Investigation of Cerium Reduction Efficiency by Grinding with Microwave Irradiation in Mechanochemical Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis
2.2. Heating Experiments by Microwave Irradiation
2.3. Grinding Experiments by Planetary Ball Mill
2.4. Grinding Experiments with Microwave Irradiation
2.5. X-ray Absorption Fine Structure Analysis of the Cerium LIII- and K-Edge
3. Results and Discussion
3.1. Characterization of Weathered Residual Rare-Earth Ore
3.2. Cerium Reduction by Heating Experiments
3.3. Cerium Reduction by Mechanochemical Processing via Planetary Ball Milling
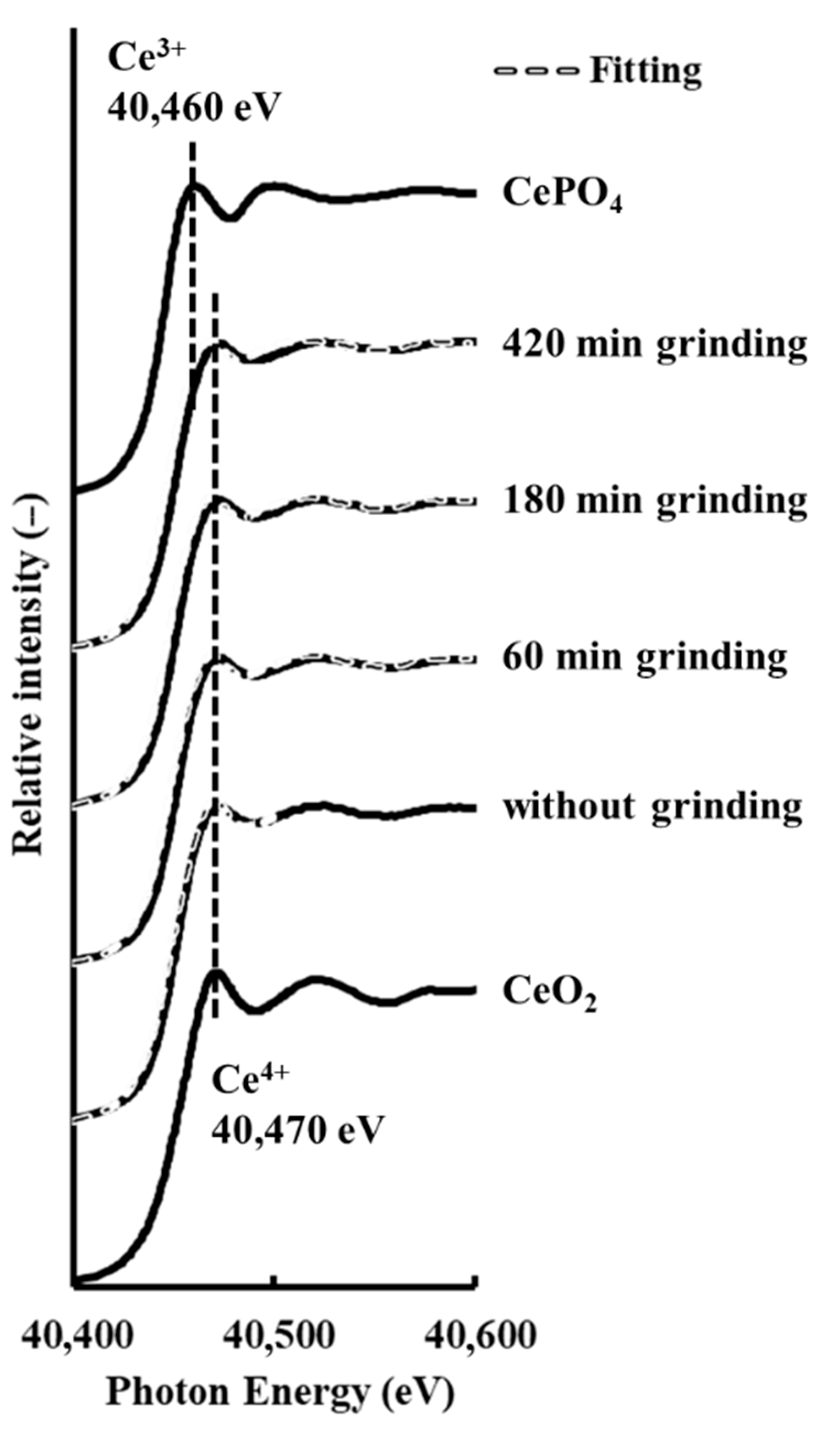
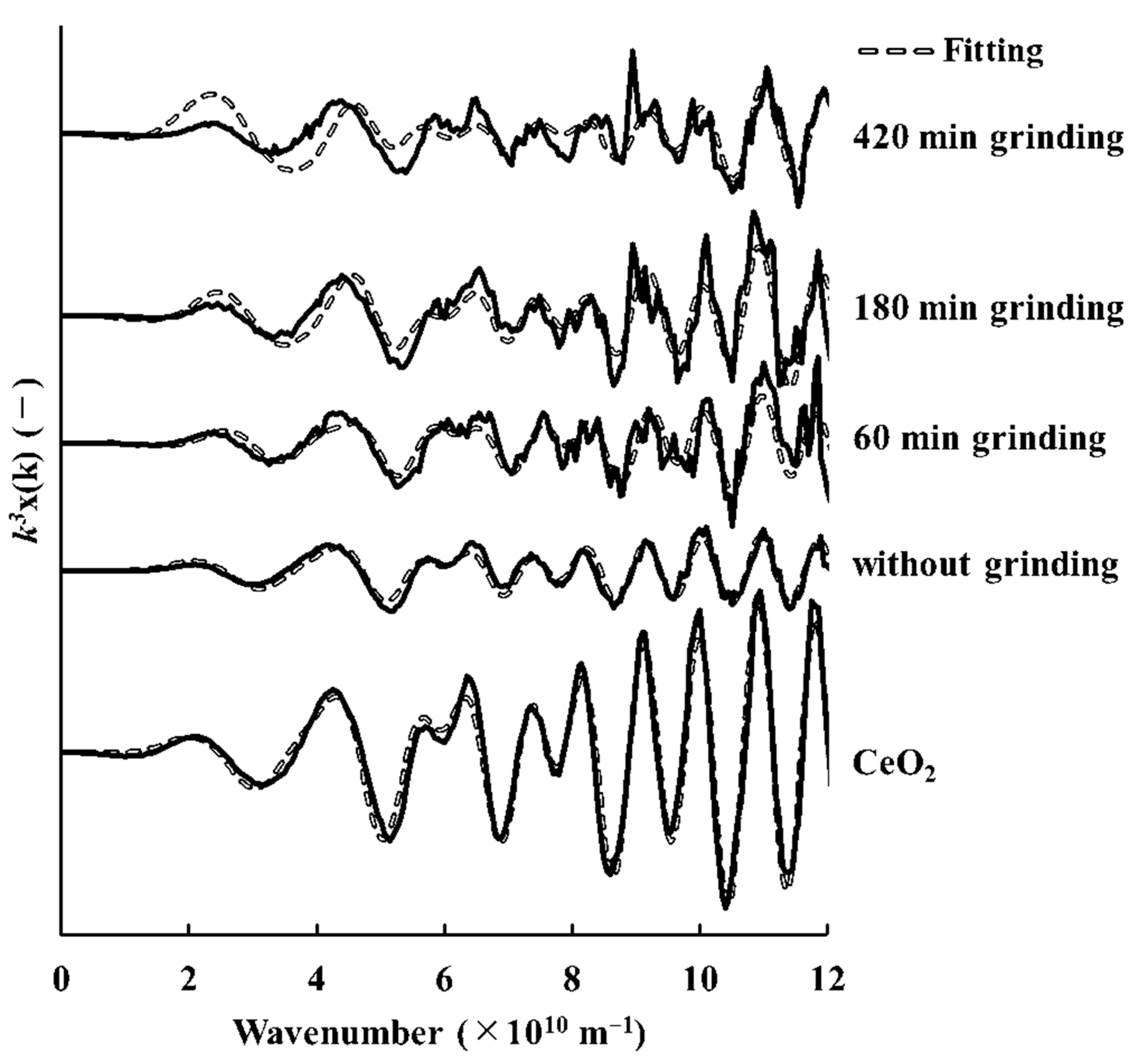
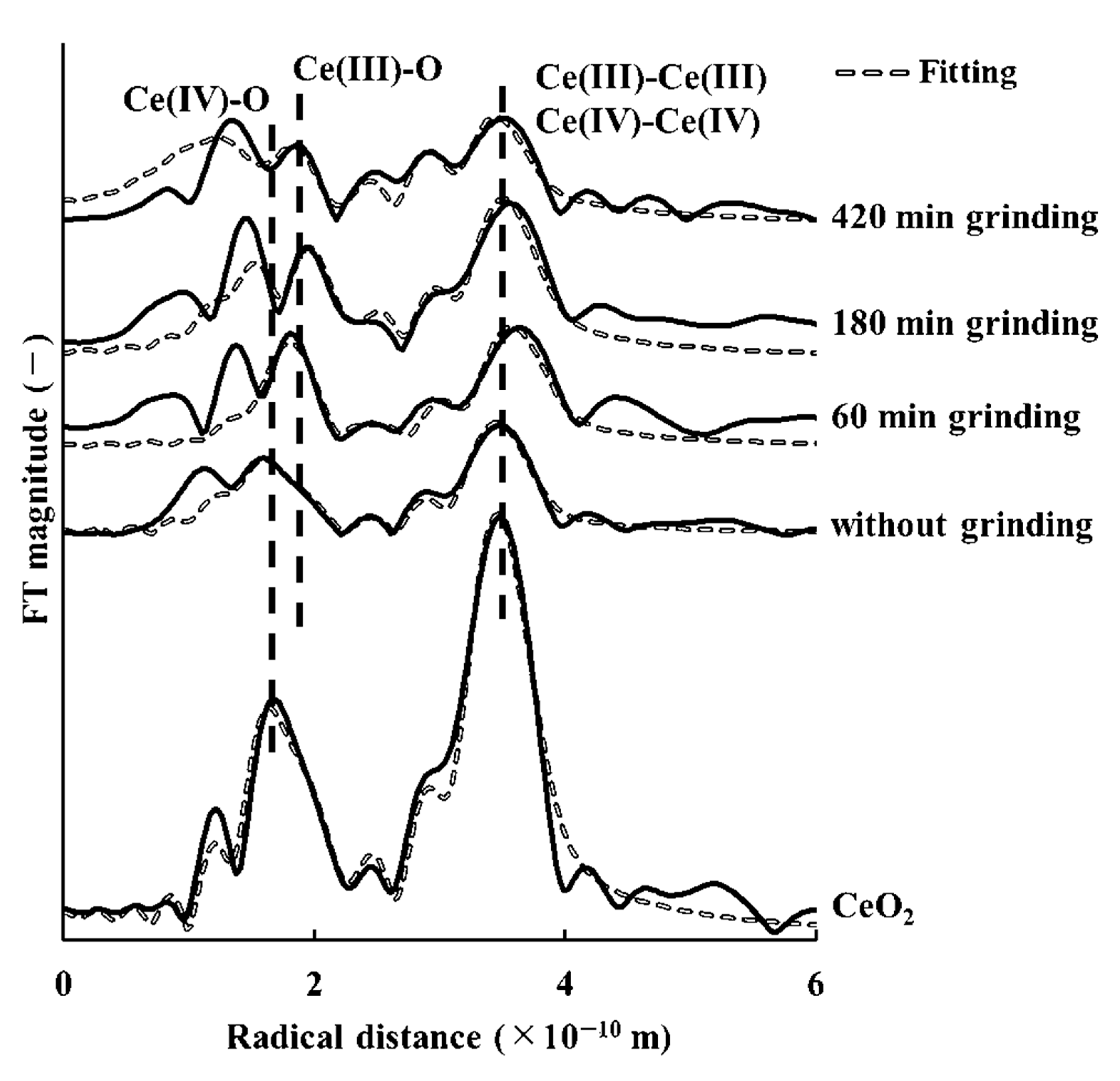
3.4. Cerium Reduction by Mechanochemical Processing via Grinding with Microwave Irradiation
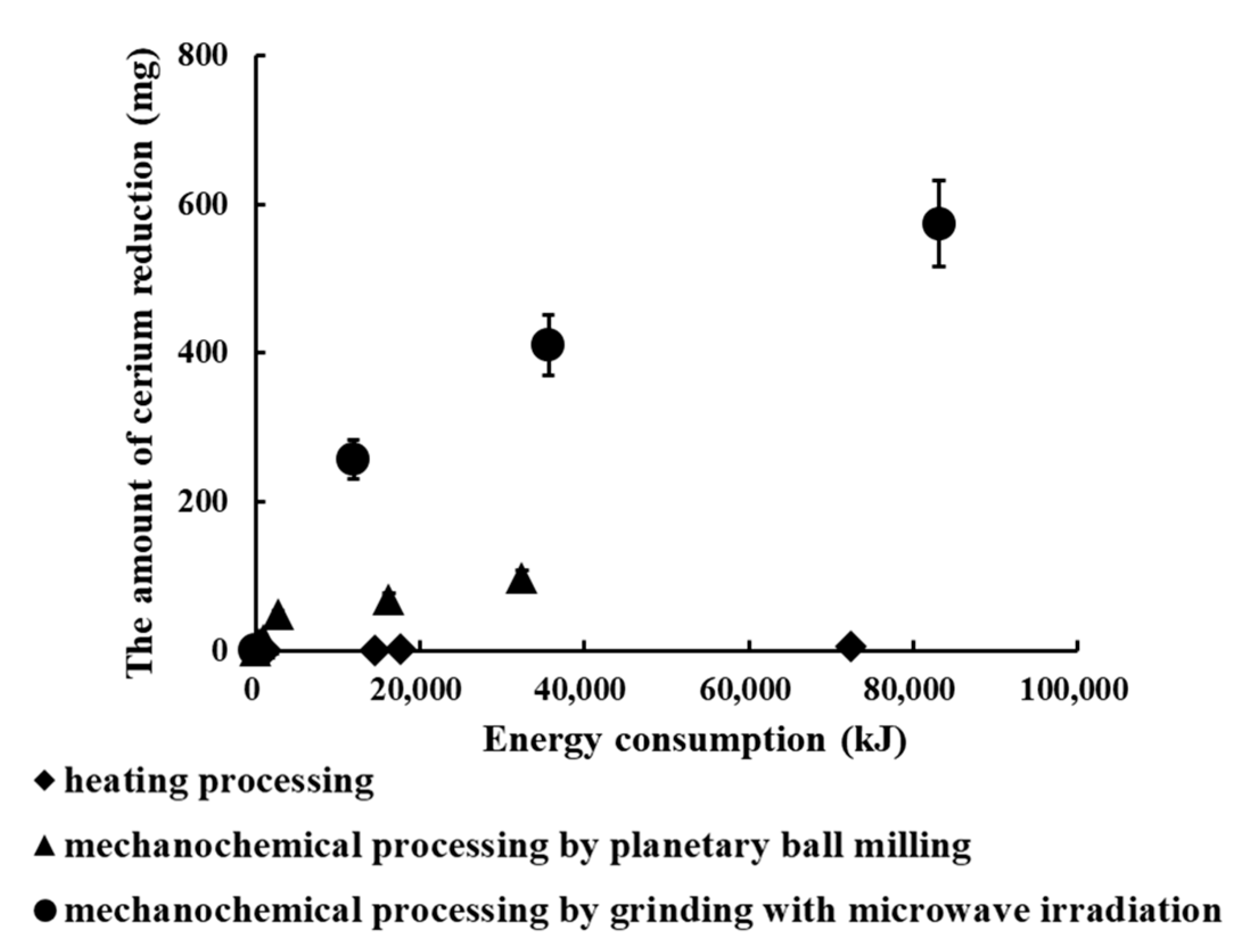
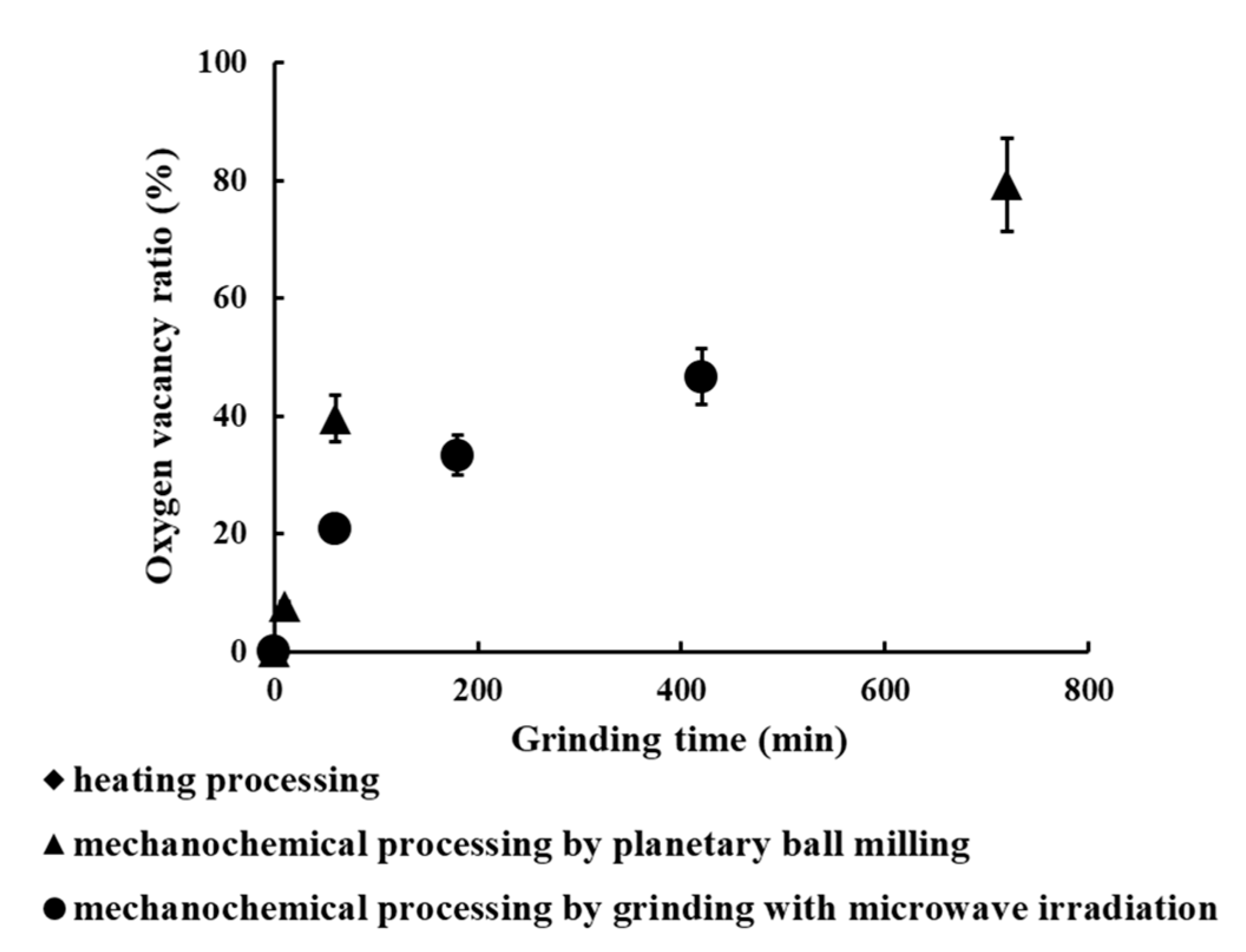
3.5. Comparison Cerium Reduction Efficiency among These Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsenis, A.D.; Puskaric, A.; Strukil, V.; Mottillo, C.; Julien, P.A.; Uzarevic, K.; Pham, M.H.; Do, T.O.; Kimber, S.A.J.; Lazic, P.; et al. In situ x-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef] [PubMed]
- Balaz, P.; Takacs, L.; Luxova, M.; Godocikova, E.; Ficeriova, J. Mechanochemical processing of sulphidic minerals. Int. J. Miner. Process. 2004, 74S, S365–S371. [Google Scholar] [CrossRef]
