Abstract
An efficient sintering process was proposed based on the autocatalytic denitrification of the sintered ore. The catalytic denitrification of sintered ore, the effect of double-layer ignition sintering process on the emission reduction in nitrogen oxides, and the impact on the quality of sintered ore were studied. The results showed that the catalyzed reduction of NO with sinter ore as a catalyst has a significant effect; when the airspeed reaches 3000 h−1, the temperature is 500 °C, and the conversion rate of NO can reach 99.58%. The sinter yield of double-layer ignition sintering is increased, solid fuel consumption is slightly reduced, falling strength is slightly increased, and drum strength is slightly decreased. Under the conditions of layer height proportion of 320/400 mm (lower/upper) and ignition time interval of 10 min, the yield, drum strength, shatter strength, and solid fuel consumption reached 61.60%, 54.82%, 46.75%, and 69.55%, respectively. NOx concentration under the 16% baseline oxygen content () in the flue gas of double-layer ignition sintering is reduced to a certain extent, and the generation time of NOx is greatly shortened. The double-layer ignition sintering process can reduce the emission of nitrogen oxides in the sintering process under the condition of guaranteeing the quality of sinter, which has great economic and environmental benefits.
1. Introduction
Nitrogen oxide (NOx) is a major air pollutant [1]. The excess emission of NOx has brought harm and trouble to human beings repeatedly due to its role in the formation of photochemical smog and acid rain [2,3]. The iron and steel industry is one of the major polluters in the industrial field; the NOx emissions from iron and steel industry approximately account for 6% of the total industrial emissions in China [4,5]. Meanwhile, sintering, as one of the most polluting processes in steel production, accounts for about 50% of the total NOx emissions of iron and steel production [6,7]. According to the formation mechanism, NOx can be divided into three types: thermal NOx, prompt NOx, and coal NOx [8,9]. In the sintering process, NOx mainly comes from coal combustion, accounting for about 80%–90% of the total NOx emissions from the sintering machine [10]. In the process of NOx production by coal combustion, NO accounts for about 95%, NO2 only accounts for about 5%, and N2O is very small [11]. In 2019, the Ministry of Ecology and Environment of the People’s Republic of China implemented “Opinions on promoting the implementation of ultra-low emissions in the steel industry”, which point out that the steel industry has begun to implement ultra-low emission policies, especially the average hourly NOx emission concentration in sintering machine flue gas, which is strictly controlled to 50 mg/m3, which has brought tremendous environmental pressure to iron and steel enterprises. Therefore, the comprehensive treatment of NOx in sintering process has become the top priority of environmental protection standards for iron and steel enterprises.
Recent and ongoing efforts aim at further reducing ambient NOx concentration globally, which mainly includes source control, process control, and end-of-pipe treatment technology. At present, resource control mainly includes the use of low-nitrogen coke powder and biomass carbon to replace high nitrogen fuel [12], the use of new low-nitrogen burners, etc. In terms of process control, it mainly includes adjusting sintering process parameters, using sintering additives [2,13], fuel pretreatment [14], gas/steam injection [15], flue gas circulation [16], etc. The main processes of the end-of-pipe treatment technology include selective non-catalytic reduction (SNCR) [17,18,19], selective catalytic reduction (SCR) [20,21,22], activated carbon adsorption [23], and oxidative absorption [24]. The NOx concentration in sintering flue gas can be reduced to below 50 mg/m3 by the end-of-pipe treatment technology, but there are many problems such as high investment in denitrification equipment, easy deactivation of catalyst, and high denitrification operation cost [10]. Therefore, the treatment of sintering flue gas should be transformed from relying on a single terminal treatment technology to process control coordinated the end-of-pipe treatment technology.
Flue gas recirculation technology is proposed by researchers aiming to reducing the exhaust gas emission and reusing waste heat in 20th century, which is developed based on a principle that parts of waste gases recycled into sintering bed [4,25]. Flue gas recirculation (FGR) is a method reported to effectively reduce NOx pollutant emissions by recirculating part of the flue gas [26,27,28,29], such as the emission-optimized sintering process—EOS [30]. Linz steelworks and Siemens VAI have developed an improved denitrification process with recycling flue gas—eposint [31]. In the sinter zone, NO–CO catalytic reduction occurs in the range of 500–900 °C. When the sinter temperature is 700 °C, the highest nitrogen reduction ratio (NRR) achieved is 8%; NOx in flue gas is mainly a product of fuel combustion in the combustion zone, as the nitrogen conversion rate reaches 50%–60%, because N-containing intermediates exist during the fuel combustion. The NRR in the combustion zone reaches a range of 18%–20% [16,32]. If sinter can be used as denitrification catalyst, it can be used in a large number of production applications. In the sintering system, the calcium ferrite reaction generated in the sintering process plays a catalytic role in the reduction reaction of NOx generated in the same system [33], but the paper only discusses the catalytic reduction of NOx in the calcium ferrite system, and it is difficult to realize the preparation and application of calcium ferrite in the actual production process. Sinter is the target product of the sintering process.
The double-layer ignition sintering method [34] makes full use of the lower sinter, itself containing a reduction in the catalyst composition, using less high-temperature sintering ore in the sintering process, as denitration catalysts, effective in the sintering process of NOx catalytic reduction, environmental protection, and synchronous sintering and denitration online, ensure the efficiency of sintering and the demand of the catalyst and greatly reduce the sintering process of denitration operation costs.
This research aims to use sintered ore as a denitrification catalyst to study the effect of sintered ore on the reduction of nitrogen oxides and to explore the autocatalytic denitrification based on the sinter bed. Meanwhile, the effect of double-layer ignition sintering process on the emission reduction of nitrogen oxides and the impact on the quality of sintered ore was studied; research results can be used to control the sintering nitrogen oxide process to reduce the production and emission of nitrogen oxides, which has very important environmental protection significance and economic prospects.
2. Experiment
2.1. Experimental Materials
2.1.1. Raw Materials for Catalytic Reduction
In this experiment, sintered ore obtained from a steel plant was used as denitrification catalyst. The main chemical compositions of sintered ores tested by ICP-AES are shown in Table 1. The results showed that the total iron content and FeO content are 56.89% and 7.32%, respectively. The binary basicity, defined as the ratio of CaO and SiO2 mass content in the sintered ores, was 1.98.

