Gibbsite Crystallinity and Morphology in Ferralsols and Bauxites
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Mineralogical Characterization
3.2. Crystallographic Characterization
3.3. Gibbsite Morphology
4. Discussion
4.1. Gibbsite Characterization
4.2. Gibbsite Crystallinity
4.3. Gibbsite Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, M.M.; Fernandes, B.; Curi, N. Mineralogia da fração argila e estrutura de Latossolos da região sudeste do Brasil. Rev. Bras. Cienc. Solo. 1999, 23, 507–514. [Google Scholar] [CrossRef]
- Vitorino, A.C.T.; Ferreira, M.M.; Curi, N.; Lima, J.M.; Silva, M.L.N.; Motta, P.E.F. Mineralogia, química e estabilidade de agregados do tamanho de silte de solos da Região Sudeste do Brasil. Pesqui. Agropecu. Bras. 2003, 38, 133–141. [Google Scholar] [CrossRef]
- Leal, J.R.; Velloso, A.C.X. Adsorção de fosfato em Latossolos sob vegetação de Cerrado. Pesqui. Agropecu. Bras. 1973, 8, 81–88. [Google Scholar]
- Fontes, M.P.F.; Weed, S.B. Phosphate adsorption by clays from Brazilian Oxisols: Relationships with specific surface area and mineralogy. Geoderma 1996, 72, 37–51. [Google Scholar] [CrossRef]
- Moreira, F.L.M.; Mota, F.O.B.; Clemente, C.A.; Azevedo, B.M.; Bomfim, G.B. Adsorção de fósforo em solos do Estado do Ceará. Cienc. Agron. 2006, 37, 7–12. [Google Scholar]
- Goldberg, S.; Lebron, I.; Seaman, J.C.; Suarez, D.L. Soil colloidal behavior. In Handbook of Soil Sciences: Properties and Processes, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Chapter 15; pp. 15-1–15-39. [Google Scholar]
- Fontes, M.P.F.; Weed, S.B. Iron oxides in selected Brazilian oxisols: I. Mineralogy. Soil. Sci. Soc. Am. J. 1991, 55, 1143–1149. [Google Scholar] [CrossRef]
