Improving the Copper-Molybdenum Ores Flotation Technology Using a Combined Collecting Agent
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chanturia, V.A.; Weisberg, L.F.; Kozlov, A.P. Priority areas of research in the field of mineral processing. Ore Benef. 2014, 2, 3–9. [Google Scholar] [CrossRef]
- Zimbovsky, I.G.; Ivanova, T.A.; Chanturia, V.A.; Chanturia, E.L. Complex-forming collector for selective flotation of chalcopyrite. Phys. Tech. Probl. Miner. Dev. 2015, 3, 124–129. [Google Scholar]
- Balaramesh, P.; Venkatesh, P.; Jabbar, A. Influence of dithiocarbamate on metal complex and thin film depositions. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15301–15309. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hope, G.A.; Lee, K.C.; Petrovic, E.A.; Woods, R. Adsorption of O-isopropyl-N-ethyl thionocarbamate on Cu sulfide ore minerals. Miner. Eng. 2014, 69, 120–132. [Google Scholar] [CrossRef]
- Bu, Y.; Hu, Y.; Sun, W.; Gao, Z.; Liu, R. Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector. Minerals 2018, 8, 115. [Google Scholar] [CrossRef]
- Ryaboy, V.I.; Shepeta, E.D. Effect of surface activity and water-repellent dialkyldithiophosphates properties on the flotation of copper arsenic-containing ores. Obogashchenie Rud. 2016, 4, 29–34. [Google Scholar] [CrossRef]
- Kondratiev, S.A. Evaluation of the flotation activity of reagents-collectors. Ore Benef. 2010, 4, 24–30. [Google Scholar]
