Magnetotactic Bacteria: From Evolution to Biomineralization and Biomedical Applications
Abstract
1. Magnetotactic Bacteria (MTB)
2. Magnetotaxis
2.1. Evolution of Magnetoreception
2.2. Evolution of Magnetotaxis
2.3. Relevance of Magnetotaxis
2.4. Magnetotaxis Functionality
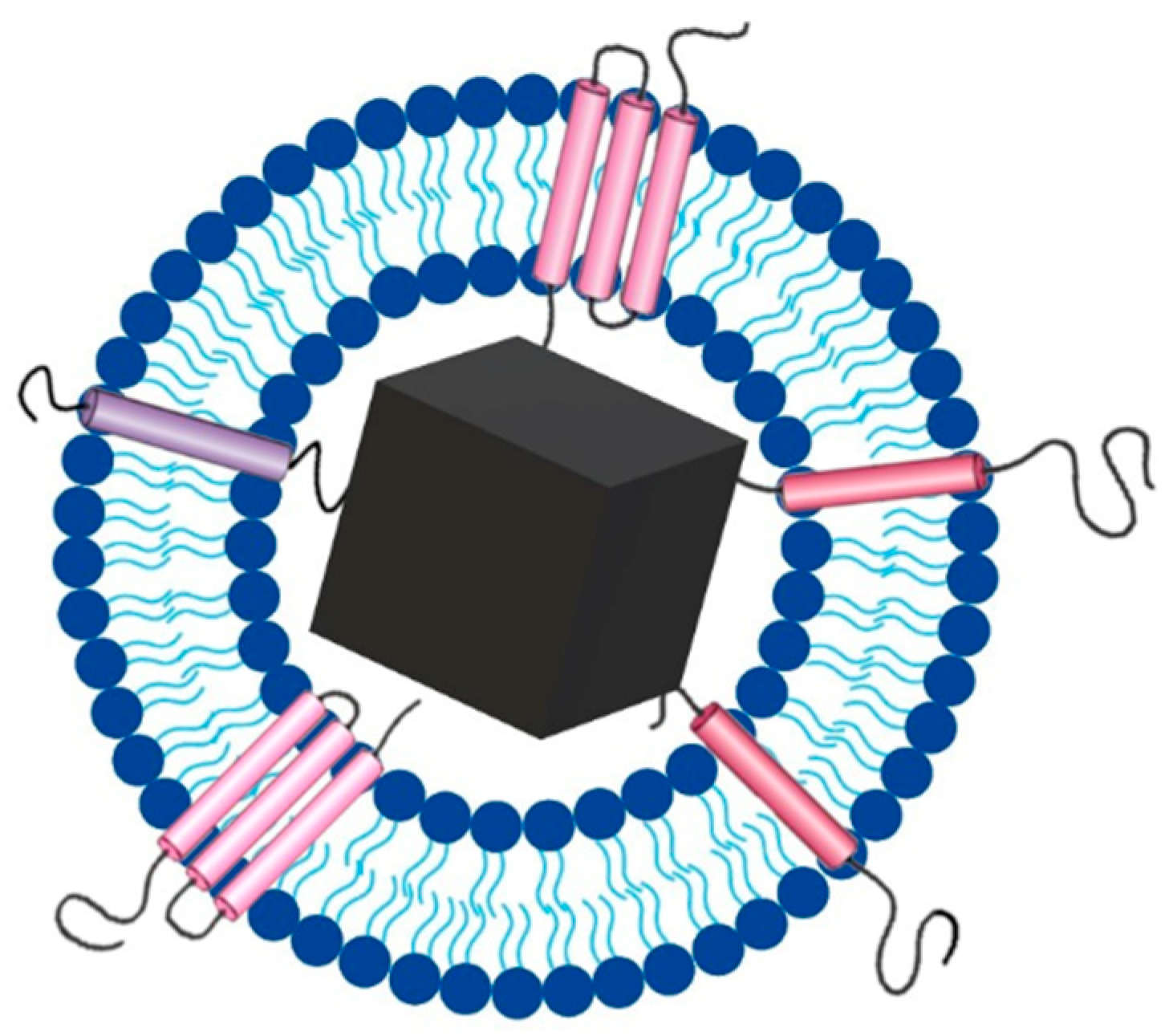
3. Magnetosomes
3.1. Evolution of Magnetosomes
3.2. Magnetosomes Biomineralization
3.3. Magnetosomes Properties
4. Iron Flow in MTB
4.1. Evolution of Iron Metabolism
4.2. Iron Uptake in MTB
4.3. Iron Transport in MTB
5. Applications of MTB
| Advantages | Disadvantages | |
|---|---|---|
| Drug delivery |
| |
| MRI contrast agents |
|
|
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boskey, A.L. Biomineralization: An overview. Connect. Tissue Res. 2003, 44, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. in Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Weiner, S. Biomineralization: A structrural perspective. J. Struct. Biol. 2008, 163, 229–234. [Google Scholar] [CrossRef]
- Nudelman, H.; Lee, Y.-Z.; Hung, Y.-L.; Kolusheva, S.; Upcher, A.; Chen, Y.-C.; Chen, J.-Y.; Sue, S.-C.; Zarivach, R. Understanding the Biomineralization Role of Magnetite-Interacting Components (MICs) From Magnetotactic Bacteria. Front. Microbiol. 2018, 9, 2480. [Google Scholar] [CrossRef] [PubMed]
- Faivre, D.; Schuler, D. Magnetotactic bacteria and magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef]
- Vargas, G.; Cypriano, J.; Correa, T.; Leao, P.; Bazylinski, D.A.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef]
- Lin, W.; Pan, Y.; Bazylinski, D.A. Diversity and ecology of and biomineralization by magnetotactic bacteria. Env. Microbiol. Rep. 2017, 9, 345–356. [Google Scholar] [CrossRef]
- Ben-Shimon, S.; Stein, D.; Zarivach, R. Current view of iron biomineralization in magnetotactic baceria. J. Struct. Biol. X 2021, 5, 100052. [Google Scholar] [CrossRef]
- Amor, M.; Tharaud, M.; Gelabert, A.; Komeili, A. Single-cell determination of iron content in magnetotactic bacteria: Implications for the iron biogeochemical cycle. Environ. Microbiol. 2020, 22, 823–831. [Google Scholar] [CrossRef]
- Vali, H.; Forster, O.; Amarantidis, G.; Petersen, N. Magnetotactic bacteria and their magnetofossils in sediments. Earth Planet. Sci. Lett. 1987, 86, 389–400. [Google Scholar] [CrossRef]
- Martins, J.L.; Silveira, T.S.; Abreu, F.; Silva, K.T.; da Silva-Neto, I.D.; Lins, U. Grazing protozoa and magnetosome dissolution in magnetotactic bacteria. Environ. Microbiol. 2007, 9, 2775–2781. [Google Scholar] [CrossRef]
- Goswami, P.; He, K.; Li, J.; Pan, Y.; Roberts, A.P.; Lin, W. Magnetotactic bacteria and magnetofossils: Ecology, evolution and environmental implications. NPJ Biofilms Microbiomes 2022, 8, 43. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kosterov, A.; Koziaeva, V.V.; Sergienko, E.S.; Shevtsov, M.A. Magnetotactic bacteria and magnetosomes: Basic properties and applications. Magnetochemistry 2021, 7, 86. [Google Scholar] [CrossRef]
- Lefevre, C.T.; Bazylinski, D.A. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 497–526. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Zarivach, R. A look into the biochemistry of magnetosome biosynthesis in magnetotactic bacteria. ACS Chem. Biol. 2017, 12, 13–22. [Google Scholar] [CrossRef]
- Mao, X.; Egli, R.; Liu, X.; Zhao, L. Magnetotactic advantage in stable sediment by long-term observations of magnetotactic bacteria in Earth’s field, zero field and alternating field. PLoS ONE 2022, 17, e0263593. [Google Scholar] [CrossRef]
- Smith, M.J.; Sheehan, P.E.; Perry, L.L.; O’Connor, K.; Csonka, L.N.; Applegate, B.M.; Whitman, L.J. Quantifiying the magnetic advantage in magnetotaxis. Biophys. J. 2006, 91, 1098–1107. [Google Scholar] [CrossRef]
- Muller, F.D.; Schuler, D.; Pfeiffer, D. A compass to boost navigation: Cell biology of bacterial magnetotaxis. J. Bacteriol. 2020, 202, e00398-20. [Google Scholar] [CrossRef]
- Bazylinski, D.; Lefevre, C. Magnetotactic bacteria from extreme environments. Life 2013, 3, 295–307. [Google Scholar] [CrossRef]
- Lin, W.; Bazylinski, D.A.; Xiao, T.; Wu, L.F.; Pan, Y. Life with compass: Diversity and biogeography of magnetotactic bacteria. Environ. Microbiol. 2014, 16, 2646–2658. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, W.; Zhao, X.; Roberts, A.P.; Paterson, G.A.; Bazylinski, D.; Pan, Y. Genomic expansion of magnetotactic bacteria reveals an early common origin of magnetotaxis with lineage-specific evolution. ISME J. 2018, 12, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, S.; Chen, P.; Liu, H.; Yin, H.; Li, H. Magnetotactic bacteria, magnetosomes and their application. Microb. Res. 2012, 167, 507–519. [Google Scholar] [CrossRef]
