Controlled Hydrothermal Precipitation of Alunite and Natroalunite in High-Aluminum Vanadium-Bearing Aqueous System
Abstract
:1. Introduction
2. Experimental Section
2.1. The Removal Behavior of Al in Acid Solution
2.2. Detection Methods
3. Results and Discussion
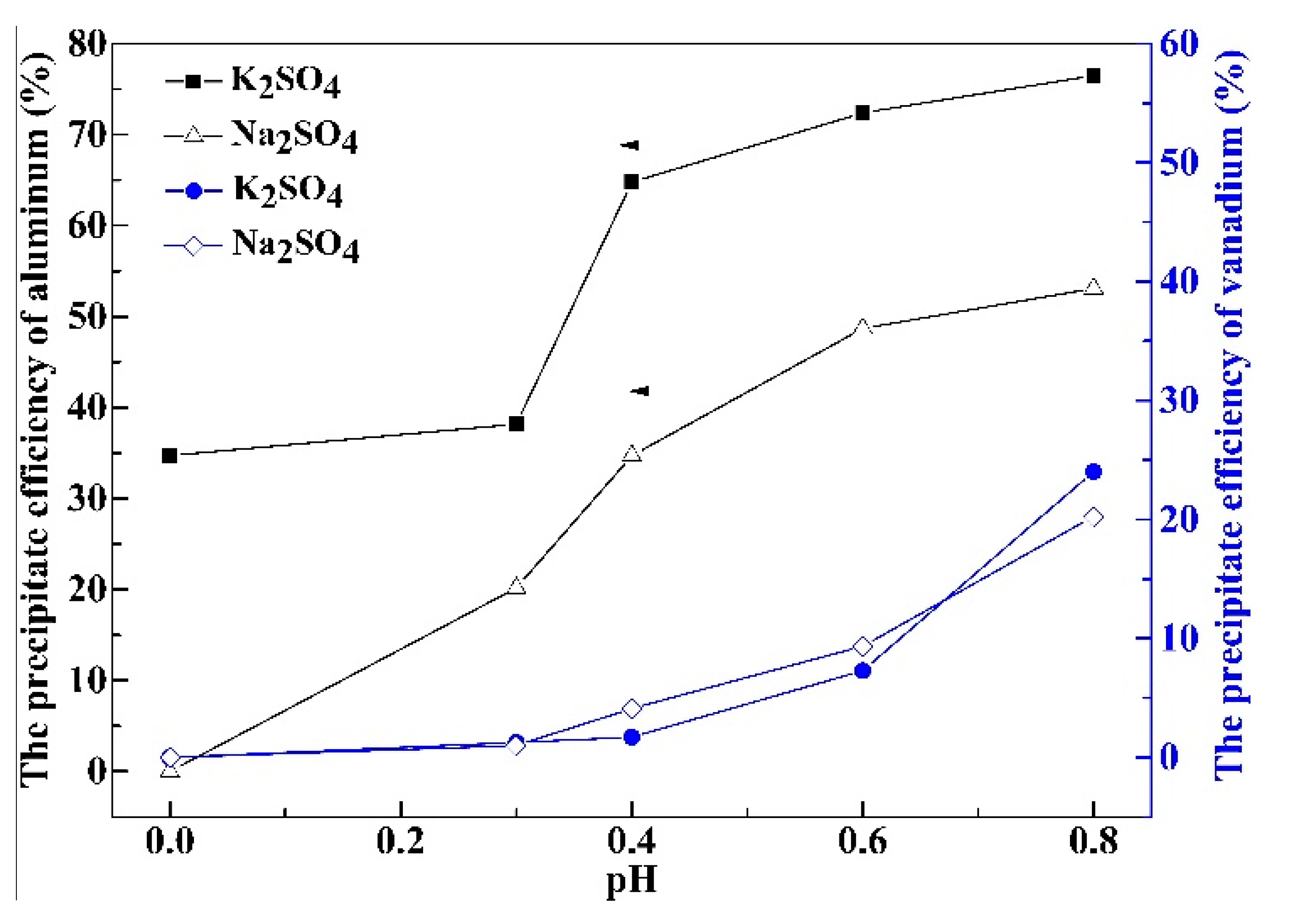
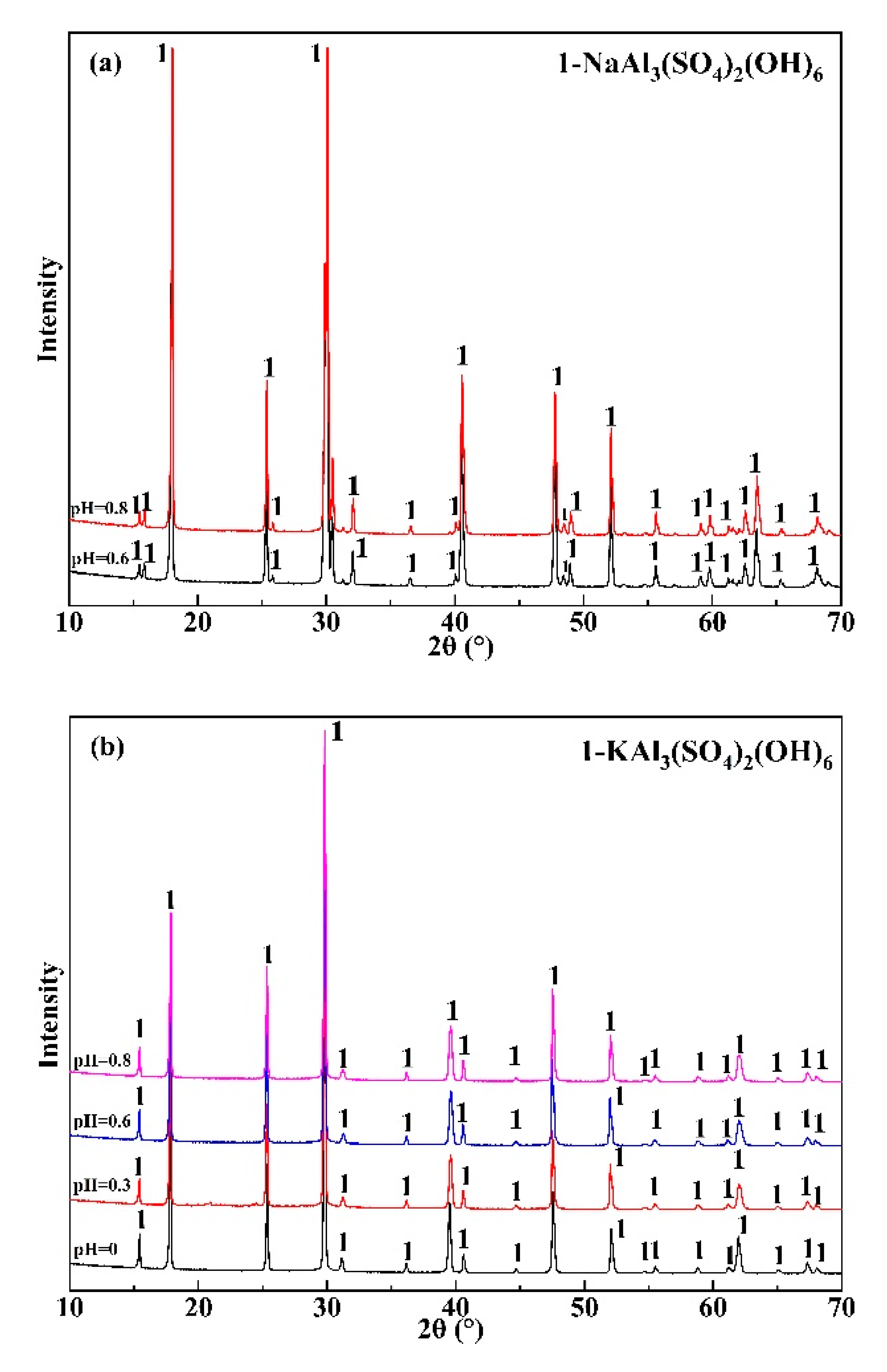
3.1. Effect of Initial pH Value on the Precipitation Efficiency of Al
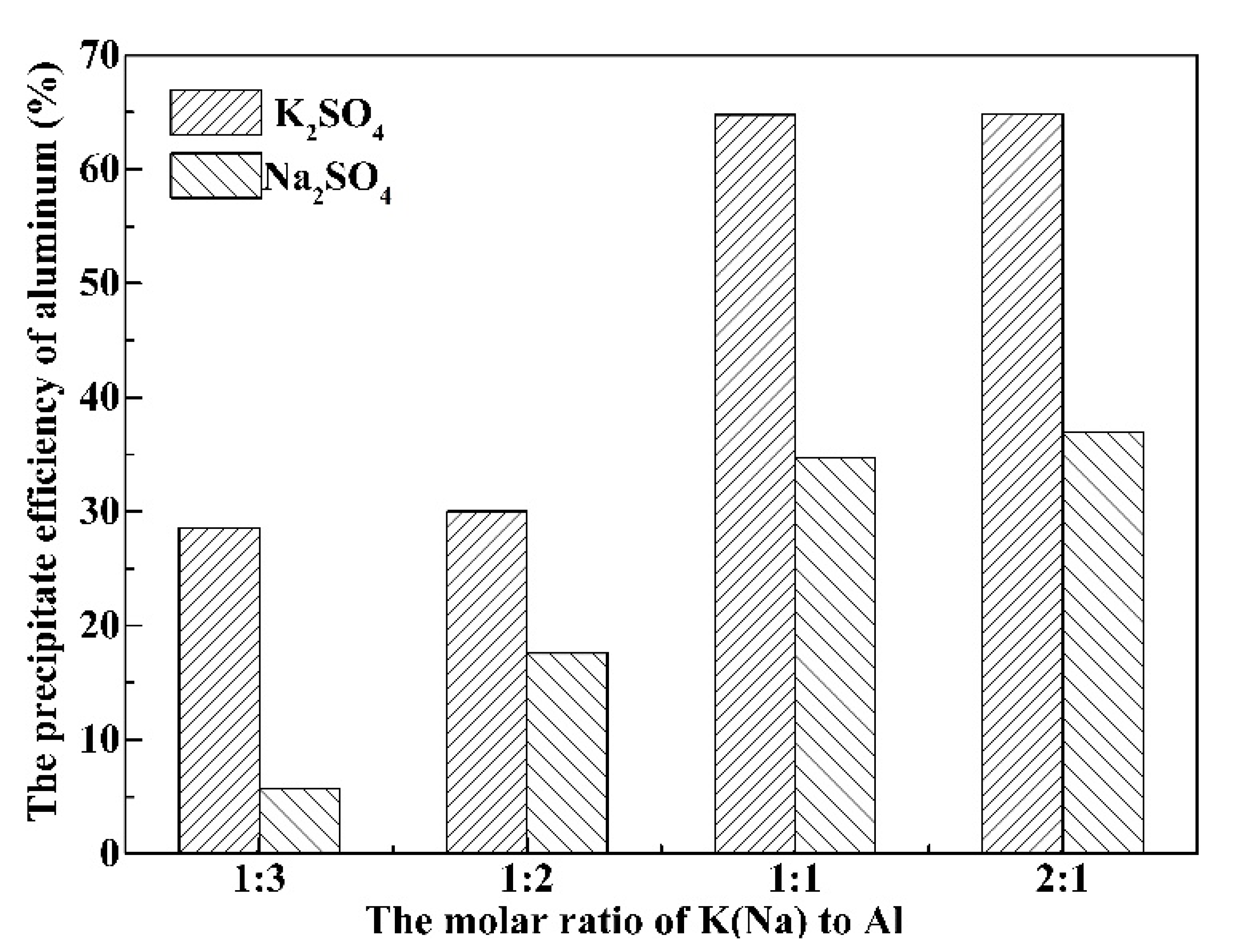
3.2. Effect of K(Na)/Al Molar Ratio on the Precipitation Efficiency of Al
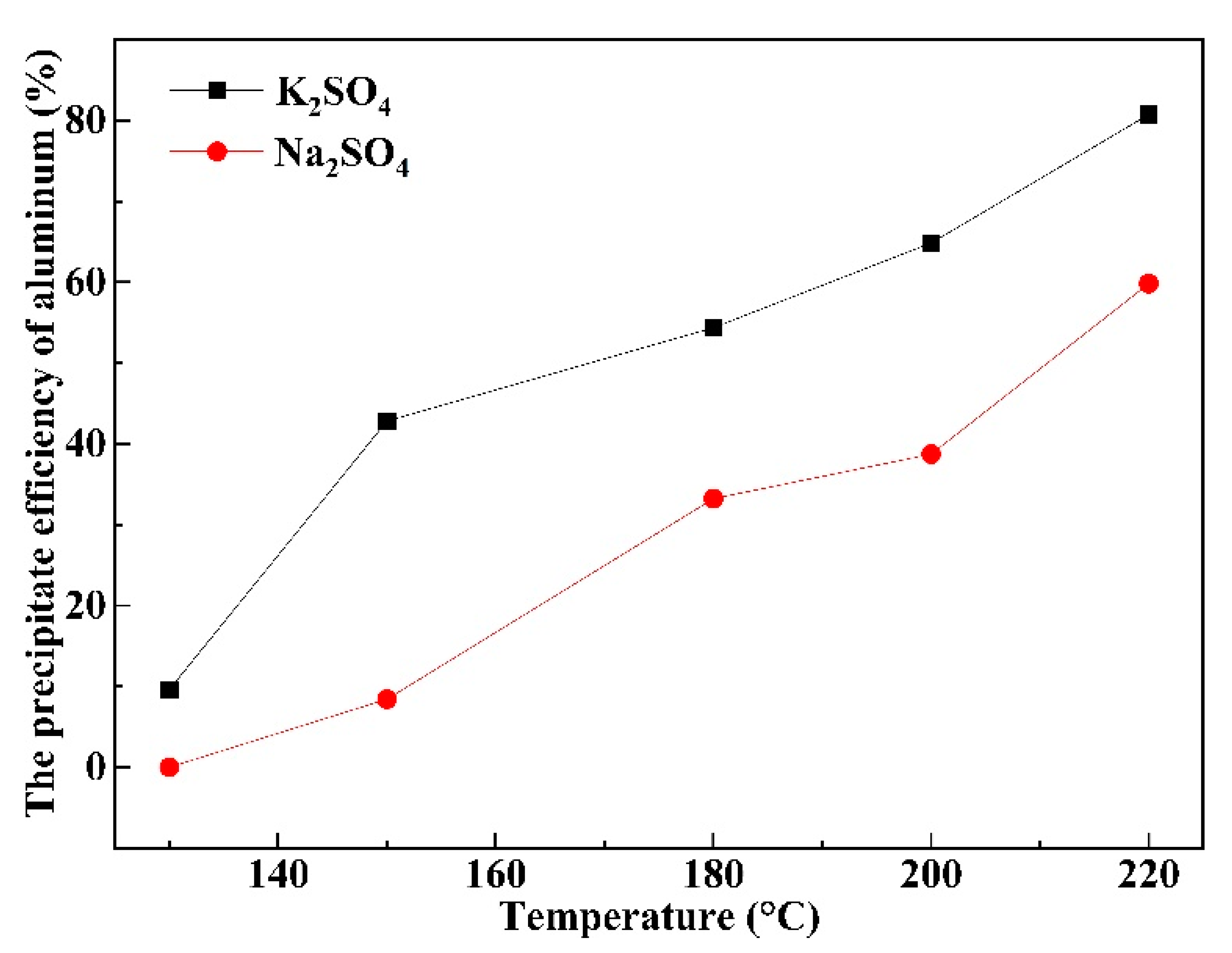
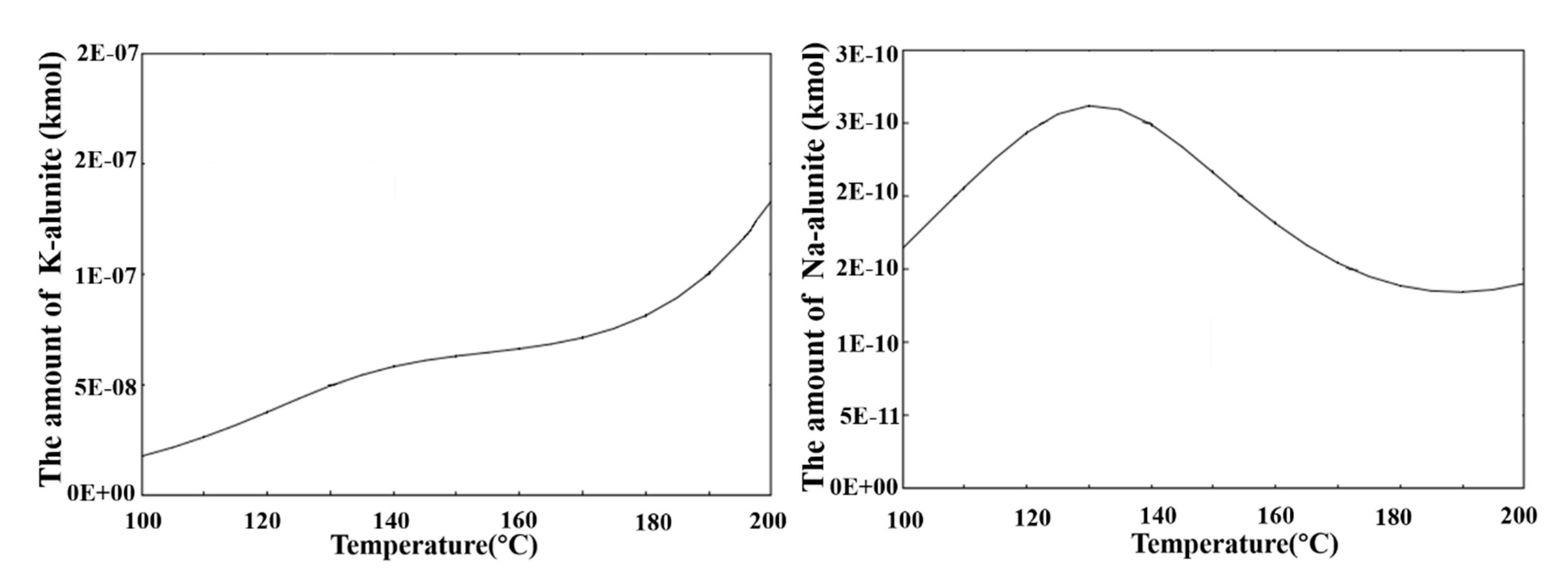
3.3. Effect of Temperature on the Precipitation Efficiency of Al
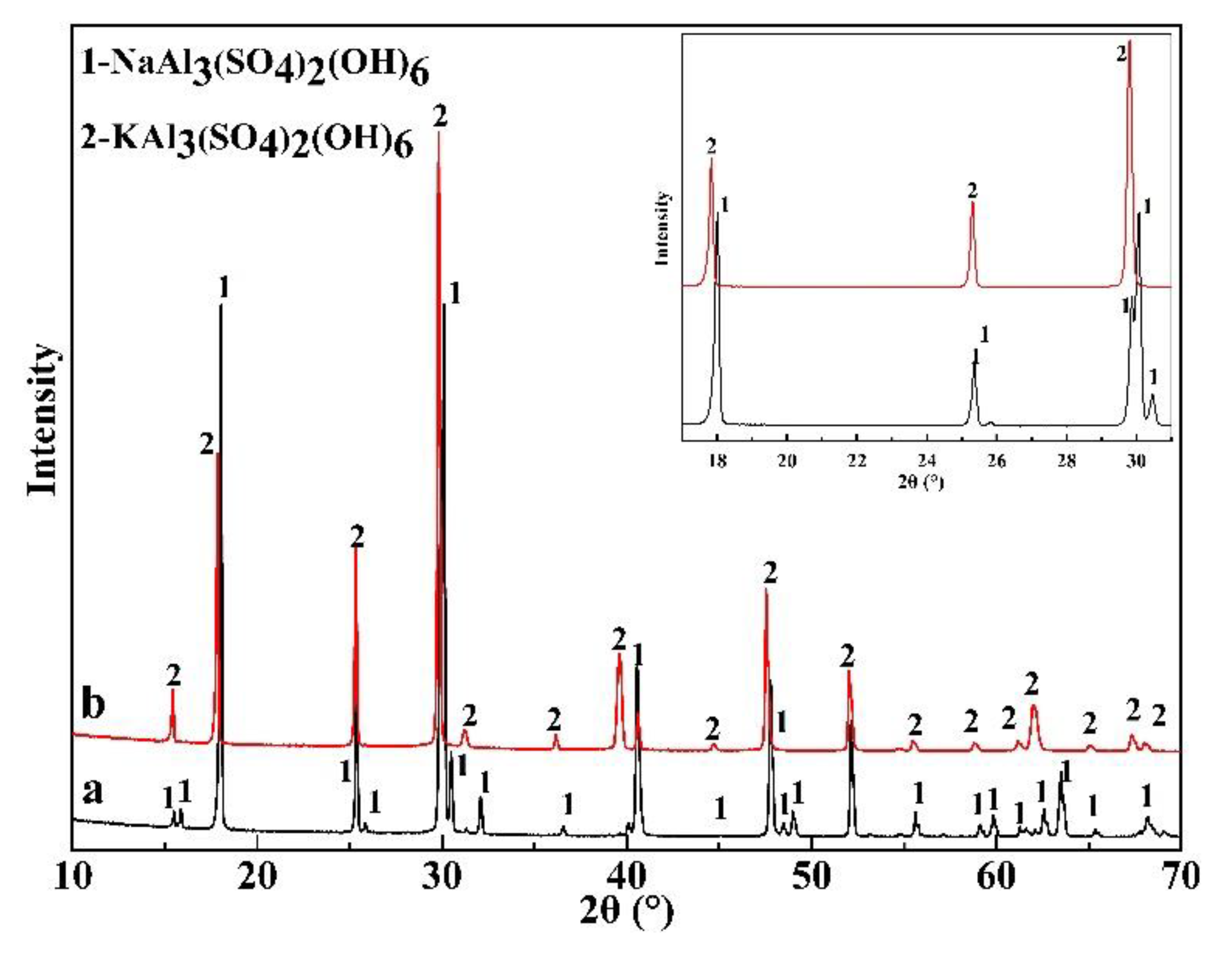
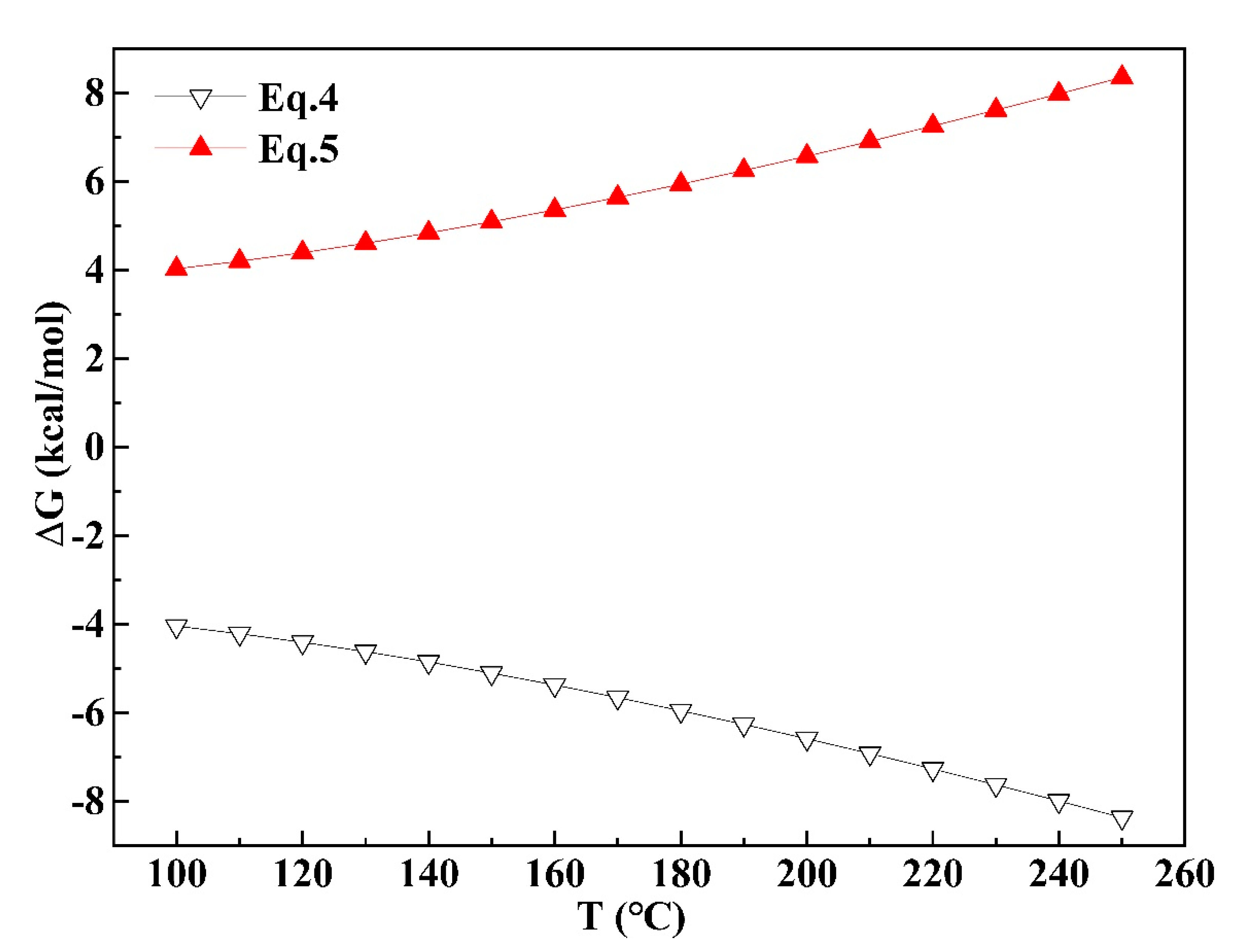
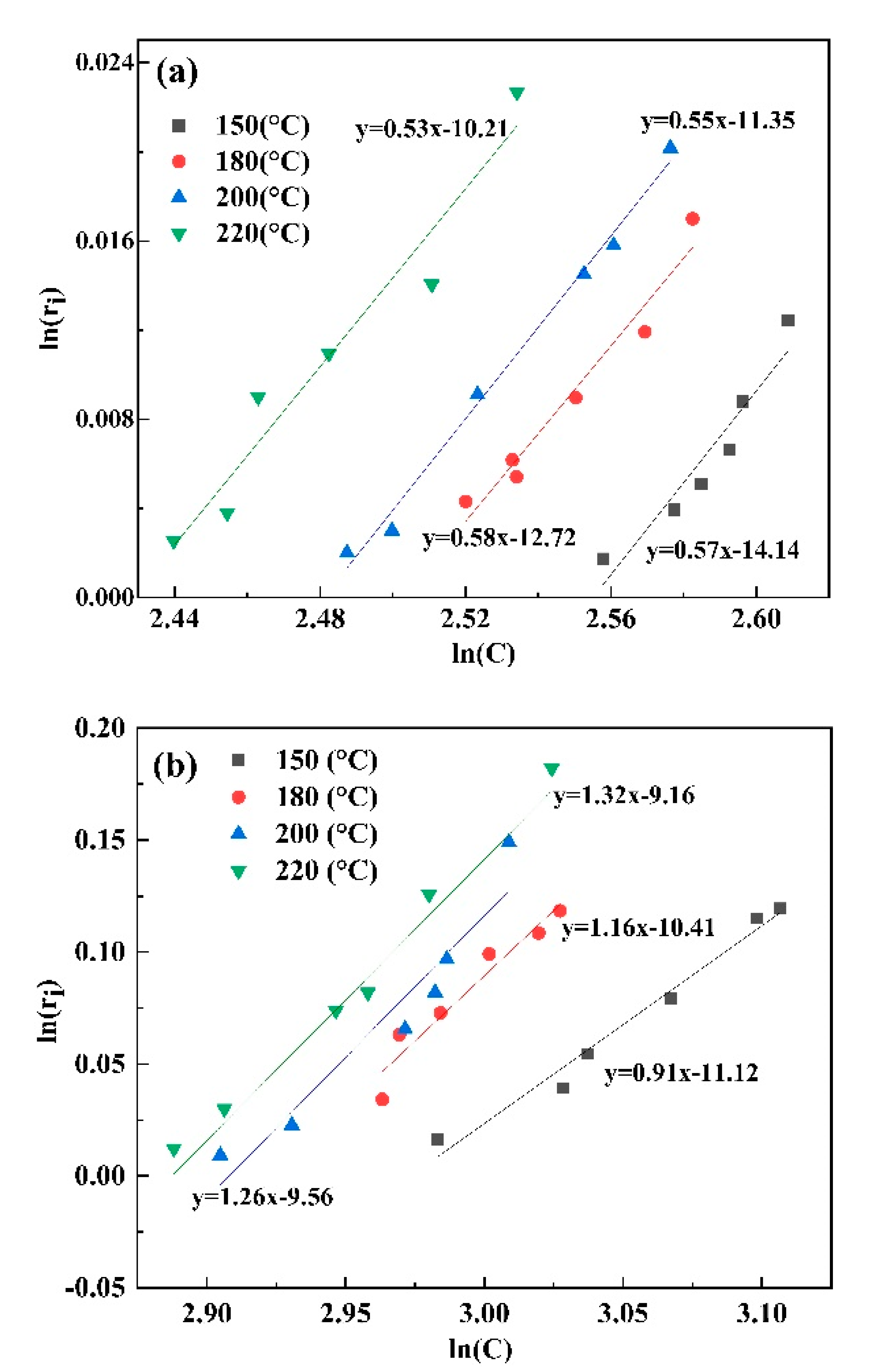
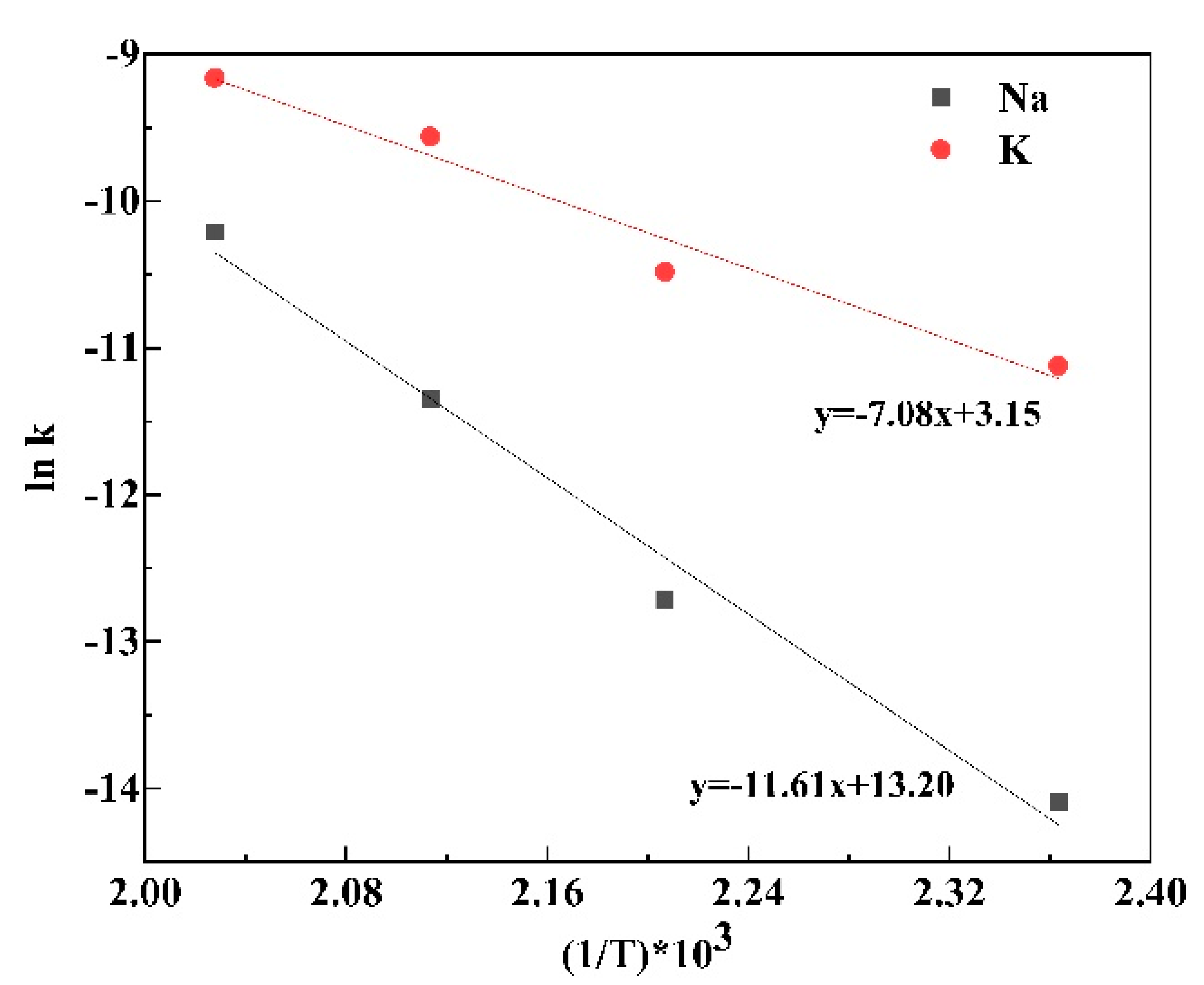
3.4. The Precipitation Thermodynamics and Kinetics of Alunite and Natroalunite
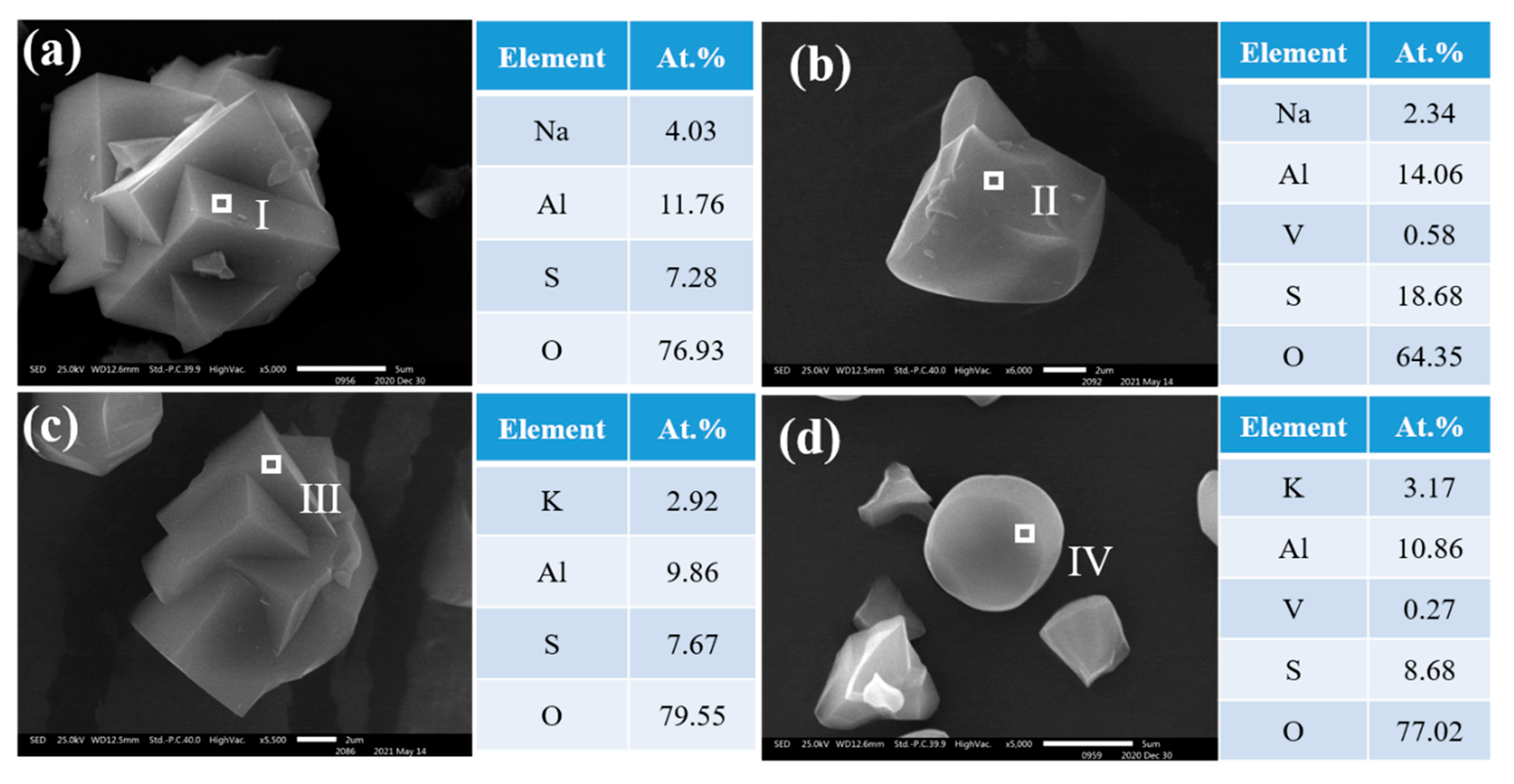
3.5. Mechanism for the Co-Precipitation of Vanadium and Alunite (Natroalunite)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.X.; Wang, H.; Yan, B.J. Sulfuric acid leaching and recovery of vanadium from a spinel concentrate beneficiated from stone coal ore. Hydrometallurgy 2020, 191, 105239. [Google Scholar] [CrossRef]
