Arsenic Remediation through Sustainable Phytoremediation Approaches
Abstract
:1. Introduction
2. Phytoremediation: A Sustainable Approach
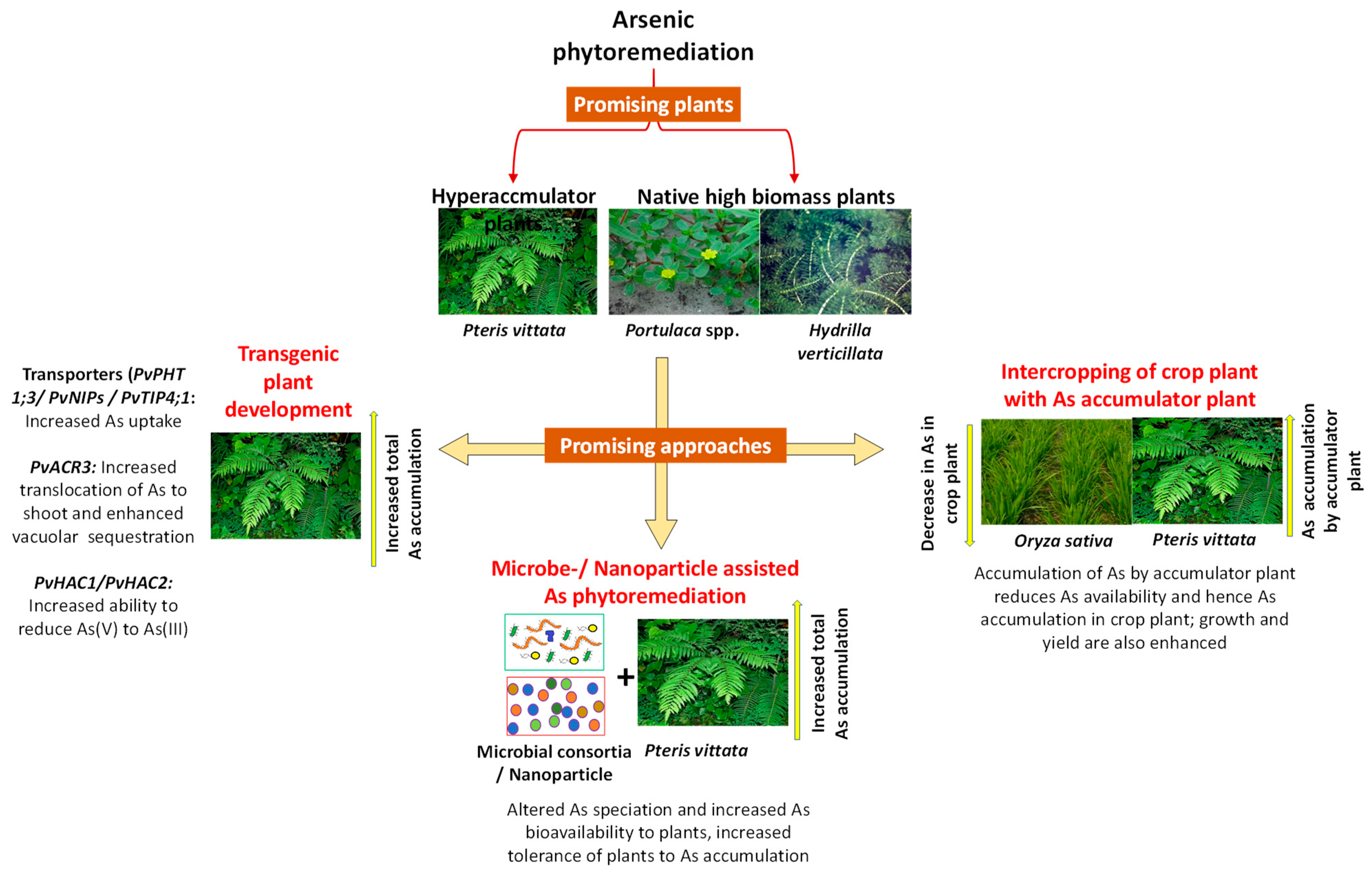
2.1. Selection of Plants for Arsenic Phytoremediation
2.1.1. Arsenic Hyperaccumulators
2.1.2. High Biomass Plants for Arsenic Cleanup
2.1.3. Plants with Bioenergy Potential and Economic Utility
2.2. Promising Approaches for Augmenting Arsenic Remediation by Plants
2.2.1. Microbe-Assisted Arsenic Phytoremediation
2.2.2. Intercropping and Co-Cultivation Methods
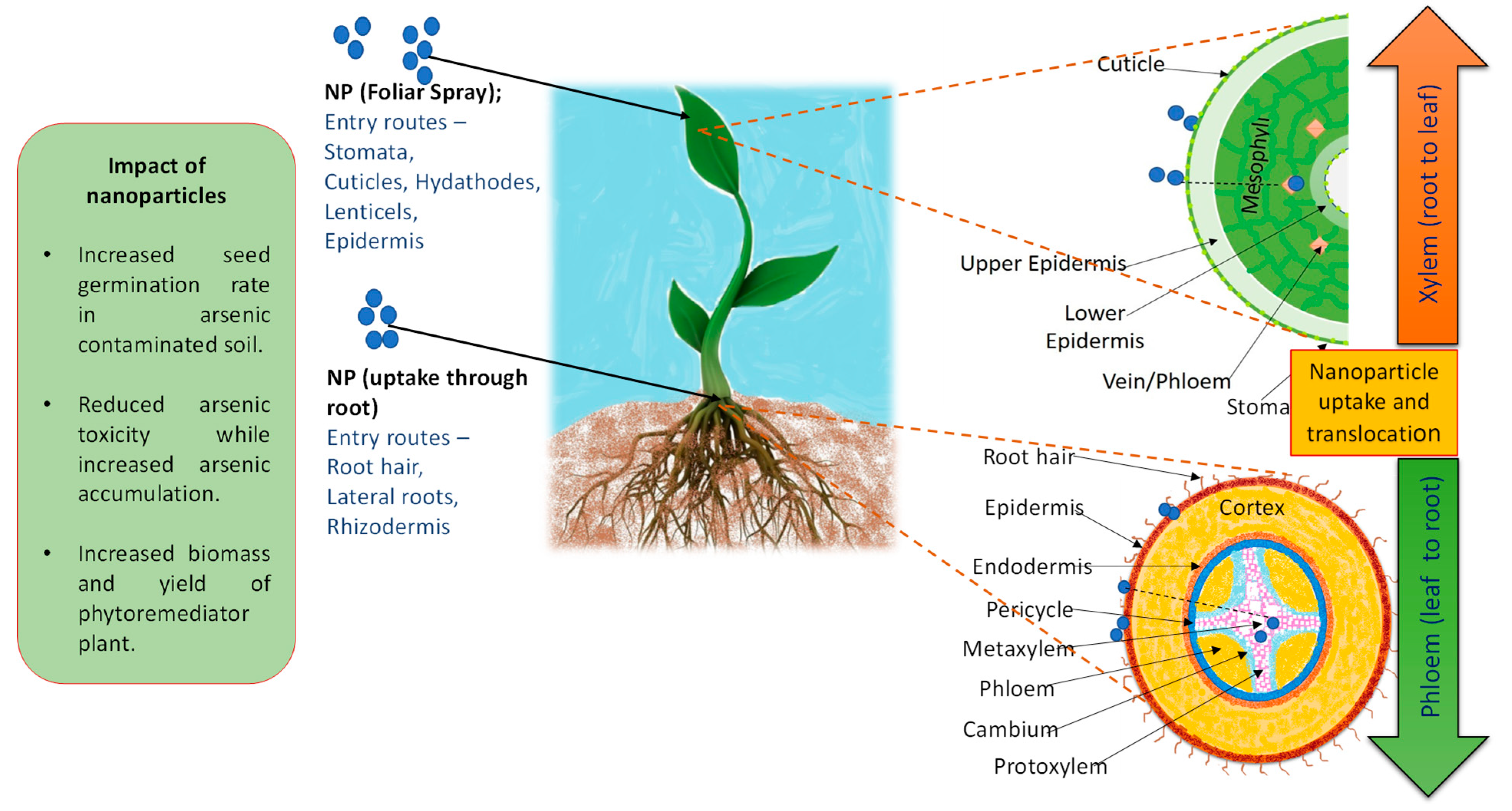
2.2.3. Nanotechnological Approaches to Enhance Phytoremediation
2.2.4. Genetic Engineering for Improving Arsenic Phytoremediation
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, S. Arsenic in Drinking Water and Food; Springer Nature: Singapore, 2020. [Google Scholar]
- Shukla, A.; Awasthi, S.; Chauhan, R.; Srivastava, S. The Status of Arsenic Contamination in India. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 1–12. [Google Scholar]
- Medunic, G.; Fiket, Z.; Ivanic, M. Arsenic Contamination Status in Europe, Australia, and Other Parts of the World. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 183–233. [Google Scholar]
- Jankovic, M.M. Arsenic Contamination Status in North America. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 41–69. [Google Scholar]
- Srivastava, S.; Pathak, S.; Ponsin, M.; Hensawang, S.; Chanpiwat, P.; Yoeurn, C.; Phan, K. Sustainable solutions to arsenic ac-cumulation in rice grown in south and southeast Asia. Crop Pasture Sci. 2021, in press. [Google Scholar] [CrossRef]
- Neumann, R.B.; Vincent, A.P.S.; Roberts, L.C.; Badruzzaman, A.B.M.; Ali, M.A.; Harvey, C.F. Rice Field Geochemistry and Hydrology: An Explanation for Why Groundwater Irrigated Fields in Bangladesh are Net Sinks of Arsenic from Groundwater. Environ. Sci. Technol. 2011, 45, 2072–2078. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, M.K.; Majumdar, A.; Kumar, J.S.; Srivastava, S. Arsenic in Rice Agro-Ecosystem: Solutions for Safe and Sustainable Rice Production. Front. Sustain. Food Syst. 2020, 4, 53. [Google Scholar] [CrossRef]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The Journey of Arsenic from Soil to Grain in Rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himeno, S.; Sumi, D.; Fujishiro, H. Toxicometallomics of Cadmium, Manganese and Arsenic with Special Reference to the Roles of Metal Transporters. Toxicol. Res. 2019, 35, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Huang, L.; Xue, S.G.; Shi, L.Z.; Hartley, W.; Cui, M.; Wong, M.H. Arsenic sorption by red mud-modified biochar pro-duced from rice straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Singh, N.; Tuli, R.; Gupta, D.K.; Maathuis, F.J. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007, 25, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2019, 14, 24. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phy-toremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kumar, A.; Kumar, R. Phytoremediation and Nanoremediation. In New Frontiers of Nanomaterials in Environmental Science; Kumar, R., Kumar, R., Kaur, G., Eds.; Springer Nature: Singapore, 2021; pp. 281–297. [Google Scholar]
- Juwarkar, A.A.; Singh, S.K.; Mudhoo, A. A comprehensive overview of elements in bioremediation. Rev. Environ. Sci. Biotechnol. 2010, 9, 215–288. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Phytoextraction of mine wastes: Opinion and impossibilities. Chem. Erde Geochem. 2005, 65, 29–42. [Google Scholar] [CrossRef]
- Tripathi, P.; Dwivedi, S.; Mishra, A.; Kumar, A.; Dave, R.; Srivastava, S.; Shukla, M.K.; Srivastava, P.K.; Chakrabarty, D.; Trivedi, P.K.; et al. Arsenic accumulation in native plants of West Bengal, India: Prospects for phytoremediation but concerns with the use of medicinal plants. Environ. Monit. Assess. 2011, 184, 2617–2631. [Google Scholar] [CrossRef]
- Mesa, V.; Navazas, A.; González-Gil, R.; González, A.; Weyens, N.; Lauga, B.; Gallego, J.L.R.; Sánchez, J.; Peláez, A.I. Use of Endophytic and Rhizosphere Bacteria to Improve Phytoremediation of Arsenic-Contaminated Industrial Soils by Autochthonous Betula celtiberica. Appl. Environ. Microbiol. 2017, 83, e03411-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, E.; Rolli, E.; Marasco, R.; Agazzi, G.; Borin, S.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G. Phytoremediation of a multi contaminated soil: Mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J. Soils Sediments 2016, 17, 1224–1236. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Minkina, T.; Bauer, T.; Chauhan, A.; Jindal, T. Nanoparticles induced stress and toxicity in plants. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100457. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2016, 110, 236–264. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-Reyes, J.; Majumdar, S.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure studies of core-shell Fe/Fe(3)O(4) and Cu/CuO NPs to lettuce (Lactuca sativa) plants: Are they a potential physiological and nutritional hazard? J. Hazard. Mater. 2014, 267, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Srivastav, A.; Yadav, K.K.; Yadav, S.; Gupta, N.; Singh, J.K.; Katiyar, R.; Kumar, V. Nano-phytoremediation of Pollutants from Contaminated Soil Environment: Current Scenario and Future Prospects. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 6, pp. 383–401. [Google Scholar]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef]
- Canatto, R.A.; De Oliveira, J.A.; Da-Silva, C.J.; Albino, B.S. Tolerance of Landoltia punctata to arsenate: An evaluation of the potential use in phytoremediation programs. Int. J. Phytoremediat. 2020, 23, 102–110. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Tsang, D.C.; Song, L.; Zhang, C.; Yin, M.; Liu, J.; Xiao, T.; Zhang, G.; Wang, J. Hyperaccumulation and transport mechanism of thallium and arsenic in brake ferns (Pteris vittata L.): A case study from mining area. J. Hazard. Mater. 2019, 388, 121756. [Google Scholar] [CrossRef]
- Singh, S.; Fulzele, D.P. Phytoextraction of arsenic using a weed plant Calotropis procera from contaminated water and soil: Growth and biochemical response. Int. J. Phytoremediat. 2021, 1–9. [Google Scholar] [CrossRef]
- Negi, S. Heavy metal accumulation in Portulaca oleracea Linn. J. Pharmacogn. Phytochem. 2018, 7, 2978–2982. [Google Scholar]
- Sahito, Z.A.; Zehra, A.; Tang, L.; Ali, Z.; Hashmi, M.L.R.; Ullah, M.A.; He, Z.; Yang, X. Arsenic and mercury uptake and accumulation in oilseed sunflower accessions selected to mitigate co-contaminated soil coupled with oil and bioenergy pro-duction. J. Clean. Prod. 2021, 291, 125226. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhen, Z.; Wang, Z.; Zeng, L.; Yan, C. Influence of environmental factors on arsenic accumulation and biotransformation using the aquatic plant species Hydrilla verticillata. J. Environ. Sci. 2019, 90, 244–252. [Google Scholar] [CrossRef]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef] [PubMed]
- Debiec-Andrzejewska, K.; Krucon, T.; Piatkowska, K.; Drewniak, L. Enhancing the plants growth and arsenic uptake from soil using arsenite-oxidizing bacteria. Environ. Pollut. 2020, 264, 114692. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.; Chandra, P.; Jeppu, G.P. Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. Int. J. Phytoremediat. 2020, 22, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Karimi, N.; Norouzi, L.; Ma, X. Elucidating the physiological mechanisms underlying enhanced arsenic hyperaccu-mulation by glutathione modified superparamagnetic iron oxide nanoparticles in Isatis cappadocica. Ecotox. Environ. Saf. 2020, 53, 111336. [Google Scholar] [CrossRef] [PubMed]
- Deromachi, Y.; Uraguchi, S.; Kiyono, M.; Kuga, K.; Nishimura, K.; Sato, M.H.; Hirano, T. Stable expression of bacterial trans-porter ArsB attached to SNARE molecule enhances arsenic accumulation in Arabidopsis. Plant Signal. Behav. 2020, 15, 1802553. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, H.; Sun, D.; Xu, G.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Heterologous Expression of Pteris vittata Phosphate Transporter PvPht1;3 Enhances Arsenic Translocation to and Accumulation in Tobacco Shoots. Environ. Sci. Technol. 2019, 53, 10636–10644. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Dunham, S.; McGrath, S.P. Arsenic hyperaccumulation by different fern species. New Phytol. 2002, 156, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, M.; Ma, L.Q.; Singh, N.; Singh, S. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J. Exp. Bot. 2005, 56, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francesconi, K.; Visoottiviseth, P.; Sridokchan, W.; Goessler, W. Arsenic species in an arsenic hyperaccumulating fern, Pityrogramm acalomelanos: A potential phytoremediator of arsenic-contaminated soils. Sci. Total Environ. 2002, 284, 27–35. [Google Scholar] [CrossRef]
- Karimi, N.; Ghaderian, S.M.; Raab, A.; Feldmann, J.; Meharg, A.A. An arsenic-accumulating, hypertolerant brassica, Isatis cap-padocica. New Phytol. 2009, 184, 41–47. [Google Scholar] [CrossRef]