- Ker, J.C. Latossolos do Brasil: Uma Revisão. Geonomos 1997, 5, 17–40. [Google Scholar] [CrossRef]
- Macías-Vazquez, F. Formation of gibbsite in soils and saprolites of temperatehumid zones. Clay Miner. 1981, 16, 43–52. [Google Scholar] [CrossRef]
- Kämpf, N.; Scheinost, A.C.; Schulze, D.G. Oxide Minerals in Soils. In Handbook of Soil Sciences: Properties and Processes, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 22-1–22-34. [Google Scholar]
- Caner, L.; Radtke, L.M.; Vignol-Lelarge, M.L.; Inda, A.V.; Bortoluzzi, E.C.; Mexias, A.S. Basalt and rhyo-dacite weathering and soil clay formation under subtropical climate in southern Brazil. Geoderma 2014, 235, 100–112. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ruan, H.D.; Frost, R.L. Thermal decomposition of bauxite minerals: Infrared emission spectroscopy of gibbsite, boehmite and diaspore. J. Mater. Sci. 2002, 37, 1121–1129. [Google Scholar] [CrossRef]
- Melfi, A.J.; Carvalho, A.; Boulange, B.; Lucas, Y. Brazilian Bauxites; USP: São Paulo, Brazil; FAPESP: São Paulo, Brazil; ORSTOM: Paris, France, 1997; 331p. [Google Scholar]
- Hsu, P.H. Aluminum Hydroxides and Oxyhydroxides. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 331–378. [Google Scholar]
- Huang, P.M.; Wang, M.K.; Kämpf, N.; Schulze, D.G. Aluminum hydroxides. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2002; pp. 261–289. [Google Scholar]
- Schulze, D.G. An Introduction to Soil Mineralogy. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2002; pp. 1–35. [Google Scholar]
- Oliveira Junior, J.C.; Souza, L.C.P.; Melo, V.F.; Rocha, H.O. Variabilidade espacial de atributos mineralógicos de solos da formação Guabirotuba, Curitiba (PR). Rev. Bras. Cienc. Solo. 2011, 35, 1481–1490. [Google Scholar] [CrossRef][Green Version]
