Response and Dynamic Change of Microbial Community during Bioremediation of Uranium Tailings by Bacillus sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. The Source of Bacteria and Medium
2.2. Remediation Experiment of Uranium Tailings
2.3. DNA Extraction, PCR Amplification
2.4. High-Throughput Sequencing
2.5. Analytical Methods
3. Results and Discussion
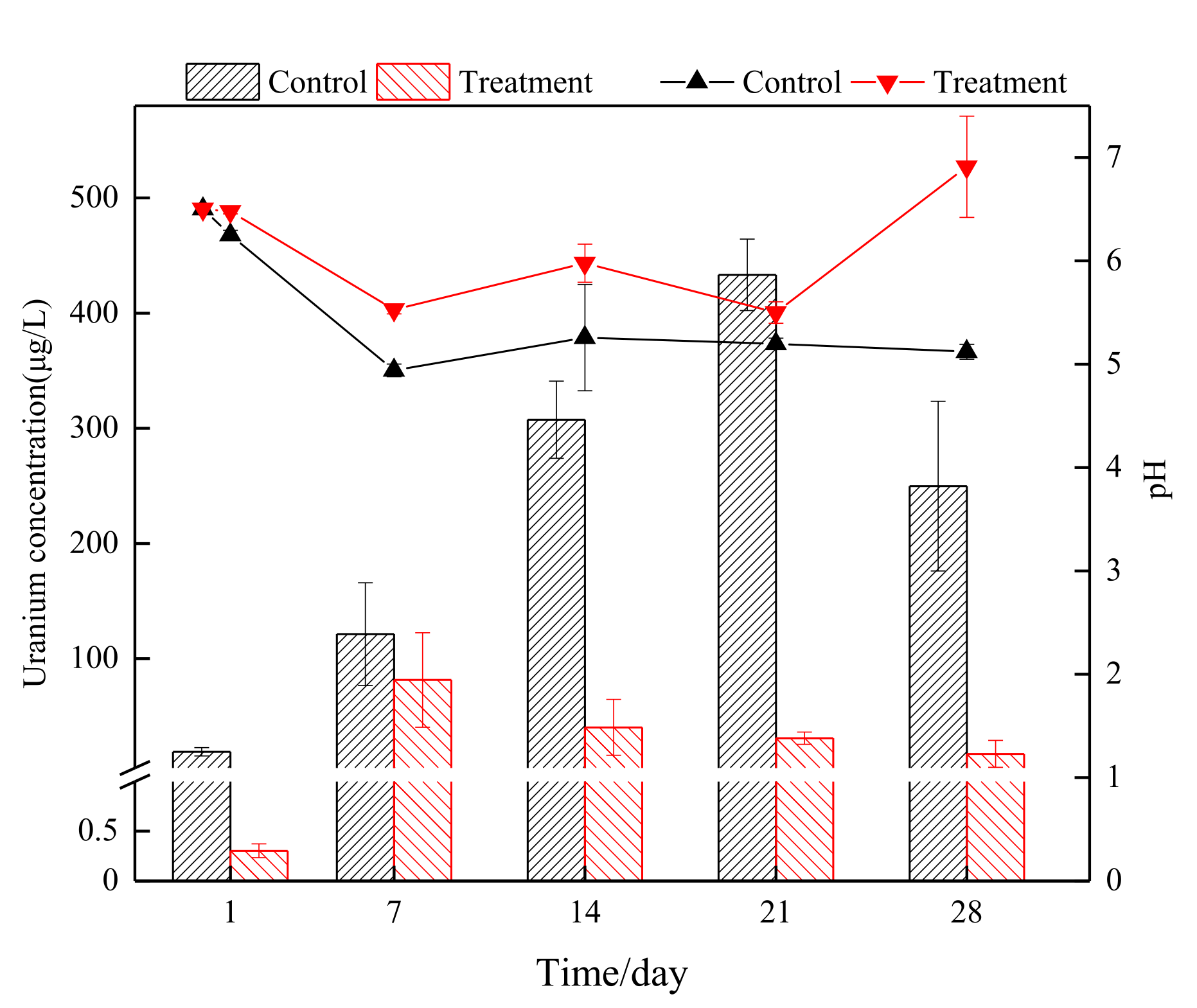
3.1. Removal Efficiency of Uranium
3.2. Microbial Diversity and Richness Assessment
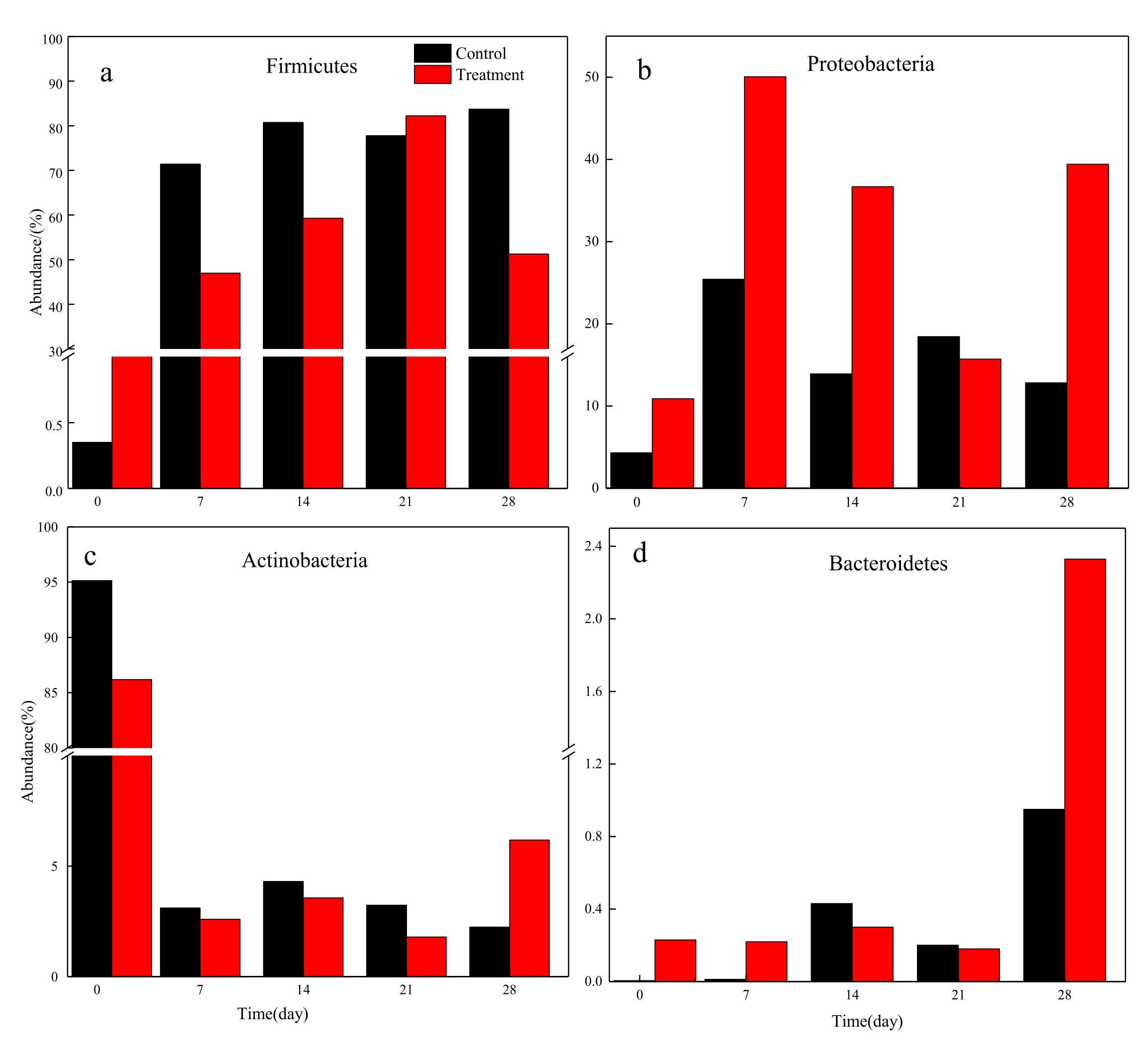
3.3. Microbial Community Composition Analysis at Phylum Level
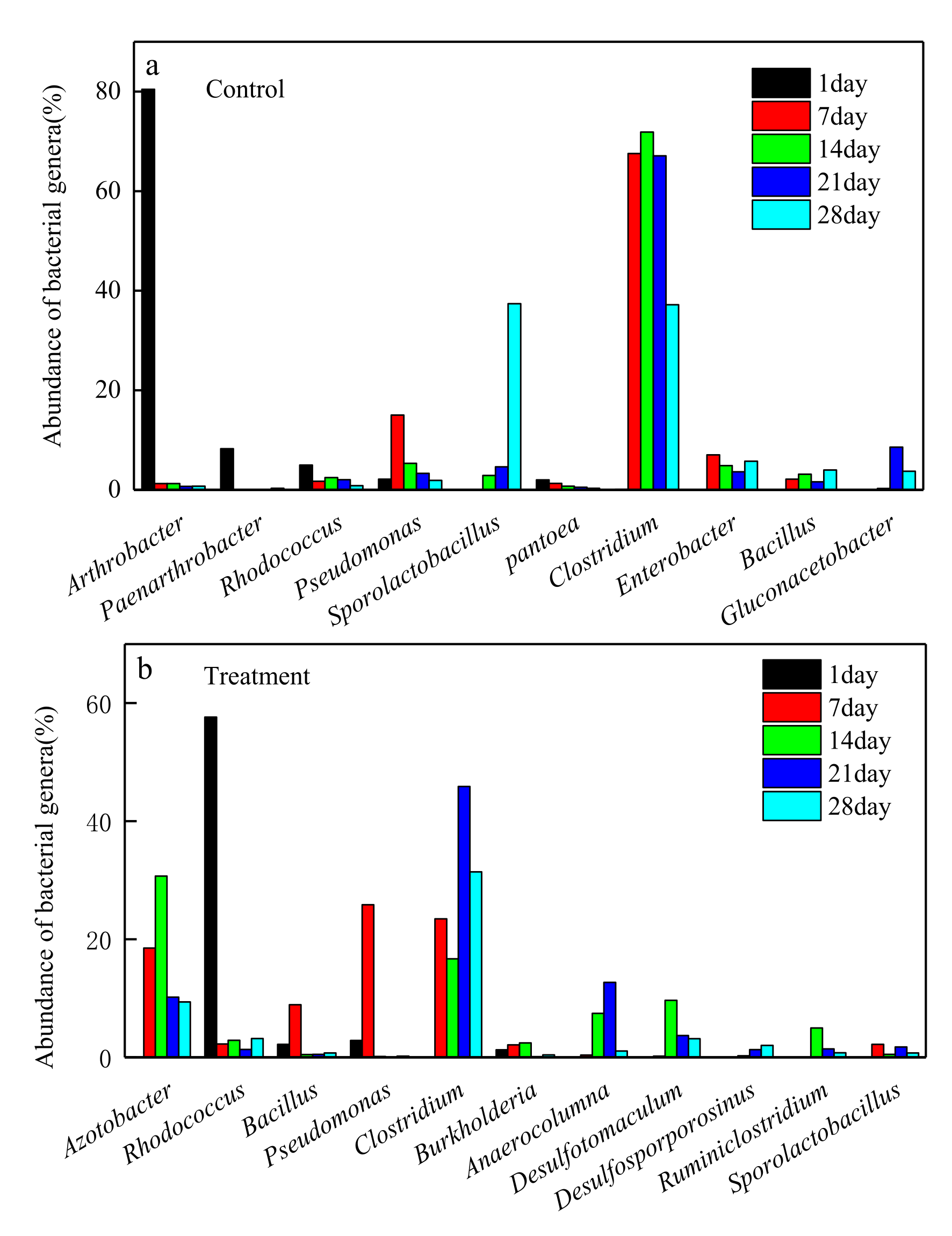
3.4. Dynamic Change of Microbial Community at Genus Level
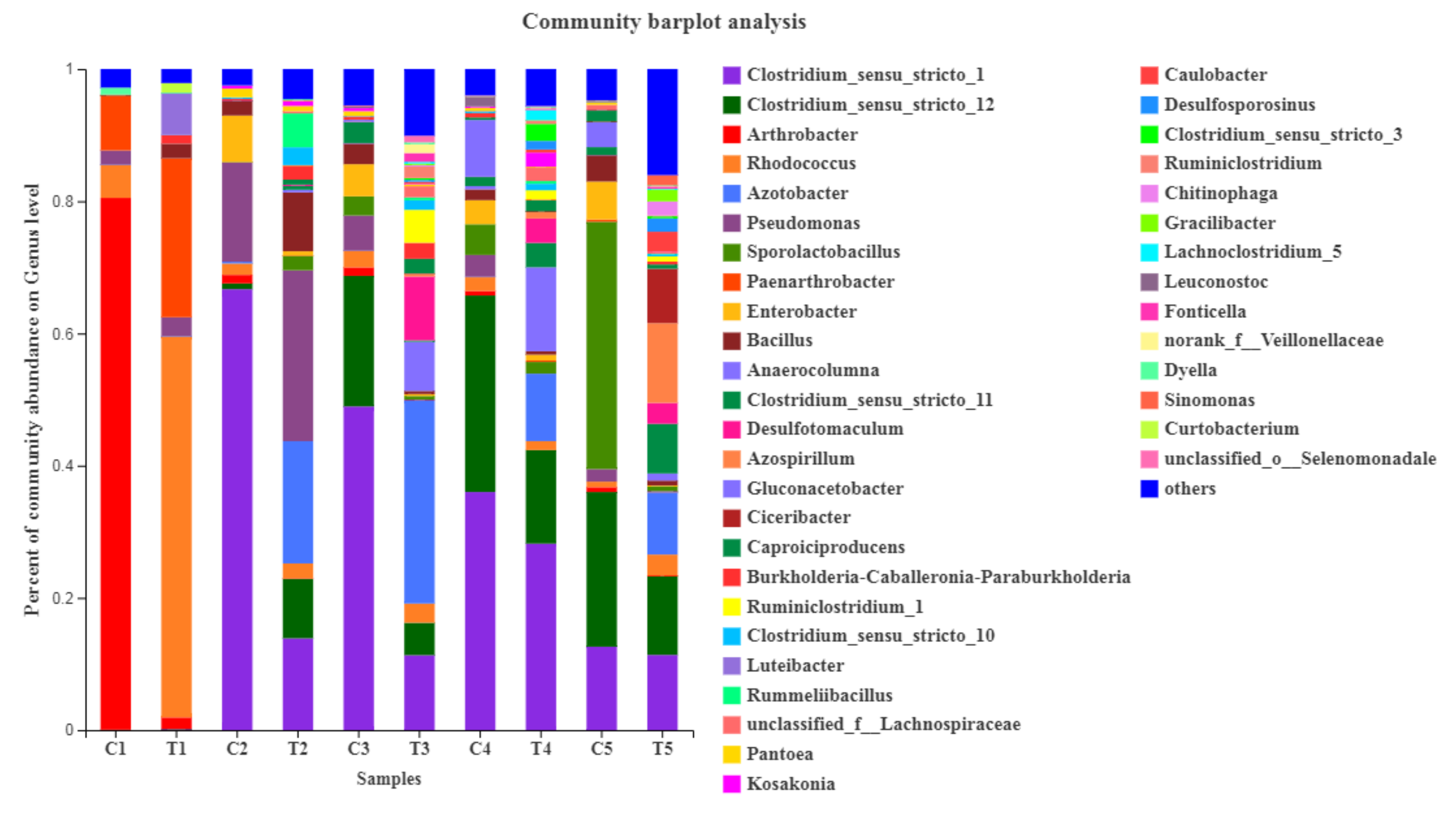
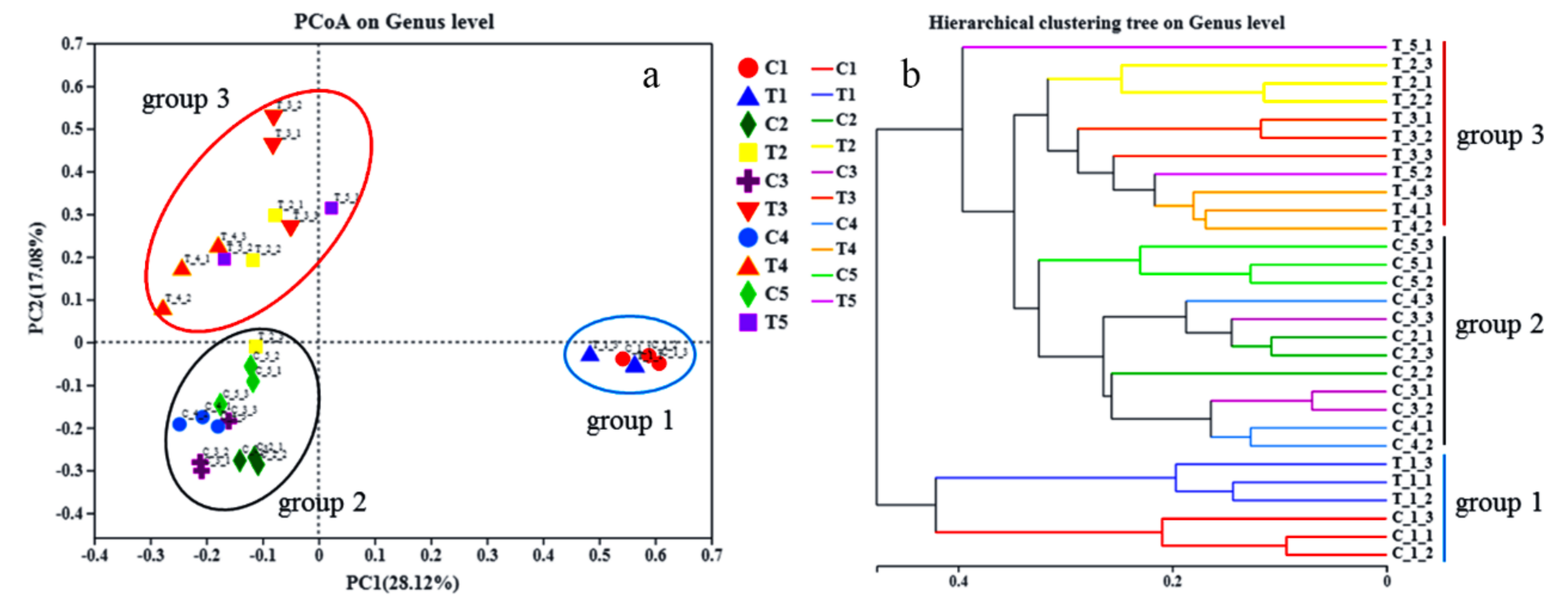
3.5. Microbial Community Structures
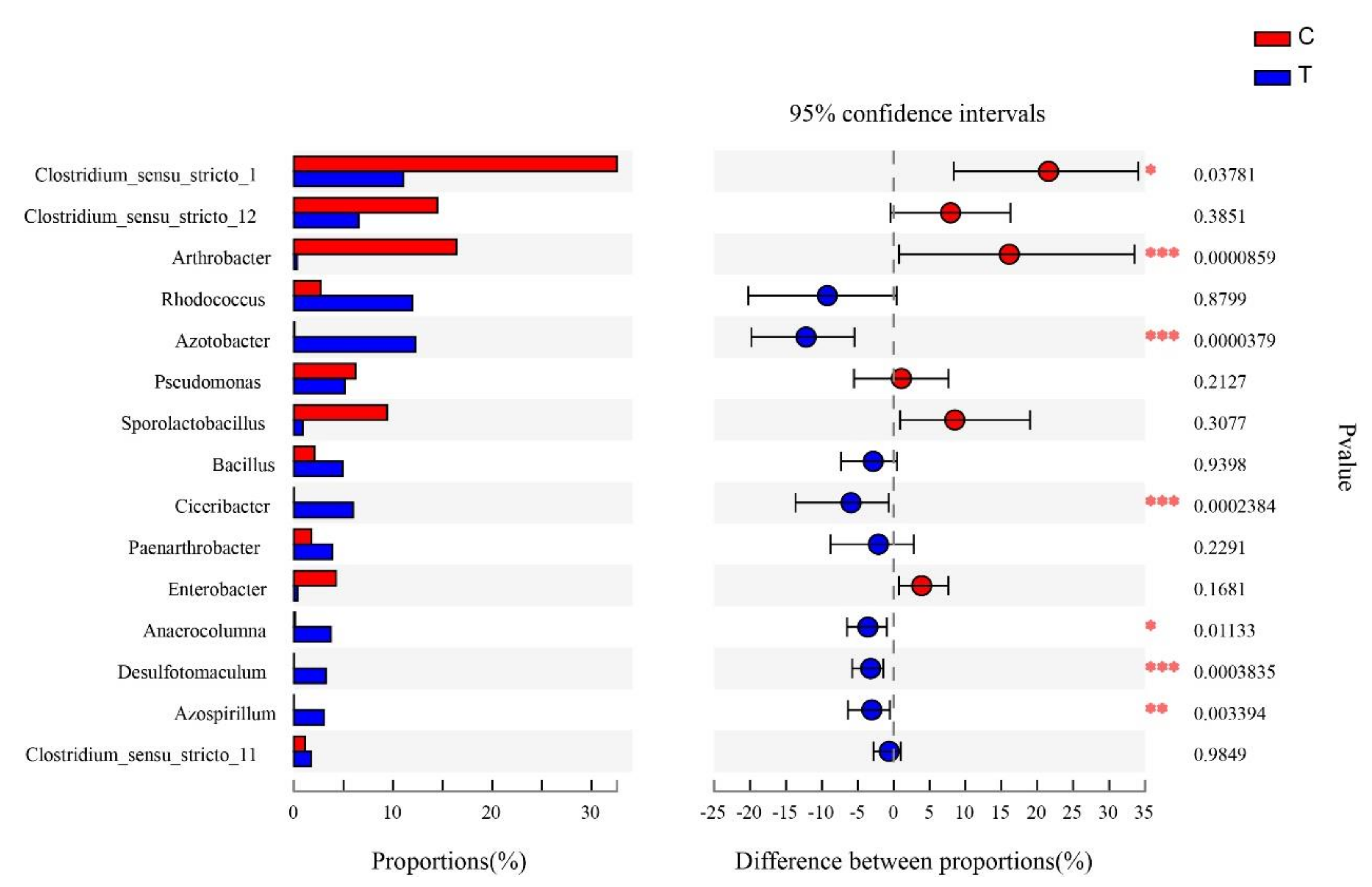
3.6. Significance Test of Differences between Control and Treatment Groups
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, M.; Sun, J.; Chen, Y.; Wang, J.; Shang, J.; Belshaw, N.; Shen, C.; Liu, J.; Li, H.; Linghu, W.; et al. Mechanism of uranium release from uranium mill tailings under long-term exposure to simulated acid rain: Geochemical evidence and environmental implication. Environ. Pollut. 2019, 244, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Sun, J.; He, H.; Liu, J.; Chen, D. Uranium re-adsorption on uranium mill tailings and environmental implications. J. Hazard. Mater. 2021, 416, 126153. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef]
- Yue, Y.; Li, M.; Wang, H..; Zhang, B.; He, W. The toxicological mechanisms and detoxification of depleted uranium exposure. Environ. Heal. Prev. Med. 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Rainer, K. Gastrointestinal absorption of uranium compounds—A review. Regul. Toxicol. Pharmacol. 2015, 71, 125–133. [Google Scholar]
- Jing, L.; Zhang, X.; Ali, I.; Chen, X.; Wang, L.; Chen, H.; Han, M.; Shang, R.; Wu, Y. Usage of microbial combination degradation technology for the remediation of uranium contaminated ryegrass. Environ. Int. 2020, 144, 106051. [Google Scholar] [CrossRef]