- Vetterlein, D.; Wesenberg, D.; Nathan, P.; Brautigam, A.; Schierhorn, A.; Mattusch, J.; Jahn, R. Pteris vittata—Revisited: Uptake of As and its speciation, impact of P.; role of phytochelatins and S. Environ. Pollut. 2013, 157, 3016–3024. [Google Scholar] [CrossRef]
- Xiyuan, X.; Tongbin, C.; Zhizhuang, A.; Mei, L.; Zechun, H.; Xiaoyong, L.; Yingru, L. Potential of Pteris vittata L. for phytore-mediation of sites co-contaminated with cadmium and arsenic: The tolerance and accumulation. J. Environ. Sci. 2008, 20, 62–67. [Google Scholar]
- Liao, X.Y.; Chen, T.B.; Xie, H.; Xiao, X.Y. Effect of application of P fertilizer on efficiency of As removal in contaminated soil using phytoremediation: Field demonstration. Acta Sci. Circumst. 2004, 24, 455–462. [Google Scholar]
- Fayiga, A.O.; Ma, L.Q. Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci. Total Environ. 2006, 359, 17–25. [Google Scholar] [CrossRef]
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Choppala, G. Phosphorus-arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci. Total Environ. 2013, 463–464, 1154–1162. [Google Scholar] [CrossRef]
- Fu, J.W.; Liu, X.; Han, Y.H.; Mei, H.; Cao, Y.; de Oliveira, L.M.; Liu, Y.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arse-nic-hyperaccumulator Pteris vittata efficiently solubilized phosphate rock to sustain plant growth and As uptake. J. Hazard. Mater. 2017, 330, 68–75. [Google Scholar] [CrossRef]
- Elless, M.P.; Poynton, C.Y.; Willms, C.A.; Doyle, M.P.; Lopez, A.C.; Sokkary, D.A.; Ferguson, B.W.; Blaylock, M.J. Pilot-scale demonstration of phytofiltration for treatment of arsenic in New Mexico drinking water. Water Res. 2005, 39, 3863–3872. [Google Scholar] [CrossRef]
- Natarajan, S.; Stamps, R.H.; Saha, U.K.; Ma, L.Q. Phytofiltration of arsenic-contaminated groundwater using Pteris vittata L.: Effect of plant density and nitrogen and phosphorus levels. Int. J. Phytoremediat. 2008, 10, 222–235. [Google Scholar] [CrossRef]
- Huang, Y.; Miyauchi, K.; Inoue, C.; Endo, G. Development of suitable hydroponics system for phytoremediation of arse-nic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci. Biotechnol. Biochem. 2016, 80, 614–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Juwarkar, A.A.; Phani Kumar, G.; Thawale, P.R.; Singh, S.K.; Chakrabarti, T. Bioaccumulation and phy-to-translocation of arsenic, chromium and zinc by Jatropha curcas L.: Impact of dairy sludge and biofertilizer. Bioresour. Technol. 2009, 100, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lei, M.; Duan, L.; Longhurst, P. Integrating phytoremediation with biomass valorisation and critical element recovery: A UK contaminated land perspective. Biomass Bioenergy 2015, 83, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J. Exp. Bot. 2009, 60, 3419–3431. [Google Scholar] [CrossRef] [Green Version]
- Piracha, M.A.; Ashraf, M.; Niaz, A. Arsenic fractionation and its impact on physiological behavior of sunflower (Helianthus annuus L.) in three texturally different soils under alkaline calcareous conditions. Environ. Sci. Pollut. Res. 2019, 26, 17438–17449. [Google Scholar] [CrossRef]
- Purdy, J.J.; Smart, L.B. Hydroponic Screening of Shrub Willow (Salix spp.) for Arsenic Tolerance and Uptake. Int. J. Phytoremediat. 2008, 10, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Ali, N.; Ahmad, A. Enhanced phytoremediation of cadmium contaminated soil by Parthenium hysterophorus plant: Effect of gibberelic acid (GA3) and synthetic chelator, alone and in combinations. Bioremediat. J. 2014, 18, 46–55. [Google Scholar] [CrossRef]
- Favas, P.J.; Pratas, J.; Prasad, M. Accumulation of arsenic by aquatic plants in large-scale field conditions: Opportunities for phytoremediation and bioindication. Sci. Total Environ. 2012, 433, 390–397. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Srivastava, S.; Patade, V.Y.; Dwivedi, S.; Tripathi, R.; Nikam, T.; Suprasanna, P. Investigation of arsenic accumulation and tolerance potential of Sesuvium portulacastrum (L.) L. Chemosphere 2011, 82, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Srivastava, S.; Mishra, S.; Dixit, B.; Kumar, A.; Tripathi, R. Screening of native plants and algae growing on fly-ash affected areas near National Thermal Power Corporation, Tanda, Uttar Pradesh, India for accumulation of toxic heavy metals. J. Hazard. Mater. 2008, 158, 359–365. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Nigam, S.; Gopal, K.; Vankar, P.S. Biosorption of arsenic in drinking water by submerged plant: Hydrilla verticilata. Environ. Sci. Pollut. Res. 2012, 20, 4000–4008. [Google Scholar] [CrossRef]
