Magnetic Preconcentration and Process Mineralogical Study of the Kiviniemi Sc-Enriched Ferrodiorite
Abstract
:1. Introduction
2. The Kiviniemi Intrusion
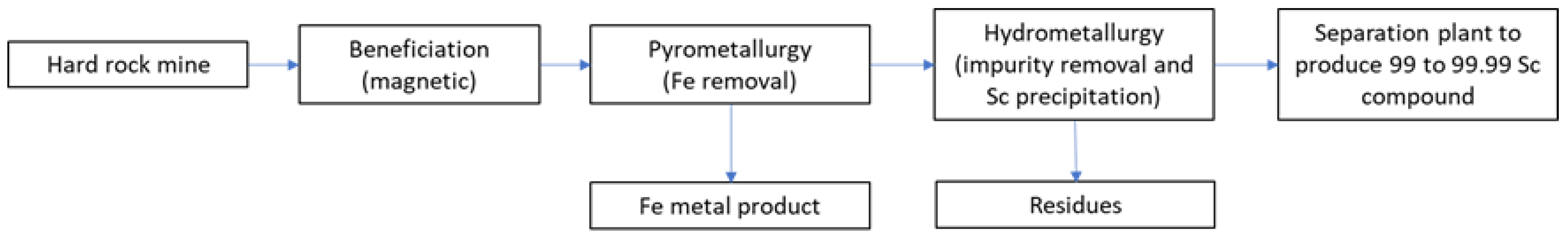
3. Materials and Methods
4. Results
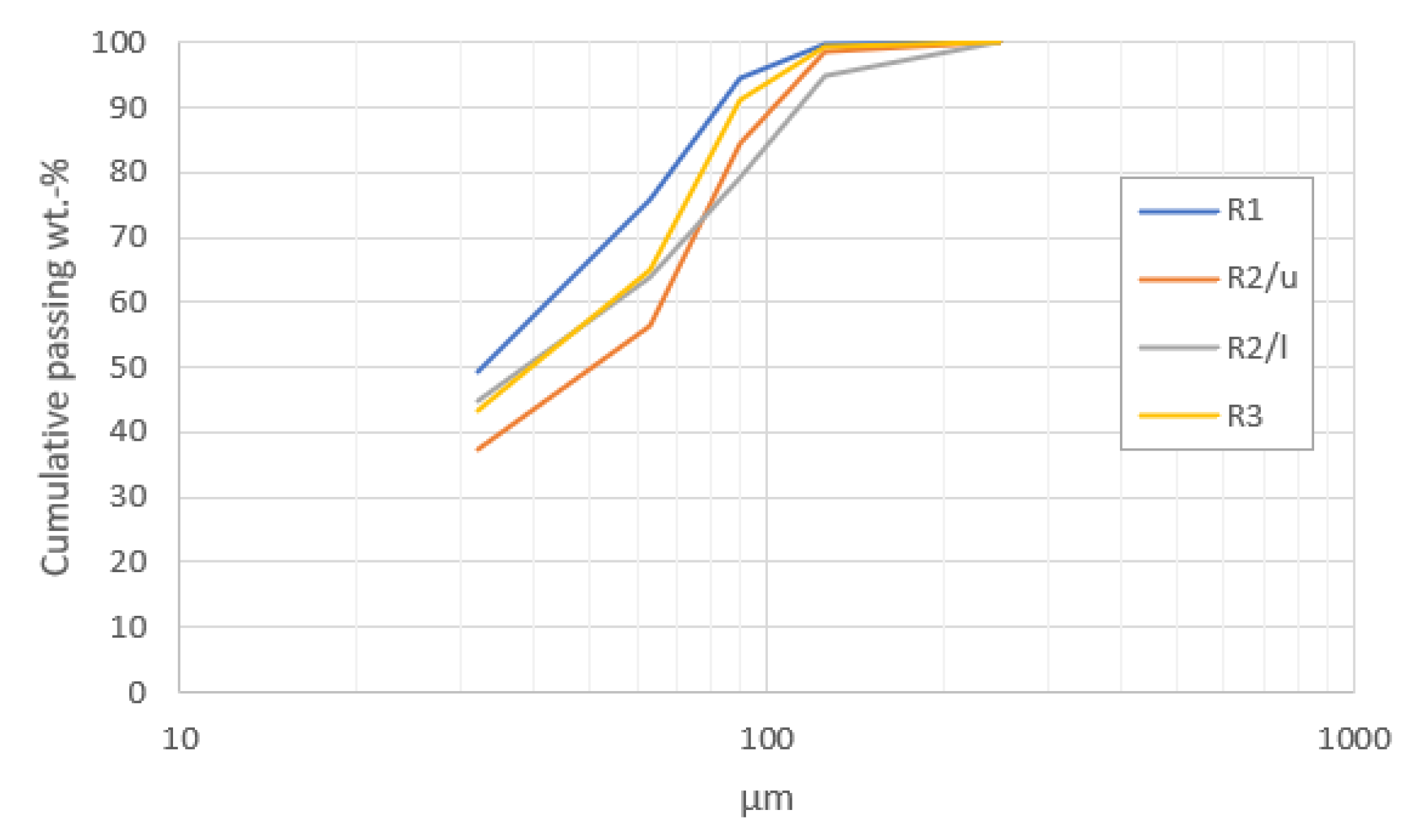
4.1. Feed Particle Size Distribution and Chemical Compositions
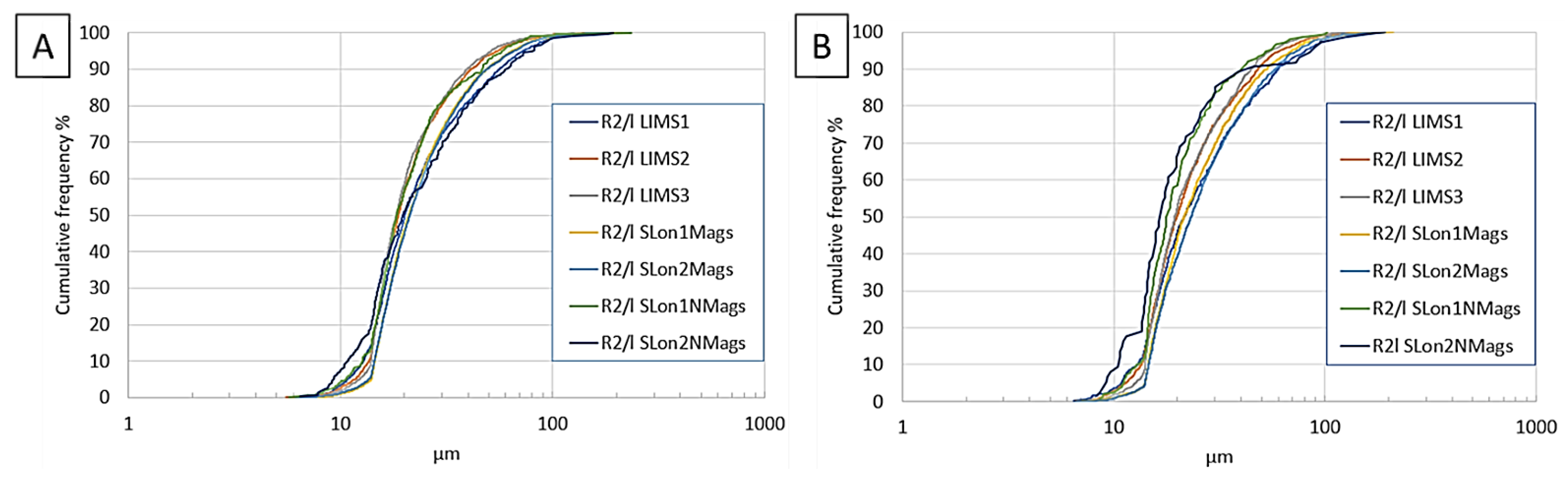
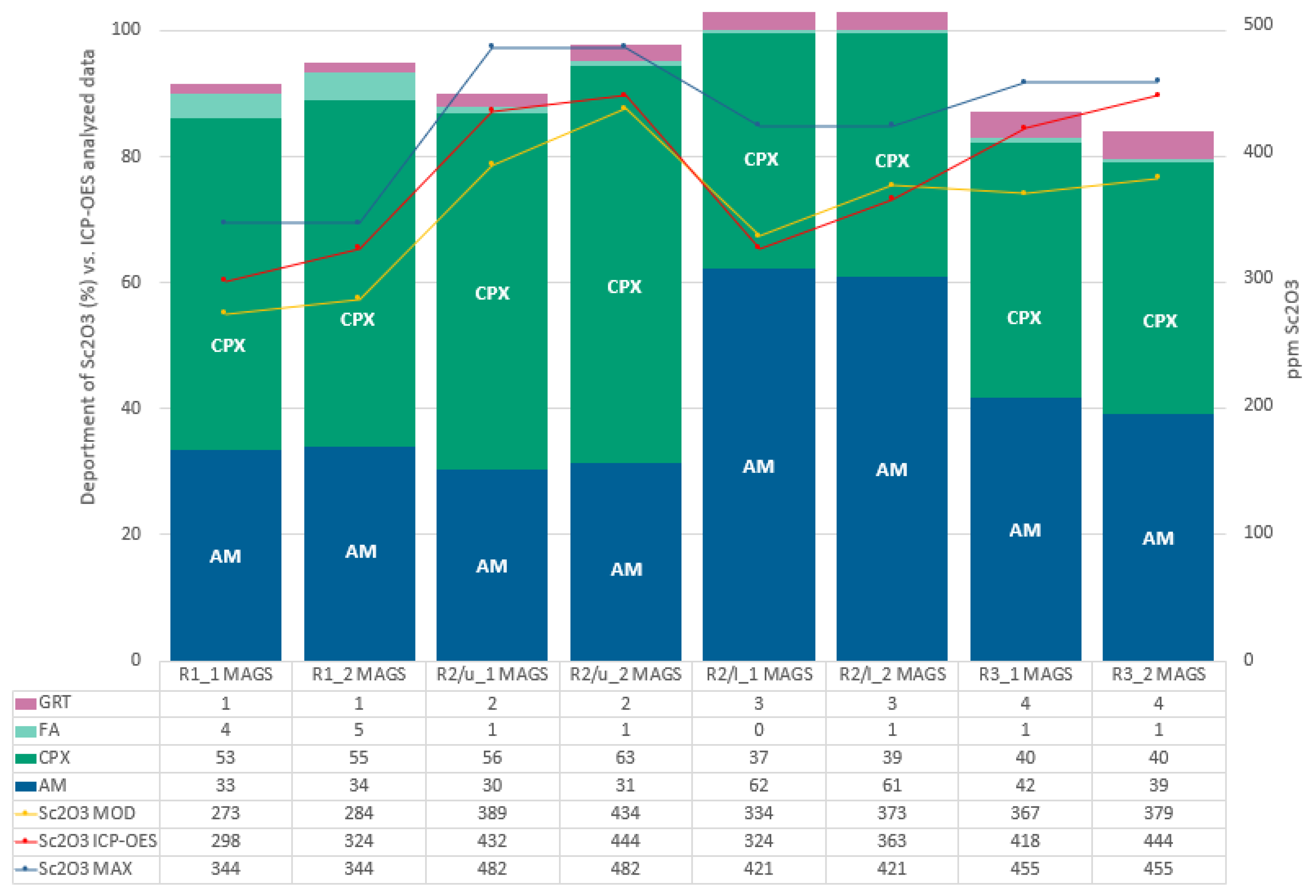
4.2. Magnetic Separation
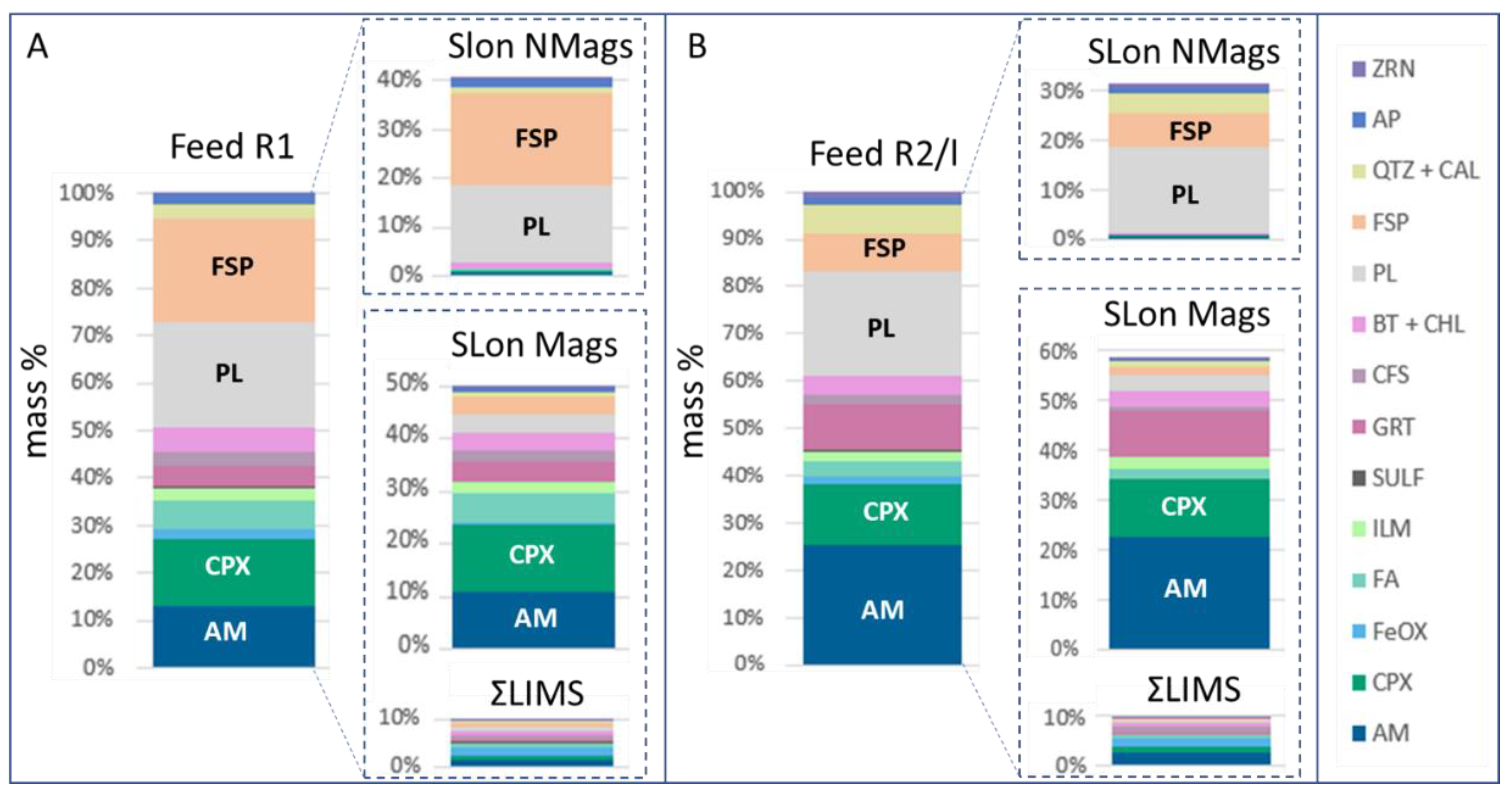
4.3. Process Mineralogical Features
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connelly, N.G.; Hartshorn, R.M.; Damhus, T.; Hutton, A.T. Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005; Royal Society of Chemistry: London, UK, 2005. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Vasyukova, O.V. The economic geology of scandium, the runt of the rare earth element litter. Econ. Geol. 2018, 113, 973–988. [Google Scholar] [CrossRef] [Green Version]
- Horovitz, C.T.; Gschneidner, K.A., Jr.; Melson, G.A.; Youngblood, D.H.; Schock, H.H. Scandium Its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology; Academic Press Inc.: London, UK, 1975. [Google Scholar]
