Influence of Biomacromolecules on Calcium Phosphate Formation on TiO2 Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
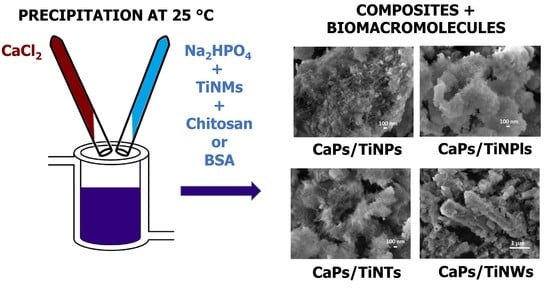
2.2. Preparation of TiO2 Nanomaterials
2.3. Precipitation Experiments
2.4. Characterization Methods
3. Results and Discussion
3.1. The Influence of the Biomacromolecules on the Induction Time for Crystalline Phase Precipitation
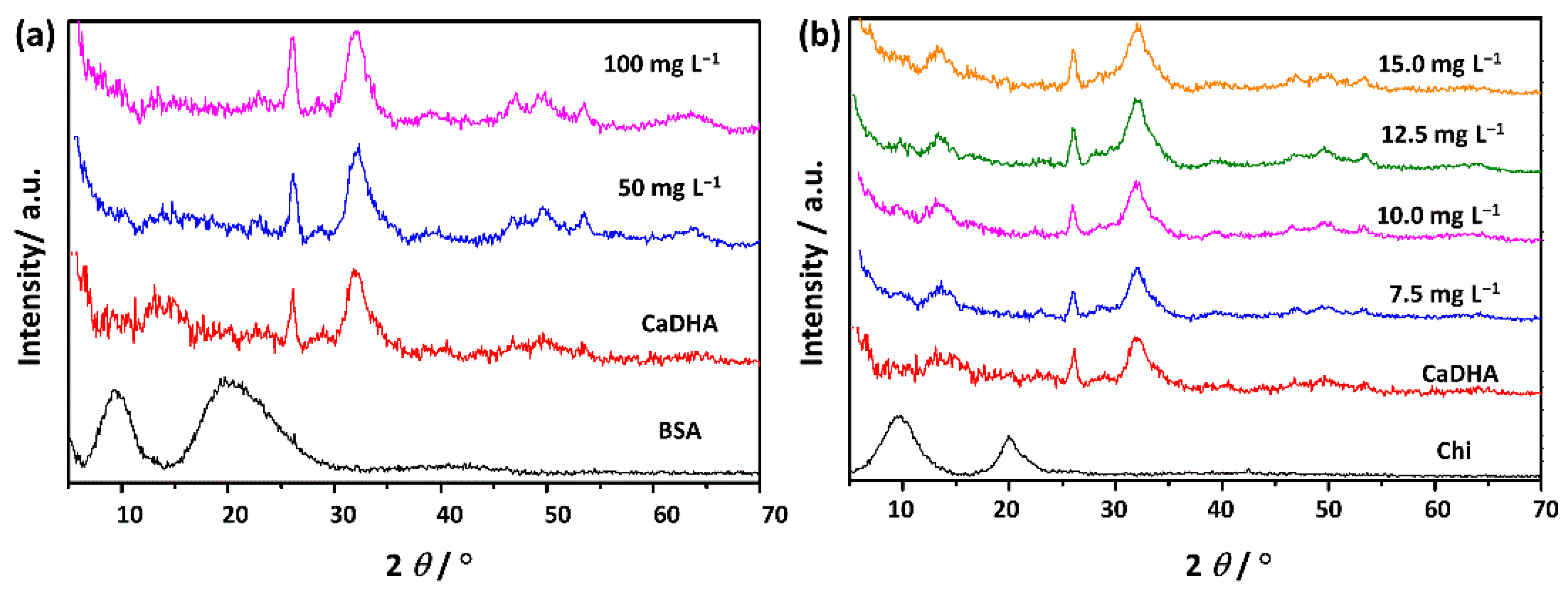
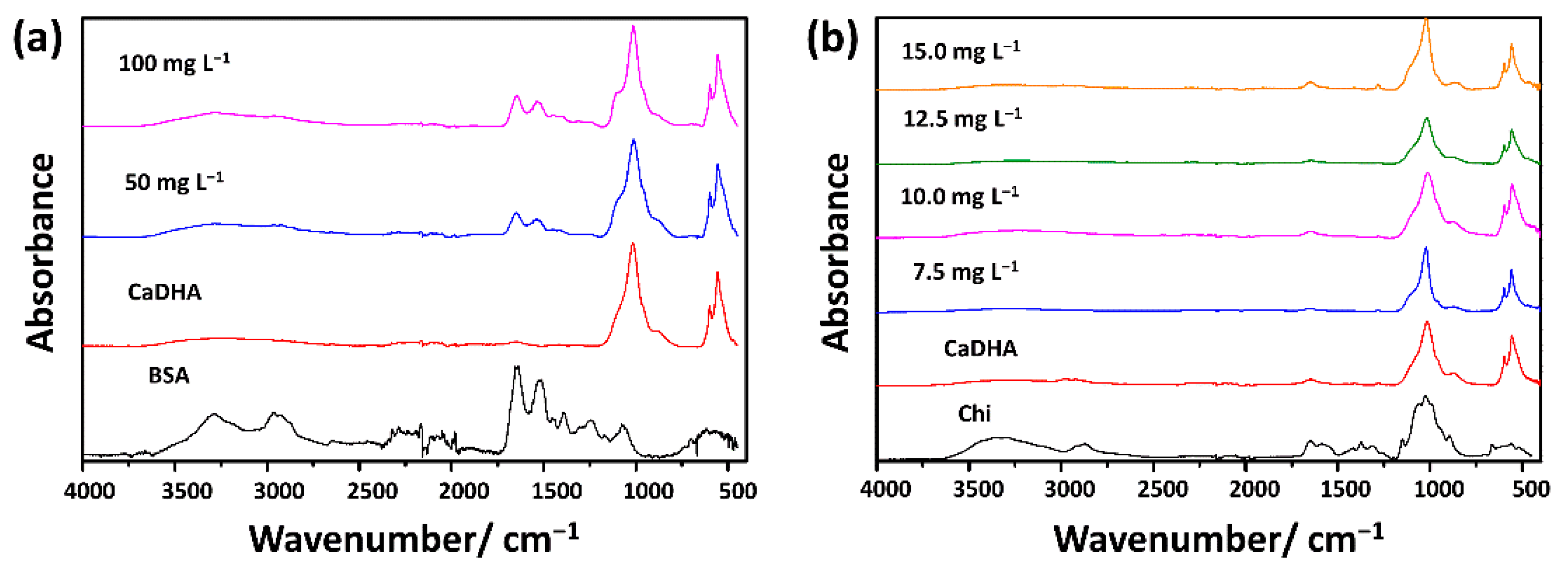
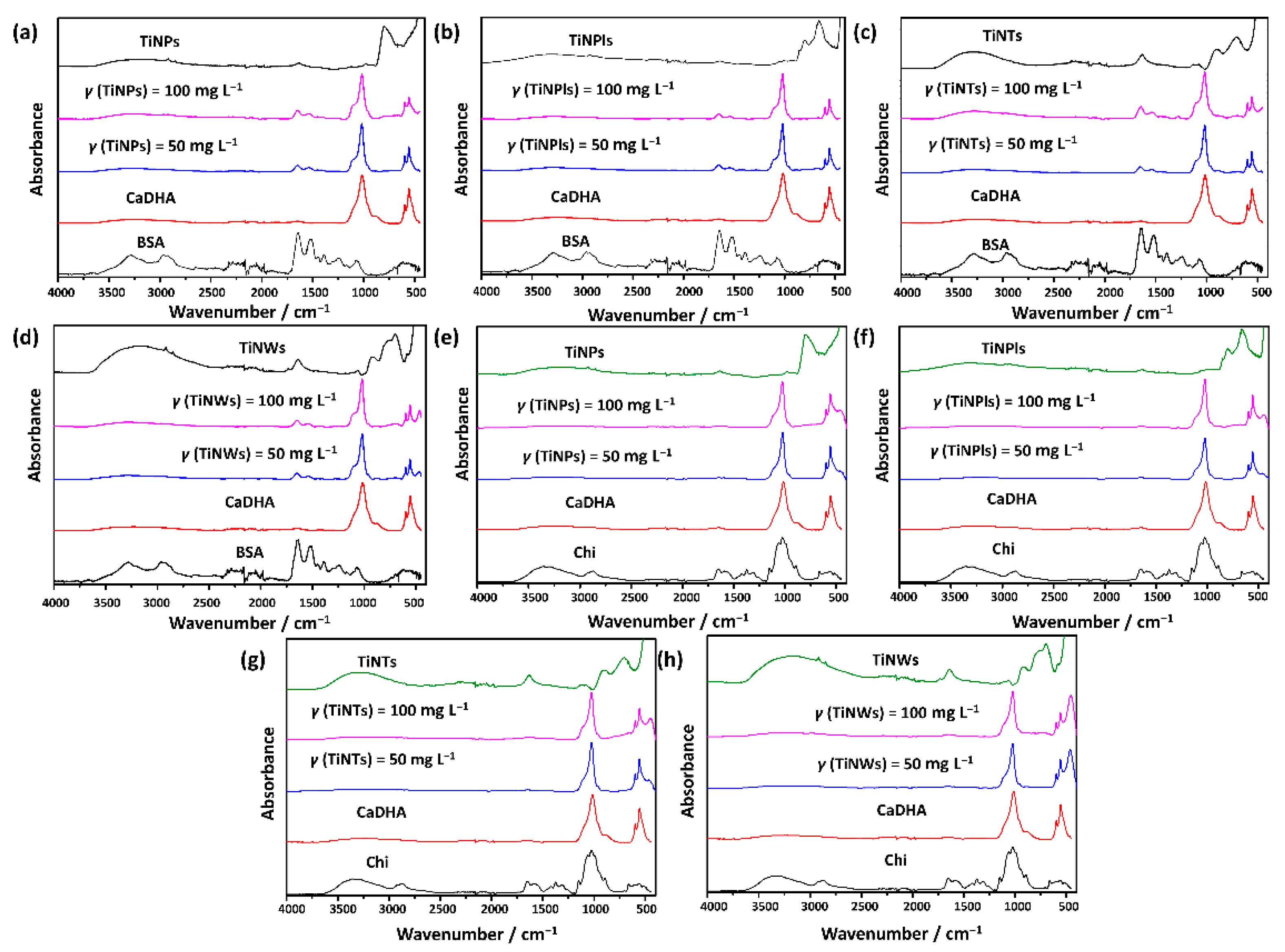
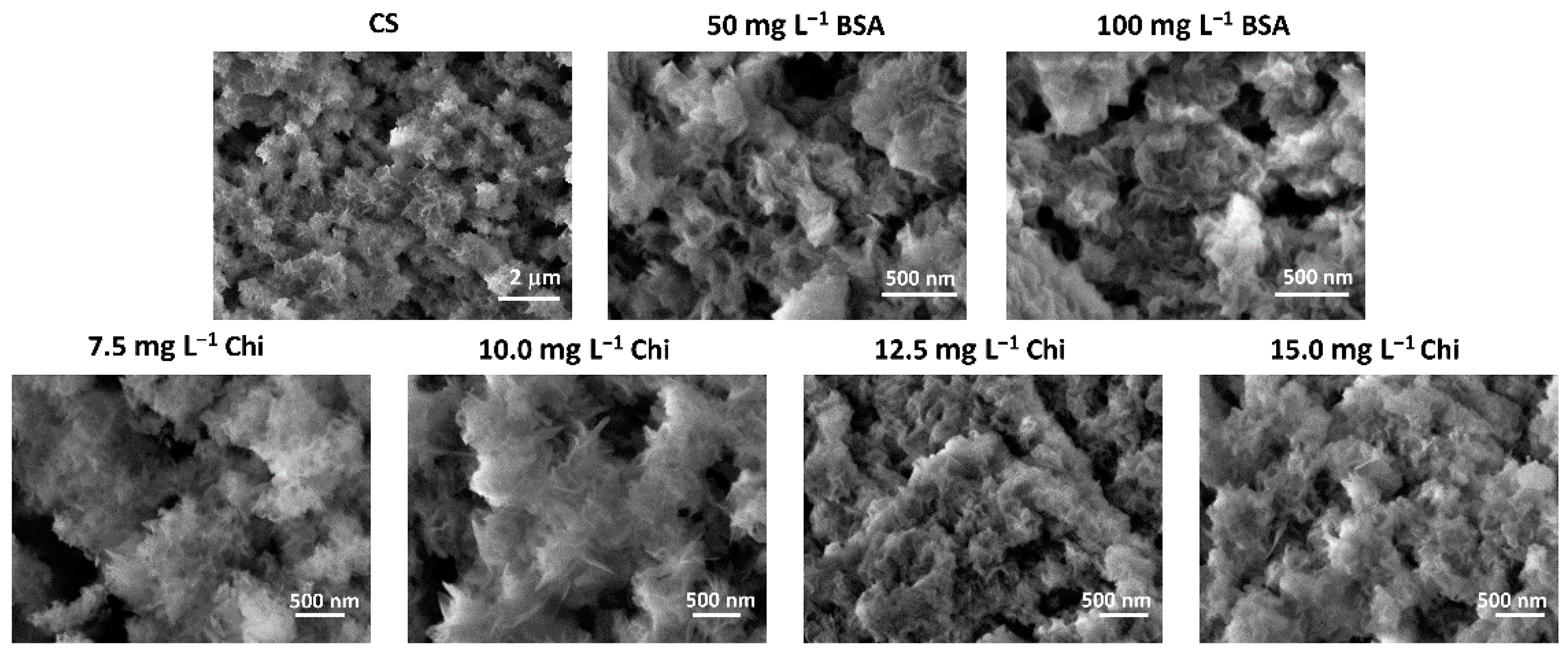
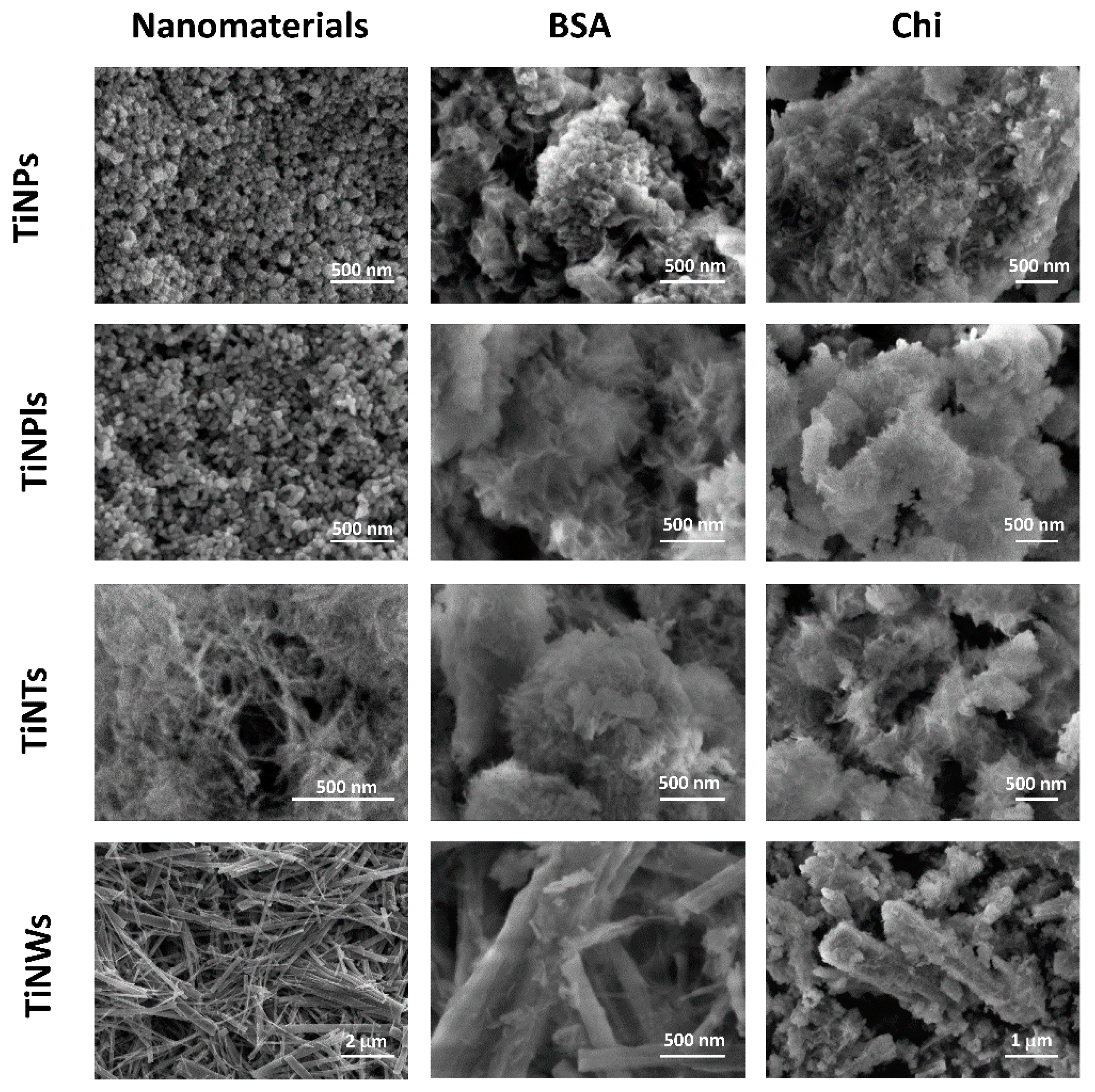
3.2. Influence of Biomacromolecules on the Properties of Crystalline Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium Orthophosphates: Applications in Nature, Biology, and Medicine; Pan Stanford: Singapore, 2012; ISBN 978-981-4316-62-0. [Google Scholar]
- Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2004; ISBN 978-0-12-582463-7. [Google Scholar]
- Dorozhkin, S.V. Functionalized Calcium Orthophosphates (CaPO4) and Their Biomedical Applications. J. Mater. Chem. B 2019, 7, 7471–7489. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E. Functionalized Biomimetic Calcium Phosphates for Bone Tissue Repair. J. Appl. Biomater. Funct. Mater. 2017, 15, e313–e325. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. THE MATERIAL BONE: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Nardecchia, S.; Gutiérrez, M.C.; Serrano, M.C.; Dentini, M.; Barbetta, A.; Ferrer, M.L.; del Monte, F. In Situ Precipitation of Amorphous Calcium Phosphate and Ciprofloxacin Crystals during the Formation of Chitosan Hydrogels and Its Application for Drug Delivery Purposes. Langmuir 2012, 28, 15937–15946. [Google Scholar] [CrossRef]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA Polymer Blends as Coatings for Hydroxyapatite Nanoparticles and Their Effect on Antimicrobial Properties, Osteoconductivity and Regeneration of Osseous Tissues. Mater. Sci. Eng. C 2016, 60, 357–364. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Farrugia, A. Albumin Usage in Clinical Medicine: Tradition or Therapeutic? Transfus. Med. Rev. 2010, 24, 53–63. [Google Scholar] [CrossRef]