Table 1.
Chemical composition of catalyst/wt%.
The phase composition of sintered ore tested by an X-ray diffractometer (XRD, D/Max-2500, Rigaku Co., Tokyo, Japan) was shown in Figure 1. The results showed that iron-bearing minerals in sintered ore are mainly calcium ferrite, hematite, magnetite, and hedenbergite. Diffraction peaks at 11.9, 27.6, 34.3, 41.5, and 60.7 reveal the presence of calcium ferrite [35,36]. Diffraction peaks at 33.2, 35.6, 41.0, 49.5, 54.1, 62.5, 64.1, and 72.0 of the sintered ore are observed, which reveal the presence of hematite [37]. Diffraction peaks at 30.1, 35.4, 43.1, 53.4, 56.9, 62.6, 73.9, and 75.0 of the sintered ore are observed, which can be attributed to the characteristic peaks of Fe3O4 [38]. Diffraction peaks at 29.5, 29.7, 35.0, and 35.3 of the sintered ore are observed, which reveal the presence of hedenbergite, which refer to JCPDS (Joint Committee on Powder Diffraction Standards) (01-071-1500).
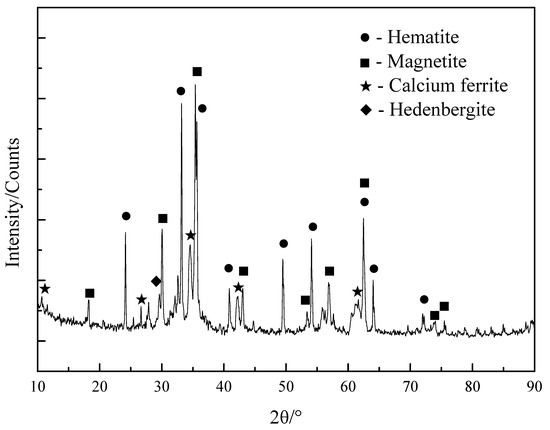
Figure 1.
XRD analysis of catalyst.
2.1.2. Raw Materials for Double-Layer Ignition Sintering
The raw materials used in the sintering experiment in this study were obtained from a sintering plant of iron and Steel Group Co., LTD. (Wuhan, China). The main chemical compositions of iron ore fines, return fines, fuel, and fluxes (including calcined lime, limestone and dolomite) tested by ICP-AES are shown in Table 2. The iron grade of iron ore fines is 61.47%, and the burning loss is 5.14%. The grade of return fines is 54.66%, and the burning loss is 1.21%. The fuel is anthracite, and its industrial analysis is shown in Table 3. The fixed carbon mass fraction is 79.86%, and ash and volatile matter are 15.06% and 3.63%, respectively.

Table 2.
Chemical composition of raw materials/wt%.

Table 3.
Proximate analyses of coal (air-dry basis)/wt%.
2.2. Experimental Methods and Devices
2.2.1. Catalytic Reduction
Catalytic reduction experiments are carried out in a tubular reduction furnace, and the experimental system is shown in Figure 2. It mainly includes three parts: gas distribution device, reaction device, and flue gas measuring device. In the gas supply system, the gas is stored in the high-pressure gas cylinder, and the flow is controlled by the rotor flowmeter. After the gas is mixed evenly in the mixing tank, it is passed into the reaction system. The reaction system is mainly composed of a quartz tube holding sinter catalyst and a reduction furnace. The inner diameter of the quartz tube is 35 mm, and the height is 700 mm. The gas used in the distribution system includes four kinds of gas, NO, CO, O2, and N2, which are, respectively, configured by high-pressure gas cylinders, controlled by glass rotor flowmeter, and mixed by a mixer and then passed into the quartz tube in the tubular reduction furnace. The composition of the gas in the high-pressure gas cylinder is as follows: NO standard gas (NO concentration is 10 vol%, balance gas is N2), CO standard gas (CO concentration is 10 vol%, balance gas is N2) (provided by Wuhan Newred Special Gas Co., LTD., Wuhan, China), O2 standard gas (purity 99.9 vol%), and N2 standard gas (purity 99.9 vol%) (provided by Wuhan Minghui Gas Technology Co., LTD., Wuhan, China).
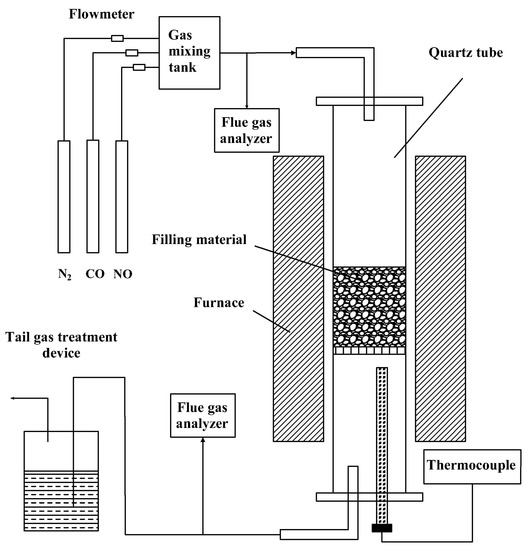
Figure 2.
Schematic diagram of catalytic reduction experiment apparatus.
The sinter catalyst is put into the quartz tube, and then the quartz tube is placed in the reduction furnace. The reduction furnace is opened, and the sinter is heated to a predetermined temperature. During the heating process, nitrogen is used to protect the sinter. After reaching the specified temperature, the gas is switched to reduction gas, which flows in from below the quartz tube, passes through the sinter layer, and then flows out from the upper outlet, and is processed in the tail gas bottle before entering the atmosphere. The analysis system mainly uses a portable flue gas analyzer (PG-250, HORIBA, Kyoto, Japan) to measure the gas composition at the entrance and exit of the reaction system. The measurement accuracy of O2 is ±2.0%, and that of other gases is ±1.0%. During the experiment, when the data of the flue gas analyzer are roughly stable for 10 min, the experimental data should be recorded to avoid interference and ensure the authenticity of the data.