- Omarova, N.K.; Sherembaeva, R.T. Flotation of sulfide copper ore with PS flotation reagent. Ore Benef. 2015, 2, 15–17. [Google Scholar] [CrossRef]
- Sherembaeva, R.T.; Omarova, N.K.; Akimbekova, B.B.; Katkeeva, G.L. The use of a new R flotation agent in the sulfide copper ores flotation. Non-Ferr. Metals 2014, 6, 12–16. [Google Scholar]
- Mitrofanova, G.V.; Chernousenko, E.V.; Bazarova, E.A.; Tyukin, A.P. Search for new complexing reagents for copper-nickel ores flotation. Non-Ferr. Metals 2019, 11, 27–33. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, G.; Zhong, H.; Huang, Y.; Cao, Z. The flotation behavior and adsorption mechanism of O-isopropyl-S-[2-(hydroxyimino)propyl] dithiocarbonate ester to chalcopyrite. J. Taiwan Inst. Chem. Eng. 2017, 71, 38–46. [Google Scholar] [CrossRef]
- Gusev, V.Y.; Radushev, A.V.; Chekanova, L.G.; Baigacheva, E.V.; Manylova, K.O.; Gogolishvili, V.O. Azo derivatives of phenol and 1-naphthol as collectors for flotation of non-ferrous metal sulfide ores. J. Appl. Chem. 2018, 4, 503–512. [Google Scholar] [CrossRef]
- Chernousenko, E.V.; Mitrofanova, G.V.; Kameneva, Y.S.; Vishnyakova, I.N. Evaluation of the action of complexing reagents in the copper-nickel ores flotation. Tsvetnye Met. 2019, 1, 7–12. [Google Scholar] [CrossRef]
- Adiguzel, E.; Yilmaz, F.; Emirik, M. Synthesis and characterization of two new hydroxamic acids derivatives and their metal complexes. An investigation on the keto/enol, E/Z and hydroxamate/hydroximate forms. J. Mol. Struct. 2017, 1127, 403–412. [Google Scholar] [CrossRef]
