A Combined Extended X-ray Absorption Fine Structure Spectroscopy and Density Functional Theory Study of Americium vs. Yttrium Adsorption on Corundum (α–Al2O3)
Abstract
1. Introduction
2. Materials and Methods
2.1. Density Functional Theory Calculations
2.2. Eu3+ Sorption Isotherms in the Absence and Presence of Y3+
2.3. Extended X-ray Absorption Fine Structure Spectroscopy (EXAFS)
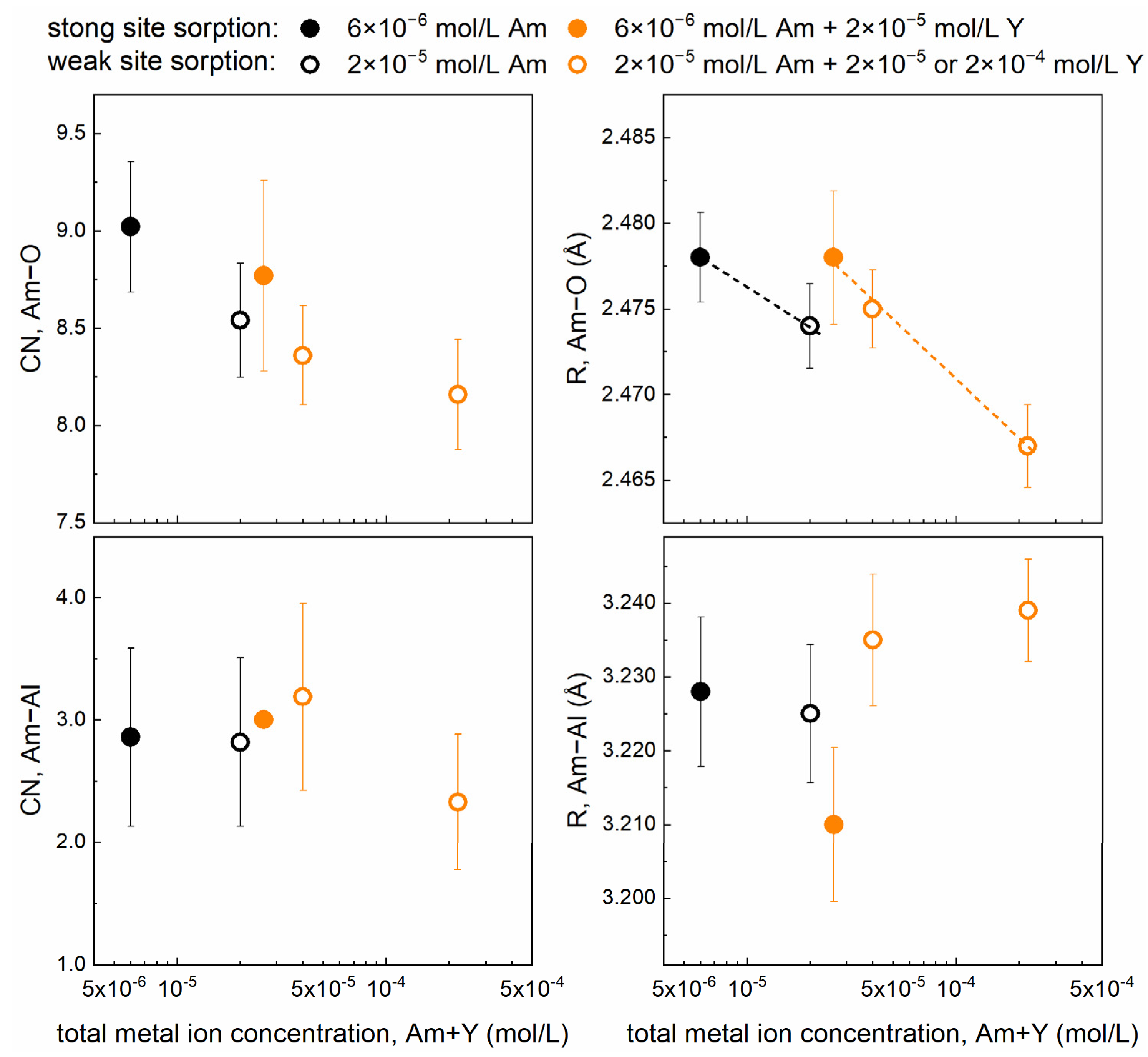
3. Results and Discussion
3.1. Density Functional Theory Calculations
3.2. Eu3+ Sorption Isotherms
3.3. Am3+ EXAFS Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lokshin, E.P.; Ivanenko, V.I.; Tareeva, O.A.; Korneikov, R.I. Sorption extraction of lanthanides from phosphoric acid solutions. Russ. J. Appl. Chem. 2009, 82, 537–544. [Google Scholar] [CrossRef]
- Tu, Y.J.; Lo, S.C.; You, C.F. Selective and fast recovery of neodymium from seawater by magnetic iron oxide Fe3O4. Chem. Eng. J. 2015, 262, 966–972. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. An overview of rare-earth recovery by ion-exchange leaching from ion-adsorption clays of various origins. Mineral. Mag. 2016, 80, 63–76. [Google Scholar] [CrossRef]
- Lozano, A.; Ayora, C.; Fernández-Martínez, A. Sorption of rare earth elements onto basaluminite: The role of sulfate and pH. Geochim. Cosmochim. Acta 2019, 258, 50–62. [Google Scholar] [CrossRef]
- Kammerlander, K.K.K.; Köhler, L.K.; Huittinen, N.; Bok, F.; Steudtner, R.; Oschatz, C.; Vogel, M.; Stumpf, T.; Brunner, E. Sorption of europium on diatom biosilica as model of a “green” sorbent for f-elements. Appl. Geochem. 2021, 126, 9. [Google Scholar] [CrossRef]
- Rabung, T.; Schild, D.; Geckeis, H.; Klenze, R.; Fanghänel, T. Cm(III) Sorption onto Sapphire (α-Al2O3) Single Crystals. J. Phys. Chem. B 2004, 108, 17160–17165. [Google Scholar] [CrossRef]
- Rabung, T.; Pierret, M.C.; Bauer, A.; Geckeis, H.; Bradbury, M.H.; Baeyens, B. Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 1: Batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim. Cosmochim. Acta 2005, 69, 5393–5402. [Google Scholar] [CrossRef]
- Hartmann, E.; Baeyens, B.; Bradbury, M.H.; Geckeis, H.; Stumpf, T. A Spectroscopic Characterization and Quantification of M(III)/Clay Mineral Outer-Sphere Complexes. Environ. Sci. Technol. 2008, 42, 7601–7606. [Google Scholar] [CrossRef]
- Huittinen, N.; Rabung, T.; Lützenkirchen, J.; Mitchell, S.C.; Bickmore, B.R.; Lehto, J.; Geckeis, H. Sorption of Cm(III) and Gd(III) onto gibbsite, α-Al(OH)3: A batch and TRLFS study. J. Colloid Interface Sci. 2009, 332, 158–164. [Google Scholar] [CrossRef]