2.2.2. Double-Layer Ignition Sintering
The experimental device of double-layer ignition sintering was designed and developed by us. Its structure is shown in Figure 3, including gas fuel, ignition device, lower sintering cup, connecting device of upper and lower sintering cups, upper sintering cup, vacuum chamber, blower, and extractor fan. In order to investigate the effect of double-layer ignition sintering on the sinter indexes and emission concentration of flue gas pollutants in the sintering process under the same granulation conditions, the single-layer sintering experiment and double-layer ignition sintering experiment were carried out in the laboratory; the specific experimental scheme is shown in Table 4.
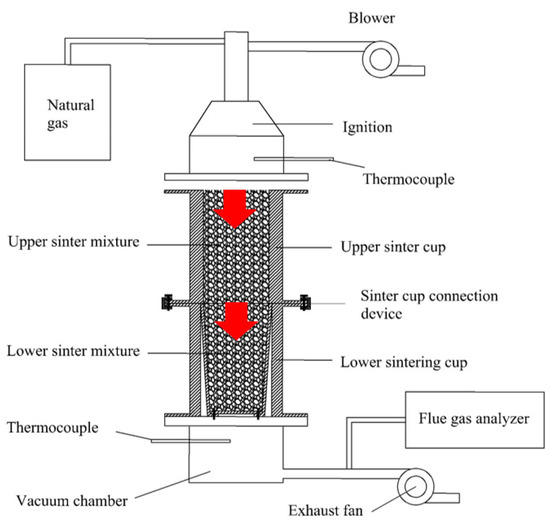
Figure 3.
Schematic diagram of double-layer ignition sintering experiment device.

Table 4.
Experimental conditions.
Sintering tests include proportioning, mixing, granulation, ignition, sintering, cooling, sieving, and quality testing of sinter. A single-layer sintering experiment adopts a conventional sintering method; the specific operation methods were as below. It took weight batching for raw materials matching, as given in Table 5 in the sintering process. After batching, the mixture was fully mixed and charged into a cylinder mixer 600 mm in diameter and 300 mm in length to granulate at 20 rpm for 4 min. The granulated mixture was then fed into the sintering pot. To protect the hearth, sintered ores with particle size of 10–16 mm were fed as hearth layer at the bottom of pot, of which the height was about 20 mm (1.0 Kg); the granulated mixture is distributed into the sinter cup, the material height of which was 720 mm; the ignition temperature was 1050 ± 50 °C, the ignition time was 2 min, and the negative pressure was 5 KPa. After the ignition, the negative pressure was adjusted to 10 KPa. After the sintering, it was cooled for 4 min under a negative pressure of 4 KPa. The experimental method of double-layer ignition and sintering is as follows: part of the granulated mixture was placed in the lower sinter cup. After loading, the upper layer mixture was ignited with natural gas for 1.5 min at 1050 °C under the suction pressure of 5 KPa. After the ignition, the negative pressure was adjusted to 10 KPa. When the lower layer was sintered for 5–10 min, the rest of the granulated mixture was quickly distributed into the upper sintering cup for the upper ignition. After loading, the upper layer mixture was ignited with natural gas for 1.5 min at 1050 °C under the suction pressure of 10 KPa. After the ignition, the negative pressure was adjusted to 10 KPa. During the sintering experiment, the flue gas analyzer (PG-250, HORIBA, Kyoto, Japan) was used to detect the composition of sintering flue gas, including the concentration of NOx, O2, CO2, CO, and other gases, and record the data every 5 s.

Table 5.
Composition of sintering mixture/wt%.
2.3. Calculation of NOx
In the process of catalytic reduction, the conversion rate of nitric oxide is calculated in Equation (1):
where is the conversion of NO, %; is the concentration of NO at the inlet, mg/m3; and is the concentration of NO at the outlet, mg/m3.
Refer to GB/T 16157-1996, “Determination of particulate matter and sampling of gaseous pollutants in stationary source exhaust” and Ministry of Ecology and Environment, PRC “Opinions on Promoting ultra-Low Emissions in Iron and Steel Industry”. In the sintering process, the nitrogen oxide concentration in flue gas is based on the NOx concentration under the 16% baseline oxygen content (), and the calculation formula is shown in Equation (2).
where, is the NOx concentration under the 16% baseline oxygen content, mg/m3; is the actual concentration of NOx in the sintering process, mg/m3; is the actual volume fraction of flue gas O2, %.
2.4. Sintering Indices
After sintering, the indexes were tested, including particle size composition, yield, drum index, and fall strength. The testing criteria are as follows: (1) particle size and particle size composition test refer to ISO4701; (2) refer to GB8209-87 for strength test of drum; (3) standard test of shatter strength (refer to JIS8711-77); The calculation methods of these sintering indices are shown in Table 6.

Table 6.
Calculation equations for yield and qualities index of sintered ore.
3. Results and Discussion
3.1. Catalytic Reduction
3.1.1. Effect of Temperature on Catalytic Reduction of NO in Sintered Ore
In order to explore the strength of sinter catalysis under different reaction temperatures, this experiment studied the influence of different temperatures on the catalytic reduction of NO in sinter under the conditions of particle size of 0.2–1 mm, space velocity ratio of 3000 h−1, gas flow rate of 1 L/min, CO concentration of 0.3%, and NO concentration of 669.87 mg/m3. The experiment without catalyst under the same conditions was conducted as a blank control, and the results are shown in Figure 4. The results showed that, in the blank controlled test, when the temperature is lower than 600 °C, CO has no reduction effect on the reduction of NO. When the temperature reaches 800 °C, the NOx conversion rate increases to 12.2%; when the temperature reaches 1000 °C, the NO conversion rate reaches 45.65%. It indicates that the reduction of NO by CO can only occur at high temperature above 600 °C, and it is difficult to proceed at low temperature.
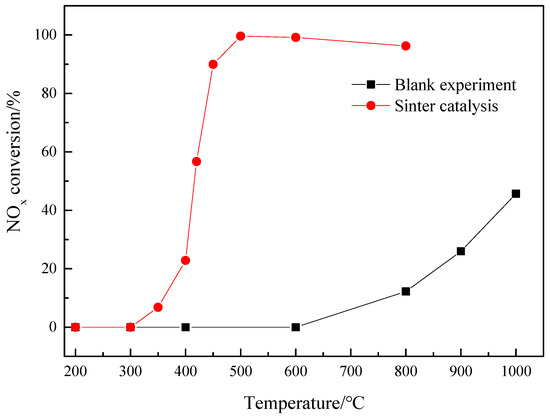
Figure 4.
