Orthogonal Test Design for the Optimization of Preparation of Steel Slag-Based Carbonated Building Materials with Ultramafic Tailings as Fine Aggregates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
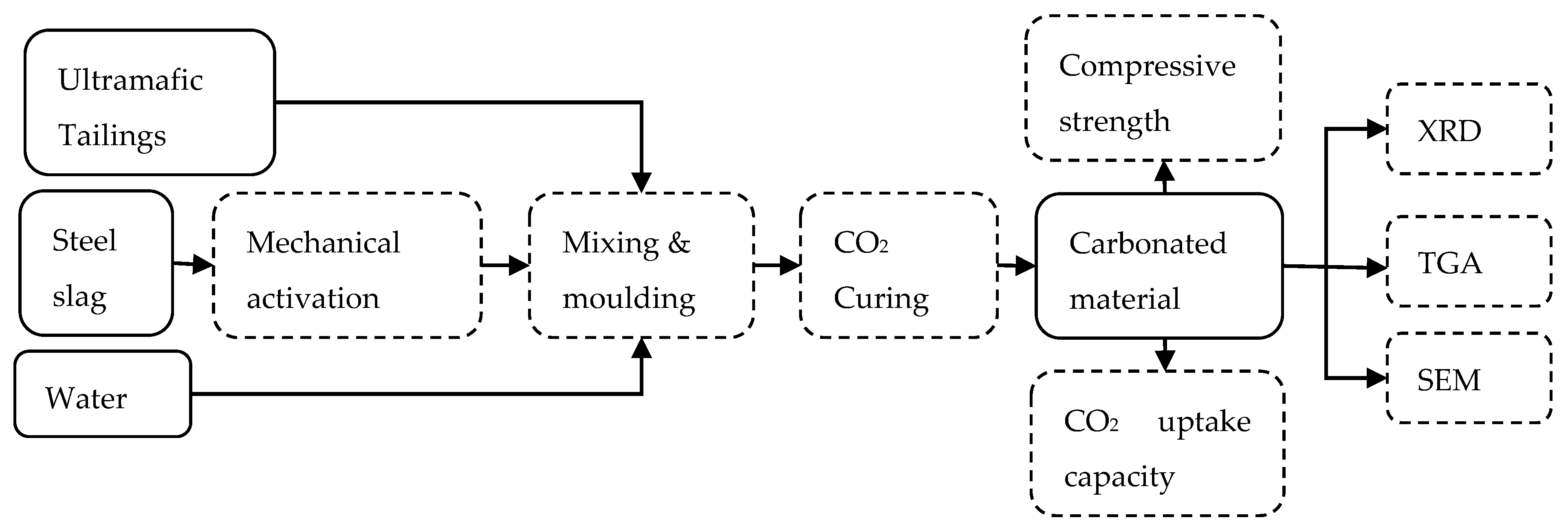
2.2. Experiments
2.2.1. Mechanical Activation
2.2.2. Mixing and Molding
2.2.3. Carbonation Curing
2.2.4. CO2 Uptake Capacity Calculation
2.2.5. Compressive Strength
2.2.6. Quantitative X-ray Diffraction
2.2.7. Thermal Gravity Analysis