- Huang, Z.L.; Chen, T.; Zhou, Y.; Xu, W.B.; Lin, H.Z.; Yan, B. Optimization of oxidative leaching for vanadium extraction from low-grade stone coal using response surface methodology. Process 2020, 8, 1534. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Sun, C.Y.; Li, H.; Yin, W.Z.; Zhou, J.J. Blank roasting kinetics of illite type vanadium bearing stone coal. J. Mater. Res. Technol. 2020, 9, 7363–7369. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhang, Q.; Cai, Y.; Wang, D.P.; Li, K.W. The occurrence state of vanadium in the black shale-hosted vanadium deposits in Shangling of Guangxi Province, China. Chin. J. Geochem. 2015, 34, 484–497. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Zhang, Y.M.; Xue, N.N.; Liu, T.; Huang, J. Vanadium occupation and its leachability differences in trioctahedral and dioctahedral mica. RSC Adv. 2019, 9, 27615–27624. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, Y.M.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.B.; Liu, J. A mechanism of calcium fluoride-enhanced vanadium leaching from stone coal. Int. J. Miner. Process 2016, 145, 87–93. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process 2012, 114–117, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhu, X.B.; Liu, T.; Huang, J.; Song, S.X. Effect of colloidal potassium alum formation on vanadium recovery from acid leach solutions of stone coal. Hydrometallurgy 2013, 138, 54–58. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.M.; Liu, T.; Huang, J.; Wang, Y. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal. Hydrometallurgy 2013, 131–132, 1–7. [Google Scholar] [CrossRef]
- Shi, Q.H.; Zhang, Y.M.; Huang, J.; Liu, T.; Liu, H.; Wang, L.Y. Synergistic solvent extraction of vanadium from leaching solution of stone coal using D2EHPA and PC88A. Sep. Purif. Technol. 2017, 181, 1–7. [Google Scholar] [CrossRef]
- Shi, Q.H.; Zhang, Y.M.; Liu, T.; Huang, J.; Liu, H. Two-stage separation of V(IV) and Al(III) by crystallization and solvent extraction from aluminum-rich sulfuric acid leaching solution of stone coal. JOM 2017, 69, 1950–1957. [Google Scholar] [CrossRef]