- Tang, L.; Eaton, J.W. Natural Responses to Unnatural Materials: A Molecular Mechanism for Foreign Body Reactions. Mol. Med. 1999, 5, 351–358. [Google Scholar] [CrossRef]
- Kato, K.; Lee, S.; Nagata, F. Preparation of Protein–Peptide–Calcium Phosphate Composites for Controlled Protein Release. Molecules 2020, 25, 2312. [Google Scholar] [CrossRef]
- Ozhukil Kollath, V.; Mullens, S.; Luyten, J.; Traina, K.; Cloots, R. Protein–Calcium Phosphate Nanocomposites: Benchmarking Protein Loading via Physical and Chemical Modifications against Co-Precipitation. RSC Adv. 2015, 5, 55625–55632. [Google Scholar] [CrossRef]
- Tomoda, K.; Ariizumi, H.; Nakaji, T.; Makino, K. Hydroxyapatite Particles as Drug Carriers for Proteins. Colloids Surf. B Biointerfaces 2010, 76, 226–235. [Google Scholar] [CrossRef]
- Xu, Z.; Grassian, V.H. Bovine Serum Albumin Adsorption on TiO2 Nanoparticle Surfaces: Effects of PH and Coadsorption of Phosphate on Protein–Surface Interactions and Protein Structure. J. Phys. Chem. C 2017, 121, 21763–21771. [Google Scholar] [CrossRef]
- Bleek, K.; Taubert, A. New Developments in Polymer-Controlled, Bioinspired Calcium Phosphate Mineralization from Aqueous Solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef]
- Sikirić, M.D.; Füredi-Milhofer, H. The Influence of Surface Active Molecules on the Crystallization of Biominerals in Solution. Adv. Colloid Interface Sci. 2006, 128–130, 135–158. [Google Scholar] [CrossRef]
- Kargupta, R.; Bok, S.; Darr, C.M.; Crist, B.D.; Gangopadhyay, K.; Gangopadhyay, S.; Sengupta, S. Coatings and Surface Modifications Imparting Antimicrobial Activity to Orthopedic Implants. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 475–495. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Macha, I.; Cazalbou, S.; Choi, A.H. Calcium Phosphate Nanocoatings and Nanocomposites, Part 2: Thin Films for Slow Drug Delivery and Osteomyelitis. Nanomedicine 2016, 11, 531–544. [Google Scholar] [CrossRef]
