Liquid-Liquid Extraction of Ferric Ions into the Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
- 1-ethyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide-[C2mim][NTf2]
- 1-butyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide-[C4mim][NTf2]
- 1-hexyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide-[C6mim][NTf2]
- Methyltrioctylammonium bis (trifluoromethanesulfonyl) imide-[N1888][NTf2]
- Tributylmethylammonium bis (trifluoromethanesulfonyl) imide-[N1444][NTf2]
2.2. Instrumentation and Methods
- HNO3 in a concentration of 0.001–1.5 mol L−1 or
- 0.2 M phosphate buffer or
- 0.1 M borate buffer or
- 0.01 M H2Ox or 0.005 M H3Cit or the mixture of 0.01 M H2Ox and 0.005 M H3Cit
- Equal volumes (1 mL) of organic and aqueous phases were contacted in Eppendorf microvials and shaken for 30 min at laboratory temperature.
- Aqueous and organic phases were separated by centrifugation (5 min at 700 RCF)
- 200 μL aliquots were taken from both phases and measured in a well-type 2″ NaI (Tl) scintillation detector connected to an NV 3102 single-channel counter.
- After the separation of phases, the equilibrium pH values of the aqueous phase were measured using a digital pH meter PHM240 equipped with a combination Red-Rod glass electrode.
3. Results and Discussions
3.1. Acetylacetone
3.2. Thenoyltrifluoroacetone
3.3. 8-Hydroxyquinoline
3.4. Extraction from Aqueous Solution Containing Common Complexing Agents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smirnova, S.V.; Samarina, T.O.; Pletnev, I.V. Novel ionic liquids for liquid-liquid extraction. In Analytical Applications od Ionic Liquids; Koel, M., Ed.; World Scientific Publishing Europe Ltd.: Singapore, 2017; pp. 139–189. ISBN 9781786340719. [Google Scholar]
- Freemantle, M. Properties of Ionic Liquids. In An Introduction to Ionic Liquids; The Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Dietz, M.L. Ionic Liquids as Extraction Solvents: Where do We Stand? Sep. Sci. 2006, 41, 2047–2063. [Google Scholar] [CrossRef]
