Abstract
Critical metallic elements in coal gangue have great utilization potential, especially due to the current shortage of these metals. This paper focused on examining the feasibility of physical separation (screening and float-sink tests) and calcination treatment for the enrichment of critical elements (Li, Ga, and rare earth elements plus yttrium (REY)) from coal gangue. The impacts of these enrichment methods on the acid leaching recovery of these elements were then studied. Screening tests indicated that Li and Ga were enriched in >0.125 mm size fraction and the content of REY was highest in <75 μm size fraction. Float-sink tests showed that high-density fractions were enriched in Li and Ga, and low-density fractions were enriched in REY. Physical separation cannot significantly improve the leaching rate of Li, Ga, and REY. Notably, Li, Ga, and REY were enriched significantly, and their acid leaching recoveries were increased by 54~68% after calcination under 400 °C. Sequential chemical extraction tests showed that the majority of insoluble Li, Ga, and REY was converted into soluble forms at the above temperature, which is attributed to the formation of amorphous metakaolinite and the decomposition of organic matter. Based on the results, a conceptually combined flowsheet was proposed for the extraction of Li and Ga from coal gangue.
1. Introduction
Strategic critical metals, defined as metallic elements critical to the national economy and security, mainly include rare metals, rare earth metals, and scattered metals, and have attracted unprecedented attention from many countries in recent years [1,2,3]. These elements play vital roles in emerging industries, such as new energy, new materials, digital technologies, clean technologies, and aerospace technologies [4,5,6]. Especially, Li is mainly used for the manufacture of lithium batteries, and Ga is an important raw material for semiconductors. The government of Japan established the Rare Metals Security Strategy list containing 31 metals in 2009 [2]. Subsequently, the United States and the European Union proposed similar strategies to deal with potential supply risks of critical metals. In China, the critical metals that are likely to face a tight supply situation mainly include Li, Be, Nb, and so on [4]. With the rapid development of modern technology, strategic critical metals will continue to gain importance. Moreover, due to the increasing demand for critical metals and the gradual exhaustion of traditional critical metal resources, several studies have evaluated the feasibility of recovering critical metals from other sources such as coal and its by-products [7,8,9,10,11,12]. There are abundant reserves of critical metals in many coalfields [13,14,15,16], which have great prospects for development and utilization.
Critical metals can be enriched in coal deposits under some specific geological and geochemical conditions [17,18,19,20,21]. The abnormal accumulations of critical metallic elements in coal deposits were reported in several countries including the United States, Russia, and China [22,23]. Some reports indicate that several coal deposits in Inner Mongolia, north China, are significantly rich in critical metals, such as Jungar coalfield [24,25] (Heidaigou mine [26], Guanbanwusu mine [27]), and Daqingshan coalfield [13]. In addition, coal seams enriched with critical elements have also been found in Sichuan [28,29], Chongqing [30], and Guizhou province [31], southwestern China. Critical metallic elements in coal can occur in organic or inorganic form, but most of them are closely associated with inorganic matter [11,24,32,33]. As a result, the concentration of critical elements in the coal gangues (e.g., floor, roof, and partings) may be several times higher than that in the coals [28,29,34]. Currently, there are relatively few studies on the enrichment and recovery of critical elements from coal gangue. Certain critical elements are enriched in the specific products during the coal separation process [35]. If the critical elements in the coal gangue can be further enriched by this similar process, it is of great significance for the recovery of these elements from coal gangue.
Physical separation methods have been widely applied in coal preparation processes for reducing mineral components in coal and increasing its heating value [36]. These methods mainly include gravity separation (also called density separation), particle size separation, and magnetic separation. In recent years, some studies have explored the distribution and occurrence of critical elements in coal fly ash using these methods [37,38,39,40,41]. However, applications of these methods in the enrichment and recovery of critical elements from coal gangue have been reported scarcely [42]. For instance, Lin et al. [42] found that rare earth elements from clay and shale samples (coal mining by-products) were more concentrated in coarser size fractions and higher density fractions. But the leachability of rare earth elements in these fractions was not analyzed. Further research into the impact of physical separation on the extraction of critical elements is crucial.
Although coal gangue is a coal mining and processing by-product, there is still some organic matter in it. Therefore, the calcination method can be used to enrich the critical elements from coal gangue by removing organic matter [43]. Meanwhile, calcination treatment can also improve the chemical activity of coal gangue, which is beneficial to the subsequent extraction of critical elements [44,45,46]. In addition, the sequential chemical extraction method has been viewed as the most appropriate method to quantitatively analyze the occurrence of trace elements [40,47,48,49]. This method can also provide some significant insight into the transformation mechanism of critical elements during calcination.
Based on the concerns mentioned above, this paper focused on studying the technical feasibility of calcination and physical separation techniques (including particle size separation and density separation, while magnetic separation was not used because the coal gangue was extremely weakly magnetic) for the enrichment of Li, Ga, and REY from coal gangue. The effects of these enrichment methods on the recovery of Li, Ga, and REY were evaluated by acid leaching tests. The positive impacts of calcination treatment for the extraction of Li, Ga, and REY were further investigated. Based on the obtained results, a conceptual “enrichment and extraction” flowsheet was proposed.
2. Materials and Methods
2.1. Materials
The coal gangue sample was collected from a coal preparation plant in Inner Mongolia. Chinese Standard GB/T 19494.1–2004 was chosen as the sampling method. The feed coal for the plant is derived from the Heidaigou Openpit Coal Mine, and the coal seam belongs to the Early Permian coal [50]. Samples were crushed and ground into fine particles with two different target particle size ranges prior to physical separation and stored in airtight plastic bags. Among them, the samples with a maximum particle size of 0.5 mm were prepared as feedstocks for particle size separation according to Chinese Standard GB/T 477–2008, and other samples with a particle size smaller than 74 μm were used for density separation, the calcination enrichment tests as well as sample characterization [44]. The reason for choosing sample sizes smaller than 74 μm for density separation is to maximize the liberation of minerals and organic matter before separation.
The chemical reagents used for the acid leaching and sequential chemical extraction tests were analytical grade. Ultrapure water (resistivity = 18.20 MΩ × cm) was utilized for all tests. Tetrachloromethane and tribromomethane were used as separation media in the float-sink tests.
2.2. Elemental Analysis
The critical elements in the samples were measured using an inductively coupled plasma mass spectrometer (ICP-MS, iCAP RQ, Thermo Scientific, Waltham, MA, USA). Before ICP-MS analysis, the solid samples need to be dissolved in mixed acid solution via microwave digestion. For the digestion process, 0.05 g sample was mixed with 5 mL of concentrated nitric acid, 4 mL of concentrated hydrofluoric acid, and 1 mL hydrogen peroxide in each Teflon digestion tank, and then these tanks were placed in the microwave digestion instrument (ETHOS UP, Milestone, Italy). Digestion was carried out according to the specific program shown in Table 1. After digestion, the tanks were transferred to an electric hot plate at 220 °C and 1 mL of concentrated perchloric acid was added to each tank. The purpose of the process is to evaporate the hydrofluoric acid to prevent it from affecting the ICP-MS tests. After approximately 3 h, the solution in the tank was concentrated to the size of a soybean (its volume corresponded approximately to the added perchloric acid), 10 mL of concentrated nitric acid was added into the tank, and then the hot plate temperature was adjusted to 80 °C and held for 2 h. After that, the digestion tanks were taken out and cooled to room temperature. Finally, the solution in the tank was transferred into a Teflon volumetric flask and diluted with ultrapure water to 100 mL for ICP-MS analysis.