- Brennan, S.A.; Ní Fhoghlú, C.; Devitt, B.M.; O’Mahony, F.J.; Brabazon, D.; Walsh, A. Silver Nanoparticles and Their Orthopaedic Applications. Bone Jt. J. 2015, 97-B, 582–589. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Balan, V.; Popa, M.I.; Lobiuc, A.; Antoniac, A.; Antoniac, I.V.; Verestiuc, L. Biopolymers—Calcium Phosphates Composites with Inclusions of Magnetic Nanoparticles for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 125, 612–620. [Google Scholar] [CrossRef]
- Keskar, M.; Sabatini, C.; Cheng, C.; Swihart, M.T. Synthesis and Characterization of Silver Nanoparticle-Loaded Amorphous Calcium Phosphate Microspheres for Dental Applications. Nanoscale Adv. 2019, 1, 627–635. [Google Scholar] [CrossRef]
- Salarian, M.; Xu, W.Z.; Wang, Z.; Sham, T.-K.; Charpentier, P.A. Hydroxyapatite–TiO2 -Based Nanocomposites Synthesized in Supercritical CO2 for Bone Tissue Engineering: Physical and Mechanical Properties. ACS Appl. Mater. Interfaces 2014, 6, 16918–16931. [Google Scholar] [CrossRef]
- Oktar, F.N. Hydroxyapatite–TiO2 Composites. Mater. Lett. 2006, 60, 2207–2210. [Google Scholar] [CrossRef]
- Joseph Nathanael, A.; Mangalaraj, D.; Chen, P.C.; Ponpandian, N. Mechanical and Photocatalytic Properties of Hydroxyapatite/Titania Nanocomposites Prepared by Combined High Gravity and Hydrothermal Process. Compos. Sci. Technol. 2010, 70, 419–426. [Google Scholar] [CrossRef]
- Que, W.; Khor, K.A.; Xu, J.L.; Yu, L.G. Hydroxyapatite/Titania Nanocomposites Derived by Combining High-Energy Ball Milling with Spark Plasma Sintering Processes. J. Eur. Ceram. Soc. 2008, 28, 3083–3090. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Todorova, N.; Romanos, G.; Vaimakis, T.; Dillert, R.; Bahnemann, D.; Trapalis, C. Composite Hydroxyapatite/TiO2 Materials for Photocatalytic Oxidation of NOx. Mater. Sci. Eng. B 2012, 177, 1046–1052. [Google Scholar] [CrossRef]
- Milella, E. Preparation and Characterisation of Titania/Hydroxyapatite Composite Coatings Obtained by Sol–Gel Process. Biomaterials 2001, 22, 1425–1431. [Google Scholar] [CrossRef]
- Duan, K.; Wang, R. Surface Modifications of Bone Implants through Wet Chemistry. J. Mater. Chem. 2006, 16, 2309. [Google Scholar] [CrossRef]