- Tan, X.; Fang, M.; Wang, X. Sorption speciation of lanthanides/actinides on minerals by TRLFS, EXAFS and DFT studies: A review. Molecules 2010, 15, 8431–8468. [Google Scholar] [CrossRef]
- Huittinen, N.; Rabung, T.; Andrieux, P.; Lehto, J.; Geckeis, H. A comparative batch sorption and time-resolved laser fluorescence spectroscopy study on the sorption of Eu(III) and Cm(III) on synthetic and natural kaolinite. Radiochim. Acta 2010, 98, 613–620. [Google Scholar] [CrossRef]
- Janot, N.; Benedetti, M.F.; Reiller, P.E. Colloidal α-Al2O3, Europium(III) and Humic Substances Interactions: A Macroscopic and Spectroscopic Study. Environ. Sci. Technol. 2011, 45, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, A.; Marsac, R.; Rabung, T.; Lützenkirchen, J.; Geckeis, H. Sorption of Cm(III) and Eu(III) onto clay minerals under saline conditions: Batch adsorption, laser-fluorescence spectroscopy and modeling. Geochim. Cosmochim. Acta 2015, 151, 192–202. [Google Scholar] [CrossRef]
- Virtanen, S.; Meriläinen, S.; Eibl, M.; Rabung, T.; Lehto, J.; Huittinen, N. Sorption competition and kinetics of trivalent cations (Eu, Y and Cm) on corundum (α-Al2O3): A batch sorption and TRLFS study. Appl. Geochem. 2018, 92, 71–81. [Google Scholar] [CrossRef]
- Eibl, M.; Virtanen, S.; Pischel, F.; Bok, F.; Lönnrot, S.; Shaw, S.; Huittinen, N. A spectroscopic study of trivalent cation (Cm3+ and Eu3+) sorption on monoclinic zirconia (ZrO2). Appl. Surf. Sci. 2019, 487, 1316–1328. [Google Scholar] [CrossRef]
- Neumann, J.; Brinkmann, H.; Britz, S.; Lutzenkirchen, J.; Bok, F.; Stockmann, M.; Brendler, V.; Stumpf, T.; Schmidt, M. A comprehensive study of the sorption mechanism and thermodynamics of f-element sorption onto K-feldspar. J. Colloid Interface Sci. 2021, 591, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Kent, D.B. Surface Complexation Modeling in Aqueous Geochemistry. Rev. Mineral. 1990, 23, 177–260. [Google Scholar]
- Ochs, M.; Davis, J.A.; Olin, M.; Payne, T.E.; Tweed, C.J.; Askarieh, M.M.; Altmann, S. Use of thermodynamic sorption models to derive radionuclide Kd values for performance assessment: Selected results and recommendations of the NEA sorption project. Radiochim. Acta 2006, 94, 779–785. [Google Scholar] [CrossRef]
- Fedoroff, M.; Lefevre, G.; Duc, M.; Milonjić, S.; Nešković, C. Sorption mechanisms and sorption models. Mater. Sci. Forum 2004, 453–454, 305. [Google Scholar] [CrossRef]
