1. Introduction
As gradual shift towards renewable energy sources on a global scale, access to particular raw materials or critical metals (CM), such as platinum-group elements (PGE), rare earth elements (REE), and scandium (Sc), with potential presence in known resources is a growing concern within the European Union (EU) and in a worldwide scale [
1,
2]. Although the production for these metals is mostly derived from magmatic deposits, volcanogenic massive sulfide (VMS) deposits, the black shale-hosted deposit in Finland and Sweden, and deposits of supergene origin associated with the release of major and trace elements from the alteration of related rocks [
1,
3,
4,
5,
6,
7], deposits of Fe–Ni ± Co laterite may be a potential resource for critical metals as well [
8,
9]. Ni laterites contain about 60–70% of the world’s nickel resources and account for about 40% of the world’s nickel production, with the remainder coming from sulfide ores [
10]. The Ni–Co laterite deposits provide one of the two major natural sources of nickel and cobalt and became of economic importance with the recent increasing industrialization of developing countries [
11]. The type of Ni–Co laterites developed by chemical weathering of ultramafic rocks with potential post redeposition enrichment of weathering products may occur above weathered bedrock. Among others, they have been described in the Philippines (Taganito/Adlay), in Western Australia (The Murrin Murrin deposit), in New Caledonia, Indonesia, and the Dominican Republic [
11,
12,
13]. Recently, the Fe–Ni ± Co type of laterites is an attractive research topic due to their large tonnage, easy exploitation (open pit mining), a significant development of a Ni and Co demand, and the technological innovations implemented by exploration companies [
11,
12]. With respect to known classifications, three mineralogical subtypes of Fe–Ni ± Co laterite deposits are recognized: the oxide, hydrous Mg–silicate, and clay types, with median Ni and Co grades, 1.14% and 0.09% of the oxide type, 1.44 and 0.06% for the Mg type, and 1.27 and 0.06% for the clay type [
11,
13]. The Fe–Ni laterite deposits in the Balkan Peninsula are associated with ophiolites, which represent a remnant of the Tethyan oceanic lithosphere, located in the Mirdita–Sub-Pelagonian and Pelagonian geotectonic zones [
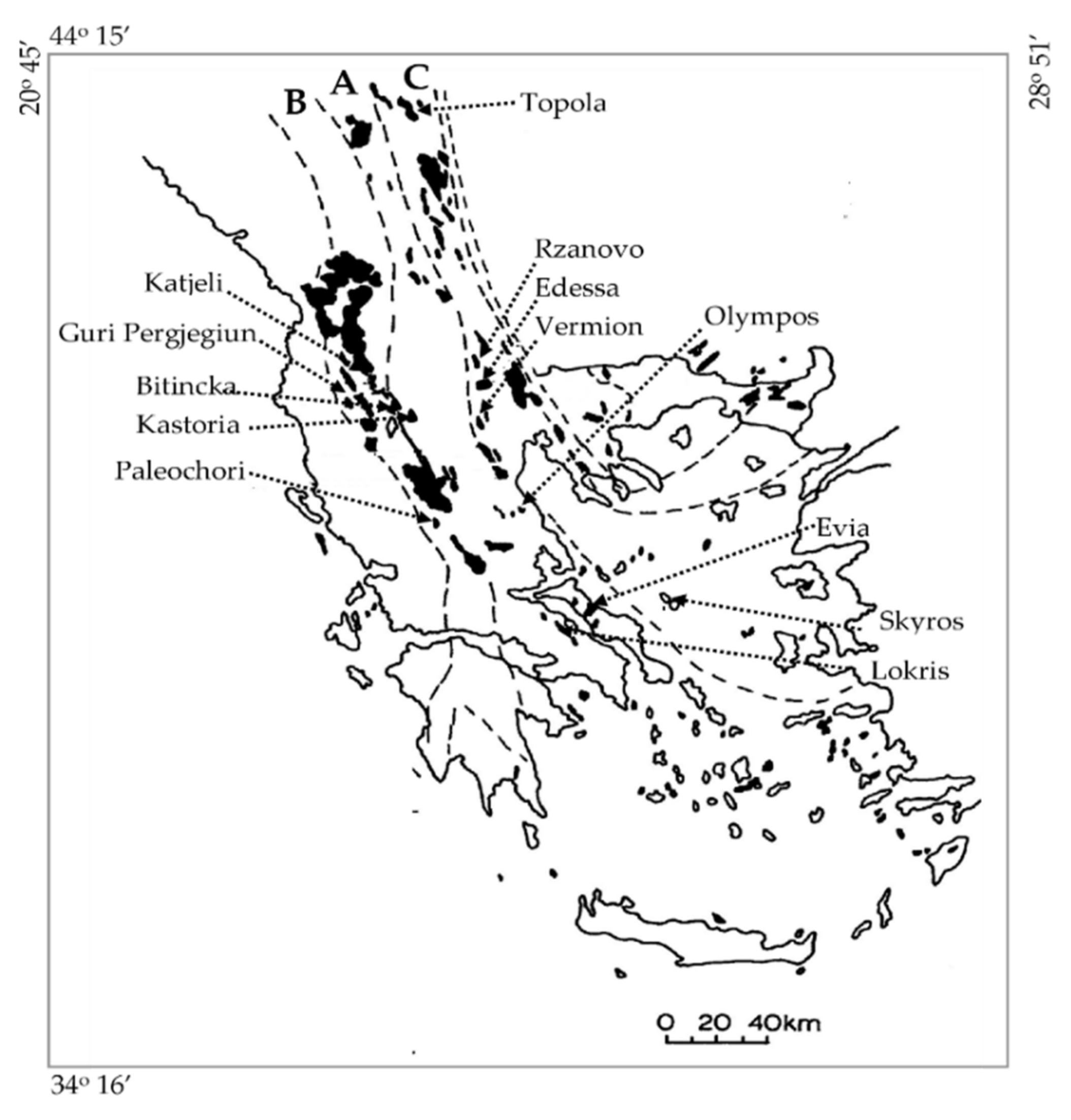
14]. They provide some 2–3% of the World’s total Ni and include deposits in Serbia (Topola), in N. Macedonia, former F.Y.R.O.M. (Rzanovo), Albania (Bitincka, Gouri-Perjuegjiun, and Katjeli) and Greece (Lokris, C. Evia, Kastoria, Vermion, Paleochori, Edessa, Olympos) (
Figure 1) [
15,
16,
17,
18,
19,
20,
21].