- Suriya, J.; Shekar, M.C.; Nathani, N.M.; Suganya, T.; Bharathiraja, S.; Krishnan, M. Assessment of bacterial community composition in response to uranium levels in sediment samples of sacred Cauvery River. Appl. Microbiol. Biotechnol. 2017, 101, 1–11. [Google Scholar] [CrossRef]
- Beazley, M.J.; Martinez, R.J.; Sobecky, P.A.; Webb, S.M.; Taillefert, M. Nonreductive Biomineralization of Uranium(VI) Phosphate Via Microbial Phosphatase Activity in Anaerobic Conditions. Geomicrobiol. J. 2009, 26, 431–441. [Google Scholar] [CrossRef]
- Jaswal, R.; Pathak, A.; Edwards, B.; Lewis, R.; Seaman, J.C.; Stothard, P.; Krivushin, K.; Blom, J.; Rupp, O.; Chauhan, A. Metagenomics-Guided Survey, Isolation, and Characterization of Uranium Resistant Microbiota from the Savannah River Site, USA. Genes 2019, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Costa, G.; Nande, M.; Botías, P.; Garcia-Cantalejo, J.; Martín, M. Pb, Cd, and Zn soil contamination: Monitoring functional and structural impacts on the microbiome. Appl. Soil Ecol. 2018, 135, 56–64. [Google Scholar] [CrossRef]
- Yuebing, S.; Shunan, Z.; Lin, W.; Xuefeng, L.; Yingming, X. Changes of Enzymatic Activities, Substrate Utilization Pattern, and Microbial Community Diversity in Heavy Metal-Contaminated Soils. Water Air Soil Pollut. 2020, 231, 1–16. [Google Scholar]
- Singh, J.P.; Vaidya, B.P.; Goodey, N.M.; Krumins, J.A. Soil microbial response to metal contamination in a vegetated and urban brownfield. J. Environ. Manag. 2019, 244, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, S.; Zhong, Q.; Gong, G.; Wang, G.; Guo, X.; Xu, X. Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci. Total. Environ. 2020, 715, 136904. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, S.; Liao, W.; Ma, H.; Xie, S. Bacterial community analysis of sulfate-reducing granular sludge exposed to high concentrations of uranium. J. Water Supply Res. Technol. Aqua 2019, 68, 645–654. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, P.; Qin, Y.; Tu, Q.; Yang, Y.; He, Z.; Schadt, C.; Zhou, J. Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ. Microbiol. 2015, 18, 205–218. [Google Scholar]