- Samson, I.M.; Chassé, M. Scandium (Sc). In Encyclopedia Geochemistry; White, W.M., Ed.; Springer: Cham, Switzerland, 2016; 7p. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Norman, J.C.; Haskin, L.A. The geochemistry of Sc: A comparison to the rare earths and Fe. Geochim. Cosmochim. Acta 1968, 32, 93–108. [Google Scholar] [CrossRef]
- Walters, A.; Lusty, P.; Chetwyn, C.; Hill, A. Rare Earth Elements. In MineralsUK; British Geological Survey: Keyworth, UK, 2010; 45p, Available online: http://nora.nerc.ac.uk/id/eprint/12583/1/Rare_Earth_Elements_profile.pdf (accessed on 5 September 2020).
- Chassé, M.; Griffin, W.L.; O’Reilly, S.Y.; Calas, G. Scandium speciation in a world-class lateritic deposit. Geochem. Perspect. Lett. 2017, 3, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, M.Y.H.; Liu, Z.R.R.; Zhou, M.F. Scandium: Ore deposits, the pivotal role of magmatic enrichment and future exploration. Ore Geol. Rev. 2020, 128, 103906. [Google Scholar] [CrossRef]
- Halkoaho, T.; Ahven, M.; Rämö, O.T.; Hokka, J.; Huhma, H. Petrography, geochemistry and geochronoloy of the Sc-enriched Kiviniemi ferrodiorite intrusion, eastern Finland. Miner. Depos. 2020, 55, 1561–1580. [Google Scholar] [CrossRef] [Green Version]
- Shimazaki, H.; Yang, Z.; Miyawaki, R.; Shigeoka, M. Scandium-bearing minerals in the Bayan Obo Nb-REE-Fe Deposit, Inner Mongolia, China. Resour. Geol. 2008, 58, 80–86. [Google Scholar] [CrossRef]
- Rangott, M.; Hutchin, S.; Basile, D.; Ricketts, N.; Duckworth, G.; Rowles, T.D. Feasibility Study-Nyngan Scandium Project; NI 43-101 Technical Report; Scandium International Min. Corporation: Sparks, NV, USA, 2016; 278p. [Google Scholar]
- Petrella, L.; Williams-Jones, A.E.; Goutier, J.; Walsh, J. The nature and origin of the rare earth element mineralization in the Misery syenitic intrusion, Northern Quebec, Canada. Econ. Geol. 2014, 109, 1643–1666. [Google Scholar] [CrossRef]
- Daigle, P.J. NI 43-101 Technical Report on the Crater Lake Sc-Nb-REE Project Québec, Canada. 2017. Available online: https://imperialmgp.com/site/assets/files/4831/crater_lake_technical_report_update_clean_pjc_nov_17-17.pdf (accessed on 23 January 2020).