- Abreu, F.; Cantão, M.E.; Nicolás, M.F.; Barcellos, F.G.; Morillo, V.; Almeida, L.G.; Do Nascimento, F.F.; Lefèvre, C.T.; Bazylinski, D.A.; de Vasconcelos, A.T.R.; et al. Common ancestry of iron oxide- and iron-sulfide-based biomineralization in magnetotactic bacteria. ISME J. 2011, 5, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Komeili, A. Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria. FEMS Microbiol. Rev. 2012, 36, 232–255. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, T.; Mickoleit, F.; Dziuba, M.; Ruckert, C.; Busche, T.; Kalinowski, J.; Faivre, D.; Uebe, R.; Schuler, D. Identification and elimination of genomic regions irrelevant for magnetosome biosynthesis by large-scale deletion in Magnetospirillum gryphiswaldense. BMC Microbiol. 2021, 21, 65. [Google Scholar] [CrossRef]
- Lin, W.; Paterson, G.A.; Zhu, Q.; Wang, Y.; Kopylova, E.; Li, Y.; Knight, R.; Bazylinski, D.A.; Zhu, R.; Kirschvink, J.L.; et al. Origin of microbial biomineralization and magnetotaxis during the Archean. Proc. Natl. Acad. Sci. USA 2017, 114, 2171–2176. [Google Scholar] [CrossRef]
- Lefevre, C.T.; Trubitsyn, D.; Abreu, F.; Kolinko, S.; de Almeida, L.G.P.; de Vasconcelos, A.T.R.; Lins, U.; Schuler, D.; Ginet, N.; Pignol, D.; et al. Monophyletic origin of magnetotaxis and the first magnetosomes. Environ. Microbiol. 2013, 15, 2267–2274. [Google Scholar] [CrossRef]
- Delong, E.F.; Frankel, R.B.; Bazylinski, D.A. Multiple evolutionary origins of magnetotaxis in bacteria. Science 1993, 259, 803–806. [Google Scholar] [CrossRef]
- Bellinger, M.R.; Wei, J.; Hartmann, U.; Cadiou, H.; Winklhofer, M.; Banks, M.A. Conservation of magnetite biomineralization genes in all domains of life and implications for magnetic sensing. Proc. Natl. Acad. Sci. USA 2022, 119, e2108655119. [Google Scholar] [CrossRef]
- Torres de Araujo, F.F.; Pires, M.A.; Frankel, C.E.; Bicudo, M. Magnetite and Magnetotaxis in Algae. Biophys. J. 1986, 50, 375–378. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Gould, J.L. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems 1981, 13, 181–201. [Google Scholar] [CrossRef]
- Fleissner, G.; Stahl, B.; Thalau, P.; Falkenberg, G.; Fleissner, G. A novel concept of Fe-mineral based magnetoreception: Histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften 2007, 94, 631–642. [Google Scholar] [CrossRef]
- Vali, H.; Kirschvink, J.L. Iron Biominerals; Frankel, R.B., Blakemore, R.P., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 97–115. [Google Scholar]
- Sukumaran, P.V. Magnetotactic bacteria, magnetofossils and the antiquity of life. Curr. Sci. 2005, 88, 879–885. [Google Scholar]
- Gorobets, O.; Gorobets, S.; Koralewski, M. Physiological origin of biogenic magnetic nanoparticles in health and disease: From bacteria to humans. Int. J. Nanomed. 2017, 12, 4371–4395. [Google Scholar] [CrossRef]
- Strbak, O.; Balejcikova, L.; Kmetova, M.; Gombos, J.; Trancikova, A.; Pokusa, M.; Kopcansky, P. Quantification of iron release from native ferritin and magnetoferritin induced by vitamns B2 and C. Int. J. Mol. Sci. 2020, 21, 6332. [Google Scholar] [CrossRef]
- Quintana, C.; Gutierrez, L. Could a dysfunction of ferritin be a determinant factor in the aetiology of some neu-rodegenerative disease? Biochim. Biophys Acta 2010, 1800, 770–782. [Google Scholar] [CrossRef]
- Yan, L.; Da, H.; Zhang, S.; Lopez, V.M.; Wang, W. Bacterial magnetosome and its potential application. Microbiol. Res. 2017, 203, 19–28. [Google Scholar] [CrossRef]
- Kuzajewska, D.; Wszolek, A.; Zwierello, W.; Kirczuk, L.; Maruszewska, A. Magnetotactic bacteria and magnetosomes as smart drug delivery systems: A new weapon on the battelfied with cancer. Biology 2020, 9, 102. [Google Scholar] [CrossRef]
- Tarduno, J.A.; Cottrell, R.D.; Davis, W.J.; Nimmo, F.; Bono, R.K. A Hadean to Paleoarchean geodynamo recorded by single zircon crystals. Science 2015, 349, 521–524. [Google Scholar] [CrossRef]
- Schopf, J.W.; Kitajima, K.; Spicuzza, M.J.; Kudryavtsev, A.B.; Valley, J.W. SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope composition. Proc. Natl. Acad. Sci. USA 2017, 115, 53–58. [Google Scholar] [CrossRef]
- Nutmann, A.P.; Bennett, V.C.; Friend, C.R.L.; van Kranendonk, M.J.; Chivas, A.R. Rapid emergence of life shown by discovery of 3700-million-year-old microbial structures. Nature 2016, 537, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Boehnke, P.; Harrison, T.M.; Mao, W.L. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl. Acad. Sci. USA 2015, 112, 14518–14521. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, W.; Kmita, H.; Kosicki, J.Z.; Kaczmarek, L. How the geomagnetic field influences life on Earth—An in-tegrated approach to geomagnetobiology. Orig. Life Evol. Biosph. 2021, 54, 231–257. [Google Scholar] [CrossRef] [PubMed]