- Ruso, J.M.; Verdinelli, V.; Hassan, N.; Pieroni, O.; Messina, P.V. Enhancing CaP Biomimetic Growth on TiO2 Cuboids Nanoparticles via Highly Reactive Facets. Langmuir 2013, 29, 2350–2358. [Google Scholar] [CrossRef]
- Erceg, I.; Selmani, A.; Gajović, A.; Panžić, I.; Iveković, D.; Faraguna, F.; Šegota, S.; Ćurlin, M.; Strasser, V.; Kontrec, J.; et al. Calcium Phosphate Formation on TiO2 Nanomaterials of Different Dimensionality. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124615. [Google Scholar] [CrossRef]
- Erceg, I.; Selmani, A.; Gajović, A.; Radatović, B.; Šegota, S.; Ćurlin, M.; Strasser, V.; Kontrec, J.; Kralj, D.; Maltar-Strmečki, N.; et al. Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis. Nanomaterials 2021, 11, 1523. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of Titania Nanosheets with a High Percentage of Exposed (001) Facets and Related Photocatalytic Properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef]
- Sofianou, M.-V.; Trapalis, C.; Psycharis, V.; Boukos, N.; Vaimakis, T.; Yu, J.; Wang, W. Study of TiO2 Anatase Nano and Microstructures with Dominant {001} Facets for NO Oxidation. Environ. Sci. Pollut. Res. 2012, 19, 3719–3726. [Google Scholar] [CrossRef]
- Selmani, A.; Špadina, M.; Plodinec, M.; Delač Marion, I.; Willinger, M.G.; Lützenkirchen, J.; Gafney, H.D.; Redel, E. An Experimental and Theoretical Approach to Understanding the Surface Properties of One-Dimensional TiO2 Nanomaterials. J. Phys. Chem. C 2015, 119, 19729–19742. [Google Scholar] [CrossRef]
- Bar-Yosef Ofir, P.; Govrin-Lippman, R.; Garti, N.; Füredi-Milhofer, H. The Influence of Polyelectrolytes on the Formation and Phase Transformation of Amorphous Calcium Phosphate. Cryst. Growth Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Selmani, A.; Coha, I.; Magdić, K.; Čolović, B.; Jokanović, V.; Šegota, S.; Gajović, S.; Gajović, A.; Jurašin, D.; Dutour Sikirić, M. Multiscale Study of the Influence of Cationic Surfactants on Amorphous Calcium Phosphate Precipitation. CrystEngComm 2015, 17, 8529–8548. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, W.; Pan, H.; Jiang, S.; Tang, R. Stabilizing Amorphous Calcium Phosphate Phase by Citrate Adsorption. CrystEngComm 2014, 16, 1864–1867. [Google Scholar] [CrossRef]