- Payne, T.E.; Brendler, V.; Comarmond, M.J.; Nebelung, C. Assessment of surface area normalisation for interpreting distribution coefficients (Kd) for uranium sorption. J. Environ. Radioact. 2011, 102, 888–895. [Google Scholar] [CrossRef]
- Stockmann, M.; Schikora, J.; Becker, D.A.; Flugge, J.; Noseck, U.; Brendler, V. Smart Kd-values, their uncertainties and sensitivities—Applying a new approach for realistic distribution coefficients in geochemical modeling of complex systems. Chemosphere 2017, 187, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kulik, D.; Berner, U.; Curti, E. Modelling Chemical Equilibrium Partitioning with the GEMS-PSI Code; Paul Scherrer Institute: Villigen, Switzerland, 2004; pp. 109–122. [Google Scholar]
- Rabung, T.; Stumpf, T.; Geckeis, H.; Klenze, R.; Kim, J.I. Sorption of Am(III) and Eu(III) onto γ-alumina: Experiment and modelling. Radiochim. Acta 2000, 88, 711–716. [Google Scholar] [CrossRef]
- Rabung, T.; Geckeis, H.; Wang, X.K.; Rothe, J.; Denecke, M.A.; Klenze, R.; Fanghänel, T. Cm(III) sorption onto γ-Al2O3: New insight into sorption mechanisms by time-resolved laser fluorescence spectroscopy and extended X-ray absorption fine structure. Radiochim. Acta 2006, 94, 609–618. [Google Scholar] [CrossRef]
- Rabung, T.; Geckeis, H.; Kim, J.I.; Beck, H.P. Sorption of Eu(III) on a Natural Hematite: Application of a Surface Complexation Model. J. Colloid Interface Sci. 1998, 208, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pourret, O.; Guo, H.; Bonhoure, J. Rare earth elements sorption to iron oxyhydroxide: Model development and application to groundwater. Appl. Geochem. 2017, 87, 158–166. [Google Scholar] [CrossRef]
- Pourret, O.; Davranche, M. Rare earth element sorption onto hydrous manganese oxide: A modeling study. J. Colloid Interface Sci. 2013, 395, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bouby, M.; Lützenkirchen, J.; Dardenne, K.; Preocanin, T.; Denecke, M.A.; Klenze, R.; Geckeis, H. Sorption of Eu(III) onto titanium dioxide: Measurements and modeling. J. Colloid Interface Sci. 2010, 350, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Ridley, M.K.; Hiemstra, T.; Machesky, M.L.; Wesolowski, D.J.; van Riemsdijk, W.H. Surface speciation of yttrium and neodymium sorbed on rutile: Interpretations using the charge distribution model. Geochim. Cosmochim. Acta 2012, 95, 227–240. [Google Scholar] [CrossRef]