- Schoen, R.; Roberson, C.E. Structures of Aluminum Hydroxide and Geochemical Implications. Am. Min. 1970, 55, 43–77. [Google Scholar]
- Freij, S.J.; Parkinson, G.M.; Reyhani, M.M. Direct observation of the growth of gibbsite crystals by atomic force microscopy. J. Cryst. Growth. 2004, 260, 232–242. [Google Scholar] [CrossRef]
- Fontes, M.P.F.; Camargo, O.A.; Sposito, G. Eletroquímica das partículas coloidais e sua relação com a mineralogia de solos altamente intemperizados. Sci. Agric. 2001, 58, 627–646. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS study of the major minerals in bauxite: Gibbsite, bayerite and (pseudo-) boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Melo, V.F.; Fontes, M.P.F.; Novais, R.F.; Singh, B.; Schaefer, C.E.G.R. Características dos óxidos de ferro e de alumínio de diferentes classes de solos. Rev. Bras. Cienc. Solo. 2001, 25, 19–32. [Google Scholar] [CrossRef]
- Sweegers, C.; Connick, H.C.; Meekes, H.; Enckervort, W.J.P.; Hiralal, I.D.K.; Rijkeboer, A. Morphology, evolution and other characteristics of gibbsite crystals grown from pure and impure aqueous sodium aluminate solutions. J. Cryst. Growth. 2001, 233, 567–582. [Google Scholar] [CrossRef]
- Braga, M.A.S.; Paquet, H.; Begonha, A. Weathering of granites in a temperate climate (NW Portugal): Granitic saprolites and arenization. Catena 2002, 49, 41–56. [Google Scholar] [CrossRef]
- Saalfeld, H.; Wedde, M. Refinement of the crystal structure of gibbsite, Al(OH)3. Z. Krist. 1974, 139, 120–135. [Google Scholar] [CrossRef]
- Ksenofontov, D.A.; Kabalov, Y.K. Structure refinement and thermal stability of gibbsite. Inorg. Mater. 2012, 48, 142–144. [Google Scholar] [CrossRef]
