Investigations on Strategic Element Recovery by an Underground Membrane Pilot Plant from In-Situ Extracted Bioleaching Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Scale Setup
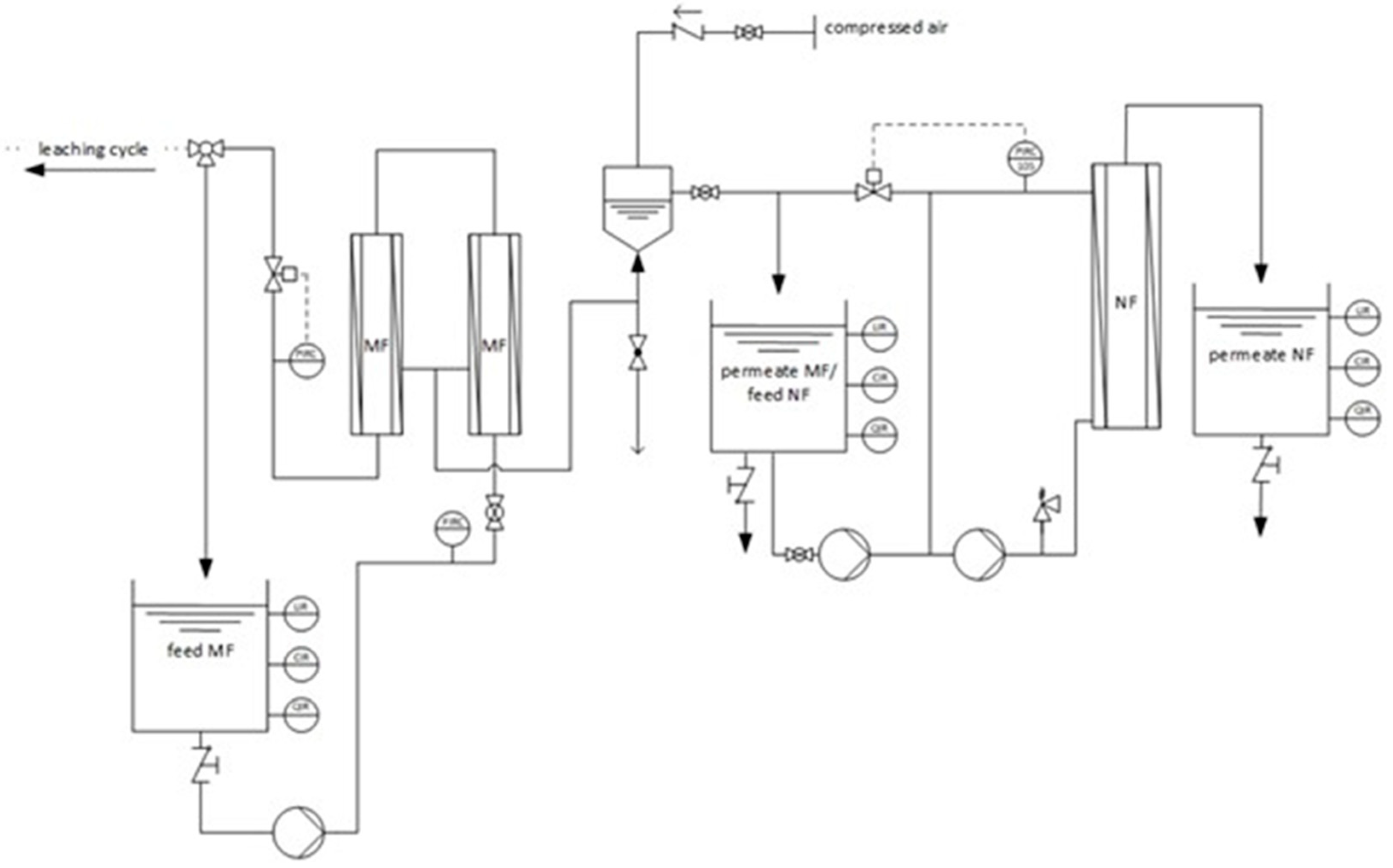
2.2. Pilot Scale Setup
2.3. Membranes
2.4. Feed Solutions
2.5. Membrane Characterization
2.6. Test Procedure
3. Results
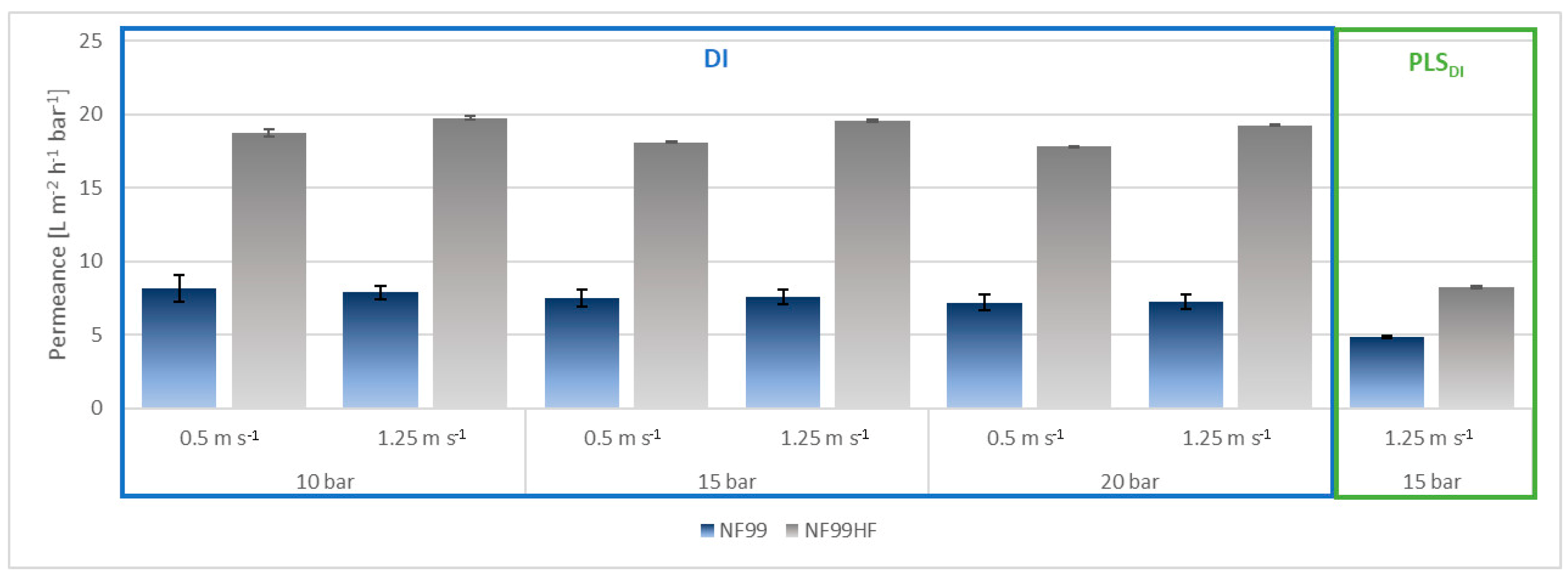
3.1. Preparatory Lab Experiments
3.2. Pilot-Scale Studies
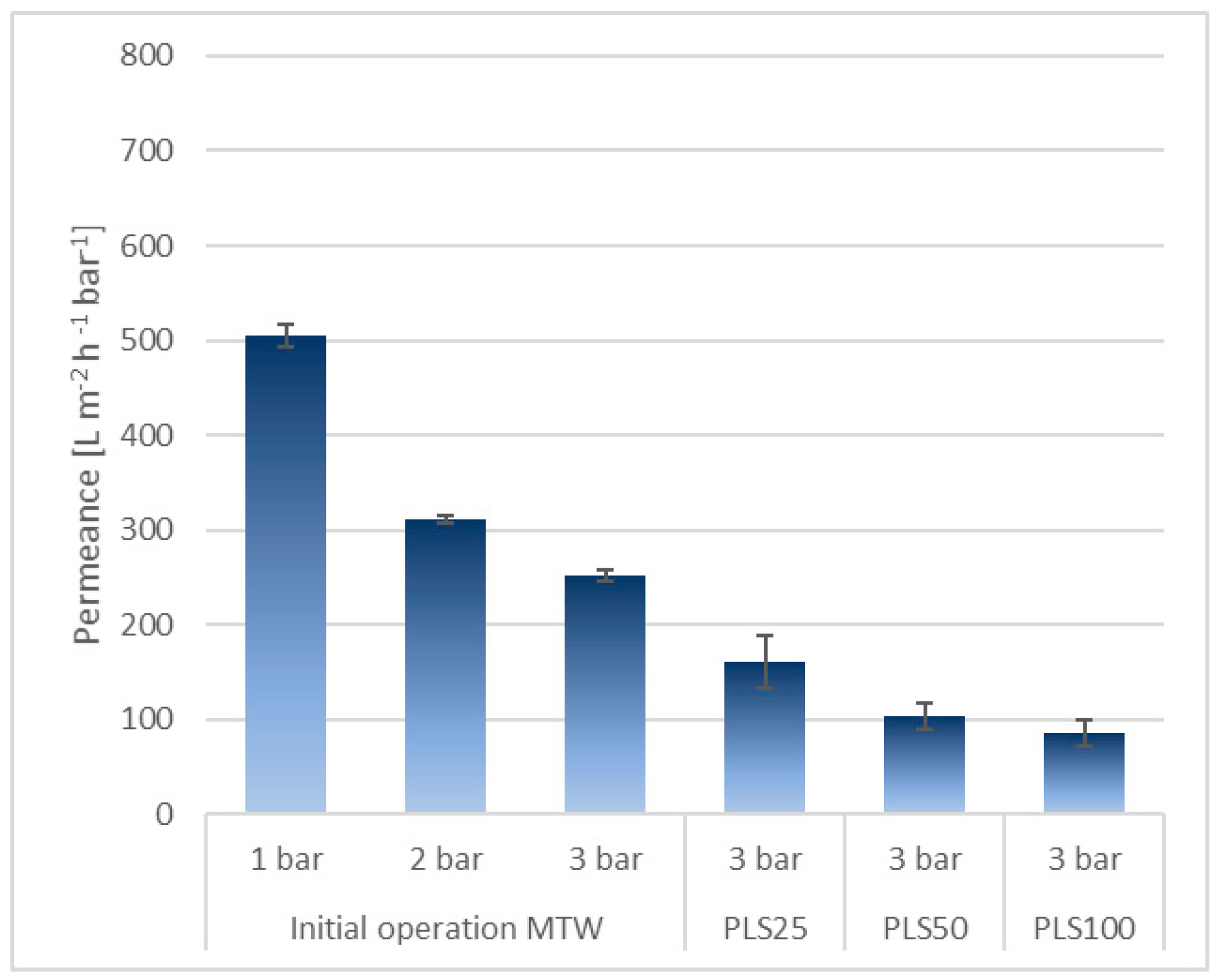
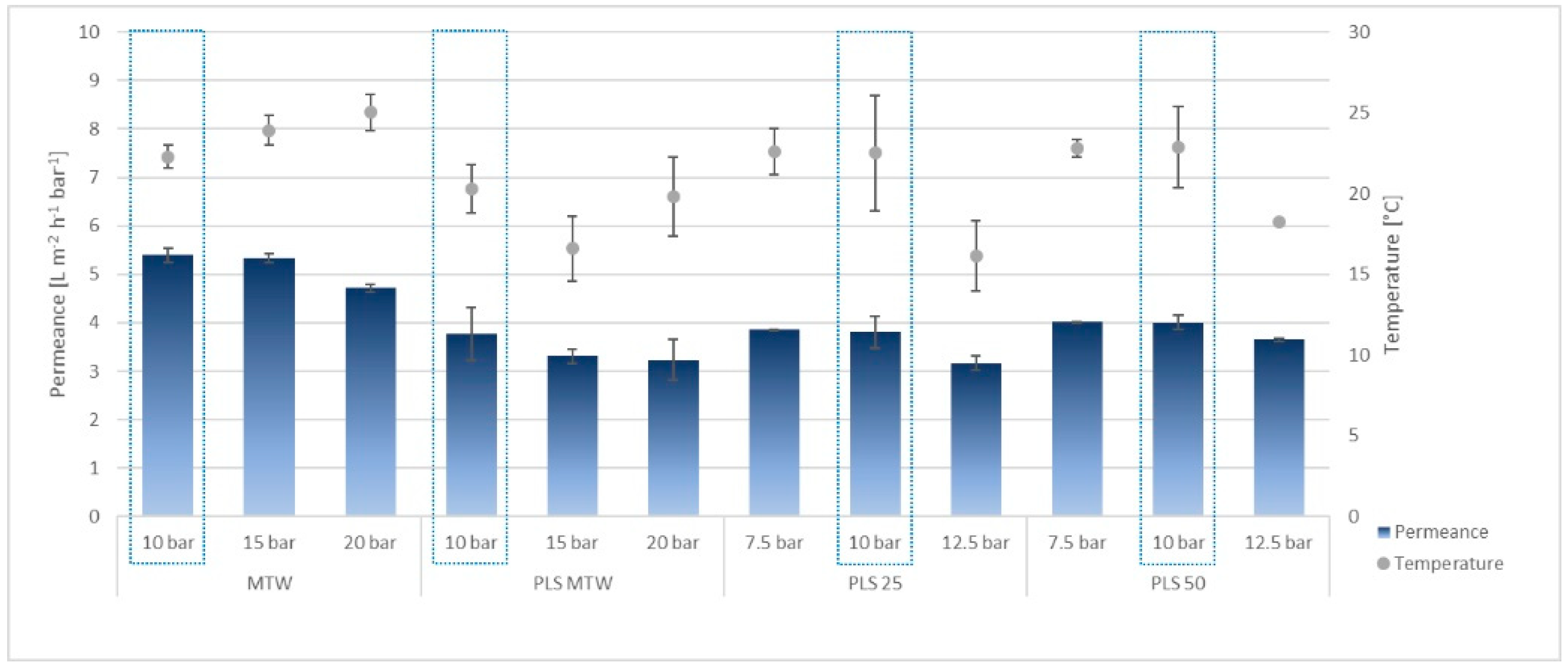
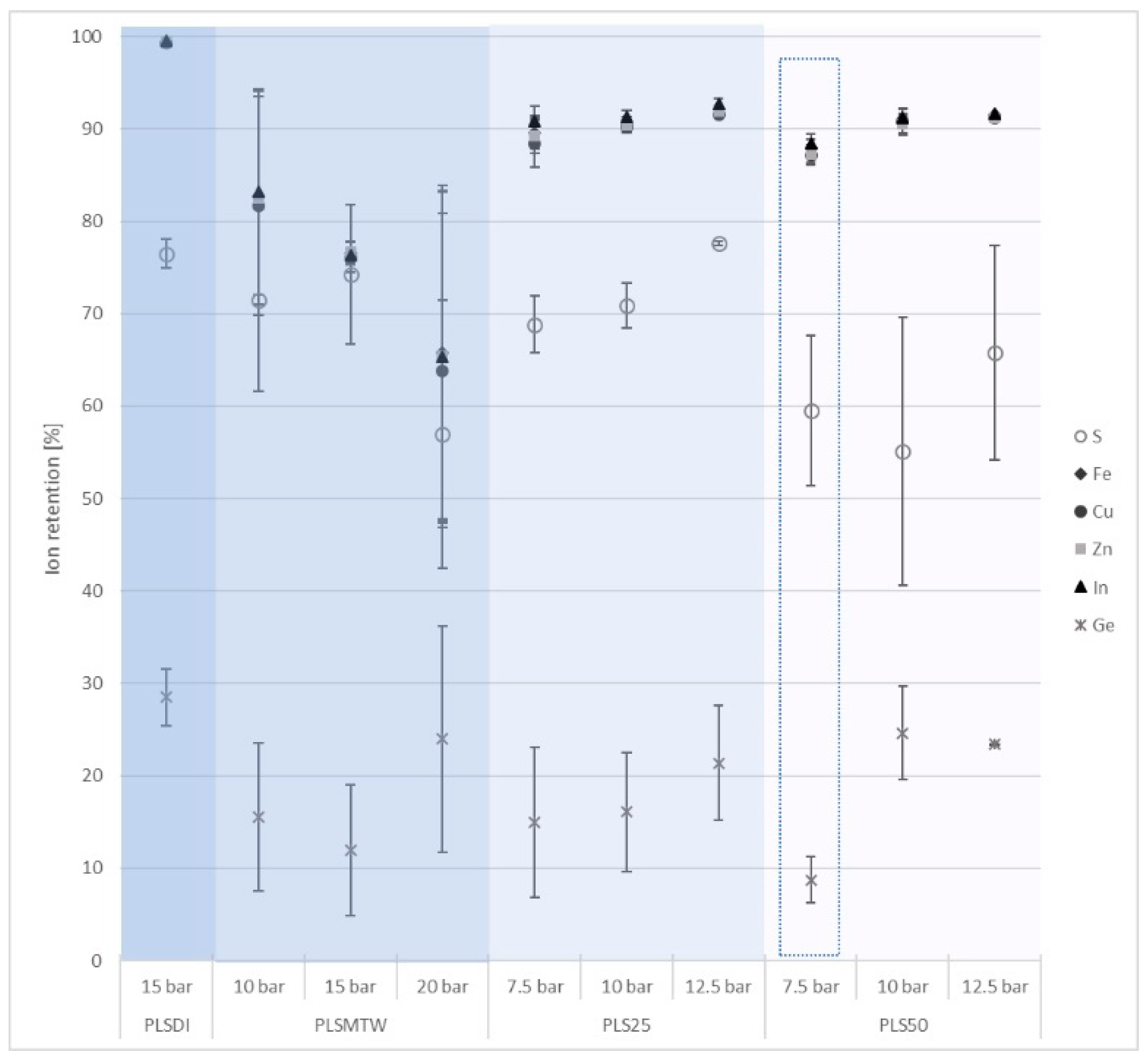
3.2.1. Microfiltration
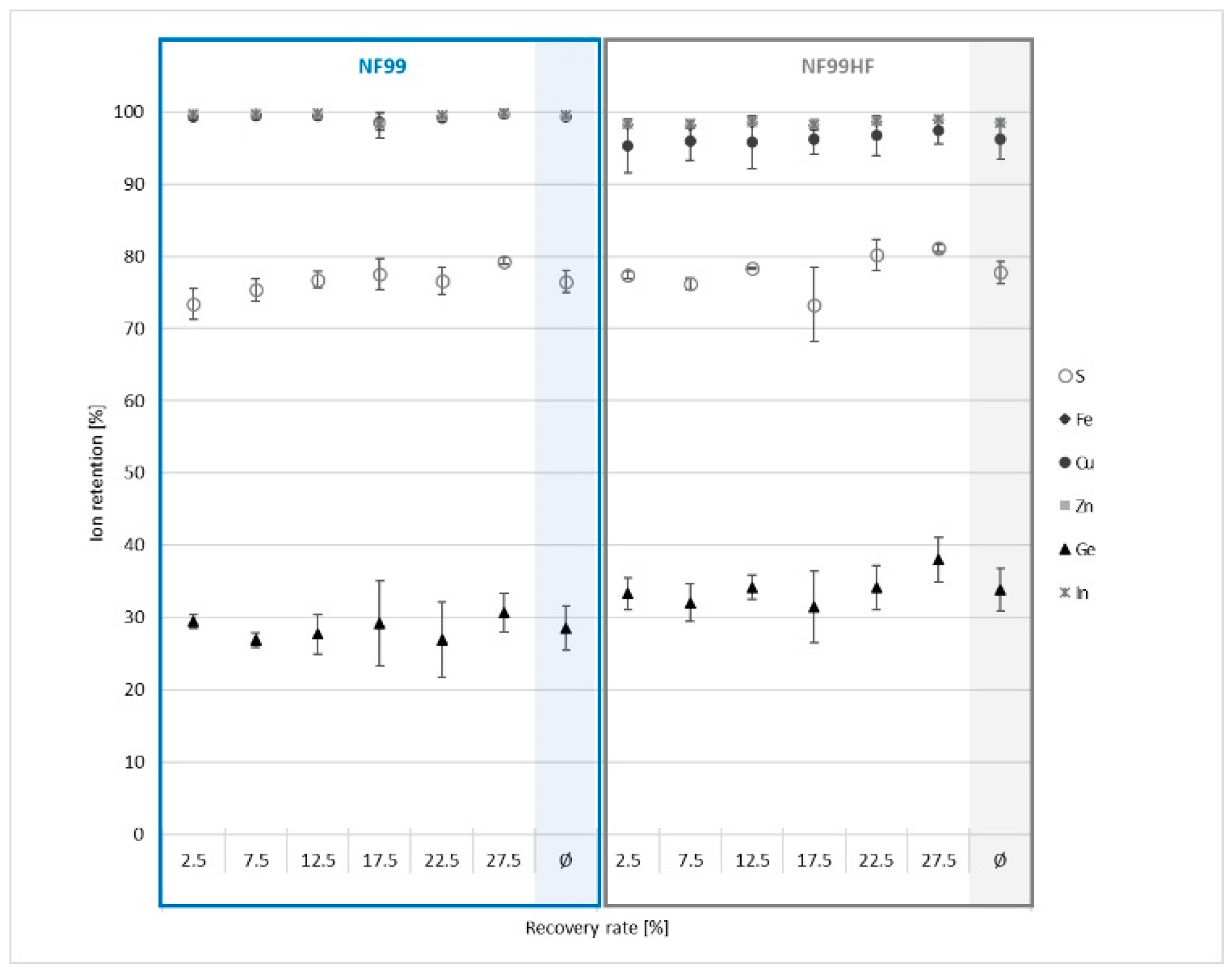