- Kasar, S.; Kumar, S.; Kar, A.S.; Godbole, S.V.; Tomar, B.S. Sorption of Eu(III) by amorphous titania, anatase and rutile: Denticity difference in surface complexes. Colloids Surf. A Physicochem. Eng. 2013, 434, 72–77. [Google Scholar] [CrossRef]
- Den Auwer, C.; Drot, R.; Simoni, E.; Conradson, S.D.; Gailhanou, M.; Mustre de Leon, J. Grazing incidence XAFS spectroscopy of uranyl sorbed onto TiO2 rutile surfaces. New J. Chem. 2003, 27, 648–655. [Google Scholar] [CrossRef]
- Marques Fernandes, M.; Scheinost, A.C.; Baeyens, B. Sorption of trivalent lanthanides and actinides onto montmorillonite: Macroscopic, thermodynamic and structural evidence for ternary hydroxo and carbonato surface complexes on multiple sorption sites. Water Res. 2016, 99, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, M.; Pashalidis, I. Competitive sorption of Cu(II), Eu(III) and U(VI) ions on TiO2 in aqueous solutions—A potentiometric study. Colloids Surf. A Physicochem. Eng. 2008, 324, 217–221. [Google Scholar] [CrossRef]
- Gou, W.; Ji, J.; Li, W. An EXAFS investigation of the mechanism of competitive sorption between Co(II) and Ni(II) at γ-alumina/solution interface. Acta Geochim. 2017, 36, 462–464. [Google Scholar] [CrossRef]
- Sun, Q.; Cui, P.-X.; Fan, T.-T.; Wu, S.; Zhu, M.; Alves, M.E.; Zhou, D.-M.; Wang, Y.-J. Effects of Fe(II) on Cd(II) immobilization by Mn(III)-rich δ-MnO2. Chem. Eng. J. 2018, 353, 167–175. [Google Scholar] [CrossRef]
- Bozena, G.; Zakrzewska, D.; Szymczycha, B. Sorption of Cr, Pb, Cu, Zn, Cd, Ni, and Co to nano-TiO2 in seawater. Water Sci. Technol. 2018, 77, 145–158. [Google Scholar] [CrossRef]
- Polly, R.; Schimmelpfennig, B.; Flörsheimer, M.; Rabung, T.; Kupcik, T.; Klenze, R.; Geckeis, H. Quantum chemical study of inner-sphere complexes of trivalent lanthanide and actinide ions on the corundum (110) surface. Radiochim. Acta 2013, 101, 561–570. [Google Scholar] [CrossRef]
- Polly, R.; Schimmelpfennig, B.; Rabung, T.; Flörsheimer, M.; Klenze, R.; Geckeis, H. Quantum chemical study of inner-sphere complexes of trivalent lanthanide and actinide ions on the corundum (0001) surface. Radiochim. Acta 2010, 98, 627–634. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–289. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Treutler, O.; Ahlrichs, R. Efficient molecular numerical integration schemes. J. Chem. Phys. 1995, 102, 346–354. [Google Scholar] [CrossRef]
- Von Arnim, M.; Ahlrichs, R. Performance of parallel TURBOMOLE for density functional calculations. J. Comput. Chem. 1998, 19, 1746–1757. [Google Scholar] [CrossRef]