- Morozov, V.V.; Pesriak, I.V.; Erdenezul, J. Influence of concentration of amylxanthogenic acid allyl ester, non-ionic collector, on copper-molybdenum ores flotation. Tsvetnye Met. 2018, 11, 14–20. [Google Scholar] [CrossRef]
- Jargalsaikhan, E.; Ishgen, K. Process optimization of grinding and flotation of copper-molybdenum ores with the use of model-based criteria. In Proceedings of the 22nd International Conference on Environment and Mineral Processing, Ostrava, Czech Republic, 31 May–2 June 2018; pp. 152–154. [Google Scholar]
- Bocharov, V.A.; Ignatkina, V.A.; Khachatryan, L.S. Basic foundations of selection and joint application of selective collectors and flotation depressants of sulfide minerals with close physicochemical properties. Russ. J. Non-Ferr. Met. 2008, 49, 1–5. [Google Scholar] [CrossRef]
- Ignatkina, V.A. The choice of selective collectors in the flotation of minerals with similar flotation properties. Proceedings of universities. Non-Ferr. Metall. 2011, 1, 1–7. [Google Scholar]
- Kondratiev, S.A.; Rostovtsev, V.I.; Bochkarev, G.R.; Pushkareva, G.I.; Kovalenko, K.A. Scientific substantiation and development of innovative technologies for the complex processing of refractory ores and technogenic raw materials. Phys. Tech. Probl. Min. 2014, 5, 187–202. [Google Scholar]