3.2.2. Nanofiltration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Study on the EU’s List of Critical Raw Materials (2020). Factsheets on Critical Raw Materials; European Commission: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- ProcessNet; Verein Deutscher Ingenieure; Deutsche Gesellschaft für Chemisches Apparatewesen, Chemische Technik und Biotechnologie. Anorganische Rohstoffe—Sicherung der Rohstoffbasis von morgen. Positionspapier des Temporären ProcessNet-Arbeitskreises “Rohstoffe und Kreislaufwirtschaft”; DECHEMA: Frankfurt am Main, Germany, 2015; ISBN 978-3-89746-177-2. [Google Scholar]
- Schlüter, R.; Mischo, H. In-situ underground bioleaching—Novel conditioning technologies. In Mining: Navigating the Global Waters, Proceedings of the 2015 SME Annual Conference & Expo and CMA 117th National Western Mining Conference, Denver, CO, USA, 15–18 February 2015; Society for Mining, Metallurgy, and Exploration; Colorado Mining Association: Denver, CO, USA, 2015; pp. 299–303. ISBN 978-151-080-124-0. [Google Scholar]
- Werner, A. Entwicklung eines Membranbasierten Trenn- und Anreicherungsverfahrens zur Selektiven Gewinnung von Indium und Germanium aus Laugungslösungen. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 13 August 2018. [Google Scholar] [CrossRef]
- Werner, A.; Haseneder, R.; Repke, J.-U. Design and Conception of a Membrane Pilot Plant for the In Situ Treatment of Bioleaching Solutions. Chem. Ing. Tech. 