- Uskoković, V. Visualizing Different Crystalline States during the Infrared Imaging of Calcium Phosphates. Vib. Spectrosc. 2020, 108, 103045. [Google Scholar] [CrossRef]
- Eans, E.D.; Gillessen, I.H.; Posne, A.S. Intermediate States in the Precipitation of Hydroxyapatite. Nature 1965, 208, 365–367. [Google Scholar] [CrossRef]
- Brečević, L.; Füredi-Milhofer, H. Precipitation of Calcium Phosphates from Electrolyte Solutions: II. The Formation and Transformation of the Precipitates. Calcif. Tissue Res. 1972, 10, 82–90. [Google Scholar] [CrossRef]
- Christoffersen, J.; Christoffersen, M.R.; Kibalczyc, W.; Andersen, F.A. A Contribution to the Understanding of the Formation of Calcium Phosphates. J. Cryst. Growth 1989, 94, 767–777. [Google Scholar] [CrossRef]
- Pan, H.; Liu, X.Y.; Tang, R.; Xu, H.Y. Mystery of the Transformation from Amorphous Calcium Phosphate to Hydroxyapatite. Chem. Commun. 2010, 46, 7415. [Google Scholar] [CrossRef]
- Jiang, S.; Jin, W.; Wang, Y.-N.; Pan, H.; Sun, Z.; Tang, R. Effect of the Aggregation State of Amorphous Calcium Phosphate on Hydroxyapatite Nucleation Kinetics. RSC Adv. 2017, 7, 25497–25503. [Google Scholar] [CrossRef]
- Li, S.; Wang, L. Phosphorylated Osteopontin Peptides Inhibit Crystallization by Resisting the Aggregation of Calcium Phosphate Nanoparticles. CrystEngComm 2012, 14, 8037. [Google Scholar] [CrossRef]
- Yang, X.; Xie, B.; Wang, L.; Qin, Y.; Henneman, Z.J.; Nancollas, G.H. Influence of Magnesium Ions and Amino Acids on the Nucleation and Growth of Hydroxyapatite. CrystEngComm 2011, 13, 1153–1158. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Du, L.-W.; Bian, S.; Gou, B.-D.; Jiang, Y.; Huang, J.; Gao, Y.-X.; Zhao, Y.-D.; Wen, W.; Zhang, T.-L.; Wang, K. Structure of Clusters and Formation of Amorphous Calcium Phosphate and Hydroxyapatite: From the Perspective of Coordination Chemistry. Cryst. Growth Des. 2013, 13, 3103–3109. [Google Scholar] [CrossRef]