- Shoja, S.M.R.; Abdouss, M.; Beigi, A.A.M. Synthesis and characterization of physicochemical properties of imidazolium-based ionic liquids and their application for simultaneous determination of sulfur compounds. J. Mol. Struct. 2021, 1230, 129917. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Dai, S. Ionic liquids-based extraction: A promising strategy for the advanced nuclear fuel cycle. Chem. Rev. 2012, 112, 2100–2128. [Google Scholar] [CrossRef] [PubMed]
- Cubova, K.; Semelova, M.; Nemec, M.; Straka, M. Separation of Co from simulated decontamination media using ionic liquids. J. Radioanal. Nucl. Chem. 2019, 322, 1849–1855. [Google Scholar] [CrossRef]
- Parmentier, D.; Valia, Y.A.; Metz, S.J.; Burheim, O.S.; Kroon, M.C. Regeneration of ionic liquid tetraoctylammonium oleate after metal extraction. Hydrometallurgy 2015, 158, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Wellens, S.; Goovaerts, R.; Moeller, C.; Luyten, J.; Thijs, B.; Binnemans, K. Continuous ionic liquid extraction process for the separation of cobalt from nickel. Green Chem. 2013, 11, 3160. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on extraction od Iron(III) Using Phosphonium and Ammonium Ionic Liquids as Extractant of Metals. In Proceedings of the 26th International Conference on Metallurgy and Materials, Brno, Czech Republic, 24–26 May 2017; pp. 199–203. [Google Scholar]
- Matsumiya, M.; Yamada, T.; Kikuchi, Y.; Kawakami, S. Removal of Iron and Boron ba Solvent Extraction with Ionic Liquids an Recovery of Neodymium Metal by Direct Electrodeposition. Solvent Extr. Ion Exch. 2016, 34, 522–534. [Google Scholar] [CrossRef]