- Bhambhani, T.; Nagaraj, D.R.; Yavuzran, O. Improving flotation extraction of oxide copper minerals. In Proceedings of the IMPC 2016: XXVIII International Mineral Processing Congress, Quebec City, QC, Canada, 11–15 September 2016; pp. 1–13. [Google Scholar]
- Ignatkina, V.A.; Bocharov, V.A.; Milovich, F.O.; Ivanova, P.G.; Khachatryan, L.S. Selective increase in the flotation activity of non-ferrous metal sulfides using combinations of sulfhydryl collectors. Ore Benef. 2015, 3, 18–24. [Google Scholar] [CrossRef]
- Ignatkina, V.A. Selective reagent modes of flotation of non-ferrous and noble metal sulfides from refractory sulfide ores. Non-Ferr. Metals 2016, 11, 27–33. [Google Scholar] [CrossRef]
- Ryaboy, V.I. Research of Mekhanobr-Orgsintez-Reagent LLC in the field of flotation reagents. Ore Benef. 2016, 5, 60–62. [Google Scholar]
- Ryaboy, V.; Shepeta, E.; Kretov, V.; Golikov, V. New dialkylditiophosphates for the flotation of copper, gold and silver containing ores. In Proceedings of the IMPC 2014 XXVII, Santiago, Chile, 20–24 October 2014; pp. 1–8. [Google Scholar]
- Ryaboy, V.I.; Shepeta, E.D.; Kretov, V.P.; Levkovets, S.E.; Ryaboy, I.V. Influence of surface-active properties of reagents containing sodium dialkyldithiophosphates on sulfide flotation. Ore Benef. 2015, 2, 18–22. [Google Scholar] [CrossRef]
- Ryaboy, V.I.; Shepeta, E.D.; Kretov, V.P.; Golikov, V.V. New dialkyldithiophosphates for flotation of copper-, gold- and silver-bearing ores. Ore Benef. 2014, 1, 29–33. [Google Scholar]