- Esmaeili, E.; Rounaghi, S.A.; Eckert, J. Mechanochemical synthesis of rosin-modified montmorillonite: A breakthrough approach to the next generation of OMMT/Rubber nanocomposites. Nanocomposites 2021, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Li, L.; Zhang, X.; Bian, Y.; Xue, Q.; Wu, J.; Wu, F.; Chen, R. Selective recovery of Li and Fe from spent lithium-ion batteries by an environmentally friendly mechanochemical approach. ACS Sustain. Chem. Eng. 2018, 6, 11029–11035. [Google Scholar] [CrossRef]
- Liu, K.; Liu, L.; Tan, Q.; Li, J. Selective extraction of lithium from a spent lithium iron phosphate battery by mechanochemical solid-phase oxidation. Green Chem. 2021, 23, 1344. [Google Scholar] [CrossRef]
- Wang, G.W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Li, W.; Lu, Y.; Meng, H.; Li, C. Efficient destruction of hexachlorobenzene by calcium carbide through mechanochemical reaction in a planetary ball mill. Chemosphere 2017, 166, 275–280. [Google Scholar] [CrossRef]
- Dallimore, M.P.; Mccormick, P.G. Dynamics of planetary ball milling: A comparison of computer simulated processing parameters with CuO/Ni displacement reaction milling kinetics. Mater. Trans. JIM 1996, 37, 1091–1098. [Google Scholar] [CrossRef]
- Patil, P.R.; Kartha, K.P.R. Application of ball milling technology to carbohydrate reactions: I. regioselective primary hydroxyl protection of hexosides and nucleoside by planetary ball milling. J. Carbohydr. Chem. 2008, 27, 279–293. [Google Scholar] [CrossRef]
- Yamamoto, K.; Garcia, S.E.B.; Muramatsu, A. Zeolite synthesis using mechanochemical reaction. Microporous Mesoporous Mater. 2007, 101, 90–96. [Google Scholar] [CrossRef]