- Doglioni, C.; Pignatti, J.; Coleman, M. Why did life develop on the surface of the Earth in the Cambrian? Geosci. Front. 2016, 7, 865–873. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Winklhofer, M.; Walker, M.M. Biophysics of magnetic orientation: Strengthening the interface between theory and experimental design. J. R. Soc. Interface 2010, 7, S179–S191. [Google Scholar] [CrossRef]
- Kirschvink, J.L. Magnetoreception: Homing in on vertebrates. Nature 1997, 390, 339–340. [Google Scholar] [CrossRef]
- Ahmad, M.; Galland, P.; Ritz, T.; Wiltschko, R.; Wiltschko, W. Magnetic intensity affects crypto-chrome-dependent responses in Arabidopsis thaliana. Planta 2007, 225, 615–624. [Google Scholar] [CrossRef]
- Winklhofer, M. Magnetoreception. J. R. Soc. Interface 2010, 7, S131–S134. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Hagadorn, J.W. The Biomineralisation of Nano- and Micro- Structures; Bäuerlein, E., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; pp. 139–150. Available online: https://resolver.caltech.edu/CaltechAUTHORS:20130118-101342177 (accessed on 23 January 2013).
- Lin, W.; Zhang, W.; Paterson, G.A.; Zhu, Q.; Zhao, X.; Knight, R.; Bazilinski, D.A.; Roberts, A.P.; Pan, Y. Expanding magnetic organelle biogenesis in the domain Bacteria. Microbiome 2020, 7, 252. [Google Scholar] [CrossRef]
- Strbak, O.; Dobrota, D. Archean iron-based metabolism analysis and the photoferrotrophy-driven hypothesis of microbial magnetotaxis origin. Geomicrobiol. J. 2019, 36, 278–290. [Google Scholar] [CrossRef]
- Lin, W.; Kirschvink, J.L.; Paterson, G.A.; Bazylinski, D.A.; Pan, Y. On the origin of microbial magnetoreception. Natl. Sci. Rev. 2020, 7, 472–479. [Google Scholar] [CrossRef]
- Sanchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef]
- Knoll, A.H.; Nowak, M.A. The timetable of evolution. Sci. Adv. 2017, 3, e1603076. [Google Scholar] [CrossRef]
- Martin, W.F.; Sousa, F.L. Early microbial evolution: The age of anaerobes. Cold Spring Harb. Perspect. Biol. 2016, 8, a018127. [Google Scholar] [CrossRef]
- Cardenas, J.P.; Quatrini, R.; Holmes, D.S. Aerobic lineage of the oxidative stress response protein rubrerythrin emerged in an ancient microaerobic, (hyper)thermophilic environment. Front. Microbiol. 2016, 7, 1822. [Google Scholar] [CrossRef]
- David, L.A.; Alm, E.J. Rapid evolutionary innovation during an Archaean genetic expansion. Nature 2011, 469, 93–96. [Google Scholar] [CrossRef]
- Andrews, S.C. The ferritin-like superfamily: Evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim. Biophys Acta 2010, 1800, 691–705. [Google Scholar] [CrossRef]
- Abreu, F.; Morillo, V.; Trubitsyn, D.; Bazylinski, D. Magnetotaxis in Prokaryotes. eLS 2020, 1–14. [Google Scholar] [CrossRef]
- Monteil, C.; Vallenet, D.; Benzerara, K.; Barbe, V.; Fouteau, S.; Cruaud, C.; Floriani, M.; Viollier, E.; Adryanczyk, G.; Leonhardt, N.; et al. A symbiotic origin of magnetoreception in unicellular eukaryotes. Nat. Microbiol. 2019, 4, 1088–1095. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Keren-Khadmy, N.; Zarivach, R. From invagination to navigation: The story of magnetosome-associated proteins in magnetotactic bacteria. Protein Sci. 2016, 25, 338–351. [Google Scholar] [CrossRef]
- Stolz, J.F.; Chang, S.-B.R.; Kirschvink, J.L. Magnetotactic bacteria and single-domain magnetite in hemipelagic sediments. Nature 1986, 321, 849–851. [Google Scholar] [CrossRef]
- Popp, F.; Armitage, J.P.; Schuler, D. Polarity of bacterial magnetotaxis is controlled by aerotaxis through a common sensory pathway. Nat. Commun. 2014, 5, 5398. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.L.; Bazylinski, D.A.; Edwards, K.J. South-seeking magnetotactic bacteria in the Northern Hemisphere. Science 2006, 311, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kirilenko, D.A.; Kosterov, A.; Koziaeva, V.V.; Levitskii, V.S.; Multhoff, G.; Nepomnyashchaya, E.K.; Nikitin, A.V.; et al. Magnetic properties of bacterial magnetosomes produced by Magnetospirillum caucaseum SO-1. Microorganisms 2021, 9, 1854. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Morin, G.; Menguy, N.; Gonzalez, T.P.; Widdrat, M.; Cosmidis, J.; Faivre, D. Magnetotactic bacteria form magnetite from a phosphate-rich ferric hydroxide via nanometric ferric (oxyhydr)oxide intermediates. Proc. Natl. Acad. Sci. USA 2013, 110, 14883–14888. [Google Scholar] [CrossRef]
- Frankel, R.B.; Zhang, J.P.; Bazylinski, D.A. Single magnetic domains in magnetotactic bacteria. J. Geophys. Res. 1998, 103, 30601–30604. [Google Scholar] [CrossRef]
- Lin, W.; Deng, A.; Wang, Z.; Li, Y.; Wen, T.; Wu, L.F.; Wu, M.; Pan, Y. Genomic insight into the uncultured genus “Candidatus Magnetobacterium” in the phylum Nitrospirae. ISME J. 2014, 8, 2463–2477. [Google Scholar] [CrossRef]
