Ion-Exchange-Induced Transformation and Mechanism of Cooperative Crystal Chemical Adaptation in Sitinakite: Theoretical and Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
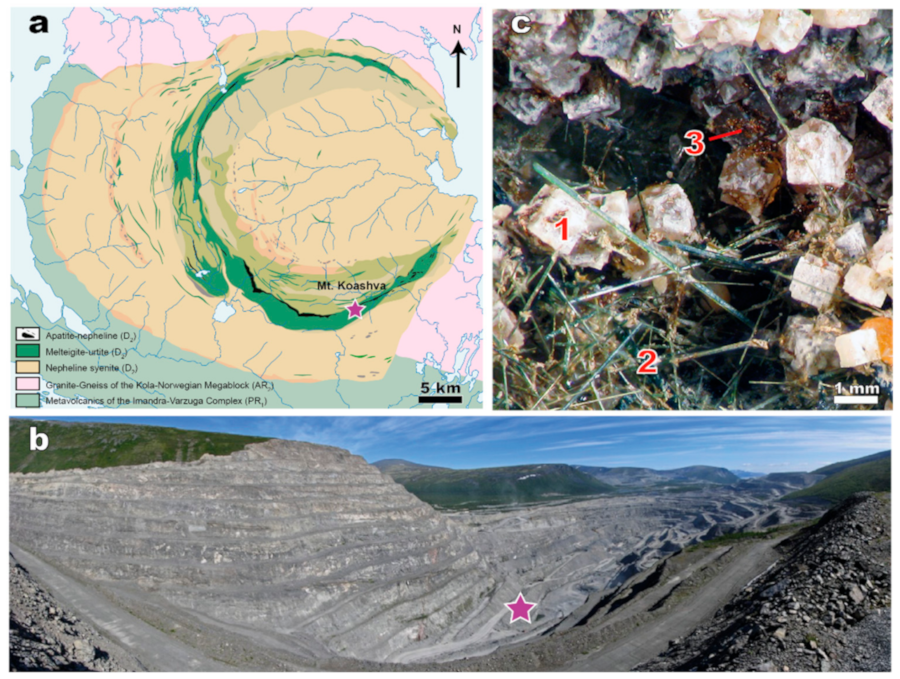
2.1. Sample
2.2. Synthesis
2.3. Ion-Exchange
2.4. Composition
2.5. Raman Spectroscopy
2.6. Powder and Single-Crystal X-Ray Diffraction
2.7. Geometrical–Topological Analysis
3. Results
3.1. Composition
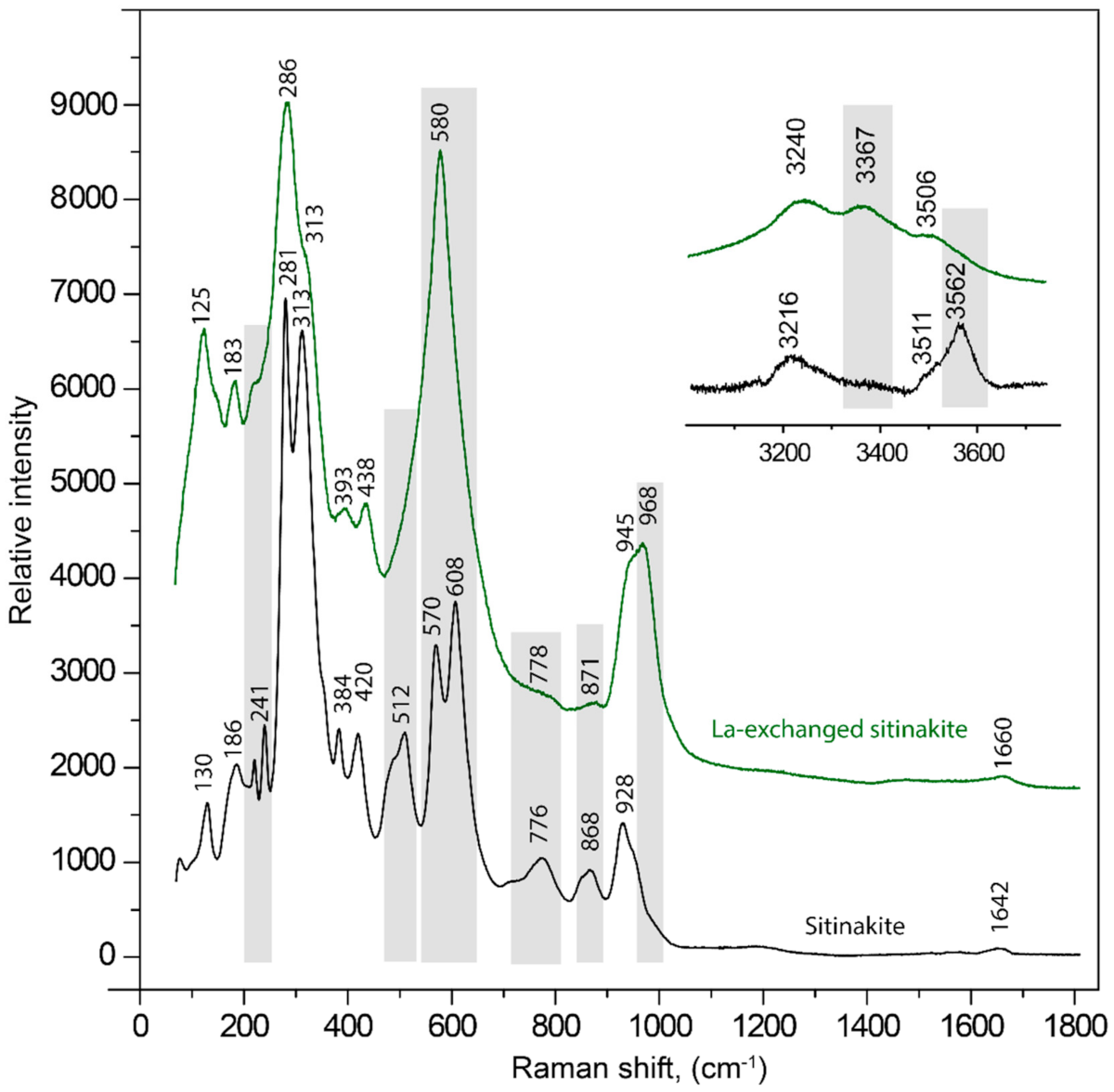
3.2. Raman Spectroscopy
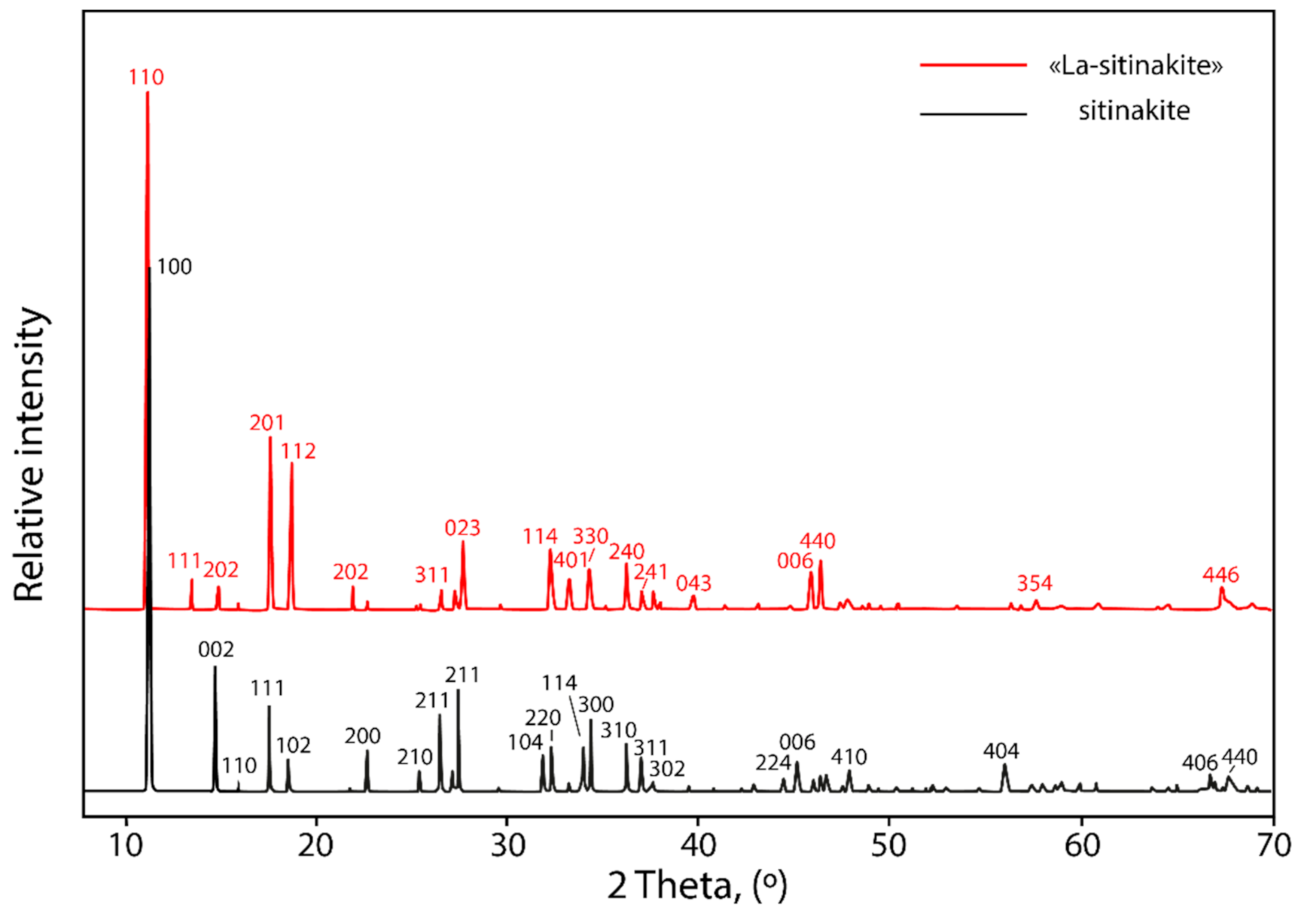
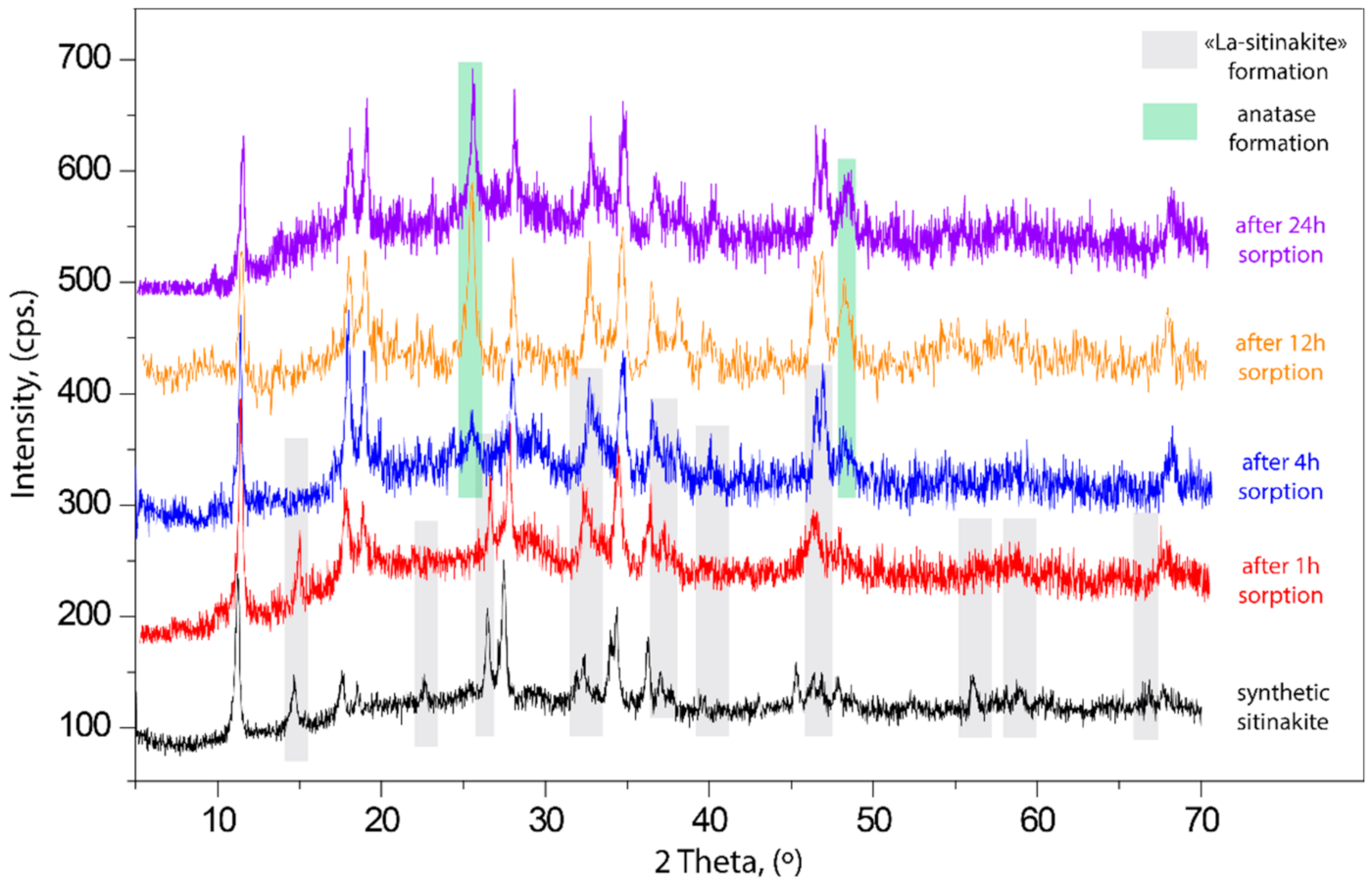
3.3. Powder X-ray Diffraction
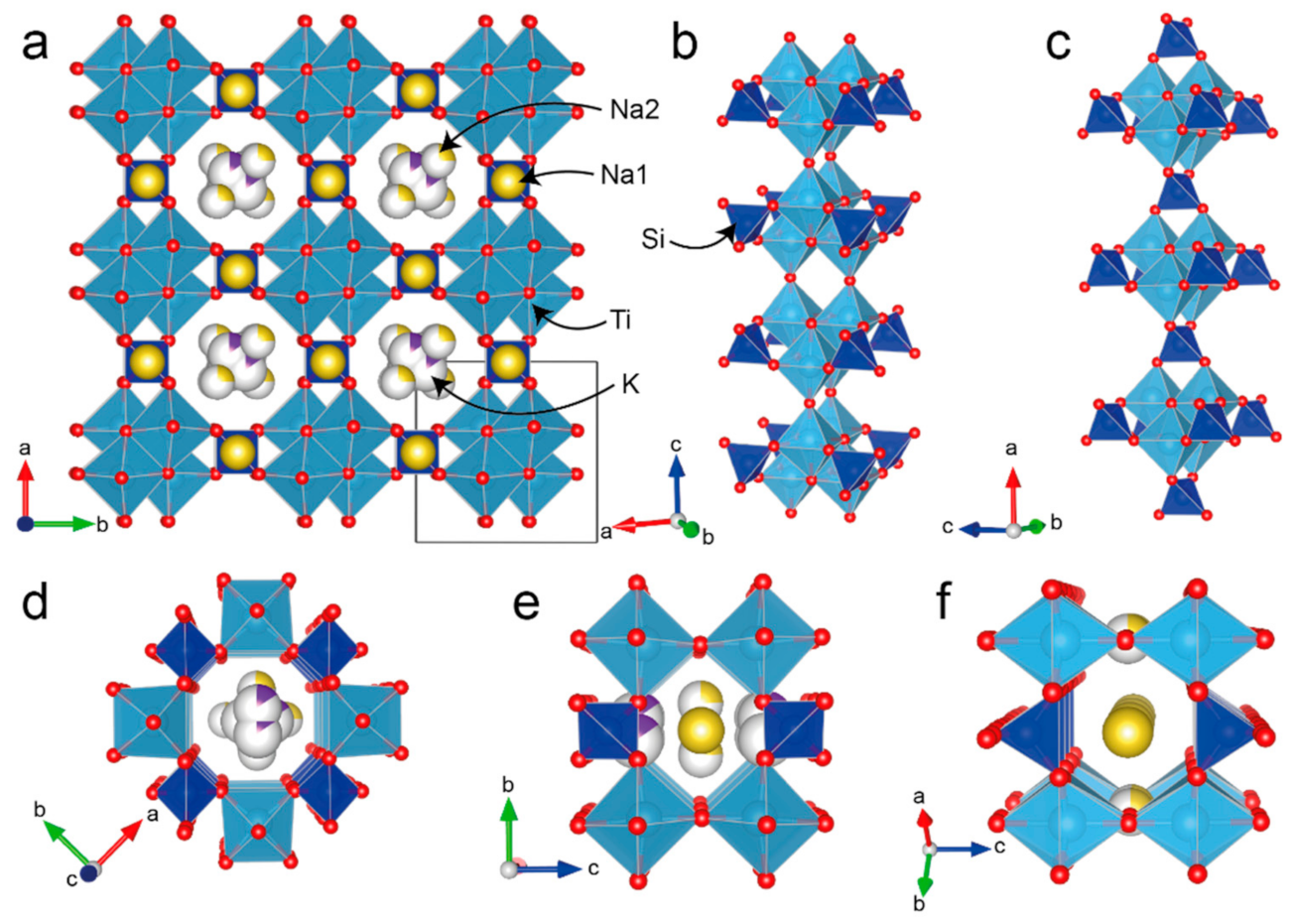
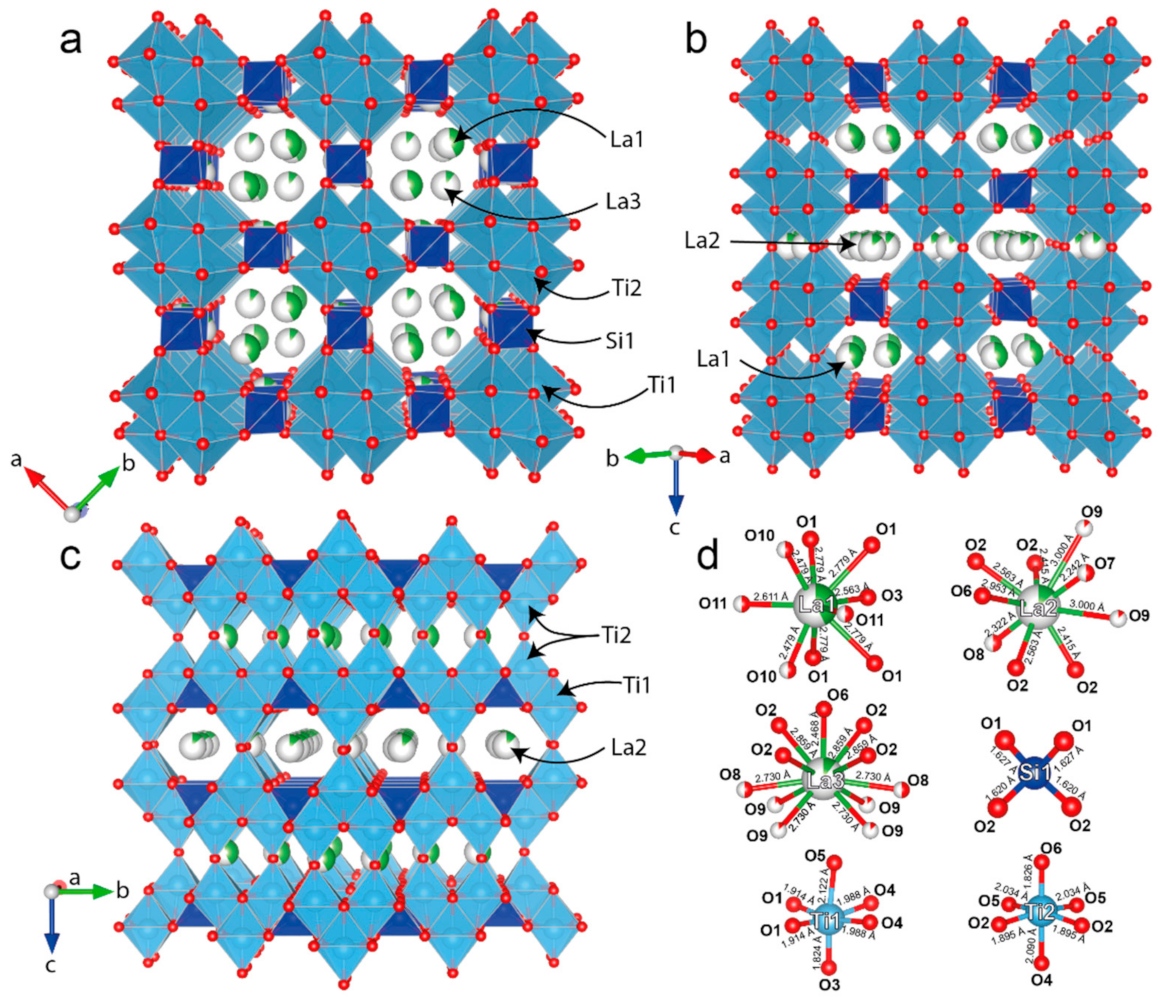
3.4. Single-Crystal X-Ray Diffraction
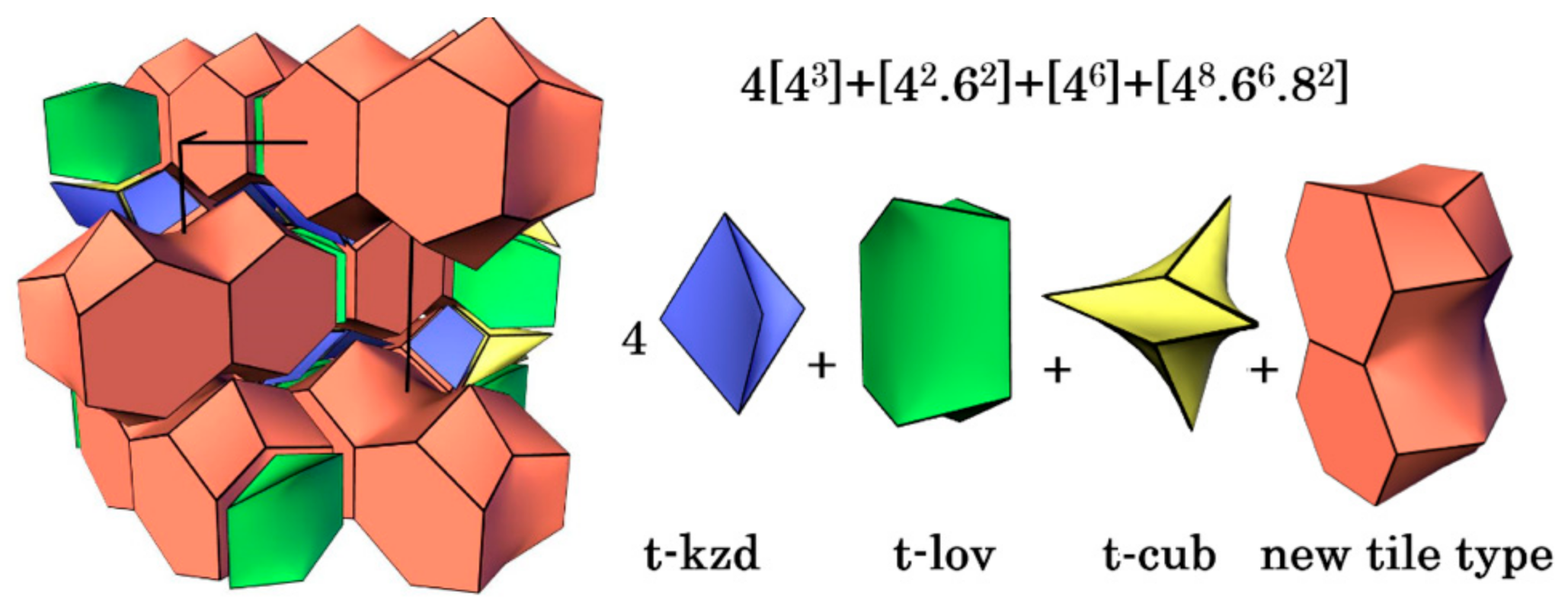
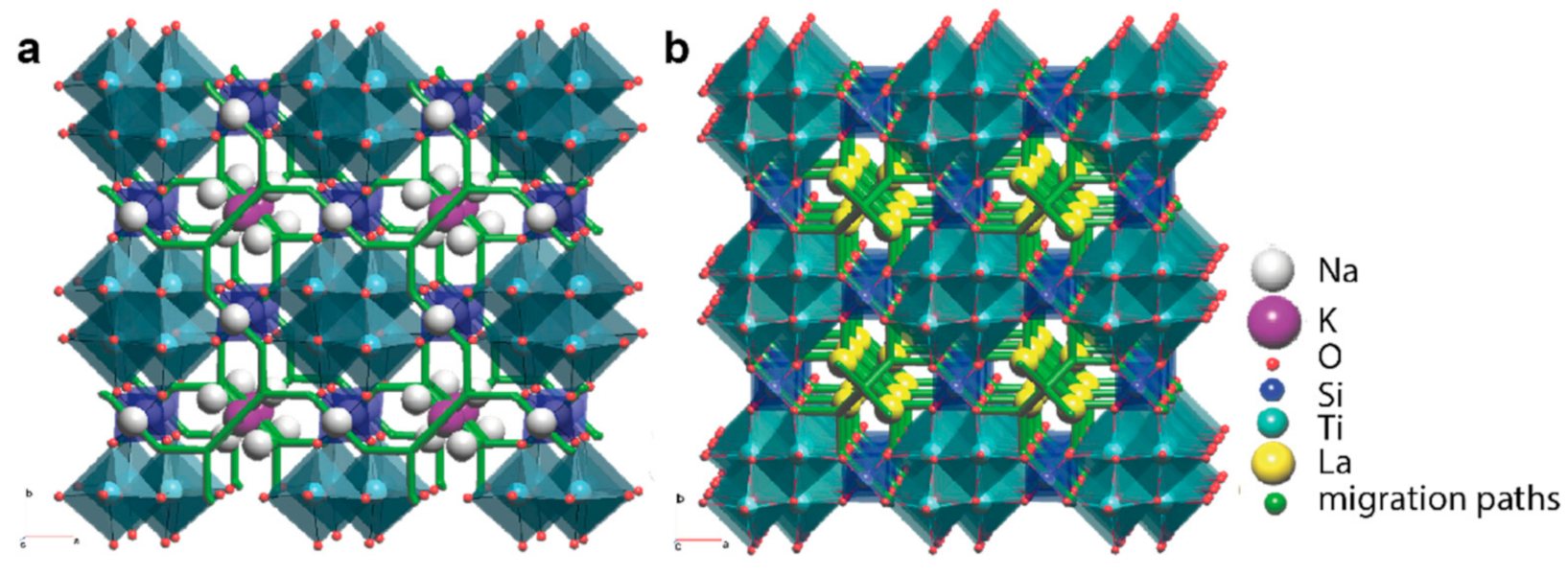
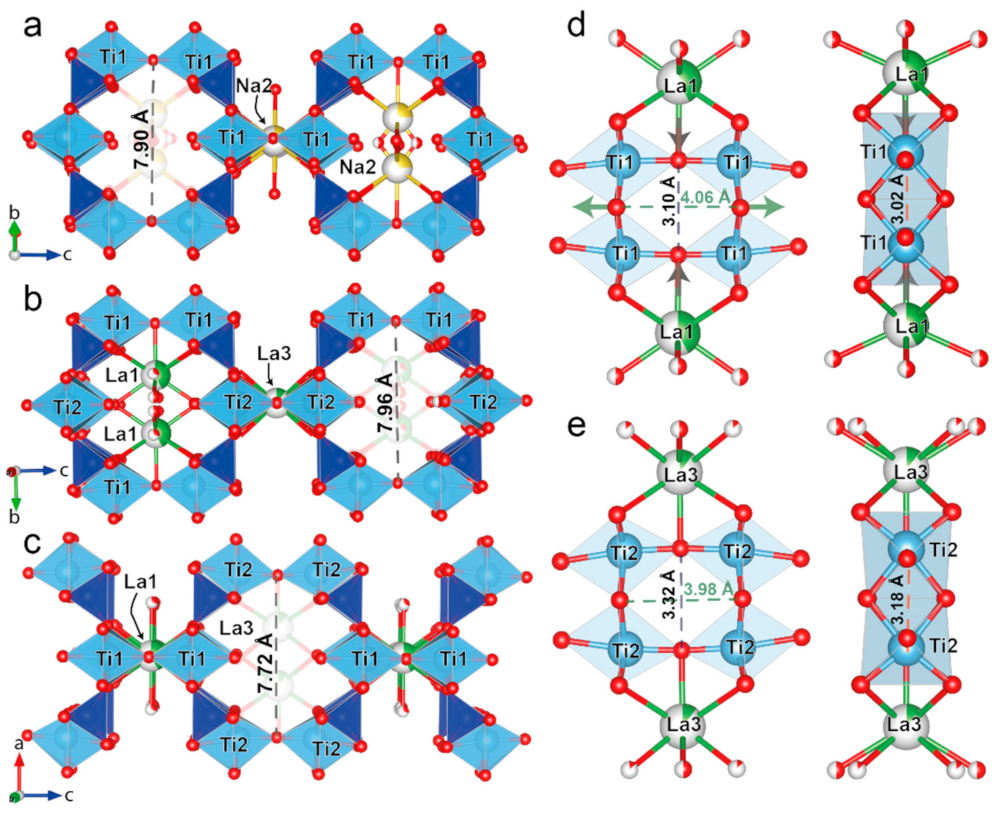
3.5. Topological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perovskiy, I.A.; Yanicheva, N.Y.; Stalyugin, V.V.; Panikorovskii, T.L.; Golov, A.A. Sorption of multivalent cations on titanosilicate obtained from natural raw materials. The mechanism and thermodynamics of sorption. Microporous Mesoporous Mater. 2021, 311, 110716. [Google Scholar] [CrossRef]
- Sizgek, G.D.; Sizgek, E. Microwave Drying Characteristics of Impregnated Synroc Ceramic Microspheres. J. Microw. Power Electromagn. Energy 1997, 32, 171–179. [Google Scholar] [CrossRef]
- Sizgek, E.; Bartlett, J.R.; Woolfrey, J.L.; Vance, E.R. Production of Synroc Ceramics from Titanate Gel Microspheres. MRS Proc. 1993, 333, 305. [Google Scholar] [CrossRef]
- Gregg, D.J.; Vance, E.R. Synroc tailored waste forms for actinide immobilization. Radiochim. Acta 2017, 105, 907–925. [Google Scholar] [CrossRef]
- Zubekhina, B.Y.; Burakov, B.E.; Petrov, Y.Y.; Britvin, S.N.; Mararitsa, V.F.; Demidov, Y.T.; Nickolsky, M.S. New route for synthesis of Synroc-like ceramic using non-selective sorbent LHT-9. MRS Adv. 2018, 3, 1111–1116. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Nekrasova, N.A.; Yanicheva, N.Y.; Kalashnikova, G.O.; Ganicheva, Y.Y. Sorption of cesium and strontium radionuclides onto crystalline alkali metal titanosilicates. Radiochemistry 2017, 59, 65–69. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Kesson, S.E.; Ware, N.G.; Hibberson, W.; Major, A. Immobilisation of high level nuclear reactor wastes in SYNROC. Nature 1979, 278, 219–223. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Kesson, S.E.; Reeve, K.D.; Lutze, W.; Ewing, R.C. Synroc. In Radioactive Waste Forms for the Future; Werner, L., Ewing, R.C., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1988; pp. 233–344. [Google Scholar]
- Minerals as Advanced Materials II; Krivovichev, S.V. (Ed.) Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-20017-5. [Google Scholar]
