National-Scale Geochemical Baseline of 69 Elements in Laos Stream Sediments
Abstract
1. Introduction
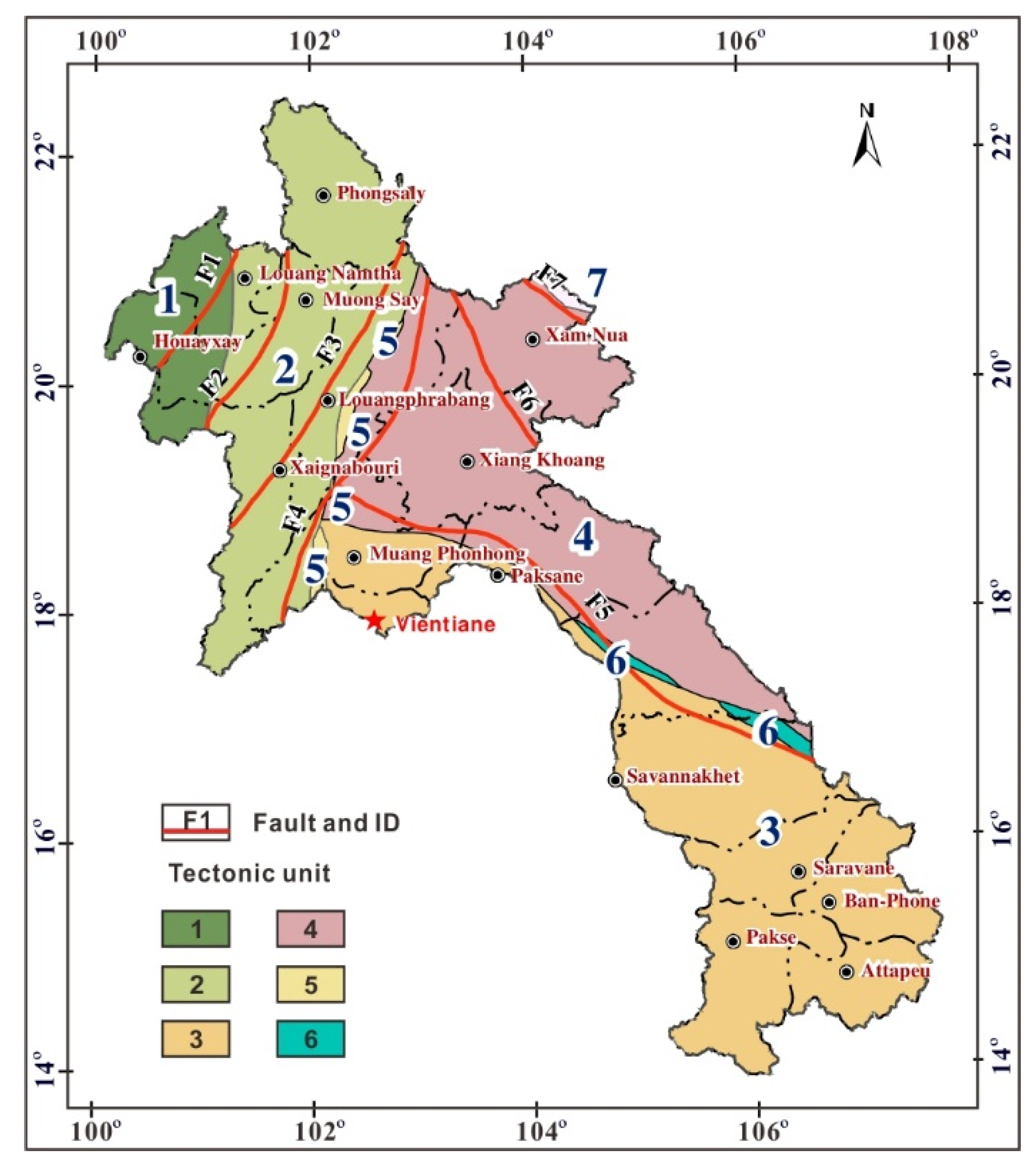
2. Geological Background
3. Materials and Methods
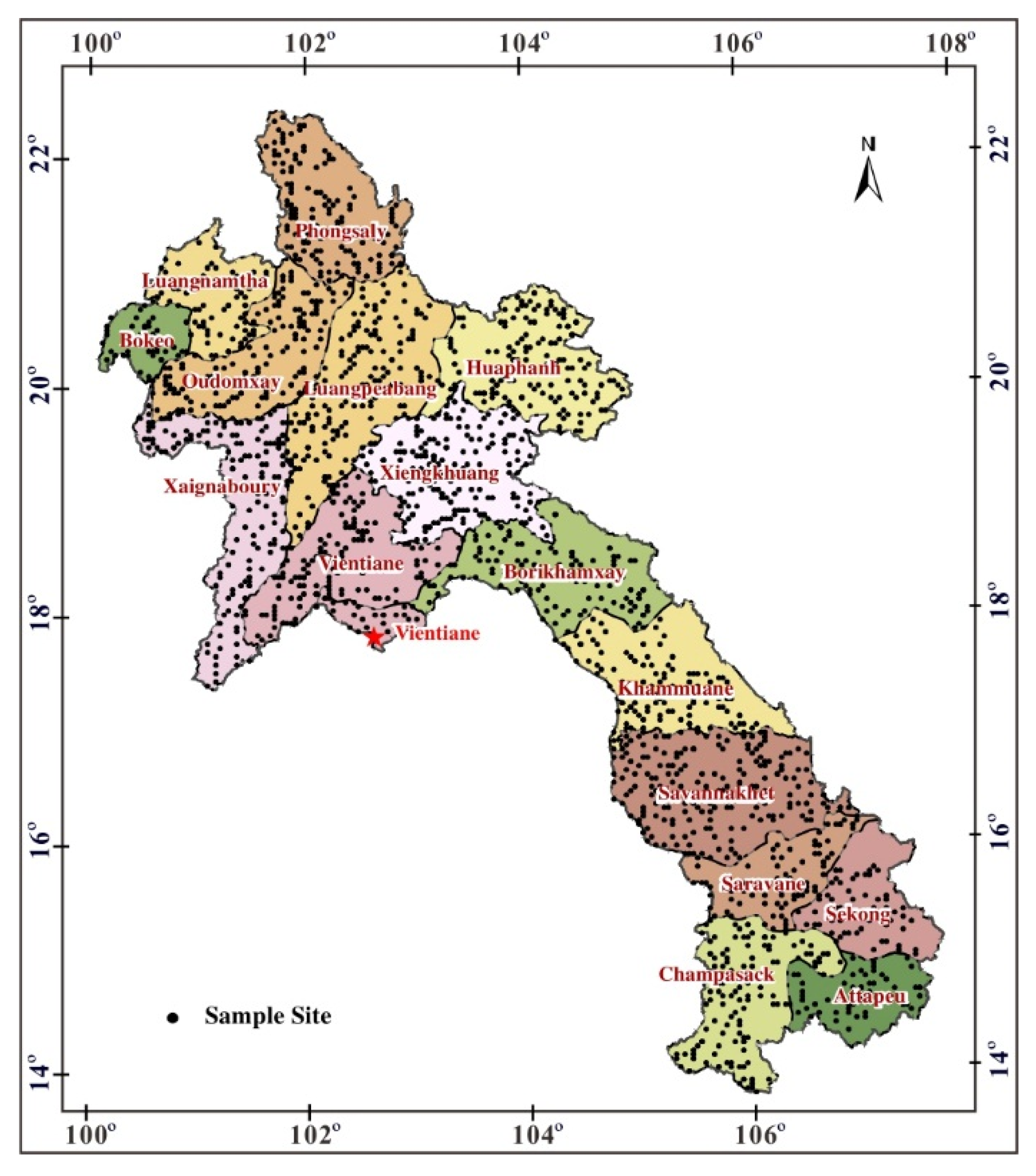
3.1. Sampling
3.2. Laboratory Chemical Analysis
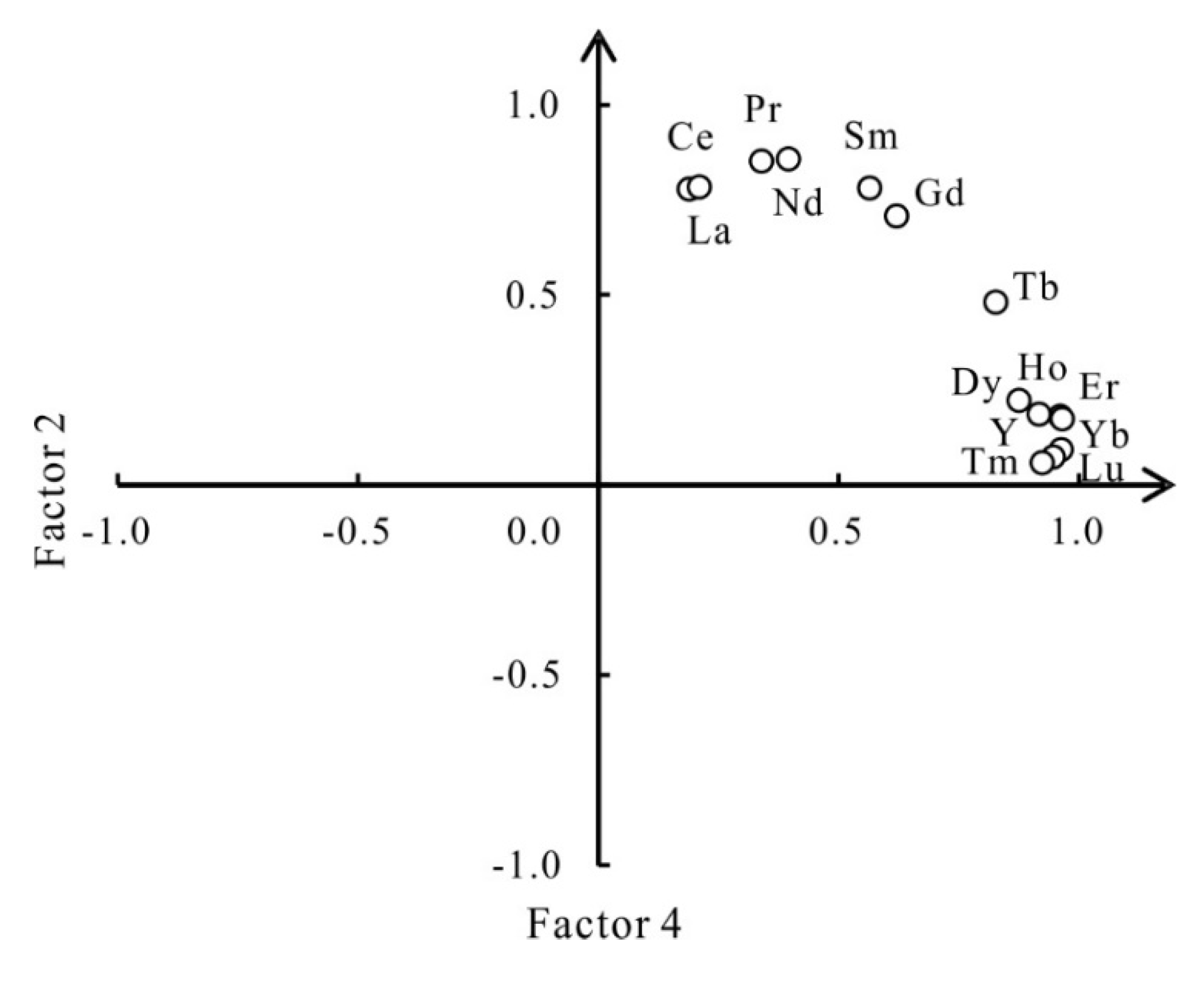
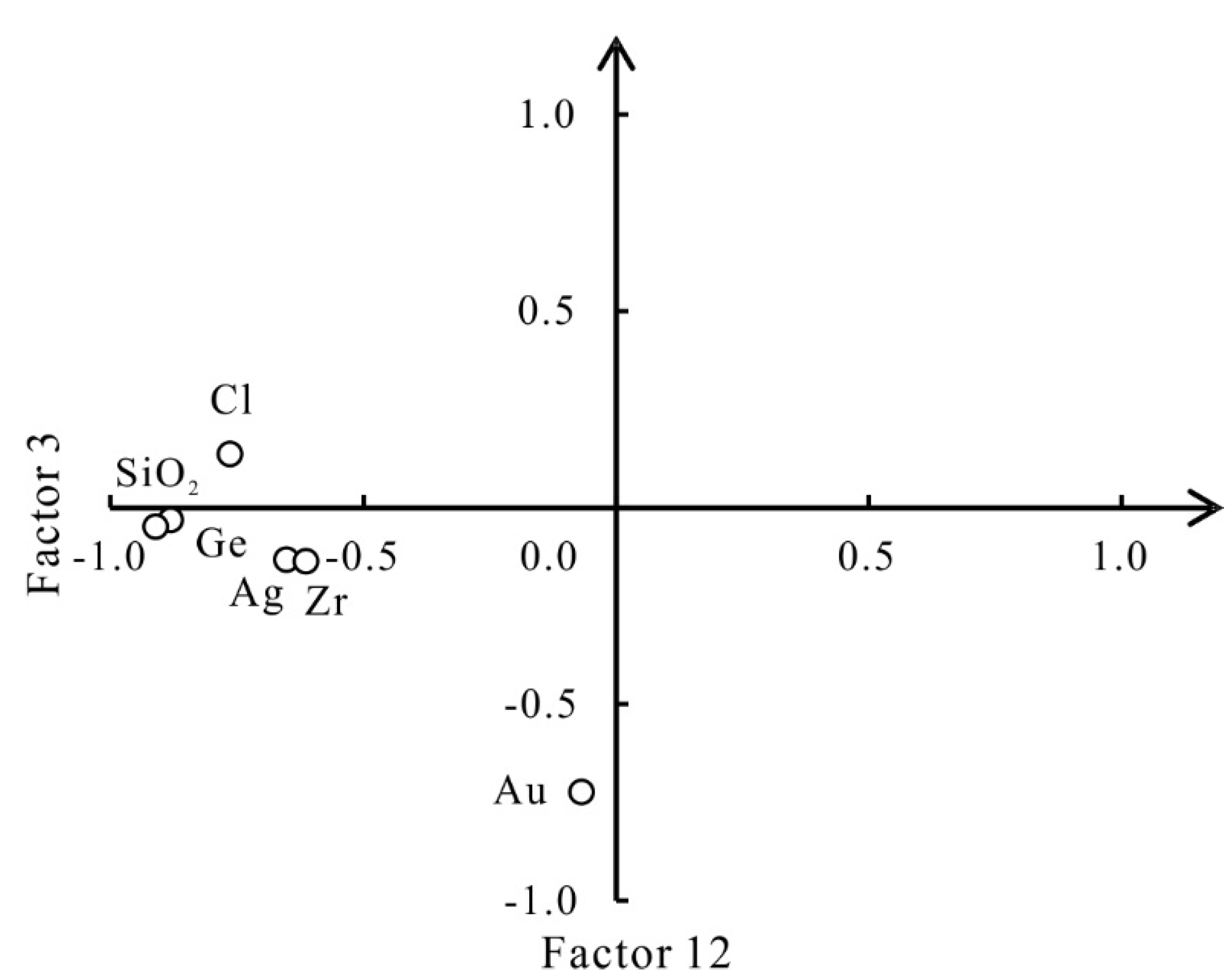
3.3. R-Mode Factor Analysis
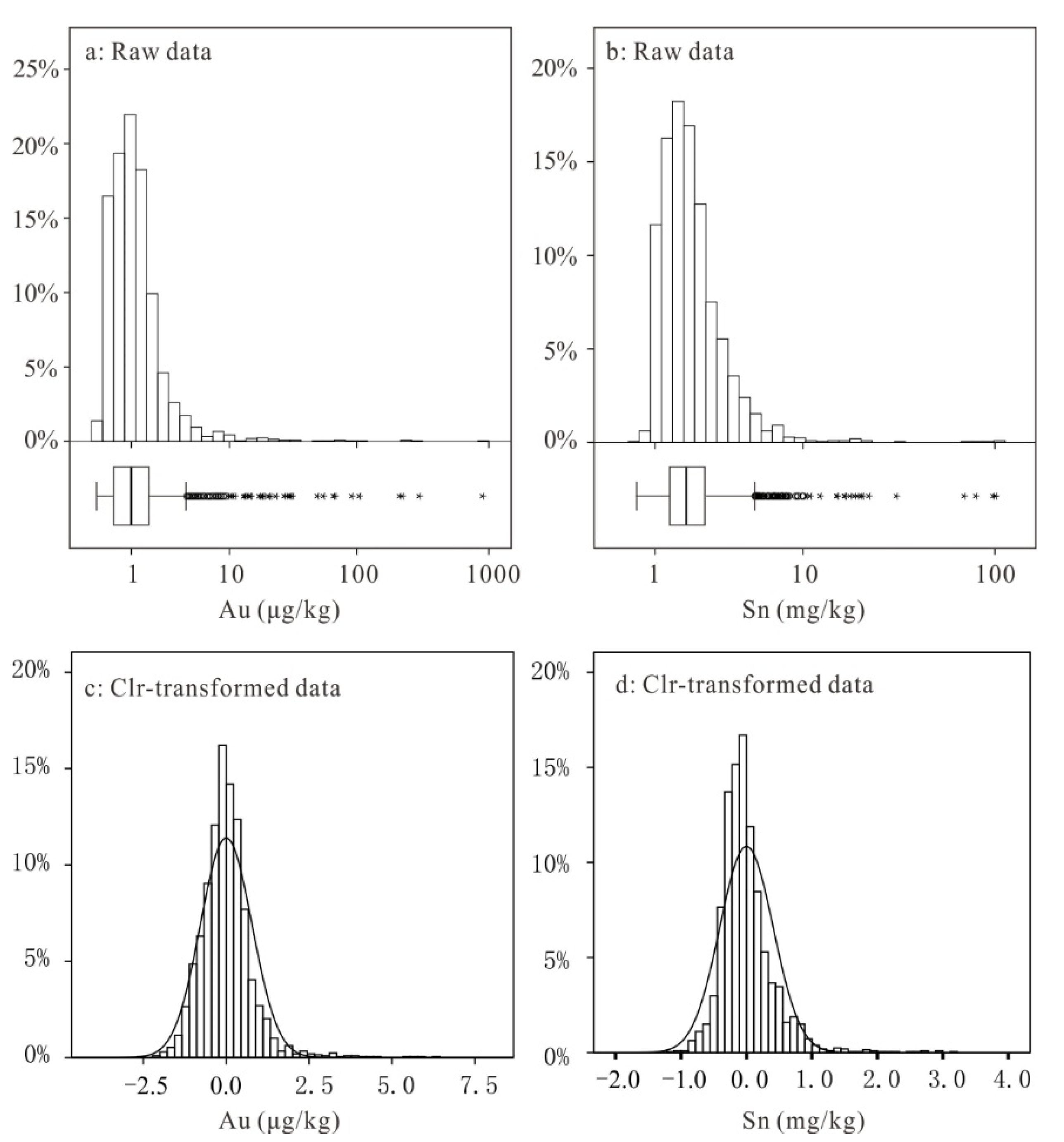
4. Results
5. Discussion
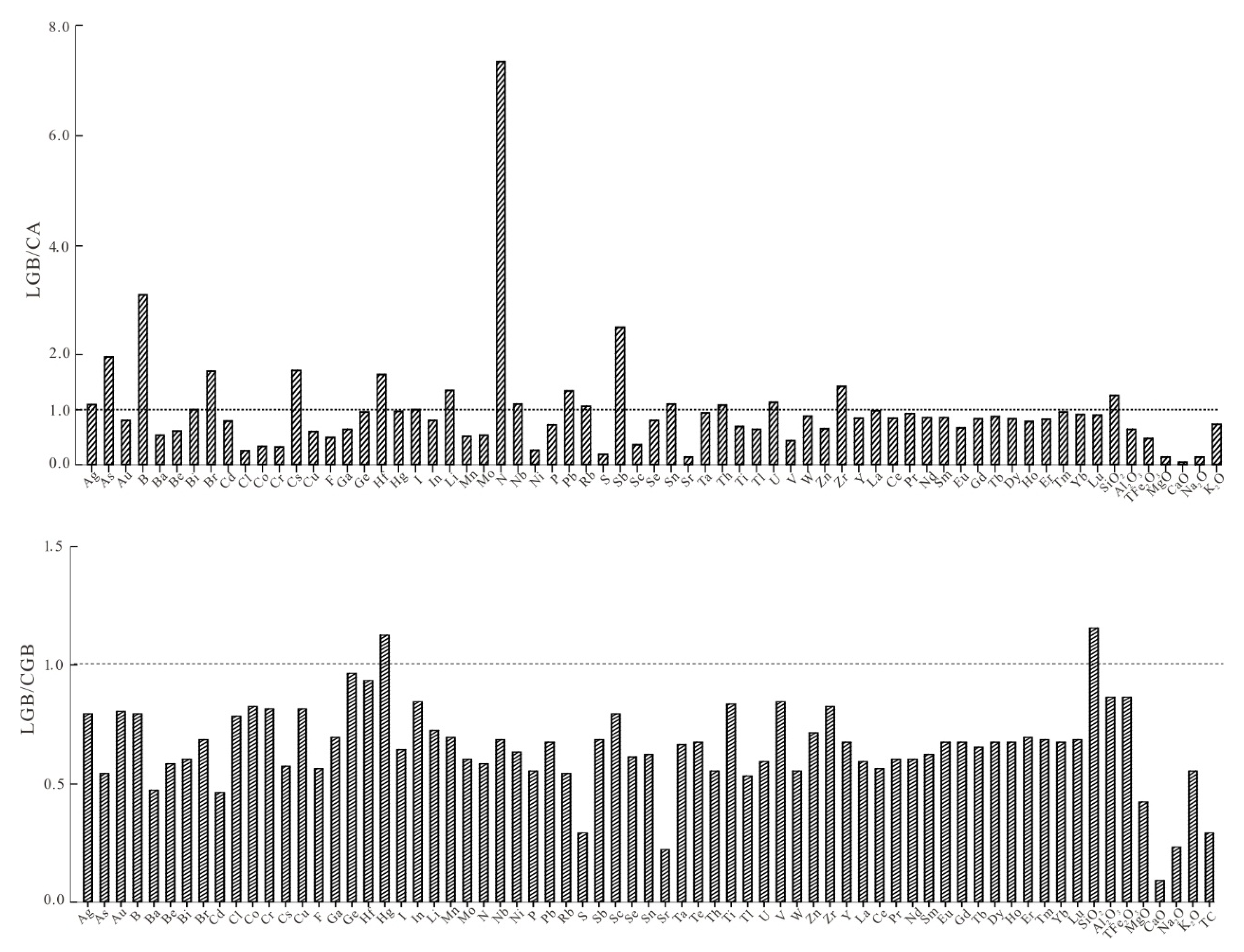
5.1. Elements Related to Bedrock
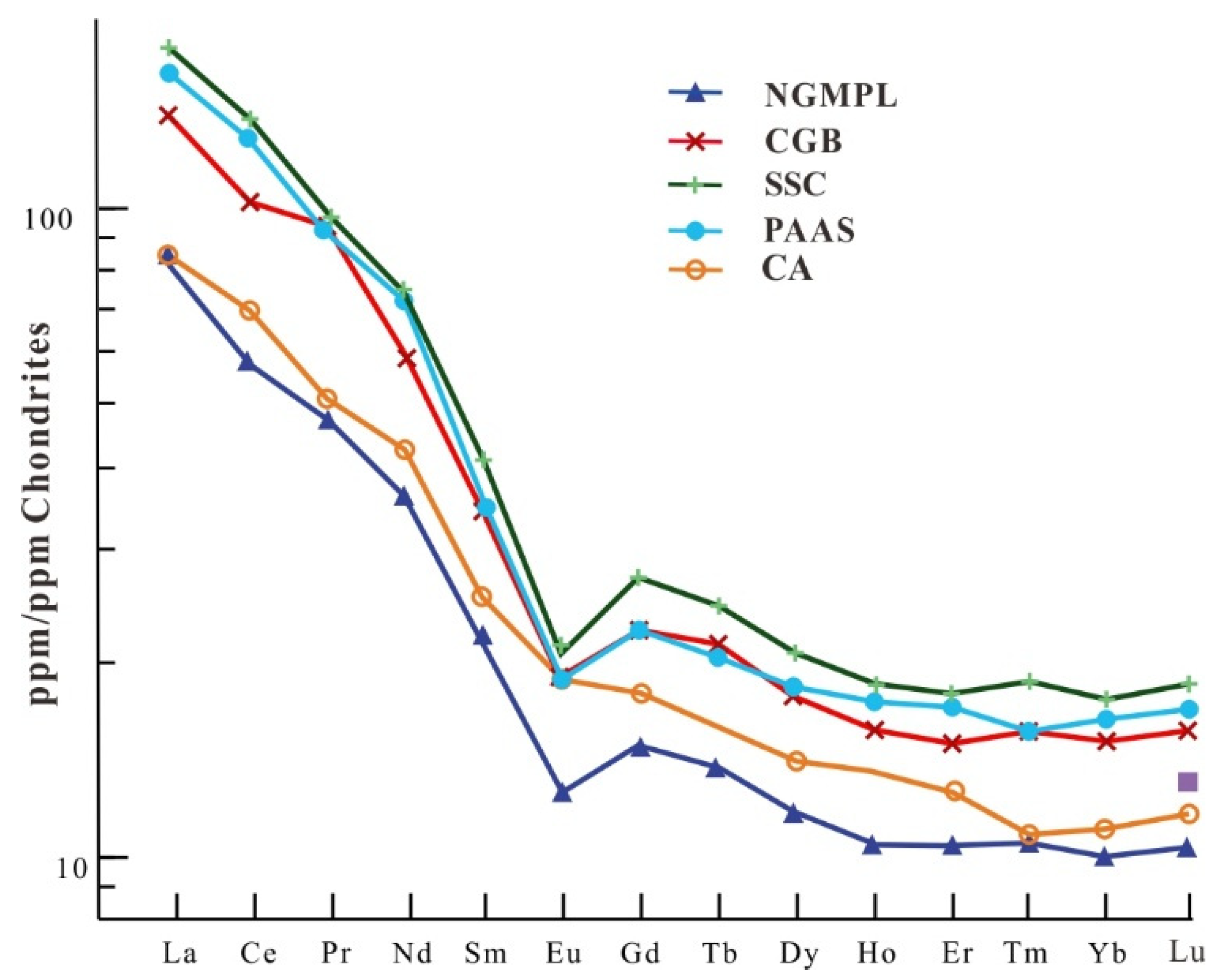
5.2. Rare Earth Elements
5.3. Elements Related to Mineralization
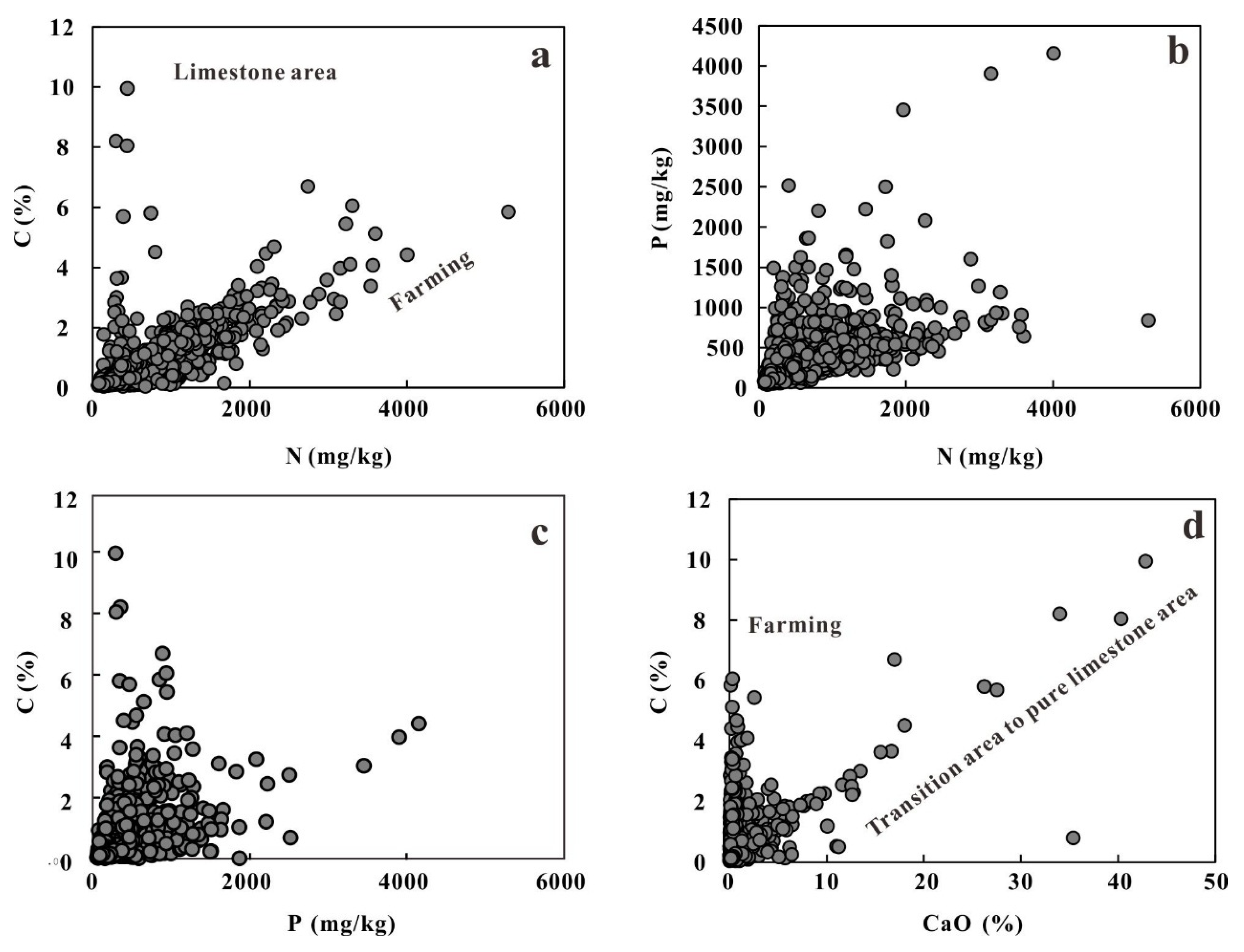
5.4. Elements Impacted by Human Activities
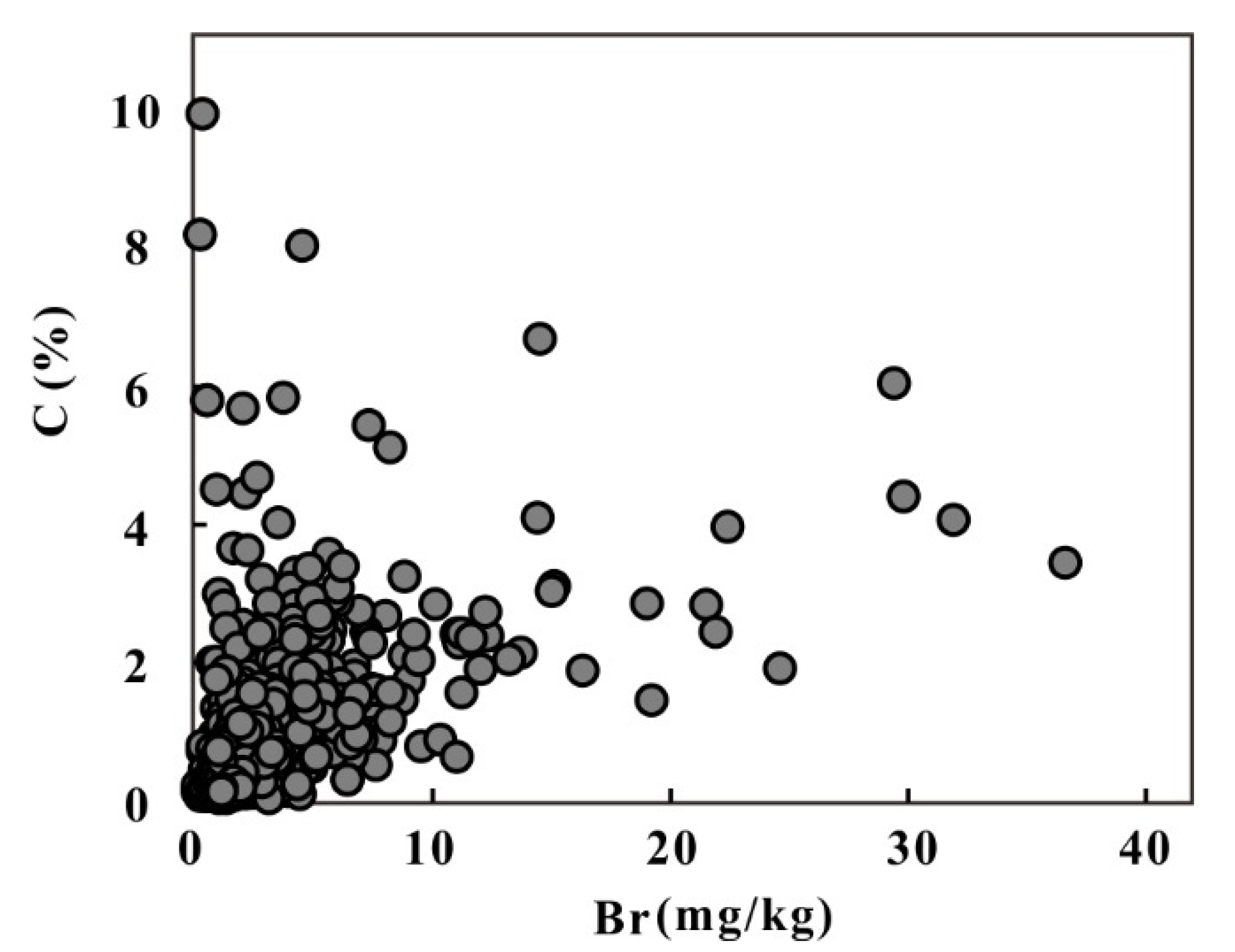
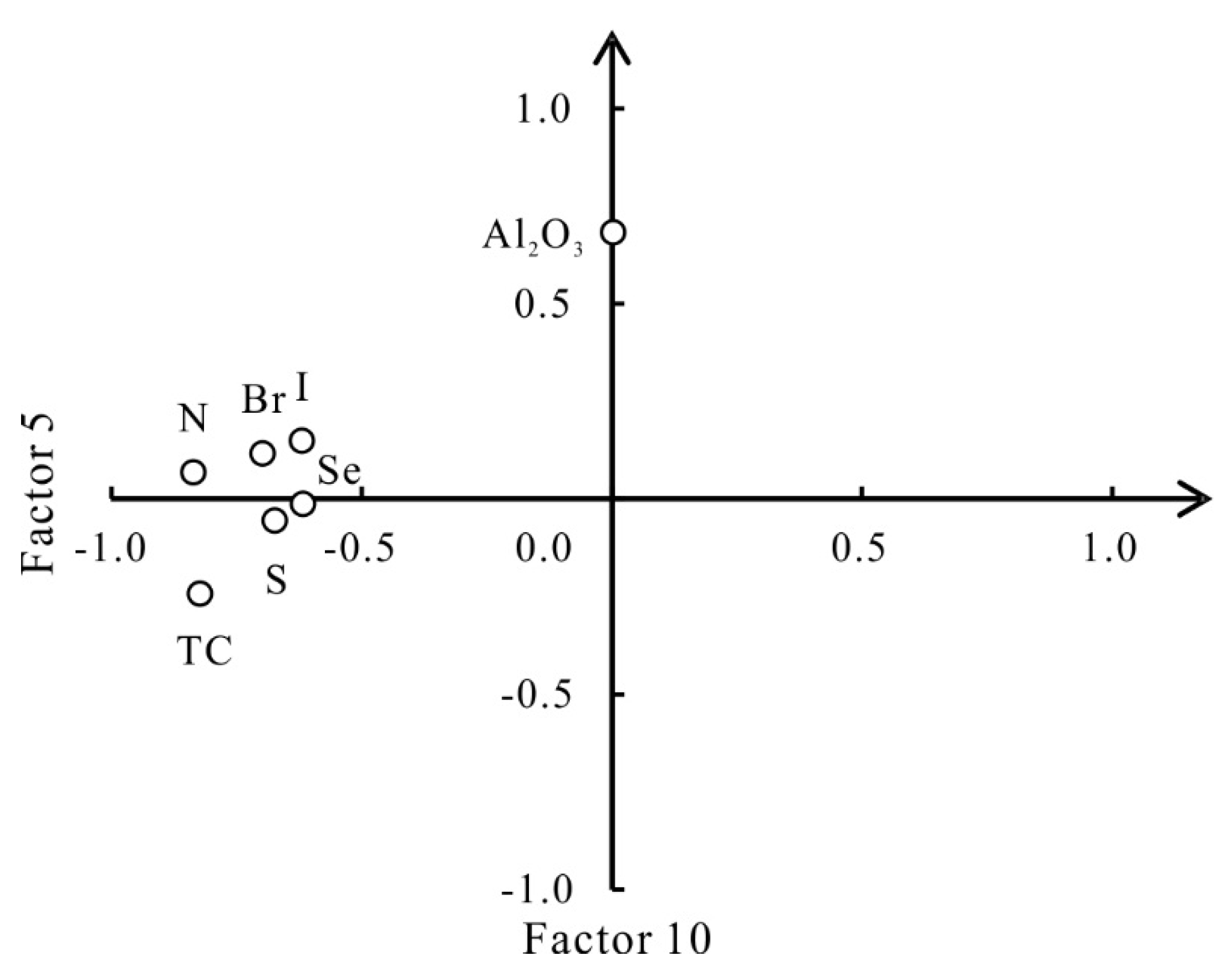
5.5. Halogen Elements Linked to Climate
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ottesen, R.T.; Bogen, J.; Bølviken, B.; Volden, T. Overbank sediment: A representative sample medium for regional geochemical mapping. J. Geochem. Explor. 1989, 32, 257–277. [Google Scholar] [CrossRef]
- Bölviken, B.; Bogen, J.; Demtriades, A.; De, V.W.; Eeeing, J.; Hindel, R.; Langedal, M.; Locutura, J.; O’Connor, P.; Ottesen, R.T.; et al. Regional geochemical mapping of Western Europe towards the year 2000. J. Geochem. Explor. 1996, 56, 141–166. [Google Scholar] [CrossRef]
- Elsenbroek, J.H.; Neser, J.A. An environmental application of regional geochemical mapping in understanding enzootic geophagia of calves in the Reivilo area, South Africa. Environ. Geochem. Health 2002, 24, 159–181. [Google Scholar] [CrossRef]
- Wang, X.Q. Exploration geochemistry: Past achievements and future challenges. Earth Sci. Front. 2003, 10, 239–248. [Google Scholar]
- Ohta, A.; Imai, N.; Terashima, S.; Tachibana, Y. Application of multi-element statistical analysis for regional geochemical mapping in Central Japan. Appl. Geochem. 2005, 20, 1017–1037. [Google Scholar] [CrossRef]
- Xie, X. Development of geochemical mapping. Geol. Bull. China 2008, 27, 163–168. [Google Scholar]
- Wang, X.Q. Landmark events of exploration geochemistry in the past 80 years. Geol. China 2013, 40, 322–330. [Google Scholar]
- Cheng, Z.; Xie, X.; Yao, W.; Feng, J.; Zhang, Q.; Fang, J. Multi-element geochemical mapping in Southern China. J. Geochem. Explor. 2014, 139, 183–192. [Google Scholar] [CrossRef]
- Lado, L.R.; Hengl, T.; Reuter, H.I. Heavy metals in European soils: A geostatistical analysis of the FOREGS Geochemical database. Geoderma 2008, 148, 189–199. [Google Scholar] [CrossRef]
- Xie, X. Global geochemical mapping. Geol. China 2003, 30, 1–9. [Google Scholar]
- Xie, X. Exploration geochemistry: Retrospect and prospect. Geol. Prospect. 2002, 38, 1–9. [Google Scholar]
- Xie, X. Field Method of Regional Geological Survey; Geological Publishing House: Beijing, China, 1979; pp. 4–10. [Google Scholar]
- Xie, X.; Ren, T.X.; Xi, X.H.; Zhang, L.S. The implementation of The Regional Geochemistry-National Reconnaissance Program (RGNR) in China in the past thirty years. Acta Geol. Sin. 2009, 30, 700–716. [Google Scholar]
- Wang, X.Q. The initiative for international cooperation project of mapping chemical earth for sustaining global resources and environments. Geol. China 2017, 44, 201–202. [Google Scholar]
- Wang, X.; The CGB Sampling Team. China geochemical baselines: Sampling methodology. J. Geochem. Explor. 2015, 154, 17–31. [Google Scholar] [CrossRef]
- Darnley, A.; Björklund, A.; Bölviken, B.; Gustavsson, N.; Koval, P.; Plant, J.; Steenfelt, A.; Tauchid, M.; Xuejing, X.; Garrett, R. A Global geochemical database for environmental and resource management. Final report of IGCP Project 259. Earth Sci. 1995, 19, 122. [Google Scholar]