- Mkandawire, M.; Lyubun, Y.V.; Kosterin, P.V.; Dudel, E.G. Toxicity of arsenic species to Lemna gibba L. and the influence of phosphate on arsenic bioavailability. Environ. Toxicol. 2004, 19, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic uptake by Lemna minor in hydroponic system. Int. J. Phytoremediat. 2014, 16, 1221–1227. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, A.-J.; Zhao, F.-J.; Xu, G.-Z.; Duan, G.-L.; Zhu, Y.-G. Arsenic accumulation by the aquatic fern Azolla: Comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ. Pollut. 2008, 156, 1149–1155. [Google Scholar] [CrossRef]
- Farnese, F.; Oliveira, J.; Lima, F.; Leão, G.; Gusman, G.; Silva, L. Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. Braz. J. Biol. 2014, 74, S108–S112. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Hasegawa, H.; Ueda, K.; Maki, T. Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans L.). Chem. Eng. J. 2008, 145, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Zimmels, Y.; Kirzhner, F.; Malkovskaja, A. Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel. J. Environ. Manag. 2006, 81, 420–428. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.D.; Borges, A.C.; de Matos, A.T.; Veloso, R.W.; Braga, A.F. Optimization of arsenic phytoremediation using Eic-chornia crassipes. Int. J. Phytoremediat. 2018, 20, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Shrivastava, M.; Suprasanna, P.; D’Souza, S. Phytofiltration of arsenic from simulated contaminated water using Hydrilla verticillata in field conditions. Ecol. Eng. 2011, 37, 1937–1941. [Google Scholar] [CrossRef]
- Islam, M.S.; Saito, T.; Kurasaki, M. Phytofiltration of arsenic and cadmium by using an aquatic plant, Micranthemum umbrosum: Phytotoxicity, uptake kinetics, and mechanism. Ecotoxicol. Environ. Saf. 2015, 112, 193–200. [Google Scholar] [CrossRef]
- Aryal, R.; Nirola, R.; Beecham, S.; Kamruzzaman, M. Impact of elemental uptake in the root chemistry of wetland plants. Int. J. Phytoremediat. 2016, 18, 936–942. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. Phytoremediation Potential of Bioenergy Plants; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Chaukura, N.; Gwenzi, W.; Tavengwa, N.; Manyuchi, M.M. Biosorbents for the removal of synthetic organics and emerging pollutants: Opportunities and challenges for developing countries. Environ. Dev. 2016, 19, 84–89. [Google Scholar] [CrossRef]
- Bote, M.A.; Naik, V.R.; Jagadeeshgouda, K.B. Review on water hyacinth weed as a potential biofuel crop to meet collective energy needs. Mater. Sci. Energy Technol. 2020, 3, 397–406. [Google Scholar]
- Nahar, K.; Sunny, S.A. Duckweed-based clean energy production dynamics (ethanol and biogas) and phyto-remediation po-tential in Bangladesh. Model. Earth Syst. Environ. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, J.; Tang, J.; Zhu, Y.; Wu, Y. Arsenic removal by periphytic biofilm and its application combined with biochar. Bioresour. Technol. 2018, 248, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Shahir, S.; Ibrahim, N.; Chai, T.-T.; Bahari, Z.M.; Manan, F.A. The role of plant growth promoting bacteria on arsenic removal: A review of existing perspectives. Environ. Technol. Innov. 2020, 17, 100602. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Zloch, M.; Kowalkowski, T.; Baum, C.; Buszewski, B. Efficiency of microbially assisted phytoremediation of heavy-metal contaminated soils. Environ. Rev. 2018, 26, 316–332. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Yadav, P.; Shukla, A.; Srivastava, S. Utilizing the potential of microorganisms for managing arsenic contam-ination: A feasible and sustainable approach. Front. Environ. Sci. 2018, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant-microbiome inter-actions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 2016, 16, 341. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Genet. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Chauhan, R.; Dwivedi, S.; Srivastava, S.; Srivastava, S.; Tripathi, R.D. A consortium of alga (Chlorella vulgaris) and bacterium (Pseudomonas putida) for amelioration of arsenic toxicity in rice: A promising and feasible approach. Environ. Exp. Bot. 2018, 150, 115–126. [Google Scholar] [CrossRef]
- Asad, S.A.; Farooq, M.; Afzal, A.; West, H. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment—A review. Chemosphere 2018, 217, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Hao, T.; Chen, S. Colonization and Maize Growth Promotion Induced by Phosphate Solubilizing Bacterial Isolates. Int. J. Mol. Sci. 2017, 18, 1253. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microor-ganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Bahari, Z.M.; Ibrahim, Z.; Jaafar, J.; Shahir, S. Draft Genome Sequence of Arsenic-Resistant Microbacterium sp. Strain SZ1 Isolated from Arsenic-Bearing Gold Ores. Genome Announc. 2017, 5, e01183-17. [Google Scholar] [CrossRef] [Green Version]
