The Mechanism of the Effect of Pre-Magnetized Butyl Xanthate on Chalcopyrite Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Research Methods
2.2.1. Magnetization of Butyl Xanthate
2.2.2. Monomineral Flotation Test
2.2.3. Adsorption Test
2.2.4. FTIR Test
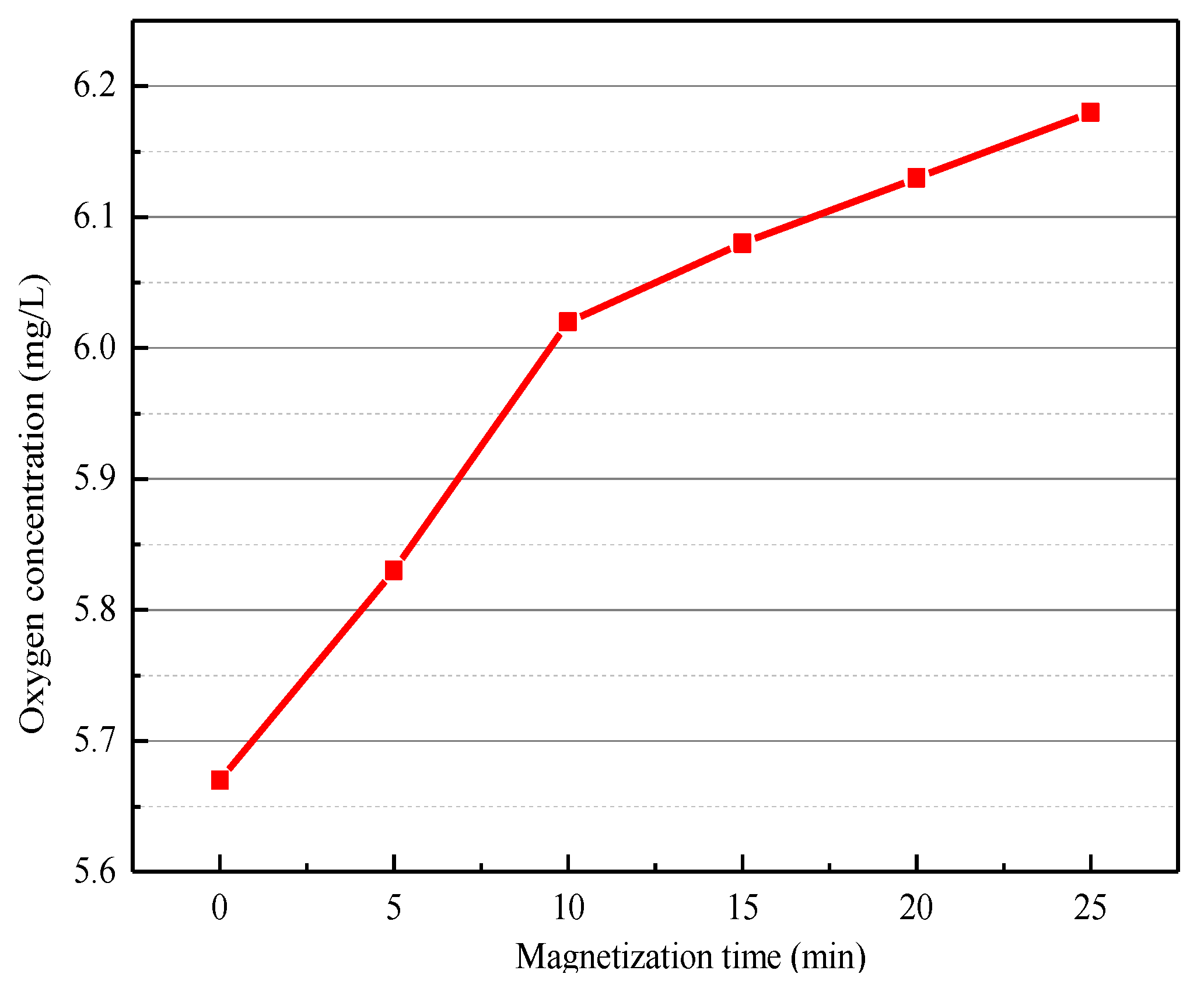
2.2.5. Conductivity and Dissolved Oxygen Test
2.2.6. Electrochemical Test
3. Results and Discussion
3.1. Effect of Pre-Magnetized Butyl Xanthate on Flotation Behavior of Chalcopyrite Monomineral
3.1.1. Effect of Magnetization Intensity and Magnetization Time
3.1.2. Effect of Butyl Xanthate Dosage
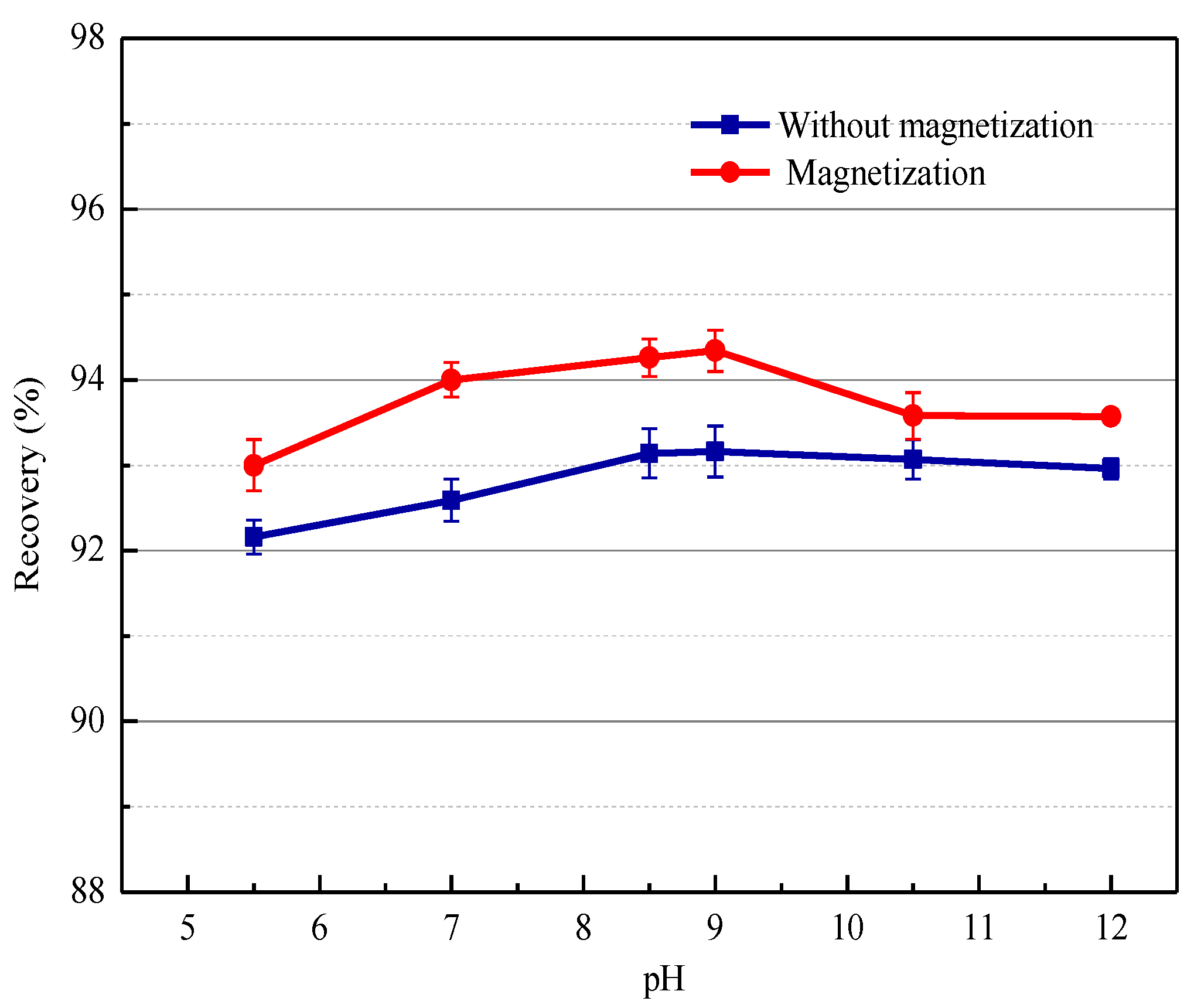
3.1.3. Effect of Pulp pH
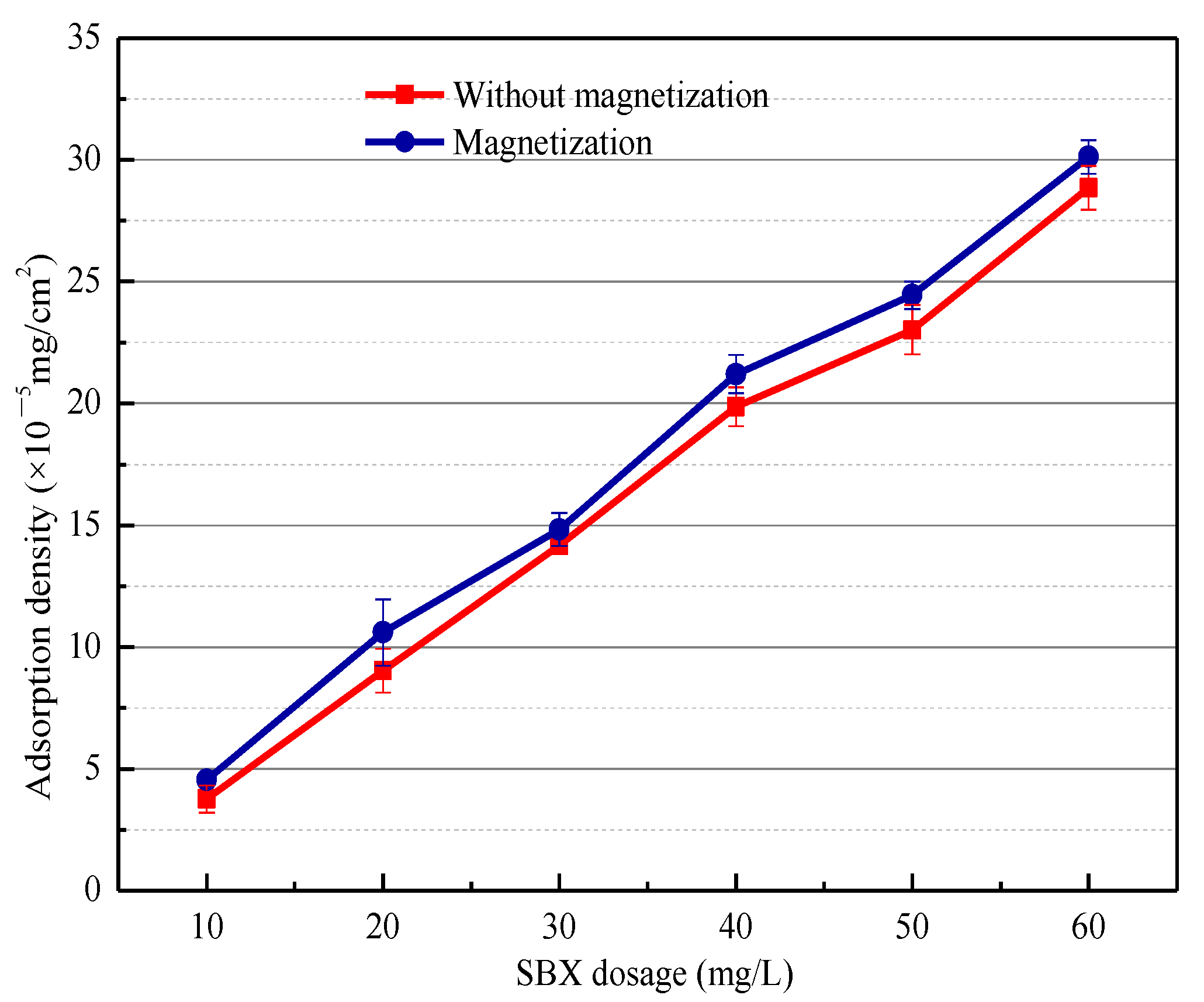
3.2. Adsorption Test
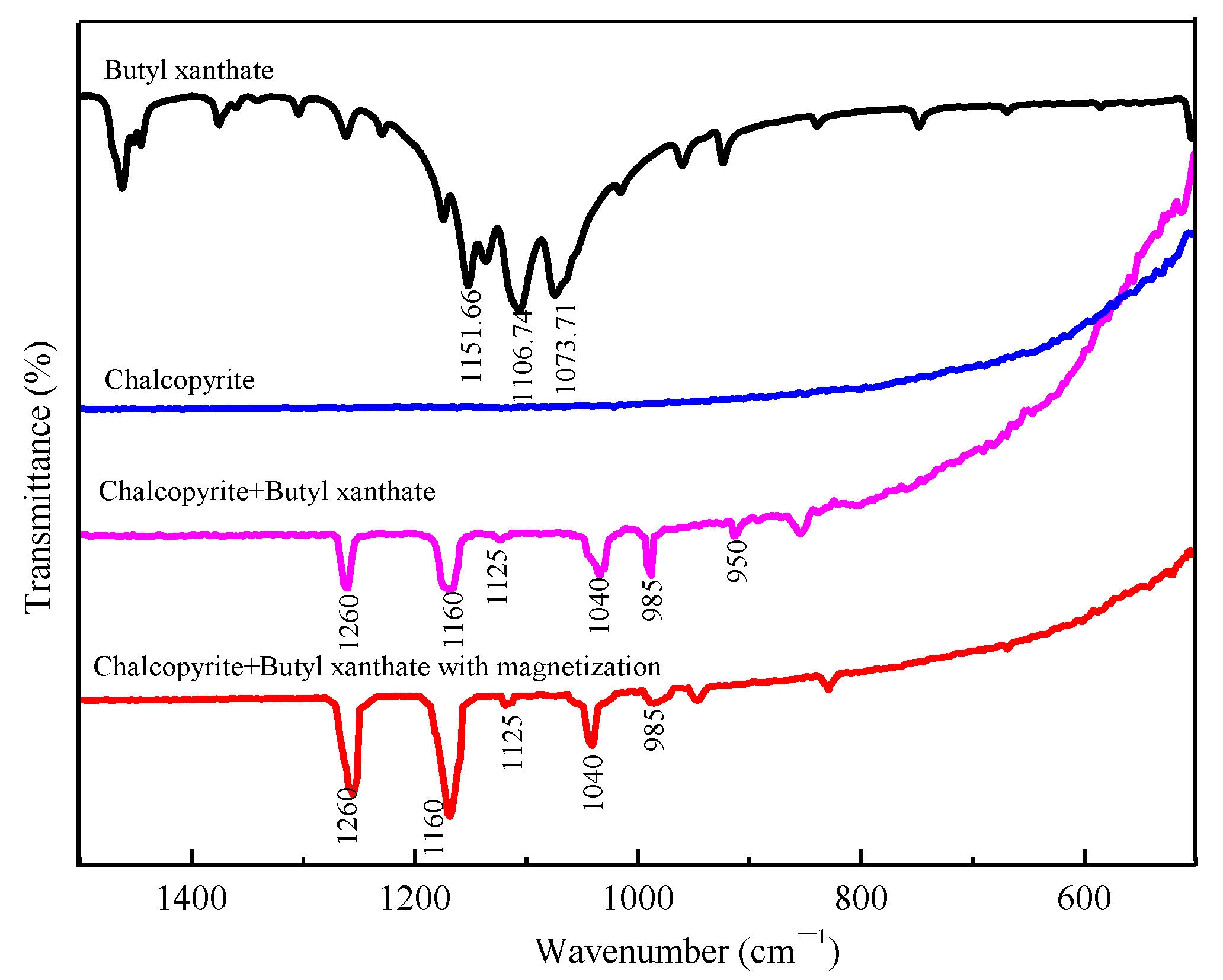
3.3. FTIR Analysis
3.4. The Mechanism of Magnetization Pretreatment to Promote the Selective Adsorption of Butyl Xanthate
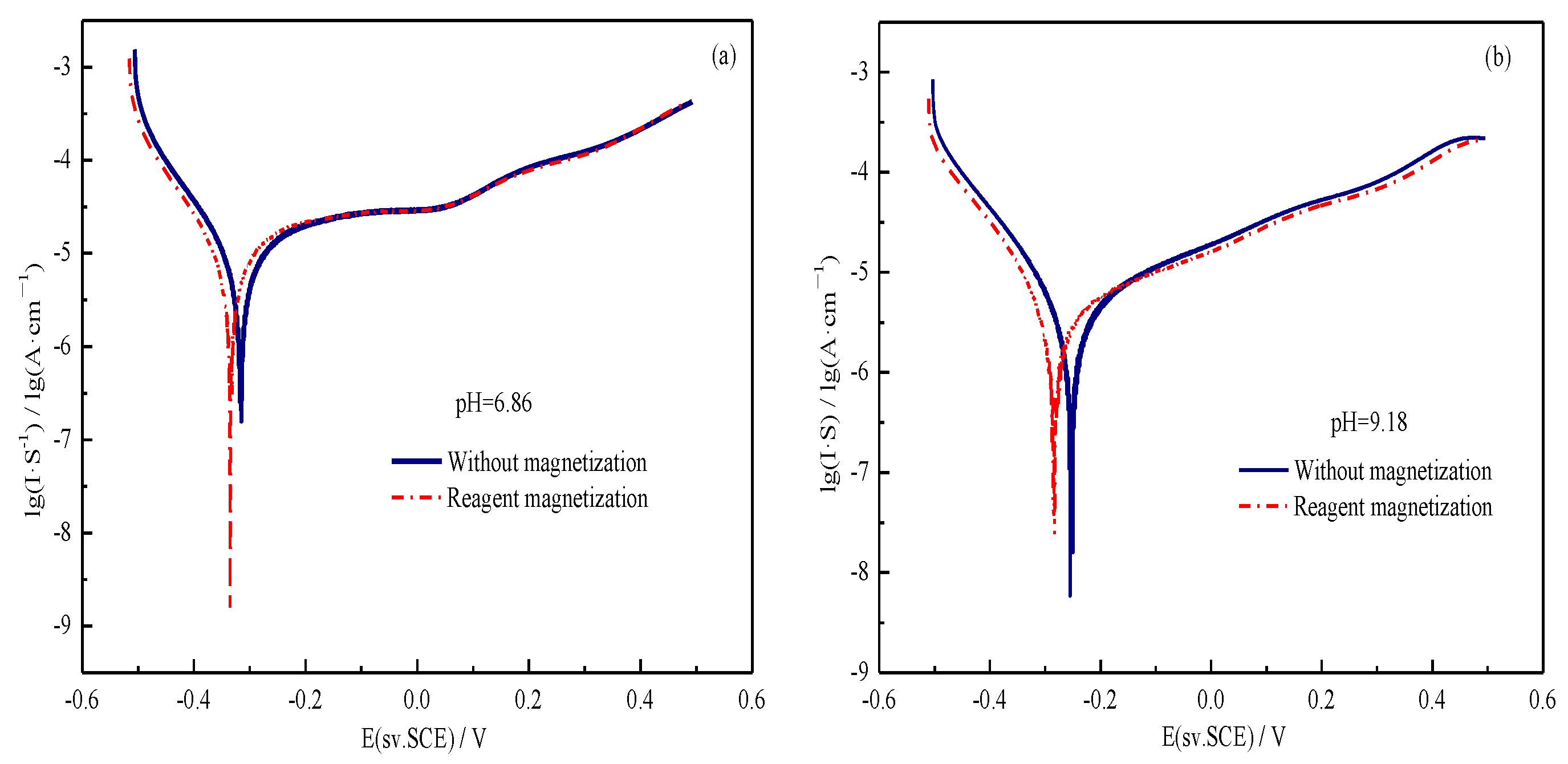
3.5. Electrochemical Property Analysis
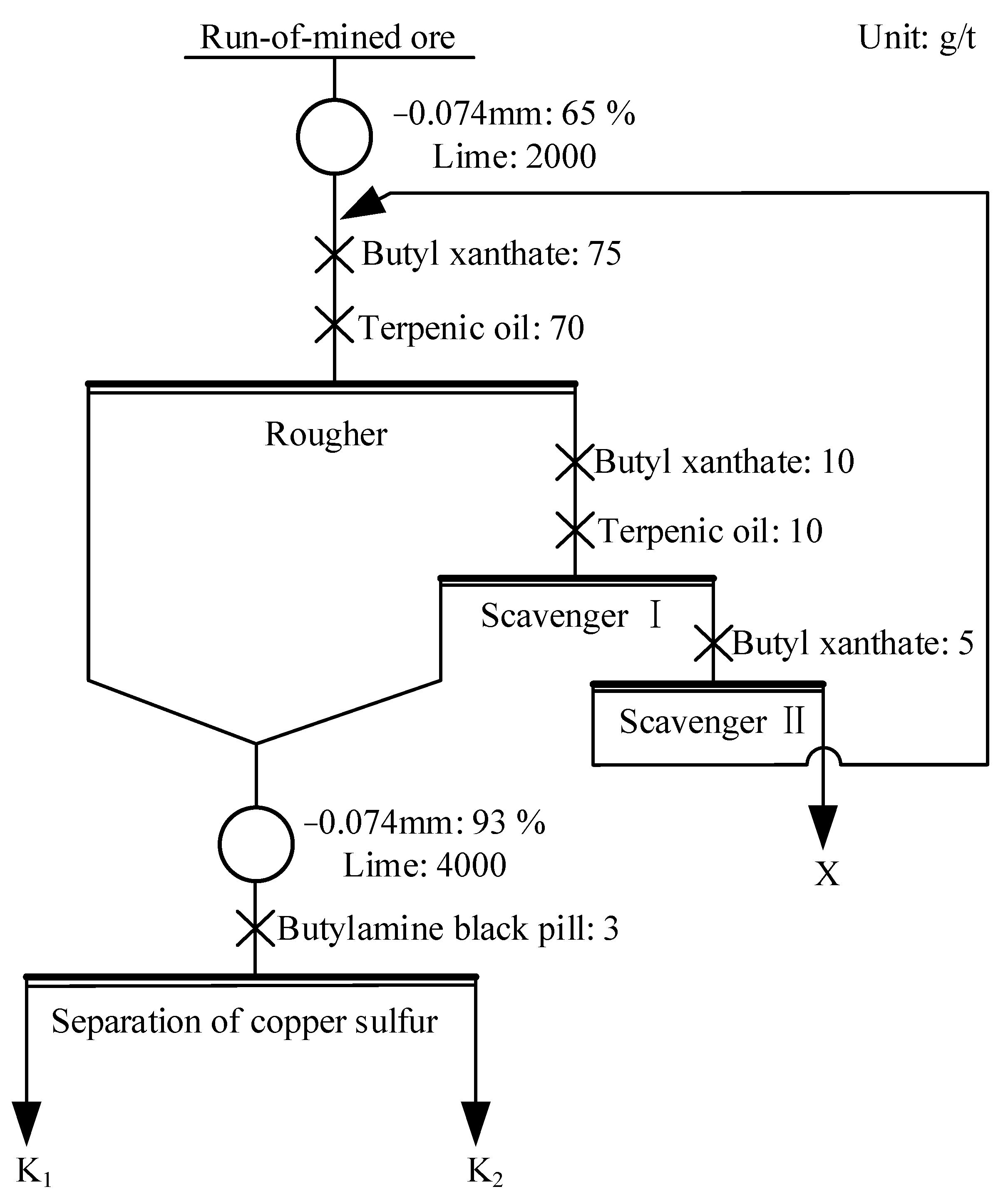
3.6. Flotation Test of Actual Copper Sulfide Ore
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, Y.; Li, F.; Jia, Q.; Li, G. Application of Magnetization Treatment in Flotation. J. Phys. Conf. Ser. 2021, 1748, 062005. [Google Scholar] [CrossRef]