- Panikorovskii, T.L.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Bazai, A.V.; Ivanyuk, G.Y.; Kalashnikova, G.O.; Yanicheva, N.Y.; Aksenov, S.M.; Nikolaev, A.I.; Chukanov, N.V.; et al. Natural titanosilicates from the Kola alkaline province as prototypes for functional materials. Proc. Fersman Sci. Sess. Geol. Inst. KSC RAS 2020, 17, 427–431. (In Russian) [Google Scholar] [CrossRef]
- Popa, K.; Pavel, C.C. Radioactive wastewaters purification using titanosilicates materials: State of the art and perspectives. Desalination 2012, 293, 78–86. [Google Scholar] [CrossRef]
- Rocha, J.; Anderson, M.W. Microporous Titanosilicates and other Novel Mixed Octahedral-Tetrahedral Framework Oxides. Eur. J. Inorg. Chem. 2000, 2000, 801–818. [Google Scholar] [CrossRef]
- Milne, N.A.; Griffith, C.S.; Hanna, J.V.; Skyllas-Kazacos, M.; Luca, V. Lithium Intercalation into the Titanosilicate Sitinakite. Chem. Mater. 2006, 18, 3192–3202. [Google Scholar] [CrossRef]
- Anson, A.; Lin, C.C.H.; Kuznicki, S.M.; Sawada, J.A. Adsorption of carbon dioxide, ethane, and methane on titanosilicate type molecular sieves. Chem. Eng. Sci. 2009, 64, 3683–3687. [Google Scholar] [CrossRef]
- Lin, C.C.H.; Dambrowitz, K.A.; Kuznicki, S.M. Evolving applications of zeolite molecular sieves. Can. J. Chem. Eng. 2012, 90, 207–216. [Google Scholar] [CrossRef]
- Oleksiienko, O.; Wolkersdorfer, C.; Sillanpää, M. Titanosilicates in cation adsorption and cation exchange–A review. Chem. Eng. J. 2017, 317, 570–585. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Yakovenchuk, V.N.; Yanicheva, N.Y.; Pakhomovsky, Y.A.; Shilovskikh, V.V.; Bocharov, V.N.; Krivovichev, S.V. Crystal chemistry of ivanyukite-group minerals, A3–xH1+x[Ti4O4(SiO4)3](H2O)n (A = Na, K, Cu), (n = 6–9, x = 0–2): Crystal structures, ion-exchange, chemical evolution. Mineral. Mag. 2021, 85, 607–619. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V. Heterosilicates with Tetrahedral-Octahedral Frameworks: Mineralogical and Crystal-Chemical Aspects. Rev. Mineral. Geochem. 2005, 57, 105–143. [Google Scholar] [CrossRef]
- Gerasimova, L.G.; Nikolaev, A.I.; Shchukina, E.S.; Maslova, M.V.; Kalashnikova, G.O.; Samburov, G.O.; Ivanyuk, G.Y. Hydrochloric Acidic Processing of Titanite Ore to Produce a Synthetic Analogue of Korobitsynite. Minerals 2019, 9, 315. [Google Scholar] [CrossRef] [Green Version]
- Ferdov, S.; Lopes, A.M.L.; Araujo, J.P.; Shivachev, B.; Titorenkova, R.; Petrova, N.; Nikolova, R. Three-Dimensional (3D) Microporous Iron Silicate with an Imandrite Type of Structure. Inorg. Chem. 2021, 60, 4563–4568. [Google Scholar] [CrossRef]
- Kalashnikova, G.O.; Zhitova, E.S.; Selivanova, E.A.; Pakhomovsky, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Kasikov, A.G.; Drogobuzhskaya, S.V.; Elizarova, I.R.; Kiselev, Y.G.; et al. The new method for obtaining titanosilicate AM-4 and its decationated form: Crystal chemistry, properties and advanced areas of application. Microporous Mesoporous Mater. 2021, 313, 110787. [Google Scholar] [CrossRef]