- Rudnick, R.; Gao, S.; Holland, H.; Turekian, K. Composition of the continental crust. Crust 2003, 3, 1–64. [Google Scholar]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhou, J.; Xu, S.F.; Chi, Q.H.; Nie, L.S.; Zhang, B.M.; Yao, W.S.; Wang, W.; Liu, H.L.; Liu, D.S. China soil geochemical baselines networks: Data characteristics. Geol. China 2016, 43, 1469–1480. [Google Scholar]
- Lin, Y.H.; Guo, M.X.; Gan, W.M. Mercury pollution from small gold mines in China. Water Air Soil Pollut. 1997, 97, 233–239. [Google Scholar] [CrossRef]
- Wakita, K.; Metcalfe, I. Ocean plate stratigraphy in east and southeast Asia. J. Asian Earth Sci. 2005, 24, 679–702. [Google Scholar] [CrossRef]
- Yin, F.G.; Pan, G.T.; Wang, F.; Li, X.Z.; Wang, F.G. Tectonic facies along the Nujiang-Lancangjiang-Jinshajiang orogenic belt in southwestern China. Sediment. Geol. Tethyan Geol. 2006, 26, 33–39. [Google Scholar]
- Zhu, Y.J.; Wu, J.; Hu, J.J.; Cui, Z.L.; Huang, X.L.; Yan, C.M. Introduction for Geology and Mineral Resources in Laos; Yunnan Science and Technology Publishing House: Kunming, China, 2009. [Google Scholar]
- Jia, R.X.; Fang, W.X.; Wei, X.Y. General introduction of geology, mineral resources and mining exploitation in Laos. Miner. Explor. 2014, 5, 826–833. [Google Scholar]
- Zaw, K.; Meffre, S.; Lai, C.-K.; Burrett, C.; Santosh, M.; Graham, I.; Manaka, T.; Salam, A.; Kamvong, T.; Cromie, P. Tectonics and metallogeny of mainland SE Asia—A review and contribution. Gondwana Res. 2014, 26, 5–30. [Google Scholar]
- Zaw, K.; Santosh, M.; Graham, I.T. Tectonics and metallogeny of mainland SE Asia: Preface. Gondwana Res. 2014, 26, 1–4. [Google Scholar]
- Wang, H.; Lin, F.C.; Li, X.Z.; Shi, M.F. The division of tectonic units and tectonic evolution in Laos and its adjacent regions. Geol. China 2015, 42, 71–84. [Google Scholar]
- Liu, S.S.; Yang, Y.F.; Guo, L.N.; Nie, F.; Peng, Z.M.; Pan, G.T. Tectonic characteristics and metallogeny in Southeast Asia. Geol. China 2018, 45, 863–889. [Google Scholar]
- Wang, W.; Wang, X.Q.; Zhang, B.M.; Han, Z.X.; Laolo, S.; Souksan, P.; Liu, H.L.; Liu, D.S. National-scale geochemical mapping and prediction of metallogenic prospective areas in Laos. Acta Geol. Sin. 2020, 41, 80–90. [Google Scholar]
- Wang, X.; Metcalfe, I.; Ping, J.; He, L.; Wang, C. The Jinshajiang–Ailaoshan Suture Zone, China: Tectonostratigraphy, Age and Evolution. J. Asian Earth Sci. 2000, 18, 675–690. [Google Scholar] [CrossRef]
- Liu, J.L.; Tang, Y.; Song, Z.J.; Dung, T.M.; Zhai, Y.F.; Wu, W.B.; Chen, W. The Ailaoshan Belt in Western Yunnna: Tectonic Framework and Tectonic Evolution. J. Jilin Univ. Earth Sci. Ed. 2011, 41, 1285–1303. [Google Scholar]
- Lepvrier, C.; Maluski, H.; Tich, V.V.; Leyreloup, A.; Thi, P.T.; Vuong, N.V. The Early Triassic Indosinian Orogeny in Vietnam (Truong Son Belt and Kontum Massif); Implications for the Geodynamic Evolution of Indochina. Tectonophysics 2004, 393, 87–118. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.Q.; Zhang, B.M.; Nie, L.S.; Cheng, X.B.; Han, Z.X.; Liu, H.L.; Liu, D.S. Geochemical background values of 69 elements in surface sediment of the Laos. Earth Sci. 2022, 47, 2765–2780. [Google Scholar]
- Zhang, Q.; Bai, J.F.; Wang, Y. Analytical scheme and quality monitoring system for China Geochemical Baselines. Earth Sci. Front. 2012, 19, 33–42. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.G. Factor analysis applied to regional geochemical data: Problems and possibilities. Appl. Geochem. 2002, 17, 185–206. [Google Scholar] [CrossRef]
