 4.431 | 1.150 | 0.217 | 1.058 | 0.158 | 0.918 | 0.180 | 0.481 | 0.067 | 0.415 | 0.061 | 7.840 |
| MJB-12 | 1.287 | 2.606 | 0.314 | 1.152 | 0.250 | 0.040 | 0.206 | 0.030 | 0.173 | 0.032 | 0.089 | 0.014 | 0.095 | 0.015 | 1.549 |
| HJL9-3 | 2.501 | 4.317 | 0.450 | 1.647 | 0.400 | 0.065 | 0.338 | 0.048 | 0.294 | 0.064 | 0.180 | 0.028 | 0.177 | 0.026 | 3.622 |
| MJB-05 | 0.360 | 0.613 | 0.068 | 0.231 | 0.055 | 0.009 | 0.045 | 0.007 | 0.043 | 0.009 | 0.026 | 0.004 | 0.025 | 0.004 | 0.586 |
| GX-13 | 3.782 | 6.513 | 0.708 | 2.609 | 0.680 | 0.121 | 0.604 | 0.090 | 0.526 | 0.099 | 0.274 | 0.039 | 0.235 | 0.033 | 8.123 |
| GX-14 | 4.600 | 8.314 | 0.987 | 3.624 | 0.905 | 0.168 | 0.843 | 0.120 | 0.696 | 0.133 | 0.347 | 0.048 | 0.305 | 0.043 | 9.116 |
| GX-15 | 4.308 | 8.009 | 0.886 | 3.257 | 0.826 | 0.151 | 0.774 | 0.109 | 0.636 | 0.122 | 0.321 | 0.046 | 0.280 | 0.039 | 8.941 |
| HJL9-1 | 3.995 | 8.229 | 0.940 | 3.353 | 0.781 | 0.136 | 0.690 | 0.099 | 0.573 | 0.115 | 0.320 | 0.048 | 0.310 | 0.045 | 5.644 |
| HJL10-2 | 3.133 | 5.916 | 0.663 | 2.512 | 0.645 | 0.092 | 0.564 | 0.084 | 0.498 | 0.103 | 0.290 | 0.042 | 0.262 | 0.038 | 5.118 |
| HJL11-3 | 3.127 | 6.107 | 0.652 | 2.386 | 0.605 | 0.100 | 0.567 | 0.080 | 0.471 | 0.090 | 0.241 | 0.032 | 0.195 | 0.028 | 6.812 |
| HJL13-2 | 3.523 | 6.248 | 0.675 | 2.607 | 0.670 | 0.116 | 0.634 | 0.095 | 0.577 | 0.113 | 0.311 | 0.043 | 0.251 | 0.034 | 10.655 |
| HJL13-7 | 5.945 | 10.872 | 1.269 | 4.810 | 1.212 | 0.215 | 1.011 | 0.155 | 0.895 | 0.170 | 0.431 | 0.060 | 0.346 | 0.048 | 12.389 |
| HJL15-1 | 6.307 | 10.989 | 1.158 | 4.331 | 1.101 | 0.188 | 0.950 | 0.137 | 0.797 | 0.155 | 0.408 | 0.058 | 0.348 | 0.050 | 11.068 |
| HJL16-1 | 20.029 | 37.769 | 4.197 | 16.199 | 4.086 | 0.791 | 4.276 | 0.674 | 4.167 | 0.803 | 2.125 | 0.301 | 1.843 | 0.264 | 41.310 |
| HJL17-1 | 7.906 | 14.217 | 1.559 | 5.317 | 1.194 | 0.190 | 1.012 | 0.146 | 0.851 | 0.171 | 0.453 | 0.069 | 0.478 | 0.073 | 8.417 |
| HJL18-1 | 2.484 | 4.317 | 0.434 | 1.535 | 0.371 | 0.063 | 0.340 | 0.051 | 0.310 | 0.060 | 0.166 | 0.024 | 0.155 | 0.023 | 4.754 |
| HJL18-2B | 5.614 | 10.273 | 1.134 | 4.163 | 1.001 | 0.184 | 0.950 | 0.142 | 0.837 | 0.158 | 0.419 | 0.060 | 0.363 | 0.052 | 11.552 |
| HJL18-4 | 8.459 | 15.028 | 1.633 | 6.064 | 1.537 | 0.274 | 1.488 | 0.217 | 1.253 | 0.247 | 0.681 | 0.097 | 0.586 | 0.081 | 19.178 |
| HJL19-2 | 10.468 | 20.141 | 2.179 | 7.319 | 1.663 | 0.258 | 1.331 | 0.188 | 1.097 | 0.220 | 0.599 | 0.091 | 0.635 | 0.096 | 9.173 |