- Hokka, J.; Halkoaho, T. 3D Modelling and Mineral Resource Estimation of the Kiviniemi Scandium Deposit, Eastern Finland; Mineral Resource Estimation Report; Geological Survey of Finland: Espoo, Finland, 2017; 21p. [Google Scholar] [CrossRef]
- Goode, J.R. Rare Earth Elements. In SME Mineral Processing and Extractive Metallurgy Handbook; Dunne, R.C., Kawatra, S.K., Young, C.A., Eds.; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 2019; pp. 2049–2075. [Google Scholar]
- Eilu, P. Critical Raw Materials Factsheets. Available online: https://www.gtk.fi/wp-content/uploads/2020/05/Critical_metals_report_Scandium.pdf (accessed on 29 August 2020).
- Laurent, A. Commodities at a glance: Special issue on rare earths. U. N. Conf. Trade Dev. 2014, 5, 1–58. [Google Scholar]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Li, G.; Ye, Q.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Extraction of scandium from scandium-rich material derived from bauxite ore residues. Hydrometallurgy 2018, 176, 62–68. [Google Scholar] [CrossRef]
- Duyvesteyn, W.P.C.; Putnam, G.F. Scandium, a Review of the Element, Its Characteristics, and Current and Emerging Commercial Applications; White Paper; EMC Metals Corporation: Sparks, NV, USA, 2014; 12p. [Google Scholar]
- Yagmurlu, B.; Alkan, G.; Xakalashe, B.; Schier, C.; Gronen, L.; Koiwa, I.; Dittrich, C.; Friedrich, B. Synthesis of Scandium Phosphate after Peroxide Assisted Leaching of Iron Depleted Bauxite Residue (Red Mud) Slags. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2005; Volume 37. [Google Scholar]
- Faris, N.; Ram, R.; Tardio, J.; Bhargava, S.; McMaster, S.; Pownceby, M. Application of ferrous pyrometallurgy to the beneficiation of rare earth bearing iron ores—A review. Miner. Eng. 2017, 110, 20–30. [Google Scholar] [CrossRef]
- Korhonen, T.; Neitola, R.; Mörsky, P.; Laukkanen, J. Preliminary Beneficiation Study on Kiviniemi Samples; Report of Investigation; Geological Survey of Finland: Espoo, Finland, 2011; 35p. (In Finnish) [Google Scholar]
- Halkoaho, T.; Niskanen, M.M. A Research Report of Scandium and Zirconium Studies Concerning the Claim Area of Kiviniemi 1 (Register Number of Claim 8777/1) in Rautalampi during Years 2008–2010; Claim Report; Geological Survey of Finland: Espoo, Finland, 2015; Volume 56, 32p. (In Finnish) [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 3rd ed.; Mineralogical Society of Great Britain: London, UK, 2013. [Google Scholar]