Table 1.
Microwave digestion program.
To verify the accuracy of this digestion method, the standard samples (GBW07109) supplied by the National Standard Material Center of China were treated with target samples under the same condition, and then the elemental content was determined using ICP-MS. All ratios (the measured content to the value provided by the supplier) were between 90% and 110% and the relative standard deviation values were less than 5%, which met the requirements.
2.3. Physical Separation
The distribution of Li, Ga, and REY in coal gangue as a function of particle size and density was studied using screening and float-sink tests to find a suitable method for the preconcentration of these elements. Particle size separation was carried out by screening tests according to Chinese Standard GB/T 477–2008. The samples were sieved into four particle size fractions of 0.5~0.25, 0.25~0.125, 0.125~0.075, and <0.075 mm. Density separation was conducted based on the conventional float-sink method. Organic liquids, namely tetrachloromethane and tribromomethane, were used as separation media to prepare heavy liquids with different specific gravity. Through float-sink tests, the fractions of different densities were obtained as <2.0, 2.0~2.2, 2.2~2.4, 2.4~2.6 and >2.6 g/cm3. All fraction samples obtained by physical separation were listed in Table 2.

Table 2.
Sample information of physical separation and calcination.
2.4. Calcination
Coal gangue samples were calcined for two hours with the aim of removing organic matter. All calcined products are shown in Table 2. For each calcination test, a crucible rack loaded with ten crucibles (each crucible contained 1 g of samples) was placed in the muffle furnace that had reached the target temperature. A static air condition was maintained throughout the calcination process. After the calcination was stopped, the crucible rack was quickly removed from the furnace, and the calcined products were naturally cooled to room temperature. Subsequently, products were weighed and stored in a desiccator.
2.5. Acid Leaching
The recoverability of critical metals in coal gangue obtained by physical separation and calcination treatment was evaluated by acid leaching tests under the same conditions. Leaching tests were conducted in a 100 mL round bottom flask, which was immersed in a constant-temperature water bath tank equipped with a magnetic stirring function. For each test, 50 mL of 2 M hydrochloric acid solution was added to each 5 g sample in a flask and then leached at 60 °C for 4 h. The above conditions were chosen based on the optimal conditions of Zhang et al. [44]. After the leaching test, slurries in the flask were filtered using filter paper, and the solid residue was washed with ultrapure water three times at the same time. The concentration of Li, Ga, and REY in the leaching solution was determined using ICP-MS.
2.6. Sequential Chemical Extraction Procedure
The sequential chemical extraction procedure was often used as an indirect method to study the modes of occurrence of trace elements in the coal and coal fly ash [51]. Since the calcination treatment could greatly improve the recovery of Li, Ga, and REY, the changes in their occurrence mode were investigated by the sequential chemical extraction tests. In this study, a four-step sequential chemical extraction procedure was formulated following the methodology described by Pan et al. [40,41]. The critical elements Li, Ga, and REY in the raw materials and calcined products were classified into the following five modes of occurrence: ion-exchangeable, carbonate-bound, metal oxide-bound, organic matter-bound, and insoluble. At the beginning of the test, 1 g sample was transferred to a 50 mL centrifuge tube and then 10 mL of 1 M magnesium chloride solution was added. Subsequently, the tube was shaken for 1 h by the shaking table operating at 180 rpm and 25 °C. The leaching suspension in the tube was centrifuged and filtered to obtain the leachate, and the remaining solid was washed three times with ultrapure water. The solid residue was submitted to the next step of this procedure, and the wash water was recycled into leachate. Similar operations were carried out sequentially to extract the rest species, according to the conditions described in Figure 1. After the organic matter-bound extraction step, the solid residue was digested as the described method in Section 2.2. The concentrations of Li, Ga and REY in the leachate of each step and the digestion solution of residue were measured using ICP-MS. For each sample, the tests were repeated three times to obtain the mean.
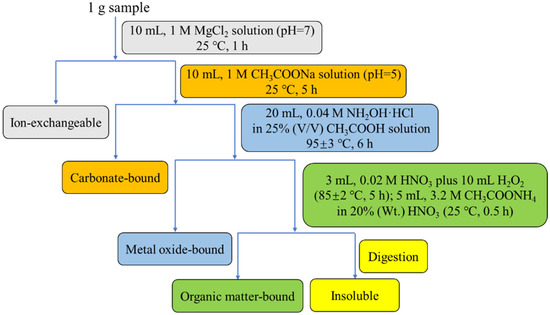
Figure 1.
Diagram of the sequential chemical extraction procedure.
2.7. Sample Characterization
Mineral phase analysis was performed using X-ray diffraction (XRD, D8 Advance, Bruker AXS Corporation, Billerica, MA, USA). The XRD patterns were recorded from 5° to 90° with a step size of 0.02°. X-ray fluorescence spectroscopy (XRF, S8 Tiger, Bruker AXS Corporation) was used to determine the content of the major chemical composition. Scanning electron microscopy (SEM, ZEISS ΣIGMA, Oberkochen, Germany) equipped with energy dispersive X-ray spectroscopy (EDS, Oxford X-MaxN 20) (collectively, SEM-EDS, Oxford, UK) was applied to characterize the morphology and elemental composition of coal gangue particles. Point mode was employed in the work for elemental analysis, and images were captured via a retractable solid-state backscatter electron detector. SEM-EDS observation samples were prepared by sprinkling the coal gangue particles onto double-sided carbon tape mounted on an SEM stub. The samples for SEM-EDS analysis were not previously covered. The functional groups of the samples were investigated by Fourier transform infrared spectroscopy (FT-IR, VERTEX 80v, Bruker, Oberkochen, Germany) results. The samples were mixed with KBr and ground for 5 min, and then the mixture was pressed into a pellet for infrared testing.
3. Results and Discussion
3.1. Raw Material Characteristics
The primary mineral compositions of raw coal gangue samples were analyzed by XRD. Results are shown in Figure 2, the crystalline minerals in the coal gangue are kaolinite, boehmite, and calcite. The major chemical compositions of the sample were measured using XRF. Table 3 presents the sample composition data (major chemical compositions were presented as oxides and were normalized to 100 wt% excluding C). This coal gangue contained much silica and aluminum oxide, accounting for 48.56% and 47.22%, respectively. Combined with the results of XRD analysis, silicon and aluminum mainly exist in the coal gangue in the form of kaolinite and boehmite minerals. The loss of ignition (LOI) in the sample was 29.62%, which includes mass loss from the combustion of organic matter and decomposition of minerals.
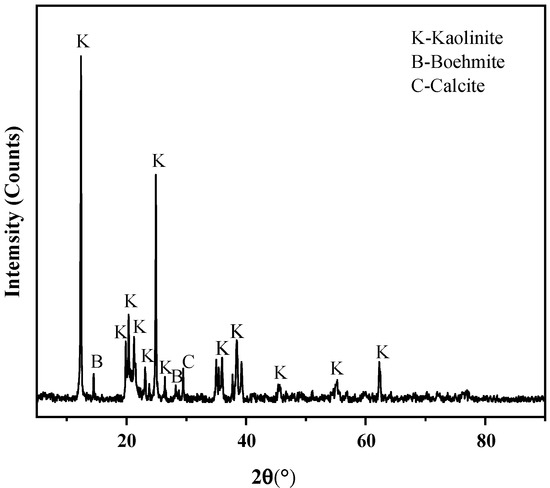
Figure 2.