- Füredi-Milhofer, H.; Sarig, S. Interactions between Polyelectrolytes and Sparingly Soluble Salts. Prog. Cryst. Growth Charact. Mater. 1996, 32, 45–74. [Google Scholar] [CrossRef]
- Bujacz, A. Structures of Bovine, Equine and Leporine Serum Albumin. Acta Cryst. D Biol Cryst. 2012, 68, 1278–1289. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Damen, J.J.M.; Ten Cate, J.M.; Ellingsen, J.E. Induction of Calcium Phosphate Precipitation by Titanium Dioxide. J. Dent. Res. 1991, 70, 1346–1349. [Google Scholar] [CrossRef]
- Serro, A.P.; Fernandes, A.C.; Saramago, B.; Lima, J.; Barbosa, M.A. Apatite Deposition on Titanium Surfaces—The Role of Albumin Adsorption. Biomaterials 1997, 18, 963–968. [Google Scholar] [CrossRef]
- Serro, A.P.; Bastos, M.; Pessoa, J.C.; Saramago, B. Bovine Serum Albumin Conformational Changes upon Adsorption on Titania and on Hydroxyapatite and Their Relation with Biomineralization. J. Biomed. Mater. Res. 2004, 70A, 420–427. [Google Scholar] [CrossRef]
- Stenport, V.; Kjellin, P.; Andersson, M.; Currie, F.; Sul, Y.-T.; Wennerberg, A.; Arvidsson, A. Precipitation of Calcium Phosphate in the Presence of Albumin on Titanium Implants with Four Different Possibly Bioactive Surface Preparations. An in Vitro Study. J. Mater. Sci. Mater. Med. 2008, 19, 3497–3505. [Google Scholar] [CrossRef]
- Lima, J.; Sousa, S.R.; Ferreira, A.; Barbosa, M.A. Interactions between Calcium, Phosphate, and Albumin on the Surface of Titanium. J. Biomed. Mater. Res. 2001, 55, 45–53. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous Calcium (Ortho)Phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Liou, S.-C.; Chen, S.-Y.; Lee, H.-Y.; Bow, J.-S. Structural Characterization of Nano-Sized Calcium Deficient Apatite Powders. Biomaterials 2004, 25, 189–196. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic Substitutions in Calcium Phosphates Synthesized at Low Temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Mochales, C.; Wilson, R.M.; Dowker, S.E.P.; Ginebra, M.-P. Dry Mechanosynthesis of Nanocrystalline Calcium Deficient Hydroxyapatite: Structural Characterisation. J. Alloys Compd. 2011, 509, 7389–7394. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds. BioMed. Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef]