- Kienko, L.A.; Voronova, O.V.; Kondratyev, S.A. Investigation of the effect of ultrasonic influences on the selectivity of flotation during the enrichment of waste products of the Yaroslavl Mining Company. Phys. Tech. Probl. Miner. Dev. 2019, 4, 174–181. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsorption studies. Miner. Eng. 2007, 20, 600–608. [Google Scholar] [CrossRef]
- Jorjani, E.; Barkhordari, H.R.; Tayebi Khorami, M.; Fazeli, A. Effects of aluminosilicate minerals on copper-molybdenum flotation from Sarcheshmeh porphyry ores. Miner. Eng. 2011, 24, 754–759. [Google Scholar] [CrossRef]

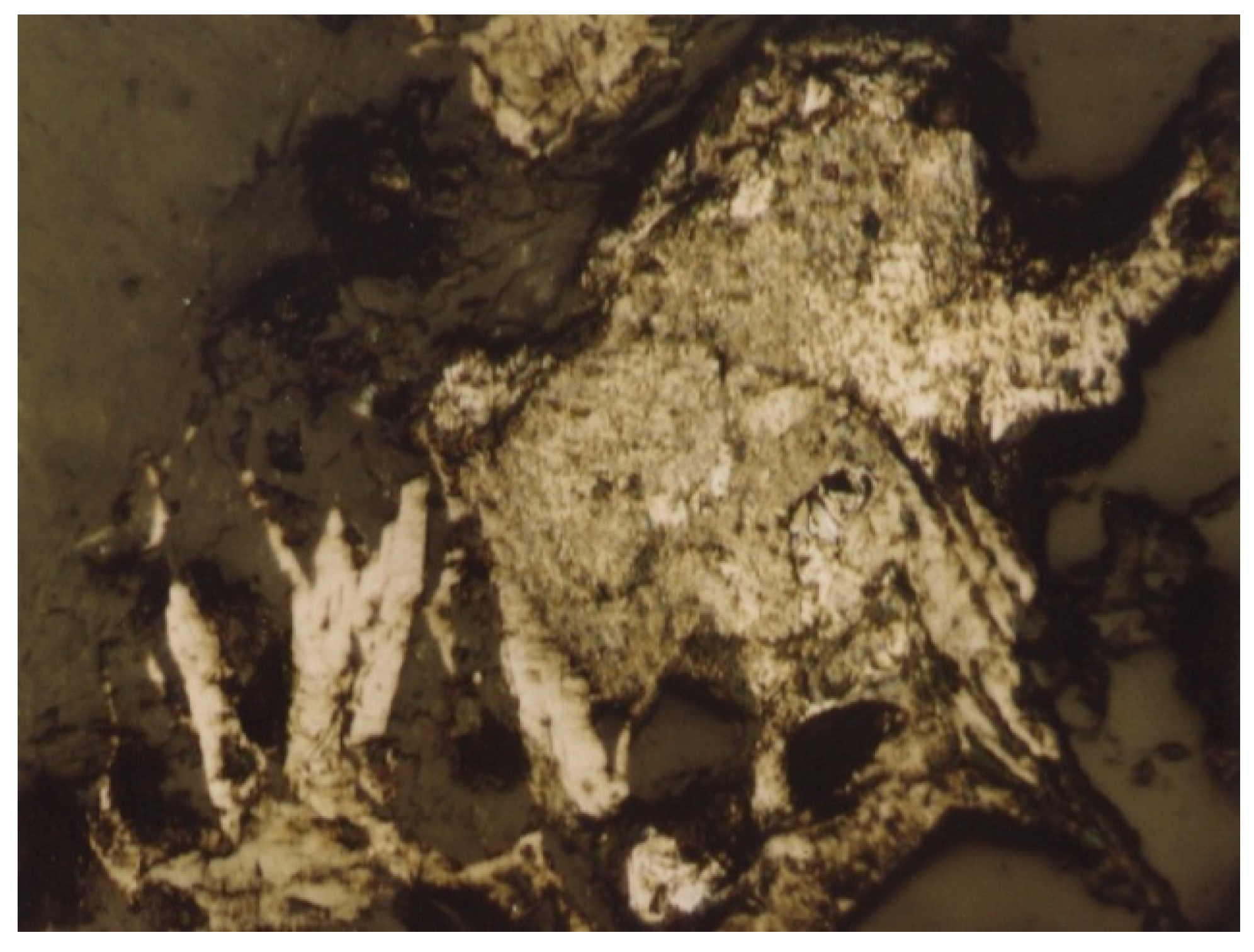
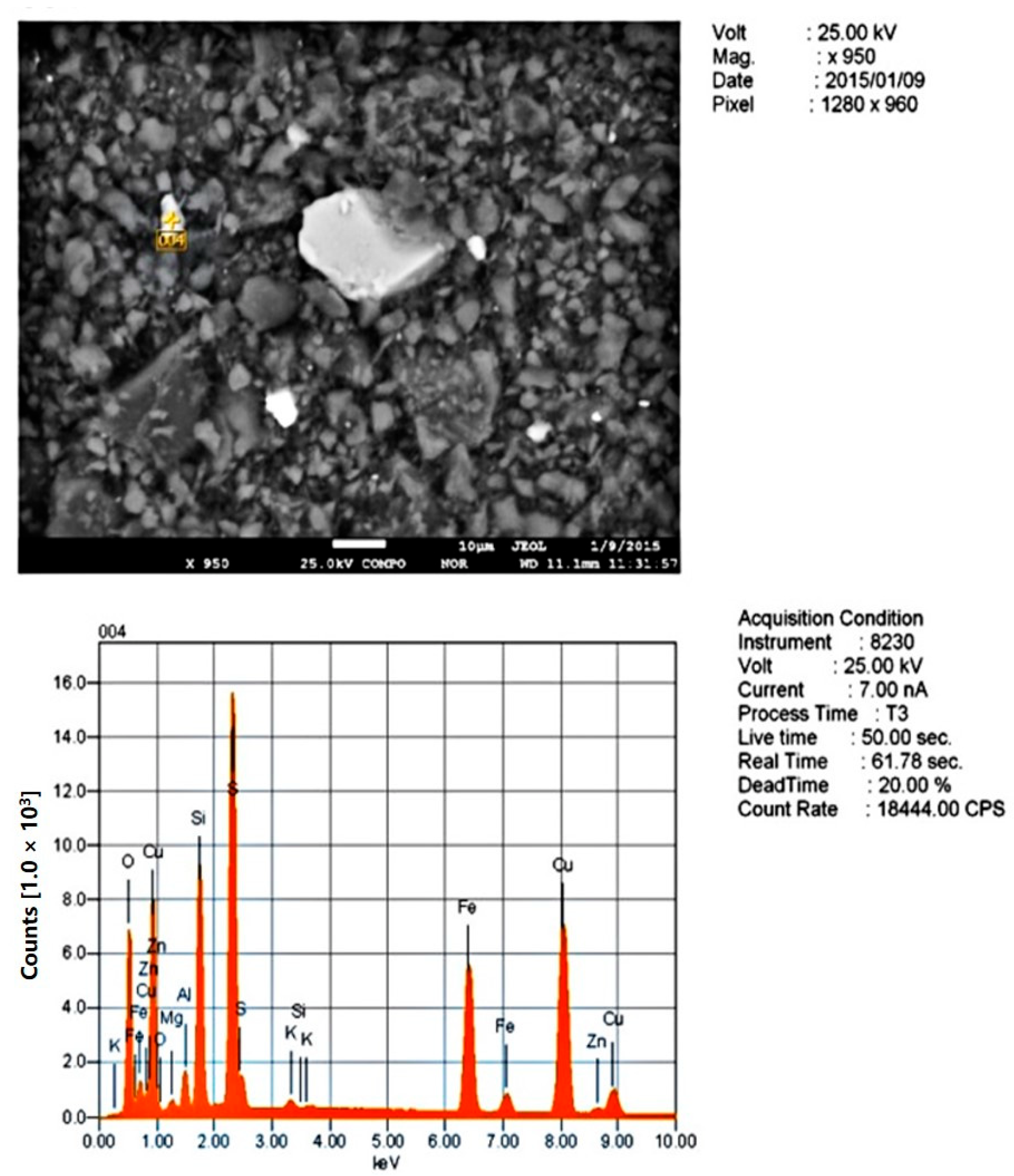


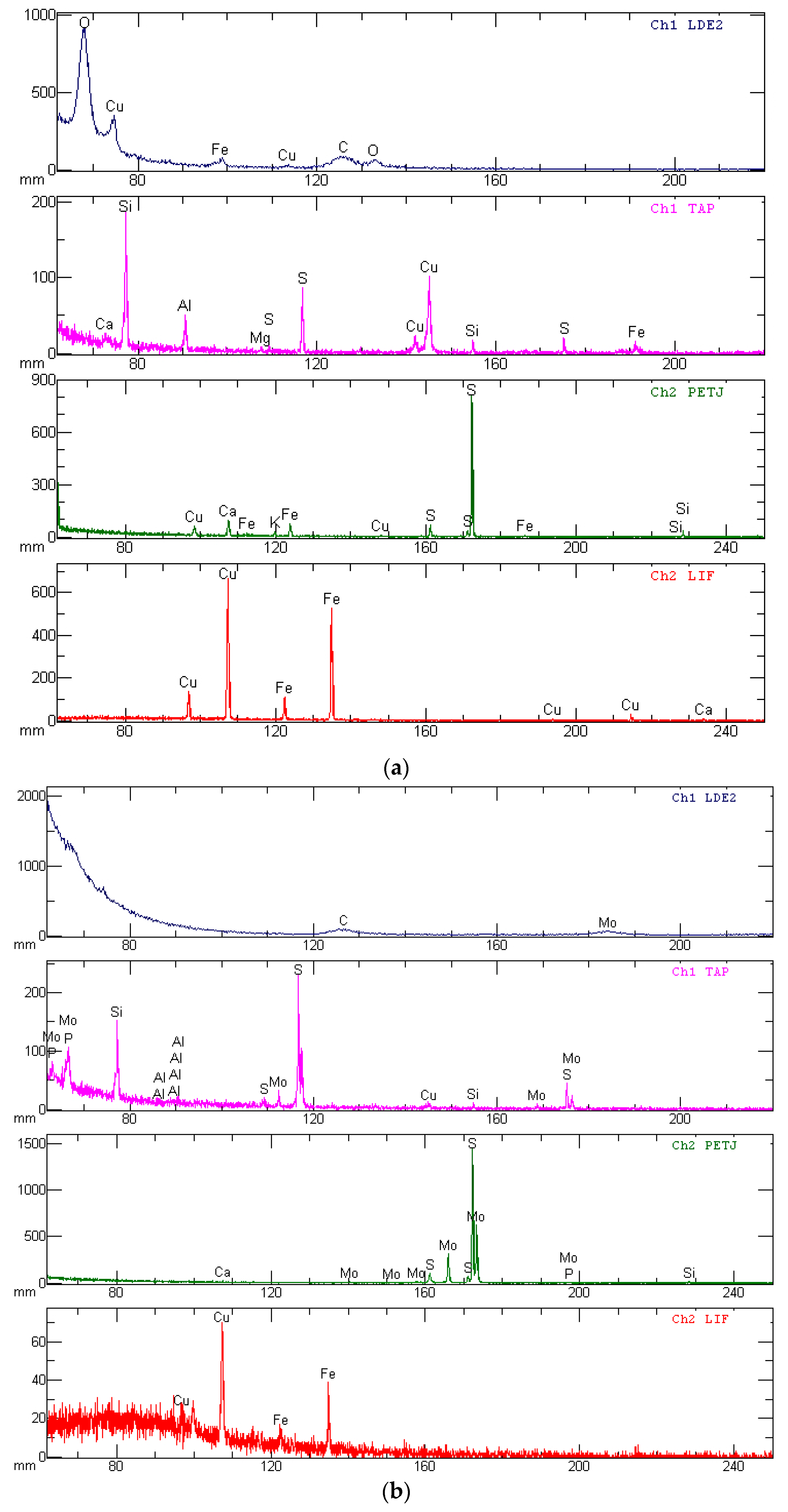
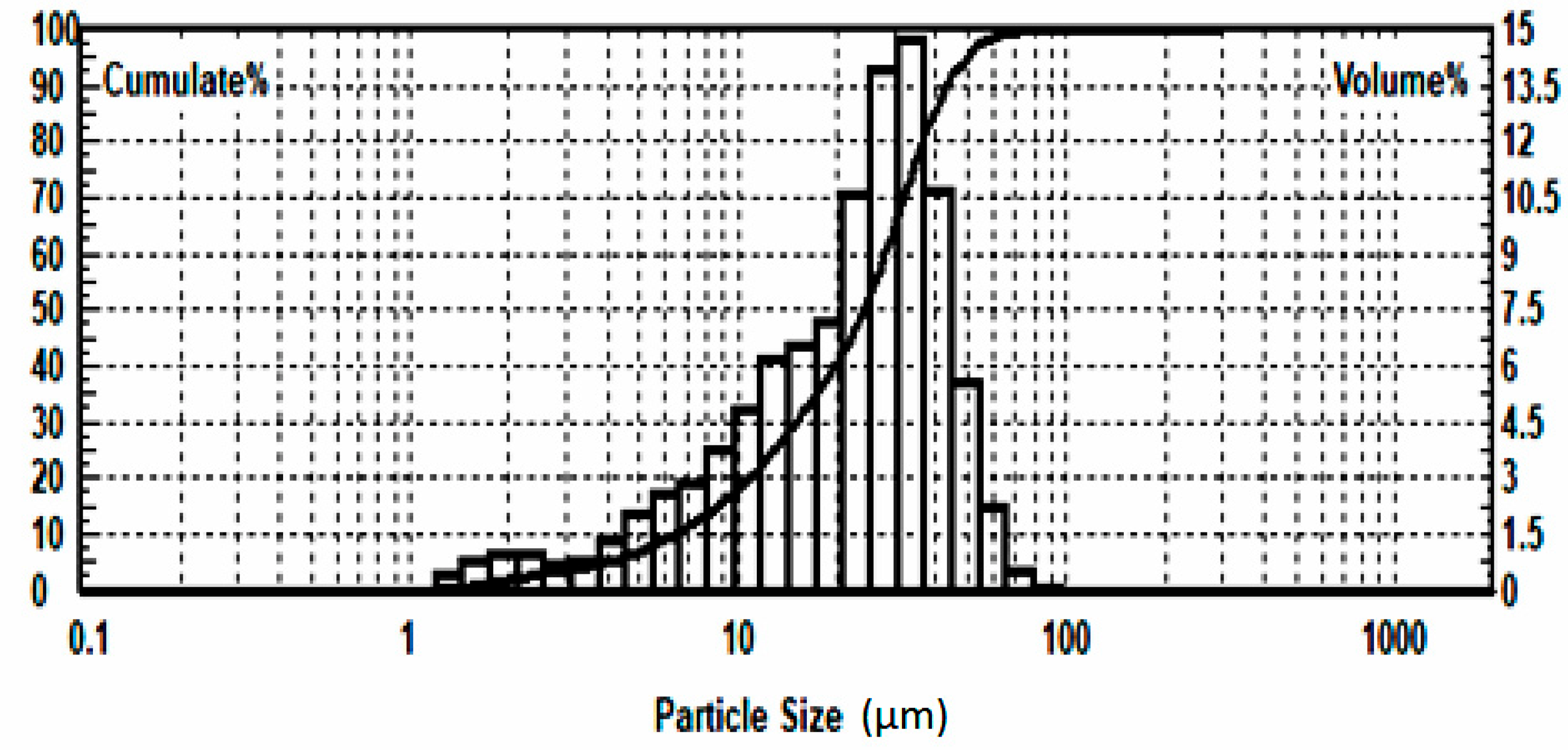
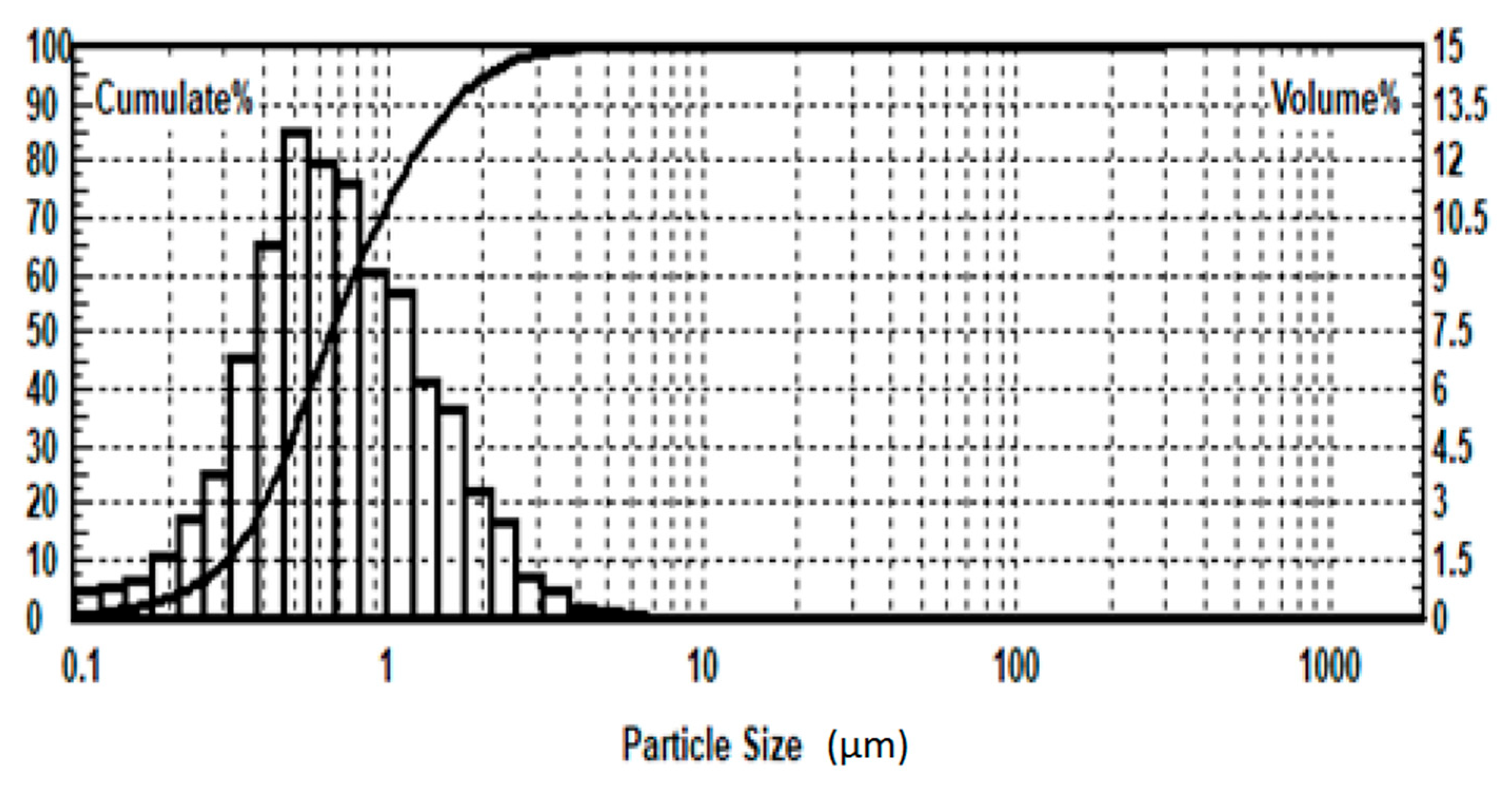
| Grinding Fineness, % −0.074 mm | 65.0 | 75.0 | 85.0 | |
| Chalcopyrite | Free, % | 60.0 | 72.0 | 78.0 |
| In intergrowths with pyrite and rock-forming minerals % | 40.0 | 28.0 | 22.0 | |
| Pyrite | Free, % | 80.0 | 88.6 | 92.0 |
| In intergrowths with pyrite and rock-forming minerals % | 20.0 | 11.4 | 8.0 |
| Size, µm | Yield, % | Content, % | Distribution, % | ||
|---|---|---|---|---|---|
| Cu | Mo | Cu | Mo | ||
| +71 | 19.00 | 0.39 | 0.015 | 19.55 | 28.36 |
| −71 + 50 | 12.10 | 0.3 | 0.008 | 9.58 | 9.63 |
| −50 + 40 | 11.90 | 0.37 | 0.01 | 11.62 | 11.84 |
| −40 + 30 | 2.93 | 0.44 | 0.01 | 3.40 | 2.92 |
| −30 + 20 | 10.70 | 0.39 | 0.009 | 11.01 | 9.58 |