- Li, B.; Wu, W.; Watson, D.; Cardenas, E.; Chao, Y.; Phillips, D.; Mehlhorn, T.; Lowe, K.; Kelly, S.; Li, P.; et al. Bacterial community shift and coexisting/coexcluding patterns revealed by network analysis in a bioreduced uranium contaminated site after reoxidation. Appl. Environ. Microbiol. 2018, 84, e02885-17. [Google Scholar] [CrossRef]
- You, W.; Peng, W.; Tian, Z.; Zheng, M. Uranium bioremediation with U(VI)-reducing bacteria. Sci. Total. Environ. 2021, 798, 149107. [Google Scholar] [CrossRef]
- Zhong, J.; Hu, X.; Liu, X.; Cui, X.; Lv, Y.; Tang, C.; Zhang, M.; Li, H.; Qiu, L.; Sun, W. Isolation and Identification of Uranium Tolerant Phosphate-Solubilizing Bacillus spp. and Their Synergistic Strategies to U(VI) Immobilization. Front. Microbiol. 2021, 12, 676391. [Google Scholar] [CrossRef]
- Tu, H.; Yuan, G.; Zhao, C.; Liu, J.; Li, F.; Yang, J.; Liao, J.; Yang, Y.; Liu, N. U-phosphate biomineralization induced by Bacillus sp. dw-2 in the presence of organic acids. Nucl. Eng. Technol. 2019, 51, 1322–1332. [Google Scholar] [CrossRef]
- Pan, X.; Chen, Z.; Chen, F.; Cheng, Y.; Lin, Z.; Guan, X. The mechanism of uranium transformation from U(VI) into nano-uramphite by two indigenous Bacillus thuringiensis strains. J. Hazard. Mater. 2015, 297, 313–319. [Google Scholar] [CrossRef]
- Conceição, M.V.R.; Costa, S.S.; Schaan, A.P.; Ribeiro-dos-Santos, Â.K.C.; Silva, A.; Graças, D.A.; Schneider, M.P.C.; Baraúna, R.A. Amazonia Seasons Have an Influence in the Composition of Bacterial Gut Microbiota of Mangrove Oysters (Crassostrea gasar). Front. Genet. 2021, 11, 602608. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Li, L.; Mo, G.; Wang, G.; Liu, H.; Xie, S. Analysis of uranium removal capacity of anaerobic granular sludge bacterial communities under different initial pH conditions. Environ. Sci. Pollut. Res. 2019, 26, 5613–5622. [Google Scholar] [CrossRef] [PubMed]