- Moens, M.; Branco, R.; Morais, P.V. Arsenic accumulation by a rhizosphere bacterial strain Ochrobactrumtritici reduces rice plant arsenic levels. World J. Microbiol. Biotechnol. 2020, 36, 23. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Ortiz, J.; Herrera, H.; Fuentes, A.; Almonacid, L.; Charles, T.C.; Arriagada, C. Enhanced arsenic tolerance in Triticum aestivuminoculated with arsenic-resistant and plant growth promoter microorganisms from a heavy metal-polluted soil. Micro-organisms 2019, 7, 348. [Google Scholar]
- Yang, C.; Ho, Y.-N.; Makita, R.; Inoue, C.; Chien, M.-F. A multifunctional rhizobacterial strain with wide application in different ferns facilitates arsenic phytoremediation. Sci. Total Environ. 2020, 712, 134504. [Google Scholar] [CrossRef] [PubMed]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilmformation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610–611, 1239. [Google Scholar] [CrossRef]
- Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi. Plants 2020, 9, 1211. [Google Scholar] [CrossRef]
- He, X.; Lilleskov, E. Arsenic Uptake and Phytoremediation Potential by Arbuscular Mycorrhizal Fungi. In Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration; Solaiman, Z.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 259–273. [Google Scholar]
- Vijaya Kumar, V.; Suprasanna, P. Mycorrhizoremediation: A Novel Tool for Bioremediation. In Rhizomicrobiome Dynamics in Bioremediation; Kumar, V., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–12. [Google Scholar]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular Mycorrhiza Augments Arsenic Tolerance in Wheat (Triticum aestivum L.) by Strengthening Antioxidant Defense System and Thiol Metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Chen, X.; Wong, M. Arbuscular mycorrhizal fungi reduced the ratios of inorganic/organic arsenic in rice grains. Chem-osphere 2016, 145, 224–230. [Google Scholar] [CrossRef]
- Pathare, V.; Srivastava, S.; Sonawane, B.V.; Suprasanna, P. Arsenic stress affects the expression profile of genes of 14-3-3 proteins in the shoot of mycorrhiza colonized rice. Physiol. Mol. Biol. Plants 2016, 22, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonam; Srivastava, S.; Pathare, V.; Suprasanna, P. Physiological and molecular insights into rice-arbuscular mycorrhizal in-teractions under arsenic stress. Plant Gene 2017, 11, 232–237. [Google Scholar] [CrossRef]
- Yin, N.; Zhang, Z.; Wang, L.; Qian, K. Variations in organic carbon, aggregation, and enzyme activities of gangue-fly ash-reconstructed soils with sludge and arbuscular mycorrhizal fungi during 6-year reclamation. Environ. Sci. Pollut. Res. 2016, 23, 17840–17849. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Christie, P.; Liu, Y.; Zhang, J.; Li, X. The arbuscular mycorrhizal fungus Glomus mosseae can enhance arsenic tolerance in Medicago truncatula by increasing plant phosphorus status and restricting arsenate uptake. Environ. Pollut. 2008, 156, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhu, Y.-G.; Chen, B.; Christie, P.; Li, X. Yield and arsenate uptake of arbuscular mycorrhizal tomato colonized by Glomus mosseae BEG167 in As spiked soil under glasshouse conditions. Environ. Int. 2005, 31, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Zhu, Y.-G.; Smith, F.A.; Wang, Y.; Chen, B. Arbuscular mycorrhiza enhanced arsenic resistance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) plants in an arsenic-contaminated soil. Environ. Pollut. 2008, 155, 174–181. [Google Scholar] [CrossRef] [PubMed]
- De Conti, L.; Ceretta, C.A.; Melo, G.W.B.; Tiecher, T.L.; Silva, L.O.S.; Garlet, L.P.; Mimmo, T.; Cesco, S.; Brunetto, G. Inter-cropping of young grapevines with native grasses for phytoremediation of Cu-contaminated soils. Chemosphere 2019, 216, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.Y.; Yan, X.L.; Liao, X.Y.; Zhang, Y.X.; Ma, X. Arsenic Accumulation in Panax notoginseng Monoculture and Intercropping with Pteris vittata. Water Air Soil Pollut. 2015, 226, 1–8. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T.; Yang, J. Intercropped Pteris vittata L. and Morus alba L. presents a safe utilization mode for arse-nic-contaminated soil. Sci. Total Environ 2017, 579, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lei, E.; Lei, M.; Liu, Y.; Chen, T. Remediation of Arsenic contaminated soil using malposed intercropping of Pteris vittata L. and maize. Chemosphere 2018, 194, 737–744. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Kwon, E.E.; Biswas, J.K.; Tack, F.M.G.; Ok, Y.S.; Rinklebe, J. Arsenic, chromium, molybdenum, and selenium: Geochemical fractions and potential mobilization in riverine soil profiles originating from Germany and Egypt. Chemosphere 2017, 180, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total. Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, X.; Lei, M. Application of arsenic hyperaccumulator Pteris vittata L. to contaminated soil in Northern China. J. Geochem. Explor. 2017, 182, 132–137. [Google Scholar] [CrossRef]