- Qiu, T.S.; Cui, L.F.; Fang, X.H. Progress in the Application Research of Magnetic Treatment Technology in Mineral Processing. Met. Min. 2008, 12, 1–4, 8. (In Chinese) [Google Scholar]
- Li, F.J.; Kong, Y.R.; Jia, Q.M.; Li, G.F. Mechanism of Magnetization Effect on Flotation and Its Application in Flotation. Mul-Tipurpose Util. Miner. Resour. 2021, 3, 120–126. (In Chinese) [Google Scholar]
- Qiu, T.S.; Cui, L.F.; Fang, X.H. Effect of magnetization on flotation reagents and process. Chin. J. Nonferrous Met. 2009, 7, 1338–1344. (In Chinese) [Google Scholar]
- Qiu, T.S.; Yin, Y.F.; Fang, X.H.; Luo, X.P. Effects of Magnetization Treatment on Pyrite Depression and Its Mechanism Probing. Multipurp. Util. Miner. Resour. 2001, 6, 5–8. (In Chinese) [Google Scholar]
- Wang, Z.; He, T.; Li, H.; Wang, Y. Influence of magnetized water on molybdenite flotation and its mechanism. Environ. Technol. 2020, 43, 107–115. [Google Scholar] [CrossRef]
- Wan, H.; Qu, J.; Li, H.; He, T.; Bu, X.; Yang, W. A Novel Method for Improving Low-Temperature Flotation Performance of Nonpolar Oil in the Molybdenite Flotation. Minerals 2019, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Zhou, W.B.; Xiong, W.; Cheng, H.; Zhu, Z.Q.; Xu, M. Study on the Effect and Mechanism of Magnetization on Fine Grain Hematite Flotation. Met. Min. 2020, 8, 76–82. (In Chinese) [Google Scholar]