- Khomdram, N.; Narzary, D. Heavy metal tolerance of bacterial isolates associated with overburden strata of an opencast coal mine of Assam (India). Environ. Sci. Pollut. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Ding, D.X.; Li, S.M.; Hu, N.; Xu, F.; Li, G.Y.; Wang, Y.D. Bioreduction of U(VI) in groundwater under anoxic conditions from a decommissioned in situ leaching uranium mine. Bioprocess. Biosyst. Eng. 2015, 38, 661–669. [Google Scholar] [CrossRef]
- Yan, X.; Luo, X.; Zhao, M. Metagenomic analysis of microbial community in uranium-contaminated soil. Appl. Microbiol. Biotechnol. 2016, 100, 299–310. [Google Scholar] [CrossRef]
- Martinez, R.; Wu, C.H.; Andersen, G.L.; Taillefert, M.; Beazley, M.J.; Hazen, T.C.; Sobecky, P.A. Uranium Biomineralization by Natural Microbial Phosphatase Activities in the Subsurface. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2015. [Google Scholar]
- Rajeev, M.; Sushmitha, J.; Aravindraja, C.; Toleti, S.R.; Pandian, S.K. Exploring the impacts of heavy metals on spatial variations of sediment-associated bacterial communities. Ecotoxicol. Environ. Saf. 2021, 209, 111808. [Google Scholar] [CrossRef]
- Mtimunye, P.J.; Chirwa, E.M. Uranium (VI) Reduction in a Fixed-Film Reactor by a Bacterial Consortium Isolated from Uranium Mining Tailing Heaps. Biochem. Eng. J. 2019, 145, 62–73. [Google Scholar] [CrossRef]
- Grigoryan, A.A.; Bondici, V.F.; Kryachko, Y.; Khan, N.H.; Lawrence, J.R.; Singh, S.; Wolfaardt, G.M.; Gamage, N.W.; Shetty, D.; Korber, D.R. Draft Genome Sequence of Arthrobacter sp. Strain 260, Isolated from a Uranium Tailings Management Facility in Northern Saskatchewan, Canada. Microbiol. Resour. Announc. 2021, 10, e0036021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dodge, C.J.; Malhotra, S.V.; Francis, A.J. Bioreduction and precipitation of uranium in ionic liquid aqueous solution by Clostridium sp. Bioresour. Technol. 2013, 136, 752–756. [Google Scholar] [CrossRef]