- Dunne, R.C.; Honaker, R.Q.; Giblett, A. Gravity Concentration. In SME Mineral Processing and Extractive Metallurgy Handbook; Dunne, R.C., Kawatra, S.K., Young, C.A., Eds.; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 2019; pp. 787–814. [Google Scholar]
- Leißner, T.; Bachmann, K.; Gutzmer, J.; Peuker, U.A. MLA-based partition curves for magnetic separation. Miner. Eng. 2016, 94, 94–103. [Google Scholar] [CrossRef]
- Rosenblum, S.; Brownfield, I. Magnetic Susceptibilities of Minerals; Open File Report; USGS: Reston, VA, USA, 2000; pp. 1–37. [CrossRef]
- Svoboda, J. Magnetic Techniques for the Treatment of Materials; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Hunt, C.P.; Moskowitz, B.M.; Banerjee, S.K. Magnetic Properties of Rocks and Minerals. In Rock Physics and Phase Relations: A Handbook of Physical Constants; Ahrens, T.J., Ed.; American Geophysical Union: Washington, DC, USA, 1995; pp. 189–204. [Google Scholar] [CrossRef]
- Nordman, T.; Kuusisto, M. Application of High Intensity Magnetic Separation for the Beneficiation of Finnish Metal and Industrial Mineral Ores; Literature Survey; Finnish Association of Mining and Metallurgical Engineers: Espoo, Finland, 1993; 63p. (In Finnish) [Google Scholar]
- Dobbins, M.; Dunn, P.; Sherrell, I. Recent Advances in Magnetic Separator Designs and Applications. In Proceedings of the 7th International Heavy Miner. Conference What Next; The Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2009; pp. 63–70. [Google Scholar]
- Outotec. SLon ® Vertically Pulsating High-Gradient Magnetic Separator; Technical Leaflet; Outotec: Espoo, Finland, 2013; 4p. [Google Scholar]
- Chen, L.; Xiong, D. Magnetic Techniques for Mineral Processing. In Progress in Filtration and Separation; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 287–324. [Google Scholar] [CrossRef]
- Liipo, J.; Lang, C.; Burgess, S.; Otterström, H.; Person, H.; Lamberg, P. Automated mineral liberation analysis using INCAMineral. In Proceedings of the Process Mineralogy’12, Cape Town, South Africa, 7–9 November 2012; Minerals Engineering International (MEI): Falmouth, UK, 2012; pp. 402–408. [Google Scholar]
- Schulz, B.; Sandmann, D.; Gilbricht, S. Sem-based automated mineralogy and its application in geo-and material sciences. Minerals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Blannin, R.; Frenzel, M.; Tuşa, L.; Birtel, S.; Ivăşcanu, P.; Baker, T.; Gutzmer, J. Uncertainties in quantitative mineralogical studies using scanning electron microscope-based image analysis. Miner. Eng. 2021, 167, 198–203. [Google Scholar] [CrossRef]