- Shi, Y.X.; Ji, H.J.; Lu, J.L.; Ma, L.; Duan, G.Z. Factor analysismenthod and application of stream sediment geochemical partition. Geol. Prospect. 2004, 40, 73–76. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.; Dutter, R. Principal component analysis (PCA) and factor analysis (FA). In Statistical Data Analysis Explained: Applied Environmental Statistics with R; Wiley: Hoboken, NJ, USA, 2008; pp. 211–232. [Google Scholar]
- Aitchison, J. The statistical analysis of compositional data. J. R. Stat. Soc. Ser. B Methodol. 1982, 44, 139–160. [Google Scholar] [CrossRef]
- Thió-Henestrosa, S.; Martín-Fernández, J.A. Dealing with compositional data: The freeware CoDaPack. Math. Geol. 2005, 37, 773–793. [Google Scholar] [CrossRef]
- Thió-Henestrosa, S.; Martín-Fernández, J.A. Detailed guide to CoDaPack: A freeware compositional software. Geol. Soc. Lond. Spec. Publ. 2006, 264, 101–118. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Guimarães, J.T.F.; Souza-Filho, P.W.M.; Powell, M.A.; da Silva, M.S.; Moraes, A.M.; Alves, R.; Leite, A.S.; Júnior, W.N.; Rodrigues, T.M. Statistical analysis of lake sediment geochemical data for understanding surface geological factors and processes: An example from Amazonian upland lakes, Brazil. Catena 2019, 175, 47–62. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Dall’Agnol, R.; Salomão, G.N.; Junior, J.D.S.F.; Silva, M.S.; Souza Filho, P.W.M.; da Costa, M.L.; Angélica, R.S.; Medeiros Filho, C.A.; da Costa, M.F. Regional-scale mapping for determining geochemical background values in soils of the Itacaiúnas River Basin, Brazil: The use of compositional data analysis (CoDA). Geoderma 2020, 376, 114504. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.Q.; Liu, H.L.; Tian, M.; Zhang, B.Y.; Li, R.H.; Yang, D.P.; Xiong, Y.X. Targeting deep-seated gold deposits: A study from the qujia gold deposit, Shandong province, China. Appl. Geochem. 2021, 130, 104982. [Google Scholar] [CrossRef]
- Hawkes, H.; Webb, J. Geochemistry in Mineral Exploration; Harper: New York, NY, USA, 1962. [Google Scholar]
- Reimann, C.; Garrett, R.G. Geochemical background—Concept and reality. Sci. Total Environ. 2005, 350, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Apollaro, C.; Di Curzio, D.; Fuoco, I.; Buccianti, A.; Dinelli, E.; Vespasiano, G.; Castrignanò, A.; Rusi, S.; Barca, D.; Figoli, A. A multivariate non-parametric approach for estimating probability of exceeding the local natural background level of arsenic in the aquifers of Calabria region (Southern Italy). Sci. Total Environ. 2022, 806, 150345. [Google Scholar] [CrossRef] [PubMed]
- Ming, C.; Ma, L.Q.; Hoogeweg, C.G.; Harris, W.G. Arsenic Background Concentrations in Florida, USA Surface Soils: Determination and Interpretation. Environ. Forensics 2001, 2, 117–126. [Google Scholar]
- Zoback, M.L. Grand challenges in earth and environmental sciences: Science, stewardship, and service for the Twenty-First Century. GSA Today 2001, 11, 41–47. [Google Scholar]
- Reimann, C.; Demetriades, A.; Eggen, O.A.; Filzmoser, P.; Schedl, A.; Reitner, H.; Haslinger, E.; Walter, D.V.; Hrvatovic, H.; Miosic, N. The EuroGeoSurveys GEochemical Mapping of Agricultural and grazing land Soils project (GEMAS)-Evaluation of quality control results of total C and S, total organic carbon (TOC), cation exchange capacity (CEC), XRF, pH, and particle size distribution (PSD) analysis. Geochem. Mapp. Agric. Soils 2011, 90. [Google Scholar]
- Stoll, H.M.; Schrag, D.P. Coccolith Sr/Ca as a new indicator of coccolithophorid calcification and growth rate. Geochem. Geophys. Geosystems 2000, 1, 1–24. [Google Scholar] [CrossRef]