- Zhang, C.; Malhotra, S.V.; Francis, A.J. Toxicity of ionic liquids to Clostridium sp. and effects on uranium biosorption. J. Hazard. Mater. 2014, 264, 246–253. [Google Scholar] [CrossRef]
- Povedano-Priego, C.; Jroundi, F.; Lopez-Fernandez, M.; Sánchez-Castro, I.; Martin-Sánchez, I.; Huertas, F.J.; Merroun, M.L. Shifts in bentonite bacterial community and mineralogy in response to uranium and glycerol-2-phosphate exposure. Sci. Total. Environ. 2019, 692, 219–232. [Google Scholar] [CrossRef]
- Shutova, V.V.; Revin, V.V.; Kalinkina, E.A.; Safonov, A.V.; Maksimov, G.V. Levan from Azotobacter vinelandii as a Component of Biosorbents for Heavy Metals and Radionuclides. Appl. Biochem. Microbiol. 2021, 57, 102–109. [Google Scholar] [CrossRef]
- Fathollahzadeh, H.; Becker, T.; Eksteen, J.J.; Kaksonen, A.H.; Watkin, E.L. Microbial contact enhances bioleaching of rare earth elements. Bioresour. Technol. Rep. 2018, 3, 102–108. [Google Scholar] [CrossRef]
- Pollmann, K.; Kutschke, S.; Matys, S.; Raff, J.; Hlawacek, G.; Lederer, F. Bio-recycling of metals: Recycling of technical products using biological applications. Biotechnol. Adv. 2018, 36, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Haghighatjoo, M.; Mohammadi, A. Bioleaching for Nanoparticle Production as a Solution for Environmental Pollution. J. Biosaf. 2021, 13, 62. [Google Scholar]
- Edberg, F.; Kalinowski, B.E.; Holmström, S.J.; Holm, K. Mobilization of metals from uranium mine waste: The role of pyoverdines produced by Pseudomonas fluorescens. Geobiology 2010, 8, 278–292. [Google Scholar] [CrossRef]
- Leigh, M.B.; Wu, W.-M.; Cardenas, E.; Uhlik, O.; Carroll, S.; Gentry, T.; Marsh, T.; Zhou, J.; Jardine, P.; Criddle, C.S.; et al. Microbial communities biostimulated by ethanol during uranium (VI) bioremediation in contaminated sediment as shown by stable isotope probing. Front. Environ. Sci. Eng. 2014, 9, 453–464. [Google Scholar] [CrossRef]