- Lykova, I.S.; Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Chervonnaya, N.A.; Zolotarev, A.A.; Giester, G. Crystal chemistry of cation-exchanged forms of epistolite-group minerals. Part II. Vigrishinite and Zn-exchanged murmanite. Eur. J. Mineral. 2015, 27, 669–682. [Google Scholar] [CrossRef]
- Lykova, I.S.; Pekov, I.V.; Zubkova, N.V.; Chukanov, N.V.; Yapaskurt, V.O.; Chervonnaya, N.A.; Zolotarev, A.A. Crystal chemistry of cation-exchanged forms of epistolite-group minerals, Part I. Ag- and Cu-exchanged lomonosovite and Ag-exchanged murmanite. Eur. J. Mineral. 2015, 27, 535–549. [Google Scholar] [CrossRef]
- Anthony, R.G.; Dosch, R.G.; Gu, D.; Philip, C.V. Use of silicotitanates for removing cesium and strontium from defense waste. Ind. Eng. Chem. Res. 1994, 33, 2702–2705. [Google Scholar] [CrossRef]
- Men’shikov, Y.P.; Sokolova, E.V.; Egorov-Tismenko, Y.K.; Khomyakov, A.P.; Polezhaeva, L.I. Sitinakite, Na2KTi4Si2O13(OH)·4H2O—A new mineral. Zap. RMO 1992, 121, 94–99. (In Russian) [Google Scholar]
- Anthony, R.G.; Philip, C.V.; Dosch, R.G. Selective adsorption and ion exchange of metal cations and anions with silico-titanates and layered titanates. Waste Manag. 1993, 13, 503–512. [Google Scholar] [CrossRef]
- Celestian, A.J.; Kubicki, J.D.; Hanson, J.; Clearfield, A.; Parise, J.B. The Mechanism Responsible for Extraordinary Cs Ion Selectivity in Crystalline Silicotitanate. J. Am. Chem. Soc. 2008, 130, 11689–11694. [Google Scholar] [CrossRef]
- Clearfield, A.; Bortun, L.; Bortun, A. Alkali metal ion exchange by the framework titanium silicate M2Ti2O3SiO4·nH2O (M=H, Na). React. Funct. Polym. 2000, 43, 85–95. [Google Scholar] [CrossRef]
- Clearfield, A.; Medvedev, D.G.; Kerlegon, S.; Bosser, T.; Burns, J.D.; Jackson, M. Rates of Exchange of Cs+ and Sr2+ for Poorly Crystalline Sodium Titanium Silicate (CST) in Nuclear Waste Systems. Solvent Extr. Ion Exch. 2012, 30, 229–243. [Google Scholar] [CrossRef]
- Anthony, R.G.; Dosch, R.G.; Philip, C.V. Method of Using Novel Silico-Titanates. U.S. Patent 6,110,378, 29 August 2000. [Google Scholar]
- Miller, J.E.; Brown, N.E. Development and Properties of Crystalline Silicotitanate (CST) Ion Exchangers for Radioactive Waste Applications; Sandia National Lab: Albuquerque, NM, USA; Livermore, CA, USA; U.S. Department of Energy Office: Washington, DC, USA, 1997. [Google Scholar]
- Lehto, J.; Koivula, R.; Leinonen, H.; Tusa, E.; Harjula, R. Removal of Radionuclides from Fukushima Daiichi Waste Effluents. Sep. Purif. Rev. 2019, 48, 122–142. [Google Scholar] [CrossRef]
- Thorogood, G.J.; Kennedy, B.J.; Griffith, C.S.; Elcombe, M.M.; Avdeev, M.; Hanna, J.V.; Thorogood, S.K.; Luca, V. Structure and Phase Transformations in the Titanosilicate, Sitinakite. The Importance of Water. Chem. Mater. 2010, 22, 4222–4231. [Google Scholar] [CrossRef]