2019, 91, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Meschke, K. Selective separation of strategic elements germanium and rhenium from multicomponent leaching solutions by nanofiltration. Ph.D. Thesis, TU Berlin, Berlin, Germany, 20 November 2020. [Google Scholar]
- Darling, P. SME Mining Engineering Handbook, 3rd ed.; Society for Mining Metallurgy and Exploration: Littleton, CO, USA, 2011; ISBN 978-0-87335-264-2. [Google Scholar]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Pakostova, E.; Grail, B.M.; Johnson, D.B. Bio-processing of a saline, calcareous copper sulfide ore by sequential leaching. Hydrometallurgy 2018, 179, 36–43. [Google Scholar] [CrossRef]
- Zhang, R.; Hedrich, S.; Römer, F.; Goldmann, D.; Schippers, A. Bioleaching of cobalt from Cu/Co-rich sulfidic mine tailings from the polymetallic Rammelsberg mine, Germany. Hydrometallurgy 2020, 197, 105443. [Google Scholar] [CrossRef]
- Elbashier, E.; Mussa, A.; Hafiz, M.; Hawari, A. Recovery of rare earth elements from waste streams using membrane processes: An overview. Hydrometallurgy 2021, 204, 105706. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Haferburg, G.; Krichler, T.; Hedrich, S. Prokaryotic communities in the historic silver mine Reiche Zeche. Extremophiles 2022, 26, 2. [Google Scholar] [CrossRef]
- Seifer, T.; Sandmann, D. Mineralogy and geochemistry of indium-bearing polymetallic vein-type deposits: Implications for host minerals from the Freiberg district, Eastern Erzgebirge, Germany. Ore Geol. Rev 2006, 28, 1–31. [Google Scholar] [CrossRef]
- Meschke, K.; Hofmann, R.; Repke, J.-U. Membrane treatment of leached mining waste- A potential process chain for the separation of the strategic elements germanium and rhenium. Chem. Eng. J. 2020, 380, 122476. [Google Scholar] [CrossRef]
- Alfa Laval Corporate, A.B. Hygienic Spiral Membranes for Nano-Filtration. Alfa Laval NF PET Series, ESE00626EN 1410. 2016. Available online: http://www.sanitaryindustry.com/products/alfalaval/spiral-membranes--nf-spiral.html (accessed on 20 December 2020).