- Junier, P.; Frutschi, M.; Wigginton, N.; Schofield, E.J.; Bargar, J.R.; Bernier-Latmani, R. Metal reduction by spores ofDesulfotomaculum reducens. Environ. Microbiol. 2009, 11, 3007–3017. [Google Scholar] [CrossRef]
- Li, D.; Hu, N.; Sui, Y.; Ding, D.; Li, K.; Li, G.; Wang, Y. Influence of bicarbonate on the abundance of microbial communities capable of reducing U(vi) in groundwater. RSC Adv. 2017, 7, 49745–49752. [Google Scholar] [CrossRef]
- Thomas, S.H.; Padilla-Crespo, E.; Jardine, P.M.; Sanford, R.A.; Loffler, F.E. Diversity and Distribution of Anaeromyxobacter Strains in a Uranium-Contaminated Subsurface Environment with a Nonuniform Groundwater Flow. Appl. Environ. Microbiol. 2009, 75, 3679–3687. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Kim, J.Y.; Song, H.S.; Kim, Y.B.; Whon, T.W.; Ahn, S.W.; Lee, S.H.; Yoo, S.; Kim, Y.J.; Myoung, J.; et al. Anaerocolumna sedimenticola sp. nov., isolated from fresh water sediment. Antonie Van Leeuwenhoek 2021, 114, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ravachol, J.; de Philip, P.; Borne, R.; Mansuelle, P.; Maté, M.J.; Perret, S.; Fierobe, H.-P. Mechanisms involved in xyloglucan catabolism by the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Sci. Rep. 2016, 6, 22770. [Google Scholar] [CrossRef]
- Agarwal, M.; Pathak, A.; Rathore, R.S.; Prakash, O.; Singh, R.; Jaswal, R.; Seaman, J.; Chauhan, A. Proteogenomic Analysis of Burkholderia Species Strains 25 and 46 Isolated from Uraniferous Soils Reveals Multiple Mechanisms to Cope with Uranium Stress. Cells 2018, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Lin, H.; Dong, Y.; Li, B.; He, Y.; Liu, C.; Chen, X. A novel constructed carbonate-mineralized functional bacterial consortium for high-efficiency cadmium biomineralization. J. Hazard. Mater. 2020, 401, 123269. [Google Scholar] [CrossRef]