- Guo, F.F.; Yang, W.; Jiang, W.; Geng, S.; Peng, T.; Li, J.L. Magnetosomes eliminate intracellular reactive oxygen species in Magnetosprllum gryphiswaldense MSR-1. Environ. Microbiol. 2012, 14, 1722–1729. [Google Scholar] [CrossRef]
- Kolinko, S.; Jogler, C.; Katzmann, E.; Wanner, G.; Peplies, J.; Schüler, D. Single-cell analysis reveals a novel uncultivated magnetotactic bacterium within the candidate division OP3. Environ. Microbiol. 2012, 14, 1709–1721. [Google Scholar] [CrossRef]
- Jogler, C.; Wanner, G.; Kolinko, S.; Niebler, M.; Amann, R.; Petersen, N.; Kube, M.; Reinhardt, R.; Schuler, D. Conservation of proteobacterial magnetosome genes and structures in an uncultivated member of the deep-branching Nitrospira phylum. Proc. Natl. Acad. Sci. USA 2011, 108, 1134–1139. [Google Scholar] [CrossRef]
- Lefèvre, C.T.; Pósfai, M.; Abreu, F.; Lins, U.; Frankel, R.B.; Bazylinski, D.A. Morphological features of elon-gated-anisotropic magnetosome crystals in magnetotactic bacteria of the Nitrospirae phylum and the Deltaproteobacteria class. Earth Planet Sci. Lett. 2011, 312, 194–200. [Google Scholar] [CrossRef]
- Crichton, R. Iron Metabolism, 4th ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–21. ISBN 978-1-118-92561-4. [Google Scholar]
- Wang, C.X.; Hilburn, I.A.; Wu, D.-A.; Mizuhara, Y.; Couste, C.P.; Abrahams, J.N.H.; Bernstein, S.E.; Matani, A.; Shimojo, S.; Kirschvink, J.L. Transduction of the geomagnetic field as evidence from aplpha-band activity in the human brain. eNeuro 2019, 6, 1–23. [Google Scholar] [CrossRef]
- Correa, T.; Taveira, I.; Presciliano de Souza Filho, R.; Fernanda, A. Biomineralization of magnetosomes: Billion-year evolution shaping modern nanotools. In Materials at the Nanoscale; Mallik, A.K., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Murat, D.; Quinlan, A.; Vali, H.; Komeili, A. Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc. Natl. Acad. Sci. USA 2010, 107, 5593–5598. [Google Scholar] [CrossRef]
- Lefevre, C.T.; Trubitsyn, D.; Abreu, F.; Kolinko, S.; Jogler, C.; de Almeida, L.G.P.; de Vasconcelos, A.T.R.; Kube, M.; Reinhardt, R.; Lins, U.; et al. Comparative genomic analysis of magnetotactic bacteria from the Deltaproteobacteria provides new insight into magnetite and greigite magnetosome genes required for magnetotaxis. Environ. Microbiol. 2013, 15, 2712–2735. [Google Scholar] [CrossRef]
- Simmons, S.L.; Edwards, K.J. Geobiology of Magnetotactic Bacteria. In Magnetoreception and Magnetosomes in Bacteria; Microbiology Monographs; Schüler, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 3. [Google Scholar] [CrossRef]
- Natan, E.; Vortman, Y. Symbiotic magnetic sensing: Raising evidence and beyond. Philos. Trans. R. Soc. Lond. B 2020, 375, 20190595. [Google Scholar] [CrossRef]
- Simon, M.R.; Ranasinghe, P.D.; Barazanji, N.; Jungestrom, M.B.; Xu, J.; Bednarska, O.; Serrander, L.; Engstrom, M.; Bazylinski, D.A.; Keita, A.V.; et al. Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain. J. Oceanol. Limnol. 2021, 39, 2044–2052. [Google Scholar] [CrossRef]
- Lower, B.H.; Bazylinski, D.A. The bacterial magnetosome: A unique prokaryotic organelle. J. Mol. Microbiol. Biotechnol. 2013, 23, 63–80. [Google Scholar] [CrossRef]
- Werckmann, J.; Cypriano, J.; Lefevre, C.T.; Dembele, K.; Ersen, O.; Bazylinski, D.A.; Lins, U.; Farina, M. Localized iron accumulation precedes nucleation and growth of magnetite crystals in magnetotactic bacteria. Sci. Rep. 2017, 7, 8291. [Google Scholar] [CrossRef]
- Nudelman, H.; Zarivach, R. Structure prediction of magnetosome-associated proteins. Front. Microbiol. 2014, 5, 9. [Google Scholar] [CrossRef]
- Uebe, R.; Schuler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637. [Google Scholar] [CrossRef]
- Tanaka, M.; Okamura, Y.; Arakaki, A.; Tanaka, T.; Takeyama, H.; Matsunaga, T. Origin of magnetosome membrane: Proteomic analysis of magnetosome membrane and comparison with cytoplasmic membrane. Proteomics 2006, 6, 5234–5247. [Google Scholar] [CrossRef] [PubMed]
- Taoka, A.; Asada, R.; Sasaki, H.; Anzawa, K.; Wu, L.F.; Fukumori, Y. Spatial localizations of Mam22 and Mam12 in the magnetosomes of Magnetospirillum magnetotacticum. J. Bacteriol. 2006, 188, 3805–3812. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Taoka, A.; Uchihashi, T.; Fukumori, Y. Visualization and structrual analysis of the bacterial magnetic organelle magnetosome using atomic force microscopy. Proc. Natl. Acad. Sci. USA 2010, 107, 9382–9387. [Google Scholar] [CrossRef]