- Suzuki, M.; Iguchi, H.; Ohtani, A.; Hirota, M.; Oshima, T. Effect of grinding condition on hydrophobic surface-treatment of quarts sand using planetary mill. Kagaku Kogaku Ronbunshu 1995, 21, 111–117. [Google Scholar] [CrossRef][Green Version]
- Hiroshi, M. Estimation of mechanochemical reaction rate and optimum design of planetary ball mill by discrete element method. J. Soc. Powder Technol. Jpn. 2005, 42, 134–139. [Google Scholar]
- Kato, T.; Granata, G.; Tsunazawa, Y.; Takagi, T.; Tokoro, C. Mechanism and kinetics of enhancement of cerium dissolution from weathered residual rare earth ore by planetary ball milling. Miner. Eng. 2019, 134, 365–371. [Google Scholar] [CrossRef]
- Kato, T.; Tsunazawa, Y.; Liu, W.; Tokoro, C. Structural change analysis of cerianite in weathered residual rare earth ore by mechanochemical reduction using x-ray absorption fine structure. Minerals 2019, 9, 267. [Google Scholar] [CrossRef]
- Kato, T.; Granata, G.; Tokoro, C. Evaluation of acids onto the light rare earth elements dissolution from weathered residual rare earth ore activated by mechanochemical treatment by grinding. J. Soc. Powder Technol. Jpn. 2019, 56, 174–180. [Google Scholar] [CrossRef]
- Granata, G.; Takahashi, K.; Kato, T.; Tokoro, C. Mechanochemical activation of chalcopyrite: Relationship between activation mechanism and leaching enhancement. Miner. Eng. 2019, 131, 280–285. [Google Scholar] [CrossRef]
- Minagawa, M.; Hisatomi, S.; Kato, T.; Granata, G.; Tokoro, C. Enhancement of copper dissolution by mechanochemical activation of copper ores: Correlation between leaching experiments and DEM simulations. Adv. Powder Technol. 2018, 29, 471–478. [Google Scholar] [CrossRef]