2.2.8. Scanning Electron Microscopy
2.3. Orthogonal Experiment Method
2.3.1. Orthogonal Experimental Design
2.3.2. Range Analysis Method
3. Results and Discussions
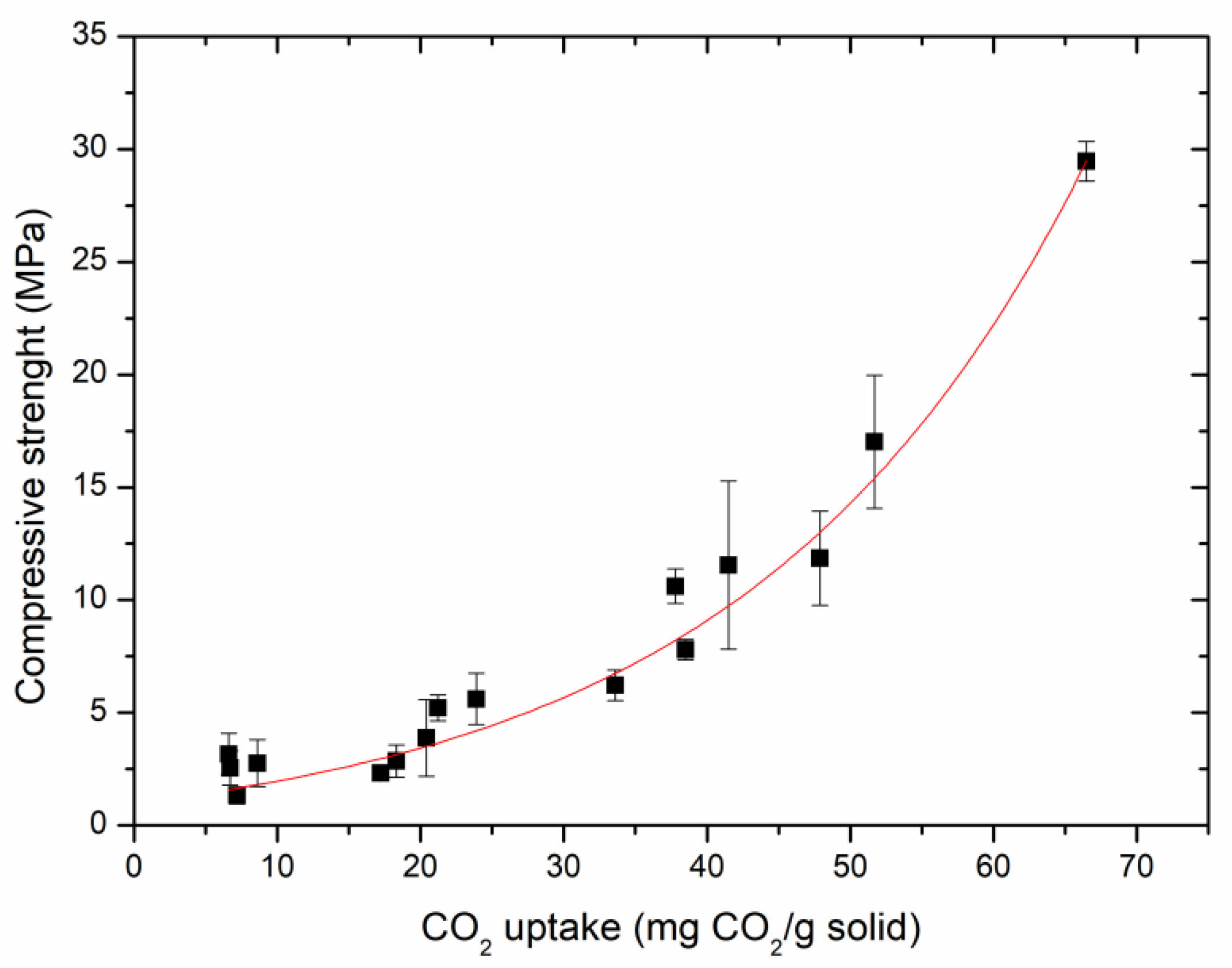
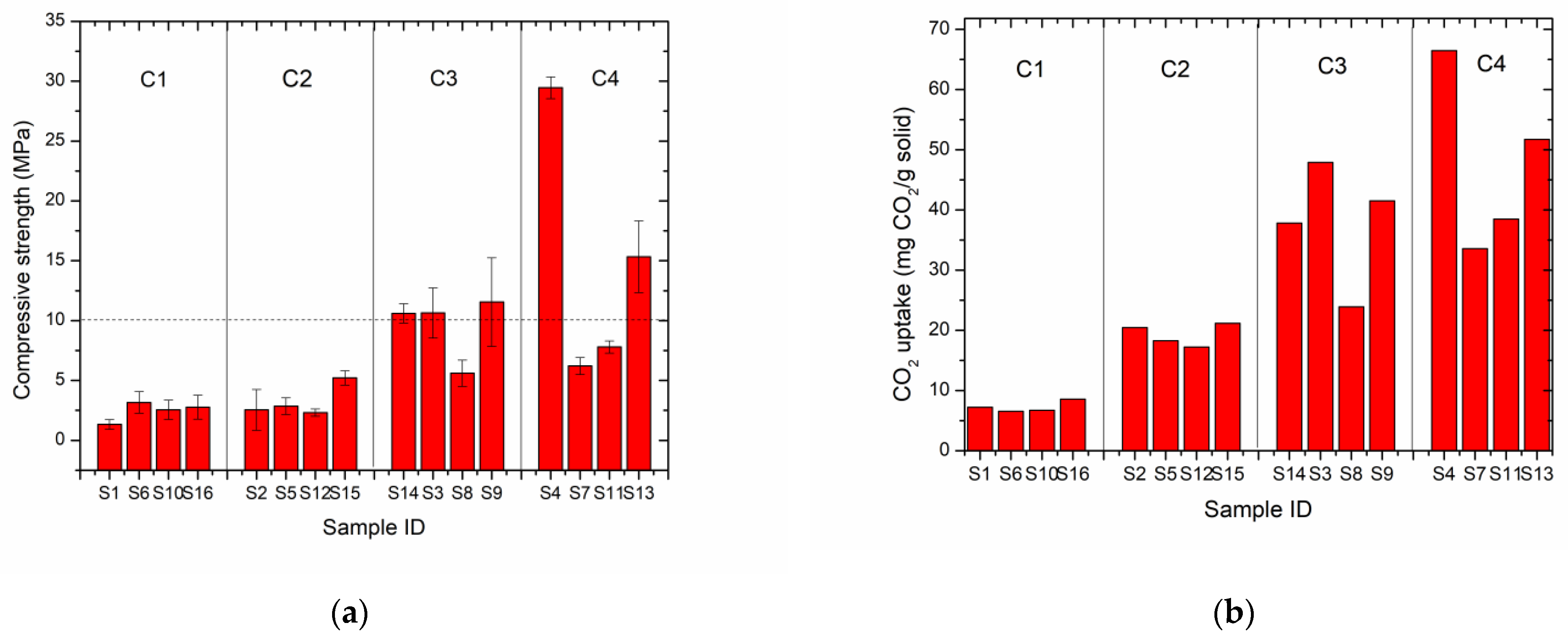
3.1. CO2 Uptake Capacity and Compressive Strength
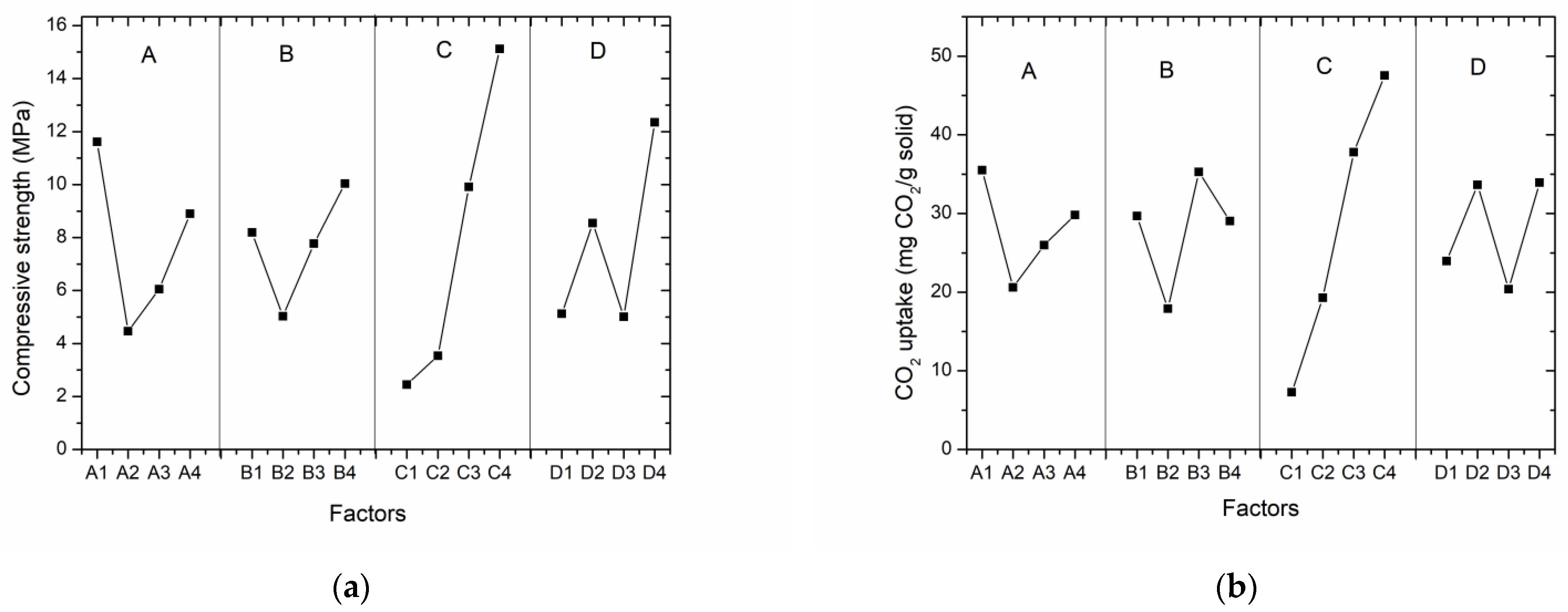
3.2. Range Analysis
3.3. Direct Analysis
3.3.1. The Effect of the Slag/Tailings Ratio on Carbonated Compacts