- Hayati Hussin, N.; Yusoff, I.; Alias, Y.; Mohamad, S.; Rahim, N.Y.; Ashraf, M.A. Ionic liquid as a medium to remove iron and other metal ions: A case study of the North Kelantan Aquifer, Malaysia. Environ. Earth Sci. 2014, 71, 2105–2113. [Google Scholar] [CrossRef]
- Knoll, G.F. (Ed.) Counting Statistics and Error Prediction. In Radiation Detection and Measurement, 4th ed.; John Willey & Sons: Hoboken, NJ, USA, 2010; pp. 94–98. [Google Scholar]
- Anatassova, M.; Billard, I. Determination of pKaIL Values of Three Chelating Extractants in ILs: Consequences for the Extraction of 4f Elements. J. Solut. Chem. 2015, 44, 606–620. [Google Scholar] [CrossRef]
- Kotrly, S.; Sucha, L. Protonation equilibrium constants and Stability constants of complexes with organic ligands. In Chemical Equilibria in Analytical Chemistry; SNTL: Prague, Czech Republic, 1988; pp. 92–133. (In Czech) [Google Scholar]
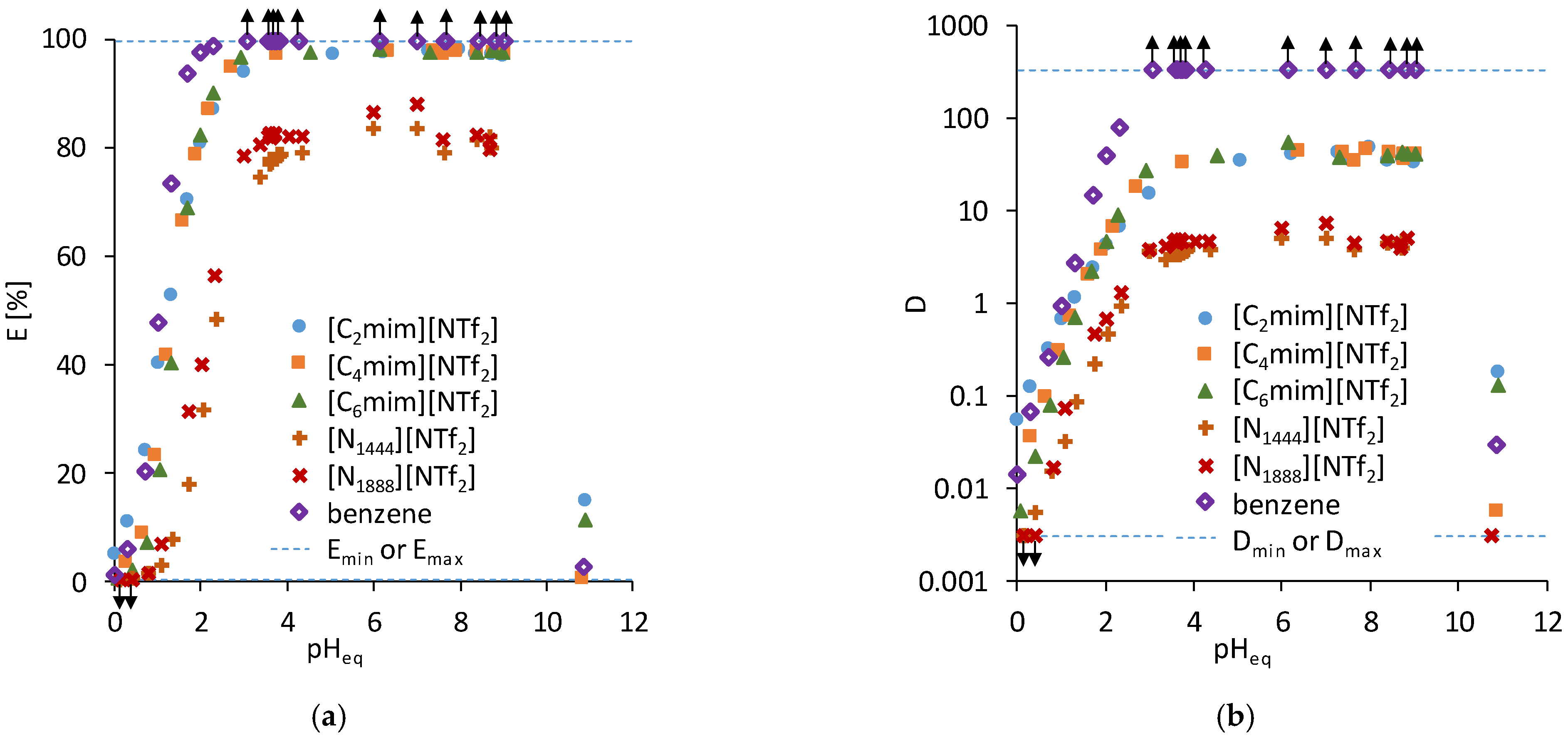
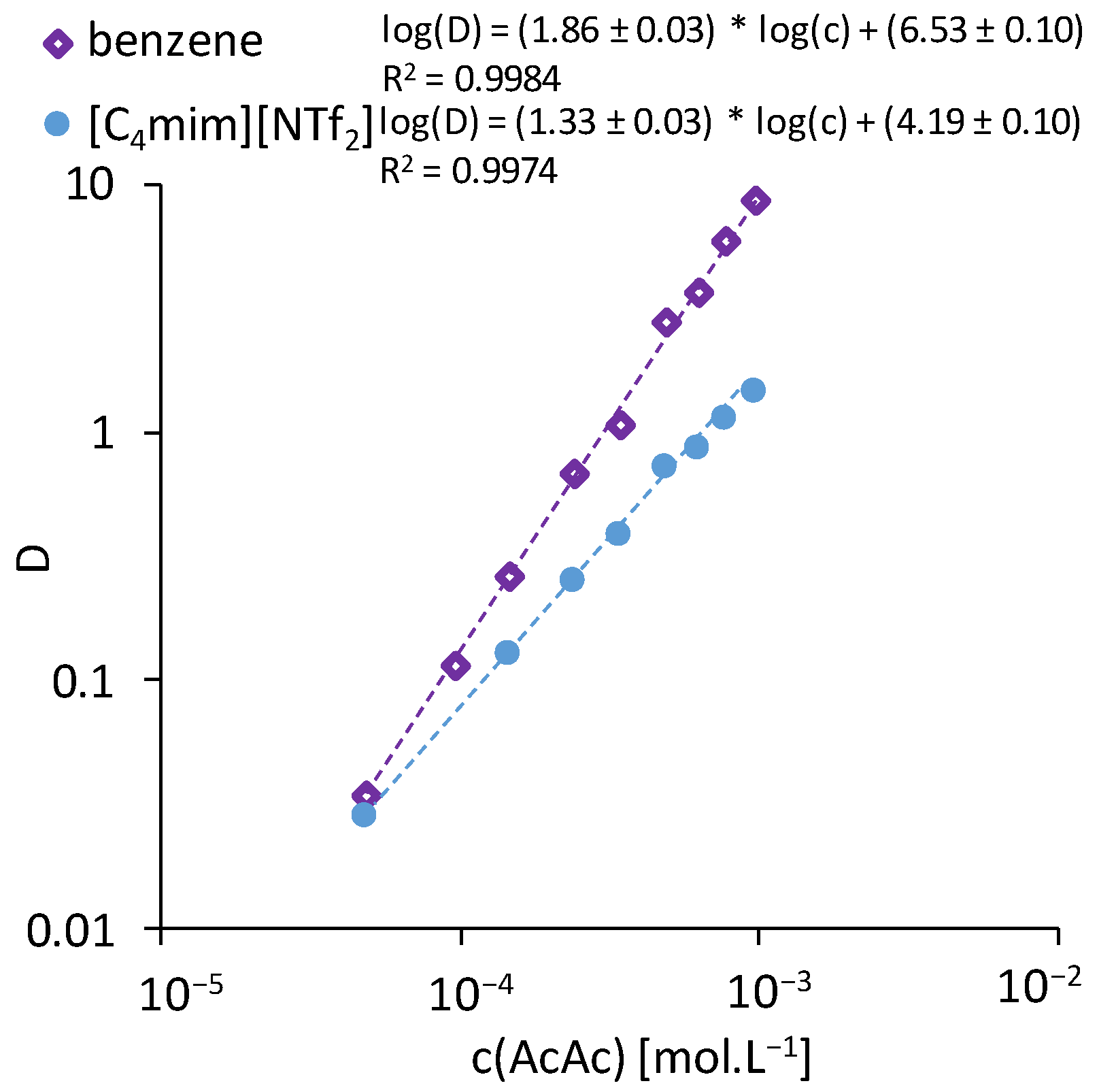




| [C2mim][NTf2] | [C4mim][NTf2] | [C6mim][NTf2] | [N1444][NTf2] | [N1888][NTf2] | Benzene | |
|---|---|---|---|---|---|---|
| Slope | 0.93 ± 0.03 | 1.19 ± 0.60 | 1.33 ± 0.08 | 1.17 ± 0.03 | 1.20 ± 0.17 | 1.64 ± 0.05 |
| pH1/2 | 1.31 ± 0.06 | 1.46 ± 0.10 | 1.59 ± 0.14 | 2.33 ± 0.08 | 2.15 ± 0.31 | 1.06 ± 0.05 |
| [C4mim][NTf2] | Benzene | |
|---|---|---|
| slope | 1.79 ± 0.24 | 1.82 ± 0.12 |
| pH1/2 | 2.78 ± 0.41 | 1.91 ± 0.19 |
| [C2mim][NTf2] | [C4mim][NTf2] | [C6mim][NTf2] | [N1444][NTf2] | [N1888][NTf2] | CHCl3 | |
|---|---|---|---|---|---|---|
| Slope pH ~ 1.2 | 3.19 ± 0.26 | 3.82 ± 0.15 | 3.38 ± 0.60 | 2.80 ± 0.19 | (2.97) | 4.96 ± 0.12 |
| Slope pH ~ 2 | (0.66) | 1.00 ± 0.06 | 1.24 ± 0.19 | 1.48 ± 0.18 | 1.44 ± 0.03 | 0.66 ± 0.03 |
| pH1/2 | 1.66 ± 0.19 | 1.37 ± 0.08 | 1.35 ± 0.34 | 1.49 ± 0.13 | 1.53 ± 0.05 | 1.19 ± 0.04 |
| No Complexant | H3Cit | H2Ox | 0.005 M H3Cit + 0.01 M H2Ox | |||
|---|---|---|---|---|---|---|
| 0.005 M | 0.01 M | 0.005 M | 0.01 M | |||
| 0.5 M AcAc in benzene | 97.5 ± 0.5% | 98.9 ± 0.4% | 95.2 ± 0.4% | 2.3 ± 0.1% | 5.2 ± 0.1% | 5.1 ± 0.1% |