- Mitani, Y.; Tsunazawa, Y.; Okura, T.; Tokoro, C. Enhancement of copper leaching from chalcopyrite using wet-type ball mill. J. Soc. Powder Technol. Jpn. 2015, 52, 723–729. [Google Scholar] [CrossRef][Green Version]
- Titscher, L.; Breitung, F.S.; Kwade, A. Experimental parametric study for dry grinding in planetary ball mills. Chem. Ing. Tech. 2016, 88, 1524–1529. [Google Scholar] [CrossRef]
- Mio, H.; Kano, J.; Saito, F. Scale-up method of planetary ball mill. Chem. Eng. Sci. 2004, 59, 5909–5916. [Google Scholar] [CrossRef]
- Stolle, A.; Schmidt, R.; Jacob, K. Scale-up of organic reactions in ball mills: Process intensification with regard to energy efficiency and economy of scale. Faraday Discuss 2014, 170, 267. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Furuya, T.; Katsuumi, N.; Haraguchi, T.; Akitsu, T. Synthesis of amino acid derivative Schiff base copper(II) complexes by microwave and wet mechanochemical methods. J. Indian Chem. Soc. 2021, 98, 100004. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Alegria, E.C.B.A.; Kopylovich, M.N.; Ferraria, A.M.; Do, R.A.M.B.; Pombeiro, A.J.L. Comparison of microwave and mechanochemical energy inputs in the catalytic oxidation of cyclohexane. Dalton Trans. 2018, 47, 8193–8198. [Google Scholar] [CrossRef] [PubMed]
- Rehr, J.J.; Albers, R.C.; Zabinsky, S.I. High-order multiple-scattering calculations of x-ray-absorption fine structure. Phys. Rev. Lett. 1992, 69, 3399–3400. [Google Scholar] [CrossRef]