XRD pattern of raw coal gangue.

Table 3.
The content of major chemical compositions and critical elements in coal gangue sample.
The content of critical elements in the raw coal gangue sample was analyzed (Table 3). Meanwhile, the enrichment characteristics of critical elements in the coal gangue are presented in Figure 3. In comparison to the mean values of China coals and world hard coals [52,53], the critical elements Li, Ga, and REY in the coal gangue were enriched. However, the total content of REY in the coal gangue was only 97.13 μg/g and lower than the mean value of China coals (136 μg/g) [53], but higher than the average level of world hard coals (64.81 μg/g) [52]. The outlook coefficient (Coutl) is applied to evaluate the market of REY, a larger Coutl means higher profitability [54]. The Coutl of the coal gangue was 0.89, indicating that the recovery of REY from this source could be potentially viable. The content of Li in the coal gangue was 437.43 μg/g, which is much higher than that of China coals (31.8 μg/g) and world hard coals (14 μg/g) [52,53]. The content of Ga was 43.02 μg/g, which is close to the cut-off grade proposed by Dai et al. [16] for the joint development and utilization of Ga and Al (Ga > 50 μg/g and Al2O3 > 40%) in coal or coal measures. In addition, compared with the world clays [55], the coal gangue was enriched in Li and slightly enriched in Ga, according to the enrichment classification proposed by Dai et al. [56].
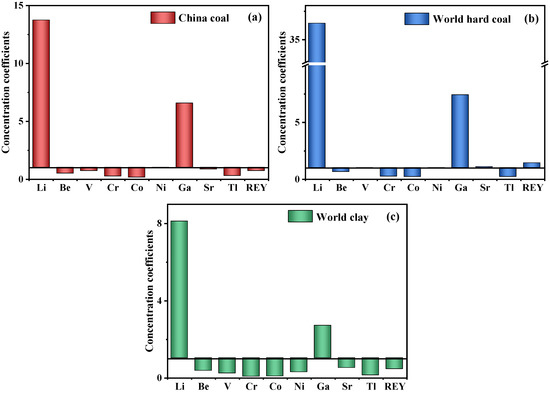
Figure 3.
Concentration coefficients (CC), the ratios of elemental content in the studied coal gangue to an average of the corresponding element in China coal (a), world hard coal (b), and world clay (c), of critical elements in coal gangue. Data for China coal, world hard coal, and world clay are from Dai et al. [53], Ketris et al. [52], and Grigoriev et al. [55], respectively.
SEM-EDS was used to identify the carrier of critical elements in coal gangue. Two particles enriched in different critical elements are shown in Figure 4. Points A and B were conducted the elemental analysis using an energy dispersive X-ray spectrometer. Based on the elemental compositions of point A shown in Figure 4a, the atomic ratio of Al to Si was 1.33, indicating that the particle was probably a clay mineral (e.g., kaolinite). The rare earth elements Ce and Nd may associate with aluminosilicate clay minerals. As suggested by many researchers [27,57], clay minerals are one of the main hosts of REY in coal. The atomic ratio of Fe to Al at point B was 2.69, which was close to the theoretical ratio of Fe to Al in almandine [Fe3Al2(SiO4)3]. In addition, the particle contained 2.33% of the critical element Cr. The substitution of Cr for other cations is common in garnet minerals [58], so it is inferred that this may be a Cr-bearing almandine. Meanwhile, this particle may be partially encapsulated by aluminosilicate minerals as observed SEM image.
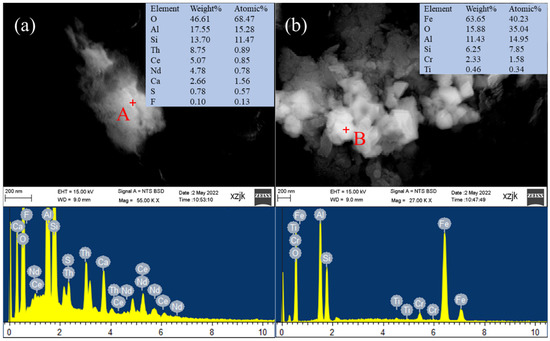
Figure 4.
Back-scattered SEM images of different coal gangues and EDS patterns of the selected points. (a) clay mineral; (b) almandine.
3.2. Enrichment Effects of Physical Separation and Calcination
To improve the grade of critical elements and remove impurities prior to leaching recovery, thereby minimizing consumption of acid and environmental concerns caused by waste acid, the distribution of critical elements in coal gangue was studied using the physical separation methods. In addition, calcination, as a unique physicochemical method, was used to remove organic matter from coal gangue to achieve the purpose of enriching critical elements. The results of these tests are discussed in the following sections.
3.2.1. Physical Separation
The screening tests were performed to study the distribution of Li, Ga, and REY in various particle size fractions, i.e., 0.5~0.25, 0.25~0.125, 0.125~0.075, and <0.075 mm. The concentration of Li, Ga, and REY in different particle-size-fractioned samples are presented in Figure 5a along with the mass yield of each particle size fraction. The mass yield of particle size fraction increased from 11.04% to 38.88% with the decrease in particle size, and the mass yield of <75 μm size fraction was the highest. The dotted line in Figure 5 represents the level of Li, Ga, and REY in raw coal gangue, and the points above it indicate these elements were enriched. There are various overall trends in these elements of different size fractions. For Li, its concentration increased a little from the first to the second fraction and then decreased gradually, and the highest concentration measured at 0.25~0.125 mm size fraction was 467.68 μg/g. According to the enrichment factor (EF, the ratio of elemental concentration between the studied fraction and raw coal gangue) proposed by Lin et al. [42], the EFs of Li in the 0.5~0.25 and 0.25~0.125 mm size fractions were 1.05 and 1.07, and Li was enriched in these two size fractions. For Ga, its concentration in coarse-size fraction was higher than that in the fine-size fraction. The concentrations of Ga in the 0.5~0.25 and 0.25~0.125 mm size fractions were higher than those of the raw sample, and the EFs were 1.05 and 1.02, respectively. The concentration of Li and Ga was 464.30 μg/g (EF = 1.06) and 44.47 μg/g (EF = 1.03) in the >0.125 mm size fraction, and the mass yield of this size fraction was 30.13%. For REY, the REY concentration increased as the particle size decreased except for the 0.25~0.125 mm size fraction. The <75 μm size fraction was enriched in REY with a concentration of 114.06 μg/g (EF = 1.17), and its mass yield was 38.87%.
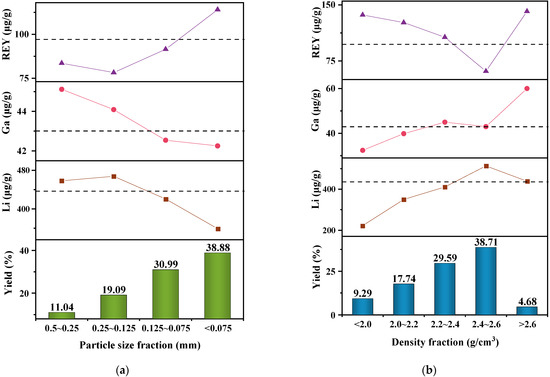
Figure 5.