- Peskleway, C.D.; Henderson, G.S.; Wicks, F.J. Dissolution of gibbsite: Direct observations using fluid cell atomic force microscopy. Am. Min. 2003, 88, 18–26. [Google Scholar] [CrossRef]
- Schwertmann, U. Properties of Goethites of Varying Crystallinity. Clays Clay Miner. 1985, 33, 369–378. [Google Scholar] [CrossRef]
- Plançon, A.; Tchoubar, C. Determination of structural defects in phyllosilicates by X-ray powder diffraction-II. Nature and proportion of defects in natural kaolinites. Clays Clay Miner. 1977, 25, 436–450. [Google Scholar] [CrossRef]
- Coffin, D.E. A method for the determination of free iron in soils and clays. Can. J. Soil Sci. 1963, 43, 7–17. [Google Scholar] [CrossRef]
- Harris, W.; White, G.N. X-ray Diffraction Techniques for Soil Mineral Identification. In Methods of Soil Analysis Part 5: Mineralogical Methods; Ulery, A.L., Drees, L.R., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2008; Volume 5, pp. 81–116. [Google Scholar]
- White, G.N. Scanning Electron Microscopy. In Methods of Soil Analysis Part 5: Mineralogical Methods; Ulery, A.L., Drees, L.R., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2008; Volume 5, pp. 81–116. [Google Scholar]
- Norrish, K.; Taylor, R.M. The isomorphous replacement of iron by aluminium in soil goethites. J. Soil Sci. 1961, 12, 294–306. [Google Scholar] [CrossRef]
- Kämpf, N.; Schwertmann, U. The 5M NaOH concentration treatment for iron oxides in soils. Clays Clay Miner. 1982, 30, 401–408. [Google Scholar] [CrossRef]
- Schwertmann, U.; Carlson, L. Aluminum Influence on Iron Oxides: XVII. Unit-Cell Parameters and Aluminum Substitution of Natural Goethites. Soil Sci. Soc. Am. J. 1994, 58, 256. [Google Scholar] [CrossRef]