- Rehr, J.J. Recent developments in multiple-scattering calculations of XAFS and XANES. Jpn. J. Appl. Phys. 1993, 32, 8–12. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Athena, artemis, hephaestus: Data analysis for x-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- HSC Chemistry 5.0. Chemical Reaction and Equilibrium Software with Extensive Hermochemical Database ver. 5.11; Outokumpu Research Oy: Piori, Finland, 2002. [Google Scholar]
- Bulfin, B.; Lowe, A.J.; Keogh, K.A.; Murphy, B.E.; Lubben, O.; Krasnikov, S.A.; Shvets, I.V. Analytical model of CeO2 oxidation and reduction. J. Phys. Chem. C 2013, 117, 24129–24137. [Google Scholar] [CrossRef]
- Trovarelli, A.; Fornasiero, P. Catalysis by Ceria and Related Materials, 2nd ed.; Imperial College Press: London, UK, 2013; pp. 1–881. [Google Scholar]
- Skorodumova, N.V.; Simak, S.L.; Lundqvist, B.L.; Abrikosov, L.A.; Johansson, B. Quantum origin of the oxygen storage capability of ceria. Phys. Rev. Lett. 2002, 89, 166601. [Google Scholar] [CrossRef]
- Silva, J.L.F.D. Stability of the Ce2O3 phases: ADFT +Uinvestigation. Phys. Rev. B 2007, 76, 193108. [Google Scholar] [CrossRef]
- Bartos, A.; Lieb, K.P.; Uhrmacher, M.; Wiarda, D. Refinement of atomic positions in bixbyite oxide using perturbed angular correlation spectroscopy. Acta Crystallogr. Sect. B 1993, 49, 165–169. [Google Scholar] [CrossRef]
- Badlducci, G.; Kaspar, J.; Fornasiero, P.; Graziani, M.; Islam, M.S.; Gale, J.D. Computer simulations studies of bulk reduction and oxygen migration in CeO2-ZrO2 solid solutions. J. Phys. Chem. 1997, 101, 1750–1753. [Google Scholar] [CrossRef]