- Evans, C.L.; Napier-Munn, T.J. Estimating error in measurements of mineral grain size distribution. Miner. Eng. 2013, 52, 198–203. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, D.; Huang, H. Pulsating high-gradient magnetic separation of fine hematite from tailings. Miner. Metall. Process. 2009, 26, 163–168. [Google Scholar] [CrossRef]


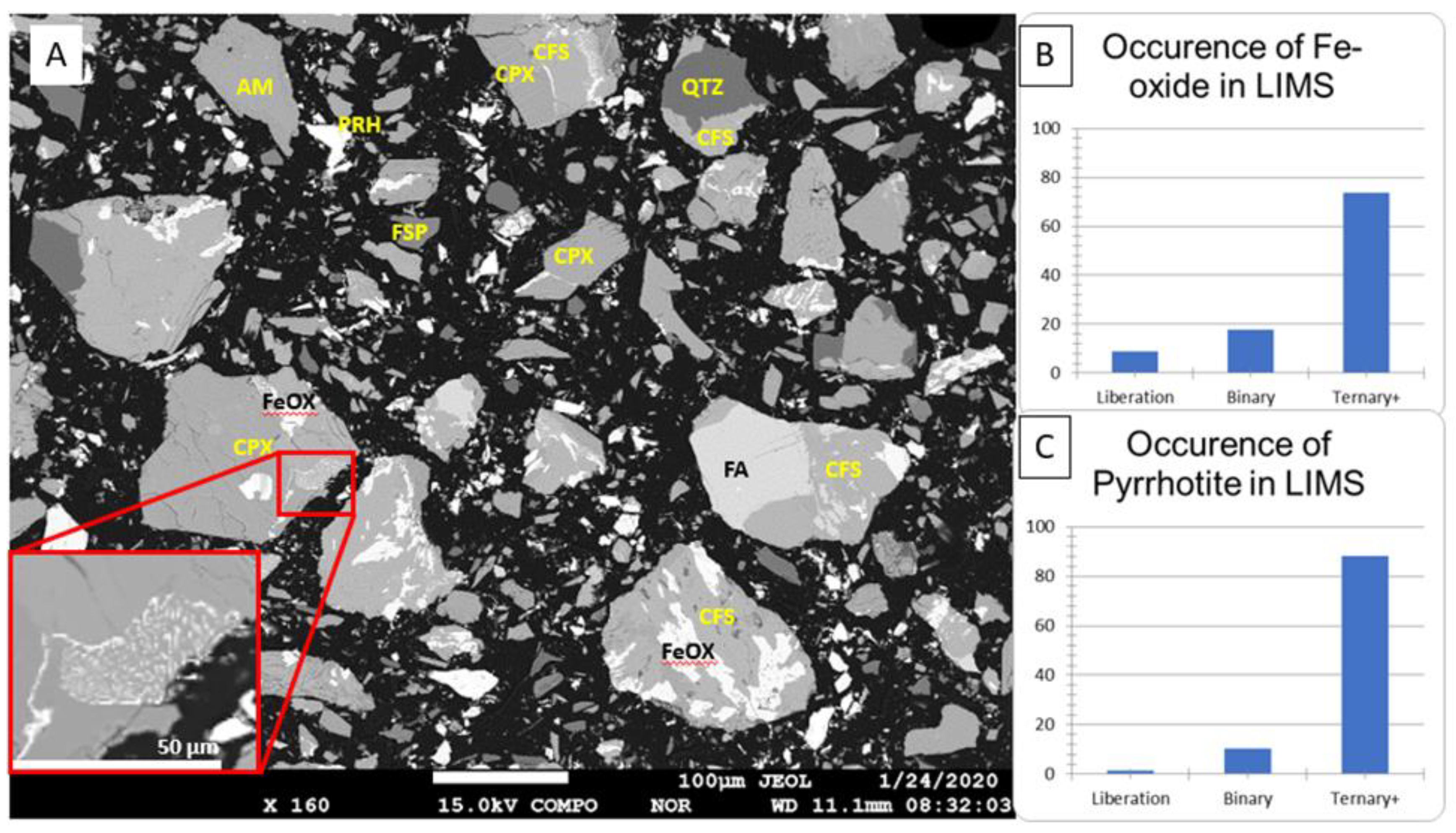
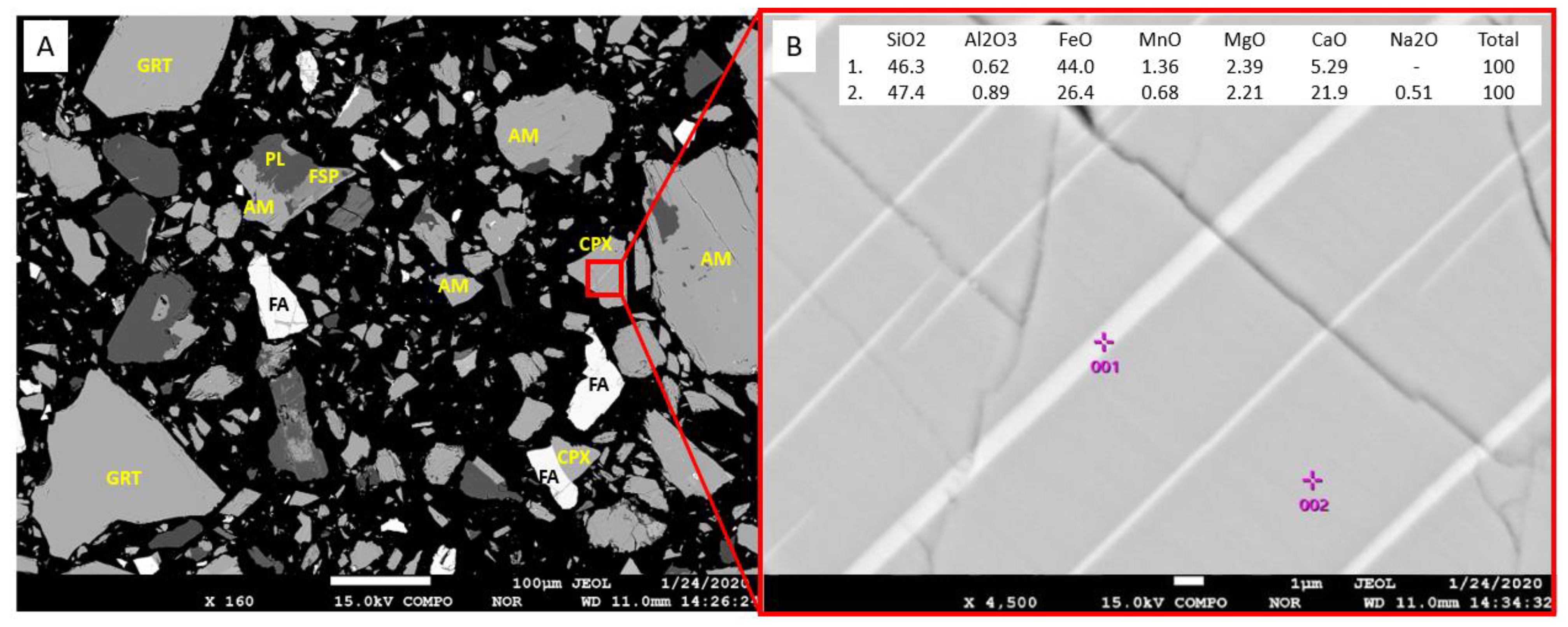
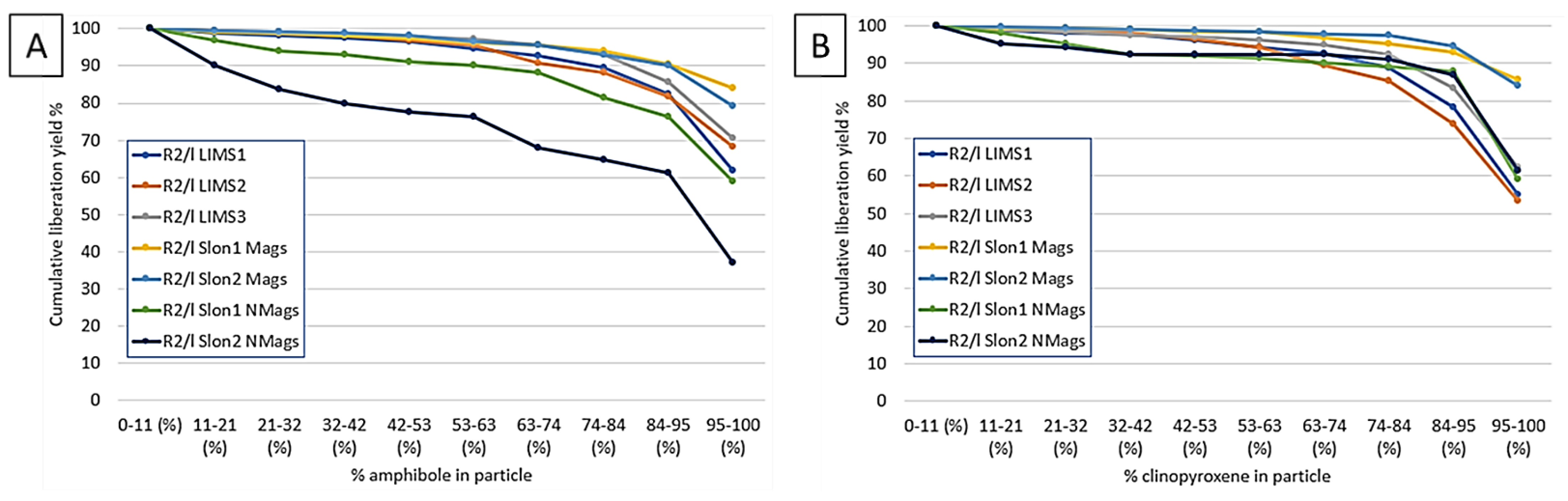
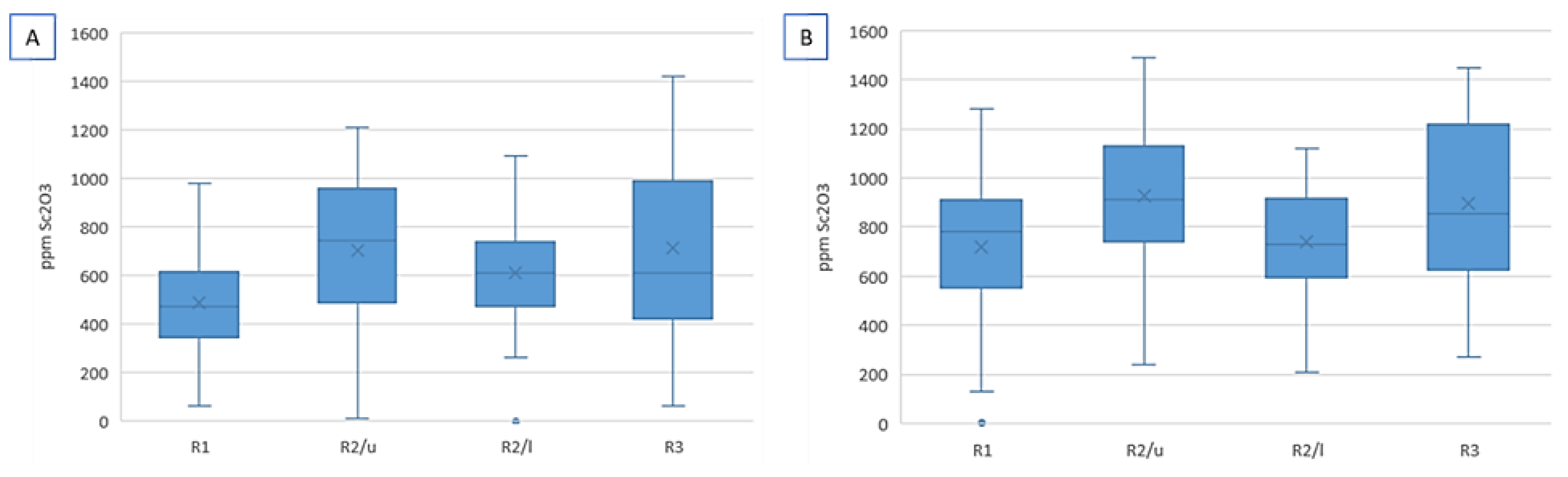
| Deposit (Country) | Resource Est. t @ Grade | Main Host Minerals for Sc | Deposit Type | References |
|---|---|---|---|---|
| Bayan Obo (China) | 140,000 t @ 200 ppm Sc | Aegirine | Carbonatite | [3,12] |
| Nyngan (Australia) | 16.9 Mt @ 235 ppm Sc | Adsorbed species on ferric oxyhydroxide in limonite and saprolite | Lateritic | [13] |
| Syerston (Australia) | 21.7 Mt @ 429 ppm Sc | Adsorbed species on goethite, hematite | Lateritic | [3,9] |
| Misery Lake (Canada) | Sc grades 150–300 ppm | Hedenbergite | Syenite | [3,14] |
| Crater Lake (Canada) | Sc grades up to 208 ppm Sc | Hedenbergite | Ferrosyenite | [15] |
| Kiviniemi (Finland) | 13.4 Mt @ 163 ppm Sc | Hedenbergite, amphibole | Ferrodiorite | [11,16] |
| Drill Core, Depth m | SiO2 | Al2O3 | FeOtot | MgO | CaO | K2O | P2O5 | S | ZrO2 | Sc2O3 ppm |
|---|---|---|---|---|---|---|---|---|---|---|
| R1 45.0–46.0 | 42.8 | 11.6 | 23.3 | 1.38 | 8.30 | 2.47 | 0.75 | 0.19 | 0.09 | 252 |
| R1 46.0–47.0 | 44.7 | 12.3 | 24.3 | 1.33 | 8.93 | 2.48 | 0.77 | 0.23 | 0.09 | 262 |
| R1 47.0–48.0 | 45.4 | 12.7 | 19.9 | 1.35 | 8.00 | 3.13 | 0.71 | 0.17 | 0.09 | 227 |
| R1 47.0–48.0 (d) | 44.7 | 12.5 | 19.8 | 1.32 | 7.89 | 3.11 | 0.68 | 0.18 | 0.09 | 222 |
| Weighted average | 44.2 | 12.2 | 22.5 | 1.35 | 8.39 | 2.69 | 0.74 | 0.20 | 0.09 | 246 |
| R2/u 131.0–132.5 | 42.6 | 10.7 | 22.9 | 0.75 | 8.19 | 2.51 | 0.72 | 0.24 | 0.60 | 307 |
| R2/u 132.5–134.0 | 41.7 | 10.5 | 22.9 | 0.78 | 8.30 | 2.14 | 0.71 | 0.23 | 0.41 | 319 |
| R2/u 134.0–135.5 | 42.8 | 11.1 | 23.3 | 0.76 | 8.34 | 2.30 | 0.77 | 0.25 | 0.32 | 304 |
| Weighted average | 42.4 | 10.8 | 23.0 | 0.76 | 8.27 | 2.31 | 0.73 | 0.24 | 0.44 | 310 |
| R2/l 210.8–212.0 | 45.4 | 13.2 | 21.7 | 0.72 | 8.62 | 1.69 | 0.66 | 0.23 | 0.53 | 230 |