Effect of temperature on catalytic reduction of NO in sinter ores.
In the temperature range of 200–800 °C, with the increase in temperature, the conversion of NO catalyzed by sintered ore firstly increases rapidly and then decreases slowly. In the temperature range of 400–500 °C, the conversion of NO is greatly affected by temperature. When the reaction temperature rises from 400 °C to 500 °C, the conversion of NO increases from 22.82% to 99.58%. The apparent reaction order of CO increases from 22.82% at 400 °C to 99.58% at 500 °C. This can be attributed to the low adsorption constants of CO and NO below 400 °C; as the temperature continues to increase, the adsorption rate of CO and NO increases, and the reaction rate accelerates [39]. When the reaction temperature exceeds 500 °C, the conversion of NO decreases slightly. It can be seen that under the catalytic action of sintered ore, the reduction temperature of CO is much lower than that without sintered ore, and the conversion rate of NO is much higher than that without sintered ore. To sum up, the catalytic reduction effect of sintered ore on NO is obvious, and the optimum temperature for catalytic reduction of NO by sinter is 500 °C.
The reaction process of NO reduction by CO on sinter surface can be divided into two parts: first, FeO generated by CO reduction reaction of Fe2O3/Fe3O4 in sinter is used as a reducing agent to reduce NO to N2; second, under the catalysis of metal oxide of sinter, CO directly reduces NO to N2. CO in the system can reduce metal oxides to lower oxides and then reduce NO. The reduction reaction mechanism of NO on iron oxide is as follows: (1) Fe-bearing minerals such as calcium ferrite were reduced by CO to form ferrous oxide; (2) NO is adsorbed on low iron oxide and REDOX reaction is carried out; (3) NO reacts with ferrous oxide to form N2 [40]. Meanwhile, the concentrations of NO and CO decreased owing to the catalytic reaction between NO–CO and Ca–Fe oxides [35]. In the process of catalytic reduction of NO to N2, there was competitiveness between CO and NO, as they were absorbed to the surface of calcium ferrite. Since calcium ferrite had the structure of spinel, and CO had fewer electrons in their valence shell than NO, it was easily absorbed on calcium ferrite, which was advantageous to reduce calcium ferrite [41]. The generation of oxygen vacancies in iron oxide had a strong activity lattice of O2−, which caused the reduction of NO [40].
3.1.2. Effect of Space Velocity on Catalytic Reduction of NO in Sintered Ore
Space velocity refers to the amount of gas treated per unit time per unit volume of catalyst, which is an important parameter to evaluate the performance of catalyst. Under the conditions of gas flow rate of 1 L/min, CO concentration of 0.3%, NO concentration of 500 ppm, and reaction temperature of 500 °C, the influence of space velocity on catalytic reduction of NO in sinter was studied with sinter of 0.2–1 mm as catalyst. The results are shown in Figure 5. The results showed that the conversion rate of NO decreases with the increase in space velocity.
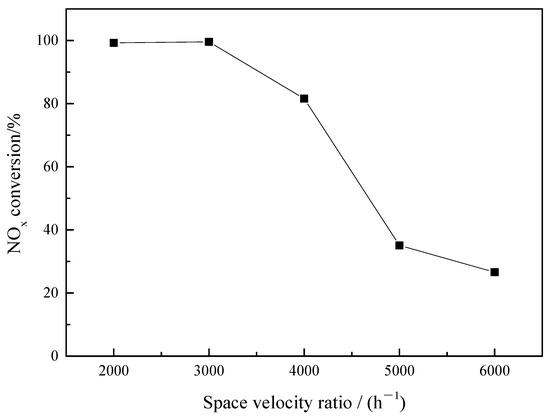
Figure 5.
Effect of Space velocity ratio on catalytic reduction of NO in sintered ore.
As can be seen from Figure 5, when the airspeed increases from 2000 h−1 to 3000 h−1, the conversion rate of NO does not change much, which is above 99%. When the airspeed increased to 5000 h−1, the NO conversion rate decreased sharply to 35.11%. When the space speed increases to 6000 h−1, the conversion rate of NO further decreases to 26.6%. This is because when the space velocity increases, the amount of NO catalyzed by sintered ore per unit volume increases, the contact between NO gas and sinter is insufficient, and the reaction time decreases, so the conversion rate of NO decreases. If the space speed is too low, the amount of catalyst is too large, and the cost is high; if the airspeed is too high, the conversion rate of NO decreases, and NO emissions cannot be effectively reduced. Therefore, 3000 h−1 airspeed is selected after comprehensive consideration.
During the sintering experiment, the CO concentration in the waste gas can reach about 2%, and the concentration of NO is 400–600 ppm [42]. However, the CO concentration in the actual sintering combustion zone can reach about 6%, which can meet the consumption of reduced NO. The temperature of combustion zone and sinter zone can meet the temperature condition of CO reduction of NO catalyzed by sintered ore [43]. At the same time, near the fuel combustion interface in the combustion zone, a large amount of oxygen is consumed to generate CO2, CO, and NOx gases. After the gas encounters the surrounding high-temperature sinter, the reduction reaction of CO and NO will occur under the catalytic action of sintered ore, which is conducive to reducing the concentration of NO in the flue gas.
3.2. Double-Layer Ignition Sintering
3.2.1. Sintering Performance
The practical effect of the layer height proportion and ignition time interval on the size composition was studied by the sintering pot experiment, and other sintering indexes were tested meanwhile. The sintering indexes in the single-layer sintering experiment were investigated. Under the conditions of layer height proportion of 320/400 mm (lower/upper), the sintering indexes under different ignition time interval were studied; under the conditions of ignition time interval of 5 min, the sintering indexes under different layer height proportion (lower/upper) were studied, and the results are shown in Table 7 and Figure 6.

Table 7.
Effect of different layer height proportions and ignition time interval on the size composition of sintered ores.
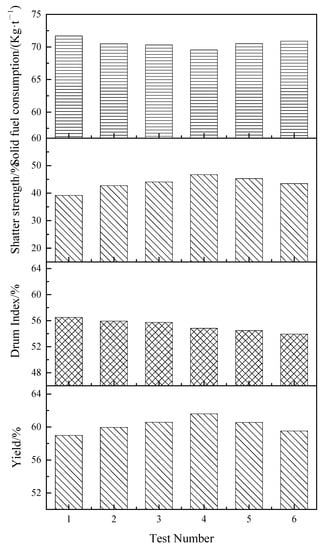
Figure 6.