| pHeq | 2.3 | 2.8 | 2.6 | 2.4 | 2.1 | 2.1 |
| 0.5 M AcAc in [C4mim][NTf2] | 94.7 ± 0.5% | 91.5 ± 0.4% | 85.8 ± 0.4% | 0.4 ± 0.1% | 0.1 ± 0.1% | 0.1 ± 0.1% |
| pHeq | 2.7 | 2.8 | 2.6 | 2.4 | 2.1 | 2.1 |
| 0.1 M TTA in [C4mim][NTf2] | 33.8 ± 0.2% | 3.3 ± 0.1% | 0.2 ± 0.1% | 4.3 ± 0.1% | 0.4 ± 0.1% | 0.2 ± 0.1% |
| pHeq | 2.3 | 2.6 | 2.4 | 2.8 | 2.0 | 2.0 |
| 0.4 M TTA in [C4mim][NTf2] | 87.0 ± 1.1% | 15.1 ± 0.1% | 2.9 ± 0.1% | 14.9 ± 0.1% | 1.1 ± 0.1% | 1.5 ± 0.1% |
| pHeq | 3.3 | 2.6 | 2.4 | 2.8 | 2.1 | 2.1 |
| 0.1 M 8HQ in CHCl3 | >99.7% | >99.7% | >99.7% | 22.3 ± 1.3% | 6.7 ± 1.2% | 5.9 ± 0.1% |
| pHeq | 3.1 | 3.8 | 3.5 | 3.8 | 3.4 | 3.3 |
| 0.1 M 8HQ in [C4mim][NTf2] | >99.7% | >99.7% | >99.7% | >99.7% | 87.2 ± 0.4% | 74.2 ± 0.3% |
| pHeq | 4.3 | 5.0 | 4.7 | 5.0 | 4.5 | 4.3 |
| 0.5 M 8HQ in CHCl3 | >99.7% | >99.7% | >99.7% | 97.9 ± 0.5% | 79.8 ± 0.4% | 82.0 ± 0.4% |
| pHeq | 4.0 | 4.2 | 4.0 | 4.1 | 3.9 | 3.8 |
| 0.5 M 8HQ in [C4mim][NTf2] | >99.7% | >99.7% | >99.7% | >99.7% | >99.7% | >99.7% |
| pHeq | 4.3 | 5.6 | 5.3 | 5.7 | 5.3 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubova, K.; Semelova, M.; Nemec, M.; Benes, V. Liquid-Liquid Extraction of Ferric Ions into the Ionic Liquids. Minerals 2022, 12, 11. https://doi.org/10.3390/min12010011
Cubova K, Semelova M, Nemec M, Benes V. Liquid-Liquid Extraction of Ferric Ions into the Ionic Liquids. Minerals. 2022; 12(1):11. https://doi.org/10.3390/min12010011
Chicago/Turabian StyleCubova, Katerina, Miroslava Semelova, Mojmir Nemec, and Vit Benes. 2022. "Liquid-Liquid Extraction of Ferric Ions into the Ionic Liquids" Minerals 12, no. 1: 11. https://doi.org/10.3390/min12010011
APA StyleCubova, K., Semelova, M., Nemec, M., & Benes, V. (2022). Liquid-Liquid Extraction of Ferric Ions into the Ionic Liquids. Minerals, 12(1), 11. https://doi.org/10.3390/min12010011