| R2/l 212.0–213.5 | 44.1 | 11.4 | 24.2 | 0.81 | 8.80 | 1.41 | 0.67 | 0.24 | 0.53 | 284 |
| R2/l 215.0–216.5 | 45.8 | 10.7 | 23.7 | 0.79 | 8.07 | 1.96 | 0.67 | 0.22 | 0.58 | 287 |
| Weighted average | 45.0 | 11.7 | 23.3 | 0.78 | 8.49 | 1.68 | 0.67 | 0.23 | 0.55 | 270 |
| R3 91.5–93.0 | 46.9 | 13.6 | 19.4 | 0.58 | 7.40 | 3.31 | 0.60 | 0.22 | 0.25 | 270 |
| R3 91.5–93.0 (d) | 46.6 | 13.5 | 19.4 | 0.58 | 7.39 | 3.30 | 0.59 | 0.22 | 0.25 | 271 |
| R3 93.0–94.5 | 47.3 | 13.7 | 17.8 | 0.56 | 6.95 | 3.87 | 0.55 | 0.21 | 0.27 | 250 |
| R3 94.5–96.0 | 48.4 | 13.3 | 19.2 | 0.58 | 7.44 | 3.53 | 0.65 | 0.22 | 0.20 | 281 |
| Weighted average | 47.4 | 13.5 | 18.8 | 0.57 | 7.26 | 3.57 | 0.60 | 0.21 | 0.24 | 267 |
| Detection limit | 0.02 | 0.02 | 0.01 | 0.02 | 0.004 | 0.004 | 0.014 | 0.006 | 0.001 | 20 |
| Rougher 1 | Rougher 2 | Rougher 3 | Rougher 4 | Rougher 5 | Rougher 1a | Cleaner 1b | Rougher 2a | Cleaner 2b | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 150 rpm | 50 rpm | 250 rpm | 150 rpm | 150 rpm | 150 rpm | 150 rpm | 150 rpm | 150 rpm | ||
| 0.5 T | 0.5 T | 0.5 T | 0.3 T | 1.0 T | 0.5 T | 1.0 T | 0.7 T | 1.0 T | ||
| Feed | mass g | 193 | 193 | 194 | 194 | 197 | 194 | 194 | ||
| Sc ppm (ICP-OES) | 170 | 170 | 170 | 170 | 170 | 170 | 170 | |||
| Sc ppm (calc.) | 172 | 169 | 168 | 179 | 177 | 180 | 174 | |||
| Mags | mass g | 116 | 135 | 101 | 111 | 128 | 101 | 108 | ||
| mass % | 60 | 70 | 52 | 57 | 65 | 52 | 56 | |||
| Sc ppm | 240 | 220 | 250 | 260 | 250 | 270 | 260 | |||
| * R Sc % | 84 | 91 | 77 | 83 | 92 | 78 | 83 | |||
| NMags | mass g | 77 | 58 | 93 | 83 | 69 | 85 | 8 | 76 | 10 |
| mass % | 40 | 30 | 48 | 43 | 35 | 44 | 4 | 39 | 5 | |
| Sc ppm | 70 | 50 | 80 | 70 | 40 | 80 | 110 | 60 | 110 | |
| Relative error % | 1.4 | 0.5 | 0.9 | 5.4 | 3.9 | 6.0 | 2.3 | |||
| R1_1 | R1_2 | R2/u_1 | R2/u_2 | R3_1 | R3_2 | ||
|---|---|---|---|---|---|---|---|
| Feed | mass g | 203 | 205 | 196 | 190 | 218 | 219 |
| Sc ppm (ICP-OES) | 150 | 150 | 210 | 210 | 180 | 180 | |
| Sc ppm (calc.) | 141 | 145 | 213 | 210 | 178 | 184 | |
| Mags | mass g | 115 | 113 | 125 | 117 | 116 | 113 |
| mass % | 57 | 55 | 64 | 61 | 53 | 51 | |
| Sc ppm | 210 | 230 | 300 | 310 | 290 | 310 | |
| * R Sc % | 85 | 88 | 90 | 91 | 87 | 87 | |
| NMags | mass g | 88 | 92 | 71 | 73 | 102 | 107 |
| mass % | 43 | 45 | 36 | 39 | 47 | 49 | |
| Sc ppm | 50 | 40 | 60 | 50 | 50 | 50 | |
| Relative error % | 6.1 | 3.3 | 1.5 | 0.1 | 1.1 | 2.1 | |