- Buljan Meić, I.; Kontrec, J.; Domazet Jurašin, D.; Njegić Džakula, B.; Štajner, L.; Lyons, D.M.; Dutour Sikirić, M.; Kralj, D. Comparative Study of Calcium Carbonates and Calcium Phosphates Precipitation in Model Systems Mimicking the Inorganic Environment for Biomineralization. Cryst. Growth Des. 2017, 17, 1103–1117. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Barinov, S.M.; Fedotov, A.Y.; Komlev, V.S. Interactions of Calcium Phosphates with Chitosan. Dokl. Chem. 2011, 441, 387–390. [Google Scholar] [CrossRef]
- Aimoli, C.G.; Beppu, M.M. Precipitation of Calcium Phosphate and Calcium Carbonate Induced over Chitosan Membranes: A Quick Method to Evaluate the Influence of Polymeric Matrices in Heterogeneous Calcification. Colloids Surf. B Biointerfaces 2006, 53, 15–22. [Google Scholar] [CrossRef]
- Bronze-Uhle, E.; Costa, B.C.; Ximenes, V.F.; Lisboa-Filho, P.N. Synthetic Nanoparticles of Bovine Serum Albumin with Entrapped Salicylic Acid. Nanotechnol. Sci. Appl. 2016, 10, 11–21. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Rohiwal, S.S.; Satvekar, R.K.; Tiwari, A.P.; Raut, A.V.; Kumbhar, S.G.; Pawar, S.H. Investigating the Influence of Effective Parameters on Molecular Characteristics of Bovine Serum Albumin Nanoparticles. Appl. Surf. Sci. 2015, 334, 157–164. [Google Scholar] [CrossRef]
- Tanase, C.E.; Popa, M.I.; Verestiuc, L. Biomimetic Chitosan-Calcium Phosphate Composites with Potential Applications as Bone Substitutes: Preparation and Characterization. J. Biomed. Mater. Res. 2012, 100B, 700–708. [Google Scholar] [CrossRef]
- Liu, Y.; Layrolle, P.; de Bruijn, J.; van Blitterswijk, C.; de Groot, K. Biomimetic Coprecipitation of Calcium Phosphate and Bovine Serum Albumin on Titanium Alloy. J. Biomed. Mater. Res. 2001, 57, 327–335. [Google Scholar] [CrossRef]
- Aimoli, C.G.; de Lima, D.O.; Beppu, M.M. Investigation on the Biomimetic Influence of Biopolymers on Calcium Phosphate Precipitation-Part 2: Chitosan. Mater. Sci. Eng. C 2008, 28, 1565–1571. [Google Scholar] [CrossRef]