- Uebe, R.; Junge, K.; Henn, V.; Poxleitner, G.; Katzmann, E.; Piltzko, J.M.; Zarivach, R.; Kasarna, T.; Wanner, G.; Posfai, M.; et al. The cation diffusion facilitator proteins MamB and MamM of Magnetospirillum gryphiswaldense have distinct and complex functions, and are involved in magnetite biomineralization and magnetosome assembly. Mol. Microbiol. 2011, 82, 818–835. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, P.; Siponen, M.I.; Lefevre, C.T.; Ginet, N.; Pignol, D. Structure and evolution of the magnetochrome domains: No longer alone. Front. Microbiol. 2014, 5, 117. [Google Scholar] [CrossRef]
- Cornejo, E.; Subramanian, P.; Li, Z.; Jensen, G.; Komeili, A. Dynamic remodeling of the magnetosome membrane is triggered by the initiation of biomineralization. mBio 2016, 7, e01898. [Google Scholar] [CrossRef]
- Byrne, M.E.; Ball, D.A.; Guerquin-Kern, J.L.; Rouiller, I.; Wu, T.D.; Downing, K.H.; Vali, H.; Komeili, A. Desulfovibrio magneticus RS-1 contains an iron- and phosphorus-rich organelle distinct from its bullet-shaped magnetosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 12263–12268. [Google Scholar] [CrossRef]
- Amor, M.; Ceballos, A.; Wan, J.; Simon, C.P.; Aron, A.T.; Chang, C.J.; Hellman, F.; Komeili, A. Magnetotactic bacteria accumulate a large pool of iron distinct from their magnetite crystals. Appl. Environ. Microbiol. 2020, 86, e01278-20. [Google Scholar] [CrossRef]
- Ahmadzadech, M.; Romero, C.; McCloy, J. Magnetic analysis of commercial hematite, magnete, and their mixtures. AIP Adv. 2018, 8, 056807. [Google Scholar] [CrossRef]
- Devouard, B.; Posfal, M.; Hua, X.; Bazylinski, D.A.; Frankel, R.B.; Buseck, P.R. Magnetite from magnetotactic bacteria: Size distributions and twinning. Am. Min. 1998, 83, 1387–1398. [Google Scholar] [CrossRef]
- Moisescu, C.; Ardelean, I.I.; Benning, L.G. The effect and role of environmental conditions on magnetosome synthesis. Front. Microbiol. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Yiriletu, I.T. Magnetic properties of magnetite synthetized by Magnetospirillum magnetotacticum MS-1 cultured with different concentrations of ferric iron. Biotechnol. Lett. 2015, 37, 2427–2433. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Schiro, G.; Reichel, V.E.; Faivre, D. Reducing conditions favor magnetosome production in Magnetospirillum magneticum. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Amskold, L.; Lalonde, S.V.; Posth, N.R.; Kappler, A.; Anbar, A. Decoupling photochemical Fe(II) oxidation from shallow-water BIF deposition. Earth Planet. Sci. Lett. 2007, 258, 87–100. [Google Scholar] [CrossRef]
- Camacho, A.; Walter, X.A.; Picazo, A.; Zopfi, J. Photoferrotrophy: Remains of an ancient photosynthesis in modern environments. Front. Microbiol. 2017, 8, 323. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lack, J.G.; Coates, J.D. Biogenic magnetite formation through anaerobic biooxidation of F(II). Appl. Environ. Microbiol. 2001, 67, 2844–2848. [Google Scholar] [CrossRef]
- Li, Z.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M.; Doi, M. Magnetite nanoparticles with high heating efficiencies for application in the hyperthermia of cancer. Mater. Sci. Eng. C 2010, 30, 990–996. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef]
- Qi, H.; Yan, B.; Lu, W.; Li, C.; Yang, Y. A non-alkoxide sol-gel method for the preparation of magnetite nanoparticles. Curr. Nanosci. 2011, 7, 381–388. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, F.; Ruoyu, H. Solvothermal synthesis of magnetic Fe3O4 microparticles via self-assembly of Fe3O4 nanoparticles. Particuology 2011, 9, 179–186. [Google Scholar] [CrossRef]
- Kiseleva, L.; Briliute, J.; Khilyas, I.V.; Simpson, D.J.W.; Fedorovich, V.; Cohen, M.; Goryanin, I. Magnet-facilitated selection of electrogenic bacteria from marine sediment. BioMed Res. Int. 2015, 2015, 582471. [Google Scholar] [CrossRef] [PubMed]
- Alphandery, E. Applications of magnetosomes synthetisized by magetotactic bacteria in medicine. Front. Bioeng. Biotechnol. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Bau, M.; Moller, P. Rare earth element systematics of the chemically precipitated component in Early Precambrian iron-formations and the evolution of the terrestrial atmosphere-hydrosphere-litosphere system. Geochim. Cosmochim. Acta 1993, 57, 2239–2249. [Google Scholar] [CrossRef]
- Alexander, B.W.; Bau, M.; Anderson, P.; Dulski, P. Continentally-derived solutes in shallow Archean seawater: Rare earth element and Nd isotope evidence in iron formation from the 2.9 Ga Pongola Supergroup, South Africa. Geochim. Cosmochim. Acta 2008, 72, 378–394. [Google Scholar] [CrossRef]
- Canfield, D.E.; Rosing, M.T.; Bjerrum, C. Early anaerobic metabolism. Philos. Trans. R. Soc. B 2006, 361, 1819–1836. [Google Scholar] [CrossRef]