Mass yield and concentration of Li, Ga, and REY in different (a) particle size fractions and (b) density fractions.
Through the density separation tests, the coal gangue samples were divided into five density fractions, including <2.0, 2.0~2.2, 2.2~2.4, 2.4~2.6 and >2.6 g/cm3. The mass yield and concentration of Li, Ga, and REY in different density fractions are shown in Figure 5b. As the density of coal gangue particles increased, the mass yield first increased to maximum values at 2.4~2.6 g/cm3 density fraction and then decreased. For Li, its concentration in density-fractioned samples generally increases as the density increase except for the >2.6 g/cm3 density fraction. The concentration of Li reached a maximum value of 513.60 μg/g (EF = 1.17) in the density range of 2.4~2.6 g/cm3, while REY concentration was lowest in this density range. The distribution of REY was opposite to that of Li, and REY was enriched in all density fractions other than the 2.4~2.6 g/cm3 density fraction. The reason for this may be that Li in coal is dominantly associated with clays, while the main hosts of REY are organic matter and authigenic minerals [30,59]. Li was enriched in the >2.4 g/cm3 density fraction with a concentration of 505.58 μg/g (EF = 1.16), and the mass yield of this density fraction was 43.39%. Because the mass yield of the >2.6 g/cm3 density fraction was only 4.68%, REY was mainly enriched in the <2.4 g/cm3 density fraction, its concentration was 117.75 μg/g (EF = 1.21), and the mass yield of this density fraction was 56.61%. For Ga, it was enriched in the 2.2~2.4 and >2.6 g/cm3 density fractions, and the highest concentration obtained in the >2.6 g/cm3 density fraction was 60.01 μg/g (EF = 1.39). As the concentration of Ga in the 2.4~2.6 g/cm3 density fraction was close to that in the raw coal gangue, Ga was considered to be enriched in the >2.2 g/cm3 density fraction. The concentration of Ga in this density fraction was 44.89 μg/g (EF = 1.04), and the mass yield of this density fraction was 72.98%.
Concentration, EF, and mass yield were used together to evaluate the enrichment effects of Li, Ga, and REY. Compared with particle size separation, density separation could produce more target products with higher Li, Ga, and REY concentrations. Therefore, density separation has a better enrichment effect.
3.2.2. Calcination
Calcination treatment could decompose organic matter in coal gangue and enrich critical elements at the same time, even if parts of individual elements may be volatilized into the atmosphere in the gaseous form [43]. In addition, the leachability of the critical elements in calcined products could be greatly improved [11], which is beneficial to the subsequent recovery of these elements. The results of calcination tests are given in Table 4. After calcination treatment, Li, Ga, and REY had been enriched to different degrees [60]. With the increase in calcination temperature, the content of Li, Ga, and REY in calcined products increased accordingly due to the decomposition of organic matter and some minerals. When the calcination temperature was increased to 950 °C, Li and REY in the calcined product were enriched by 1.32 times and 1.25 times, respectively, and their content reached 577.99 and 120.98 μg/g. However, the content of Ga was reduced by 11.08 μg/g compared to the calcined product at 800 °C, which may be caused by the formation of more gaseous Ga-bearing volatile compounds (e.g., Ga(g), GaO(g), and GaCl(g)) [61].

Table 4.
Content and enrichment factor (EF *) of Li, Ga, and REY in different calcined coal gangues.
3.3. The Leaching Characteristics of Li, Ga, and REY
Since physical separation and calcination treatment were found to be effective methods for the enrichment of Li, Ga, and REY, the hydrochloric acid leaching tests were performed on all fractions and products to assess the leachabilities of these elements. All leaching conditions were the same, see Section 2.5 for details.
The analysis results of acid leaching tests for different particle size fractions are given in Figure 6a. These data showed the same trend, Li, Ga, and REY associated with fine-grained fractions were more easily extracted relative to the coarse-grained fractions. This finding agreed well with the currently recognized results that fractions with finer particle sizes were more soluble [62]. A reduction in particle size may expose more surface area and release more micro-sized minerals containing critical elements. The leaching efficiency of REY was significantly higher than that of Li and Ga, and Li had the lowest recovery rate.
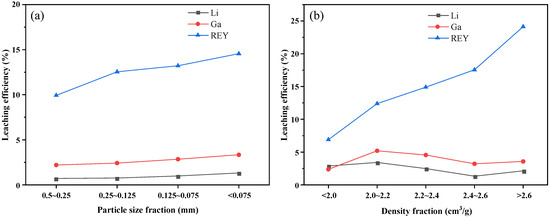
Figure 6.
Leaching characteristics of Li, Ga, and REY in different (a) particle size fractions, and (b) density fractions.
Figure 6b shows that the acid leaching results of different density fractions. There were differences in the leachabilities of Li, Ga, and REY at different density fractions, REY was easier to extract compared to Li and Ga. With the increase in density, the leaching efficiencies of Li and Ga increased to maximum values at 2.0~2.2 g/cm3 density fraction and then decreased, but finally increased slightly again. As the density increases from <2.0 g/cm3 to >2.6 g/cm3, the leaching rate of REY increases gradually from 6.90% to 24.13%, which can be explained by the presence of rare earth minerals in high-density fractions.
The recoveries of Li, Ga, and REY in different calcined products were obtained by acid leaching tests, and the results are shown in Figure 7. The leaching efficiencies of Li, Ga, and REY were significantly increased when the coal gangue was calcined under a temperature of 400 °C. For example, less than 20% of the REY was leached from raw coal gangue, whereas nearly 80% of REY was extracted from the 400 °C calcined product. The leaching efficiencies of Li and Ga were increased by 68% and 54%, respectively. With the increase in calcination temperature, the leaching recovery of Li, Ga, and REY decreased. When the calcination temperature was increased to 950 °C, the leaching rate of Li, Ga, and REY dropped sharply to 27.74%,13.44%, and 45.82%, respectively. The leaching behaviors of Li and Ga were similar to those of REY, but the leaching rates of both were lower than those of REY.
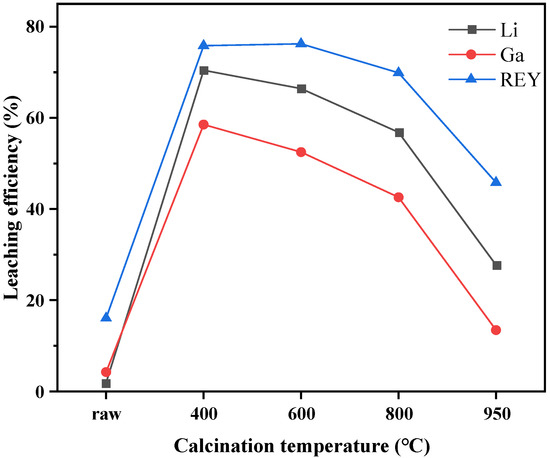
Figure 7.
Effects of calcination on the recovery of Li, Ga, and REY from coal gangue.