- Stoll, H.M.; Schrag, D.P. Effects of Quaternary sea level cycles on strontium in seawater. Geochim. Cosmochim. Acta 1998, 62, 1107–1118. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Liu, Y.J.; Cao, L.M.; Li, Z.L.; Wang, H.N.; Chu, T.Q.; Zhang, J.R. Elemental Geochemistry; Science Press: Beijing, China, 1984. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Mclennan, S.M. Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes. In Geochemistry and Mineralogy of Rare Earth Elements; De Gruyter: Berlin, Germany, 2018; pp. 169–200. [Google Scholar]
- Cheng, Z.Z.; Xie, X.J.; Pan, H.J.; Yang, R.; Shang, Y.T. Abundance of elements in stream sediment in South China. Earth Sci. 2011, 18, 289–295. [Google Scholar]
- Balashov, Y.A. The effects of climate and facies environment on the fractionation of rare earths during sedimentation. Geochem. Int. 1964, 10, 951–969. [Google Scholar]
- Bhatia, M.R. Rare earth element geochemistry of Australian Paleozoic graywackes and mudrocks: Provenance and tectonic control. Sediment. Geol. 1985, 45, 97–113. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wang, D.H.; Xu, Z.G. Important Mineral Resources and Regional Metallogenic Regularity in China; Tang, J.C., Bai, T., Lv, J., Han, B., Li, J., Yu, C.L., Eds.; Geological Publishing House: Beijing, China, 2015; pp. 344–368. [Google Scholar]
- Wang, X.Q.; Zhou, J.; Chi, Q.H.; Wang, W.; Zhang, B.M.; Nie, L.S.; Liu, D.S.; Xu, S.F.; Wu, H.; Gao, Y.F. Geochemical background and distribution of rare earth elements in China: Implications for potential prospects. Acta Geosci. Sin. 2020, 41, 747–758. [Google Scholar]
- Lu, L.; Wang, D.H.; Wang, C.H.; Zhao, Z.; Feng, W.J.; Xu, X.C.; Chen, C.; Zhong, H.R. The metallogenic regularity of ion-adsorption type REE deposit in Yunnan Provice. Acta Geosci. Sin. 2020, 94, 184–196. [Google Scholar]
- Zhao, H.J.; Chen, Y.Q.; Lu, Y.X. Ore-forming model for Cu-Au-Fe ore deposits associated with granites in the Truongson ore-forming belt of Laos. Geol. Bull. China 2011, 30, 1619–1627. [Google Scholar]
- Deng, J.; Wang, Q.F.; Li, G.J.; Hou, Z.Q.; Jiang, C.Z.; Danyushevsky, L. Geology and genesis of the giant Beiya porphyry–skarn gold deposit, northwestern Yangtze Block, China. Ore Geol. Rev. 2015, 70, 457–485. [Google Scholar] [CrossRef]
- Zhu, Y.F.; An, F.; Tan, J.J. Geochemistry of hydrothermal gold deposits: A review. Geosci. Front. 2011, 2, 367–374. [Google Scholar] [CrossRef]
- Hu, R.Z.; Su, W.C.; Bi, X.W.; Tu, G.Z.; Hofstra, A.H. Geology and geochemistry of Carlin-type gold deposits in China. Miner. Depos. 2002, 37, 378–392. [Google Scholar]
- Morrison, G.W.; Rose, W.J.; Jaireth, S. Geological and geochemical controls on the silver content (fineness) of gold in gold-silver deposits. Ore Geol. Rev. 1991, 6, 333–364. [Google Scholar] [CrossRef]
- Hough, R.M.; Butt, C.R.; Fischer-Bühner, J. The crystallography, metallography and composition of gold. Elements 2009, 5, 297–302. [Google Scholar] [CrossRef]
- Moles, N.R.; Chapman, R.J. Integration of detrital gold microchemistry, heavy mineral distribution, and sediment geochemistry to clarify regional metallogeny in glaciated terrains: Application in the Caledonides of southeast Ireland. Econ. Geol. 2019, 114, 207–232. [Google Scholar] [CrossRef]
- Knight, J.B.; Mortensen, J.K.; Morison, S.R. Lode and placer gold composition in the Klondike District, Yukon Territory, Canada; implications for the nature and genesis of Klondike placer and lode gold deposits. Econ. Geol. 1999, 94, 649–664. [Google Scholar] [CrossRef]
- Outridge, P.M.; Doherty, W.; Gregoire, D.C. Determination of trace elemental signatures in placer gold by laser ablation–inductively coupled plasma–mass spectrometry as a potential aid for gold exploration. J. Geochem. Explor. 1998, 60, 229–240. [Google Scholar] [CrossRef]
- Wang, X.Q.; Xie, X.J.; Ye, S.Y. Concepts for geochemical gold exploration based on the abundance and distribution of ultrafine gold. J. Geochem. Explor. 1995, 55, 93–101. [Google Scholar] [CrossRef]