- Sierka, M.; Hogekamp, A.; Ahlrichs, R. Fast evaluation of the Coulomb potential for electron densities using multipole accelerated resolution of identity approximation. J. Chem. Phys. 2003, 118, 9136–9148. [Google Scholar] [CrossRef]
- Moritz, A.; Cao, X.Y.; Dolg, M. Quasirelativistic energy-consistent 5f-in-core pseudopotentials for trivalent actinide elements. Theor. Chem. Acc. 2007, 117, 473–481. [Google Scholar] [CrossRef]
- Kupcik, T.; Rabung, T.; Lützenkirchen, J.; Finck, N.; Geckeis, H.; Fanghänel, T. Macroscopic and spectroscopic investigations on Eu(III) and Cm(III) sorption onto bayerite (β-Al(OH)3) and corundum α-Al2O3). J. Colloid Interface Sci. 2016, 461, 215–224. [Google Scholar] [CrossRef]
- Roques, J.; Veilly, E.; Simoni, E. Periodic density functional theory investigation of the uranyl ion sorption on three mineral surfaces: A comparative study. Int. J. Mol. Sci. 2009, 10, 2633–2661. [Google Scholar] [CrossRef]
- Geckeis, H.; Lützenkirchen, J.; Polly, R.; Rabung, T.; Schmidt, M. Mineral-water interface reactions of actinides. Chem. Rev. 2013, 113, 1016–1062. [Google Scholar] [CrossRef]
- Perron, H.; Vandenborre, J.; Domain, C.; Drot, R.; Roques, J.; Simoni, E.; Ehrhardt, J.J.; Catalette, H. Combined investigation of water sorption on TiO2 rutile (110) single crystal face: XPS vs. periodic DFT. Surf. Sci. 2007, 601, 518–527. [Google Scholar] [CrossRef]
- Kremleva, A.; Krüger, S.; Rösch, N. Uranyl adsorption at (010) edge surfaces of kaolinite: A density functional study. Geochim. Cosmochim. Acta 2011, 75, 706–718. [Google Scholar] [CrossRef]
- Janeček, J.; Netz, R.R.; Flörsheimer, M.; Klenze, R.; Schimmelpfennig, B.; Polly, R. Influence of hydrogen bonding on the structure of the (001) corundum-water interface. Density functional theory calculations and Monte Carlo simulations. Langmuir 2014, 30, 2722–2728. [Google Scholar] [CrossRef]
- Martorell, B.; Kremleva, A.; Krüger, S.; Rösch, N. Density Functional Model Study of Uranyl Adsorption on the Solvated (001) Surface of Kaolinite. J. Phys. Chem. C 2010, 114, 13287–13294. [Google Scholar] [CrossRef]
- Virtanen, S.; Bok, F.; Ikeda-Ohno, A.; Rossberg, A.; Lützenkirchen, J.; Rabung, T.; Lehto, J.; Huittinen, N. The specific sorption of Np(V) on the corundum (α-Al2O3) surface in the presence of trivalent lanthanides Eu(III) and Gd(III): A batch sorption and XAS study. J. Colloid Interface Sci. 2016, 483, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Kretzschmar, J.; Drobot, B. Not just a