- Haneda, M.; Mizushima, T.; Kakuta, N.; Ueno, A.; Sato, Y.; Matsuura, S.; Kasahara, K.; Sato, M. Structural characterization and catalytic behavior of Al2O3-supported cerium oxides. Bull. Chem. Soc. Jpn. 1993, 66, 1279–1288. [Google Scholar] [CrossRef]
- Ghigna, P.; Spinolo, G.; Scavini, M.; Tamburini, U.A.; Chadwick, A.V. The atomic and electronic structure of cerium substitutional defects in Nd2-xCexCuO4+δan XAS study. Phys. C Supercond. 1995, 253, 147–155. [Google Scholar] [CrossRef]
- Koettgen, J.; Martin, M. Coordination numbers in Sm-doped ceria using x-ray absorption spectroscopy. J. Phys. Chem. C 2019, 123, 6333–6339. [Google Scholar] [CrossRef]
- Lee, J.F.; Tang, M.T.; Shih, W.C.; Liu, R.S. Ce K-edge EXAFS study of nanocrystalline CeO2. Mater. Res. Bull. 2002, 37, 555–562. [Google Scholar] [CrossRef]


| SiO2 | Fe2O3 | Al2O3 | Na2O | MgO | P2O5 | K2O | CaO | TiO2 | MnO | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| 68.6 | 16.2 | 7.3 | 0.3 | 0.4 | 0.3 | 2.1 | 0.2 | 0.3 | 0.2 | 4.1 |
| Sc | Y | La | Ce | Pr | Nd | Sm | Eu |
|---|---|---|---|---|---|---|---|
| 5.3 | 1384.1 | 926.5 | 2495.5 | 236.8 | 903.3 | 200.0 | 11.2 |
| Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
| 224.2 | 37.4 | 236.2 | 50.4 | 153.7 | 23.1 | 137.9 | 19.6 |
| Temperature (°C) | Concentration of Trivalent Cerium | Concentration of Tetravalent Cerium |
|---|---|---|
| 25 | 50.7 | 49.3 |
| 700 | 50.8 | 49.2 |
| 900 | 51.4 | 48.6 |
| 950 | 61.0 | 39.0 |
| 1100 | 75.9 | 24.1 |
| Grinding Time (min) | Concentration of Trivalent Cerium | Concentration of Tetravalent Cerium |
|---|---|---|
| 0 | 30.5 | 69.5 |
| 10 | 31.9 | 68.1 |
| 60 | 50.0 | 50.0 |
| 720 | 74.4 | 25.6 |