Sintering indexes at different ignition time intervals and layer height proportions.
Table 7 showed that as the ignition time interval lengthened and the height of the lower layer increased, the particle size distribution of sinter is more reasonable, with the content of coarse grains (>5 mm) increasing and that of fine grains (<5 mm) decreasing, which has a favorable effect on blast furnace smelting [44,45]. The production practice of blast furnace in China shows that when sinter powder content (<5 mm) increases by 10%, blast furnace output decreases by 6–8%, and coke ratio increases by 0.5% [46]. At the same time, the particle size distribution of sintered ore is more uniform, and the content of coarse grains (5–40 mm) increases, indicating that the physical strength of sintered ore has been improved to a certain extent.
Figure 6 showed that, compared with single-layer sintering, on the whole, the sinter yield of double-layer ignition sintering is increased, solid fuel consumption is slightly reduced, falling strength is slightly increased, and drum strength is slightly decreased. This indicated that double-layer ignition sintering had a certain effect on reducing sintering solid consumption and improving the yield of sinter but was not conducive to the drum strength of sinter. This is because, in the double-layer ignition sintering process, the upper sintering and lower sintering processes were carried out at the same time, and the heat carried by the upper sintering flue gas entered the lower sintering layer, increasing the temperature of the lower sintering material layer and making the utilization rate of the heat in the flue gas inside the material layer increasing, which was beneficial to improve the quality of the sintered ore, but due to the simultaneous existence of two combustion zones, the air permeability in the sintering material layer was reduced, the lower sintering fuel was not fully burned, and the heat was not completely released, so that the temperature of the lower sintering combustion zone cannot be increased. The amount of liquid phase generated in sinter was reduced, which was not conducive to improving the quality of sinter.
As the ignition time interval lengthens from 5 to 10 min, the yield increases slightly, the solid fuel consumption decreases slowly, the shatter strength increases slightly, and the drum index decreases slowly. When the ignition time interval is 5 min, the yield, drum strength, shatter strength, and solid fuel consumption were 59.96%, 55.93%, 42.69%, and 70.49%, respectively. When the ignition time interval increased to 10 min, the yield, drum strength, shatter strength, and solid fuel consumption reached 61.60%, 54.82%, 46.75%, and 69.55%, respectively. It means that appropriately prolonging the ignition time interval is conducive to improving the yield and falling strength of sinter and reducing the solid burn up of sinter, but the drum index of sinter decreases. Therefore, on the condition that the drum index of sinter is guaranteed, the ignition time interval can be appropriately prolonged.
As the height of the lower layer increases from 320 to 400 mm, the yield decreases slightly, the solid fuel consumption increases slowly, the shatter strength increases slightly, and the drum index decreases slowly. This indicates that the increase in the height of the lower layer is not conducive to the progress of the double-layer ignition sintering process, which will lead to a decrease in the sinter yield, the shatter strength, and the drum index and also make the sintering solid fuel consumption slightly increase. Therefore, in order to improve the sintering index, the proportion of the lower layer height should be appropriately reduced.
3.2.2. Sintering Flue Gas
In order to investigate the effect of double-layer ignition sintering on the emission concentration of flue gas pollutants in the sintering process, the emission concentration of flue gas pollutants in the single-layer sintering experiment were investigated. Under the conditions of layer height proportion of 320/400 mm (lower/upper), the emission concentration of flue gas pollutants under different ignition time interval were studied. Under the conditions of ignition time interval of 5 min, the emission concentration of flue gas pollutants under different layer height proportion (lower/upper) were studied, and the results are shown in Figure 7, Figure 8, Figure 9 and Figure 10.
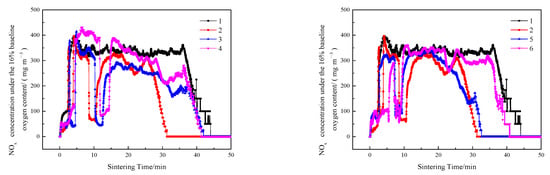
Figure 7.
NOx emissions of sintering flue gas at different ignition time intervals and layer height proportions.
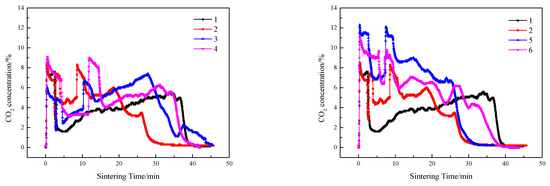
Figure 8.
CO2 emissions of sintering flue gas at different ignition time intervals and layer height proportions.
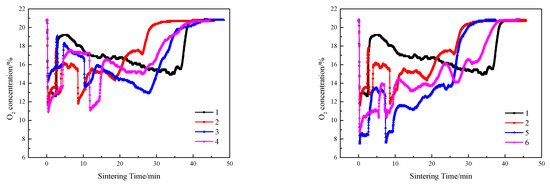
Figure 9.
O2 emissions of sintering flue gas at different ignition time intervals and layer height proportions.
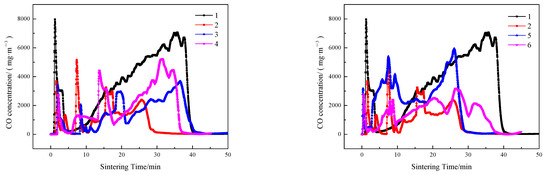
Figure 10.
CO emissions of sintering flue gas at different ignition time intervals and layer height proportions.
