Quantification of Lithium and Mineralogical Mapping in Crushed Ore Samples Using Laser Induced Breakdown Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crushed Lithium Ore Samples
2.2. ECORE LIBS Analyzer
2.3. Quantitative Analysis and Data Processing
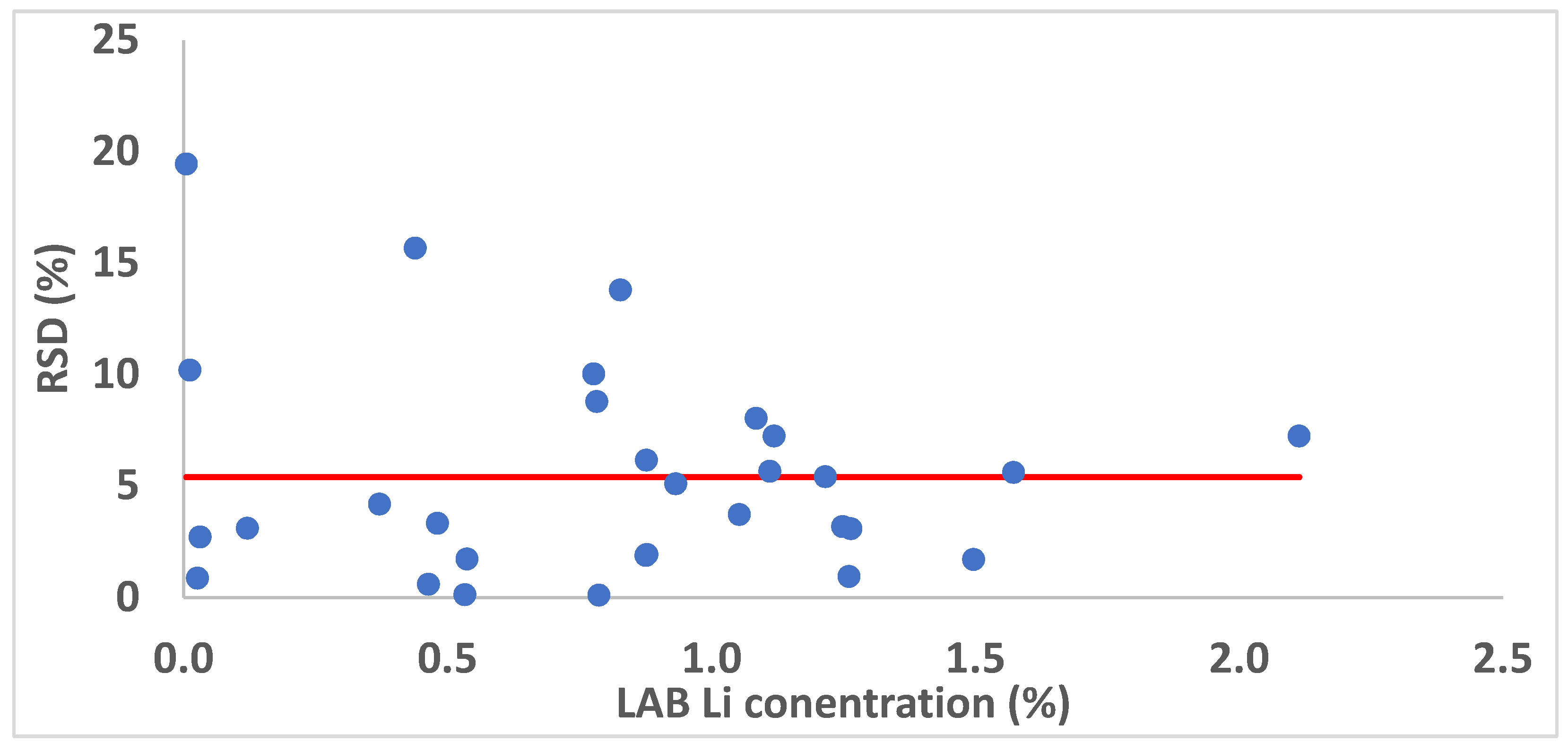
3. Results and Discussion
3.1. Quantitative Analysis Based on Mineral Composition
3.2. Matrix-Matched Univariate Calibration
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz, K.J.; DeYoung, J.H., Jr.; Seal, R.R., II; Bradley, D.C. (Eds.) Critical Mineral Resources of the United States-Economic and Environmental Geology and Prospects for Future Supply; USGS: Reston, VA, USA, 2017; 797p. [CrossRef]
- Australian Critical Minerals Prospectus 2020. Australian Government, Australian Trade and Investment Commission, Geoscience Australia, Dept. of Industry, Science, Energy and Resources. 2020; 172p. Available online: www.austrade.gov.au/ArticleDocuments/5572/Australian_Critical_Minerals_Prospectus.pdf (accessed on 2 February 2022).