| Sample | Shell | CN | R [× m−10] | σ2 [× m−20] | ΔE0 |
|---|---|---|---|---|---|
| CeO2 | Ce(IV)-O | 7.7 | 2.35 | 0.009 | −10.3 |
| Ce(III)-O | - | ||||
| Ce(III)-Ce(III) | - | ||||
| Ce(IV)-Ce(IV) | 12.2 | 3.84 | 0.005 | ||
| Without grinding | Ce(IV)-O | 8.7 | 2.35 | 0.02 | −9.3 |
| Ce(III)-O | 0.2 | 2.46 | 0.04 | ||
| Ce(III)-Ce(III) | 0.3 | 3.81 | 0.002 | ||
| Ce(IV)-Ce(IV) | 12.1 | 3.83 | 0.008 | ||
| 10 min grinding | Ce(IV)-O | 8.0 | 2.36 | 0.02 | −10.6 |
| Ce(III)-O | 0.06 | 2.47 | −0.01 | ||
| Ce(III)-Ce(III) | 0.2 | 3.81 | 0.001 | ||
| Ce(IV)-Ce(IV) | 11.4 | 3.84 | 0.008 | ||
| 60 min grinding | Ce(IV)-O | 4.0 | 2.31 | 0.006 | 2.4 |
| Ce(III)-O | 3.9 | 2.42 | 0.02 | ||
| Ce(III)-Ce(III) | 7.4 | 3.77 | 0.008 | ||
| Ce(IV)-Ce(IV) | 5.4 | 3.79 | 0.009 | ||
| 720 min grinding | Ce(IV)-O | 0.1 | 2.30 | 0.009 | −3.4 |
| Ce(III)-O | 8.4 | 2.42 | 0.03 | ||
| Ce(III)-Ce(III) | 10.0 | 3.76 | 0.04 | ||
| Ce(IV)-Ce(IV) | 2.6 | 3.79 | 0.0009 | ||
| Grinding Time (min) | Concentration of Trivalent Cerium | Concentration of Tetravalent Cerium |
|---|---|---|
| 0 | 30.5 | 69.5 |
| 60 | 40.7 | 59.3 |
| 180 | 46.9 | 53.1 |
| 420 | 53.4 | 46.6 |
| Sample | Shell | CN | R [× m−10] | σ2 [× m−20] | ΔE0 |
|---|---|---|---|---|---|
| CeO2 | Ce(IV)-O | 7.7 ± 2.0 | 2.35 | 0.009 | −10.3 |
| Ce(III)-O | - | ||||
| Ce(III)-Ce(III) | - | ||||
| Ce(IV)-Ce(IV) | 12.2 ± 12.0 | 3.84 | 0.005 | ||
| Without grinding | Ce(IV)-O | 8.7 ± 4.8 | 2.35 | 0.02 | −9.3 |
| Ce(III)-O | 0.2 ± 1.8 | 2.46 | 0.04 | ||
| Ce(III)-Ce(III) | 0.3 ± 3.8 | 3.81 | 0.002 | ||
| Ce(IV)-Ce(IV) | 12.1 ± 11.9 | 3.83 | 0.008 | ||