| −20 + 10 | 23.65 | 0.41 | 0.011 | 25.59 | 25.89 |
| −10 + 0 | 19.72 | 0.37 | 0.006 | 19.25 | 11.77 |
| Source ore | 100.00 | 0.38 | 0.010 | 100.00 | 100.00 |
| Product Name | Yield, % | Content, % | Extraction, % | Note | ||
|---|---|---|---|---|---|---|
| Cu | Mo | Cu | Mo | |||
| Cu–Mo concentrate | 2.30 | 16.25 | 0.45 | 77.79 | 79.38 | Basic mode with butyl xanthate |
| Intermediate product 1 | 5.55 | 0.183 | 0.004 | 2.11 | 1.70 | |
| Intermediate product 2 | 1.56 | 0.435 | 0.031 | 1.41 | 3.71 | |
| Intermediate product 3 | 0.50 | 1.84 | 0.019 | 1.91 | 0.73 | |
| Control flotation concentrate | 2.98 | 0.31 | 0.005 | 1.92 | 1.14 | |
| Tailings | 87.10 | 0.082 | 0.002 | 14.85 | 13.34 | |
| Source ore | 100.0 | 0.48 | 0.01 | 100.0 | 100.0 | |
| Cu–Mo concentrate | 2.42 | 18.37 | 0.495 | 80.68 | 84.84 | Combined reagent without dispersion |
| Intermediate product 1 | 5.54 | 0.183 | 0.001 | 1.84 | 0.39 | |
| Intermediate product 2 | 1.56 | 0.435 | 0.008 | 1.23 | 0.88 | |
| Intermediate product 3 | 0.50 | 1.85 | 0.038 | 1.68 | 1.35 | |
| Control flotation concentrate | 2.98 | 0.30 | 0.001 | 1.62 | 0.21 | |
| Tailings | 87.0 | 0.082 | 0.002 | 12.95 | 12.32 | |
| Source ore | 100.0 | 0.55 | 0.01 | 100.0 | 100.0 | |
| Cu–Mo concentrate | 2.46 | 18.1 | 0.487 | 80.33 | 86.52 | 1 min dispersion time |
| Intermediate product 1 | 8.36 | 0.169 | 0.005 | 2.55 | 3.02 | |
| Intermediate product 2 | 2.40 | 0.446 | 0.008 | 1.93 | 1.39 | |