3.3.2. The Effect of the Water–Solid Ratio on Carbonated Compacts
3.3.3. The Effect of the Carbonation Time on Carbonated Compacts
3.3.4. The Effect of the Grinding Time for Steel Slag on Carbonated Compacts
3.4. Microstructure Analysis on the Optimal Samples
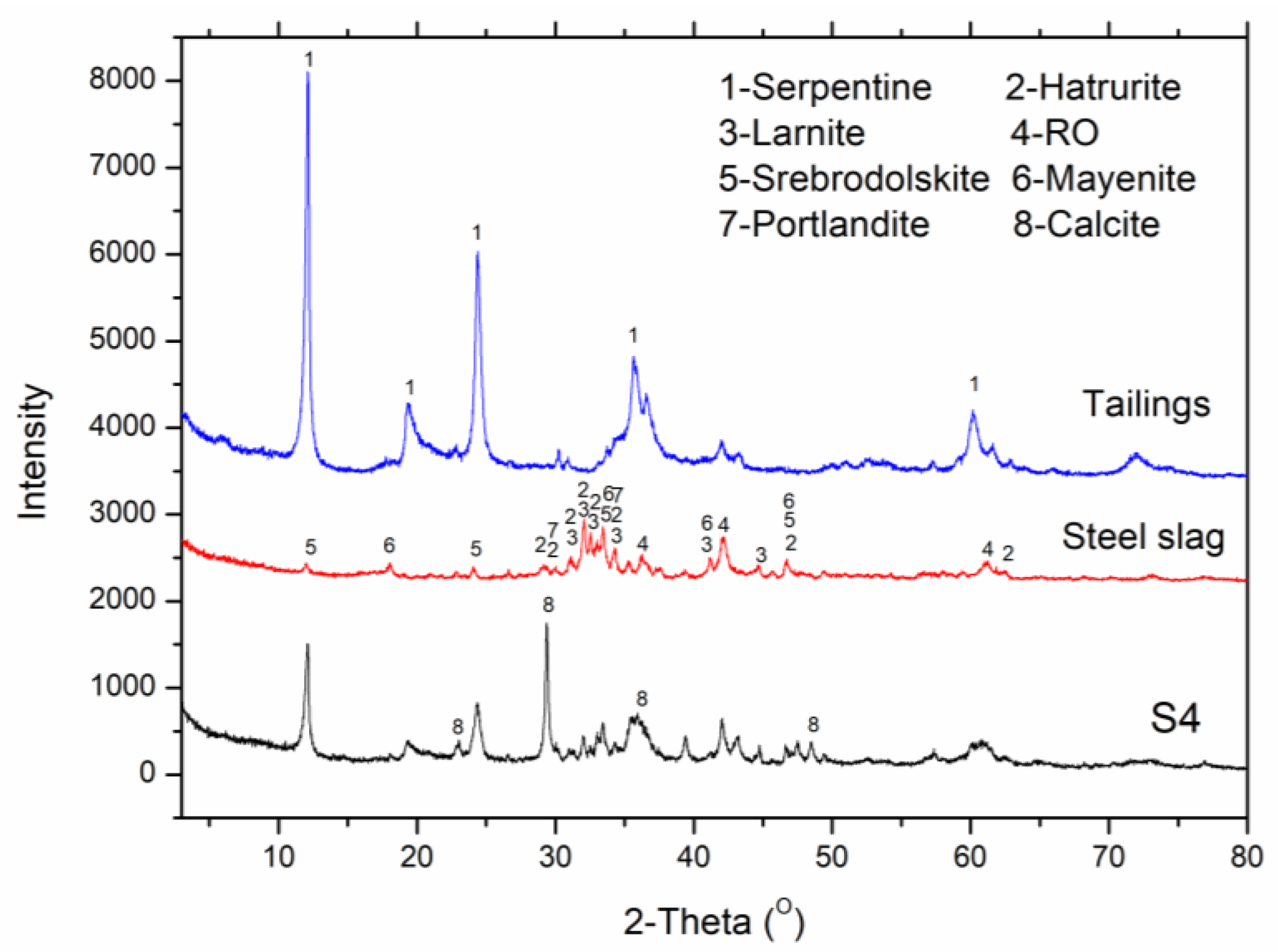
3.4.1. X-ray Diffraction Analysis
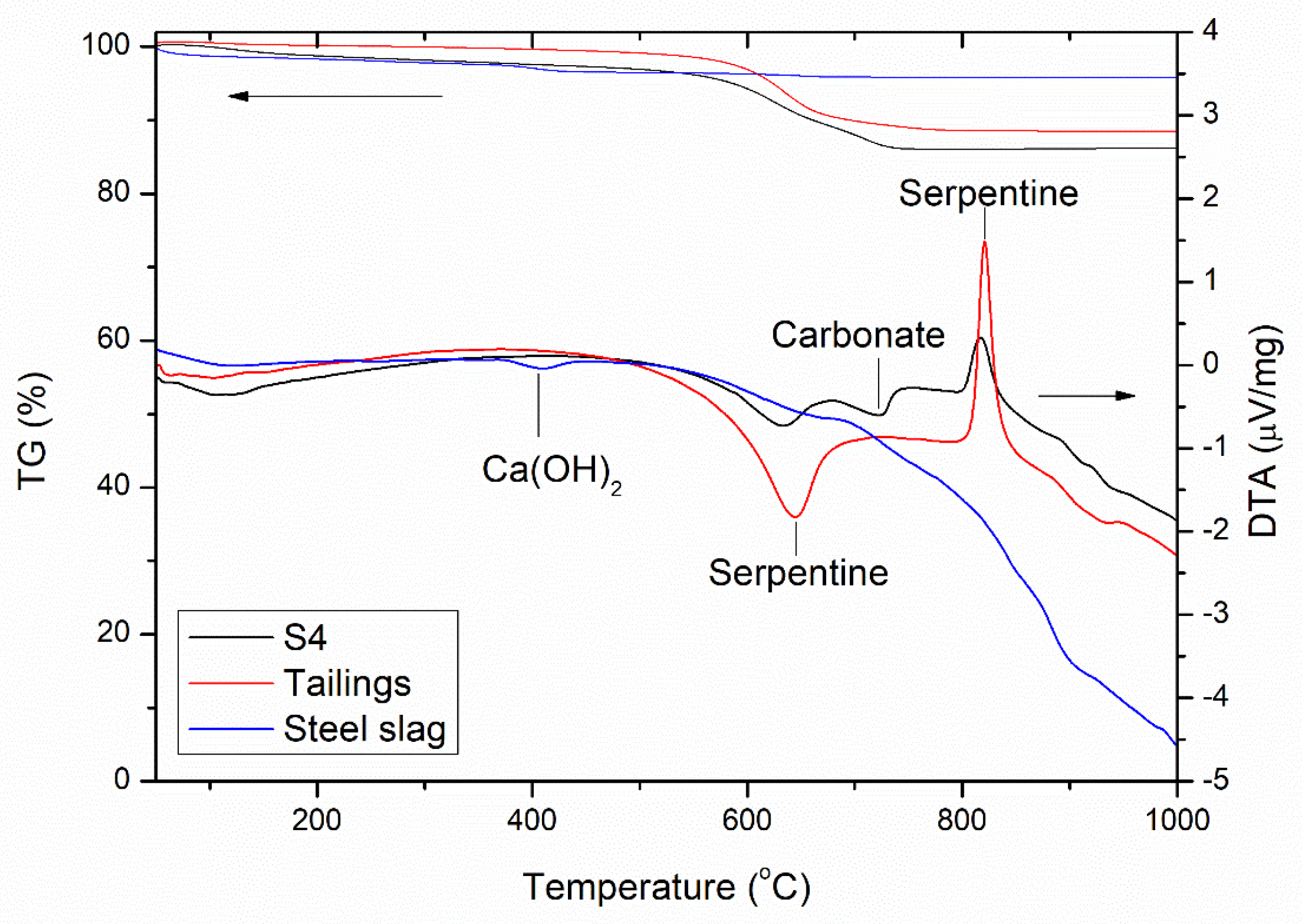
3.4.2. Thermal Analysis
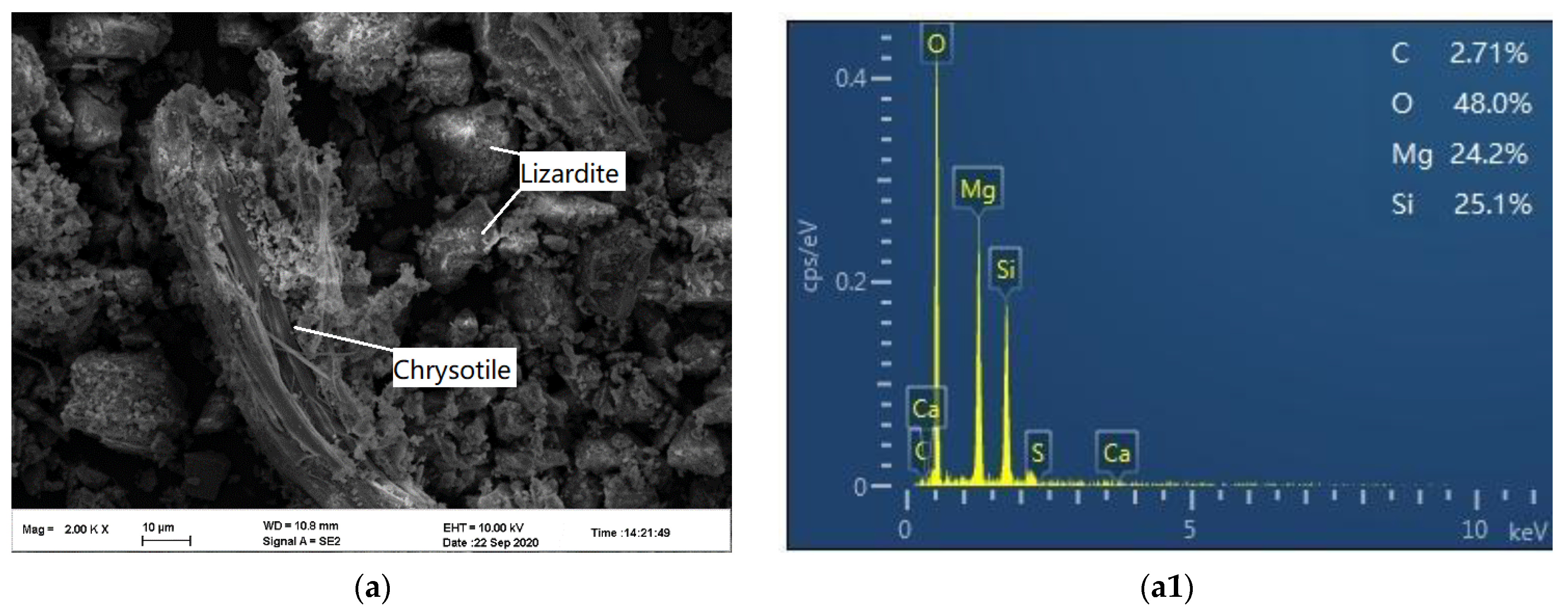
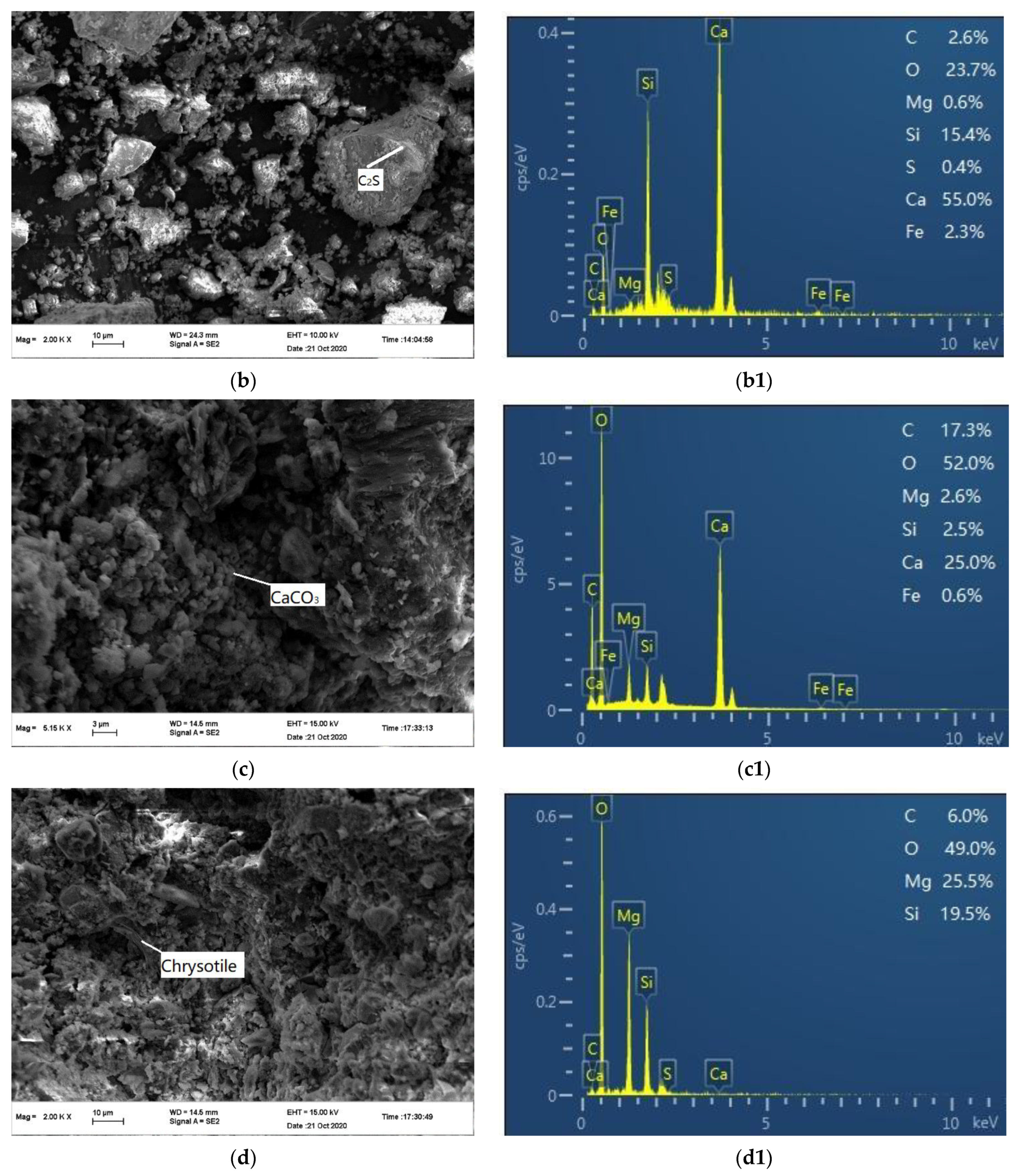
3.4.3. Morphology and Chemical Analysis
4. Conclusions
- (1)
- The compressive strength is positively exponentially related to the CO2 uptake capacity in the carbonated tailings-steel slag compacts. The optimal reaction conditions are steel slag grinding for 0 min, a water/solid ratio of 2.5:10, a slag/tailings ratio of 5:5, and carbonation curing for 12 h. The compressive strength of the carbonated compact reaches 29 MPa under the optimal reaction conditions.
- (2)
- The range analysis results of the orthogonal test show that the degree of the effects on the compressive strength of carbonated tailings-steel slag compacts ordered from large to small is slag–tailings ratio > carbonation time > grinding time for steel slag > water–solid ratio. The degree of the effects on the CO2 uptake capacity of carbonated tailings-steel slag compacts ordered from large to small is slag–tailings ratio > water–solid ratio > carbonation time > grinding time for steel slag.