- Manouchehri, H.-R. Magnetic conditioning of sulfide minerals to improve recovery of fines in flotation—A plant practice. Miner. Met. Process. 2018, 35, 46–54. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- Wang, J.B.; Li, X.; Gu, S.L. Comparative Analysis of Copper Resource Supply and Demand Development Trend in China and the United States. Adv. Mater. Res. 2014, 962–965, 1953–1960. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Interaction mechanism of tannic acid with pyrite surfaces and its response to flotation separation of chalcopyrite from pyrite in a low-alkaline medium. J. Mater. Res. Technol. 2020, 9, 4421–4430. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Wang, A.J.; Chen, Q.S. China∙s copper resources supply the challenge and countermeasures. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; Volume 295, pp. 3089–3093. [Google Scholar]
- Sarquís, P.; Menéndez-Aguado, J.; Mahamud, M.M.; Dzioba, R. Tannins: The organic depressants alternative in selective flotation of sulfides. J. Clean. Prod. 2014, 84, 723–726. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Qin, W.; Jiao, F.; Wang, X.-J.; Pei, B.; Yang, Y.-J.; Lai, C.-H. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate. Trans. Nonferrous Met. Soc. China 2016, 26, 265–271. [Google Scholar] [CrossRef]
- Ma, X.; Xia, L.Y.; Wang, S.; Zhong, H.; Jia, H. Structural Modification of Xanthate Collectors to Enhance the Flotation Selec-tivity of Chalcopyrite. Ind. Eng. Chem. Res. 2017, 56, 6307–6316. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Zhong, H. Effective production of sodium isobutyl xanthate using carbon disulfide as a solvent: Reaction kinetics, calorimetry and scale-up. J. Clean. Prod. 2018, 200, 444–453. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, Y.; Qu, Q.; Zhang, J.; Mu, Y. Separation of bastnäsite from fluorite using ethylenediamine tetraacetic acid as depressant. Miner. Eng. 2019, 134, 134–141. [Google Scholar] [CrossRef]
- Wang, X.; Qin, W.; Jiao, F.; Wu, J. The influence of galvanic interaction on the dissolution and surface composition of galena and pyrite in flotation system. Miner. Eng. 2020, 156, 106525. [Google Scholar] [CrossRef]
- Qin, W.-Q.; Wang, X.-J.; Ma, L.-Y.; Jiao, F.; Liu, R.-Z.; Gao, K. Effects of galvanic interaction between galena and pyrite on their flotation in the presence of butyl xanthate. Trans. Nonferrous Met. Soc. China 2015, 25, 3111–3118. [Google Scholar] [CrossRef]