background: pH buffers do interact with lanthanide ions-a Europium(III) case study. J. Biol. Inorg. Chem. 2022, 27, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Scheinost, A.C.; Claussner, J.; Exner, J.; Feig, M.; Findeisen, S.; Hennig, C.; Kvashnina, K.O.; Naudet, D.; Prieur, D.; Rossberg, A.; et al. ROBL-II at ESRF: A synchrotron toolbox for actinide research. J. Synchrotron Rad. 2021, 28, 333–349. [Google Scholar] [CrossRef] [PubMed]
- George, G.N.; Pickering, I.J. EXAFSPAK: A Suite of Computer Programs for Analysis of X-ray Absorption Spectra; Stanford Synchrotron Radiation Laboratory, Stanford Linear Accelerator Center: Menlo Park, CA, USA, 1995. [Google Scholar]
- Ressler, T. WinXAS: A Program for X-ray Absorption Spectroscopy Data Analysis under MS-Windows. J. Synchrotron Rad. 1998, 5, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- David, F.H.; Vokhmin, V. Thermodynamic properties of some tri- and tetravalent actinide aquo ions. New J. Chem. 2003, 27, 1627–1632. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Baeyens, B.; Bradbury, M.H. A mechanistic description of Ni and Zn sorption on Na-montmorillonite Part I: Titration and sorption measurements. J. Contam. Hydrol. 1997, 27, 199–222. [Google Scholar] [CrossRef]
- Kosmulski, M. The Effect of the Ionic Strength on the Adsorption Isotherms of Nickel on Silica. J. Colloid Interface Sci. 1997, 190, 212–223. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B. Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montmorillonite: Linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochim. Cosmochim. Acta 2005, 69, 875–892. [Google Scholar] [CrossRef]
- Dähn, R.; Baeyens, B.; Marques Fernandes, M. Zn uptake by illite and argillaceous rocks. Geochim. Cosmochim. Acta 2021, 312, 180–193. [Google Scholar] [CrossRef]
- Li, G.G.; Bridges, F.; Booth, C.H. X-ray-absorption fine structure standards: A comparison of experiment and theory. Phys. Rev. B 1995, 52, 6332–6348. [Google Scholar] [CrossRef] [PubMed]
- Aidhy, D.S.; Zhang, Y.W.; Weber, W.J. Radiation damage in cubic ZrO2 and yttria-stabilized zirconia from molecular dynamics simulations. Scr. Mater. 2015, 98, 16–19. [Google Scholar] [CrossRef][Green Version]
- Purans, J.; Afify, N.D.; Dalba, G.; Grisenti, R.; De Panfilis, S.; Kuzmin, A.; Ozhogin, V.I.; Rocca, F.; Sanson, A.; Tiutiunnikov, S.I.; et al. Isotopic effect in extended x-ray-absorption fine structure of germanium. Phys. Rev. Lett. 2008, 100, 055901. [Google Scholar] [CrossRef]