- Ye, W.; Khan, M.A.; McGrath, S.; Zhao, F.-J. Phytoremediation of arsenic contaminated paddy soils with Pteris vittata markedly reduces arsenic uptake by rice. Environ. Pollut. 2011, 159, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Poonam; Upadhyay, M.K.; Gautam, A.; Mallick, S.; Srivastava, S. A successive application approach for effective utilization of three aquatic plants in arsenic removal. Water Air Soil Pollut. 2017, 228, 54. [Google Scholar] [CrossRef]
- Srivastava, S.; Sounderajan, S.; Udas, A.; Suprasanna, P. Effect of combinations of aquatic plants (Hydrilla, Ceratophyllum, Eichhornia, Lemna and Wolffia) on arsenic removal in field conditions. Ecol. Eng. 2014, 73, 297–301. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Ma, Y.; Huang, Y. Can phosphate compounds be used to reduce the plant uptake of Pb and resist the Pb stress in Pb-contaminated soils? J. Environ. Sci. (China) 2009, 21, 360–365. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Chen, S.; Ma, Y. Alleviation of cadmium-induced root growth inhibition in crop seedlings by nanoparticles. Ecotoxicol. Environ. Saf. 2012, 79, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M.G. Use of Nan-otechnology for the bioremediation of contaminants: A review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
- Desai, M.; Haigh, M.; Walkington, H. Phytoremediation: Metal decontamination of soils after the sequential forestation of former opencast coal land. Sci. Total Environ. 2018, 656, 670–680. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Semenkov, I.; Klink, G.; Tarigholizadeh, S.; Sushkova, S. Phylogenetic analysis of hyperaccumulator plant species for heavy metals and polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2020, 43, 1629–1654. [Google Scholar] [CrossRef]
- Bao-Shan, L.; Shao-Qi, D.; Chun-Hui, L.; Li-Jun, F.; Shu-Chun, Q.; Min, Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Song, B.; Xu, P.; Chen, M.; Tang, W.; Zeng, G.; Gong, J.; Zhang, P.; Ye, S. Using nanomaterials to facilitate the phytoremediation of contaminated soil. Crit. Rev. Environ. Sci. Technol. 2019, 49, 791–824. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N.; Sarmadi, M.; Rostami, E. Salicylic acid nanoparticles (SANPs) improve growth and phytoremediation efficiency of Isatis cappadocica Desv., under As stress. IET Nanobiotechnol. 2017, 11, 650–655. [Google Scholar] [CrossRef]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2019, 239, 124794. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Bai, X.; Zheng, L.; Zhao, J.; Li, Y.-F.; Zhang, Z.; Gao, Y. Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation of Hg(ii) in soybean (Glycine max). Environ. Sci. Nano 2020, 7, 1807–1817. [Google Scholar] [CrossRef]
- Jiang, M.; Dai, S.; Wang, B.; Xie, Z.; Li, J.; Wang, L.; Li, S.; Tan, Y.; Tian, B.; Shu, Q.; et al. Gold nanoparticles synthesized using melatonin suppress cadmium uptake and alleviate its toxicity in rice. Environ. Sci. Nano 2021, 8, 1042–1056. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Wu, F.; Zhang, X.; Wang, B.; Yang, Y.; Shen, G.; Liu, J.; Tao, S.; Wang, X. Application of TiO2 nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryza sativa L.): A mechanistic study. J. Hazard. Mater. 2020, 405, 124047. [Google Scholar] [CrossRef]
- Katiyar, P.; Yadu, B.; Korram, J.; Satnami, M.L.; Kumar, M.; Keshavkant, S. Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J. Environ. Sci. 2020, 92, 18–27. [Google Scholar] [CrossRef]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Ben Fekih, I.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of Arsenic Resistance Genes in Prokaryotes. Front. Microbiol. 2018, 9, 2473. [Google Scholar] [CrossRef]
- Mosa, K.A.; Saadoun, I.; Kumar, K.; Helmy, M.; Dhankher, O.P. Potential Biotechnological Strategies for the Cleanup of Heavy Metals and Metalloids. Front. Plant Sci. 2016, 7, 303. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Choudhary, S.; Majeed, A.; Singh, A.; Bhardwaj, P. Insights into the molecular mechanism of arsenic phytoreme-diation. J. Plant Growth Regul. 2020, 39, 532–543. [Google Scholar] [CrossRef]