Furthermore, the weathering/alteration of laterite deposits and parent ultramafic rocks, mining, and large volumes of smelting residues (slag) are potential sources of environmental hazards for terrestrial and aquatic ecosystems, food quality, and socioeconomic problems [
1,
2,
3,
4,
10,
11]. An evaluation of the environmental risks associated with a possible exploitation of the deposits may contribute to the potential ways forward to protect soil and groundwater for human health and ecosystems. The present study focuses on the combination of Ni–Co–Mn mineral chemistry with geochemical characteristics of laterites from the Balkan Peninsula, which offer a variety of laterite types. The aim is the delineation of geochemical constraints as a contribution from the origin, genesis and exploration of critical metals (REE, PGE, Co, Sc) in laterites to the environmental impact from mining and plant processes of laterite ores.
2. Geological Outline of Fe–Ni ± Co Deposits and Occurrences in the Balkan Peninsula
Based on national mineral resource agencies, approximately 500 Co-bearing deposits and occurrences have been identified in 25 countries in Europe [
9]. The measured mineral resources in Greece are 220,000,000 tons [
9,
11]. The Ržanovo deposit, located close to the contact between the Axios zone and the Pelagonian massif, is one of the largest Fe–Ni ± Co deposits in the Axios zone [
20]. In general, large Fe–Ni–Co laterite deposits, which have been exploited for many years, are mainly related to the extensive lateritization of ultramafic ophiolites in the Balkan Peninsula (Shebenik–Pogradec Massif at the central and southern part of the Mirdita ophiolites—Sub-Pelagonian and Pelagonian geotectonic zones) into Greece and by extension the Anatolides zone of western Turkey [
20,
22]. Major deposits show a complete weathering profile that comprises: (1) a lower zone of saprolitized peridotite with or without partially weathered core stones; (2) a transition zone which may be dominated by quartz or smectite clays; (3) a ferruginous saprolitic limonite zone; (4) a recrystallized and locally transported limonite zone; and (5) a goethite–hematite zone [
23]. Although Fe–Ni ± Co laterites in the Balkan Peninsula, related to Upper Jurassic–Lower Cretaceous serpentinized ultramafic ophiolites, may be partially preserved in situ, they are mostly allochthonous, redeposited as marine sediments, and buried by later sedimentary rocks [
22]. The laterite ores have commonly been transported and redeposited either onto peridotites, such as at Kastoria, W. Vermion, and Palaiochori (Greece), Bitincka and Guri–Pergjegjum (Albania), or onto limestones, such as at Lokris (Aghios Ioannis), Katjeli (Albania) (
Figure 1 and
Figure 2A). Those laterite deposits are often overlain by a sequence of mudstone, limestones, and organic carbon-rich mudstones, which have been attributed to a stagnant reducing marine environment during tectonic down warping [
15]. In particular, the Katjeli Fe–Ni deposit lies on karstified Jurassic limestone, and it is conformably overlain by a Tertiary series consisting of small beds of lignite and sandstone intercalation and molasses. The exploited ores have been reworked due to repeated marine transgression and regression, deposited in a shallow marine environment, and affected by thrust faulting [
15,
17,
22,
24]. They can be divided into those which are unconformably overlain by Early Eocene shallow marine sediments, such as the Kastoria and Katjeli deposits in the Balkan Peninsula, and those overlain by Cretaceous limestones, such as the Lokris [
15,
17]. Specifically, the Kastoria Fe–Ni deposit is located in northern Greece (
Figure 1), NNW of Kastoria town, is developed on Upper Jurassic–Lower Cretaceous serpentinized ultramafic ophiolites of the Mirdita–Sub-Pelagonian zone, and is overlain by Tertiary molasses. A goethite zone, 5-m thick, consisting mainly of goethite and hematite overlies a weathering crust [
18,
25]. At the W. Vermion (Profitis Elias), ophiolites are mainly comprised of large peridotite masses of harzburgite, dunite, and orthopyroxene–dunite. The weathering crust is overlain by a highly silicified zone, while the ore is pelitic at its lowest part and becomes pisolitic towards the top. The Fe–Ni ore is mainly composed of goethite, hematite, Ni-bearing chlorite, quartz, calcite, and chromite. Talc is common in the lowest part of the ore, while illite is dominant in its upper part. In a distance of less than 1 km north of the Profitis Elias Ni laterite deposit, the Parhari deposit of bauxite laterite is located in an area where mafic rocks (diabases) are the dominant rock types [
18]. More attention is paid to the Lokris (Aghios Ioannis) Fe–Ni laterite deposits (
Figure 2) of allochthonous origin because of the association of Fe–Ni ore with the bauxite laterite at the Nissi–Patitira location, and the relatively high contents of critical metals [
22,
24,
26].
Small (1 m × 15 m) lens-like Fe–Ni occurrences, metamorphosed to amphibolite facies (probably erosional remnants of redeposited laterites), are found in the E. Vermion, Edessa, Olympos, and Skyros islands of Greece (
Figure 1 and
Figure 2A) and are overlain by Cretaceous limestones [
19]. The dismembered ophiolite masses of Upper Jurassic–Lower Cretaceous age, which consist of mainly serpentinized harzburgite and, to a lesser extent, crustal magmatic rocks (pyroxenites and gabbros), outcrop along the eastern margin of the Pelagonian massif [
28]. Due to intense tectonism, the Fe–Ni laterite occurrences are often entirely enclosed within serpentinized harzburgites [
19].
3. Methods of Investigation
Due to the heterogeneous character of most Ni laterite deposits, samples of a minimum 2 kg weight were collected from surface exposures. Although the analytical methods applied for the determination of major and trace elements in rocks, laterite ores, groundwater, and water leachates, including Cr stable isotopes and As speciation, is provided in the relative publications [
29,
30,
31], a brief outline is given here. Two samples of metallurgical residue (slag samples, Slag96 and Slag14) from the Larymna plant, kindly provided by the mining company GMM LARCO (March 1996 and November 2014, respectively), and representative samples of ultramafic altered rocks were analyzed. Major and trace elements were determined by atomic absorption at the Institute of Geology and Mineral Exploration, Greece, and minor and trace elements were obtained by Inductively Coupled Plasma Mass Spectrometry (ICP–MS) analysis, after multi-acid digestion (HNO
3–HClO
4–HF–HCl) at the ACME Laboratories Ltd., Vancouver, BC, Canada. Platinum-group element (PGE) analyses were carried out using the Ni-sulfide fire-assay pre-concentration technique, with the nickel fire-assay technique from large (30 g) samples at Genalysis Laboratory Services, Perth, Australia. This method allows for complete dissolution of samples. The detection limits were 1 ppb for Pd, 10 ppb for Pt, and 5 ppb Au. CDN–PGMS–23 was used as standard.