- Zhang, C.; He, T.; Li, H.; Bu, X. Adsorption Thermodynamics and Kinetics of Xanthate at Chalcopyrite Surface Based on Ultraviolet Spectrophotometry. Spectrosc. Spectr. Anal. 2019, 39, 3172–3178. (In Chinese) [Google Scholar]
- Mu, Y.; Peng, Y.; Lauten, R.A. The galvanic interaction between chalcopyrite and pyrite in the presence of lignosulfonate-based biopolymers and its effects on flotation performance. Miner. Eng. 2018, 122, 91–98. [Google Scholar] [CrossRef]
- Hicyilmaz, C.; Altun, N.E.; Ekmekci, Z.; Gokagac, G. Quantifying hydrophobicity of pyrite after copper activation and DTPI addition under electrochemically controlled conditions. Miner. Eng. 2004, 17, 879–890. [Google Scholar] [CrossRef]
- Abramov, A.A. Technology of Processing and Enrichment of Non-Ferrous Metal Ores; Publishing House of the Moscow State Mining University: Moscow, Russia, 2005. [Google Scholar]
- Peng, X.F.; Deng, B. Changes of features of water under action of magnetic-field and its mechanism of change. J. At. Mol. Phys. 2007, 24, 281–290. [Google Scholar]
- Wei, Q.; Dong, L.; Jiao, F.; Qin, W.; Pan, Z.; Cui, Y. The synergistic depression of lime and sodium humate on the flotation separation of sphalerite from pyrite. Miner. Eng. 2021, 163, 106779. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhu, Y.; Han, Y. Adsorption and depression mechanism of an eco-friendly depressant PCA onto chalcopyrite and pyrite for the efficiency flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126574. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, J. The Chemical Principle of Flotation Reagent; Central South University of Technology Press: Changsha, China, 1996; pp. 10–30. (In Chinese) [Google Scholar]
- Zhu, Y.S. Chemistry Principles of Flotation Reagents; Central South University of Technology Press: Changsha, China, 1996; pp. 13–17. (In Chinese) [Google Scholar]
- Jian, B.X. Flotation Reagents; Metallurgical Industry Press: Beijing, China, 1981; pp. 17–21. [Google Scholar]
- Hu, Y.; Sun, W.; Wang, D. Corrosive Electrochemistry of Oxidation-Reduction of Sulphide Minerals. In Electrochemistry of Flotation of Sulphide Minerals; Springer: Berlin/Heidelberg, Germany, 2009; pp. 167–200. [Google Scholar] [CrossRef]
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Estimation of corrosion current by the analysis of polarization curves: Elec-trochemical kinetics mode. Russ. J. Electrochem. 2014, 50, 108–113. [Google Scholar] [CrossRef]