- Neumann, R.; Avelar, A.N.; Costa, G.M. Refinement of the isomorphic substitutions in goethite and hematite by the Rietveld method, and relevance to bauxite characterisation and processing. Miner. Eng. 2014, 55, 80–86. [Google Scholar] [CrossRef]
- Singh, B.; Gilkes, R.J. Properties of soil kaolinites from south-western Australia. J. Soil Sci. 1992, 43, 645–667. [Google Scholar] [CrossRef]
- Ghidin, A.A.; Melo, V.D.F.; Lima, V.C.; Lima, J.M.J.C. Toposseqüências de Latossolos originados de rochas basálticas no Paraná: I-mineralogia da fração argila. Rev. Bras. Cienc. Solo. 2006, 30, 293–306. [Google Scholar] [CrossRef]
- Pacheco, A.A.; Ker, J.C.; Schaefer, C.E.G.R.; Fontes, M.P.F.; Andrade, F.V.; Martins, E.D.S.; Oliveira, F.S.D. Mineralogy, micromorphology, and genesis of soils with varying drainage along a hillslope on granitic rocks of the Atlantic Forest Biome, Brazil. Rev. Bras. Cienc. Solo. 2018, 42, e0170291. [Google Scholar] [CrossRef]
- Schwertmann, U. Goethite and hematite formation in the presence of clay minerals and gibbsite at 25 C. Soil Sci. Soc. Am. J. 1988, 52, 288–291. [Google Scholar] [CrossRef]
- Camargo, L.A.; Júnior, J.M.; Pereira, G.T.; Horvat, R.A. Variabilidade Espacial de Atributos Mineralógicos De Um Latossolo Sob Diferentes Formas Do Relevo. I–Mineralogia da Fração Argila. Rev. Bras. Cienc. Solo. 2008, 32, 2279–2288. [Google Scholar] [CrossRef][Green Version]
- Montanari, R.; Júnior, J.M.; Campos, M.C.C.; Souza, Z.M.; Camargo, L.A. Caracterização mineralógica de Latossolos em diferentes feições do relevo na região de Jaboticabal, SP. Cienc. Agron. 2010, 41, 191–199. [Google Scholar] [CrossRef]