- U.S. Geological Survey. Mineral Commodity Summaries 2021; USGS: Reston, VA, USA, 2021; 200p. [CrossRef]
- Rifai, K.; Laflamme, M.; Constantin, M.; Vidal, F.; Sabsabi, M.; Blouin, A.; Bouchard, P.; Fytas, K.; Castello, M.; Kamwa, B.N. Analysis of gold in rock samples using laser-induced breakdown spectroscopy: Matrix and heterogeneity effects. Spectrochim. Acta B 2017, 134, 33–41. [Google Scholar] [CrossRef]
- Jolivet, L.; Leprince, M.; Moncayo, S.; Sorbier, L.; Lienemann, C.-P.; Motto-Ros, V. Review of the recent advances and applications of LIBS based imaging. Spectrochim. Acta B 2019, 151, 41–53. [Google Scholar] [CrossRef]
- Fabre, C. Advances in Laser-Induced Breakdown Spectroscopy analysis for geology: A critical review. Spectrochim. Acta B 2020, 166, 105799. [Google Scholar] [CrossRef]
- Harmon, R.S.; Senesi, G.S. Laser-induced breakdown spectroscopy—A geochemical tool for the 21st century. Appl. Geochem. 2021, 120, 104929. [Google Scholar] [CrossRef]
- Harmon, R.S.; Lawley, C.J.M.; Watts, J.; Harraden, C.L.; Somers, A.M.; Hark, R.R. Laser-Induced Breakdown Spectroscopy—An emerging analytical tool for mineral exploration. Minerals 2019, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Ytsma, C.R.; Knudson, C.A.; Dyar, M.D.; McAdam, A.C.; Michaud, D.D.; Rollosson, L.M. Accuracies and detection limits of major, minor, and trace element quantification in rocks by portable laser-induced breakdown spectroscopy. Spectrochim. Acta B 2020, 171, 105946. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectrochim. Acta B 2021, 175, 106013. [Google Scholar] [CrossRef]
- Ytsma, C.R.; Dyar, M.D. Accuracies of lithium, boron, carbon, and sulfur quantification in geological samples with laser-induced breakdown spectroscopy in Mars, Earth, and vacuum conditions. Spectrochim. Acta B 2019, 162, 105715. [Google Scholar] [CrossRef]
- Schirmer, T.; Wahl, M.; Bock, W.; Kopnarski, M. Determination of the Li distribution in synthetic recycling slag with SIMS. Metals 2021, 11, 825. [Google Scholar] [CrossRef]
- Fabre, C.; Boiron, M.C.; Dubessy, J.; Chabiron, A.; Charoy, B.; Crespo, T.M. Advances in lithium analysis in solids by means of laser-induced breakdown spectroscopy: An exploratory study. Geochem. Cosmochim. Acta 2002, 66, 1401–1407. [Google Scholar] [CrossRef]
- Fabre, C.; Maurice, S.; Cousin, A.; Wiens, R.C.; Forni, O.; Sautter, V.; Guillaume, D. Onboard calibration igneous targets for the Mars Science Laboratory Curiosity rover and the Chemistry Camera laser induced breakdown spectroscopy instrument. Spectrochim. Acta B 2011, 66, 280–289. [Google Scholar] [CrossRef]
- Sweetapple, M.T.; Tassios, S. Laser-induced breakdown spectroscopy (LIBS) as a tool for in situ mapping and textural interpretation of lithium in pegmatite minerals. Am. Min. 2015, 100, 2141–2151. [Google Scholar] [CrossRef]
- Lawley, C.J.M.; Somers, A.M.; Kjarsgaard, B.A. Rapid geochemical imaging of rocks and minerals with handheld laser-induced breakdown spectroscopy (LIBS). J. Geochem. Explor. 2021, 222, 106694. [Google Scholar] [CrossRef]
- Romppanen, S.; Pölönen, I.; Häkkänen, H.; Kaski, S. Optimization of spodumene identification by statistical approach for laser-induced breakdown spectroscopy data of lithium pegmatite ores. Appl. Spectrosc. Rev. 2021. [Google Scholar] [CrossRef]
- Rifai, K.; Doucet, F.; Özcan, L.; Vidal, F. LIBS core imaging at kHz speed: Paving the way for real-time geochemical applications. Spectrochim. Acta B 2018, 150, 43–48. [Google Scholar] [CrossRef]
- Rifai, K.; Özcan, L.; Doucet, F.; Vidal, F. Quantification of copper, nickel and other elements in copper-nickel ore samples using laser-induced breakdown spectroscopy. Spectrochim. Acta B 2020, 165, 105766. [Google Scholar] [CrossRef]
- Rifai, K.; Özcan, L.Ç.; Doucet, F.R.; Rhoderick, K.; Vidal, F. Ultrafast elemental mapping of platinum group elements and mineral identification in platinum-palladium ore using Laser Induced Breakdown Spectroscopy. Minerals 2021, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Rifai, K.; Michaud-Paradis, M.-C.; Swierczek, Z.; Doucet, F.R.; Özcan, L.Ç.; Fayad, A.; Li, J.; Vidal, F. Emergences of new technology for ultrafast automated mineral phase identification and quantitative analysis using the CORIOSITY Laser-Induced Breakdown Spectroscopy (LIBS) system. Minerals 2021, 10, 918. [Google Scholar] [CrossRef]
- Mohamed, N.; Rifai, K.; Selmani, S.; Constantin, M.; Doucet, F.; Özcan, L.Ç.; Sabsabi, M.; Vidal, F. Chemical and mineralogical mapping of platinum-group elements ore samples using LIBS and µ-XRF. Geostand. Geoanal. Res. 2021, 45, 539–550. [Google Scholar] [CrossRef]
- Michaud-Paradis, M.-C.; Doucet, F.R.; Rifai, K.; Özcan, L.Ç.; Azami, N.; Vidal, F. ECORE: A new fast automated quantitative mineral and elemental core scanner. Minerals 2021, 11, 859. [Google Scholar] [CrossRef]
- Champion, D. Australian Resource Reviews: Lithium 2018; Geoscience Australia: Canberra, Australia, 2019; 12p. Available online: https://d28rz98at9flks.cloudfront.net/126759/ARR_Lithium_2018.pdf (accessed on 2 February 2022).
- Critical Minerals Projects in Australia 2020. Australian Government, Australian Trade and Investment Commission, Geoscience Australia, Dept. of Industry, Science, Energy and Resources. 2020; 62p. Available online: www.austrade.gov.au/ArticleDocuments/5572/Critical_Minerals_Projects_in_Australia.pdf (accessed on 2 February 2022).