- Deng, X.X.; Liao, D.H. Study on Magnetic Flotation Technique of a Refractory Scheetite. Min. R D 2015, 35, 55–59. (In Chinese) [Google Scholar]
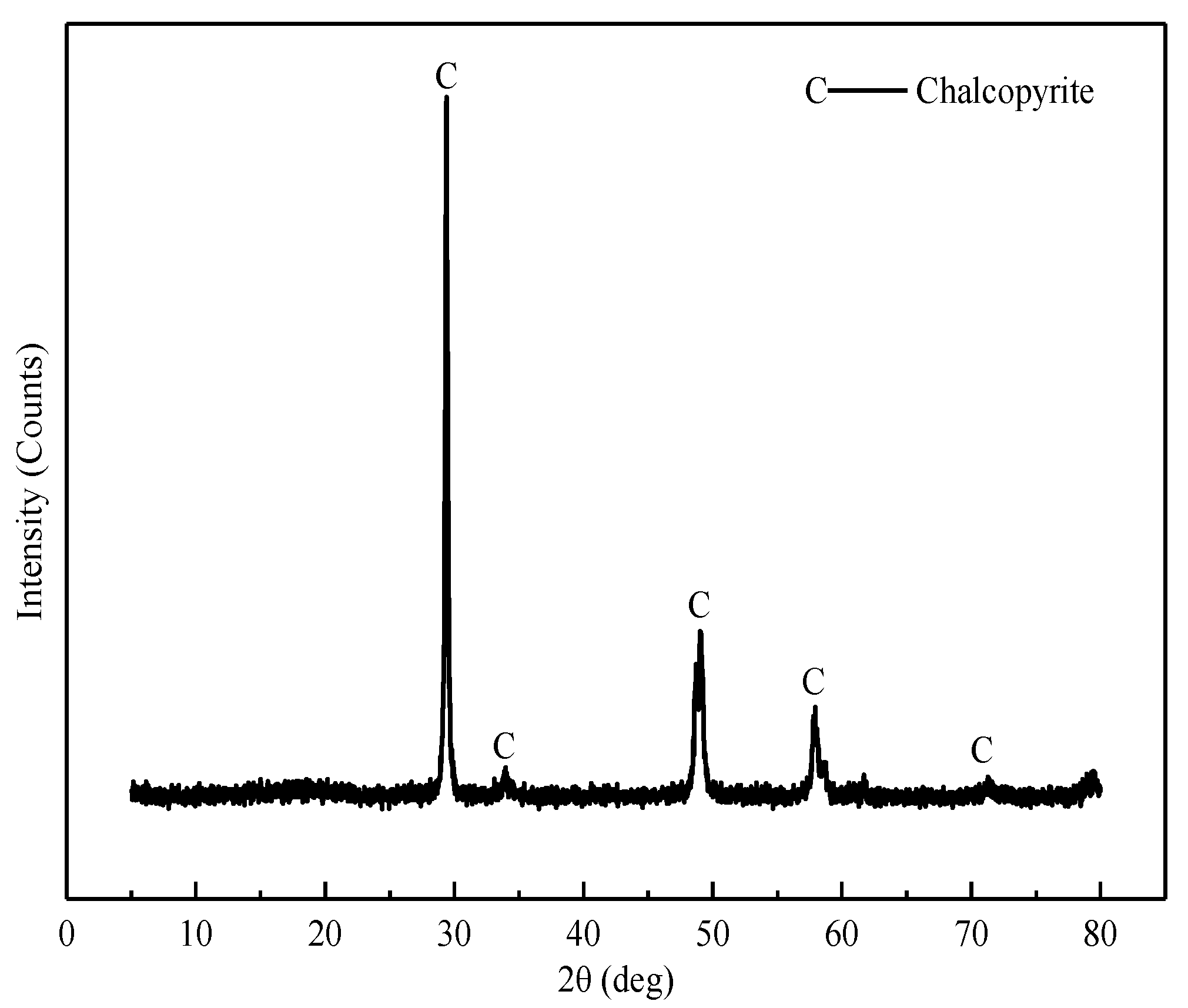
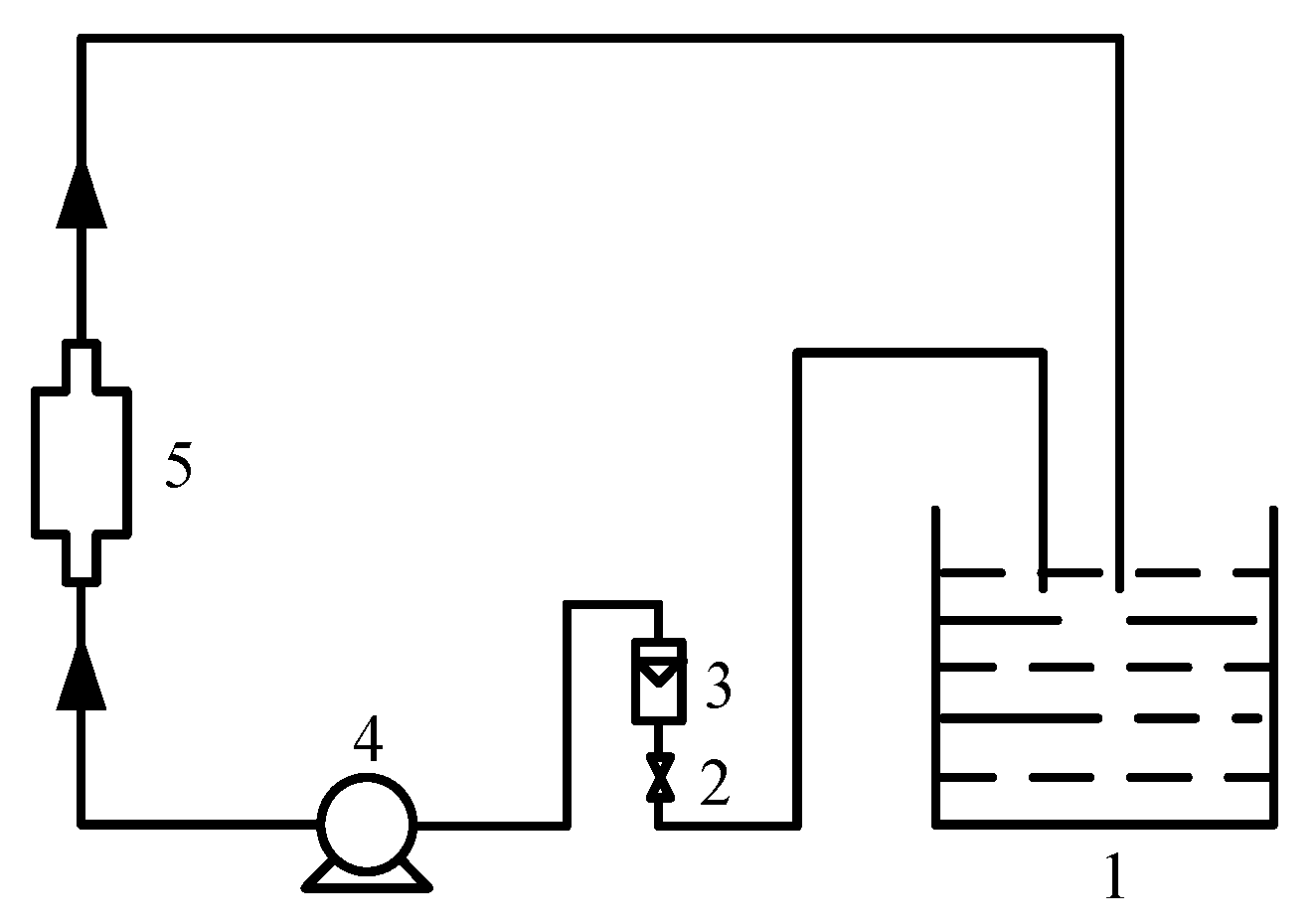
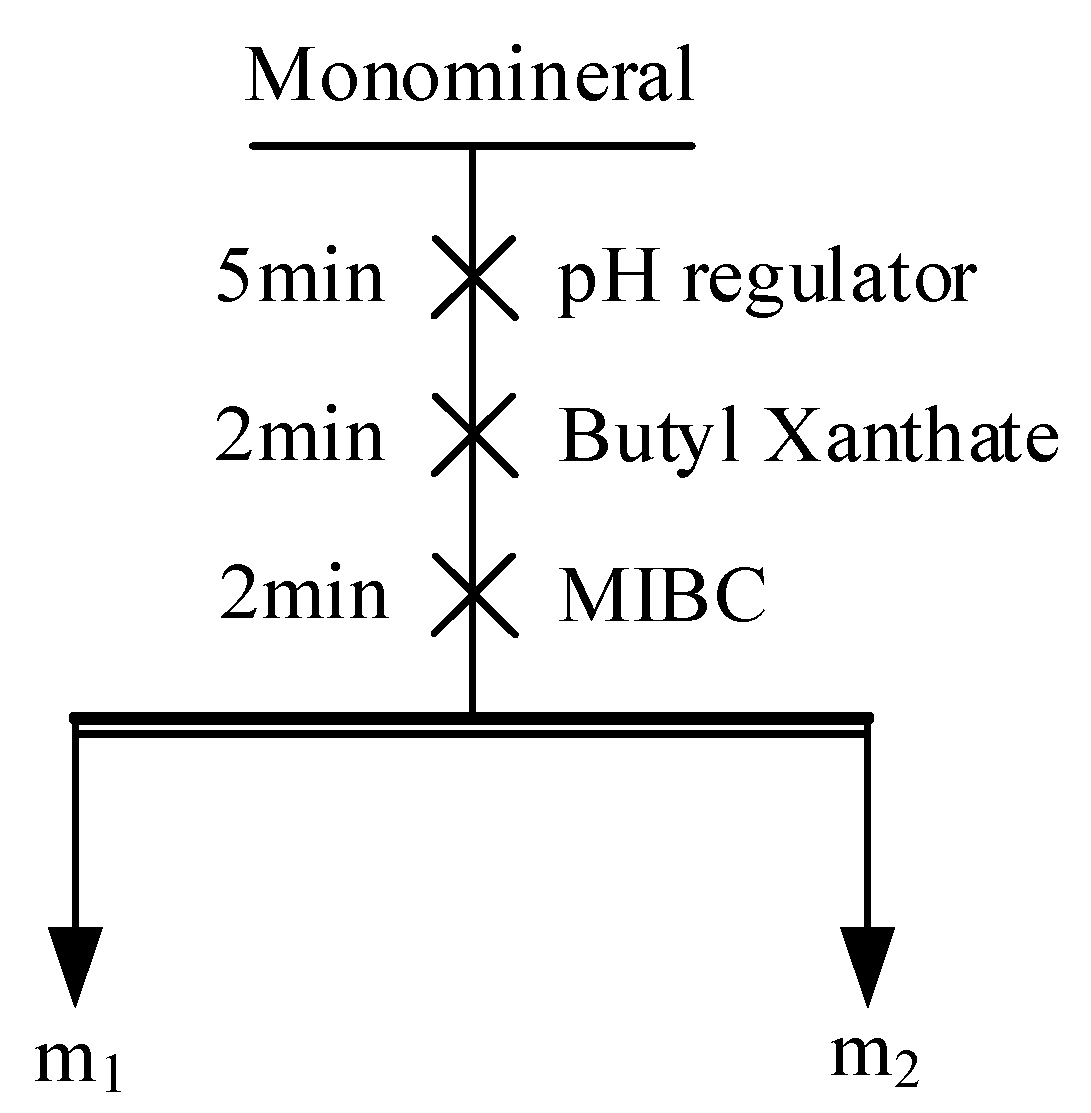
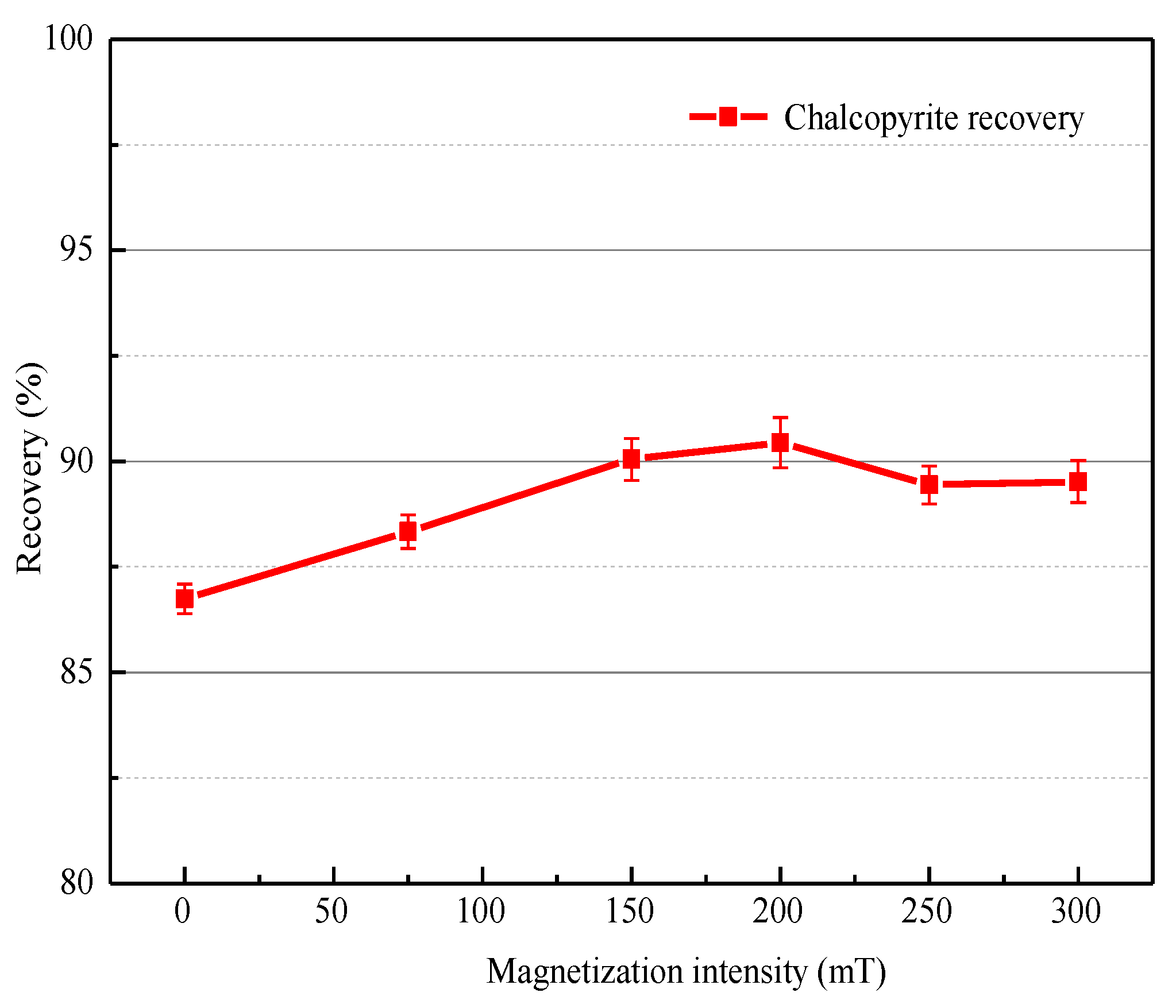



| Mineral | Fe | S | Cu | Purity |
|---|---|---|---|---|
| Chalcopyrite | 30.41 | 35.11 | 33.15 | 95.31 |
| pH | Condition | Ecorr (mV) | Icorr (μA·cm−2) |
|---|---|---|---|
| 6.86 | Without magnetization | −0.316 | 9.700 |
| Reagent magnetization | −0.335 | 9.674 | |
| 9.18 | Without magnetization | −0.253 | 4.727 |
| Reagent magnetization | −0.284 | 4.261 |
| Element | Cu | Pb | Zn | Mo | S | Fe | As | Al2O3 | SiO2 | Ca | MgO | Au * | Ag * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.34 | 0.003 | 0.0032 | 0.006 | 3.18 | 4.72 | 0.0018 | 14.23 | 62.37 | 0.93 | 0.66 | 0.26 | 0.79 |
| Condition | Product | Yield (%) | Grade (%) | Recovery (%) | ||
|---|---|---|---|---|---|---|
| Cu | S | Cu | S | |||
| Without magnetization | K1 | 3.39 | 7.92 | 11.24 | 79.01 | 11.98 |
| K2 | 6.38 | 0.32 | 37.34 | 6.00 | 74.91 | |