- Mesquita Filho, M.V.; Torrent, J. Phosphate sorption as related to mineralogy of a hydrosequence of soils from the Cerrado region (Brazil). Geoderma 1993, 58, 107–123. [Google Scholar] [CrossRef]
- Fitzpatrick, R.W.; Schwertmann, U. Al-substituted goethite-An indicator of pedogenic and other weathering environments in South Africa. Geoderma 1982, 27, 335–347. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, J.T.; Russel, S.C.; Szetu, J.L. Vibrational spectroscopy and dehydroxylation of aluminum (oxo)hydroxides: Gibbsite. Appl. Spectrosc. 1999, 53, 423–434. [Google Scholar] [CrossRef]

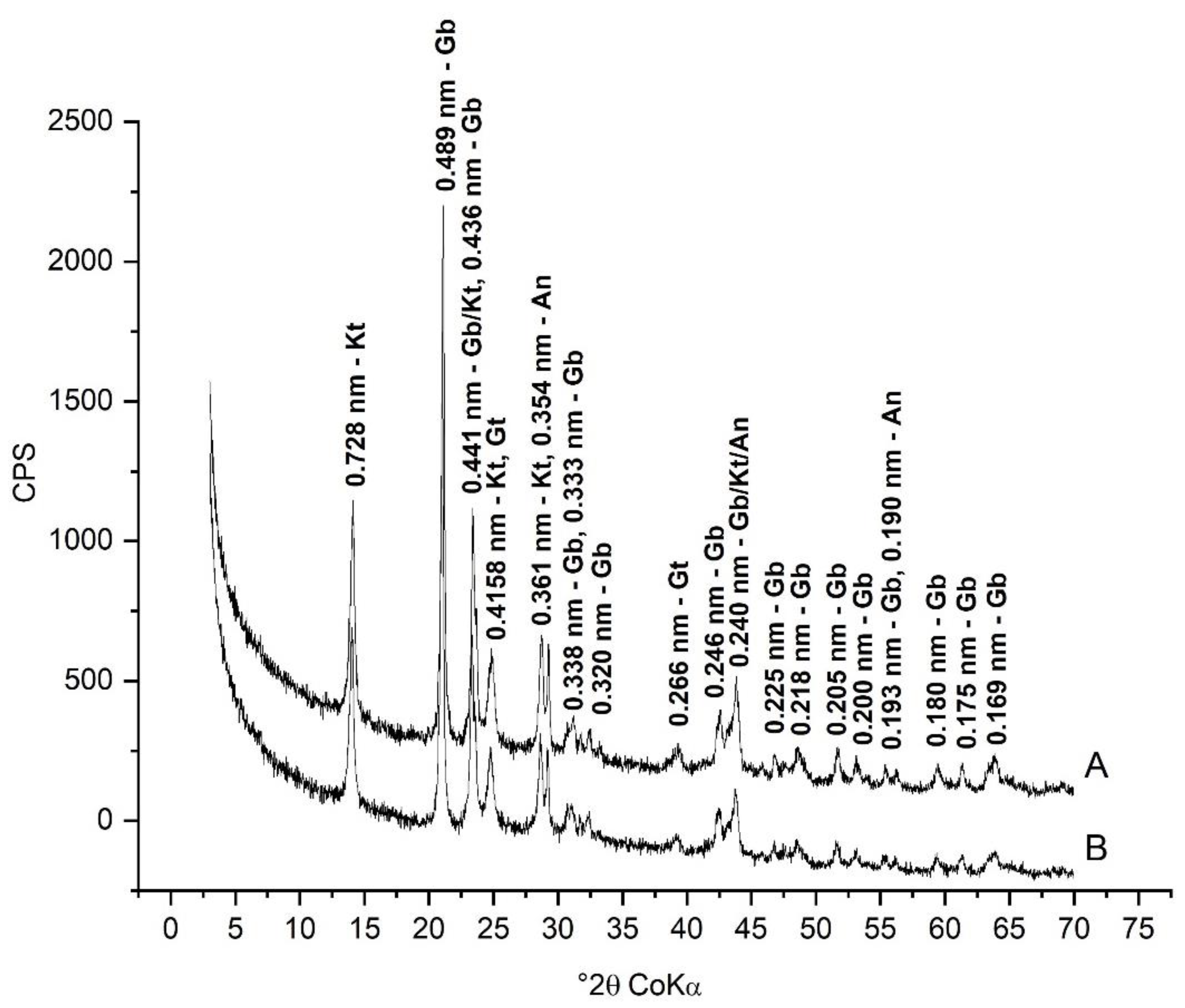

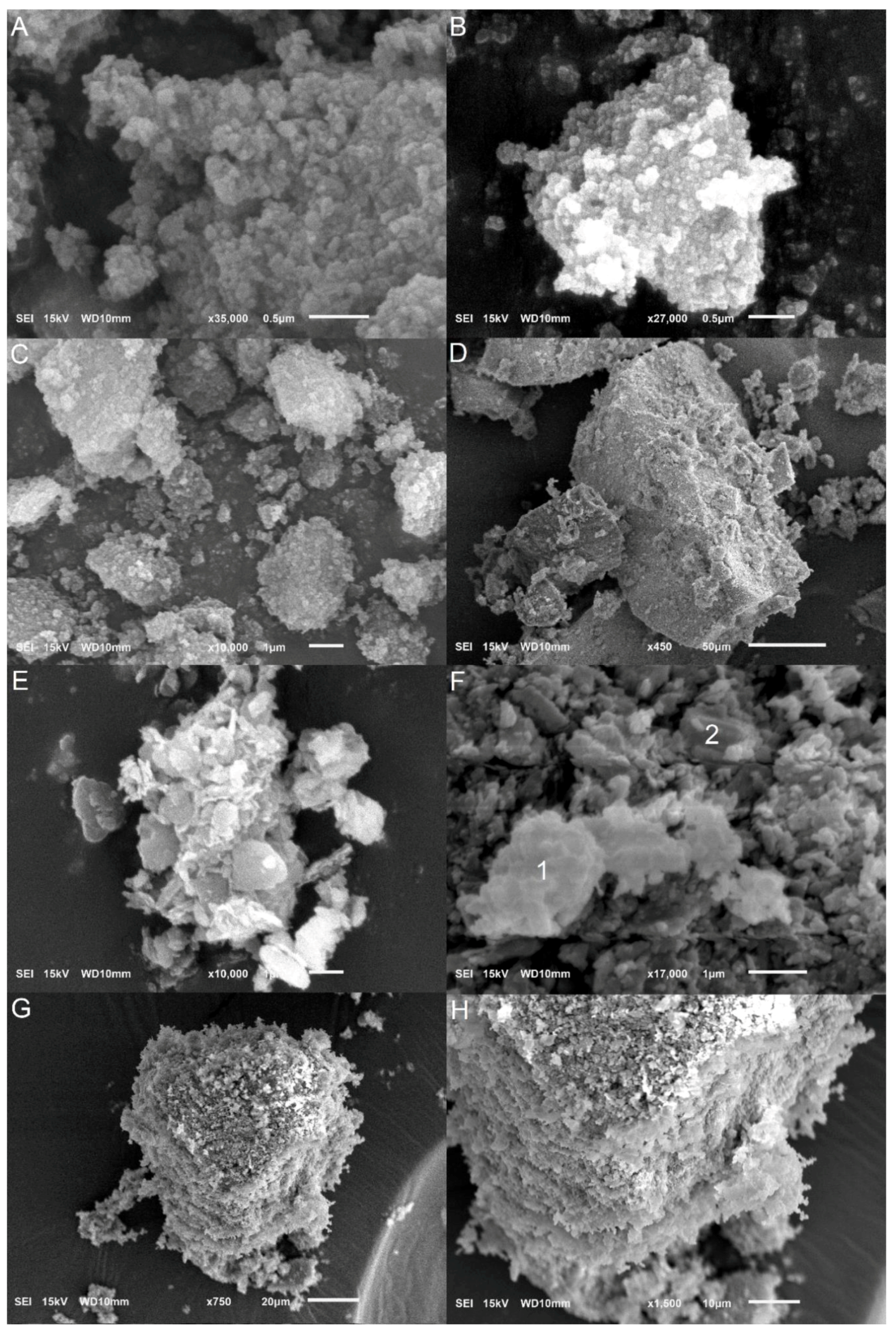
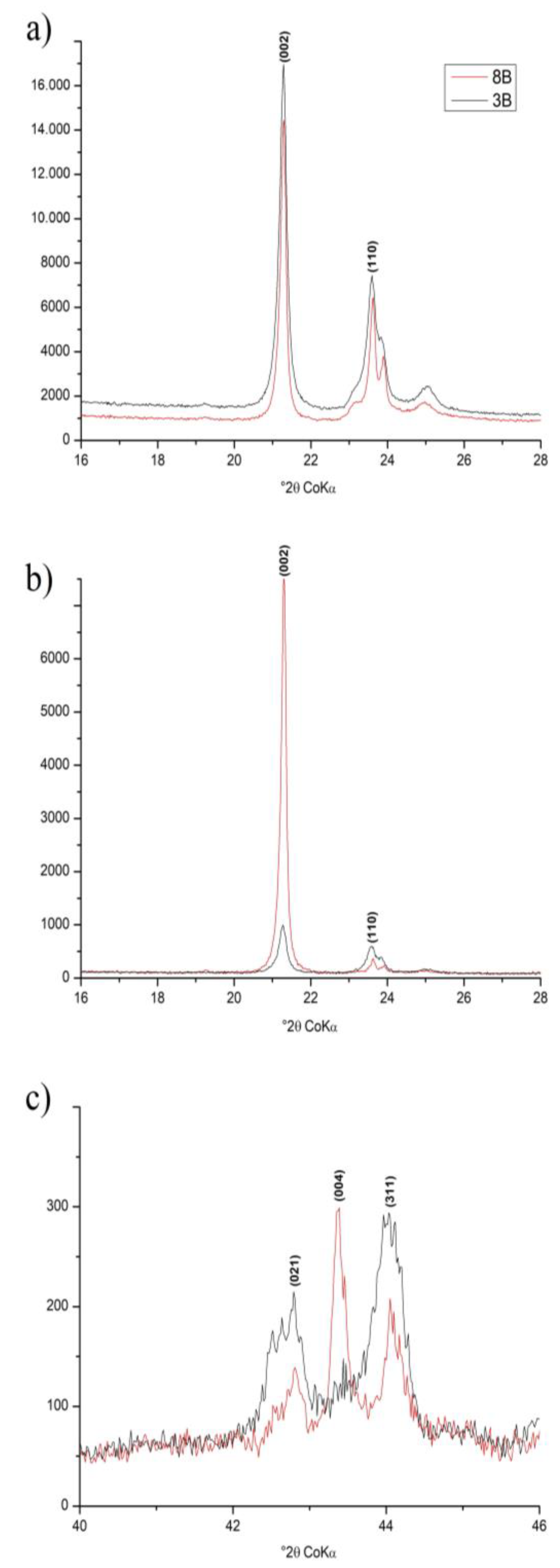
| Identification | Minerals in the Fine Fraction | WRB 1 | Soil Taxonomy | SiBCS 2 |
|---|---|---|---|---|
| Southeast and Midwest Brazilian Soils | ||||
| 1 | Gb, Kt, An, Hm, Gt, Mh | Gibbsic Ferralsol | Acrudox | LV Acriférrico |
| 2 | Gb, Kt, An, Hm, Gt, Mh | Gibbsic Ferralsol | Acrudox | LV Acriférrico |
| 3 | Gb, Kt, An, Gt | Gibbsic Ferralsol | Acrudox | LA Ácrico |
| 4 | Gb, Kt, An, 2:1, Il, Hm, Gt | Gibbsic Ferralsol | Acrudox | LVA Acriférrico |
| 5 | Gb, Kt, An, Hm, Gt | Gibbsic Ferralsol | Acrudox | LVA Ácrico |
| Amazon Soils | ||||
| 6 | Gb, Kt, Gt, An | Haplic Ferralsol | Hapludox | LA Distrófico |
| 7 | Gb, Kt, Gt, An | Haplic Ferralsol | Hapludox | LA Distrófico |
| 8 | Gb, Kt, Gt, An | Haplic Ferralsol | Hapludox | LVA Distrófico |
| Soil over bauxite | ||||
| 9 | Gb, Kt, Gt, Hm, An | - | - | - |
| Bauxites | ||||
| 10 | Gb, Kt, Il, Gt, Hm, Dp | - | - | - |
| 11 | Gb, Kt, An, Gt, Hm | - | - | - |
| 12 | Gb, Kt, An, Gt, Bh | - | - | - |
| 13 | Gb, Kt, Gt, Hm, An, Bh | - | - | - |
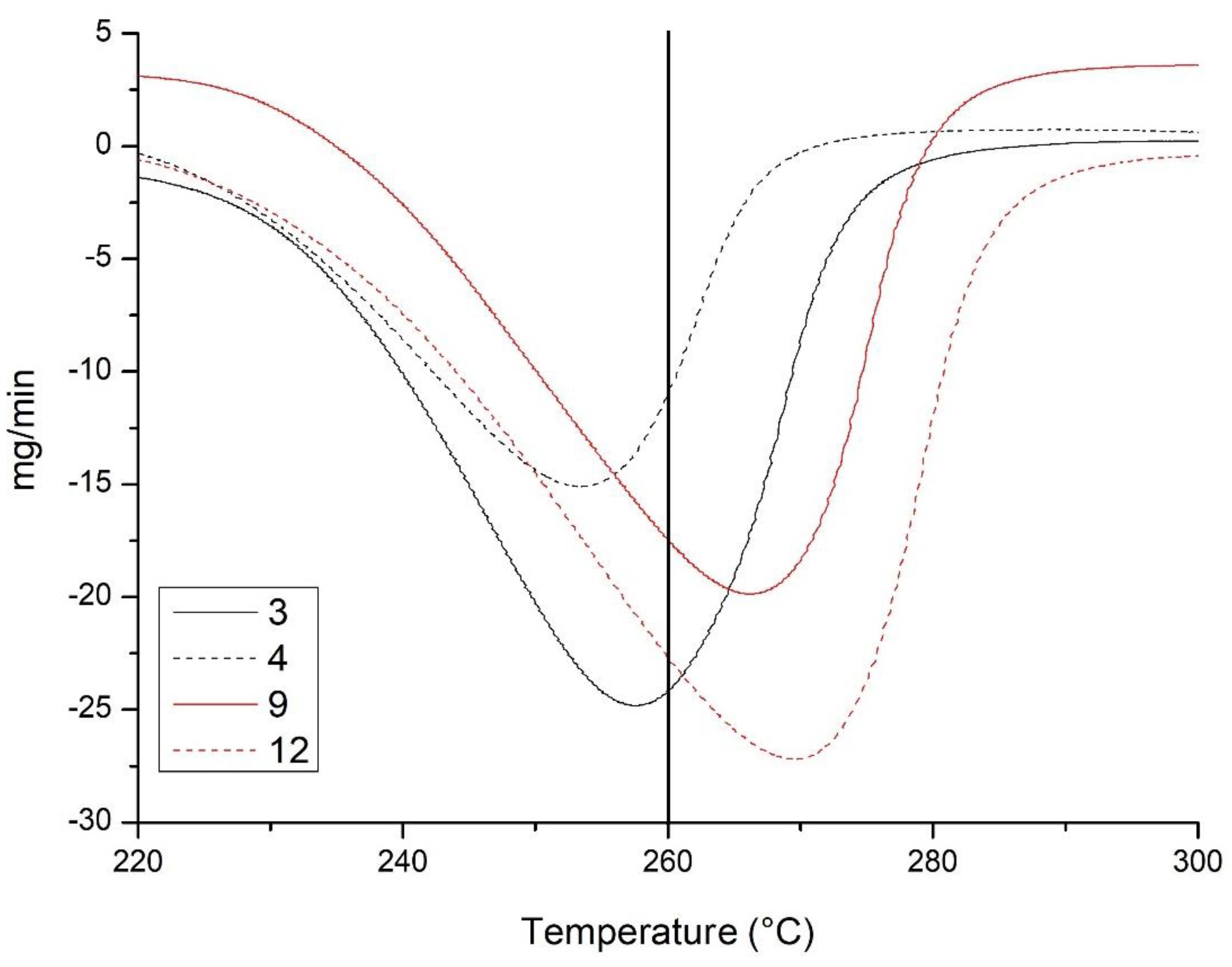