- Tripathi, A.; Medvedev, D.G.; Clearfield, A. The crystal structures of strontium exchanged sodium titanosilicates in relation to selectivity for nuclear waste treatment. J. Solid State Chem. 2005, 178, 253–261. [Google Scholar] [CrossRef]
- Celestian, A.J.; Parise, J.B.; Smith, R.I.; Toby, B.H.; Clearfield, A. Role of the Hydroxyl−Water Hydrogen-Bond Network in Structural Transitions and Selectivity toward Cesium in Cs 0.38 (D 1.08 H 0.54 )SiTi 2 O 7·(D 0.86 H 0.14 ) 2 O Crystalline Silicotitanate. Inorg. Chem. 2007, 46, 1081–1089. [Google Scholar] [CrossRef]
- Nilchi, A.; Maalek, B.; Khanchi, A.; Ghanadi Maragheh, M.; Bagheri, A.; Savoji, K. Ion exchangers in radioactive waste management: Natural Iranian zeolites. Appl. Radiat. Isot. 2006, 64, 138–143. [Google Scholar] [CrossRef]
- Zouad, S.; Jeanjean, J.; Loos-Neskovic, C.; Fedoroff, M.; Piffard, Y. Sorption of strontium and lanthanum on polyantimonic acid and two phosphatoantimonic acids. J. Radioanal. Nucl. Chem. Artic. 1994, 182, 193–204. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Chukanov, N.V.; Aksenov, S.M.; Mazur, A.S.; Avdontseva, E.Y.; Shilovskikh, V.V.; Krivovichev, S.V. Alumovesuvianite, Ca19Al(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group member from the Jeffrey mine, asbestos, Estrie region, Québec, Canada. Mineral. Petrol. 2017, 111, 833–842. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Shilovskikh, V.V.; Avdontseva, E.Y.; Zolotarev, A.A.; Karpenko, V.Y.; Mazur, A.S.; Yakovenchuk, V.N.; Bazai, A.V.; Krivovichev, S.V.; Pekov, I.V. Magnesiovesuvianite, Ca19Mg(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group mineral. J. Geosci. (Czech Repub.) 2017, 62, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Krivovichev, S.V.; Panikorovskii, T.L.; Yakovenchuk, V.N.; Selivanova, E.A.; Ivanyuk, G.Y. Trigonal variation in the garnet supergroup: The crystal structure of nikmelnikovite, Ca12Fe2+Fe3+3Al3(SiO4)6(OH)20, from Kovdor massif, Kola Peninsula, Russia. Mineral. Mag. 2021, 85, 620–626. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Mikhailova, J.A.; Pakhomovsky, Y.A.; Bazai, A.V.; Aksenov, S.M.; Kalashnikov, A.O.; Krivovichev, S.V. Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia. Minerals 2021, 11, 982. [Google Scholar] [CrossRef]
- Perovskiy, I.A.; Khramenkova, E.V.; Pidko, E.A.; Krivoshapkin, P.V.; Vinogradov, A.V.; Krivoshapkina, E.F. Efficient extraction of multivalent cations from aqueous solutions into sitinakite-based sorbents. Chem. Eng. J. 2018, 354, 727–739. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Blatova, O.A.; Ivanov-Schits, A.K.; Dem’yanets, L.N. Migration maps of Li+ cations in oxygen-containing compounds. Solid State Ion. 2008, 179, 2248–2254. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Men’shikov, Y.P. Khibiny; Wall, F., Ed.; Laplandia Minerals: Apatity, Russia, 2005. [Google Scholar]
- Snyatkova, O.L.; Mikhnyak, N.K. Report on the Results of a Geological Study and Geochemical Exploration for Rare Metals and Apatite on the Scale 1:50000, Carried Out within the Khibiny Massif and Its Surrounding Area during 1979–1983; Rosgeolfond: Apatity, Russia, 1983. [Google Scholar]