- Wang, Y.D.; Li, J.H.; Gao, Y.; Yang, Y.; Gao, Y.; Xu, Z.F. Removal of aluminum from rare-earth leaching solutions via a complexation-precipitation process. Hydrometallurgy 2020, 191, 105220. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, H.G.; Gao, D.X.; Chen, B.F.; Meng, Y.Q.; Wang, M.Y. A clean technology to separate and recover vanadium and chromium from chromate solutions. Hydrometallurgy 2018, 177, 94–99. [Google Scholar] [CrossRef]
- Wang, M.Y.; Chen, B.F.; Huang, S.; Wang, X.W.; Liu, B.; Ge, Q.; Xie, S.S. A novel technology for vanadium and chromium recovery from V-Cr-bearing reducing slag. Hydrometallurgy 2017, 171, 116–122. [Google Scholar] [CrossRef]
- Guo, Y.X.; Lv, H.B.; Yang, X.; Cheng, F.Q. AlCl3⋅6H2O recovery from the acid leaching liquor of coal gangue by using concentrated hydrochloric inpouring. Sep. Purif. Technol. 2015, 151, 177–183. [Google Scholar] [CrossRef]
- Frost, R.L.; Wills, R.; Weier, M.L.; Martens, W.; Theo Kloprogge, J. A Raman spectroscopic study of alunites. J. Mol. Struct. 2006, 785, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Maubec, N.; Lahfid, A.; Lerouge, C.; Wille, G.; Michel, K. Characterization of alunite supergroup minerals by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and their application in hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Rudolph, W.W.; Mason, R.; Schmidt, P. Synthetic alunites of the potassium-oxonium solid solution series and some other members of the group: Synthesis, thermal and X-ray characterization. Eur. J. Miner. 2003, 15, 913–924. [Google Scholar] [CrossRef]
- Viñals, J.; Sunyer, A.; Molera, P.; Cruells, M.; Llorca, N. Arsenic stabilization of calcium arsenate waste by hydrothermal precipitation of arsenical natroalunite. Hydrometallurgy 2010, 104, 247–259. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, B.; Ren, X.; Shao, D.; Tan, X.; Chen, C. Controlled synthesized natroalunite microtubes applied for cadmium(II) and phosphate co–removal. J. Hazrad. Mater. 2016, 314, 249–259. [Google Scholar] [CrossRef]
- Zhu, B.S.; Jia, Y.; Jin, Z.; Sun, B.; Luo, T.; Yu, X.Y.; Kong, L.T.; Huang, X.J.; Liu, J.H. Controlled synthesis of natroalunite microtubes and spheres with excellent fluoride removal performance. Chem. Eng. J. 2015, 271, 240–251. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, L.Y. Separation of impurities aluminum and iron during pressure acid leaching of vanadium from stone coal. J. Clean Prod. 2017, 166, 1265–1273. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.M.; Huang, J.; Liu, T.; Cai, Z.L.; Xue, N.N. Selective leaching of vanadium from roasted stone coal by dilute sulfuric acid dephosphorization-two-stage pressure acid leaching. Minerals 2016, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Tester, J.W.; Cline, J.A. Hydrolysis and oxidation in subcritical and supercritical water: Connecting process engineering science to molecular interactions. Cor. Eng. Sect. 1999, 55, 1088–1100. [Google Scholar] [CrossRef]
- Marshall, W.L.; Franck, E.U. Ion product of water substance, 0–1000 °C, 1–10,000 bars new international formulation and its background. J. Phys. Chem. Ref. Data 1981, 10, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Sunyer, A.; Viñals, J. Arsenate substitution in natroalunite: A potential medium for arsenic immobilization. Part 2: Cell parameters and stability tests. Hydrometallurgy 2011, 109, 106–115. [Google Scholar] [CrossRef]
- Croker, D.; Loan, M.; Hodnett, B.K. Kinetics and mechanisms of the hydrothermal crystallization of calcium titanate species. Cryst. Growth Des. 2009, 9, 2207–2213. [Google Scholar] [CrossRef]
- Du, G.C.; Sun, Z.H.; Xian, Y.; Jing, H.; Chen, H.J.; Yin, D.F. The nucleation kinetics of ammonium metavanadate precipitated by ammonium chloride. J. Cryst. Growth 2016, 441, 117–123. [Google Scholar] [CrossRef]
- Le, T.K.; Kang, M.; Tran, V.T.; Kim, S.W. Relation of photoluminescence and sunlight photocatalytic activities of pure V2O5 nanohollows and V2O5/RGO nanocomposites. Mat. Sci. Semicon. Proc. 2019, 100, 159–166. [Google Scholar] [CrossRef]
- Vaschetti, V.M.; Eimer, G.A.; Cánepa, A.L.; Casuscelli, S.G. Catalytic performance of V-MCM-41 nanocomposites in liquid phase limonene oxidation: Vanadium leaching mitigation. Micropor. Icropor. Mesopor. Mat. 2021, 311, 110678. [Google Scholar] [CrossRef]
- NIST XPS Database. Available online: https://srdata.nist.gov/xps/intro.aspx (accessed on 15 May 2021).
- Zhou, X.J.; Wei, C.; Li, M.T.; Qiu, S.; Li, X.B. Thermodynamics of vanadium–Sulfur–Water systems at 298K. Hydrometallurgy 2011, 106, 104–112. [Google Scholar] [CrossRef]
- Sato, E.; Nakai, I.; Miyawaki, R.; Matsubara, S. Crystal structures of alunite family minerals: Beaverite, corkite, alunite, natroalunite, jarosite, svanbergite, and woodhouseite. Neues Jahrb. Mineral. Abh. 2009, 185, 313–322. [Google Scholar] [CrossRef]