- Taube, F.; Drobot, B.; Rossberg, A.; Foerstendorf, H.; Acker, M.; Patzschke, M.; Trumm, M.; Taut, S.; Stumpf, T. Thermodynamic and Structural Studies on the Ln(III)/An(III) Malate Complexation. Inorg. Chem. 2019, 58, 368–381. [Google Scholar] [CrossRef]
- Stumpf, T.; Hennig, C.; Bauer, A.; Denecke, M.A.; Fanghänel, T. An EXAFS and TRLFS study of the sorption of trivalent actinides onto smectite and kaolinite. Radiochim. Acta 2004, 92, 133–138. [Google Scholar] [CrossRef]
- Stumpf, S.; Stumpf, T.; Dardenne, K.; Hennig, C.; Foerstendorf, H.; Klenze, R.; Fanghänel, T. Sorption of Am(III) onto 6-line-Ferrihydrite and Its Alteration Products: Investigations by EXAFS. Environ. Sci. Technol. 2006, 40, 3522–3528. [Google Scholar] [CrossRef]
- Morelova, N.; Finck, N.; Lutzenkirchen, J.; Schild, D.; Dardenne, K.; Geckeis, H. Sorption of americium/europium onto magnetite under saline conditions: Batch experiments, surface complexation modelling and X-ray absorption spectroscopy study. J. Colloid Interface Sci. 2020, 561, 708–718. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, D.; Jin, Q.; Chen, Z.; Wang, D.; Guo, Z.; Wu, W. Multi-scale study of Am(III) adsorption on Gaomiaozi bentonite: Combining experiments, modeling and DFT calculations. Chem. Geol. 2021, 581, 120414. [Google Scholar] [CrossRef]
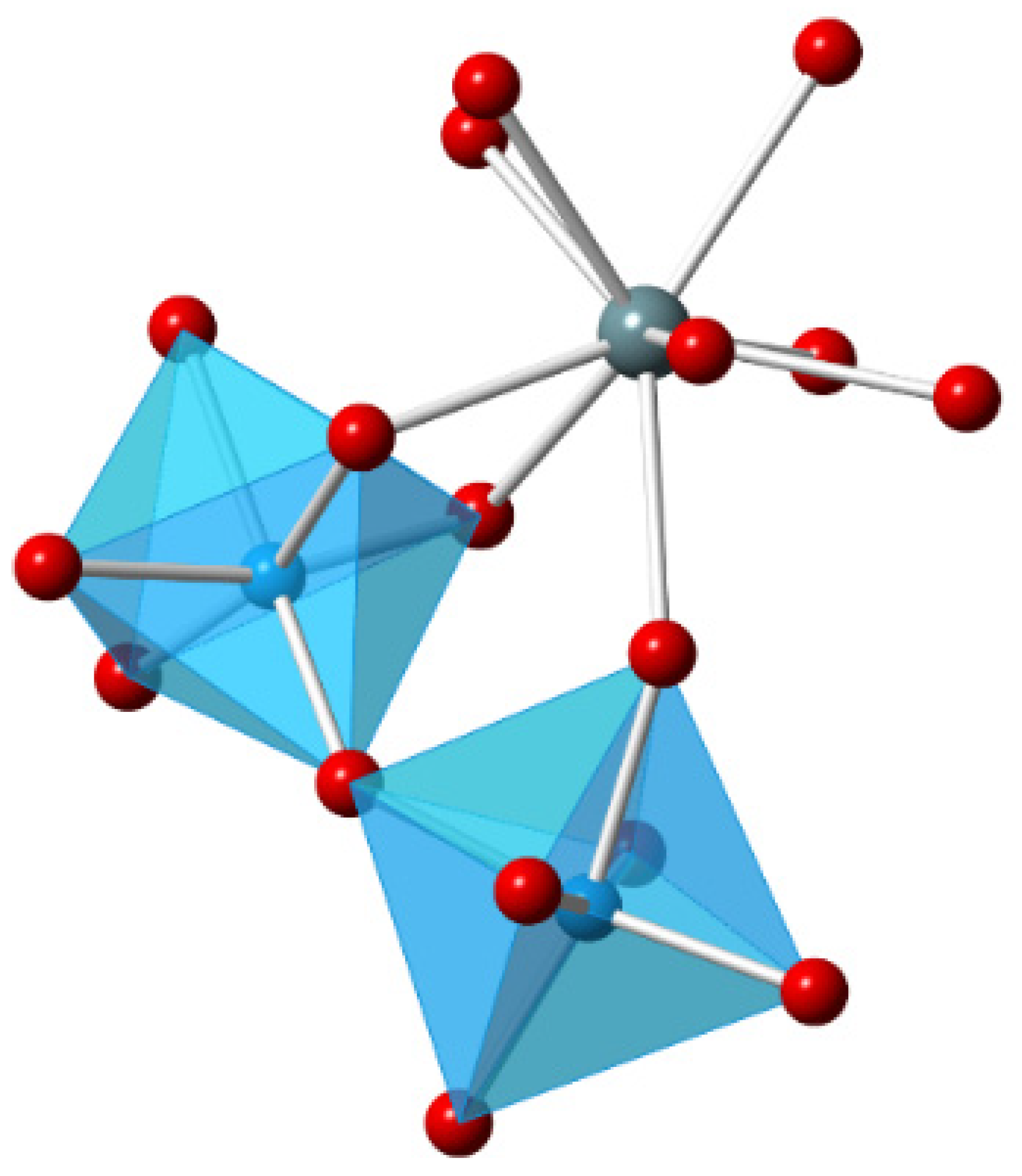
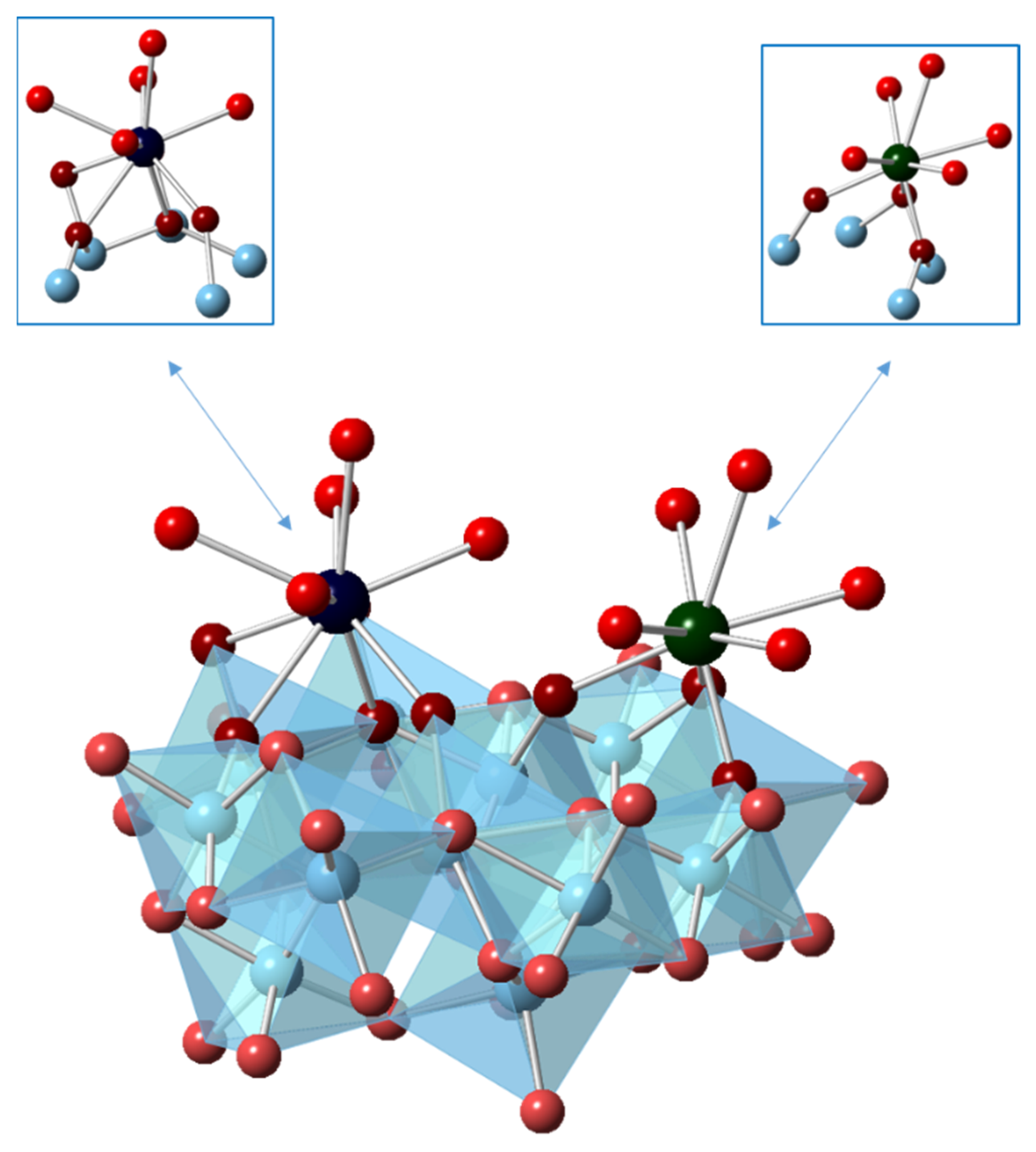
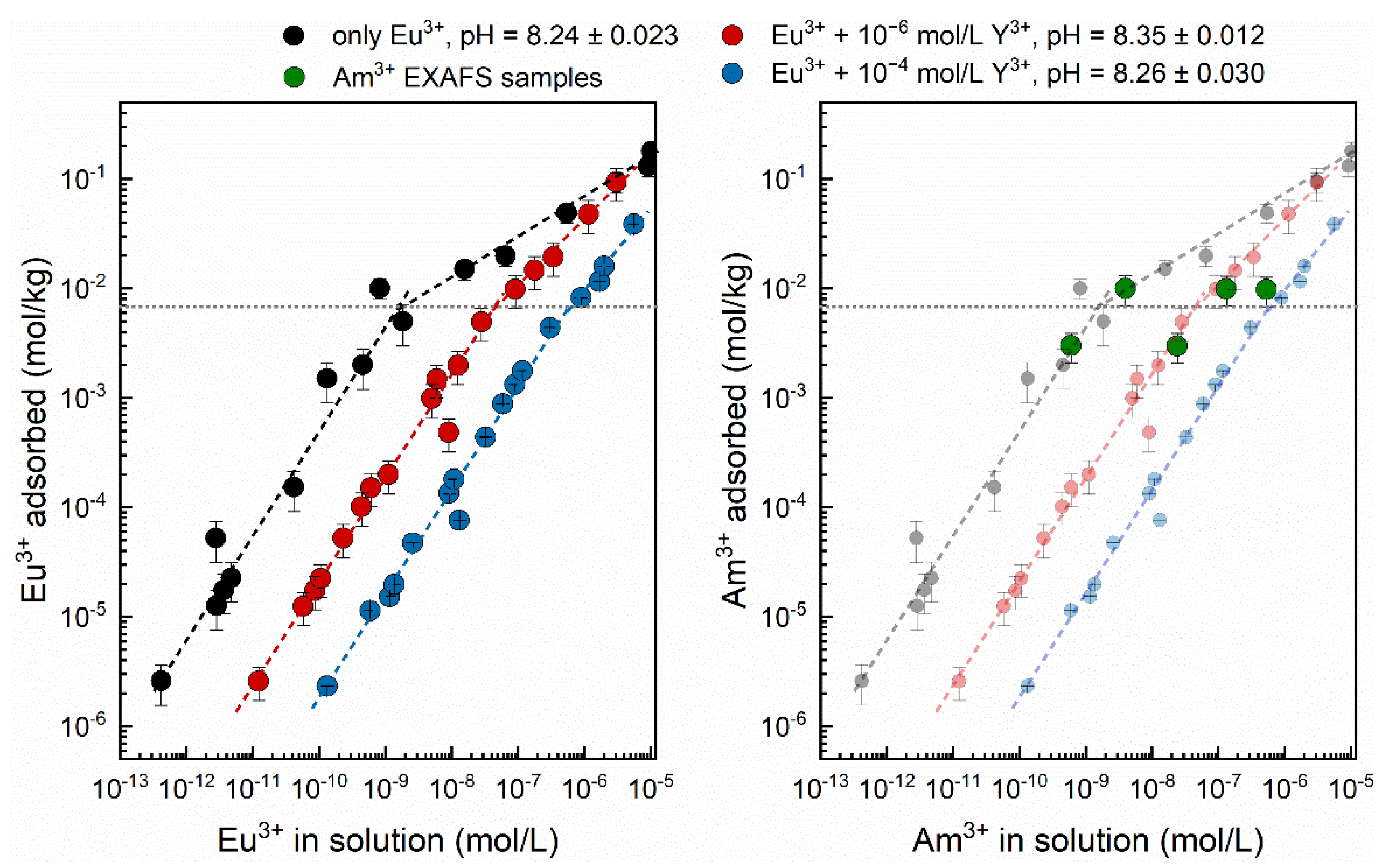
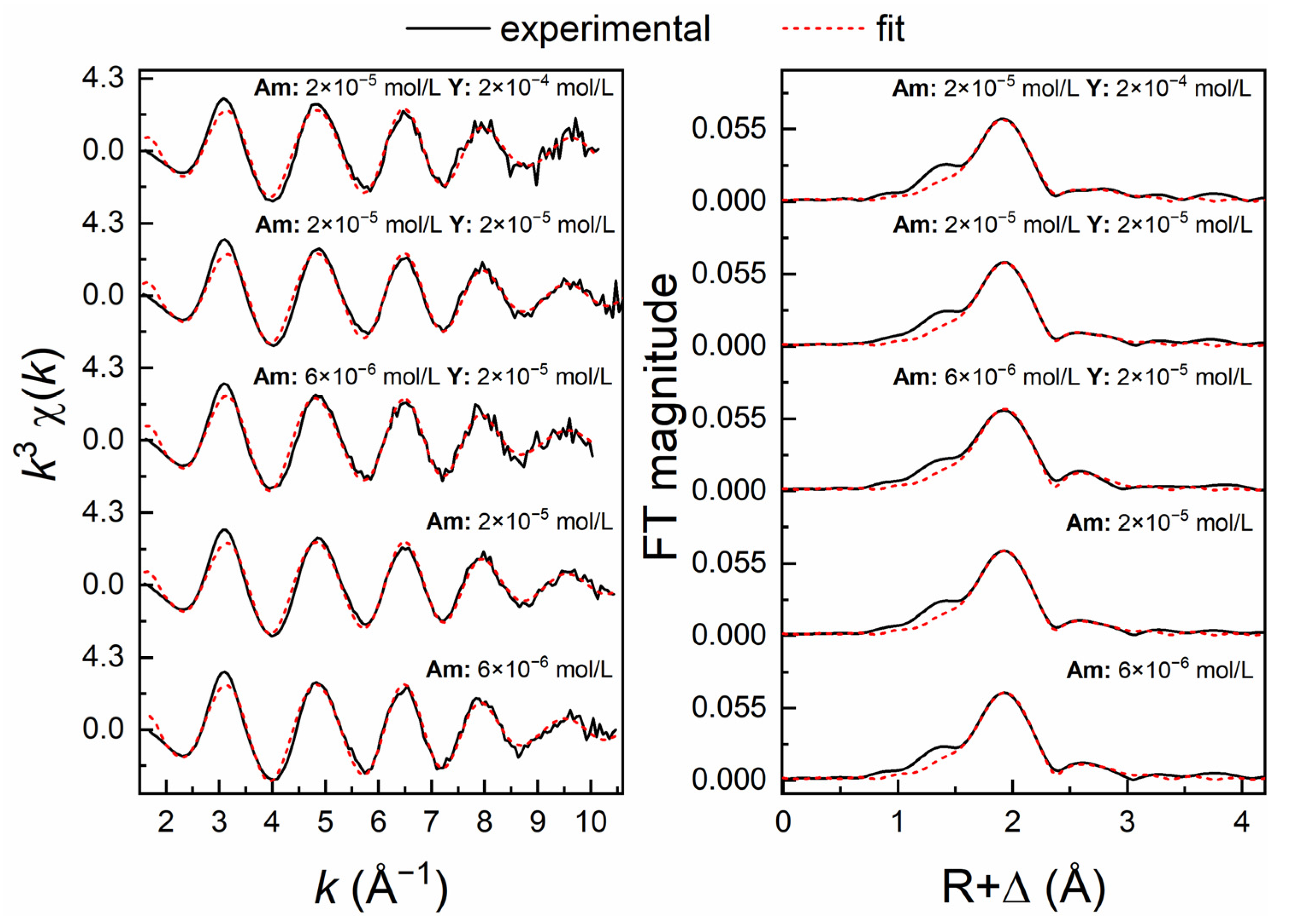
| Isotherm | c(Eu3+) (mol/L) | c(Y3+) (mol/L) | n(M3+tot)/m(Al2O3) (mol/g) | pHeq |
| 1 | 1.3 × 10−9–1.0 × 10−4 | 0 | 2.6 × 10−9–2.0 × 10−4 | 8.24 ± 0.023 |
| 2 | 1.3 × 10−9–5.0 × 10−5 | 1 × 10−6 | 2.0 × 10−6–1.02 × 10−4 | 8.35 ± 0.012 |
| 3 | 1.3 × 10−9–2.5 × 10−5 | 1 × 10−4 | 2.0 × 10−4–2.5 × 10−4 | 8.26 ± 0.030 |
| EXAFS Sample | c(243Am3+) (mol/L) | c(Y3+) (mol/L) | n(M3+tot)/m(Al2O3) (mol/g) | pHeq |
| 1 | 6 × 10−6 | 0 | 3 × 10−6 | 8.41 |
| 2 | 2 × 10−5 | 0 | 1 × 10−5 | 8.46 |
| 3 | 6 × 10−6 | 2 × 10−5 | 1.3 × 10−5 | 8.47 |
| 4 | 2 × 10−5 | 2 × 10−5 | 2 × 10−5 | 8.48 |
| 5 | 2 × 10−5 | 2 × 10−4 | 1.1 × 10−4 | 8.50 |
| Tetradentate Am Complex (CN 9) | Tridentate Am Complex (CN 8) | ||
|---|---|---|---|
| Am–Owater (Å) | Am–Osurface (Å) | Am–Owater (Å) | Am–Osurface (Å) |
| 2.46 | 2.39 | 2.45 | 2.27 |
| 2.46 | 2.46 | 2.523 | 2.33 |
| 2.52 | 2.57 | 2.57 | 2.44 |
| 2.56 | 2.59 | 2.60 | |
| 2.64 | 2.66 | ||
| Ø 2.53 | Ø 2.50 | Ø 2.56 | Ø 2.35 |
| Øall 2.52 | Øall 2.49 | ||