| MJB-02 | 0.596 | 1.127 | 0.130 | 0.464 | 0.118 | 0.020 | 0.106 | 0.015 | 0.093 | 0.019 | 0.054 | 0.008 | 0.051 | 0.007 | 1.342 |
| MJB-04 | 1.610 | 3.069 | 0.329 | 1.190 | 0.290 | 0.050 | 0.269 | 0.039 | 0.237 | 0.048 | 0.128 | 0.018 | 0.118 | 0.017 | 3.493 |
| MJB-09 | 1.369 | 2.811 | 0.313 | 1.079 | 0.250 | 0.040 | 0.211 | 0.030 | 0.171 | 0.032 | 0.081 | 0.012 | 0.076 | 0.011 | 1.597 |
| MJB-11 | 0.586 | 1.102 | 0.124 | 0.432 | 0.101 | 0.016 | 0.090 | 0.013 | 0.075 | 0.014 | 0.037 | 0.005 | 0.033 | 0.004 | 0.828 |
| GX-06 | 3.521 | 6.006 | 0.676 | 2.564 | 0.680 | 0.119 | 0.639 | 0.095 | 0.575 | 0.112 | 0.300 | 0.041 | 0.248 | 0.035 | 10.765 |
| GX-08 | 3.008 | 5.146 | 0.540 | 1.904 | 0.481 | 0.087 | 0.450 | 0.071 | 0.440 | 0.088 | 0.231 | 0.031 | 0.190 | 0.027 | 8.385 |
| GX-10 | 3.118 | 5.563 | 0.672 | 2.492 | 0.607 | 0.109 | 0.596 | 0.086 | 0.514 | 0.101 | 0.270 | 0.039 | 0.239 | 0.034 | 7.914 |
| HJL12-2 | 3.962 | 6.504 | 0.699 | 2.604 | 0.698 | 0.113 | 0.632 | 0.089 | 0.528 | 0.100 | 0.270 | 0.038 | 0.226 | 0.033 | 8.368 |
| HJL13-3A | 3.954 | 7.405 | 0.884 | 3.180 | 0.800 | 0.128 | 0.714 | 0.106 | 0.633 | 0.122 | 0.326 | 0.048 | 0.309 | 0.045 | 6.989 |
| HJL13-5 | 9.680 | 19.013 | 2.354 | 9.098 | 2.301 | 0.454 | 2.206 | 0.342 | 1.972 | 0.379 | 0.945 | 0.133 | 0.832 | 0.115 | 20.192 |
| HJL14-1 | 2.982 | 5.703 | 0.669 | 2.513 | 0.662 | 0.110 | 0.600 | 0.089 | 0.542 | 0.107 | 0.294 | 0.041 | 0.238 | 0.032 | 9.297 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Hou, M.; Chen, A.; Xu, S.; Zhang, B.; Deng, Y.; Yu, Y. Fluid Properties and Genesis of Dolomites in the Devonian Guanwushan Formation of Upper Yangtze Platform, SW China. Minerals 2022, 12, 317. https://doi.org/10.3390/min12030317
Huang S, Hou M, Chen A, Xu S, Zhang B, Deng Y, Yu Y. Fluid Properties and Genesis of Dolomites in the Devonian Guanwushan Formation of Upper Yangtze Platform, SW China. Minerals. 2022; 12(3):317. https://doi.org/10.3390/min12030317
Chicago/Turabian StyleHuang, Shuguang, Mingcai Hou, Anqing Chen, Shenglin Xu, Benjian Zhang, Yuwei Deng, and Yu Yu. 2022. "Fluid Properties and Genesis of Dolomites in the Devonian Guanwushan Formation of Upper Yangtze Platform, SW China" Minerals 12, no. 3: 317. https://doi.org/10.3390/min12030317
APA StyleHuang, S., Hou, M., Chen, A., Xu, S., Zhang, B., Deng, Y., & Yu, Y. (2022). Fluid Properties and Genesis of Dolomites in the Devonian Guanwushan Formation of Upper Yangtze Platform, SW China. Minerals, 12(3), 317. https://doi.org/10.3390/min12030317