- Brito, E.M.S.; Romero-Núñez, V.M.; Caretta, C.A.; Bertin, P.; Valerdi-Negreros, J.C.; Guyoneaud, R.; Goñi-Urriza, M. The bacterial diversity on steam vents from Paricutín and Sapichu volcanoes. Extremophiles 2019, 23, 249–263. [Google Scholar] [CrossRef]
- Pathak, A.; Chauhan, A.; Stothard, P.; Green, S.; Maienschein-Cline, M.; Jaswal, R.; Seaman, J. Genome-centric evaluation of Burkholderia sp. strain SRS-W-2-2016 resistant to high concentrations of uranium and nickel isolated from the Savannah River Site (SRS), USA. Genom. Data 2017, 12, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Lv, Y.; Liu, X.; Zhong, J.; Cui, X.; Zhang, M.; Ma, D.; Yan, X.; Zhu, X. Effects of Heavy Metals/Metalloids and Soil Properties on Microbial Communities in Farmland in the Vicinity of a Metals Smelter. Front. Microbiol. 2021, 12, 2347. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, Y.; Zhang, H.; Wang, R.; Tao, X.; Zhang, L.; Zuo, Y.; Zhang, L.; Wei, Y.; Li, J. Inoculation of phosphate-solubilizing bacteria (Bacillus) regulates microbial interaction to improve phosphorus fractions mobilization during kitchen waste composting. Bioresour. Technol. 2021, 340, 125714. [Google Scholar] [CrossRef]
| Composition | Content |
|---|---|
| U (mg/kg) | 320~1820 |
| Mn (mg/kg) | 360~520 |
| Fe (mg/kg) | 1730~3300 |
| Ca (mg/kg) | 6460~11,470 |
| SO42− (mg/kg) | 6240~10,430 |
| pH | 6.50~7.20 |
| Sample | Chao 1 Index | ACE Index | Shannon Index | Simpson Index | Coverage |
|---|---|---|---|---|---|
| C1 | 128.5 ± 61.8 | 137.1 ± 55.8 | 0.55 ± 0.03 | 0.77 ± 0.02 | 0.999 ± 0.0003 |
| C2 | 193.9 ± 45.1 | 187.8 ± 31.5 | 1.14 ± 0.34 | 0.51 ± 0.11 | 0.999 ± 0.0001 |
| C3 | 241.0 ± 42.2 | 239.1 ± 45.6 | 1.92 ± 0.33 | 0.30 ± 0.09 | 0.999 ± 0.0004 |
| C4 | 291.9 ± 25.5 | 286.6 ± 18.4 | 2.25 ± 007 | 0.20 ± 0.03 | 0.999 ± 0.0002 |
| C5 | 455.4 ± 102.8 | 516.8 ± 4.6 | 2.79 ± 0.06 | 0.11 ± 0.01 | 0.998 ± 0.0001 |
| T1 | 317.5 ± 76.1 | 475.2 ± 73.0 | 1.31 ± 0.35 | 0.45 ± 0.19 | 0.997 ± 0.0006 |
| T2 | 310.4 ± 86.1 | 310.5 ± 79.5 | 2.52 ± 0.39 | 0.16 ± 0.06 | 0.998 ± 0.0003 |
| T3 | 317.6 ± 34.2 | 325.0 ± 76.0 | 2.91 ± 0.11 | 0.18 ± 0.01 | 0.999 ± 0.0004 |