| Sample | DT-Gibbsite | Gibbsite | Kaolinite |
|---|---|---|---|
| °C | dag kg−1 | ||
| 2B | 255.13 | 51.73 | 34.14 |
| 3B | 257.42 | 62.02 | 26.64 |
| 4B | 253.33 | 39.46 | 47.21 |
| 5B | 256.30 | 41.15 | 51.21 |
| 8B | 266.30 | 55.45 | 35.00 |
| 9A | 261.02 | 46.03 | 33.21 |
| 10 | 263.62 | 40.29 | 36.64 |
| 11 | 269.52 | 73.40 | 22.86 |
| 12 | 265.36 | 68.59 | 30.57 |
| 13 | 267.75 | 60.67 | 29.00 |
| Sample | dc (nm) | WHH (°2θ) | MCD (nm) | I110/002 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (002) | (110) | (002) | (021) | (313) | (31-4) | (002) | (021) | (313) | (31-4) | O | R | |
| Soils | ||||||||||||
| 1A | 0.4842 | 0.4377 | 0.23 | 0.56 | 0.56 | 0.55 | 83.61 | 22.50 | 23.10 | 24.68 | 0.42 | 0.46 |
| 1B | 0.4844 | 0.4369 | 0.24 | 0.63 | 0.65 | 0.59 | 104.78 | 20.92 | 20.68 | 24.57 | 0.43 | 0.44 |
| 2A | 0.4838 | 0.4373 | 0.20 | 0.59 | 0.43 | 0.47 | 81.78 | 19.48 | 30.11 | 27.93 | 0.48 | 0.50 |
| 2B | 0.4846 | 0.4380 | 0.23 | 0.58 | 0.48 | 0.53 | 186.28 | 24.65 | 33.86 | 30.83 | 0.49 | 0.48 |
| 3A | 0.4844 | 0.4377 | 0.20 | 0.59 | 0.44 | 0.47 | 204.99 | 22.83 | 35.66 | 33.68 | 0.55 | 0.41 |
| 3B | 0.4839 | 0.4369 | 0.22 | 0.60 | 0.40 | 0.50 | 129.31 | 21.67 | 40.23 | 30.08 | 0.56 | 0.39 |
| 4B | 0.4838 | 0.4371 | 0.24 | 0.68 | 0.47 | 0.57 | 146.23 | 19.68 | 34.86 | 27.37 | 0.50 | 0.54 |
| 5B | 0.4838 | 0.4370 | 0.19 | 0.64 | 0.36 | 0.46 | 228.99 | 20.28 | 49.39 | 34.28 | 0.48 | 0.51 |
| 6A | 0.4842 | 0.4367 | 0.19 | 0.47 | 0.31 | 0.36 | 305.80 | 31.63 | 65.63 | 51.97 | 0.08 | 0.46 |
| 6B | 0.4840 | 0.4371 | 0.18 | 0.48 | 0.34 | 0.36 | 533.39 | 31.15 | 57.08 | 54.14 | 0.07 | 0.48 |
| 7A | 0.4846 | 0.4373 | 0.21 | 0.48 | 0.38 | 0.38 | 321.51 | 33.31 | 52.07 | 54.86 | 0.04 | 0.51 |