As shown schematically in Figure 7, Figure 8, Figure 9 and Figure 10, in contrast to the single-layer sintering, NOx concentration under the 16% baseline oxygen content () in the flue gas of double-layer ignition sintering is reduced to a certain extent. Under the conditions of layer height proportion of 320/400 mm (lower/upper), with the extension of the ignition time interval, the total sintering time increases gradually, increases, and the generation time of nitrogen oxide also increases gradually, indicating that the extension of the ignition time is not conducive to the emission reduction of NOx in the whole sintering process. When the ignition time interval is 5 min, in the early stage of double-layer ignition and sintering process is slightly lower than that of single-layer sintering, and the generation time of NOx is greatly shortened. This is because, in the process of double-layer ignition and sintering, the upper and lower sintering material layers are sintered down at the same time, resulting in the overall sintering time is greatly shortened. The NOx generated in the upper sintering process are partially decomposed and reduced when passing through the lower sintering combustion zone, which reduces the emission of nitrogen oxides [4]. At the same time, the oxygen content in the upper sintering flue gas is low and contains a certain concentration of CO and a large amount of CO2, leading to a reduction in NOx production in the lower sintering process [47,48]. Metal oxides in sinter, such as Fe2O3, Fe3O4, and calcium ferrite, have different degrees of catalytic effect on the removal of NO by CO reduction [35,40,49]. When the ignition time interval is extended to 10 min, the formation time of nitrogen oxide is gradually prolonged, which is due to the late ignition time of the upper sintering, while the lower part has been a large part of the sintering, and the material permeability of the lower layer is gradually reduced, resulting in the upper sintering speed slowed down, so the overall sintering time is longer than the ignition time interval of 5 min.
Compared with single-layer sintering, the CO2 concentration in flue gas of double-layer ignition sintering was higher, and the O2 concentration was lower. In the early stage of sintering, because only the lower sintering process was carried out, the height of the material layer was lower, the permeability of the material layer was better, and the fuel combustion rate was increased. When the upper layer was ignited, the sintering processes of the upper and lower layers were carried out at the same time, the permeability of the sintering material layer decreased, resulting in a decline in fuel combustion rate, and upper sintering flue gas entered the lower sintering, which caused the lower sintering of oxygen to decrease, the content of CO2 and CO to increase, the oxidizing atmosphere in the sintering material layer to weaken, and the lower combustion zone to form a local reducing atmosphere, which has a significant effect on decreasing NO [50]. The NOx in the material layer is reduced and decomposed by CO under the catalysis of the hot sintered ore, thereby reducing the emission concentration of NOx [4]. At the same time, CO generated by sintering in the upper layer is burned again and consumed by REDOX reaction when passing through the lower layer sintering process; therefore, the content of carbon monoxide in the flue gas of double-layer ignition sintering is lower.
3.2.3. Mechanism of NOx Degradation in Double-Layer Ignition Sintering
A possible mechanism for the degradation of NOx by double-layer ignition sintering is shown in Figure 11. This study changed the traditional single-layer ignition sintering to double-layer ignition sintering. In the double-layer ignition sintering process, the upper and lower sintering materials are sintered at the same time, and two combustion zones exist at the same time, which increases the width of the combustion zone inside the entire sintering material layer. The oxidizing atmosphere in the upper sintering flue gas is weakened, and the high-temperature hot sintered ore in the lower layer provides favorable conditions for catalytic reduction of NOx. The use of sintered ore to catalytically reduced the NOx in the flue gas at high temperatures enables the NOx in the upper sintering flue gas to be reduced and decomposed when passing through the lower combustion zone. At the same time, when the upper layer sintering flue gas with lower oxygen content passed through the lower layer sintering, the NOx conversion rate of the lower layer sintering fuel was reduced, thereby reducing the nitrogen oxide concentration during the entire sintering process.
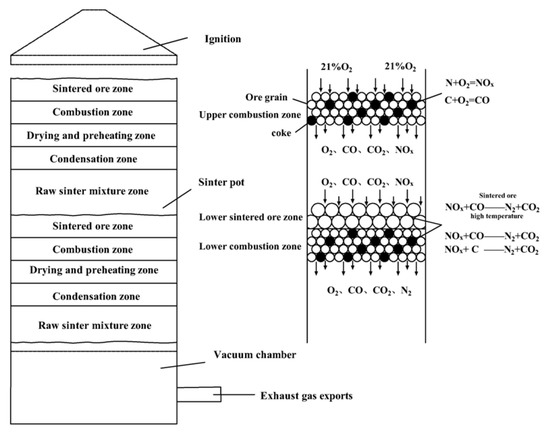
Figure 11.
Schematic diagram of NOx degradation mechanism of double-layer ignition sintering.
4. Conclusions
- (1)
- The sintered ores are beneficial to promote the reaction process of CO reducing NO. The reduction of NO by CO can only occur at high temperature above 600 °C, and it is difficult to proceed at low temperature. When sinter is used as catalyst, the conversion rate of NO reduced by sintered ore increased significantly, reaching 99.58% at 500 °C.
- (2)
- Compared with single-layer sintering, the sinter yield of double-layer ignition sintering is increased, solid fuel consumption is slightly reduced, falling strength is slightly increased, and drum strength is slightly decreased. Under the conditions of layer height proportion of 320/400 mm (lower/upper) and ignition time interval of 10 min, the yield, drum strength, shatter strength, and solid fuel consumption were reached 61.60%, 54.82%, 46.75%, and 69.55%, respectively.
- (3)
- In contrast to the single-layer sintering, NOx concentration under the 16% baseline oxygen content (c(NOx)) in the flue gas of double-layer ignition sintering is reduced. The CO2 concentration in flue gas of double-layer ignition sintering was higher, and the O2 concentration was lower. The oxidizing atmosphere in the upper sintering flue gas is weakened, and the high-temperature hot sintered ore in the lower layer provides favorable conditions for catalytic reduction of NOx.
The double-layer ignition sintering can reduce NOx emission in sintering process under the condition of guaranteeing the sintering index, which provides a way to reduce NOx emission in the sintering process.
Author Contributions
Conceptualization, T.C. and X.Z.; methodology, J.W. and Y.L.; software, J.W.; validation, T.C., X.Z. and B.S.; formal analysis, Z.W.; resources, T.C.; data curation, J.W., J.L. and M.H.; writing—original draft preparation, J.W.; writing—review and editing, T.C., X.Z. and Y.L.; project administration, X.Z.; funding acquisition, B.S. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [the Basic Research Fund of Zhongye Changtian International Engineering Co., Ltd.] grant number [No. 2020JCYJ06], the APC was funded by [Wuhan University of Science and Technology].