- Lakin, H.W.; Curtin, G.C.; Hubert, A.E. Geochemistry of Gold in the Weathering Cycle: A Study of the Solubilization of Gold by the Formation of Chloride, Bromide, Iodide, Thiosulfate, Thiocyanide, and Cyanide Gold Complexes and Related Topics; US Government Printing Office: Washington, DC, USA, 1974. [Google Scholar]
- Reith, F.; Etschmann, B.; Dart, R.C.; Brewe, D.L.; Vogt, S.; Mumm, A.S.; Brugger, J. Distribution and speciation of gold in biogenic and abiogenic calcium carbonates–Implications for the formation of gold anomalous calcrete. Geochim. Cosmochim. Acta 2011, 75, 1942–1956. [Google Scholar] [CrossRef]
- Fairbrother, L.; Brugger, J.; Shapter, J.; Laird, J.S.; Southam, G.; Reith, F. Supergene gold transformation: Biogenic secondary and nano-particulate gold from arid Australia. Chem. Geol. 2012, 320, 17–31. [Google Scholar] [CrossRef]
- Lintern, M.J. The association of gold with calcrete. Ore Geol. Rev. 2015, 66, 132–199. [Google Scholar] [CrossRef]
- Fan, P.F. Accreted terranes and mineral deposits of Indochina. J. Asian Earth Sci. 2000, 18, 343–350. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.Q.; Zhang, B.M.; Nie, L.S.; Sounthone, L.; Phomsylalai, S.; Zhou, J.; Liu, H.L.; Han, Z.X.; Liu, D.S.; et al. Geochemical background and anomalies of copper in Laos. Acta Geol. Sin. 2020, 41, 861–867. [Google Scholar]
- Garwin, S.; Hall, R.; Watanabe, Y. Tectonic setting, geology, and gold and copper mineralization in Cenozoic magmatic arcs of Southeast Asia and the West Pacific. Econ. Geol. 100th Anniv. Vol. 2005, 891, 930. [Google Scholar]
- Fan, R.; Qian, G.J.; Li, Y.B.; Short, M.D.; Schumann, R.C.; Chen, M.; Smart, R.S.C.; Gerson, A.R. Evolution of pyrite oxidation from a 10-year kinetic leach study: Implications for secondary mineralisation in acid mine drainage control. Chem. Geol. 2022, 588, 120653. [Google Scholar] [CrossRef]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B.; Apollaro, C. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manag. 2022, 301, 113796. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Wang, X.Q.; Chi, Q.H.; Tian, M. Spatial variations in soil organic carbon, nitrogen, phosphorus contents and controlling factors across the “Three Rivers” regions of southwest China. Sci. Total Environ. 2021, 794, 148795. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Lamborg, C.H. Environmental Geochemistry; Lollar, B.S., Ed.; Elsevier, Ltd.: Amsterdam, The Netherlands, 2004; Volume 9, pp. 108–148. [Google Scholar]
- Wang, W.; Wang, X.Q.; Chi, Q.H.; Wu, H.; Zhang, B.M.; Xu, S.F.; Han, Z.X.; Nie, L.S.; Liu, H.L.; Liu, D.S. Geochemical characteristics of fluorine (F) in mainland China’s pedosphere: On the basis of the China Geochemical Baselines project. J. Geochem. Explor. 2020, 219, 106635. [Google Scholar] [CrossRef]
- Fuge, R. Fluorine in the environment, a review of its sources and geochemistry. Appl. Geochem. 2019, 100, 393–406. [Google Scholar] [CrossRef]
- Garrett, R.G. Essentials of Medical Geology; Selinus, O., Alloway, B.J., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., Smedley, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–57. [Google Scholar]
- Battaleb-Looie, S.; Moore, F.; Jacks, G.; Ketabdari, M.R. Geological sources of fluoride and acceptable intake of fluoride in an endemic fluorosis area, southern Iran. Environ. Geochem. Health 2012, 34, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, J.; Wang, X.Q.; Zhang, B.M.; Nie, L.S.; Liu, H.L.; Liu, D.S.; Han, Z.X.; Chi, Q.H.; Xu, S.F. Progress in national scale and geochemical mapping of Laos:taking fluorine element as an example. J. GuiLin Univ. Technol. 2019, 39, 335–340. [Google Scholar]