Representative surface (up to 20 cm) soil samples from the rhizosphere of plants and corresponding plants/crops covering some sites surrounding laterite deposits have been analyzed by inductively coupled plasma mass spectroscopy (ICP/MS) after aqua regia digestion at ACME Laboratories Ltd., Vancouver, BC, Canada.
A series of batch leaching experiments have been carried out, using natural water in order to study the long-term leaching responses of Cr under atmospheric conditions [
31]. For these experiments, 20 g of a crushed ore, metallurgical residue (slag), rock, or soil sample and 150 mL of natural water were transferred in a 200 mL Erlenmeyer flask at room temperature. The reaction flask was shaken at approximately 120 rpm by a reciprocal shaker for 5 weeks. After the period of shaking, the slurries were filtered through a 0.45 μm polyamide membrane filter. Furthermore, following the same methodology, except the shaker time, which was 24 h in this case, water leaching experiments were carried out in the present study.
Since oxidative weathering of Cr-bearing ultramafic rocks facilitate the oxidation of Cr(III) into water-soluble Cr(VI) and back again, the chromium isotopes have been applied in the investigation of contaminated groundwater and water leachates. Water and water leachates in an appropriate amount to have approximately 1 μg of Cr
total were used for the determination of the Cr isotope composition following the method described by [
29]. Both Cr concentrations and isotope ratios were analyzed using an IsotopX/GV IsoProbe T thermal ionization mass spectrometer (TIMS) equipped with eight Faraday cups at the University of Copenhagen, Denmark. Four Cr beams (
50Cr+,
52Cr+,
53Cr+, and
54Cr+) were analyzed simultaneously with
49Ti+,
51V+, and
56Fe+ beams, which were used to monitor interfering ions. The final isotope composition of a sample was determined as the average of the repeated analyses and reported relative to the certified SRM 979 standard as follows: δ
53Cr(‰) = [(
53Cr/
52Cr
sample/
53Cr/
52CrSRM
979) − 1] × 1000. The raw data were corrected for naturally and instrumentally induced isotope fractionation using the double spike routine. To assess the precision of the analyses, a double spike-treated, certified standard reference material (NIST SRM
979) was used.
A representative ore sample from the Nissi (Lokris) bauxite laterite deposit with remarkably high As content (350 mg/kg in bulk) was investigated by Synchrotron Radiation (SR). The SR micro-X-ray Fluorescence (μ-XRF) elemental mapping and micro-X-ray Absorption Fine Structure (μ–XAFS) spectra were both obtained in the X-ray beamline of the Laboratory for Environmental Studies (SUL-X) of the ANKA Synchrotron Radiation Facility (Karlsruhe Institute of Technology/KIT, Karlsruhe, Germany). For this purpose, solid fragments of the ore were embedded into resin and polished, whereas powders of reference minerals and compounds were pressed with cellulose to pellets. The SR μ-XRF elemental maps and the As K-edge of the μ–XAFS spectra revealed that As is exclusively correlated to Fe, occurring as As
5+ in the form of arsenate anions (AsO
43−) [
30].
Thin polished sections of Fe–Ni laterites were investigated using a reflected light microscope, a scanning electron microscope (SEM), and energy dispersive spectroscopy (EDS). The SEM–EDS semi-quantitative analyses were carried out at the Department of Geology and Geoenvironmemt, National and Kapodistrian University of Athens (NKUA), using a JEOL JSM 5600 SEM (JEOL, Tokyo, Japan), equipped with the ISIS 300 OXFORD automated energy dispersive X-ray analysis system. Analytical conditions were 20 kV accelerating voltage, 0.5 nA beam current.
4. Mineralogical Characteristic Features
The texture characteristics of Fe–Ni ± Co ores from the Balkan Peninsula reflect a multistage evolution of the mineralogy and mineral chemistry of the mineralization. Specifically, the Lokris Fe–Ni ± Co laterite deposits are mostly composed of goethite, limonite, hematite, Ni-bearing chlorite, Fe-chlorite, rutile, quartz, calcite, and chromite, while AlOOH polymorphs boehmite and diaspore are dominant minerals in the bauxite laterite; gibbsite, illite, kaolinite, montmorillonite, smectite, takovite, and Ni–Co lithiophorite (13 wt% Ni and 9.2 wt% Co) replaced commonly by asbolane, manganomelane (6.2 wt% Ni and 4.9 wt% Co), halloysite, and Al–Ni–Co–Mn silicates are more abundant towards fractures and the lowest parts of the deposit [
15,
17,
18,
24,
25,
26]. A characteristic feature of the transitional zone between the overlying sequence of limestones and the carbonate basement of the laterite ores and organic carbon-rich mudstones is the presence of organic matter (
Figure 3c,d) [
24]. In addition, the lowermost part of the Lokris bauxite laterite deposit lying on karstified Jurassic limestone is characterized by the presence of abundant rare earth minerals (
Figure 3e,f), such as authigenic (hydroxyl)bastnaesite-(Nd) and (La,Nd,Y)-bastnaesite, [
26,
29]. The Kastoria laterites, overlying a weathering crust, are composed of a pelitic matrix (goethite, hematite), clastic grains of quartz and chromite, as well as silicates (Fe–serpentine, talc), carbonates (calcite, siderite) and Mn-oxides (pyrolusite, lithiophorite), occurring as more than one type: rounded fragments of goethite occur in a subsequently formed matrix of (Fe, Mn, Ni)-hydrous oxides associated with detrital quartz and chromite, while siderite and calcite cross-cut all previous formations (
Figure 3g,h). Fine-grained goethite, growing over the vestiges of microorganism cells (
Figure 3i) suggests the involvement of microorganisms in the deposition of goethite [
24,
26].