| pH Value | Na2SO4 | K2SO4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before reaction | 0 | 0.3 | 0.4 | 0.6 | 0.8 | 0 | 0.3 | 0.4 | 0.6 | 0.8 |
| After reaction | 0 | 0.29 | 0.21 | 0.41 | 0.39 | −0.10 | 0.27 | 0.25 | 0.51 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xue, N.; Zhang, Y.; Hu, P. Controlled Hydrothermal Precipitation of Alunite and Natroalunite in High-Aluminum Vanadium-Bearing Aqueous System. Minerals 2021, 11, 892. https://doi.org/10.3390/min11080892
Wang L, Xue N, Zhang Y, Hu P. Controlled Hydrothermal Precipitation of Alunite and Natroalunite in High-Aluminum Vanadium-Bearing Aqueous System. Minerals. 2021; 11(8):892. https://doi.org/10.3390/min11080892
Chicago/Turabian StyleWang, Luyao, Nannan Xue, Yimin Zhang, and Pengcheng Hu. 2021. "Controlled Hydrothermal Precipitation of Alunite and Natroalunite in High-Aluminum Vanadium-Bearing Aqueous System" Minerals 11, no. 8: 892. https://doi.org/10.3390/min11080892
APA StyleWang, L., Xue, N., Zhang, Y., & Hu, P. (2021). Controlled Hydrothermal Precipitation of Alunite and Natroalunite in High-Aluminum Vanadium-Bearing Aqueous System. Minerals, 11(8), 892. https://doi.org/10.3390/min11080892