| T4 | 281.6 ± 64.0 | 285.8 ± 61.6 | 2.94 ± 0.15 | 0.12 ± 0.03 | 0.999 ± 0.0005 |
| T5 | 340.7 ± 36.2 | 331.5 ± 123.9 | 3.27 ± 0.12 | 0.09 ± 0.02 | 0.999 ± 0.0003 |
| Genus | Desulfotomaculum | Desulfosporporosinus | Anaerocolumna | Caproiciproducens | Burkholderia |
|---|---|---|---|---|---|
| C1 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 1 ± 1 |
| T1 | 1 ± 1 | 0 ± 0 | 1 ± 1 | 1 ± 1 | 415 ± 1.41 |
| C2 | 12 ± 19 | 2 ± 2 | 16 ± 10 | 10 ± 7 | 22 ± 7 |
| T2 | 45 ± 35 | 1 ± 1 | 121 ± 47 | 223 ± 61 | 626 ± 15 |
| C3 | 14 ± 22 | 2 ± 0 | 26 ± 22 | 9 ± 12 | 42 ± 33 |
| T3 | 2970 ± 221 | 82 ± 55 | 2164 ± 354 | 638 ± 80 | 951 ± 716 |
| C4 | 8 ± 12 | 0 ± 0 | 30 ± 24 | 111 ± 45 | 209 ± 59 |
| T4 | 1315 ± 439 | 417 ± 30 | 4809 ± 2183 | 495 ± 50 | 36 ± 23 |
| C5 | 1 ± 1 | 7 ± 9 | 11 ± 6 | 179.5 ± 9 | 43 ± 23 |
| T5 | 946 ± 494 | 604 ± 426 | 322 ± 136 | 198 ± 32 | 122 ± 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Zhong, J.; Lv, Y.; Liu, X.; Li, Y.; Zhang, M.; Yan, X.; Sun, W. Response and Dynamic Change of Microbial Community during Bioremediation of Uranium Tailings by Bacillus sp. Minerals 2021, 11, 967. https://doi.org/10.3390/min11090967
Tang C, Zhong J, Lv Y, Liu X, Li Y, Zhang M, Yan X, Sun W. Response and Dynamic Change of Microbial Community during Bioremediation of Uranium Tailings by Bacillus sp. Minerals. 2021; 11(9):967. https://doi.org/10.3390/min11090967
Chicago/Turabian StyleTang, Chuiyun, Juan Zhong, Ying Lv, Xingyu Liu, Yongbin Li, Mingjiang Zhang, Xiao Yan, and Weimin Sun. 2021. "Response and Dynamic Change of Microbial Community during Bioremediation of Uranium Tailings by Bacillus sp." Minerals 11, no. 9: 967. https://doi.org/10.3390/min11090967
APA StyleTang, C., Zhong, J., Lv, Y., Liu, X., Li, Y., Zhang, M., Yan, X., & Sun, W. (2021). Response and Dynamic Change of Microbial Community during Bioremediation of Uranium Tailings by Bacillus sp. Minerals, 11(9), 967. https://doi.org/10.3390/min11090967