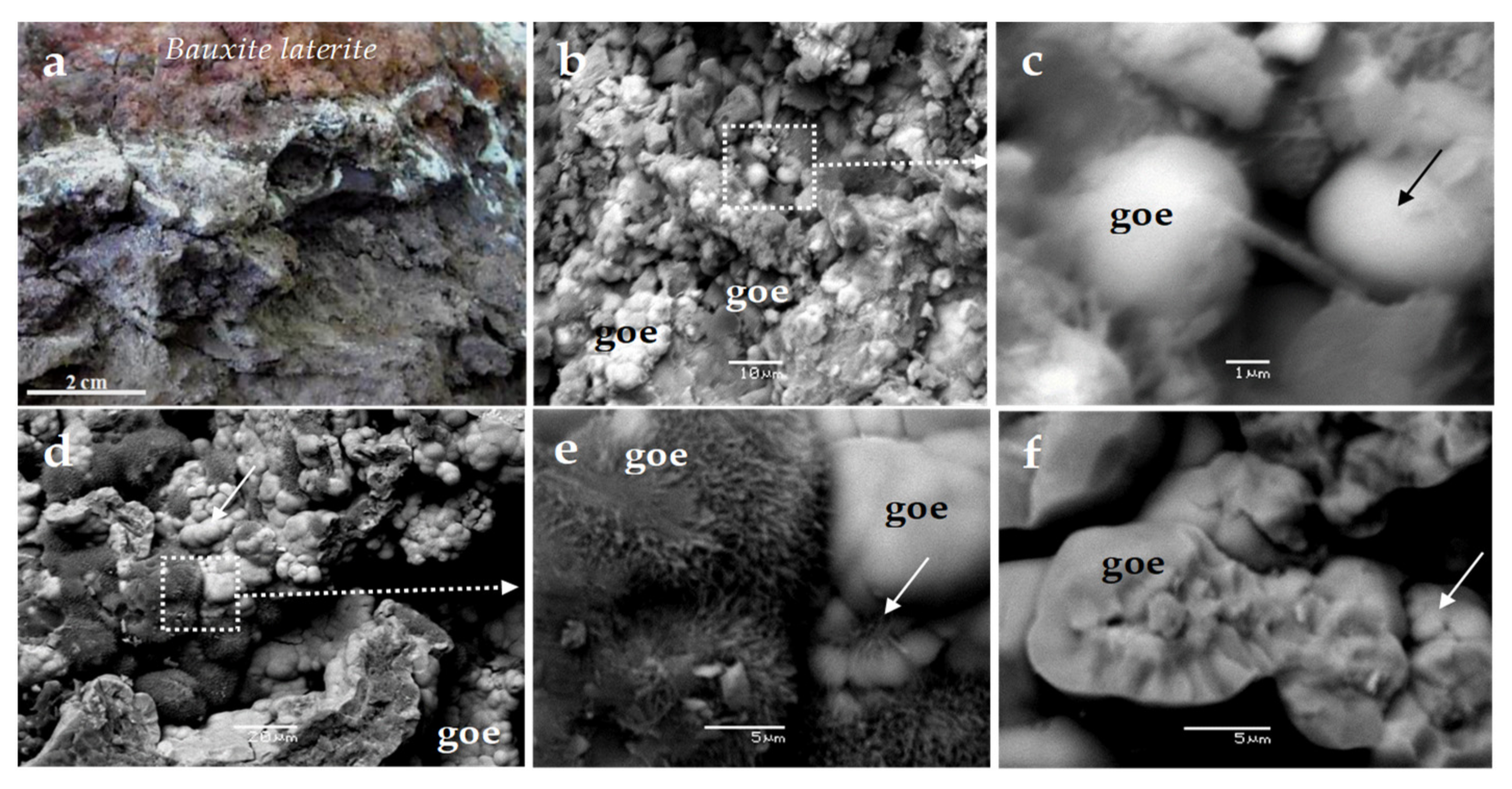
In addition, a salient feature of the Nissi–Patitira deposit at Lokris is the existence of transitional zones between a typical brown-red laterite ore, gradually grading to grey-white, grey-black, pinkish-white, and pale green zones (
Figure 4a), as well as different goethite morphologies, with sub-micron spherulitic aggregates being the dominant micro-textures (
Figure 4b–f). The occurrence of As-bearing bacteriomorphic goethite in samples with significant organic matter and the presence of C, N, Fe, Al, K, Si, Ca, Mn, and Ni in goethite, probably due to the presence of microorganisms, has been emphasized in previous studies [
24,
26].
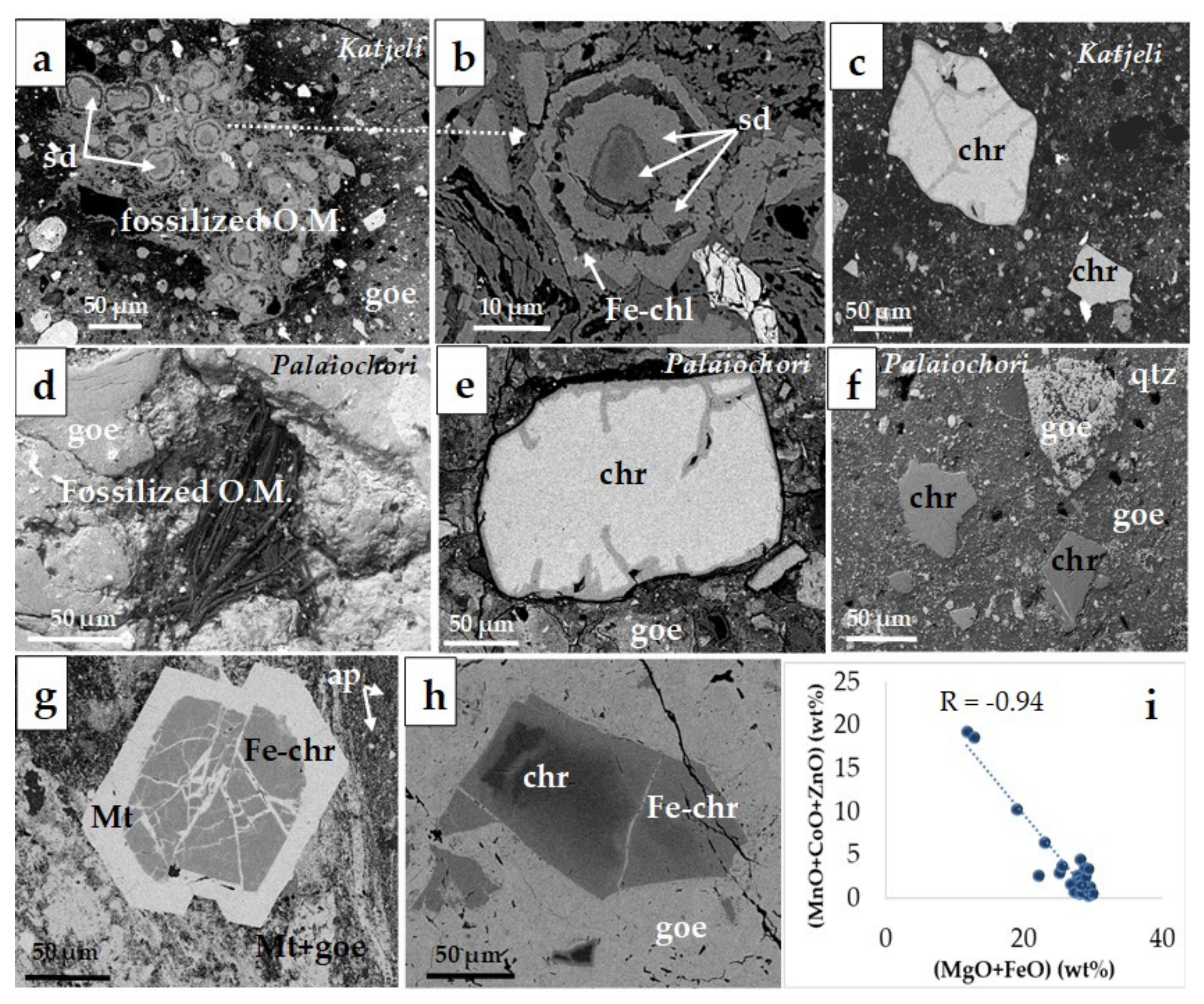
The groundmass of pisolitic and pelitomorphic ore in the Palaiochori and Katjeli deposits is fine grained, and it is mainly composed of iron oxides (goethite, magnetite, and hematite), clastic grains of chromite, quartz, Fe-chlorite (chamosite), calcite, Mn-bearing siderite, and Mn-oxides (pyrolusite) (
Figure 4) [
19]. Chromite grains are present in a relatively small proportion, whereas the pisolitic ore overlies the pelitomorphic ore [
32]. The occurrence of fossilized organic matter and different morphologies of goethite and/or carbonate minerals (siderite) are common in the Lokris, Kastoria, Palaiochori, Katjeli, and other deposits (
Figure 3 and
Figure 5). The occurrence of chromite grains, inherited from the parent ophiolitic rocks, is a common feature of all Fe–Ni laterite ores and bauxite-laterites. They usually show a cataclastic texture, cemented by magnetite, or occur as chromite cores surrounded by a zone of Fe chromite, showing a gradual decrease of Cr, Al, and Mg as Fe increases outward from the core [
33,
34]. However, the alteration zone along cracks and peripheral parts of chromite from the Palaiochori and Katjeli deposits is very limited, and it is commonly darker in the BSE images (
Figure 5c,e,f). A small amount of apatite is also present in the ores from Edessa (
Figure 5g), while the presence of organic matter is common in Palaiochori and Katjeli deposits (
Figure 5a,b,d).