- Wang, W.; Wang, X.Q.; Zhang, B.M.; Chi, Q.H.; Liu, H.L.; Liu, X.M.; Zhou, J.; Liu, D.S.; Cheng, X.B.; Xu, S.F.; et al. Concentrations and spatial distribution of chlorine in the pedosphere in China: Based on the China Geochemical Baselines Project. J. Geochem. Explor. 2022, 242, 107089. [Google Scholar] [CrossRef]



| Analysis Method | Items | Elements Measured |
|---|---|---|
| Inductively coupled plasma optical emission spectrometry (ICP-OES) | 7 | CaO, MgO, Na2O, Be, Li, Mn, Sr |
| X-ray fluorescence spectrometry (XRF) | 20 | SiO2, Al2O3, Tot. Fe2O3, KO2, Ba, Br, Cl, Co, Cr, Cu, Ga, Nb, Ni, P, Rb, S, Ti, V, Zn, Zr |
| Inductively coupled plasma mass spectrometry (ICP-MS) | 29 | Bi, Cd, Cs, Hf, In, Mo, Pb, Sc, Ta, Te, Th, Tl, U, W, La, Y, Ce, Dy, Er, Eu, Gd, Ho, Lu, Nd, Pr, Sm, Tb, Tm, Yb |
| Atomic fluorescence spectrometry (AFS) | 5 | As, Hg, Sb, Se, Ge |
| Emission spectrometry (ES) | 3 | Ag, B, Sn |
| Ion selective electrode (ISE) | 1 | F |
| Colorimetry (COL) | 1 | I |
| Oxidative combustion and gas chromatography (GC) | 2 | C, N |
| Graphite furnace/Flame atomic absorption spectrometry (GF-AAS) | 1 | Au |
| ID | Element | Minimum Requirement | ID | Element | Minimum Requirement | ||
|---|---|---|---|---|---|---|---|
| Analysis Method | LoD | Analysis Method | LoD | ||||
| 1 | Ag | ES | 20 | 36 | Sr | ICP-OES | 5 |
| 2 | As | AFS | 1 | 37 | Ta | ICP-MS | 0.1 |
| 3 | Au | GF-AAS | 0.2 | 38 | Te | ICP-MS | 0.01 |
| 4 | B | ES | 1 | 39 | Th | ICP-MS | 1.0 |
| 5 | Ba | XRF | 5 | 40 | Ti | XRF | 10 |
| 6 | Be | ICP-OES | 0.5 | 41 | Tl | ICP-MS | 0.1 |
| 7 | Bi | ICP-MS | 0.05 | 42 | U | ICP-MS | 0.1 |
| 8 | Br | XRF | 1.0 | 43 | V | XRF | 5 |
| 9 | Cd | ICP-MS | 20 | 44 | W | ICP-MS | 0.2 |
| 10 | Cl | XRF | 20 | 45 | Zn | XRF | 2 |
| 11 | Co | XRF | 1 | 46 | Zr | XRF | 2 |
| 12 | Cr | XRF | 5 | 47 | Y | ICP-MS | 1 |
| 13 | Cs | ICP-MS | 1 | 48 | La | ICP-MS | 1 |
| 14 | Cu | XRF | 1 | 49 | Ce | ICP-MS | 1 |
| 15 | F | ISE | 100 | 50 | Pr | ICP-MS | 0.1 |
| 16 | Ga | XRF | 2 | 51 | Nd | ICP-MS | 0.1 |
| 17 | Ge | AFS | 0.1 | 52 | Sm | ICP-MS | 0.1 |
| 18 | Hf | ICP-MS | 0.2 | 53 | Eu | ICP-MS | 0.1 |
| 19 | Hg | AFS | 2 | 54 | Gd | ICP-MS | 0.1 |
| 20 | I | COL | 0.5 | 55 | Tb | ICP-MS | 0.1 |
| 21 | In | ICP-MS | 0.02 | 56 | Dy | ICP-MS | 0.1 |
| 22 | Li | ICP-OES | 1 | 57 | Ho | ICP-MS | 0.1 |
| 23 | Mn | ICP-OES | 10 | 58 | Er | ICP-MS | 0.1 |
| 24 | Mo | ICP-MS | 0.2 | 59 | Tm | ICP-MS | 0.1 |
| 25 | N | GC | 20 | 60 | Yb | ICP-MS | 0.1 |
| 26 | Nb | XRF | 2 | 61 | Lu | ICP-MS | 0.1 |
| 27 | Ni | XRF | 2 | 62 | SiO2 | XRF | 0.1 |
| 28 | P | XRF | 10 | 63 | Al2O3 | XRF | 0.05 |
| 29 | Pb | ICP-MS | 2 | 64 | Tot. Fe2O3 | XRF | 0.05 |
| 30 | Rb | XRF | 5 | 65 | MgO | ICP-OES | 0.05 |
| 31 | S | XRF | 30 | 66 | CaO | ICP-OES | 0.05 |
| 32 | Sb | AFS | 0.05 | 67 | Na2O | ICP-OES | 0.05 |
| 33 | Sc | ICP-MS | 1 | 68 | K2O | XRF | 0.05 |
| 34 | Se | AFS | 0.01 | 69 | TC | GC | 0.1 |
| 35 | Sn | ES | 1.0 | ||||
| Element | LGB Values of 69 Elements | CA | CGB | Enrichment/Depletion Coefficient | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Q25 | Q50 | Q75 | Q85 | Max. | LGB/CA | LGB/ CGB | |||
| Ag | 19 | 50 | 61 | 75 | 87 | 10,000 | 56 | 77 | 1.1 | 0.8 |
| As | 1 | 2 | 5 | 9 | 12 | 2755 | 3 | 9 | 1.7 | 0.6 |
| Au | 0.1 | 0.6 | 1.0 | 1.5 | 2.0 | 914.0 | 1.3 | 1.3 | 0.8 | 0.8 |
| B | 2 | 21 | 34 | 52 | 63 | 334 | 11 | 43 | 3.1 | 0.8 |
| Ba | 11 | 151 | 243 | 345 | 421 | 4673 | 456 | 512 | 0.5 | 0.5 |
| Be | 0.1 | 0.8 | 1.2 | 1.7 | 2.0 | 7.1 | 1.9 | 2.0 | 0.6 | 0.6 |
| Bi | 0.03 | 0.10 | 0.18 | 0.29 | 0.36 | 46.62 | 0.18 | 0.30 | 1.0 | 0.6 |
| Br | 0.1 | 1.1 | 1.5 | 2.1 | 2.8 | 36.6 | 0.9 | 2.2 | 1.7 | 0.7 |