- (3)
- A high water–solid ratio hinders the early carbonation reaction (1–3 h) and early strength development, but promotes the long-term (12 h) carbonation reaction and long-term strength development of carbonated tailings-steel slag compacts.
- (4)
- Steel slag are the main component for the hardening of carbonated tailings-steel slag compacts, while the fibrous minerals in the ultramafic tailings enforces the strength of the compacts.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. IPCC Special REPORT on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; 2019; in press; Available online: https://www.ipcc.ch/site/assets/uploads/sites/4/2021/07/210714-IPCCJ7230-SRCCL-Complete-BOOK-HRES.pdf (accessed on 10 February 2022).
- GOSAT. The Whole-Atmosphere Monthly Mean CO2 Concentration Based on GOSTA Observation. Available online: http://www.gosat.nies.go.jp/en/recent-global-co2.html (accessed on 15 February 2020).
- NOAA National Centers for Environmental Information. State of the Climate: Global Climate Report for 2021. Available online: https://www.ncdc.noaa.gov/sotc/global/202113/supplemental/page-1 (accessed on 15 February 2020).
- UNFCCC. Paris Agreement. Unitted Nations. 2015. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 15 February 2020).
- Tu, Z.; Guo, M.Z.; Poon, C.S.; Shi, C. Effects of limestone powder on CaCO3 precipitation in CO2 cured cement pastes. Cem. Concr. Compos. 2016, 72, 9–16. [Google Scholar] [CrossRef]
- Lord, M. Rethinking Cement; Beyound Zero Emissions Inc.: Victoria, Austrilia, 2017; Available online: https://bze.org.au/wp-content/uploads/2020/12/rethinking-cement-bze-report-2017.pdf (accessed on 15 February 2020).
- Huang, X.; Wang, Z.; Liu, Y.; Hu, W.; Ni, W. On the use of blast furnace slag and steel slag in the preparation of green artificial reef concrete. Costr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.C.; Pan, S.Y. Towards carbon-neutral construction materials: Carbonation of cement-based materials and the future perspective. J. Build. Eng. 2020, 28, 101062. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Younsi, A.; Turcry, P.; Rozire, E.; Aït-Mokhtar, A.; Loukili, A. Performance-based design and carbonation of concrete with high fly ash content. Cem. Concr. Compos. 2011, 33, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Qiu, Q. A state-of-the-art review on the carbonation process in cementitious materials: Fundamentals and characterization techniques. Constr. Build. Mater. 2020, 247, 118503. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, W. Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Librandi, P.; Nielsen, P.; Costa, G.; Snellings, R.; Quaghebeur, M.; Baciocchi, R. Mechanical and environmental properties of carbonated steel slag compacts as a function of mineralogy and CO2 uptake. J. CO2 Util. 2019, 33, 201–214. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- World Steel Association. Global Crude Steel Output Increases by 3.4% in 2019. Available online: https://worldsteel.org/media-centre/press-releases/2020/global-crude-steel-output-increases-by-3-4-in-2019/ (accessed on 10 February 2022).
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011, 463633. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Li, K.; Hu, W. Use of CO2 to cure steel slag and gypsum-based material. Energies 2021, 14, 5174. [Google Scholar] [CrossRef]
- Humbert, P.S.; Castro-Gomes, J. CO2 activated steel slag-based materials: A review. J. Clean. Prod. 2019, 208, 448–457. [Google Scholar] [CrossRef]
- Mo, L.; Hao, Y.; Liu, Y.; Wang, F.; Deng, M. Preparation of calcium carbonate binders via CO2 activation of magnesium slag. Cem. Concr. Res. 2019, 121, 81–90. [Google Scholar] [CrossRef]
- Mahoutian, M.; Ghouleh, Z.; Shao, Y. Carbon dioxide activated ladle slag binder. Constr. Build. Mater. 2014, 66, 214–221. [Google Scholar] [CrossRef]
- Mahoutian, M.; Shao, Y.; Mucci, A.; Fournier, B. Carbonation and hydration behavior of EAF and BOF steel slag binders. Mater. Struct. 2014, 3075–3085. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. Carbon sequestration through accelerated carbonation of BOF slag: Influence of particle size characteristics. Chem. Eng. J. 2016, 298, 26–35. [Google Scholar] [CrossRef]
- Li, J.; Ni, W.; Wang, X.; Zhu, S.; Wei, X.; Jiang, F.; Zeng, H.; Hitch, M. Mechanical activation of medium basicity steel slag under dry condition for carbonation curing. J. Build. Eng. 2022, 50, 104123. [Google Scholar] [CrossRef]
- Chang, J.; Xiong, C.; Zhang, Y.; Wang, D. Foaming characteristics and microstructure of aerated steel slag block prepared by accelerated carbonation. Constr. Build. Mater. 2019, 209, 222–233. [Google Scholar] [CrossRef]