The Fe–Ni ore in the W. Vermion is mainly comprised of goethite, hematite, Ni-bearing chlorite, quartz, calcite, and chromite. Talc is common in the lowest part of the deposit, while illite is dominant in its upper parts. Although the majority of the W. Vermion laterites are classified as Fe–Ni–Co laterites, bauxite-laterite has been located lying on serpentinized peridotites, at the Parchari area, in a distance of less than 1 km north of the Profitis Ilias Ni laterite deposit [
18]. The bauxite laterite ore is mainly comprised of goethite, hematite, boehmite, Ni–Fe chlorite, illite, quartz, calcite, chromite, rutile, and small amounts of pyrite [
18]. Apart from the dominant peridotites, mafic rocks (diabases) are also present in the Parchari area.
Most Fe–Ni ± Co deposits are dominated by Fe-oxyhydroxides as the main nickel carrier, while the Mn-oxides are commonly enriched in Co and Ni, and zoned chromite grains may contain Mn, Co, and Zn as well [
19]. A common feature of the E. Vermion, Olympos, Edessa, and Skyros small laterite occurrences, which are found on serpentinized harzburgites, is the gradual increase of Mn, Co, and Zn outwards of the chromite, attaining the greatest values at the periphery of chromite cores and in the Fe-chromite, reaching values up to 13.0, 4.1 and 2.1 wt%, respectively, and dropping off to negligible values in magnetite, as is exemplified by the plot of (MnO + CoO + ZnO) versus (MgO + FeO) content and the strong negative correlation between them (
Figure 5i) [
19].
At the area of E. Vermio, there is abundant garnet (grossular) and calcite, while Ni is mainly hosted in chlorite, serpentine, and theophrastite (
Figure 6a), the latter containing 80 wt% NiO [
35]. A (Co,Mn,Ni)-hydroxide with a wide compositional range occurs in a spatial association with theophrastite and organic matter (
Figure 6a,b,c). Hydroxides dominated by Co, occur towards the central parts of the concentring development, while the peripheral parts are dominated by Mn (
Figure 6c–f and
Figure 7,
Table 1). The association of (Co,Mn,Ni)(OH)
2 and theophrastite with silicate minerals (mostly Ni-serpentine), garnet, and magnetite, all cross-cutting earlier deformation events (
Figure 6), may have a common origin in space. The theophrastite coexists with magnetite and frequently encloses tiny grains of magnetite, both showing an orientation parallel to the general direction of the schistosity (
Figure 6a).