- Lindsay, E.; Maathuis, F.J.M. Arabidopsis thalianaNIP7;1 is involved in tissue arsenic distribution and tolerance in response to arsenate. FEBS Lett. 2016, 590, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Rastogi, A.; Shukla, A.; Srivastava, S.; Yadav, S. Prospects of genetic engineering utilizing potential genes for reg-ulating arsenic accumulation in plants. Chemosphere 2018, 211, 397–406. [Google Scholar] [CrossRef] [PubMed]
- DiTusa, S.F.; Fontenot, E.B.; Wallace, R.W.; Silvers, M.; Steele, T.N.; Elnagar, A.H.; Dearman, K.M.; Smith, A.P. A member of the Phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol. 2015, 209, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Feng, H.-Y.; Li, X.-Y.; Ai, H.; Sun, S.; Chen, Y.; Xu, G.; Rathinasabapathi, B.; Cao, Y.; Ma, L.Q. Expression of New Pteris vittata Phosphate Transporter PvPht1;4 Reduces Arsenic Translocation from the Roots to Shoots in Tobacco Plants. Environ. Sci. Technol. 2019, 54, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Indriolo, E.; Na, G.; Ellis, D.; Salt, D.E.; Banks, J.A. A Vacuolar Arsenite Transporter Necessary for Arsenic Tolerance in the Arsenic Hyperaccumulating Fern Pteris vittata Is Missing in Flowering Plants. Plant Cell 2010, 22, 2045–2057. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, W.; Shen, H.; Yan, H.; Xu, W.; He, Z.; Ma, M. Engineering Arsenic Tolerance and Hyperaccumulation in Plants for Phytoremediation by a PvACR3 Transgenic Approach. Environ. Sci. Technol. 2013, 47, 9355–9362. [Google Scholar] [CrossRef]
- Wang, C.; Na, G.; Bermejo, E.S.; Chen, Y.; Banks, J.A.; Salt, D.E.; Zhao, F. Dissecting the components controlling root-to-shoot arsenic translocation in Arabidopsis thaliana. New Phytol. 2017, 217, 206–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhankher, O.P.; Li, Y.; Rosen, B.P.; Shi, J.; Salt, D.; Senecoff, J.F.; Sashti, N.A.; Meagher, R.B. Engineering tolerance and hy-peraccumulation of arsenic in plants by combining arsenate reductase and gamma-glutamyl cysteine synthetase expression. Nat. Biotechnol. 2002, 20, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Rosen, B.P.; McKinney, E.C.; Meagher, R.B. Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc. Natl. Acad. Sci. USA 2006, 103, 5413–5418. [Google Scholar] [CrossRef] [Green Version]
- Nahar, N.; Rahman, A.; Nawani, N.N.; Ghosh, S.; Mandal, A. Phytoremediation of arsenic from the contaminated soil using transgenic tobacco plants expressing ACR2 gene of Arabidopsis thaliana. J. Plant Physiol. 2017, 218, 121–126. [Google Scholar] [CrossRef]
- Li, X.; Sun, D.; Feng, H.; Chen, J.; Chen, Y.; Li, H.; Cao, Y.; Ma, L.Q. Efficient arsenate reduction in As-hyperaccumulator Pteris vittata are mediated by novel arsenate reductases PvHAC1 and PvHAC2. J. Hazard. Mater. 2020, 399, 122895. [Google Scholar] [CrossRef]
- Cai, C.; Lanman, N.A.; Withers, K.A.; DeLeon, A.M.; Wu, Q.; Gribskov, M.; Salt, D.E.; Banks, J.A. Three genes define a bac-terial-like arsenic tolerance mechanism in the arsenic hyperaccumulating fern Pteris vittata. Curr. Biol. 2019, 29, 1625–1633. [Google Scholar] [CrossRef]
| Plants | Arsenic Stress | Results | Ref. |
|---|---|---|---|
| Arsenic Hyperaccumulator Plants | |||
| Landolita punctata | As(V) (0.5–3.0 mg/L ) | Plants showed As hyperaccumulation (>1000 mg/kg As) at or more than 1 mg/L As; however, higher than 1 mg/L As levels were toxic | [27] |
| Pteris vittata | As (average 8885 mg/kg) and thallium (3.91 to 178 mg/kg) contaminated mining area | Pteris vittata accumulated around 7215–11,110 mg/kg As, and 6.47–111 mg/kg of thallium | [28] |
| High Biomass Producing Plants | |||
| Calatropis prosera | Arsenic given in hydroponic and soil | C. procera reduced As concentration by 45% and 58% in hydroponics and by 30% and 36% in soil, after 15 and 30 days, respectively. | [29] |
| Portulaca oleracea | As (154 mg/kg and 193 mg/kg at site-I and site-II); other metals (Cd, Pb, Cu) were also present | At site I, As accumulation in stem was around 94.5 mg/kg, whereas at site II, it was 73.6 mg/kg | [30] |
| Plants with Economic Utiliity | |||
| Helianthus annus | Farmland soil containing As (84.85 mg/kg) | The mean As level 49.04 mg/kg in the above-ground parts. Average seed yield (45.90 kg/m2) and oil production (34.65%) | [31] |
| Hydrilla verticillata | As(V) (15–375 μg/L) | Total As accumulation was 197.2 μg/g dry weight when As(V) was 375 μg/L | [32] |