- Restolho, J.A.; Prates, A.; de Pinho, M.N.; Afonso, M.D. Sugars and lignosulphonates recovery from eucalyptus spent sulphite liquor by membrane processes. Biomass Bioenergy 2009, 33, 1558–1566. [Google Scholar] [CrossRef]
- Afonso, M.D. Assessment of NF and RO for the potential concentration of acetic acid and furfural from the condensate of eucalyptus spent sulphite liquor. Sep. Purif. 2012, 99, 86–90. [Google Scholar] [CrossRef]
- Kelewou, H.; Lhassani, A.; Merzouki, M.; Drogui, P.; Sellamuthu, B. Salts retention by nanofiltration membranes: Physicochemical and hydrodynamic approaches and modelling. Desalination 2011, 277, 106–112. [Google Scholar] [CrossRef]
- Melin, T.; Rautenbach, R. Membranfverfahren. Grundlagen der Modul- und Anlagenauslegung, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 3-540-00071-2. [Google Scholar]
- Van der Bruggen, B. Nanofiltration. In Encyclopedia of Membrane Science and Technology, 2nd ed.; Hoek, E.M.V., Tarabara, V.V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Volkov, A. Membrane Compaction. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Madsen, H.T.; Søgaard, E.G. Applicability and modelling of nanofiltration and reverse osmosis for remediation of groundwater polluted with pesticides and pesticide transformation products, Sep. Purif. Technol. 2014, 125, 111–119. [Google Scholar] [CrossRef]
- Park, H.-J.; Tavlarides, L.L. Germanium(IV) adsorption from aqueous solution using a Kelex-100 functional adsorbent. Ind. Eng. Chem. Res. 2009, 48, 4014–4021. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Tobiason, J.E.; Howe, K.J. Fouling indices for low pressure hollow fiber membrane performance assessment. Water Res. 2011, 45, 2627–2637. [Google Scholar] [CrossRef]
- Andrade, L.H.; Aguiar, A.O.; Pires, W.L.; Grossi, L.B.; Amaral, M.C.S. Comprehensive bench- and pilot-scale investigation of NF for gold mining effluent treatment: Membrane performance and fouling control strategies. Sep. Purif. Technol. 2017, 174, 44–56. [Google Scholar] [CrossRef]
- Afonso, M.; Pinho, M. Transport of MgSO4, MgCl2, and Na2SO4 across an amphoteric nanofiltration membrane. J. Membr. Sci. 2000, 179, 137–154. [Google Scholar] [CrossRef]
- Schipolowski, T. Experimentelle und Theoretische Untersuchungen zur Auslegung von Ultrafiltrationsverfahren. Ph.D. Thesis, TU Berlin, Berlin, Germany, 25 January 2007. [Google Scholar] [CrossRef]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Ambiado, K.; Bustos, C.; Schwarz, A.; Bórquez, R. Membrane technology applied to acid mine drainage from copper mining. Water Sci. Technol. 2017, 75, 705–715. [Google Scholar] [CrossRef]
- Ricci, B.C.; Ferreira, C.D.; Aguiar, A.O.; Amaral, M.C.S. Integration of nanofiltration and reverse osmosis for metal separation and sulfuric acid recovery from gold mining effluent. Sep. Purif. Technol. 2015, 154, 11–21. [Google Scholar] [CrossRef]
- Aguiar, A.O.; Andrade, L.H.; Ricci, B.C.; Pires, W.L.; Miranda, G.A.; Amaral, M.C.S. Gold acid mine drainage treatment by membrane separation processes: An evaluation of the main operational conditions. Sep. Purif. Technol. 2016, 170, 360–369. [Google Scholar] [CrossRef]
- Pino, L.; Vargas, C.; Schwarz, A.; Borquez, R. Influence of operating conditions on the removal of metals and sulfate from copper acid mine drainage by nanofiltration. Chem. Eng. J. 2018, 345, 114–125. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J.; Nyström, M. The role of membrane charge on nanofiltration performance. J. Membr. Sci. 2005, 265, 160–166. [Google Scholar] [CrossRef]