| Tetradentate Am Complex | Am–Al (Å) | Am–Al (Å) | Am–Al (Å) | Tridentate Am Complex | Am–Al (Å) | Am–Al (Å) |
|---|---|---|---|---|---|---|
| Am–O1 | 3.12 | Am–O1 | 3.32 | 3.73 | ||
| Am–O2 | 3.12 | 4.26 | Am–O2 | 3.32 | 4.23 | |
| Am–O3 | 3.12 | 3.93 | 3.97 | Am–O3 | 3.91 | |
| Am–O4 | 4.20 | |||||
| weighted Ø | 3.67 | weighted Ø | 3.74 | |||
| Sample Composition | Shell | CN | R (Å) | σ2 (Å2) | ΔE0 (eV) | R (%) |
|---|---|---|---|---|---|---|
| Sample 1 6 × 10−6 mol/L Am | Am–O | 9.0 (3) | 2.478 (3) | 0.0109 (6) | 5.07 (17) | 5.06 |
| Am–Al | 2.9 (7) | 3.228 (10) | 0.0122(30) | |||
| Sample 2 2 × 10−5 mol/L Am | Am–O | 8.5 (3) | 2.474 (2) | 0.0107 (5) | 4.67 (15) | 4.18 |
| Am–Al | 2.8 (7) | 3.225 (9) | 0.0140 (30) | |||
| Sample 3 6 × 10−6 mol/L Am + 2 × 10−5 mol/L Y | Am–O | 8.8 (5) | 2.478 (4) | 0.0116 (7) | 4.83 (16) | 7.35 |
| Am–Al | 3.0 (7) | 3.210 (10) | 0.0134 (33) | |||
| Sample 4 2 × 10−5 mol/L Am + 2 × 10−5 mol/L Y | Am–O | 8.4 (3) | 2.475 (2) | 0.0107 (4) | 4.81 (14) | 3.59 |
| Am–Al | 3.2 (8) | 3.235 (9) | 0.0165 (33) | |||
| Sample 5 2 × 10−5 mol/L Am + 2 × 10−4 mol/L Y | Am–O | 8.2 (3) | 2.467 (2) | 0.0105 (5) | 4.01 (15) | 4.17 |
| Am–Al | 2.3 (6) | 3.239 (7) | 0.0115 (27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huittinen, N.; Virtanen, S.; Rossberg, A.; Eibl, M.; Lönnrot, S.; Polly, R. A Combined Extended X-ray Absorption Fine Structure Spectroscopy and Density Functional Theory Study of Americium vs. Yttrium Adsorption on Corundum (α–Al2O3). Minerals 2022, 12, 1380. https://doi.org/10.3390/min12111380
Huittinen N, Virtanen S, Rossberg A, Eibl M, Lönnrot S, Polly R. A Combined Extended X-ray Absorption Fine Structure Spectroscopy and Density Functional Theory Study of Americium vs. Yttrium Adsorption on Corundum (α–Al2O3). Minerals. 2022; 12(11):1380. https://doi.org/10.3390/min12111380
Chicago/Turabian StyleHuittinen, Nina, Sinikka Virtanen, André Rossberg, Manuel Eibl, Satu Lönnrot, and Robert Polly. 2022. "A Combined Extended X-ray Absorption Fine Structure Spectroscopy and Density Functional Theory Study of Americium vs. Yttrium Adsorption on Corundum (α–Al2O3)" Minerals 12, no. 11: 1380. https://doi.org/10.3390/min12111380
APA StyleHuittinen, N., Virtanen, S., Rossberg, A., Eibl, M., Lönnrot, S., & Polly, R. (2022). A Combined Extended X-ray Absorption Fine Structure Spectroscopy and Density Functional Theory Study of Americium vs. Yttrium Adsorption on Corundum (α–Al2O3). Minerals, 12(11), 1380. https://doi.org/10.3390/min12111380