- Anbar, A.D.; Duan, Y.; Lyons, T.W.; Arnold, G.L.; Kendall, B.; Creaser, R.A.; Kaufman, A.J.; Gordon, G.W.; Scott, C.; Garvin, J.; et al. A whiff of oxygen before the great oxidation event? Science 2007, 317, 1903–1906. [Google Scholar] [CrossRef]
- Williams, R.J.P. Iron in evolution. FEBS Lett. 2012, 586, 479–484. [Google Scholar] [CrossRef]
- Cnossen, I.; Sanz-Forcada, J.; Favata, F.; Witasse, O.; Zegers, T.; Arnold, N.F. Habitat of early life: Solar X-ray and UV radiation at Earth’s surface 4-3.5 billion years ago. J. Geophys. Res. 2007, 112, E02008. [Google Scholar] [CrossRef]
- Tarduno, J.A.; Cottrell, R.D.; Watkeys, M.K.; Hofmann, A.; Doubrovine, P.V.; Mamajek, E.E.; Liu, D.; Sibeck, D.G.; Neukrich, P.L.; Usui, Y. Geodynamo, solar wind, and magnetopause 3.4 to 3.45 billion years ago. Science 2010, 327, 1238–1240. [Google Scholar] [CrossRef]
- Pierre, J.L.; Fontecave, M.; Crichton, R.R. Chemistry for an essential biological process: The reduction of ferric iron. BioMetals 2002, 15, 341–346. [Google Scholar] [CrossRef]
- Ilbert, M.; Bonnefoy, V. Insight the evolution of the iron oxidation pathways. Biochim. Biophys. Acta 2013, 1827, 161–175. [Google Scholar] [CrossRef]
- Archibald, F.S. Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiol. Lett. 1983, 19, 29–32. [Google Scholar] [CrossRef]
- Amor, M.; Busigny, V.; Louvat, P.; Gelabert, A.; Cartigny, P.; Durand-Dubief, M.; Ona-Nguema, G.; Alphandery, E.; Chebbi, I.; Guyot, F. Mass-dependent and –independent signature of Fe isotopes in magnetotactic bacteria. Science 2016, 352, 705–708. [Google Scholar] [CrossRef]
- Amor, M.; Busigny, V.; Louvat, P.; Tharaud, M.; Gelabert, A.; Cartigny, P.; Carlut, J.; Isambert, A.; Durand-Dubief, M.; Ona-Nguema, G.; et al. Iron uptake and magnetite biomineralization in the magnetotactic bacterium Magnetospirillum magneticum strain AMB-1: An iron isotope study. Geochym. Cosmochim. Acta 2018, 232, 225–243. [Google Scholar] [CrossRef]
- Fdez-Gubieda, M.L.; Muela, A.; Alonso, J.; Garcia-Prieto, A.; Olivi, L.; Fernandez-Pacheco, R.; Barandiaran, J.M. Magnetite biomineralization in Magnetospirillum gryphiswaldense: Time resolved magnetic and structural studies. ACS Nano 2013, 7, 3297–3305. [Google Scholar] [CrossRef]
- Faivre, D.; Bottger, L.H.; Matzanke, B.F.; Schuler, D. Intracellular magnetite biomineralzation in bacteria proceeds by a distinct pathway involving membrane-bound ferritin and an iron(II) species. Angew. Chem. Int. Ed. Eng. 2007, 46, 8495–8499. [Google Scholar] [CrossRef]
- Staniland, S.; Ward, B.; Harrianson, A.; Van der Laan, G.; Telling, N. Rapid magnetosome formation shown by real-time X-ray magnetic circular dichroism. Proc. Natl. Acad. Sci USA 2007, 104, 19524–19528. [Google Scholar] [CrossRef]
- Schuler, D.; Naeuerlein, E. Dynamics of iron uptake and Fe3O4 biomineralization during aerobic and microaerobic growth of Magnetospirillum gryphiswaldense. J. Bacteriol. 1998, 180, 159–162. [Google Scholar] [CrossRef]
- Andrade, A.L.; Souza, D.M.; Pereira, M.C.; Fabris, J.D.; Domingues, R.Z. pH effect on the synthesis of magnetite nanoparticles by the chemical reduction-precipitation method. Quim. Nova. 2010, 33, 524–527. [Google Scholar] [CrossRef]
- Komeili, A.; Vali, H.; Beveridge, T.J.; Newman, D.K. Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc. Natl. Acad. Sci USA 2004, 101, 3839–3844. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharma. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, E.; Scheffel, A.; Gruska, M.; Plitzko, J.M.; Schuler, D. Loss of the actin-like protein MamK has pleiotropic effects on magnetosome formation and chain assembly in Magnetospirillum gryphiswaldense. Mol. Microbiol. 2010, 77, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Raschdorf, O.; Forstner, Y.; Kolinko, I.; Uebe, R.; Plitzko, J.M.; Schuler, D. Genetic and ultrastructural analysis reveals the key players and initial steps of bacterial magnetosome membrane biogenesis. PLoS Genet. 2016, 12, e1006101. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Kikuchi, D.; Tanaka, M.; Yamagishi, A.; Yoda, T.; Matsunaga, T. Comparative Subcellular Localization Analysis of Magnetosome Proteins Reveals a Unique Localization Behavior of Mms6 Protein onto Magnetite Crystals. J. Bacteriol. 2016, 198, 2794–2802. [Google Scholar] [CrossRef]
- Hershey, D.M.; Browne, P.J.; Iavarone, A.T.; Teyra, J.; Lee, E.H.; Sidhu, S.S.; Komeili, A. Magnetite biomineralization in Magnetospirillum magneticum as regulated by a switch-like behavior in the HtrA protease MamE. J. Biol. Chem. 2016, 291, 17941–17952. [Google Scholar] [CrossRef]
- Abreu, N.; Mannoubi, S.; Ozymak, E.; Pignol, D.; Ginet, N.; Komeili, A. Interplay between two bacterial actin homologs, MamK and MamK like, is required for the alignment of magnetosome organelles in Magnetospirillum magneticum AMB-1. J. Bacteriol. 2014, 196, 3111–3121. [Google Scholar] [CrossRef]