- Perovskiy, I.A.; Ignat’ev, G.V. Ammonium fluoride method of desilication of leucoxene concentrate of Yarega deposit. Predictive estimate of technologicalproperties of minerals by applied mineralogy methods. Proceedings of 7th Russian Seminar of Process Mineralogy, Karelian Scientific Centre RAS, Petrozavodsk, Russia, 9 April 2013; pp. 110–116. (In Russian). [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-Dimensional Visualization in Powder Diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Agilent Technologies CrysAlis CCD and CrysAlis RED; Oxford Diffr. Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Eremin, R.A.; Kabanova, N.A.; Morkhova, Y.A.; Golov, A.A.; Blatov, V.A. High-throughput search for potential potassium ion conductors: A combination of geometrical-topological and density functional theory approaches. Solid State Ion. 2018, 326, 188–199. [Google Scholar] [CrossRef]
- Fedotov, S.S.; Kabanova, N.A.; Kabanov, A.A.; Blatov, V.A.; Khasanova, N.R.; Antipov, E.V. Crystallochemical tools in the search for cathode materials of rechargeable Na-ion batteries and analysis of their transport properties. Solid State Ion. 2018, 314, 129–140. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P. Analysis of voids in crystal structures: The methods of “dual” crystal chemistry. Acta Crystallogr. Sect. A Found. Crystallogr. 2003, 59, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.C. Atomic radii in crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- O‘Keeffe, M.; Yaghi, O.M. Deconstructing the crystal structures of Metal-Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Proserpio, D.M. Natural tilings for zeolite-type frameworks. J. Phys. Chem. C 2010, 114, 10160–10170. [Google Scholar] [CrossRef]
- Kabanova, N.A.; Panikorovskii, T.L.; Shilovskikh, V.V.; Vlasenko, N.S.; Yakovenchuk, V.N.; Aksenov, S.M.; Bocharov, V.N.; Krivovichev, S.V. The Na2−nHn[Zr(Si2O7)]∙mH2O Minerals and Related Compounds (n = 0–0.5; m = 0.1): Structure Refinement, Framework Topology, and Possible Na+-Ion Migration Paths. Crystals 2020, 10, 1016. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Kabanova, N.A.; Chukanov, N.V.; Panikorovsky, T.L.; Blatov, V.A.; Krivovichev, S.V. The role of local heteropolyhedral substitutions on the stoichiometry, topology, and ion-migration paths in the eudialyte-type structures: A quantitative analysis. IUCrJ 2021. In print. [Google Scholar]
- Blatov, V.A.; Delgado-Friedrichs, O.; O’Keeffe, M.; Proserpio, D.M. Three-periodic nets and tilings: Natural tilings for nets. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 63, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Blatov, V.A. Methods for topological analysis of atomic nets. J. Struct. Chem. 2009, 50, 160–167. [Google Scholar] [CrossRef]