3.4. The Mechanism Analysis of the Calcination
Sequential chemical extraction tests were carried out to analyze the modes of occurrence of Li, Ga and REY varied with the change in the calcination temperature. The results are shown in Figure 8, a considerable portion of the Li, Ga, and REY in the raw coal gangue occurred as the insoluble form (95.01%, 88.49%, and 80.85%, respectively), which explained the low extraction efficiencies in the leaching tests. After the coal gangue was calcined in the temperature range of 400 to 800 °C, some insoluble forms of Li, Ga, and REY were converted to more soluble forms. For example, the proportion of insoluble Li was significantly reduced to 57.75% at 400 °C, and the ion-exchangeable, carbonate-bound, metal oxide-bound and organic matter-bound fractions increased (from 0.77% to 4.18%, 0.82% to 2.04%, 2.43% to 32.40% and 0.96% to 3.63%, respectively). Compared with Li, the proportion of insoluble rare earth was relatively low in this calcination temperature range, and the proportion of insoluble Ga was higher. When the calcination temperature was increased to 950 °C, the proportion of Li, Ga, and REY in the insoluble form increased again to 78.75%, 88.59%, and 69.81%, respectively. These findings were consistent with the results of the acid leaching tests.
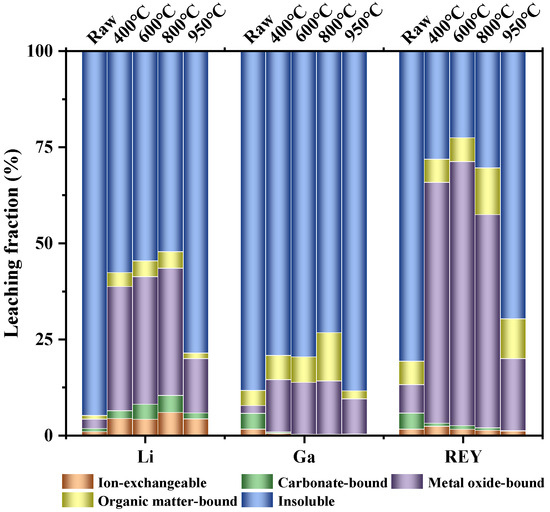
Figure 8.
The distribution of Li, Ga, and REY in different modes of occurrence in raw and calcined coal gangues.
As shown in Figure 9, the characteristic diffraction peaks of calcined products gradually weakened and finally disappeared as the calcination temperature increased. After the coal gangue was calcined at 400 °C, the intensity of diffraction peaks belonging to kaolinite weakened, and the characteristic peaks of boehmite disappeared due to the destruction of the crystal structure. When the calcination temperature reached 600 °C, the diffraction peaks of kaolinite completely vanished and a broad diffraction peak was formed in the range of 20~26°, which was caused by the breakages of Al–OH bonds and the formation of metakaolinite phase [45]. The calcite diffraction peak also disappeared at this temperature, which was attributed to the decomposition of this mineral [63]. At the calcination temperature of 950 °C, several additional weak peaks were observed at 45.86°, 60.90°, and 67.03°, respectively, due to the formation of γ-Al2O3 [64]. The substance can hinder the leaching of Li, Ga, and REY because it is more stable in the acid environment [65,66], which was consistent with the results of the acid leaching tests. Compared to substances with crystal structure, Li, Ga, and REY were more easily extracted from amorphous metakaolinite.
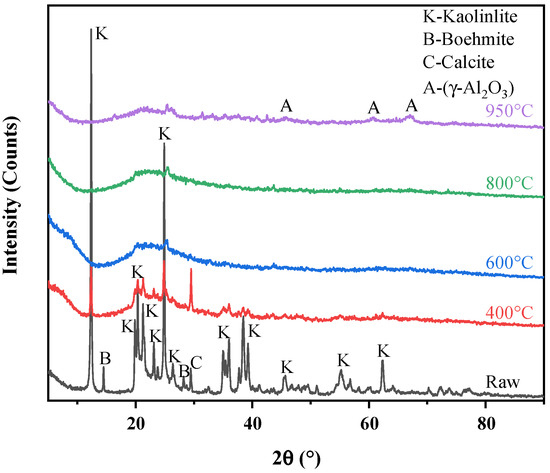
Figure 9.
XRD patterns of calcined coal gangues at different temperatures.
The infrared spectrums of coal gangue before and after calcination at different temperatures are shown in Figure 10. According to the results of infrared analysis, the raw coal gangue was consistent with the infrared spectral characteristics of kaolinite except for several infrared bands of adsorbed water and organic matter. The absorption bands at 3692 and 3620 cm−1 are hydroxyl stretching vibration bands of kaolinite, while those at 914 cm−1 are the bending vibration band of Al–OH [67,68]. The absorption bands at 1034 and 1012 cm−1 correspond to the stretching vibration bands of Si–O [45]. The absorption bands at 790 and 753 cm−1 belong to the hydroxyl translation bands of kaolinite [67]. The absorption band at 753 cm−1 is also a superposition band of Al–O stretching vibration, which corresponds to boehmite [69]. The absorption band at 692 cm−1 is the perpendicular deformation band of Si–O, and the bands at 541 and 471 cm−1 are the bending vibration bands of Si–O–Al [68]. In addition, the adsorption bands at 3441 and 1623 cm−1 are attributed to hydroxyl stretching and bending vibration bands of adsorbed water, and the band at 1433 cm−1 belongs to the methyl or methylene vibration band of organic matter and vibration band of calcite [45,70].
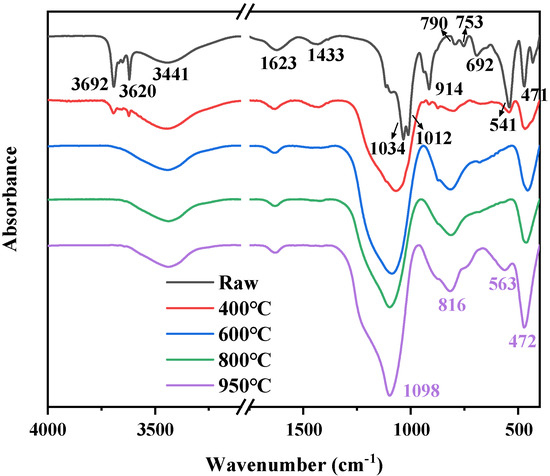
Figure 10.
FT-IR patterns of calcined coal gangues at different temperatures.
After the coal gangue was calcined at 400 °C, the intensity of hydroxyl stretching and bending vibration bands of kaolinite were significantly reduced compared to the raw sample, indicating that the dehydroxylation process of kaolinite had begun. The adsorption bands belonging to Si–O and Si–O–Al disappeared, shifted, or weakened, which means that the layered skeleton of kaolinite was destroyed. The superposition band vanished due to the disruption of the Al–O bond in boehmite [69]. When the calcination temperature reached 600 °C, the adsorption bands corresponding to the -OH groups completely disappeared. The significant broadening of Si–O stretching bands and the appearance of 816 cm−1 adsorption band demonstrates that the silica tetrahedrons were depolymerized and the metakaolinite phase formed. When the calcination temperature was increased to 950 °C, the adsorption band appeared at 563 cm−1 was the Al–O stretching vibration band of γ-Al2O3 [71], which indicated that a new mineral phase formed in the calcined product. The adsorption band at 1433 cm−1 disappeared at 600 °C, which was attributed to the combustion of organic matter and decomposition of calcite in coal gangue. The decomposition of difficult-to-dissolve minerals into soluble forms due to calcination treatment explained the results of acid leaching tests and sequential chemical extraction tests.