| Cd | 1 | 37 | 63 | 107 | 150 | 41,650 | 80 | 137 | 0.8 | 0.5 |
| Cl | 21 | 51 | 61 | 73 | 81 | 5615 | 244 | 78 | 0.3 | 0.8 |
| Co | <1 | 5 | 9 | 14 | 18 | 243 | 27 | 11 | 0.3 | 0.8 |
| Cr | 2 | 26 | 43 | 62 | 78 | 24,101 | 135 | 53 | 0.3 | 0.8 |
| Cs | <1 | 2 | 3 | 5 | 7 | 26 | 2 | 6 | 1.5 | 0.5 |
| Cu | 1 | 10 | 16 | 26 | 33 | 459 | 27 | 20 | 0.6 | 0.8 |
| F | 21 | 182 | 271 | 374 | 441 | 2717 | 553 | 488 | 0.5 | 0.6 |
| Ga | 1 | 7 | 10 | 14 | 16 | 44 | 16 | 15 | 0.6 | 0.7 |
| Ge | 0.3 | 1.1 | 1.3 | 1.4 | 1.5 | 2.5 | 1.3 | 1.3 | 1.0 | 1.0 |
| Hf | 0.8 | 3.9 | 6.1 | 9.8 | 12.4 | 76.2 | 3.7 | 6.5 | 1.6 | 0.9 |
| Hg | 3 | 18 | 29 | 50 | 68 | 806 | 30 | 26 | 1.0 | 1.1 |
| I | 0.2 | 0.5 | 0.7 | 1.2 | 1.8 | 32.6 | 0.7 | 1.1 | 1.0 | 0.6 |
| In | <0.01 | 0.03 | 0.04 | 0.06 | 0.07 | 10.16 | 0.05 | 0.05 | 0.8 | 0.8 |
| Li | 1 | 14 | 22 | 30 | 35 | 134 | 16 | 30 | 1.4 | 0.7 |
| Mn | 75 | 262 | 391 | 622 | 809 | 14,212 | 774 | 569 | 0.5 | 0.7 |
| Mo | 0.1 | 0.3 | 0.4 | 0.7 | 0.9 | 25.4 | 0.8 | 0.7 | 0.5 | 0.6 |
| N | 67 | 266 | 411 | 725 | 989 | 5290 | 56 | 707 | 7.3 | 0.6 |
| Nb | <1 | 6 | 9 | 12 | 14 | 64 | 8 | 13 | 1.1 | 0.7 |
| Ni | 1 | 9 | 15 | 24 | 30 | 237 | 59 | 24 | 0.3 | 0.6 |
| P | 42 | 199 | 312 | 439 | 526 | 4157 | 436 | 570 | 0.7 | 0.5 |
| Pb | 2 | 10 | 15 | 22 | 27 | 4695 | 11 | 22 | 1.4 | 0.7 |
| Rb | 3 | 31 | 52 | 83 | 103 | 381 | 49 | 96 | 1.1 | 0.5 |
| S | 19 | 52 | 72 | 113 | 153 | 2346 | 404 | 245 | 0.2 | 0.3 |
| Sb | 0.05 | 0.30 | 0.50 | 0.88 | 1.23 | 1284.94 | 0.20 | 0.73 | 2.5 | 0.7 |
| Sc | <1 | 5 | 8 | 12 | 14 | 49 | 22 | 10 | 0.4 | 0.8 |
| Se | 0.02 | 0.06 | 0.10 | 0.19 | 0.25 | 3.17 | 0.13 | 0.17 | 0.8 | 0.6 |
| Sn | 0.6 | 1.4 | 1.9 | 2.5 | 3.2 | 100.0 | 1.7 | 3.0 | 1.1 | 0.6 |
| Sr | 1 | 25 | 43 | 66 | 82 | 584 | 320 | 197 | 0.1 | 0.2 |
| Ta | 0.1 | 0.5 | 0.7 | 1.0 | 1.1 | 5.4 | 0.7 | 1.0 | 1.0 | 0.7 |
| Te | <0.01 | 0.02 | 0.04 | 0.04 | 0.05 | 1.25 | -- | 0.04 | -- | 1.0 |
| Th | 0.3 | 3.6 | 6.0 | 9.3 | 11.7 | 51.1 | 5.6 | 11.0 | 1.1 | 0.5 |
| Ti | 229 | 1921 | 2888 | 4009 | 4771 | 32,015 | 4200 | 3498 | 0.7 | 0.8 |
| Tl | <0.1 | 0.2 | 0.3 | 0.5 | 0.7 | 2.6 | 0.5 | 0.6 | 0.6 | 0.5 |
| U | 0.2 | 1.0 | 1.5 | 2.3 | 2.8 | 17.8 | 1.3 | 2.5 | 1.2 | 0.6 |
| V | 4 | 36 | 59 | 89 | 109 | 569 | 138 | 70 | 0.4 | 0.8 |
| W | 0.1 | 0.6 | 0.9 | 1.4 | 1.8 | 37.2 | 1.0 | 1.6 | 0.9 | 0.6 |
| Zn | 4 | 28 | 47 | 66 | 77 | 2524 | 72 | 66 | 0.7 | 0.7 |
| Zr | 23 | 119 | 188 | 268 | 323 | 1886 | 132 | 230 | 1.4 | 0.8 |
| Y | 2 | 11 | 16 | 23 | 26 | 340 | 19 | 24 | 0.8 | 0.7 |
| La | 2 | 14 | 20 | 28 | 33 | 319 | 20 | 33 | 1.0 | 0.6 |
| Ce | 4 | 25 | 36 | 53 | 63 | 385 | 43 | 64 | 0.8 | 0.6 |
| Pr | 0.5 | 3.2 | 4.5 | 6.6 | 7.7 | 57.7 | 4.9 | 7.6 | 0.9 | 0.6 |
| Nd | 1.7 | 11.9 | 17 | 24.1 | 28.3 | 177.6 | 20.0 | 28.2 | 0.9 | 0.6 |
| Sm | 0.3 | 2.3 | 3.3 | 4.7 | 5.5 | 37.6 | 3.9 | 5.3 | 0.8 | 0.6 |
| Eu | 0.1 | 0.5 | 0.7 | 1.0 | 1.2 | 7.8 | 1.1 | 1.1 | 0.6 | 0.6 |
| Gd | 0.3 | 2.1 | 3.1 | 4.3 | 4.9 | 34.9 | 3.7 | 4.6 | 0.8 | 0.7 |
| Tb | 0.1 | 0.4 | 0.5 | 0.7 | 0.8 | 6.1 | 0.6 | 0.8 | 0.8 | 0.6 |
| Dy | 0.3 | 2.0 | 3.0 | 4.2 | 4.8 | 38.3 | 3.6 | 4.5 | 0.8 | 0.7 |
| Ho | 0.1 | 0.4 | 0.6 | 0.8 | 0.9 | 7.4 | 0.8 | 0.9 | 0.8 | 0.7 |
| Er | 0.2 | 1.2 | 1.7 | 2.4 | 2.7 | 20.3 | 2.1 | 2.5 | 0.8 | 0.7 |
| Tm | <0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 2.8 | 0.3 | 0.4 | 1.0 | 0.8 |
| Yb | 0.2 | 1.2 | 1.7 | 2.4 | 2.7 | 16.7 | 1.9 | 2.6 | 0.9 | 0.7 |
| Lu | <0.1 | 0.2 | 0.3 | 0.4 | 0.4 | 2.4 | 0.3 | 0.4 | 1.0 | 0.8 |
| ΣLREE | 9.16 | 56.68 | 81.09 | 117.89 | 138.88 | 984.62 | 92.90 | 139.20 | 0.9 | 0.6 |
| ΣHREE | 2.79 | 18.42 | 27.19 | 38.13 | 43.71 | 469.14 | 32.25 | 40.70 | 0.8 | 0.7 |
| ΣREE | 11.95 | 75.10 | 108.28 | 156.02 | 182.95 | 1453.76 | 125.15 | 179.90 | 0.9 | 0.6 |