| 7B | 0.4842 | 0.4374 | 0.17 | 0.46 | 0.34 | 0.38 | 1031.66 | 32.72 | 54.79 | 48.12 | 0.02 | 0.46 |
| 8A | 0.4843 | 0.4374 | 0.17 | 0.45 | 0.33 | 0.35 | 383.19 | 32.16 | 54.94 | 52.63 | 0.05 | 0.46 |
| 8B | 0.4846 | 0.4374 | 0.19 | 0.49 | 0.33 | 0.37 | 279.39 | 29.34 | 56.91 | 49.21 | 0.04 | 0.44 |
| Soil over bauxite | ||||||||||||
| 9A | 0,4844 | 0.4374 | 0.19 | 0.54 | 0.32 | 0.41 | 261.50 | 25.64 | 60.38 | 41.92 | 0.07 | 0.44 |
| Bauxites | ||||||||||||
| 10 | 0.4842 | 0.4373 | 0.21 | 0.53 | 0.53 | 0.57 | 1618.46 | 29.10 | 29.79 | 27.86 | 0.11 | 0.37 |
| 11 | 0.4843 | 0.4371 | 0.18 | 0.51 | 0.36 | 0.41 | 502.02 | 28.38 | 50.41 | 42.42 | 0.07 | 0.43 |
| 12 | 0.4846 | 0.4374 | 0.20 | 0.53 | 0.46 | 0.50 | 204.98 | 26.03 | 32.74 | 30.34 | 0.06 | 0.39 |
| 13 | 0.4846 | 0.4374 | 0.16 | 0.49 | 0.40 | 0.41 | 530.39 | 29.00 | 40.51 | 40.76 | 0.02 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparini, A.S.; Fontes, M.P.F.; Pacheco, A.A.; Ker, J.C. Gibbsite Crystallinity and Morphology in Ferralsols and Bauxites. Minerals 2022, 12, 1441. https://doi.org/10.3390/min12111441
Gasparini AS, Fontes MPF, Pacheco AA, Ker JC. Gibbsite Crystallinity and Morphology in Ferralsols and Bauxites. Minerals. 2022; 12(11):1441. https://doi.org/10.3390/min12111441
Chicago/Turabian StyleGasparini, Arthur Stefanelli, Maurício Paulo Ferreira Fontes, Anderson Almeida Pacheco, and João Carlos Ker. 2022. "Gibbsite Crystallinity and Morphology in Ferralsols and Bauxites" Minerals 12, no. 11: 1441. https://doi.org/10.3390/min12111441
APA StyleGasparini, A. S., Fontes, M. P. F., Pacheco, A. A., & Ker, J. C. (2022). Gibbsite Crystallinity and Morphology in Ferralsols and Bauxites. Minerals, 12(11), 1441. https://doi.org/10.3390/min12111441