- Takabatake, H.; Taniguchi, M.; Kurihara, M. Advanced Technologies for Stabilization and High Performance of Seawater RO Membrane Desalination Plants. Membranes 2021, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, A.S.; Webber, M.E. Predicting the Specific Energy Consumption of Reverse Osmosis Desalination. Water 2016, 8, 601. [Google Scholar] [CrossRef]



| NF99 | NF99HF | |
|---|---|---|
| Recommended Operating Conditions 1 | ||
| pH value | 3–9 (25 °C) | |
| Temperature (°C) | 5–50 | |
| Pressure (bar) | 15–35, max. 55 | |
| Specific Membrane Characteristics | ||
| MWCO (Da) | 200 | |
| Water permeance (l m−2 h−1 bar−1) | 10 (25 °C, 4–30 bar) 2 | 9–18 (25 °C, 18 bar) 3 |
| 22 ± 2 (25 °C, 15 bar) 4 | ||
| Feed | Description |
|---|---|
| DI | Deionized Water |
| MTW | Mine tap water, percolation, and fissure water collected and stored 80 m above the pilot testing site, subject to natural fluctuations |
| PLSDI | Synthetic pregnant leach solution based on DI |
| PLSMTW | Synthetic pregnant leach solution based on MTW |
| PLS100 | 100% real pregnant leach solution, originating from the underground leaching testing site, subject to natural fluctuations |
| PLS50 | 50 wt.% of PLS100 and 50 wt.% of PLSMTW |
| PLS25 | 25 wt.% of PLS100 and 75 wt.% of PLSMTW |
| Element | Concentration (mg·L−1) | Manufacturer, Purity | |
|---|---|---|---|
| Zn | ZnSO4·H2O | 2600 | Alfa Aesar (Kandel, Germany) |
| Fe | FeSO4·7 H2O | 620 | Acros Organics (Kandel, Germany), 99+% |
| Cu | CuSO4 | 15 | Sigma-Aldrich (St. Louis, MI, USA), ≥99.0% |
| In | In2(SO4)3 | 1 | Alfa Aesar (Kandel, Germany), 99.9% |
| Ge | GeO2 | 1 | Alfa Aesar (Kandel, Germany), 99.999% |
| pH | Conductivity | TOC | Turbidity | Elements | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | Zn | Fe | Cu | Ge | In | |||||
| (-) | (mS cm−1) | (mg L−1) | (FNU) | (mg L−1) | (µg L−1) | |||||
| PLSDI | 2.0 ± 0.1 | 12.7 ± 0.1 | <1 * | 0 | 2350 ± 139 | 256 ± 78 | 643 ± 22 | 19 ± 1 | 900 ± 20 | 960 ± 30 |
| PLSMTW | 2.0 ± 0.1 | 8.9 ± 1.3 | <1 * | 3.5 ± 1.8 | 1504 ± 202 | 1859 ± 108 | 442 ± 32 | 11 ± 1 | 600 ± 100 | 600 ± 4 |
| PLS100 | 2.1 ± 0.2 | 10.4 ± 1.9 | 4.0 ± 0.2 | 126.6 ± 10.6 | 2920 ± 75 | 727 ± 49 | 959 ± 76 | 22 ± 2 | 70 ± 30 | 40 ± 0 |
| MTW | 4.8 ± 0.1 | 1.9 ± 0.2 | 1.1 ± 0.2 | 3.7 ± 1.2 | 408 ± 51 | 90 ± 8 | 1 ± 0 | 1 ± 0 | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Götze, K.; Haseneder, R.; Braeuer, A.S. Investigations on Strategic Element Recovery by an Underground Membrane Pilot Plant from In-Situ Extracted Bioleaching Solutions. Minerals 2022, 12, 46. https://doi.org/10.3390/min12010046
Götze K, Haseneder R, Braeuer AS. Investigations on Strategic Element Recovery by an Underground Membrane Pilot Plant from In-Situ Extracted Bioleaching Solutions. Minerals. 2022; 12(1):46. https://doi.org/10.3390/min12010046
Chicago/Turabian StyleGötze, Katja, Roland Haseneder, and Andreas Siegfried Braeuer. 2022. "Investigations on Strategic Element Recovery by an Underground Membrane Pilot Plant from In-Situ Extracted Bioleaching Solutions" Minerals 12, no. 1: 46. https://doi.org/10.3390/min12010046
APA StyleGötze, K., Haseneder, R., & Braeuer, A. S. (2022). Investigations on Strategic Element Recovery by an Underground Membrane Pilot Plant from In-Situ Extracted Bioleaching Solutions. Minerals, 12(1), 46. https://doi.org/10.3390/min12010046