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rocha, L.; Kim, H.; Lee, C.; Jung, S.M. Mechanism of NOx Formation from Nitrogen in the Combustion of the Coals Used in Sintering Process. Metall. Mater. Trans. B 2020, 51, 2068–2078. [Google Scholar] [CrossRef]
- Chen, Y.G.; Guo, Z.C.; Wang, Z. Influence of CeO2 on NOx emission during iron ore sintering. Fuel Processing Technol. 2009, 90, 933–938. [Google Scholar] [CrossRef]
- Lu, P.; Hao, J.T.; Yu, W.; Zhu, X.M.; Dai, X. Effects of water vapor and Na/K additives on NO reduction through advanced biomass reburning. Fuel 2015, 170, 60–66. [Google Scholar] [CrossRef]
- Fan, X.H.; Yu, Z.Y.; Gan, M.K.H.; Chen, X.L.; Chen, Q.; Liu, S.; Huang, Y.S. Elimination Behaviors of NOx in the Sintering Process with Flue Gas Recirculation. ISIJ Int. 2015, 55, 2074–2081. [Google Scholar] [CrossRef]
- Liu, D.J.; Wei, Y.Q.; Yang, L.Q. Research of emission reduction of nitrogen oxide in Chinese iron and steel enterprises. Environ. Eng. 2012, 5, 118–123. [Google Scholar]
- Zhu, T.Y. Sintering Flue Gas Purification Technology; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Ni, W.J.; Li, H.F.; Zhang, Y.Y.; Zou, Z.S. Effects of Fuel Type and Operation Parameters on Combustion and NOx Emission of the Iron Ore Sintering Process. Energies 2019, 12, 213. [Google Scholar] [CrossRef]
- Speth, K.; Murer, M.; Spliethoff, H. Experimental Investigation of Nitrogen Species Distribution in Wood Combustion and Their Influence on NOx Reduction by Combining Air Staging and Ammonia Injection. Energy Fuels 2016, 30, 5816–5824. [Google Scholar] [CrossRef]
- Xu, M.X.; Li, S.Y.; Wu, Y.H.; Jia, L.S.; Lu, Q.G. Effects of CO2 on the fuel nitrogen conversion during coal rapid pyrolysis. Fuel 2016, 184, 430–439. [Google Scholar] [CrossRef]
- Gan, M.; Fan, X.H.; Lv, W.; Chen, X.L.; Yu, Z.Y.; Zhou, Y. Fuel pre-granulation for reducing NOx emissions from the iron ore sintering process. Powder Technol. 2016, 301, 478–485. [Google Scholar] [CrossRef]
- Wo, C.N. NOx Formation of Pulverized Coal under Pressure Oxygen-Enriched Combustion. Master’s Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar]
- Gan, M.; Fan, X.; Ji, Z.; Jiang, T.; Chen, X.; Yu, Z.; Li, G.; Yin, L. Application of biomass fuel in iron ore sintering: Influencing mechanism and emission reduction. Ironmak. Steelmak. Processes Prod. Appl. 2014, 42, 27–33. [Google Scholar] [CrossRef]
- Mo, C.L.; Teo, C.S.; Hamilton, I.; Morrison, J. Admixing Hydrocarbons in Raw Mix to Reduce NOx Emission in Iron Ore Sintering Process. ISIJ Int. 1997, 37, 350–357. [Google Scholar] [CrossRef]
- Chen, Y.G.; Guo, Z.C.; Wang, Z. Application of Modified Coke to NOx Reduction with Recycling Flue Gas during Iron Ore Sintering Process. ISIJ Int. 2008, 11, 1517–1523. [Google Scholar] [CrossRef][Green Version]
- Cheng, Z.L.; Wang, J.Y.; Wei, S.S.; Guo, Z.G.; Yang, J.; Wang, Q.W. Optimization of gaseous fuel injection for saving energy consumption and improving imbalance of heat distribution in iron ore sintering. Appl. Energy 2017, 207, 230–242. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Fan, X.H.; Gan, M.; Chen, X.L.; Lv, W. NOx Reduction in the Iron Ore Sintering Process with Flue Gas Recirculation. JOM 2017, 69, 1570–1574. [Google Scholar] [CrossRef]
- Locci, C.; Vervisch, L.; Farcy, B.; Domingo, P.; Perret, N. Selective Non-Catalytic Reduction (SNCR) of Nitrogen Oxide Emissions: A Perspective from Numerical Modeling. Flow Turbul. Combust. 2018, 100, 301–340. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Baeyens, J.; Seville, J. NOx formation and selective non-catalytic reduction (SNCR) in a fluidized bed combustor of biomass. Biomass Bioenergy 2010, 34, 1393–1409. [Google Scholar] [CrossRef]
- Procházka, L.; Mec, P. Possibility of using fly ash after denitrification by SNCR as admixture in alkali-activated materials. Mater. Today Proc. 2020, 37, 42–47. [Google Scholar] [CrossRef]
- Chen, X.P.; Liu, Q.; Wu, Q.; Luo, Z.K.; Zhao, W.T.; Chen, J.J.; Li, J.H. A hollow structure WO3@CeO2 catalyst for NH3-SCR of NOx. Catal. Commun. 2021, 149, 106252. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Zhao, S.; Wang, Y.L.; Wenren, Y.S.; Zhang, X.M. Plasma-enhanced low temperature NH3-SCR of NOx over a Cu-Mn/SAPO-34 catalyst under oxygen-rich conditions. Appl. Catal. B Environ. 2021, 286, 119886. [Google Scholar] [CrossRef]
- Xu, G.Y.; Guo, X.L.; Cheng, X.X.; Yu, J.; Fang, B.Z. A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale 2021, 13, 7052–7080. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.; Moreno-Pirajan, J.C. Adsorption microcalorimetry Characterisation of activated carbons and their application in the study of NOx retention. J. Therm. Anal. Calorim. 2015, 121, 245–255. [Google Scholar]
- Song, Z.J.; Wang, B.; Yang, W.; Chen, T.; Sun, L. Research on NO and SO2 removal using TiO2 supported iron catalyst with vaporized H2O2 in a catalytic oxidation combined with absorption process. Environ. Sci. Pollut. R 2020, 27, 18329–18344. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z.; Tuti, M.; Durgun, O. Experimental investigation of the effects of water adding to the intake air on the engine performance and exhaust emissions in a DI automotive diesel engine. Fuel 2014, 115, 884–895. [Google Scholar] [CrossRef]
- Xiao-Hui, J.I. Influence of O2 Content in Circulating Flue Gas on Iron Ore Sintering. J. Iron Steel Res. Int. 2013, 20, 1–6. [Google Scholar]
- Menad, N.; Tayibi, H.; Carcedo, F.G.; Hernandez, A. Minimization methods for emissions generated from sinter strands: A review. J. Clean. Prod. 2006, 14, 740–747. [Google Scholar] [CrossRef]
- Fleischander, A.; Aichinger, C.; Zwittag, E. New developments for achieving environmentally friendly sinter production-Eposint and MEROS. China Metall. 2008, 18, 41–46. [Google Scholar]
- Sidorkin, V.T.; Tugov, A.N.; Moshnikov, A.N.; Vereshchetin, V.A.; Bersenev, K.G. Effect of Flue Gas Recirculation on the Technical and Environmental Performance of a Boiler. Power Technol. Eng. 2016, 49, 354–358. [Google Scholar] [CrossRef]
- Vanderheyden, B.; Borlee, J.; Brouhon, M. Impact of Waste Gas Recycling on Sintering Performances and Emissions; U.S. Department of Energy, Office of Scientific and Technical Information: Pittsburgh, PA, USA, 1996; Volume 55, p. 807.