3.5. Implications for Li and Ga Recovery
The above analyses show that Li, Ga, and REY in the coal gangue are enriched, in which Li is thirteen times the Chinese average of coal, and Ga is close to the cut-off grade for the combined utilization of Ga and Al. In addition, Li and Ga have great potential for co-recovery due to their consistency during physical separation, calcination treatment, and acid leaching process. Comparing the two physical separation methods, it was found that the EFs of the two methods were close, but density separation could obtain more fractions enriched in Li and Ga. Therefore, density separation was chosen to pre-enrich Li and Ga from raw coal gangue in the next conceptual process. Calcination treatment was employed to further increase the Li and Ga content in the product and to enhance the leachability of Li and Ga. The acid leaching method was applied to extract Li and Ga from calcined products.
Based on the above discussion, a conceptual process flowsheet was proposed for the recovery of Li and Ga from coal gangue, as shown in Figure 11. The raw coal gangue is crushed and ground to a particle size of fewer than 75 μm before separation. In the gravity separation stage, the separation density was set to 2.2 g/cm3. Through gravity separation, a >2.2 g/cm3 density fraction enriched in Li and Ga will be obtained. After dehydration, the >2.2 g/cm3 density fraction will be calcined at 400 °C. Then, Li and Ga will be extracted from the calcined product by acid leaching. The leaching solution will be sent to the next step for separation and purification.
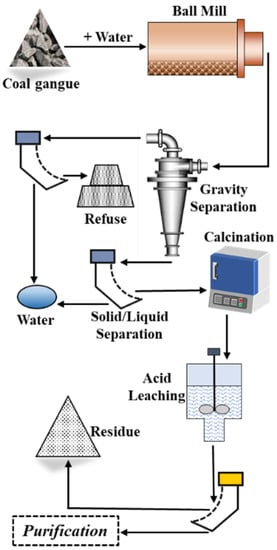
Figure 11.
The combined flowsheet for co-extraction of Li and Ga from coal gangue.
4. Conclusions
This paper focused on analyzing the feasibility of physical separation and calcination treatment for the enrichment of Li, Ga, and REY from coal gangue, and then the impacts of these enrichment methods on the acid leaching recovery of these elements were investigated. The major conclusions of this study are as follows:
(1) The contents of Li, Ga, and REY in the coal gangue were 473.43, 43.02, and 97.13 μg/g, respectively, among which Li was thirteen times the average content in Chinese coal, and Ga was close to the cut-off grade for the combined utilization of Ga and Al;
(2) The Li, Ga, and REY were enriched in the particle size fractions of the >0.125, >0.125, and <0.075 mm (EF = 1.06, 1.03, and 1.17) as well as the density fractions of the >2.4, >2.2, and <2.4 g/cm3 (EF = 1.16, 1.04, and 1.21), respectively. The physical separation had a slight effect on the leaching of Li, Ga, and REY;
(3) The Li, Ga, and REY were significantly enriched, and their acid leaching recoveries were increased by 54~68% when the sample was calcined under 400 °C. Those were because the majority of insoluble Li, Ga, and REY was converted into soluble forms. However, at 950 °C, the formation of crystal material caused a sharp decline in leaching recovery;
(4) The Li and Ga can be enriched together into high-density fractions due to their consistency. A conceptually combined flowsheet of density separation, calcination, and acid leaching was proposed for the extraction of Li and Ga from coal gangue.
Author Contributions
Conceptualization, L.Z. and J.P.; Methodology, L.Z.; Validation, L.Z., H.C. and X.L.; Formal Analysis, L.Z.; Investigation, S.S.; Resources, J.P.; Data Curation, L.Z.; Writing—Original Draft Preparation, L.Z.; Writing—Review and Editing, J.P., Z.W. and S.S.; Visualization, L.Z.; Supervision, C.Z.; Project Administration, C.Z.; Funding Acquisition, C.Z. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 92062109, 52204292, and 51974309); and National Postdoctoral Program for Innovative Talents (Grant No. BX2021362).
Data Availability Statement
The data presented in this work are available on request from the corresponding author.
Acknowledgments
We are very grateful to all the editors and reviewers who have helped us improve and publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jowitt, S.M.; Mudd, G.M.; Werner, T.T.; Weng, Z.; Barkoff, D.W.; McCaffrey, D. The Critical Metals: An Overview and Opportunities and Concerns for the Future. In Metals, Minerals, and Society; Society-of-Economic-Geologists: Littleton, CO, USA, 2018; pp. 25–38. [Google Scholar]
- Liu, W.; Li, X.; Wang, M.; Liu, L. Research trend and dynamical development of focusing on the global critical metals: A bibliometric analysis during 1991–2020. Environ. Sci. Pollut. Res. Int. 2022, 29, 26688–26705. [Google Scholar] [CrossRef]
- Løvik, A.N.; Hagelüken, C.; Wäger, P. Improving supply security of critical metals: Current developments and research in the EU. Sustain. Mater. Technol. 2018, 15, 9–18. [Google Scholar] [CrossRef]
- Zhai, M.; Wu, F.; Hu, R.; Jiang, S.; Li, W.; Wang, R.; Wang, D.; Qi, T.; Qin, K.; Wen, H. Critical metal mineral resources: Current research status and scientific issues. Bull. Natl. Nat. Sci. Found. China 2019, 33, 106–111. [Google Scholar]
- Arunachalam, S.; Kirubasankar, B.; Pan, D.; Liu, H.; Yan, C.; Guo, Z.; Angaiah, S. Research progress in rare earths and their composites based electrode materials for supercapacitors. Green Energy Environ. 2020, 5, 259–273. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Yang, C.; Xu, Z. Global supply risk assessment of the metals used in clean energy technologies. J. Clean. Prod. 2022, 331, 129602. [Google Scholar] [CrossRef]
- Can Sener, S.E.; Thomas, V.M.; Hogan, D.E.; Maier, R.M.; Carbajales-Dale, M.; Barton, M.D.; Karanfil, T.; Crittenden, J.C.; Amy, G.L. Recovery of Critical Metals from Aqueous Sources. ACS Sustain. Chem. Eng. 2021, 9, 11616–11634. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C.C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef]
- Hedin, B.C.; Hedin, R.S.; Capo, R.C.; Stewart, B.W. Critical metal recovery potential of Appalachian acid mine drainage treatment solids. Int. J. Coal Geol. 2020, 231, 103610. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Characterization and recovery of rare earth elements and other critical metals (Co, Cr, Li, Mn, Sr, and V) from the calcination products of a coal refuse sample. Fuel 2020, 267, 117236. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. Lithium leaching recovery and mechanisms from density fractions of an Illinois Basin bituminous coal. Fuel 2020, 268, 117319. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Nechaev, V.P.; Nechaeva, E.V.; Graham, I.T.; French, D.; Sun, J. Enrichment of critical elements (Nb-Ta-Zr-Hf-REE) within coal and host rocks from the Datanhao mine, Daqingshan Coalfield, northern China. Ore Geol. Rev. 2019, 111, 102951. [Google Scholar] [CrossRef]