| SiO2 | 12.1 | 69.3 | 76.4 | 82.8 | 85.7 | 94.3 | 60.7 | 66.7 | 1.3 | 1.1 |
| Al2O3 | 0.55 | 6.50 | 10.21 | 12.89 | 14.42 | 27.72 | 15.88 | 11.90 | 0.6 | 0.9 |
| Tot. Fe2O3 | 0.20 | 2.10 | 3.60 | 5.41 | 6.56 | 29.09 | 7.59 | 4.20 | 0.5 | 0.9 |
| MgO | 0.02 | 0.39 | 0.60 | 0.89 | 1.09 | 7.00 | 4.72 | 1.43 | 0.1 | 0.4 |
| CaO | 0.01 | 0.15 | 0.24 | 0.46 | 0.78 | 42.83 | 6.39 | 2.74 | 0.0 | 0.1 |
| Na2O | 0.03 | 0.20 | 0.41 | 0.65 | 0.82 | 3.31 | 3.10 | 1.75 | 0.1 | 0.2 |
| K2O | 0.07 | 0.80 | 1.30 | 1.95 | 2.36 | 6.88 | 1.79 | 2.36 | 0.7 | 0.6 |
| TC | <0.1 | 0.2 | 0.4 | 0.8 | 1.2 | 10.0 | -- | 1.3 | -- | 0.3 |
| Depletion | 0.7–1.3 | Enrichment | |
|---|---|---|---|
| <0.7 | >1.3 | ||
| LGB/CA | Ba, Be, Cl, Co, Cr, Cu, F, Ga, Mn, Mo, Ni, S, Sc, Sr, Ti, Tl, V, Zn, Eu, Al2O3, Tot. Fe2O3, MgO, CaO, Na2O | Ag, Au, Bi, Cd, Ge, Hg, I, In, Nb, P, Rb, Se, Sn, Ta, Th, U, W, Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, K2O | As, B, Br, Cs, Hf, Li, N, Pb, Sb, Zr, SiO2 |
| LGB/CGB | Ba, Cl, Co, Cr, Cu, F, Ga, Mn, Ni, P, S, Sc, Sr, Te, V, Zn, La, Ce, Pr, Nd, Sm, Eu, Al2O3, TFe2O3, MgO, CaO, Na2O, K2O | Ag, Au, Be, Bi, Cd, Ge, In, Li, Mo, Nb, Pb, Rb, Ta, Th, Ti, Tl, U, Zr, Y, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, SiO2 | As, B, Br, Cs, Hf, Hg, I, N, Sb, Se, Sn, W |
| Factor | Element Association | Relations | Eigenvalue | % of Variance | Cumulative % |
|---|---|---|---|---|---|
| Factor 1 | Bi-Th-U-W-Cs-K2O-Rb-Tl-(V-Cr-Cu-Ni-Co-Ti-Tot. Fe2O3) | Acid rocks, Basic volcanic rocks | 11.02 | 15.98 | 15.98 |
| Factor 2 | Er-Tm-Ho-Yb-Lu-Y-Dy-Tb-Gd | Bedrocks | 8.83 | 12.79 | 28.77 |
| Factor 3 | SiO2-Ge-Cl-Ag-Zr | Mineralizations | 6.55 | 9.49 | 38.26 |
| Factor 4 | La-Ce-Pr-Nd-Sm-Gd | Bedrocks | 5.54 | 8.03 | 46.29 |
| Factor 5 | TC-N-S-Br-I-Se | Farming activities | 5.07 | 7.35 | 53.64 |
| Factor 6 | Sr-Na2O | Bedrocks | 3.15 | 4.57 | 58.20 |
| Factor 7 | Ta-Nb | Bedrocks | 3.14 | 4.55 | 62.75 |
| Factor 8 | Li-B | Bedrocks | 2.91 | 4.22 | 66.97 |
| Factor 9 | Sb-As | Mineralizations | 2.34 | 3.39 | 70.36 |
| Factor 10 | Al2O3 | Bedrocks | 2.24 | 3.25 | 73.61 |
| Factor 11 | Mo | Mo mineralizations | 1.57 | 2.27 | 75.88 |
| Factor 12 | Au | Au mineralizations | 1.41 | 2.05 | 77.93 |
| ΣREE (mg/kg) | ΣLREE (mg/kg) | ΣHREE (mg/kg) | L/H | δEu | δCe | (La/Sm)N | (Gd/Yb)N | (La/Yb)N | |
|---|---|---|---|---|---|---|---|---|---|
| NGMPL | 108.28 | 81.09 | 27.19 | 2.98 | 0.71 | 0.94 | 3.80 | 1.47 | 8.09 |
| CGB | 179.90 | 139.20 | 40.70 | 3.42 | 0.68 | 0.99 | 4.02 | 1.46 | 9.10 |
| Crust average | 125.15 | 92.90 | 32.25 | 2.88 | 0.89 | 1.06 | 3.31 | 1.61 | 7.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, X.; Zhang, B.; Wang, Q.; Liu, D.; Han, Z.; LAOLO, S.; SOUKSAN, P.; Liu, H.; Zhou, J.; et al. National-Scale Geochemical Baseline of 69 Elements in Laos Stream Sediments. Minerals 2022, 12, 1360. https://doi.org/10.3390/min12111360
Wang W, Wang X, Zhang B, Wang Q, Liu D, Han Z, LAOLO S, SOUKSAN P, Liu H, Zhou J, et al. National-Scale Geochemical Baseline of 69 Elements in Laos Stream Sediments. Minerals. 2022; 12(11):1360. https://doi.org/10.3390/min12111360
Chicago/Turabian StyleWang, Wei, Xueqiu Wang, Bimin Zhang, Qiang Wang, Dongsheng Liu, Zhixuan Han, Sounthone LAOLO, Phomsylalai SOUKSAN, Hanliang Liu, Jian Zhou, and et al. 2022. "National-Scale Geochemical Baseline of 69 Elements in Laos Stream Sediments" Minerals 12, no. 11: 1360. https://doi.org/10.3390/min12111360
APA StyleWang, W., Wang, X., Zhang, B., Wang, Q., Liu, D., Han, Z., LAOLO, S., SOUKSAN, P., Liu, H., Zhou, J., Cheng, X., & Nie, L. (2022). National-Scale Geochemical Baseline of 69 Elements in Laos Stream Sediments. Minerals, 12(11), 1360. https://doi.org/10.3390/min12111360