- Marques, P.A.A.P.; Serro, A.P.; Saramago, B.J.; Fernandes, A.C.; Magalhães, M.C.F.; Correia, R.N. Mineralisation of Two Phosphate Ceramics in HBSS: Role of Albumin. Biomaterials 2003, 24, 451–460. [Google Scholar] [CrossRef]

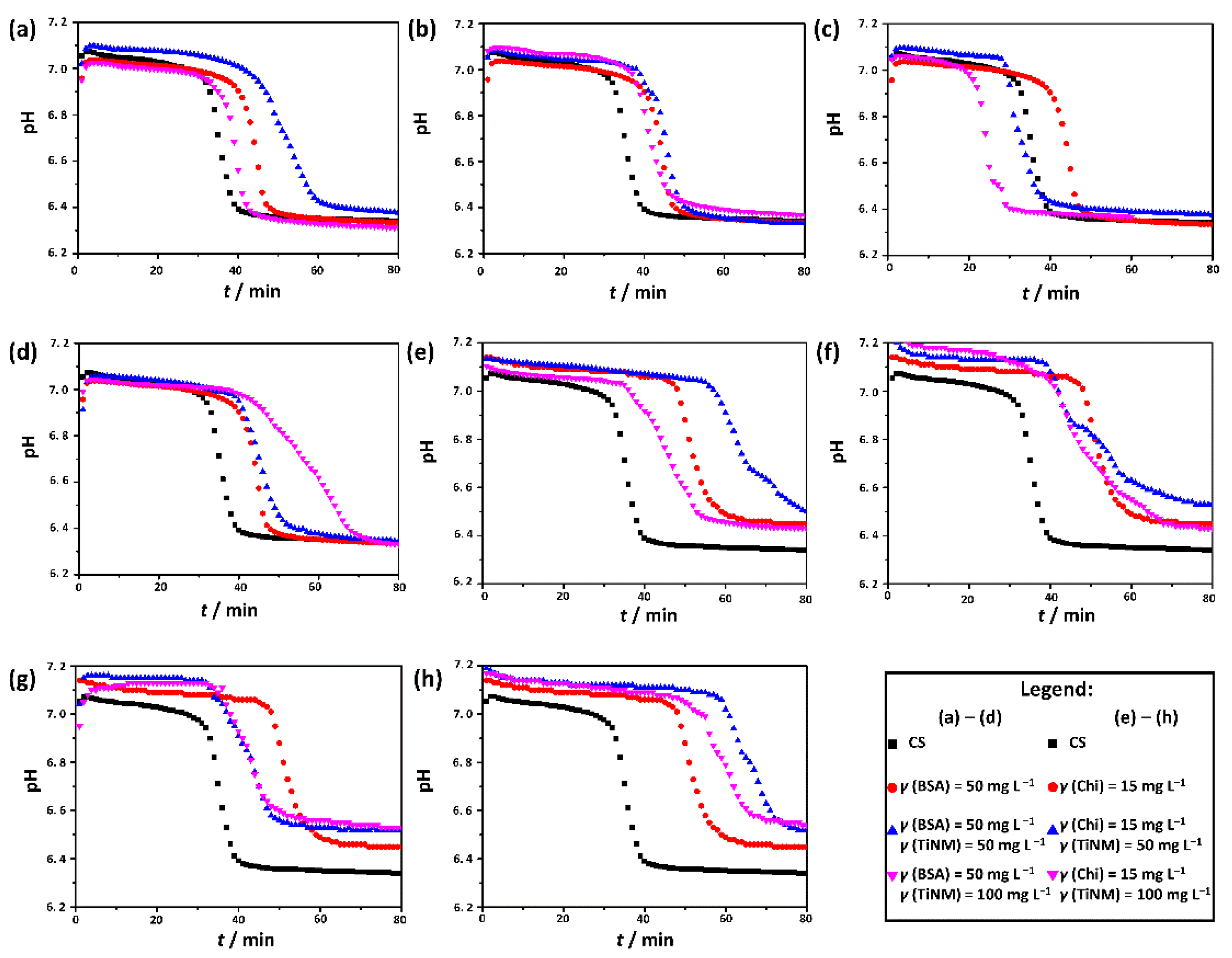

| ti/min | |||||||
|---|---|---|---|---|---|---|---|
| γ/mg L−1 | 0 | 7.5 | 10.0 | 12.5 | 15.0 | 50.0 | 100.0 |
| BSA | 29.4 ± 3.2 | 39.7 ± 1.0 | 45.1 ± 0.9 | ||||
| Chi | 45.1± 2.1 | 34.9 ± 1.2 | 49.1 ± 1.1 | 46.4 ± 1.1 | |||
| ti/min | ||||
|---|---|---|---|---|
| BSA | Chi | |||
| TiNMs | γ/mg L−1 | 0 * | 50 | 15 |
| TiNPs | 50 | 28.4 ± 0.8 | 40.7 ± 5.3 | 55.6 ± 2.1 |
| 100 | 22.4 ± 1.2 | 34.9 ± 0.5 | 37.0 ± 1.1 | |
| TiNPls | 50 | 27.1 ± 0.6 | 37.9 ± 2.5 | 37.5 ± 1.4 |
| 100 | 25.3 ± 0.9 | 35.9 ± 1.0 | 34.0 ± 1.6 | |
| TiNTs | 50 | 17.4 ± 0.9 | 28.2 ± 1.1 | 31.9 ± 0.9 |
| 100 | 17.9 ± 2.0 | 20.3 ± 1.5 | 33.4 ± 1.5 | |
| TiNWs | 50 | 29.2 ± 1.3 | 37.3 ± 1.7 | 57.6 ± 2.3 |
| 100 | 23.2 ± 1.6 | 38.8 ± 4.2 | 50.2 ± 0.3 | |
| ζ/mV | |||
|---|---|---|---|
| Sample | Anionic | Anionic + BSA | Anionic + Chi |
| TiNPs | −45.9 ± 2.2 a | −32.6 ± 1.3 | 3.13 ± 0.56 |
| TiNPls | −53.0 ± 2.5 b | −34.3 ± 2.0 | 3.61 ± 1.22 |
| TiNTs | −40.5 ± 1.6 a | −31.2 ±1.7 | 3.01 ± 0.46 |
| TiNWs | −55.0 ± 2.0 b | −39.5 ± 1.9 | 3.50 ± 0.14 |
| BSA | −15.9 ± 4.7 | / | / |
| Chi | −0.04 ± 0.11 | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erceg, I.; Dutour Sikirić, M. Influence of Biomacromolecules on Calcium Phosphate Formation on TiO2 Nanomaterials. Minerals 2022, 12, 1557. https://doi.org/10.3390/min12121557
Erceg I, Dutour Sikirić M. Influence of Biomacromolecules on Calcium Phosphate Formation on TiO2 Nanomaterials. Minerals. 2022; 12(12):1557. https://doi.org/10.3390/min12121557
Chicago/Turabian StyleErceg, Ina, and Maja Dutour Sikirić. 2022. "Influence of Biomacromolecules on Calcium Phosphate Formation on TiO2 Nanomaterials" Minerals 12, no. 12: 1557. https://doi.org/10.3390/min12121557
APA StyleErceg, I., & Dutour Sikirić, M. (2022). Influence of Biomacromolecules on Calcium Phosphate Formation on TiO2 Nanomaterials. Minerals, 12(12), 1557. https://doi.org/10.3390/min12121557