- Hower, J.C.; Dai, S. Petrology and chemistry of sized Pennsylvania anthracite, with emphasis on the distribution of rare earth elements. Fuel 2016, 185, 305–315. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; Ward, C.R.; Yan, X.; Guo, W.; French, D.; Graham, I.T. Anomalies of rare metals in Lopingian super-high-organic-sulfur coals from the Yishan Coalfield, Guangxi, China. Ore Geol. Rev. 2017, 88, 235–250. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Sun, B.; Zeng, F.; Moore, T.A.; Rodrigues, S.; Liu, C.; Wang, G. Geochemistry of two high-lithium content coal seams, Shanxi Province, China. Int. J. Coal Geol. 2022, 260, 104059. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Yerin, Ü.O.; Oskay, R.G.; Bulut, Y.; Córdoba, P. Enrichment and distribution of elements in the middle Miocene coal seams in the Orhaneli coalfield (NW Turkey). Int. J. Coal Geol. 2021, 247, 103854. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Atalay, M.; Oskay, R.G.; Córdoba, P.; Querol, X.; Bulut, Y. Variations in elemental and mineralogical compositions of Late Oligocene, Early and Middle Miocene coal seams in the Kale-Tavas Molasse sub-basin, SW Turkey. Int. J. Coal Geol. 2020, 218, 103366. [Google Scholar] [CrossRef]
- Hower, J.C.; Eble, C.F.; Hopps, S.D.; Morgan, T.D. Aspects of rare earth element geochemistry of the Pond Creek coalbed, Pike County, Kentucky. Int. J. Coal Geol. 2022, 261, 104082. [Google Scholar] [CrossRef]
- Di, S.; Dai, S.; Nechaev, V.P.; Zhang, S.; French, D.; Graham, I.T.; Spiro, B.; Finkelman, R.B.; Hou, Y.; Wang, Y.; et al. Granite-bauxite provenance of abnormally enriched boehmite and critical elements (Nb, Ta, Zr, Hf and Ga) in coals from the Eastern Surface Mine, Ningwu Coalfield, Shanxi Province, China. J. Geochem. Explor. 2022, 239, 107016. [Google Scholar] [CrossRef]
- Seredin, V.V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Dai, S.; Chekryzhov, I.Y.; Seredin, V.V.; Nechaev, V.P.; Graham, I.T.; Hower, J.C.; Ward, C.R.; Ren, D.; Wang, X. Metalliferous coal deposits in East Asia (Primorye of Russia and South China): A review of geodynamic controls and styles of mineralization. Gondwana Res. 2016, 29, 60–82. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, C.; Li, Y.; Wang, J.; Zhang, J.; Jin, Z.; Lin, M.; Kalkreuth, W. Further Information of the Associated Li Deposits in the No.6 Coal Seam at Jungar Coalfield, Inner Mongolia, Northern China. Acta Geol. Sin. Engl. Ed. 2013, 87, 1097–1108. [Google Scholar]
- Dai, S.; Ren, D.; Li, S. Discovery of the superlarge gallium ore deposit in Jungar, Inner Mongolia, North China. Chin. Sci. Bull. 2006, 51, 2243–2252. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Li, S.; Zhao, L.; Zhang, Y. Coal facies evolution of the main minable coal-bed in the Heidaigou Mine, Jungar Coalfield, Inner Mongolia, northern China. Sci. China Ser. D Earth Sci. 2007, 50, 144–152. [Google Scholar] [CrossRef]
- Dai, S.; Jiang, Y.; Ward, C.R.; Gu, L.; Seredin, V.V.; Liu, H.; Zhou, D.; Wang, X.; Sun, Y.; Zou, J.; et al. Mineralogical and geochemical compositions of the coal in the Guanbanwusu Mine, Inner Mongolia, China: Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, L.; Wang, X.; Nechaev, V.P.; French, D.; Spiro, B.F.; Graham, I.T.; Hower, J.C.; Dai, S. Mineralogy and geochemistry of the Late Triassic coal from the Caotang mine, northeastern Sichuan Basin, China, with emphasis on the enrichment of the critical element lithium. Ore Geol. Rev. 2021, 139, 104582. [Google Scholar] [CrossRef]
- Dai, S.; Luo, Y.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhao, L.; Liu, S.; Zhao, C.; Tian, H.; Zou, J. Revisiting the late Permian coal from the Huayingshan, Sichuan, southwestern China: Enrichment and occurrence modes of minerals and trace elements. Int. J. Coal Geol. 2014, 122, 110–128. [Google Scholar] [CrossRef]
- Zou, J.; Cheng, L.; Guo, Y.; Wang, Z.; Tian, H.; Li, T. Mineralogical and Geochemical Characteristics of Lithium and Rare Earth Elements in High-Sulfur Coal from the Donggou Mine, Chongqing, Southwestern China. Minerals 2020, 10, 627. [Google Scholar] [CrossRef]
- Liu, J.; Song, H.; Dai, S.; Nechaev, V.P.; Graham, I.T.; French, D.; Nechaeva, E.V. Mineralization of REE-Y-Nb-Ta-Zr-Hf in Wuchiapingian coals from the Liupanshui Coalfield, Guizhou, southwestern China: Geochemical evidence for terrigenous input. Ore Geol. Rev. 2019, 115, 103190. [Google Scholar] [CrossRef]
- Hussain, R.; Luo, K. Geochemical Evaluation of Enrichment of Rare-Earth and Critical Elements in Coal Wastes from Jurassic and Permo-Carboniferous Coals in Ordos Basin, China. Nat. Resour. Res. 2019, 29, 1731–1754. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Dai, S.; French, D. The importance of minerals in coal as the hosts of chemical elements: A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Wei, Y.; He, W.; Qin, G.; Fan, M.; Cao, D. Lithium Enrichment in the No. 21 Coal of the Hebi No. 6 Mine, Anhe Coalfield, Henan Province, China. Minerals 2020, 10, 521. [Google Scholar] [CrossRef]
- Duan, P.; Wang, W.; Liu, X.; Sang, S.; Ma, M.; Zhang, W. Differentiation of rare earth elements and yttrium in different size and density fractions of the Reshuihe coal, Yunnan Province, China. Int. J. Coal Geol. 2019, 207, 1–11. [Google Scholar] [CrossRef]
- Sriramoju, S.K.; Dash, P.S.; Majumdar, S. Extraction of clean coal from washery rejects and its effect on coking properties: An approach toward sustainable development. Int. J. Coal Prep. Util. 2021, 1–23. [Google Scholar] [CrossRef]
- Xu, F.; Qin, S.; Li, S.; Wang, J.; Qi, D.E.; Lu, Q.; Xing, J. Distribution, occurrence mode, and extraction potential of critical elements in coal ashes of the Chongqing Power Plant. J. Clean. Prod. 2022, 342, 130910. [Google Scholar] [CrossRef]
- Wu, G.; Shi, N.; Wang, T.; Cheng, C.M.; Wang, J.; Tian, C.; Pan, W.P. Enrichment and occurrence form of rare earth elements during coal and coal gangue combustion. Environ. Sci. Pollut. Res. 2022, 29, 44709–44722. [Google Scholar] [CrossRef] [PubMed]