| Microbe-Assisted Arsenic Remediation | |||
| Arundo donax + consortia of two strains of Stenotrophomonas maltophilia and one strains of Agrobacterium sp. | As(III) (2–20 mg/L) | In the presence of bacterial consortium, 11.37 mg/kg As was volatilized by transpiration | [33] |
| Alfalfa + Ensifer sp. M14 | Soil As(III) (10 mg/kg) | As concentration in leaves of inoculated plants was 11% higher than those cultivated without microorganisms. | [34] |
| Nano-Phytoremediation Approaches | |||
| Eucalyptus leaf extract mediated synthesis iron oxide NPs | Arsenic | Arsenic adsorption capacity was found to be 39.84 mg/g | [35] |
| Isatis cappadocica + glutathione modified superparamagnetic iron oxide NPs {nFe3O4@GSH} | Soil As (1000 μM) | nFe3O4@GSH treatment increased growth of plants and As tolerance by reducing As accumulation in plants | [36] |
| Genetic Engineering Approaches | |||
| Arabidopsis thaliana transformed with bacterial As transporter (ArsB) targeted to vacuolar membrane | As(III) (5 μM) | Transgenic plants showed higher As accumulation in shoots compared to wild type plants | [37] |
| Nicotiana tabaccum transformed with PvPht1;3 from P. vittata | As(V) (20 μM) Soil As (9.66 mg/kg) | Arsenic accumulation in shoot tissues of transgenic tobacco increased in both hydroponic and soil experiments | [38] |
| Merits | Limitations |
|---|---|
| Arsenic Hyperaccumulator Plants | |
| Owing to As hyperaccumulation, large amount of As is concentrated in above-ground harvestable tissues | The biomass of hyperaccumulator plants is generally low and hence, total As removed in one cycle/harvest is low |
| Hyperaccumulator plants do not need much care and additional inputs for sustaining their growth | The habitat of hyperaccumulator plants may be limited and their application may not be practiced in all environment |
| High Biomass Producing Plants | |
| High biomass of plants allows large As removal in a single crop | For sustained growth of high biomass plants, additional nutrient (fertilizer) inputs and efforts may be required. |
| Native high biomass plants may be chosen to avoid habitat related issues | Native plants may be preferable feed for native wild/pet animals and may therefore pose risk |
| Plants with Economic Utiliity | |
| Plants with economic utility like oil-seed plants which restrict As accumulation in oil would allow farmers to dedicate fields for phytoremediation | For such plants also, animal consumption of leaves and shoot portion of plants must be avoided |
| Plants may find applications for bioenergy, biofuel and biochar preparation | The research on practical utility and problems is limited; volatile nature of some As species may be of concern |
| Microbe-Assisted Arsenic Remediation | |
| Arsenic tolerant and plant growth promoting microorganisms may enhace plants potential for As removal per crop cycle | Microbial supplementation might interfere with natural microbiome of plants and soil and thus, it still needs research |
| Nano-Phytoremediation Approaches | |
| NPs mediated plant growth improvement and increased As bioavilability would enhance As removal per crop cycle | The accumualtion of NPs may intself cause toxicity to plants |
| Genetic Engineering Approaches | |
| Genetic modification of plants as per the need would allow the generation of high biomass superhyperaccumulators of economic utilizability and would allow speedy phytoremediation | The issues related to approval and public acceptance of genetically modified plants are of concern |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.; Shukla, A.; Rajput, V.D.; Kumar, K.; Minkina, T.; Mandzhieva, S.; Shmaraeva, A.; Suprasanna, P. Arsenic Remediation through Sustainable Phytoremediation Approaches. Minerals 2021, 11, 936. https://doi.org/10.3390/min11090936
Srivastava S, Shukla A, Rajput VD, Kumar K, Minkina T, Mandzhieva S, Shmaraeva A, Suprasanna P. Arsenic Remediation through Sustainable Phytoremediation Approaches. Minerals. 2021; 11(9):936. https://doi.org/10.3390/min11090936
Chicago/Turabian StyleSrivastava, Sudhakar, Anurakti Shukla, Vishnu D. Rajput, Kundan Kumar, Tatiana Minkina, Saglara Mandzhieva, Antonina Shmaraeva, and Penna Suprasanna. 2021. "Arsenic Remediation through Sustainable Phytoremediation Approaches" Minerals 11, no. 9: 936. https://doi.org/10.3390/min11090936
APA StyleSrivastava, S., Shukla, A., Rajput, V. D., Kumar, K., Minkina, T., Mandzhieva, S., Shmaraeva, A., & Suprasanna, P. (2021). Arsenic Remediation through Sustainable Phytoremediation Approaches. Minerals, 11(9), 936. https://doi.org/10.3390/min11090936