- Schmid, H.; Zwittag, E.; Reidetschlager, J.; Kainz, K.; Guan, Y. Eposint—A New Waste—Gas Recycling System for Sinter Plants. World Iron Steel 2007, 3, 6–9. [Google Scholar]
- Pourhoseini, S.H.; Taghvaei, I.; Moghiman, M.; Baghban, M. Tangential Flue Gas Recirculation (TFGR) technique for enhancement of radiation characteristics and reduction of NOx emission in natural gas burners. J. Nat. Gas Sci. Eng. 2021, 94, 104130. [Google Scholar] [CrossRef]
- Pan, J. Research on Basic Theory and Technology of Reduction Emission of Sintering Flue Gas in Iron Ore. Ph.D. Thesis, Central South University, Changsha, China, 2007. [Google Scholar]
- Zhong, Q.; Liu, H.B.; Xu, L.P.; Zhang, X.; Rao, M.J.; Peng, Z.W.; Li, G.H.; Jiang, T. An efficient method for iron ore sintering with high-bed layer: Double-layer sintering. J. Iron Steel Res. Int. 2021, 28, 1366–1374. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Fan, X.H.; Gan, M.; Chen, X.L. Effect of Ca-Fe oxides additives on NOx reduction in iron ore sintering. J. Iron Steel Res. Int. 2017, 24, 18–23. [Google Scholar] [CrossRef]
- Yunas, J.; Sulaiman, N.H.; Ghazali, M.J. Comparative Study of the Calcium Ferrite Nanoparticles (CaFe2O4-NPs) Synthesis Process. In Proceedings of the 2018 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 15–17 August 2018; pp. 101–103. [Google Scholar]
- Wang, X.; Liu, Y.; Han, H.; Zhao, Y.; Ma, W.; Sun, H. Polyaniline coated Fe3O4 hollow nanospheres as anode materials for lithium ion batteries. Sustain. Energy Fuels 2017, 1, 915–922. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Zhou, R.X. Ce doping effect on performance of the Fe/b catalyst for NOx reduction by NH3. UEL Processing Technol. 2015, 133, 220–226. [Google Scholar] [CrossRef]
- Frank, B.; Renken, A. Kinetics and deactivation of the NO reduction by CO on Pt-supported catalysts. Chem. Eng. Technol. 1999, 22, 490–494. [Google Scholar] [CrossRef][Green Version]
- Gan, M.; Fan, X.H.; Yu, Z.Y.; Chen, X.L.; Ji, Z.Y.; Lv, W.; Liu, S.; Huang, Y.-S. A laboratory-based investigation into the catalytic reduction of NOx in iron ore sintering with flue gas recirculation. Ironmak. Steelmak. 2016, 43, 441–449. [Google Scholar] [CrossRef]
- Ge, X.; Chen, J.Q.; Zhang, H.L. Preparation, characterization and catalytic activity of the spinel ferrites. Chin. J. Inorg. Chem. 1999, 15, 730–731. [Google Scholar]
- Fan, X.H.; Yu, Z.Y.; Gan, M.; Chen, X.-L.; Ji, Z.Y. Combustion behavior and influence mechanism of CO on iron ore sintering with flue gas recirculation. J. Cent. South Univ. 2014, 21, 2391–2396. [Google Scholar] [CrossRef]
- Wu, S.L.; Chen, D.F.; Zhao, C.X.; Zhang, L.H.; Xue, F. Influence of Bed Depth on Sinter Flue Gas Composition and Emission. In Proceedings of the 5th International Congress on the Science and Technology of Ironmaking, Shanghai, China, 20–22 October 2009; pp. 519–523. [Google Scholar]
- Liu, F.Q.; Wang, S.L.; Gu, A.J. Analysis of influence factors of sinter strength and grain size composition. Ironmak. Technol. Newsl. 2009, 4, 27–29. [Google Scholar]
- Jiang, D.J. Influence of fuel structure on sintering character and comprehensive effect. Iron Steel 2020, 55, 31–41. [Google Scholar]
- Xu, B.; Zhuang, J.M.; Bai, G.H.; Liang, J.S. On Improving the Particle Size of Sinter. Sinter. Pelletizing 2001, 26, 19–21. [Google Scholar]
- Qian, F.; Chyang, C.S.; Chiou, J.B.; Tso, J. Effect of Flue Gas Recirculation (FGR) on NOx Emission in a Pilot-Scale Vortexing Fluidized-Bed Combustor. Energy Fuels 2011, 25, 5639–5646. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, M.X.; Liu, Z.H.; Cheng, M.; Chen, J.Z. Modeling NOx emission of coke combustion in iron ore sintering process and its experimental validation. Fuel 2016, 179, 322–331. [Google Scholar] [CrossRef]
- Xu, C.B.; Wu, S.L.; Cang, D.Q.; GeXi, R.F. Catalytical performance of several metallic oxides on elimination of NO in NO-CO-CO2-N2 system. Chin. Environ. Sci. 1998, 18, 1–6. [Google Scholar]
- Okazakf, K.; Ando, T. NOx reduction mechanism in coal combustion with recycled CO2. Energy 1997, 22, 207–215. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).