- Cadore, D.E.; Angulski da Luz, C.; Farias de Medeiros, M.H. An investigation of the carbonation of alkaline activated cement made from blast furnace slag generated by charcoal. Constr. Build. Mater. 2019, 226, 117–125. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Li, K. Study on Mineral Compositions of Direct Carbonated Steel Slag by QXRD, TG, FTIR, and XPS. Energies 2021, 14, 4489. [Google Scholar] [CrossRef]
- Wei, X.; Ni, W.; Zhang, S.; Wang, X.; Li, J.; Du, H. Influence of the key factors on the performance of steel slag-desulphurisation gypsum-based hydration-carbonation materials. J. Build. Eng. 2022, 45, 103591. [Google Scholar] [CrossRef]
- Humbert, P.S.; Castro-Gomes, J.P.; Savastano, H. Clinker-free CO2 cured steel slag based binder: Optimal conditions and potential applications. Constr. Build. Mater. 2019, 210, 413–421. [Google Scholar] [CrossRef]
- Nielsen, P.; Boone, M.A.; Horckmans, L.; Snellings, R.; Quaghebeur, Q. Accelerated carbonation of steel slag monoliths at low CO2 pressure-microstructure and strength development. J. CO2 Util. 2020, 36, 124–134. [Google Scholar] [CrossRef]
- Wang, D.; Chang, J.; Ansari, W.S. The effects of carbonation and hydration on the mineralogy and microstructure of basic oxygen furnace slag products. J. CO2 Util. 2019, 34, 87–98. [Google Scholar] [CrossRef]
- He, Z.; Wang, S.; Mahoutian, M.; Shao, Y. Flue gas carbonation of cement-based building products. J. CO2 Util. 2020, 37, 309–319. [Google Scholar] [CrossRef]
- Wang, C.; Liang, Q.; Wang, Y.; Ni, W.; Qiao, C. Experimental study on perparation of autoclaved aerated concrete with iron ore tailings in Dashihe Iron Ming of Shougang. Met. Mine 2013, 2, 4–8. [Google Scholar]
- Li, J.; Hitch, M.; Power, I.M.; Pan, Y. Integrated mineral carbonation of ultramafic mine, A review. Minerals 2018, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation, a review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Carbon dioxide adsorption isotherm study on mine waste for integrated CO2 capture and sequestration processes. Powder Technol. 2016, 291, 408–413. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon mineralization: From natural analogues to engineered systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. The role of brucite, ground granulated blastfurnace slag, and magnesium silicates in the carbonation and performance of MgO cements. Constr. Build. Mater. 2015, 94, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hitch, M. Characterization of the microstructure of mechanically-activated olivine using X-ray diffraction pattern analysis. Miner. Eng. 2016, 86, 24–33. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Ultra-fine grinding and mechanical activation of mine waste rock using a planetary mill for mineral carbonation. Int. J. Miner. Process. 2017, 158, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yang, J.; Yan, P. Cementitious properties of super-fine steel slag. Powder Technol. 2013, 245, 35–39. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Structural and chemical changes in mine waste mechanically-activated invarious milling environments. Powder Technol. 2017, 308, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Hao, J. Orthogonal test design for optimization of synthesis of super early strength anchoring material. Constr. Build. Mater. 2018, 181, 42–48. [Google Scholar] [CrossRef]
- Li, Z.; He, Z.; Chen, X. The Performance of Carbonation-Cured Concrete. Materials 2019, 12, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yan, P. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Chen, Z.; Li, R.; Zheng, X.; Liu, J. Carbon sequestration of steel slag and carbonation for activating RO phase. Cem. Concr. Res. 2021, 139, 106271. [Google Scholar] [CrossRef]
- Wilson, S.A.; Raudsepp, M.; Dipple, G.M. Verifying and quantifying carbon fixation in minerals from serpentine-rich mine tailings using the Rietveld method with X-ray powder diffraction data. Am. Mineral. 2006, 91, 1331–1341. [Google Scholar] [CrossRef]
- Harrison, A.L.; Dipple, G.M.; Power, I.M.; Ulrich Mayer, K. Influence of surface passivation and water content on mineral reactions in unsaturated porous media: Implications for brucite carbonation and CO2 sequestration. Geochim. Cosmochim. Acta 2015, 148, 477–495. [Google Scholar] [CrossRef]
- Viti, C. Serpentine minerals discrimination by thermal analysis. Am. Mineral. 2010, 95, 631–638. [Google Scholar] [CrossRef]
- Hrsak, D.; Sucik, G.; Lazic, L. The thermophysical properties of serpentinite. Metalurgija 2008, 47, 29–31. [Google Scholar]
- Wang, Q.; Wang, D.; Zhuang, S. The soundness of steel slag with different free CaO and MgO contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Smarzewski, P. Influence of basalt-polypropylene fibres on fracture properties of high performance concrete. Compos. Struct. 2019, 209, 23–33. [Google Scholar] [CrossRef]