| Sample | N | SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | Sc2O3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphibole | R1 | 60 | Avg | 38.3 | 2.16 | 10.7 | 28.0 | 0.25 | 2.16 | 10.7 | 1.43 | 1.71 | 489 |
| Stdev | 1.02 | 0.33 | 0.81 | 0.75 | 0.05 | 0.22 | 0.22 | 0.14 | 0.28 | 214 | |||
| R2/u | 42 | Avg | 38.2 | 2.11 | 10.4 | 29.4 | 0.27 | 1.38 | 10.8 | 1.50 | 1.62 | 703 | |
| Stdev | 0.78 | 0.35 | 0.83 | 0.47 | 0.08 | 0.21 | 0.60 | 0.12 | 0.19 | 309 | |||
| R2/l | 59 | Avg | 39.0 | 1.95 | 10.4 | 30.4 | 0.35 | 1.37 | 11.1 | 1.53 | 0.48 | 608 | |
| Stdev | 1.15 | 0.50 | 1.04 | 0.63 | 0.13 | 0.20 | 0.49 | 0.17 | 0.12 | 224 | |||
| R3 | 27 | Avg | 38.2 | 1.84 | 10.7 | 29.6 | 0.25 | 1.31 | 10.8 | 1.33 | 1.64 | 710 | |
| Stdev | 0.55 | 0.37 | 0.65 | 0.56 | 0.08 | 0.18 | 0.12 | 0.15 | 0.12 | 361 | |||
| Clinopyroxene | R1 | 67 | Avg | 48.1 | 0.19 | 1.18 | 25.4 | 0.53 | 3.21 | 19.5 | 0.39 | 0.02 | 717 |
| Stdev | 0.72 | 0.13 | 0.68 | 1.23 | 0.12 | 0.22 | 1.14 | 0.13 | 0.05 | 268 | |||
| R2/u | 71 | Avg | 47.3 | 0.24 | 1.40 | 26.9 | 0.49 | 2.16 | 19.3 | 0.44 | 0.07 | 929 | |
| Stdev | 0.64 | 0.15 | 0.74 | 0.52 | 0.10 | 0.23 | 0.89 | 0.12 | 0.06 | 258 | |||
| R2/l | 60 | Avg | 48.2 | 0.13 | 0.84 | 27.8 | 0.64 | 2.18 | 20.4 | 0.37 | 0.01 | 741 | |
| Stdev | 0.47 | 0.09 | 0.42 | 0.90 | 0.12 | 0.17 | 0.87 | 0.07 | 0.01 | 207 | |||
| R3 | 40 | Avg | 47.8 | 0.13 | 0.86 | 26.9 | 0.50 | 1.99 | 20.1 | 0.35 | 0.02 | 898 | |
| Stdev | 0.57 | 0.09 | 0.49 | 0.67 | 0.11 | 0.24 | 0.63 | 0.07 | 0.06 | 357 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallio, R.; Tanskanen, P.; Luukkanen, S. Magnetic Preconcentration and Process Mineralogical Study of the Kiviniemi Sc-Enriched Ferrodiorite. Minerals 2021, 11, 966. https://doi.org/10.3390/min11090966
Kallio R, Tanskanen P, Luukkanen S. Magnetic Preconcentration and Process Mineralogical Study of the Kiviniemi Sc-Enriched Ferrodiorite. Minerals. 2021; 11(9):966. https://doi.org/10.3390/min11090966
Chicago/Turabian StyleKallio, Rita, Pekka Tanskanen, and Saija Luukkanen. 2021. "Magnetic Preconcentration and Process Mineralogical Study of the Kiviniemi Sc-Enriched Ferrodiorite" Minerals 11, no. 9: 966. https://doi.org/10.3390/min11090966
APA StyleKallio, R., Tanskanen, P., & Luukkanen, S. (2021). Magnetic Preconcentration and Process Mineralogical Study of the Kiviniemi Sc-Enriched Ferrodiorite. Minerals, 11(9), 966. https://doi.org/10.3390/min11090966