- Baumgartner, J.; Menguy, N.; Gonzalez, T.P.; Morin, G.; Widdrat, M.; Faivre, D. Elongated magnetite nanoparticle formation from a solid ferrous precursor in a magnetotactic bacterium. J. R. Soc. Interface 2016, 13, 20160665. [Google Scholar] [CrossRef]
- Zeytuni, N.; Uebe, R.; Maes, M.; Davidov, G.; Baram, M.; Raschdorf, O.; Nadav-Tsubery, M.; Kolusheva, S.; Bitton, R.; Goobes, G.; et al. Cation Diffusion Facilitators Transport Initiation and Regulation Is Mediated by Cation Induced Conformational Changes of the Cytoplasmic Domain. PLoS ONE 2014, 9, e92141. [Google Scholar] [CrossRef]
- Jones, S.R.; Wilson, T.D.; Brown, M.E.; Rahn-Lee, L.; Yu, Y.; Fredriksen, L.L.; Ozyamak, E.; Komeili, A.; Chang, M.C.Y. Genetic and biochemical investigations of the role of MamP in redox control of iron biomineralization in Magnetospirillum magneticum. Proc. Natl. Acad. Sci. USA 2015, 112, 3904–3909. [Google Scholar] [CrossRef]
- Siponen, M.I.; Legrand, P.; Widdrat, M.; Jones, S.R.; Zhang, W.J.; Chang, M.C.Y.; Faivre, D.; Arnoux, P.; Pignol, D. Structural insight into magnetochrome-mediated magnetite biomineralization. Nature 2013, 502, 681–684. [Google Scholar] [CrossRef]
- Mathuriya, A.S. Magnetotactic bacteria: Nanodrivers of the future. Crit. Rev. Biotechnol. 2016, 36, 788–802. [Google Scholar] [CrossRef]
- Kotakadi, S.M.; Borelli, D.P.R.; Nannepaga, J.S. Therapeutic applications of magnetotacit bacteria and magnetosomes: A review emphasizing on the cancer treatment. Front. Bioeng. Biotechnol. 2022, 10, 789016. [Google Scholar] [CrossRef]
- Boucher, M.; Geffroy, F.; Preveral, S.; Bellanger, L.; Selingue, E.; Adryanczyk-Perrier, G.; Pean, M.; Lefervre, C.T.; Pignol, D.; Ginet, N.; et al. Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 2017, 121, 167–178. [Google Scholar] [CrossRef]
- Fdez-Gubieda, M.L.; Alonso, J.; Garcia-Prieto, A.; Garcia-Arribas, A.; Barquin, L.F.; Muela, A. Magnetotactic bacteria for cancer therapy. J. Appl. Phys. 2020, 128, 070902. [Google Scholar] [CrossRef]
- Nan, X.; Lai, W.; Li, D.; Tian, J.; Zhiyuan, H.; Fang, Q. Biocompatibility of bacterial magnetosomes as MRI contrast agent: A long-term in vivo follow-up study. Nanomaterials 2021, 11, 1235. [Google Scholar] [CrossRef]
- Mannucci, S.; Tambalo, S.; Conti, G.; Ghin, L.; Milanese, A.; Carboncino, A.; Nicolato, E.; Marinozzi, M.R.; Benati, D.; Bassi, R.; et al. Magnetosomes extracted from Magnetospisillum gryphiswaldense as theranostic agents in an experimental model of glioblastoma. Contrast Media Mol. Imaging 2018, 2018, 2198703. [Google Scholar] [CrossRef]
- Grouzdev, D.S.; Dziuba, M.V.; Kurek, D.V.; Ovchinnikov, A.I.; Zhigalova, N.A.; Kuznetsov, B.B.; Skryabin, K.G. Optimized method for preparation of IgG-binding bacterial magnetic nanoparticles. PLoS ONE 2014, 9, e109914. [Google Scholar] [CrossRef]
- Arakaki, A.; Nakazawa, H.; Nemoto, M.; Mori, T.; Matsunaga, T. Formation of magnetite by bacteria and its application. J. R. Soc. Interface 2008, 5, 977–999. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhao, J.; Yao, H.; Chu, S.; Song, Z.; He, Z.; Zhang, W. Magnetotactic bacteria: Characteristics and environmantal applications. Front. Environ. Sci. Eng. 2020, 14, 56. [Google Scholar] [CrossRef]
- Xu, J.; Hu, J.; Liu, L.; Li, L.; Wang, X.; Zhang, H.; Jiang, W.; Tian, J.; Li, Y.; Li, J. Surface expression of protein A on magnetosomes and capture of pathogenic bacteria by magnetosome/antibody complexes. Front. Microbiol. 2014, 5, 136. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Nash, C.; Kirschvink, J.L.; Leadbetter, J. A possible magnetite/maghemite electrochemical battery in the magnetotactic bacteria. AGU Fall Meet. Abstr. 2004, 2004, GP34A-06. [Google Scholar]
- Strbak, O.; Kopcansky, P.; Timko, M.; Frollo, I. Single biogenic magnetite nanoparticle physical characteristics—A biological impact study. IEEE Trans. Magn. 2013, 49, 457–462. [Google Scholar] [CrossRef]
- Guo, L.; Huang, J.; Zhang, X.; Li, Y.; Zheng, L. Bacterial magnetic nanoparticles as drug carriers. J. Mater. Chem. 2008, 18, 5993–5997. [Google Scholar] [CrossRef]
- Sun, J.B.; Duan, J.H.; Dai, S.L.; Ren, J.; Guo, L.; Jiang, W.; Li, Y. Preparation and antitumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: Magnetic nanoparticles as drug carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol. Bioeng. 2008, 101, 1313–1320. [Google Scholar] [CrossRef]
- Zhang, Z.; King, M.R. Nanomaterials for the capture and therapeutic targeting of circulating tumor cells. Cell. Mol. Bioeng. 2017, 10, 275–294. [Google Scholar] [CrossRef]