- Chapman, D.M.; Roe, A.L. Synthesis, characterization and crystal chemistry of microporous titanium-silicate materials. Zeolites 1990, 10, 730–737. [Google Scholar] [CrossRef]
- Celestian, A.J.; Powers, M.; Rader, S. In situ Raman spectroscopic study of transient polyhedral distortions during cesium ion exchange into sitinakite. Am. Mineral. 2013, 98, 1153–1161. [Google Scholar] [CrossRef]
- Yakovenchuk, V.; Pakhomovsky, Y.; Panikorovskii, T.; Zolotarev, A.; Mikhailova, J.; Bocharov, V.; Krivovichev, S.; Ivanyuk, G. Chirvinskyite, (Na,Ca)13(Fe,Mn,□)2(Ti,Nb)2(Zr,Ti)3-(Si2O7)4(OH,O,F)12, a New Mineral with a Modular Wallpaper Structure, from the Khibiny Alkaline Massif (Kola Peninsula, Russia). Minerals 2019, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Pakhomovsky, Y.A.; Panikorovskii, T.L.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Mikhailova, J.A.; Krivovichev, S.V.; Bocharov, V.N.; Kalashnikov, A.O. Selivanovaite, NaTi3(Ti,Na,Fe,Mn)4[(Si2O7)2O4(OH,H2O)4]·nH2O, a new rock-forming mineral from the eudialyte-rich malignite of the Lovozero alkaline massif (Kola Peninsula, Russia). Eur. J. Mineral. 2018, 30, 525–535. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.; Mihailova, B.; Kalvachev, Y.; Tarassov, M. Atomic arrangements in amorphous sodium titanosilicate precursor powders. Microporous Mesoporous Mater. 2005, 86, 223–230. [Google Scholar] [CrossRef]
- Ferdov, S.; Lin, Z.; Sá Ferreira, R.A.; Correia, M.R. Hydrothermal synthesis, structural, and spectroscopic studies of vanadium substituted ETS-4. Microporous Mesoporous Mater. 2008, 110, 436–441. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Waclawik, E.R.; Ke, X.; Zheng, Z.; Zhu, H.; Frost, R.L. A Raman spectroscopic study on the active site of sodium cations in the structure of Na2Ti3O7 during the adsorption of Sr2+ and Ba2+ cations. J. Raman Spectrosc. 2010, 41, 1792–1796. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Montejo, M.; López González, J.J. Structure and Vibrational Spectra of Ti(IV) Hydroxides and Their Clusters with Expanded Titanium Coordination. DFT Study. J. Phys. Chem. A 2007, 111, 7973–7979. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Li, G.; Liu, Y.; Withers, R.L. Atomic-scale control of TiO6 octahedra through solution chemistry towards giant dielectric response. Sci. Rep. 2015, 4, 6582. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.-Y.; Yu, C.-C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef]
- Środek, D.; Dulski, M.; Galuskina, I. Raman imaging as a new approach to identification of the mayenite group minerals. Sci. Rep. 2018, 8, 13593. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Rastsvetaeva, R.K.; Andrianov, V.I.; Egorov-Tismenko, Y.K.; Men’shikov, Y.P. The crystal structure of a new natural sodium titanosilicate. Sov. Phys. Dokl. 1989, 34, 583–585. [Google Scholar]