| X | 90.43 | 0.056 | 0.46 | 14.99 | 13.10 | |
| Run-of-mined ore | 100.00 | 0.34 | 3.18 | 100.00 | 100.00 | |
| Reagent magnetization | K1 | 3.58 | 7.79 | 9.49 | 82.07 | 10.68 |
| K2 | 6.06 | 0.32 | 40.80 | 5.70 | 77.75 | |
| X | 90.36 | 0.046 | 0.41 | 12.23 | 11.57 | |
| Run-of-mined ore | 100.00 | 0.34 | 3.18 | 100.00 | 100.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Yang, L.; Yan, H.; Zhang, H.; Cui, L.; Liu, X. The Mechanism of the Effect of Pre-Magnetized Butyl Xanthate on Chalcopyrite Flotation. Minerals 2022, 12, 209. https://doi.org/10.3390/min12020209
Qiu T, Yang L, Yan H, Zhang H, Cui L, Liu X. The Mechanism of the Effect of Pre-Magnetized Butyl Xanthate on Chalcopyrite Flotation. Minerals. 2022; 12(2):209. https://doi.org/10.3390/min12020209
Chicago/Turabian StyleQiu, Tingsheng, Liu Yang, Huashan Yan, Hongliang Zhang, Lifeng Cui, and Xiaohe Liu. 2022. "The Mechanism of the Effect of Pre-Magnetized Butyl Xanthate on Chalcopyrite Flotation" Minerals 12, no. 2: 209. https://doi.org/10.3390/min12020209
APA StyleQiu, T., Yang, L., Yan, H., Zhang, H., Cui, L., & Liu, X. (2022). The Mechanism of the Effect of Pre-Magnetized Butyl Xanthate on Chalcopyrite Flotation. Minerals, 12(2), 209. https://doi.org/10.3390/min12020209