4.1. Chemical Composition of Fe–Ni ± Co Laterite Ores
Detailed studies on major Fe–Ni laterite deposits of Greece, in the frame of the European GeoNickel project, reported several typical bulk compositions for major and trace elements [
36]. Representative analyses of various Fe–Ni laterite deposits and occurrences from Greece, as well as Albania, Serbia, and N. Macedonia (former F.Y.R.O.M.), completed for some critical element contents are shown in the
Table 2,
Table 3,
Table 4 and
Table 5. Cobalt contents in laterite samples from Fe–Ni laterite deposits of Greece (Lokris, Kastoria, Palaiochori, W, Vermion, Olympos, Edessa, E. Vermion, and Skyros), Albania (Bitincka, Guri Pergjegjum, and Katjeli), Serbia (Topola), and N. Macedonia, former F.Y.R.O.M. (Rzanovo), range from 180 to 1600 mg/kg, reaching values over 3100 mg/kg Co at the lowest contact of the Lokris bauxite laterite deposit with the carbonate basement (
Table 2). In addition, it is clear that the REE, U, and Th are mostly associated with bauxite-laterites and to a lesser degree with Fe–Ni laterites of karstic-type, whereas in Fe–Ni laterites with in situ features, such as the Kastoria and Bitincka, the ΣREE content is <10 mg/kg, which appears to be consistent with their mineralogical composition (
Figure 3e,f). In particular, the lowest part of the deposit lying on karstified Jurassic limestone is accompanied by an enrichment in ΣREE, reaching values over 6000 mg/kg ΣREE, 66 mg/kg U, 25 mg/kg Th, and 1800 mg/kg As (
Table 2). In general, the Co grades in the Balkan Peninsula range between 0.02 and 0.16% wt% Co and show a positive correlation with Ni and Mn (
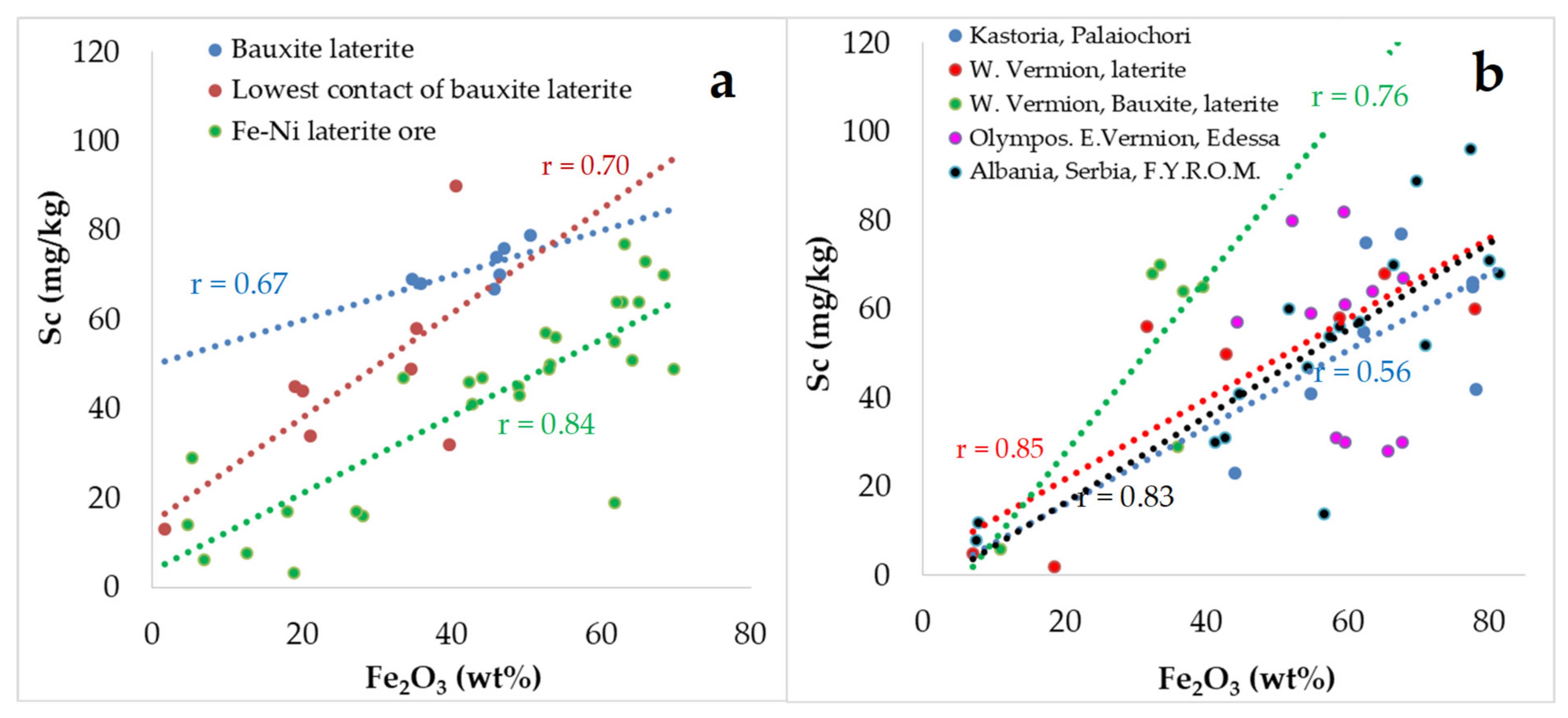
Figure 8). The majority of the Sc contents of lanthanides were 32–90 mg/kg in the Lokris deposits; 41–77 mg/kg Sc in the Kastoria, Palaiochori, and W. Vermion deposits; 30–82 mg/kg Sc in the Olympos, Edessa, E. Vermion, and Skyros Fe–Ni laterite occurrences; and 12–96 mg/kg Sc in the Albania, Serbia, and N. Macedonia, former F.Y.R.O.M., deposits (