- Liu, Y.G.; Dai, Q.L.; Wang, S.B.; Deng, Q.J.; Wu, W.G.; Chen, A.Z. Preparation and in vitro antitumor effects of cytosine arabinsoide-loaded genpin-poly-L-glutamic acid-modified bacterial magnetosomes. Int. J. Nanomed. 2015, 10, 1387–1397. [Google Scholar] [CrossRef]
- Tang, Y.-S.; Wang, D.; Zhou, C.; Zhang, S. Preparation and antitumor efficiency evaluation of bacterial magnetosome–anti-4-1BB antibody complex: Bacterial magnetosome as antibody carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol. Appl. Biochem. 2019, 66, 290–297. [Google Scholar] [CrossRef]
- Cypriano, J.; Werckmann, J.; Vargas, G.; Lopes dos Santos, A.; Silva, K.T.; Leão, P.; Almeida, F.P.; Bazylinski, D.A.; Farina, M.; Lins, U.; et al. Uptake and persistence of bacterial magnetite magnetosomes in a mammalian cell line: Implications for medical and biotechnological applications. PLoS ONE 2019, 14, e0215657. [Google Scholar] [CrossRef]
- Zhao, D. Bacteriogenic magnetic nanoparticles as magnetic resonance imaging contrast agents. Transl. Cancer Res. 2017, 6, S512–S514. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Tachibana, A.; Goldstone, A.B.; Woo, Y.J.; Chakraborty, P.; Lee, K.R.; Foote, C.S.; Piecewicz, S.; Barrozo, J.C.; Wakeel, A.; et al. Novel MRI contrast agent from Magnetotactic bacteria enables in vivo tracking of iPSC-derived cadiomyocytes. Sci. Rep. 2016, 6, 26960. [Google Scholar] [CrossRef]
- Yoshino, T.; Hirabe, H.; Takahashi, M.; Kuhara, M.; Takeyama, H.; Matsunaga, T. Magnetic cell separation using nano-sized bacterial magnetic particles with reconstructed magnetosome membrane. Biotechnol. Bioeng 2008, 101, 470–477. [Google Scholar] [CrossRef]
- Matsunaga, T.; Suzuki, T.; Tanaka, M.; Arakaki, A. Molecular analysis of magnetotactic bacteria and development of functional bacterial magnetic particles for nano-biotechnology. Trends Biotechnol. 2007, 25, 182–188. [Google Scholar] [CrossRef]

| Step | Murat et al., 2010 [77] | Komeili, 2012 [24] | Crichton 2016 [74] | Ben-Shimon et al., 2021 [8] |
|---|---|---|---|---|
| 1. | invagination of the inner membrane (regulated by MamI, MamL, MamQ, MamB) | creation of the magnetosome membrane by invagination of the inner cell membrane (mediated by MamI, MamL, MamQ, MamB) | a reductive uptake of iron from the external environment of the bacterial cell and its transport (ferritin?) across the magnetosome membrane | protein sorting and membrane invagination |
| 2. | targeting the magnetosome proteins to the newly created vesicles (regulated by MamE) | MamE is incorporated into the newly created magnetosome membrane | an accumulation of iron within the precursor of the vesicular structure of the magnetosome | magnetosomes alignment into a single or multiple chains |
| 3. | release of vesicles from the inner membrane and their alignment along the created actin-like filament (regulated by MamJ and MamK) | MamE recruits other proteins to the newly created magnetosome membrane | transformation of the initial iron deposit (ferrihydrite) into magnetite | ion transport and magnetosome inner environment control |
| 4. | iron uptake into aligned vesicles and initiation of crystal formation (regulated by MamM, MamN, and MamO) | MamK and MamJ organize newly created magnetosomes into chains (it can take place before or after crystal formation) | crystallization of the magnetite mineral to give a particle within the vesicle of a specific size and orientation | iron nucleation and crystal shape and size control |
| 5. | crystal maturation (regulated by MamA, MamGFDC, MamP, MamR, MamS, MamT) | initiation of biomineralization—formation of small crystals of magnetite (mediated by MamM, MamN, MamO, MagA, MamA) | ||
| 6. | crystal growing (MamR, MamS, MamT, MamP, MamE, Mms6, MamC, MamD, MamF, MamG are employed) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strbak, O.; Hnilicova, P.; Gombos, J.; Lokajova, A.; Kopcansky, P. Magnetotactic Bacteria: From Evolution to Biomineralization and Biomedical Applications. Minerals 2022, 12, 1403. https://doi.org/10.3390/min12111403
Strbak O, Hnilicova P, Gombos J, Lokajova A, Kopcansky P. Magnetotactic Bacteria: From Evolution to Biomineralization and Biomedical Applications. Minerals. 2022; 12(11):1403. https://doi.org/10.3390/min12111403
Chicago/Turabian StyleStrbak, Oliver, Petra Hnilicova, Jan Gombos, Alica Lokajova, and Peter Kopcansky. 2022. "Magnetotactic Bacteria: From Evolution to Biomineralization and Biomedical Applications" Minerals 12, no. 11: 1403. https://doi.org/10.3390/min12111403
APA StyleStrbak, O., Hnilicova, P., Gombos, J., Lokajova, A., & Kopcansky, P. (2022). Magnetotactic Bacteria: From Evolution to Biomineralization and Biomedical Applications. Minerals, 12(11), 1403. https://doi.org/10.3390/min12111403