- Bowell, R.J.; Lagos, L.; de LosHoyos, C.R.; Declercq, J. Classification and characteristics of natural lithium resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Feasibility Study Update NI 43-101 Technical Report Quebec Lithium Project La Corne Township; Canada Lithium Corp.: Québec, QC, Canada, 2011; 184p.
- Ibarra-Gutiérrez, S.; Bouchard, J.; Laflamme, M.; Fytas, K. The potential of lithium in Québec for the electric vehicle market: State of the art, opportunities and challenges. Int. J. Min. Reclam. Envirion. 2021, 36, 68–80. [Google Scholar] [CrossRef]

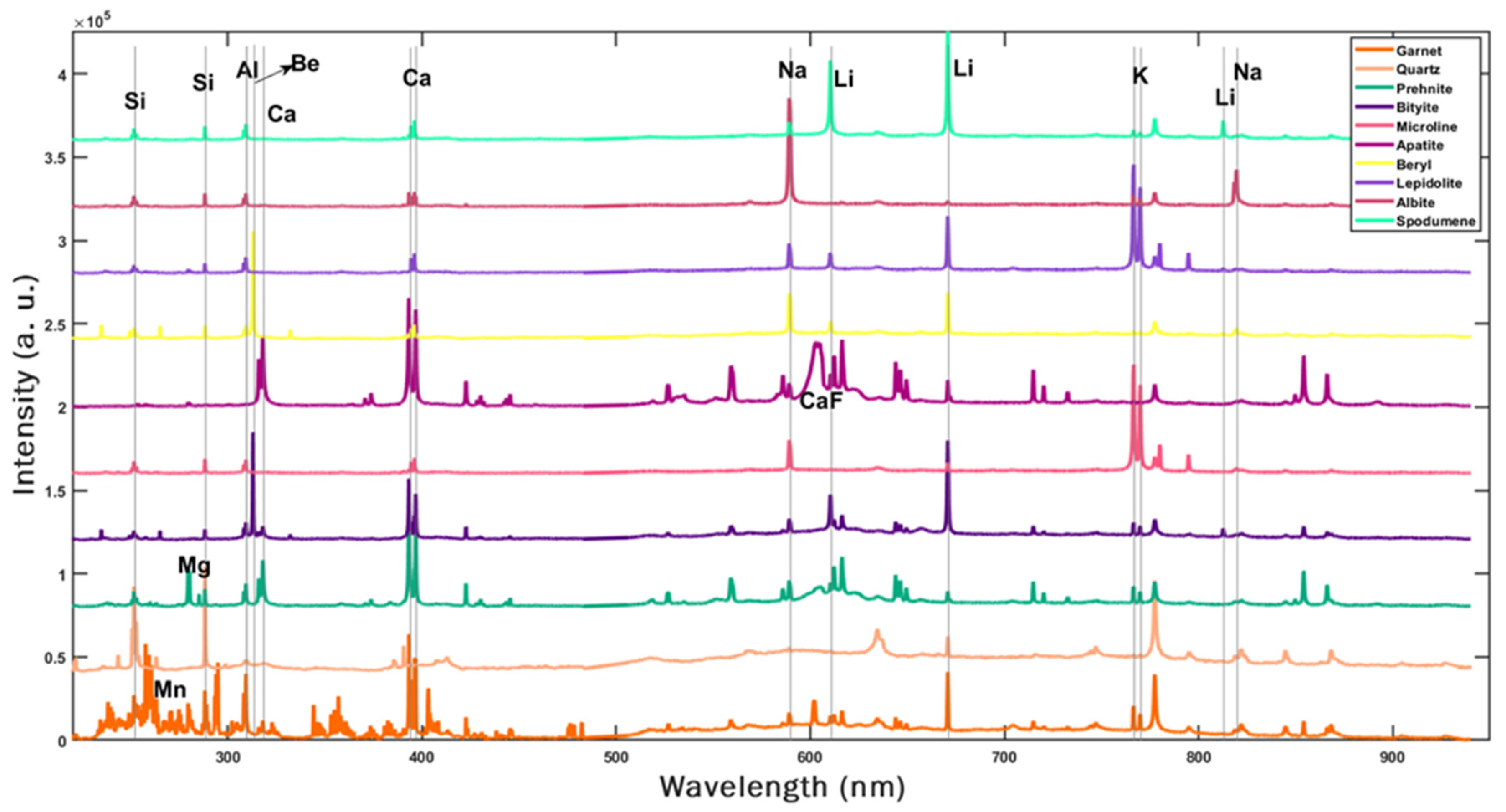
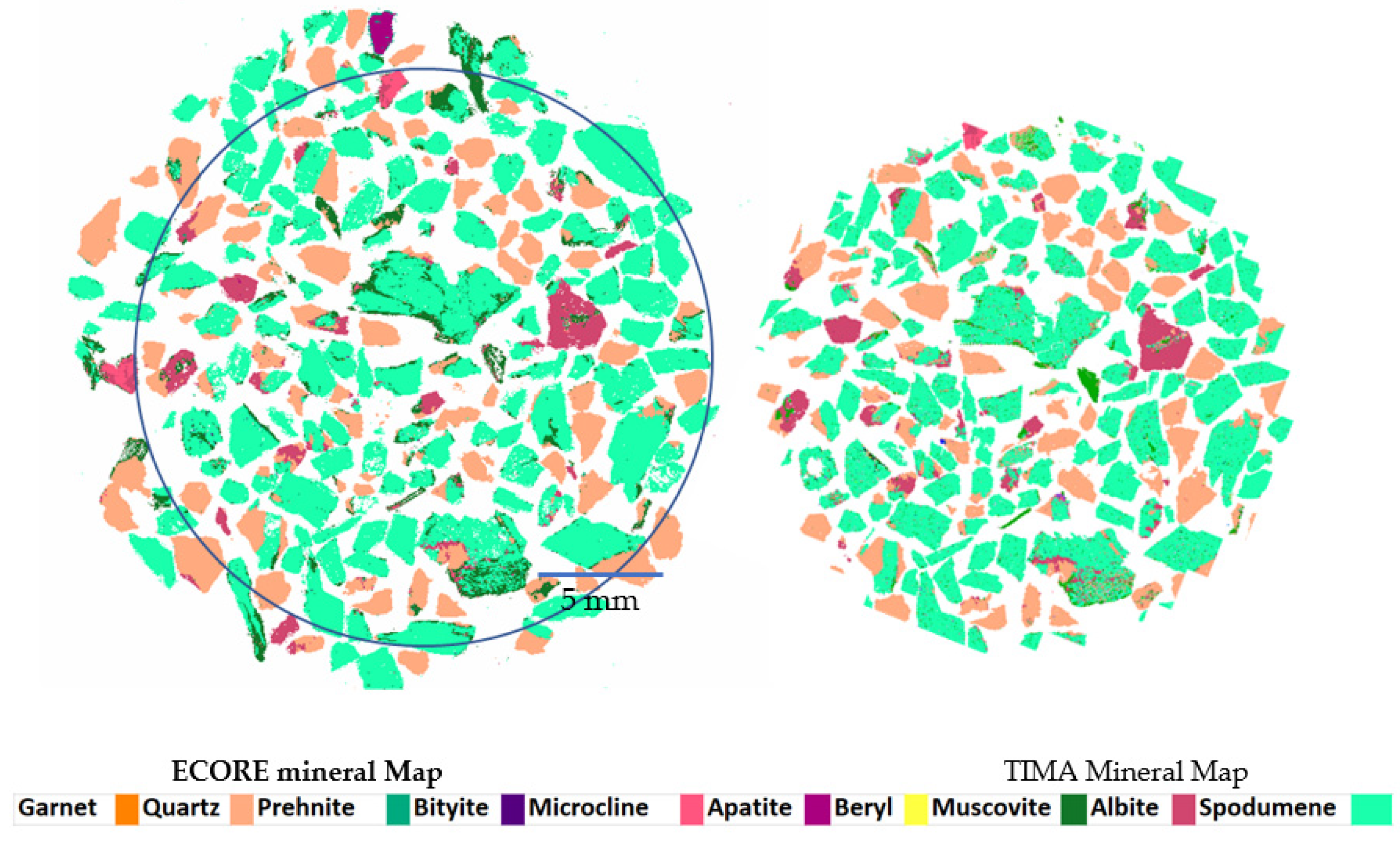
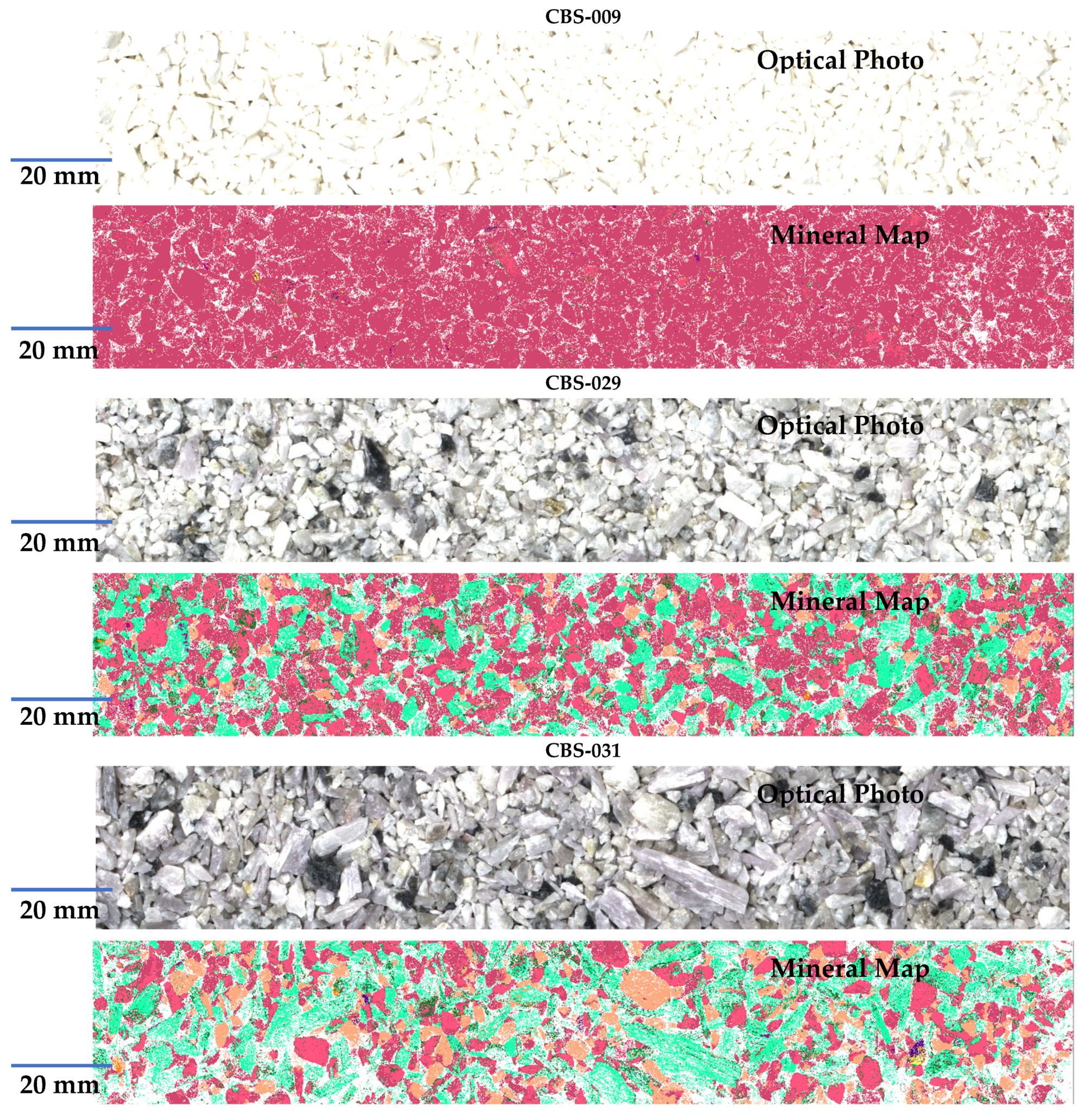
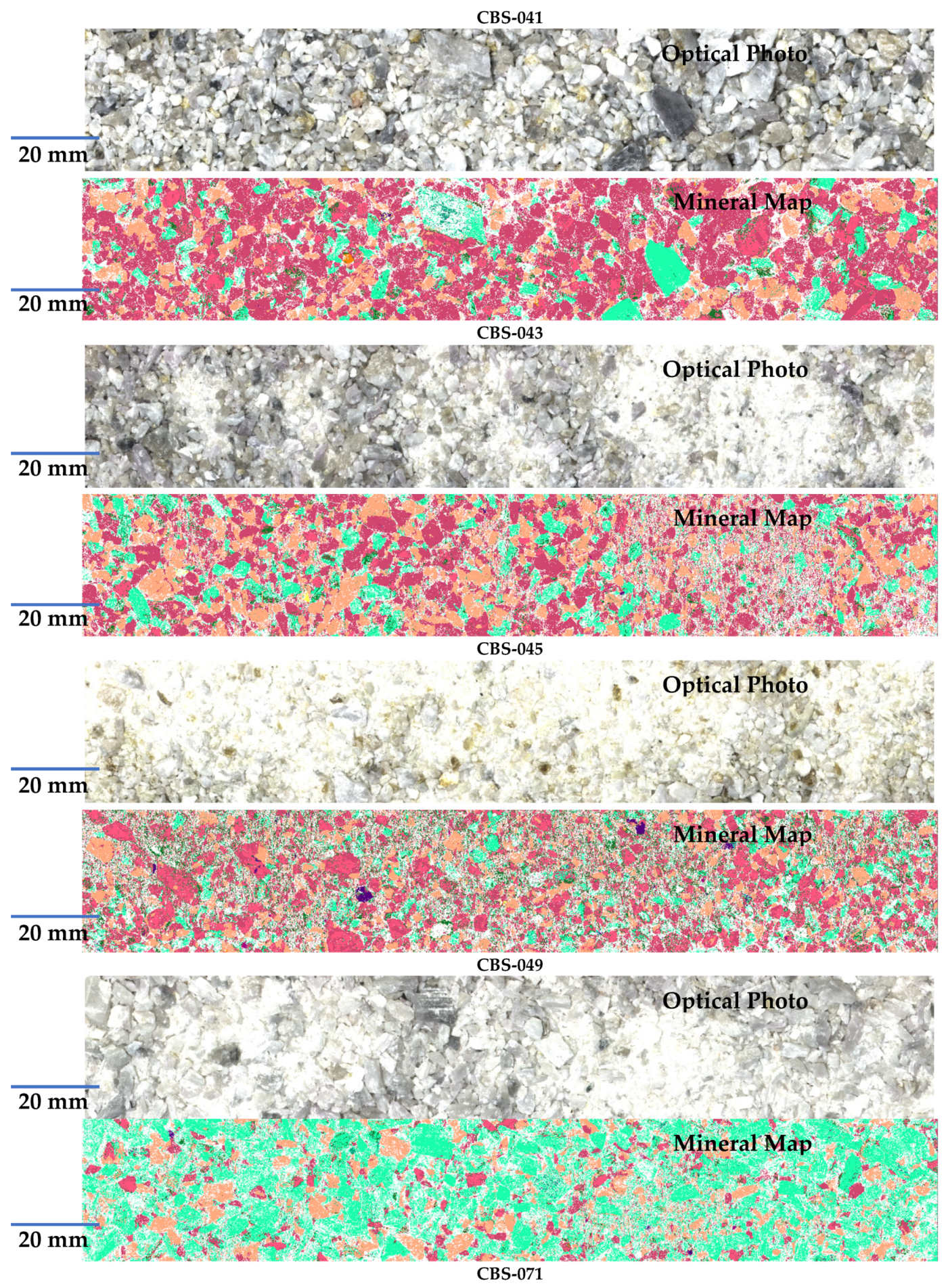
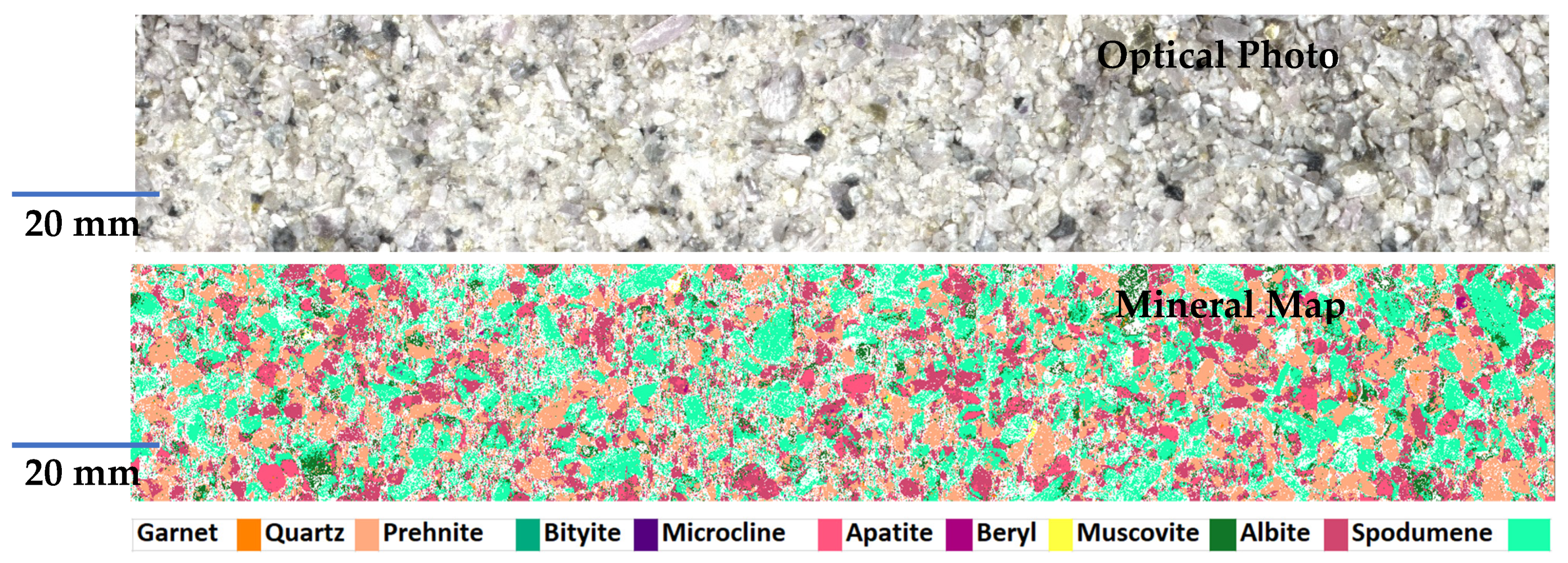
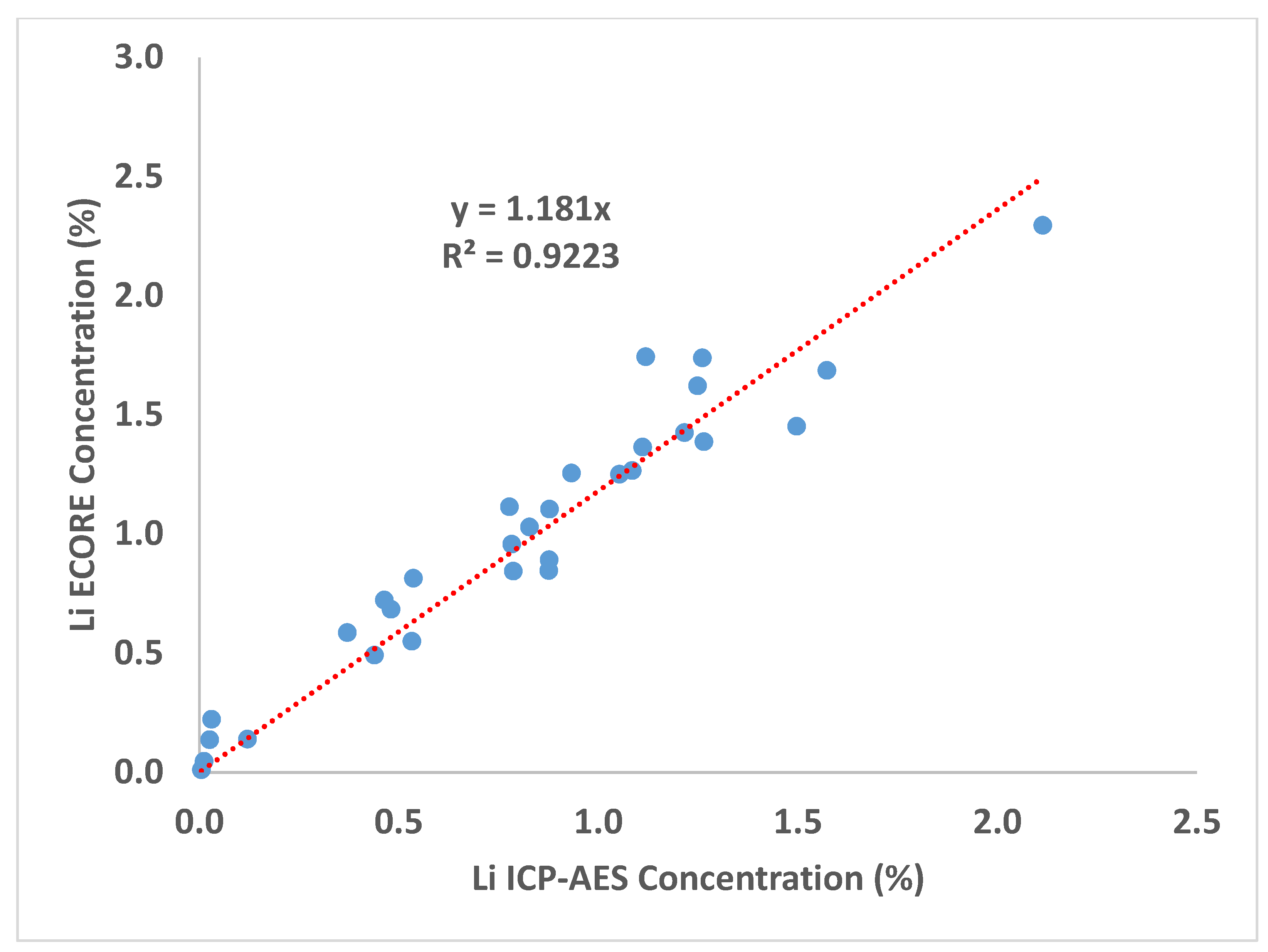
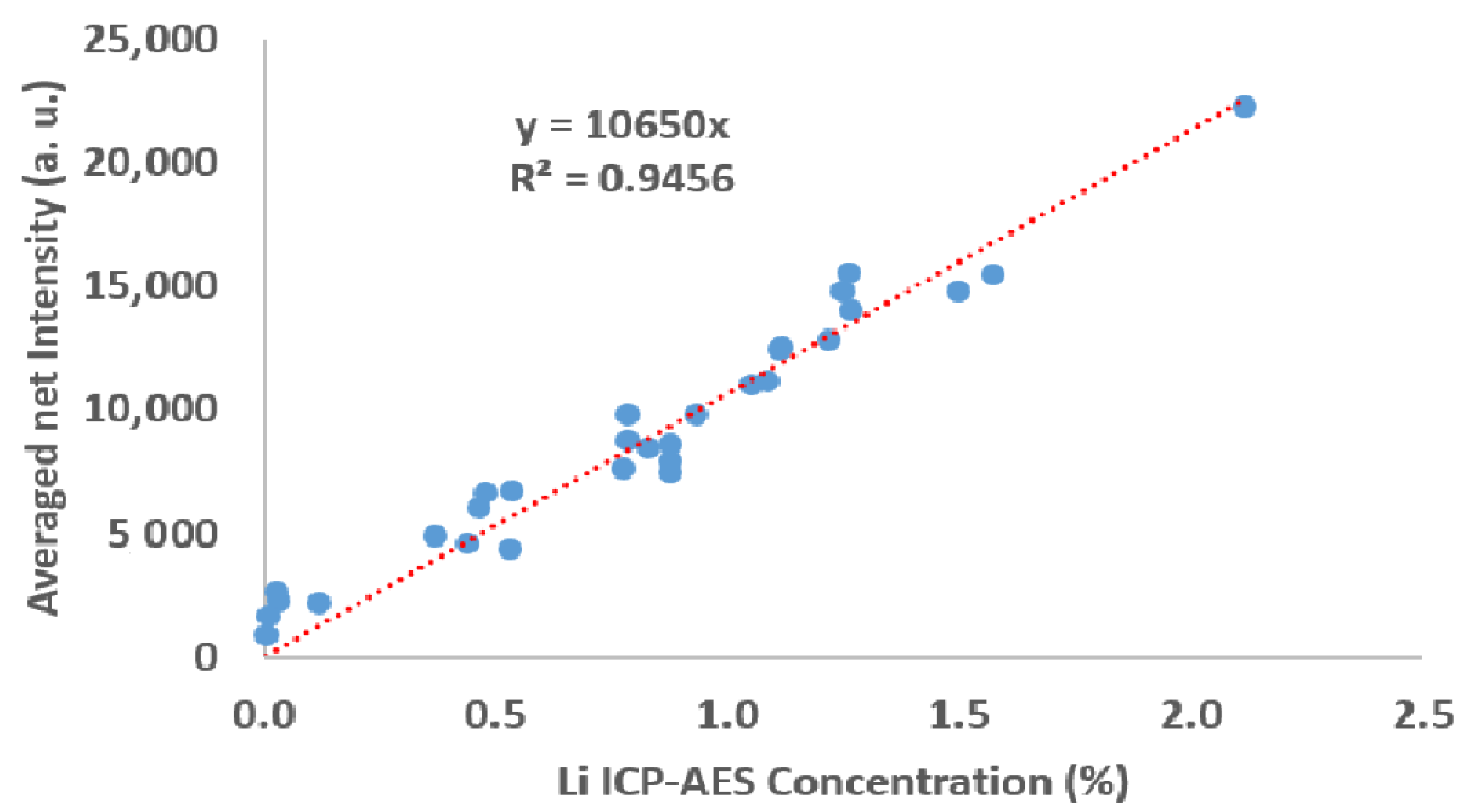
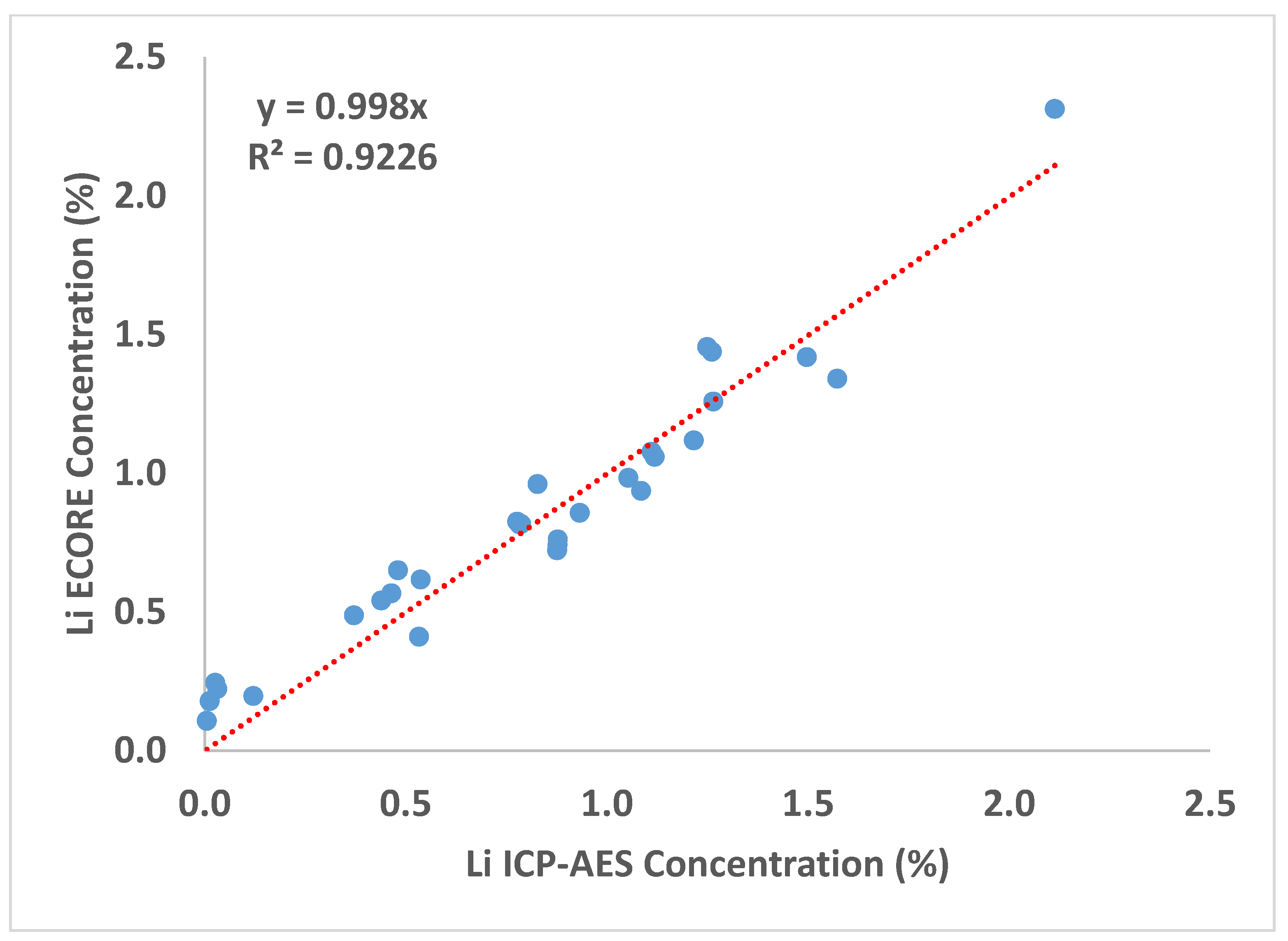
| Sample ID | CBS-087 | CBS-085 | CBS-080 | CBS-083 | CBS-091 | CBS-077 | CBS-078 | CBS-075 | CBS-079 | CBS-065 |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference [Li] (%) | 0.121 | 0.031 | 1.265 | 0.371 | 1.053 | 0.533 | 1.085 | 1.261 | 0.012 | 0.876 |