Table 2,
Table 3 and
Table 4). Increasing Fe contents are accompanied by elevated Sc, the best positive correlation between Sc and Fe being evidenced in the Lokris deposit and W. Vermion (
Table 2,
Table 3,
Table 4 and
Table 5 and
Figure 9).
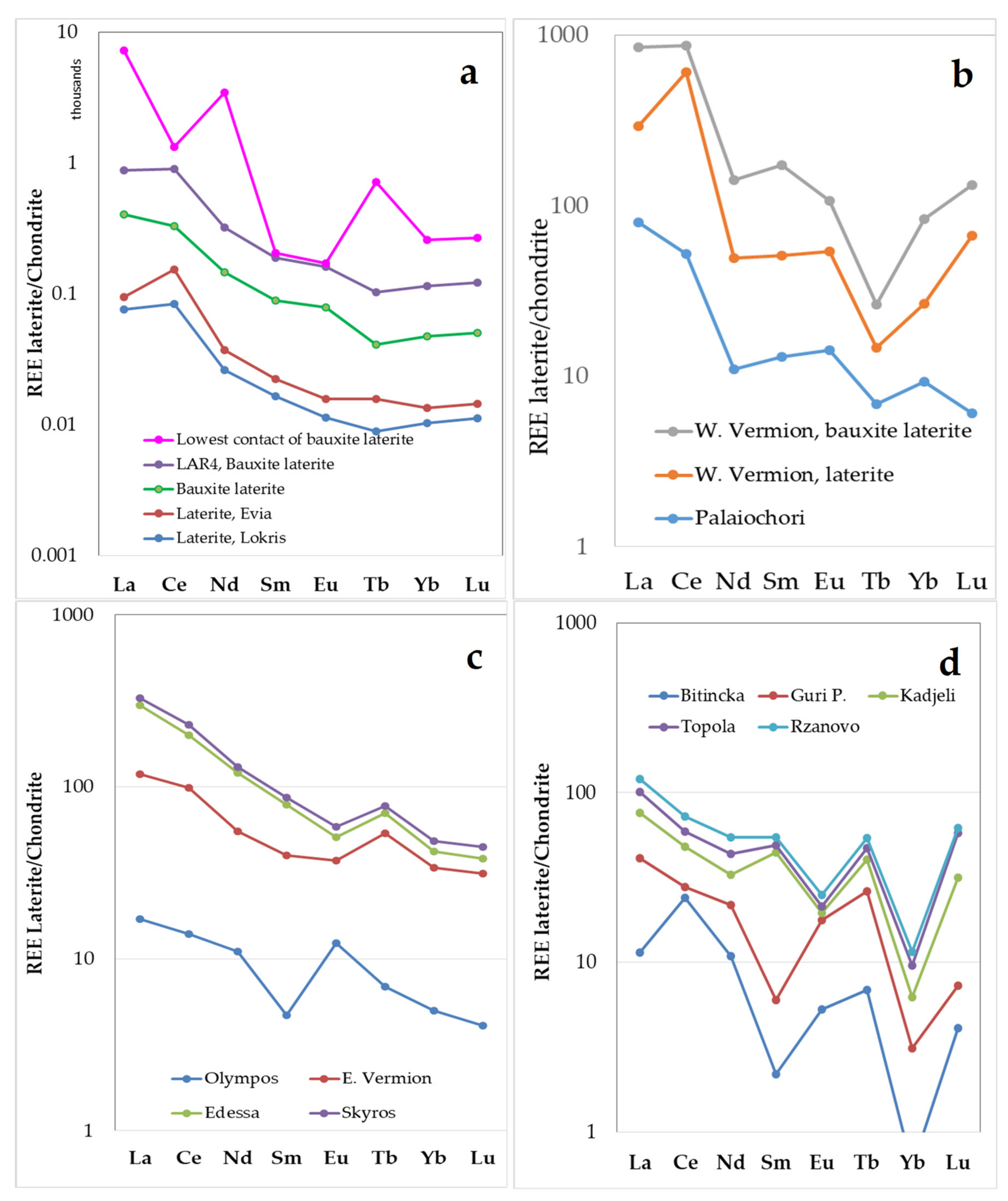
Assuming that values >1 and <1 are called positive and negative anomalies, respectively, chondrite normalized REE patterns show in general negative slopes from La–Eu and slight positive slopes from Tb–Lu for the Fe–Ni laterites and bauxite laterite samples from the Lokris and Evia (
Figure 10a) and W. Vermion (
Figure 10b), except the samples from the lowest part of the Lokris deposit, showing a negative Ce anomaly (
Figure 10a). In addition, a positive Nd anomaly in the later samples is observed that seems to be consistent with the occurrence of (hydroxyl)bastnaesite-(Nd) and (La,Nd,Y)-bastnaesite (
Figure 3e,f) [
26,
29,
30]. Laterites from W. Vermion are characterized by positive Ce and Eu anomalies. In general, negative slopes are a common feature of the chondrite normalized REE patterns of laterites from northern Greece (
Figure 10c), Albania, Serbia, and N. Macedonia, former F.Y.R.O.M. (
Figure 10d), with varying positive and negative anomalies.
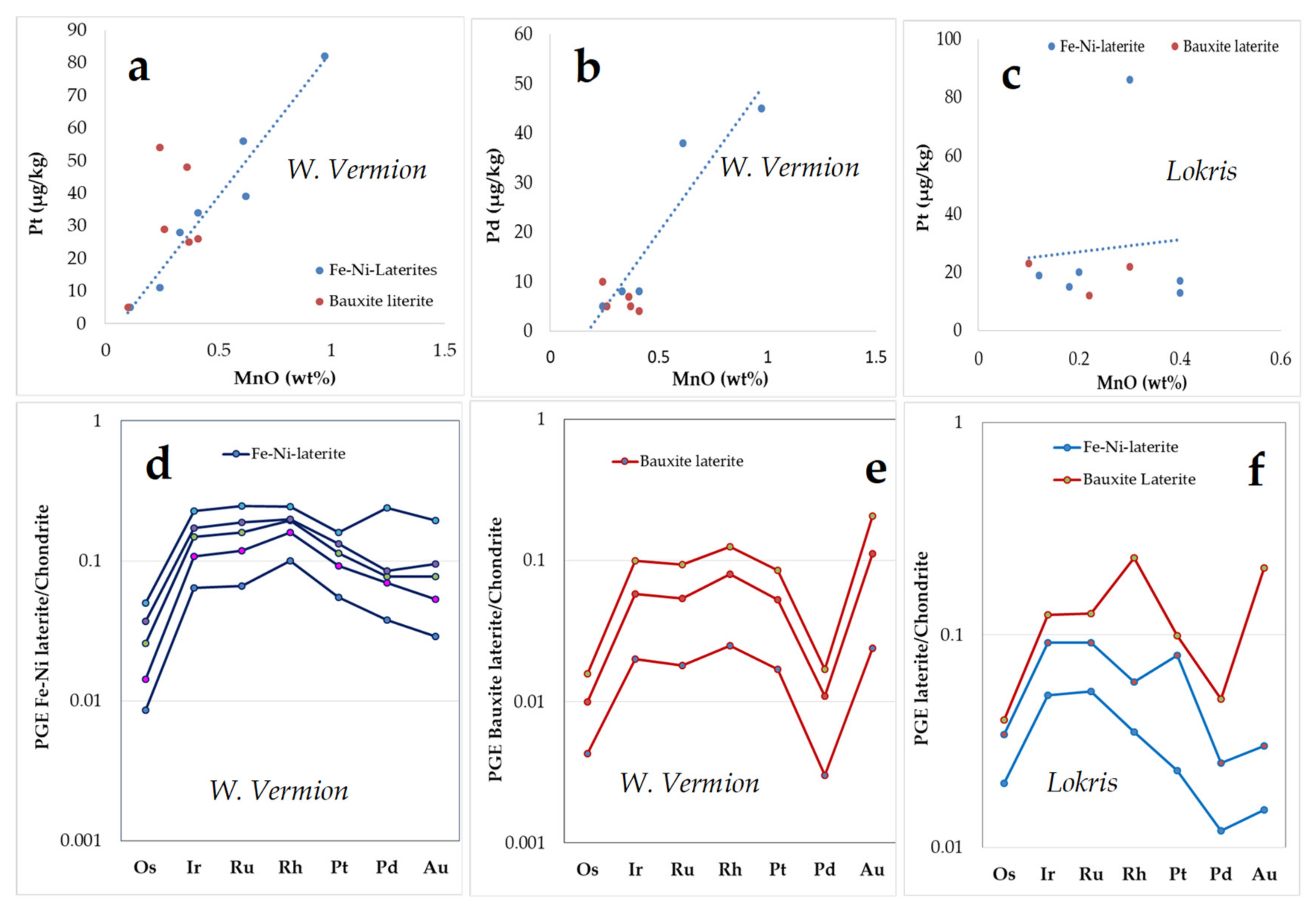
In general, the PGE content, reaching up to 88 μg/kg Pt and 45 μg/kg Pd (up to 186 μg/kg Pd in one sample), is higher in Fe–Ni ± Co laterites than in bauxite laterites (
Table 2,
Table 3,
Table 4 and
Table 5 and
Figure 11d–f) and bauxites [
37]. A common feature of the studied deposits from the Balkan Peninsula, including two Fe–Ni laterite profiles from the Gouri-Perjuegjiun and Bitincka deposits of Albania, the Kastoria, and W. Vermion laterites, is the higher Pt than Pd contents in ores compared to parent peridotites, their concentration mostly occuring in the pelitomorphic and pisolitic–oolitic types (
Table 2,
Table 3,
Table 4 and
Table 5) [
16,
18].