- Krivovichev, S. Topology of Microporous Structures. Rev. Mineral. Geochem. 2005, 57, 17–68. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Selivanova, E.A.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Spiridonova, D.V.; Krivovichev, S.V. First Natural Pharmacosiderite-Related Titanosilicates and Their Ion-Exchange Properties. In Minerals as Advanced Materials I; Springer: Berlin/Heidelberg, Germany, 2008; pp. 27–35. [Google Scholar]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts. Microporous Mesoporous Mater. 2003, 58, 3–13. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Filatov, S.K.; Bubnova, R.S. The nature of special points on unit cell parameters temperature dependences for crystal substances. Z. Krist. Suppl. 2007, 2007, 447–452. [Google Scholar] [CrossRef]
- Spiridonova, D.V.; Krivovichev, S.V.; Yakovenchuk, V.N.; Pakhomovsky, Y.A. Crystal structures of the Rb- and Sr-exchanged forms of ivanyukite-Na-T. Geol. Ore Depos. 2011, 53, 670–677. [Google Scholar] [CrossRef]




| Parameter | Data | |
|---|---|---|
| sitinakite | La-exchanged sitinakite | |
| Temperature/K | 293(2) | 293(2) |
| Crystal system | tetragonal | orthorhombic |
| Space group | P42/mcm | Cmmm |
| a/Å | 7.8159(2) | 11.0339(10) |
| b/Å | 7.8159(2) | 11.0598(8) |
| c/Å | 12.0167(3) | 11.8430(7) |
| Volume/Å3 | 734.08(4) | 1445.23(19) |
| Z | 2 | 4 |
| ρcalc/g/cm3 | 2.763 | 2.990 |
| μ/mm−1 | 2.602 | 4.670 |
| F(000) | 592.0 | 1230.0 |
| Crystal size/mm3 | 0.15 × 0.14 × 0.14 | 0.15 × 0.14 × 0.14 |
| Radiation | Mo Kα (λ = 0.71073) | |
| 2Θ range for data collection/° | 6.782 to 52.976 | 6.882 to 52.97 |
| Index ranges | −6 ≤ h ≤ 9, −6 ≤ k ≤ 9, −12 ≤ l ≤ 15 | −12 ≤ h ≤ 12, −13 ≤ k ≤ 11, −14 ≤ l ≤ 14 |
| Reflections collected | 1660 | 3559 |
| Independent reflections | 444 [Rint = 0.0291, Rsigma = 0.0248] | 841 [Rint = 0.0182, Rsigma = 0.0122] |
| Data/restraints/parameters | 444/0/54 | 841/24/104 |
| Goodness-of-fit on F2 | 1.185 | 1.136 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0343, wR2 = 0.0918 | R1 = 0.0372, wR2 = 0.1135 |
| Final R indexes [all data] | R1 = 0.0366, wR2 = 0.0933 | R1 = 0.0373, wR2 = 0.1135 |
| Largest diff. peak/hole/e Å−3 | 0.74/−0.62 | 1.06/−0.94 |
| Sample | Sitinakite, nat. | Sitinakite, syn. | La-Sitinakite, syn. 12 h Exchanged Form | La-Sitinakite, nat. 24 h Exchanged Form |
|---|---|---|---|---|
| SiO2 | 20.20 | 21.61 | 17.80 | 19.66 |
| TiO2 | 44.70 | 46.92 | 45.36 | 40.55 |
| Al2O3 | 0.43 | 0.42 | ||
| FeO | 0.15 | 2.42 | 0.84 | 0.13 |
| Na2O | 12.17 | 13.48 | 0.77 | |
| K2O | 2.15 | 0.47 | 0.08 | |
| SrO | 0.92 | |||
| Nb2O5 | 5.49 | 4.64 | ||
| La2O3 | 22.83 | 21.99 | ||
| H2O 1 | 12.90 | 14.28 | 11.98 | 12.18 |
| Total | 99.11 | 99.18 | 99.58 | 99.65 |
| Atoms per formula unit normalized on the basis of 6 Si+Ti+Nb+Fe+Al atoms | ||||
| Si4+ | 2.11 | 2.20 | 2.03 | 2.21 |
| Ti4+ | 3.52 | 3.59 | 3.89 | 3.43 |
| Al3+ | 0.10 | 0.11 | ||
| Fe2+ | 0.01 | 0.21 | 0.08 | 0.01 |