| 60 min grinding | Ce(IV)-O | 7.5 ± 1.8 | 2.35 | 0.003 | 2.6 |
| Ce(III)-O | 0.6 ± 0.6 | 2.47 | 0.016 | ||
| Ce(III)-Ce(III) | 1.0 ± 1.2 | 3.81 | 0.005 | ||
| Ce(IV)-Ce(IV) | 11.4 ± 6.6 | 3.84 | 0.006 | ||
| 180 min grinding | Ce(IV)-O | 6.9 ± 4.8 | 2.29 | 0.025 | −4.0 |
| Ce(III)-O | 1.1 ± 0.7 | 2.41 | −0.002 | ||
| Ce(III)-Ce(III) | 2.5 ± 1.0 | 3.75 | 0.008 | ||
| Ce(IV)-Ce(IV) | 10.2 ± 3.8 | 3.78 | 0.005 | ||
| 420 min grinding | Ce(IV)-O | 6.7 ± 13.1 | 2.21 | 0.063 | −14.7 |
| Ce(III)-O | 1.8 ± 12.0 | 2.32 | 0.00006 | ||
| Ce(III)-Ce(III) | 9.0 ± 4.9 | 3.66 | 0.004 | ||
| Ce(IV)-Ce(IV) | 3.4 ± 2.2 | 3.69 | 0.014 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Iwamoto, M.; Tokoro, C. Investigation of Cerium Reduction Efficiency by Grinding with Microwave Irradiation in Mechanochemical Processing. Minerals 2022, 12, 189. https://doi.org/10.3390/min12020189
Kato T, Iwamoto M, Tokoro C. Investigation of Cerium Reduction Efficiency by Grinding with Microwave Irradiation in Mechanochemical Processing. Minerals. 2022; 12(2):189. https://doi.org/10.3390/min12020189
Chicago/Turabian StyleKato, Tatsuya, Motonori Iwamoto, and Chiharu Tokoro. 2022. "Investigation of Cerium Reduction Efficiency by Grinding with Microwave Irradiation in Mechanochemical Processing" Minerals 12, no. 2: 189. https://doi.org/10.3390/min12020189
APA StyleKato, T., Iwamoto, M., & Tokoro, C. (2022). Investigation of Cerium Reduction Efficiency by Grinding with Microwave Irradiation in Mechanochemical Processing. Minerals, 12(2), 189. https://doi.org/10.3390/min12020189