| Sample ID | CBS-067 | CBS-055 | CBS-073 | CBS-051 | CBS-039 | CBS-043 | CBS-049 | CBS-071 | CBS-009 | CBS-093 |
| Reference [Li] (%) | 0.878 | 0.464 | 0.026 | 1.111 | 0.877 | 0.787 | 2.114 | 1.249 | 0.005 | 0.933 |
| Sample ID | CBS-069 | CBS-033 | CBS-057 | CBS-045 | CBS-031 | CBS-041 | CBS-029 | CBS-007 | CBS-027 | CBS-021 |
| Reference [Li] (%) | 1.497 | 0.828 | 0.537 | 0.777 | 1.573 | 0.481 | 1.119 | 0.439 | 0.783 | 1.216 |
| Mineral | Formula | [Li] % |
|---|---|---|
| Garnet | Mn2+3Al2Si3O12 + Fe2+3Al2Si3O12 | 0 |
| Quartz | SiO2 | 0 |
| Prehnite | Ca2Al2Si3O10(OH)2 | 0 |
| Bityite | CaLiAl3Be(Si2O10)(OH)2 | 1.79 |
| Microcline | KAlSi3O8 | 0 |
| Apatite | Ca5(PO4)3F | 0 |
| Beryl | Be3Al2Si6O18 | 0 |
| Muscovite | KAl3Si3O10(OH)1.8F0.2 | 0 |
| Albite | Na0.95Ca0.05Al1.05Si2.95O8 | 0 |
| Spodumene | LiAl(Si2O6) | 3.73 |
| Mineral | LIBS (%) | TIMA (%) |
|---|---|---|
| Spodumene | 62.5 | 64.9 |
| Quartz | 22 | 22.9 |
| Albite | 5.2 | 5.7 |
| Muscovite | 4.5 | 3.5 |
| Microcline | 2 | 0.85 |
| Apatite | 1 | 0.017 |
| beryl | 0.01 | 0.018 |
| Garnet | 0.02 | 0.03 |
| Prehnite | 0.1 | 0.12 |
| Bityite | 0 | 0 |