| Intermediate product 3 | 0.92 | 1.009 | 0.018 | 1.67 | 1.20 | |
| Control flotation concentrate | 4.66 | 0.266 | 0.006 | 2.24 | 2.02 | |
| Tailings | 81.20 | 0.077 | 0.001 | 11.28 | 5.86 | |
| Source ore | 100.0 | 0.55 | 0.014 | 100.0 | 100.0 | |
| Cu–Mo concentrate | 2.50 | 18.2 | 0.490 | 83.58 | 88.46 | 2 min dispersion time |
| Intermediate product 1 | 8.06 | 0.171 | 0.003 | 2.53 | 1.75 | |
| Intermediate product 2 | 2.62 | 0.332 | 0.006 | 1.60 | 1.14 | |
| Intermediate product 3 | 1.50 | 0.766 | 0.015 | 2.11 | 1.62 | |
| Control flotation concentrate | 4.02 | 0.206 | 0.004 | 1.52 | 1.16 | |
| Tailings | 81.30 | 0.058 | 0.001 | 8.66 | 5.87 | |
| Source ore | 100.0 | 0.54 | 0.0138 | 100.0 | 100.0 | |
| Cu–Mo concentrate | 2.74 | 17.8 | 0.491 | 83.02 | 88.96 | 3 min dispersion time |
| Intermediate product 1 | 7.96 | 0.18 | 0.001 | 2.44 | 0.53 | |
| Intermediate product 2 | 3.22 | 0.431 | 0.007 | 2.36 | 1.49 | |
| Intermediate product 3 | 1.48 | 0.854 | 0.035 | 2.15 | 3.43 | |
| Control flotation concentrate | 5.02 | 0.27 | 0.001 | 2.31 | 0.33 | |
| Tailings | 79.58 | 0.057 | 0.001 | 7.72 | 5.26 | |
| Source ore | 100.0 | 0.59 | 0.015 | 100.0 | 100.0 | |
| Size Class, mm | Yield, % | Cu Content, % | Cu Distribution, % | Note |
|---|---|---|---|---|
| −0.071 + 0.050 | 29.15 | 0.05 | 22.61 | Flotation tailings in basic mode |
| −0.050 + 0.040 | 10.9 | 0.05 | 9.55 | |
| −0.040 + 0.030 | 17.3 | 0.04 | 11.66 | |
| −0.030 + 0.020 | 19.65 | 0.05 | 16.23 | |