| Nb5+ | 0.26 | 0.24 | ||
| Sum O | 3.89 | 3.80 | 3.97 | 3.79 |
| K+ | 0.29 | 0.06 | 0.01 | |
| Na+ | 2.47 | 2.66 | 0.17 | |
| Sr2+ | 0.06 | |||
| La3+ | 0.96 | 0.91 | ||
| Sum A | 2.83 | 2.72 | 1.13 | 0.92 |
| OH− | 1.00 | 1.70 | 1.11 | 1.15 |
| H2O | 4.00 | 4.00 | 4.00 | 4.00 |
| Raman Shift, cm−1 | ||
|---|---|---|
| Sitinakite | La-Exchanged Sitinakite | Assignment |
| 130 | 125 | lattice vibrations |
| 186 | 183 | lattice vibrations |
| 241 | TiO6 | |
| 281s | 286s | TiO6 |
| 313s | 313sh | TiO6 |
| 384 | 393 | SiO4 |
| 370 | 378 | SiO4 |
| 420 | 438 | TiO6 |
| 512 | TiO6 | |
| 570s | 580 | SiO4, TiO6 |
| 608s | SiO4, TiO6 | |
| 776 | 778w | SiO4 |
| 868 | 871w | SiO4 |
| 928s | 945 | SiO4 |
| 965 | SiO4 | |
| 1642 | 1660 | H2O |
| 3216 | 3240 | OH |
| 3367 | OH | |
| 3511 | 3506 | OH |
| 3562 | H2O | |
| La-Exchanged Sitinakite | Sitinakite | ||||||
|---|---|---|---|---|---|---|---|
| site | Ti1 | Ti2 | Si1 | sum/sum * | Ti1 | Si1 | sum/sum * |
| O1 | 0.765↓×2 | 0.992↓×2 | 1.76/1.96 | 0.751 | 0.987↓×4 | 1.74/1.98 | |
| O2 | 0.803↓×2 | 1.014↓×2 | 1.82/1.93 | 0.602↓×2→×2 0.430 | 1.63/1.63 | ||
| O3 | 0.976→×2 | 1.95/2.03 | 0.926→×2 | 1.85/1.90 | |||
| O4 | 0.627↓×2→×3 | 0.477 | 1.88/1.92 | ||||
| O5 | 0.436 | 0.553↓×2→×2 | 1.54/1.56 | ||||
| O6 | 0.971→×2 | 1.94/1.96 | |||||
| Sum | 4.20 | 4.16 | 4.01 | 4.24 | 3.95 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panikorovskii, T.L.; Kalashnikova, G.O.; Nikolaev, A.I.; Perovskiy, I.A.; Bazai, A.V.; Yakovenchuk, V.N.; Bocharov, V.N.; Kabanova, N.A.; Krivovichev, S.V. Ion-Exchange-Induced Transformation and Mechanism of Cooperative Crystal Chemical Adaptation in Sitinakite: Theoretical and Experimental Study. Minerals 2022, 12, 248. https://doi.org/10.3390/min12020248
Panikorovskii TL, Kalashnikova GO, Nikolaev AI, Perovskiy IA, Bazai AV, Yakovenchuk VN, Bocharov VN, Kabanova NA, Krivovichev SV. Ion-Exchange-Induced Transformation and Mechanism of Cooperative Crystal Chemical Adaptation in Sitinakite: Theoretical and Experimental Study. Minerals. 2022; 12(2):248. https://doi.org/10.3390/min12020248
Chicago/Turabian StylePanikorovskii, Taras L., Galina O. Kalashnikova, Anatoly I. Nikolaev, Igor A. Perovskiy, Ayya V. Bazai, Victor N. Yakovenchuk, Vladimir N. Bocharov, Natalya A. Kabanova, and Sergey V. Krivovichev. 2022. "Ion-Exchange-Induced Transformation and Mechanism of Cooperative Crystal Chemical Adaptation in Sitinakite: Theoretical and Experimental Study" Minerals 12, no. 2: 248. https://doi.org/10.3390/min12020248
APA StylePanikorovskii, T. L., Kalashnikova, G. O., Nikolaev, A. I., Perovskiy, I. A., Bazai, A. V., Yakovenchuk, V. N., Bocharov, V. N., Kabanova, N. A., & Krivovichev, S. V. (2022). Ion-Exchange-Induced Transformation and Mechanism of Cooperative Crystal Chemical Adaptation in Sitinakite: Theoretical and Experimental Study. Minerals, 12(2), 248. https://doi.org/10.3390/min12020248