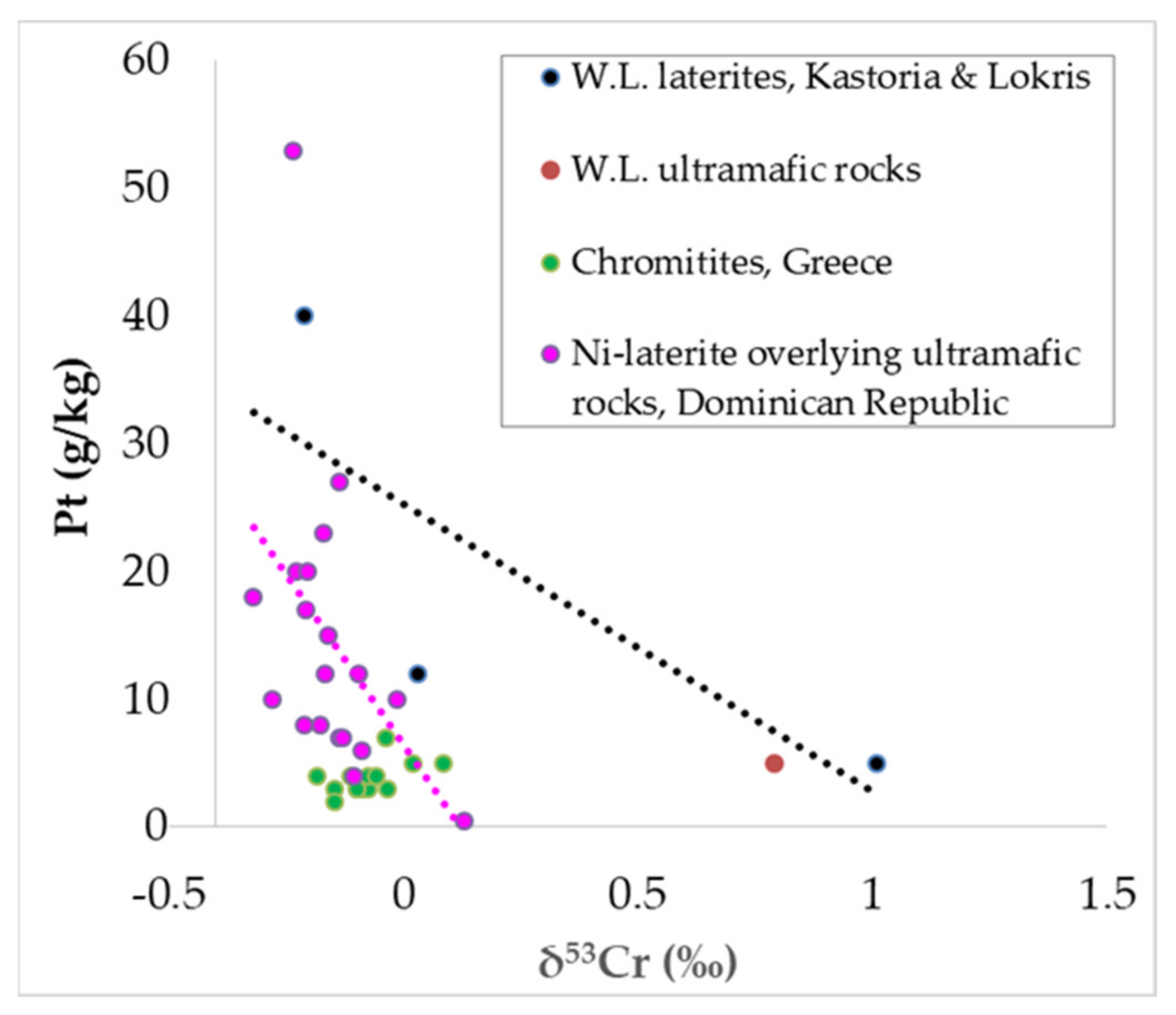
Any relationship between Sc and Pt or Pd is not clear, but there is a positive trend in the laterite samples from W. Vermion (
Figure 12a,b), whereas both Pt and Pd exhibit a negative trend with the ΣREE (
Figure 12b). In contrast, there is a positive trend between the ΣREE and Th, U, and P
2O
5 contents (
Figure 12c–e), as well as between Sc–P
2O
5 contents (
Figure 12f).
4.2. Environmental Impact from Mining and Smelting of Fe–Ni Laterites
Integrated approaches to the soil–groundwater–plant/crops compositional variations related to the weathering of rocks/ores, mining, and smelting of laterites and the preliminary results of leaching experiments of Fe–Ni laterite have been presented in previous publications and will be discussed. Furthermore, BSE images from the unpolished parts of the Nissi (Lokris) bauxite laterite ore are given in the present study (
Figure 4) in order to discuss the role of organic matter into heavy metal biomobilization and biomineralization in soil and the contamination of groundwater affected by rock/ore weathering and mining of laterite deposits.
4.2.1. Metallurgical Residues (Slag)
The result of the reductive smelting of the laterite is the formation of two separate phases, the metallic Fe–Ni and the slag residue. Bulk chemical analyses of representative samples of pyro-metallurgical residue (slag96 and slag14 samples) from the Larymna plant, indicated a significant Cr content (
Table 6).
However, preliminary results have shown that the Cr(VI) concentrations in water leachates from the metallurgical residue (slag) of Fe–Ni laterite samples from Lokris were very low (below 1 μg/L) [
31].
4.2.2. Concentrations of Cr(VI) in Water Leachates for Fe–Ni Laterites and Bauxite Laterites
Preliminary results of the water leaching experiments for Fe–Ni laterite ores have shown very low Cr(VI) concentrations in the samples from Lokris and up to 1200 μg/L Cr(VI) in samples from the Kastoria deposit [
31]. Further leaching experiments carried out in the present study for Fe–Ni laterite deposits from the Balkan Peninsula showed significant Cr(VI) concentrations in certain laterites from W. Vermion, the Bitincka, and Guri– Pergjegjum (Albania) deposits and bauxite laterite samples from the lowest parts of the Nissi deposit at Lokris (
Table 7).