| −0.020 + 0.010 | 15.3 | 0.09 | 22.95 | |
| −0.010 + 0 | 7.7 | 0.13 | 17.00 | |
| Source tails | 100.0 | 0.06 | 100.0 | |
| −0.071 + 0.050 | 30.6 | 0.08 | 32.88 | Flotation tailings using combined flotation reagent |
| −0.050 + 0.040 | 14.3 | 0.07 | 12.44 | |
| −0.040 + 0.030 | 13.2 | 0.06 | 9.62 | |
| −0.030 + 0.020 | 18.0 | 0.07 | 15.20 | |
| −0.020 + 0.010 | 17.7 | 0.09 | 21.06 | |
| −0.010 + 0 | 6.2 | 0.11 | 8.80 | |
| Source tails | 100.0 | 0.08 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semushkina, L.; Abdykirova, G.; Mukhanova, A.; Mukhamedilova, A. Improving the Copper-Molybdenum Ores Flotation Technology Using a Combined Collecting Agent. Minerals 2022, 12, 1416. https://doi.org/10.3390/min12111416
Semushkina L, Abdykirova G, Mukhanova A, Mukhamedilova A. Improving the Copper-Molybdenum Ores Flotation Technology Using a Combined Collecting Agent. Minerals. 2022; 12(11):1416. https://doi.org/10.3390/min12111416
Chicago/Turabian StyleSemushkina, Larissa, Gulnar Abdykirova, Aynur Mukhanova, and Aynur Mukhamedilova. 2022. "Improving the Copper-Molybdenum Ores Flotation Technology Using a Combined Collecting Agent" Minerals 12, no. 11: 1416. https://doi.org/10.3390/min12111416
APA StyleSemushkina, L., Abdykirova, G., Mukhanova, A., & Mukhamedilova, A. (2022). Improving the Copper-Molybdenum Ores Flotation Technology Using a Combined Collecting Agent. Minerals, 12(11), 1416. https://doi.org/10.3390/min12111416