| Others | 2.67 | 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rifai, K.; Constantin, M.; Yilmaz, A.; Özcan, L.Ç.; Doucet, F.R.; Azami, N. Quantification of Lithium and Mineralogical Mapping in Crushed Ore Samples Using Laser Induced Breakdown Spectroscopy. Minerals 2022, 12, 253. https://doi.org/10.3390/min12020253
Rifai K, Constantin M, Yilmaz A, Özcan LÇ, Doucet FR, Azami N. Quantification of Lithium and Mineralogical Mapping in Crushed Ore Samples Using Laser Induced Breakdown Spectroscopy. Minerals. 2022; 12(2):253. https://doi.org/10.3390/min12020253
Chicago/Turabian StyleRifai, Kheireddine, Marc Constantin, Adnan Yilmaz, Lütfü Ç. Özcan, François R. Doucet, and Nawfel Azami. 2022. "Quantification of Lithium and Mineralogical Mapping in Crushed Ore Samples Using Laser Induced Breakdown Spectroscopy" Minerals 12, no. 2: 253. https://doi.org/10.3390/min12020253
APA StyleRifai, K., Constantin, M., Yilmaz, A., Özcan, L. Ç., Doucet, F. R., & Azami, N. (2022). Quantification of Lithium and Mineralogical Mapping in Crushed Ore Samples Using Laser Induced Breakdown Spectroscopy. Minerals, 12(2), 253. https://doi.org/10.3390/min12020253