| Fe2O3 | Al2O3 | SiO2 | CaO | MgO | P2O5 | C | S | LOI | Others | |
|---|---|---|---|---|---|---|---|---|---|---|
| Tailings | 5.13 | 0.58 | 38.00 | 1.52 | 36.08 | 0.03 | 0.16 | 0.03 | 11.50 | 6.79 |
| Steel slag | 27.6 | 5.2 | 11.63 | 37.13 | 7.5 | 1.29 | 0.41 | 0.10 | 4.24 | 4.9 |
| Sample ID | Levels | Factors | ||||||
|---|---|---|---|---|---|---|---|---|
| A (Grinding Time for Steel Slag) | B (Water–Solid Ratio) | C (Slag–Tailings Ratio) | D (Carbonation Time) | A (min) | B | C | D (h) | |
| S1 | 1 | 1 | 1 | 1 | 0 | 1:10 | 0:1 | 1 |
| S2 | 1 | 2 | 2 | 2 | 0 | 1.5:10 | 1:9 | 3 |
| S3 | 1 | 3 | 3 | 3 | 0 | 2:10 | 3:7 | 6 |
| S4 | 1 | 4 | 4 | 4 | 0 | 2.5:10 | 5:5 | 12 |
| S5 | 2 | 1 | 2 | 3 | 30 | 1:10 | 1:9 | 6 |
| S6 | 2 | 2 | 1 | 4 | 30 | 1.5:10 | 0:1 | 12 |
| S7 | 2 | 3 | 4 | 1 | 30 | 2:10 | 5:5 | 1 |
| S8 | 2 | 4 | 3 | 2 | 30 | 2.5:10 | 3:7 | 3 |
| S9 | 3 | 1 | 3 | 4 | 60 | 1:10 | 3:7 | 12 |
| S10 | 3 | 2 | 1 | 3 | 60 | 1.5:10 | 0:1 | 6 |
| S11 | 3 | 3 | 4 | 2 | 60 | 2:10 | 5:5 | 3 |
| S12 | 3 | 4 | 2 | 1 | 60 | 2.5:10 | 1:9 | 1 |
| S13 | 4 | 1 | 4 | 2 | 120 | 1:10 | 5:5 | 3 |
| S14 | 4 | 2 | 3 | 1 | 120 | 1.5:10 | 3:7 | 1 |
| S15 | 4 | 3 | 2 | 4 | 120 | 2:10 | 1:9 | 12 |
| S16 | 4 | 4 | 1 | 3 | 120 | 2.5:10 | 0:1 | 6 |
| Sample ID | Compressive Strength (MPa) | CO2 Uptake (mg CO2/g Solid) |
|---|---|---|
| S1 | 1.3 | 7.2 |
| S2 | 3.8 | 20.4 |
| S3 | 11.9 | 47.9 |
| S4 | 29.5 | 66.5 |
| S5 | 2.9 | 18.3 |
| S6 | 3.2 | 6.6 |
| S7 | 6.2 | 33.6 |
| S8 | 5.6 | 23.9 |
| S9 | 11.6 | 41.5 |
| S10 | 2.6 | 6.7 |
| S11 | 7.8 | 38.5 |
| S12 | 2.3 | 17.2 |
| S13 | 17.0 | 51.7 |
| S14 | 10.6 | 37.8 |
| S15 | 5.2 | 21.2 |
| S16 | 2.8 | 8.6 |
| Factors | Compressive Strength (MPa) | CO2 Uptake (mg CO2/g Solid) | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| K1 | 46.42 | 32.76 | 9.80 | 20.46 | 142.03 | 118.74 | 29.09 | 95.84 |
| K2 | 17.84 | 20.10 | 14.16 | 34.20 | 82.33 | 71.59 | 77.11 | 134.54 |
| K3 | 24.20 | 31.06 | 39.62 | 20.02 | 103.95 | 141.13 | 151.19 | 81.49 |
| K4 | 35.60 | 40.14 | 60.48 | 49.38 | 119.30 | 116.16 | 190.22 | 135.74 |
| k1 | 11.61 | 8.19 | 2.45 | 5.12 | 35.51 | 29.69 | 7.27 | 23.96 |
| k2 | 4.46 | 5.03 | 3.54 | 8.55 | 20.58 | 17.90 | 19.28 | 33.64 |
| k3 | 6.05 | 7.77 | 9.91 | 5.01 | 25.99 | 35.28 | 37.80 | 20.37 |
| k4 | 8.90 | 10.04 | 15.12 | 12.35 | 29.83 | 29.04 | 47.56 | 33.94 |
| Range | 7.15 | 5.01 | 12.67 | 7.23 | 9.52 | 17.39 | 40.28 | 13.56 |
| Ranking | C > D > A > B | C > B > D > A | ||||||
| Optimum theme | A1B4C4D4 | A1B3C4D4 | ||||||
| Phases | Steel Slag | Ultramafic Tailings | Mixture (S:T = 1:1) a | S4 |
|---|---|---|---|---|
| larnite (C2S) | 36.7% | 18.4% | 2.8% | |
| hatrurite (C3S) | 11.9% | 6.0% | 0.1% | |
| portlandite (Ca(OH)2) | 12.5% | 6.3% | 0.0% | |
| mayenite (C12A7) | 4.8% | 2.4% | 0.5% | |
| srebrodolskite (C2F) | 17.6% | 8.8% | 9.8% | |
| RO(FeO, MgO, MnO) | 15.0% | 7.7% | 11.4% | 10% |
| calcite (CaCO3) | 0.6% | 0.3% | 23.6% | |
| lizardite | 92.3% | 46.2% | 53.3% |
| 50–145 °C | 145–330 °C | 330–500 °C | 500–800 °C | 800–1000 °C | LOI | |
|---|---|---|---|---|---|---|
| Tailings | - | 0.1 | 0.8 | 10.5 | 0.1 | 11.5 |
| Steel slag | 1.4 | 0.9 | 1.2 | 0.7 | 0.1 | 4.3 |
| Mixture (S:T = 1:1) a | 0.7 | 0.5 | 1.0 | 5.6 | 0.1 | 7.9 |
| S4 | 0.7 | 1.3 | 1.1 | 10.9 | −0.1 | 13.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, C.; Ni, W.; Zhu, S.; Mao, S.; Jiang, F.; Zeng, H.; Sun, X.; Huang, B.; Hitch, M. Orthogonal Test Design for the Optimization of Preparation of Steel Slag-Based Carbonated Building Materials with Ultramafic Tailings as Fine Aggregates. Minerals 2022, 12, 246. https://doi.org/10.3390/min12020246
Li J, Wang C, Ni W, Zhu S, Mao S, Jiang F, Zeng H, Sun X, Huang B, Hitch M. Orthogonal Test Design for the Optimization of Preparation of Steel Slag-Based Carbonated Building Materials with Ultramafic Tailings as Fine Aggregates. Minerals. 2022; 12(2):246. https://doi.org/10.3390/min12020246
Chicago/Turabian StyleLi, Jiajie, Chengzhou Wang, Wen Ni, Sitao Zhu, Shilong Mao, Fuxing Jiang, Hui Zeng, Xikui Sun, Bingxiang Huang, and Michel Hitch. 2022. "Orthogonal Test Design for the Optimization of Preparation of Steel Slag-Based Carbonated Building Materials with Ultramafic Tailings as Fine Aggregates" Minerals 12, no. 2: 246. https://doi.org/10.3390/min12020246
APA StyleLi, J., Wang, C., Ni, W., Zhu, S., Mao, S., Jiang, F., Zeng, H., Sun, X., Huang, B., & Hitch, M. (2022). Orthogonal Test Design for the Optimization of Preparation of Steel Slag-Based Carbonated Building Materials with Ultramafic Tailings as Fine Aggregates. Minerals, 12(2), 246. https://doi.org/10.3390/min12020246