- Rosita, W.; Bendiyasa, I.M.; Perdana, I.; Anggara, F. Sequential particle-size and magnetic separation for enrichment of rare-earth elements and yttrium in Indonesia coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103575. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, C.; Tang, M.; Cao, S.; Liu, C.; Zhang, N.; Wen, M.; Luo, Y.; Hu, T.; Ji, W. Study on the modes of occurrence of rare earth elements in coal fly ash by statistics and a sequential chemical extraction procedure. Fuel 2019, 237, 555–565. [Google Scholar] [CrossRef]
- Pan, J.; Nie, T.; Vaziri Hassas, B.; Rezaee, M.; Wen, Z.; Zhou, C. Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere 2020, 248, 126112. [Google Scholar] [CrossRef]
- Lin, R.; Howard, B.H.; Roth, E.A.; Bank, T.L.; Granite, E.J.; Soong, Y. Enrichment of rare earth elements from coal and coal by-products by physical separations. Fuel 2017, 200, 506–520. [Google Scholar] [CrossRef]
- Zhou, C.; Du, J.; Zhang, Y.; Sun, J.; Wu, W.; Liu, G. Redistribution and transformation mechanisms of gallium and germanium during coal combustion. Fuel 2021, 305, 121532. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Pan, J.; Yang, F.; Liu, H.; Zhou, C.; Zhang, N. Extraction of lithium from coal gangue by a roasting-leaching process. Int. J. Coal Prep. Util. 2022, 1–16. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, Y.; Sun, J.; Hao, Z. The thermal activation process of coal gangue selected from Zhungeer in China. J. Therm. Anal. Calorim. 2016, 126, 1559–1566. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Calcination pretreatment effects on acid leaching characteristics of rare earth elements from middlings and coarse refuse material associated with a bituminous coal source. Fuel 2019, 249, 130–145. [Google Scholar] [CrossRef]
- Nie, T.; Zhou, C.; Pan, J.; Wen, Z.; Yang, F.; Jia, R. Study on the Occurrence of Rare Earth Elements in Coal Refuse Based on Sequential Chemical Extraction and Pearson Correlation Analysis. Min. Metall. Explor. 2022, 39, 669–678. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Lim, Y.; Yu, J.; Chen, S.; Woo, S.W.; Yoon, S.; Bae, S.; Kim, H.S. Characterization of rare earth elements present in coal ash by sequential extraction. J. Hazard. Mater. 2021, 402, 123760. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Nie, T.; Zhou, C.; Yang, F.; Jia, R.; Zhang, L.; Liu, H. The effect of calcination on the occurrence and leaching of rare earth elements in coal refuse. J. Environ. Chem. Eng. 2022, 10, 108355. [Google Scholar] [CrossRef]
- Liu, C.; Chang, X.; Sun, B.; Zeng, F. New Insight into the Depositional Age of No. 6 Coal in Heidaigou Mine, Late Paleozoic Jungar Coalfield, Inner Mongolia, China. Sustainability 2022, 14, 6297. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Grigoriev, N.A. Chemical Element Distribution in the Upper Continental Crus; UB RAS: Ekaterinburg, Russia, 2009; Volume 382, p. 383. [Google Scholar]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2014, 50, 159–186. [Google Scholar] [CrossRef]
- Zhang, W.; Rezaee, M.; Bhagavatula, A.; Li, Y.; Groppo, J.; Honaker, R. A Review of the Occurrence and Promising Recovery Methods of Rare Earth Elements from Coal and Coal By-Products. Int. J. Coal Prep. Util. 2015, 35, 295–330. [Google Scholar] [CrossRef]
- Izawa MR, M.; Cloutis, E.A.; Rhind, T.; Mertzman, S.A.; Poitras, J.; Applin, D.M.; Mann, P. Spectral reflectance (0.35–2.5 µm) properties of garnets: Implications for remote sensing detection and characterization. Icarus 2018, 300, 392–410. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Nechaev, V.P.; Nechaeva, E.V.; Graham, I.T.; French, D. Enrichment origin of critical elements (Li and rare earth elements) and a Mo-U-Se-Re assemblage in Pennsylvanian anthracite from the Jincheng Coalfield, southeastern Qinshui Basin, northern China. Ore Geol. Rev. 2019, 115, 103184. [Google Scholar] [CrossRef]
- Nowak, J.; Kokowska-Pawłowska, M. Changes in the Concentration of Some Rare Earth Elements in Coal Waste. Arch. Min. Sci. 2017, 62, 495–507. [Google Scholar] [CrossRef][Green Version]
- Frandsen, F.; Dam-Johansen, K.; Rasmussen, P. Trace elements from combustion and gasification of coal—An equilibrium approach. Prog. Energy Combust. Sci. 1994, 20, 115–138. [Google Scholar] [CrossRef]
- Yang, X.; Honaker, R.Q. Leaching Kinetics of Rare Earth Elements from Fire Clay Seam Coal. Minerals 2020, 10, 491. [Google Scholar] [CrossRef]
- Reifenstein, A.P.; Kahraman, H.; Coin CD, A.; Calos, N.J.; Miller, G.; Uwins, P. Behaviour of selected minerals in an improved ash fusion test: Quartz, potassium feldspar, sodium feldspar, kaolinite, illite, calcite, dolomite, siderite, pyrite and apatite. Fuel 1999, 78, 1449–1461. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Hu, Y. Investigation of the thermal behaviour and decomposition kinetics of kaolinite. Clay Miner. 2018, 50, 199–209. [Google Scholar]
- Liu, Y.; Lei, S.; Lin, M.; Li, Y.; Ye, Z.; Fan, Y. Assessment of pozzolanic activity of calcined coal-series kaolin. Appl. Clay Sci. 2017, 143, 159–167. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.-Y.; Lei, S.-M.; Ye, Z.; Pei, Z.-Y.; Li, B. High-efficiency extraction of Al from coal-series kaolinite and its kinetics by calcination and pressure acid leaching. Appl. Clay Sci. 2018, 161, 215–224. [Google Scholar] [CrossRef]
- Cheng, H.; Frost, R.L.; Yang, J.; Liu, Q.; He, J. Infrared and infrared emission spectroscopic study of typical Chinese kaolinite and halloysite. Spectrochim. Acta Part A Mol. Biomol. 2010, 77, 1014–1020. [Google Scholar] [CrossRef]
- Ptáček, P.; Kubátová, D.; Havlica, J.; Brandštetr, J.; Šoukal, F.; Opravil, T. The non-isothermal kinetic analysis of the thermal decomposition of kaolinite by thermogravimetric analysis. Powder Technol. 2010, 204, 222–227. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, Y.; Sun, J.; Wang, Z. The thermal transmission behavior analysis of two coal gangues selected from inner Mongolia in China. Therm. Sci. 2018, 22, 1111–1119. [Google Scholar] [CrossRef]
- Cetinkaya, S.; Yurum, Y. Oxidative pyrolysis of Turkish lignites in air up to 500 degrees C. Fuel Process. Technol. 2000, 67, 177–189. [Google Scholar] [CrossRef]
- Voll, D.; Lengauer, C.; Beran, A.; Schneider, H. Infrared band assignment and structural refinement of Al-Si, Al-Ge, and Ga-Ge mullites. Eur. J. Mineral. 2001, 13, 591–604. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).