Availability of Trace Elements in Soil with Simulated Cadmium, Lead and Zinc Pollution
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Subject of the Study
2.2. Analytical Methods
2.3. Properties and Chemical Composition of the Starting Soil
2.4. Experiment Description
2.5. Calculation and Statistical Methods
3. Results and Discussion
3.1. The Content of Mobile Forms of Trace Elements in Soil
3.2. Availability Factor (AF)
3.3. Correlations between Content Available Forms of Trace Elements in Soil
3.4. Physico-Chemical Properties against the Background of Soil Pollution
3.4.1. Sorptions Properties of Soils
3.4.2. Soil Reaction (pH)
3.4.3. Soil Salinity (EC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef]
- Massas, I.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil pollution indexes. Environ. Monit. Assess. 2013, 185, 6751–6766. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Jain, V.K.; Gupta, V.K.; Sharma, L.K. Comparative studies of physico-chemical properties of the roadside soil at Morena-(M.P.). Curr World Environ. 2014, 9, 220–222. [Google Scholar] [CrossRef][Green Version]
- Radziemska, M.; Fronczyk, J. Level and contamination assessment of soil along an expressway in an ecologically valuable area in central Poland. Curr World Environ. 2015, 12, 13372–13387. [Google Scholar] [CrossRef]
- Świercz, A.; Smorzewska, E. Variations in the zinc and lead content in surface layers of urban soils in Kielce (Poland) with regard to land use. J. Elem. 2015, 20, 449–461. [Google Scholar]
- Khan, M.A.; Khan, A.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef]
- Wieczorek, J.; Baran, A.; Urbański, K.; Mazurek, R.; Klimowicz-Pawlas, A. Assessment of the pollution and ecological risk od lead and cadmium in soils. Environ. Geochem. Health 2018, 40, 2325–2342. [Google Scholar] [CrossRef] [PubMed]
- Rolka, E.; Żołnowski, A.C.; Sadowska, M.M. Assessment of heavy metal content in soils adjacent to the DK16-Route in Olsztyn (North-Eastern Poland). Pol. J. Environ. Stud. 2020, 29, 4303–4311. [Google Scholar] [CrossRef]
- Fajardo, C.; Costa, G.; Nande, M.; Botίas, P.; Garcίa-Cantalejo, J.; Martίn, M. Pb, Cd, and Zn soil contamination: Monitoring functional and structural impacts on the microbiome. Appl. Soil Ecol. 2018, 13, 56–64. [Google Scholar] [CrossRef]
- Anju, M.; Banerjee, D.K. Associations of cadmium, zinc, and lead in soils from a lead and zinc mining area as studied by single and sequential extractions. Environ. Monit. Assess. 2011, 176, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Czech, T.; Wieczorek, J. Chemical properties and toxicity of soils contaminated by mining activity. Ecotoxicology 2014, 23, 1234–1244. [Google Scholar]
- Wierzbowska, J.; Kovačik, P.; Sienkiewicz, S.; Krzebietke, S.; Bowszys, T. Determination of heavy metals and their availability to plants in soil fertilized with different waste substances. Environ. Monit. Assess. 2018, 190, 1–12. [Google Scholar] [CrossRef]
- Jaafar, R.S.; Yousif, A.; Abdulnabi, Z.A.; Alhello, A.Z.; Al-Saad, H.T. An integrative study to determine the bioavailability of heavy metals in the soil. Eco. Environ. Cons. 2019, 25, 35–42. [Google Scholar]
- Draszawka-Bołzan, B. Heavy metals in soils. WNOFNS 2015, 2, 20–37. [Google Scholar]
- Bartkowiak, A.; Lemanowicz, J. Effect of forest fire on changes in the content of total and available forms of selected heavy metals and catalase activity in soil. Soil Sci. Ann. 2017, 68, 140–148. [Google Scholar] [CrossRef]
- Zajęcka, E.; Świercz, A. Biomonitoring of the urban environment of Kielce and Olsztyn (Poland) based on studies of total and bioavailable lead content in soils and common dandelion (Taraxacum officinale agg.). Minerals 2021, 11, 52. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.A.; Senila, L.R. Assessment of metals bioavailability to vegetables under field conditions using DGT, single extractions and multivariate statistics. Chem. Cent. J. 2012, 6, 119. [Google Scholar] [CrossRef]
- Nunes, J.R.; Ramos-Miras, J.; Lopez-Piñeiro, A.; Loures, L.; Gil, C.; Coelho, J.; Loures, A. Concentrations of available heavy metals in Mediterranean agricultural soils and their relation with some soil selected properties: A case study in typical Mediterranean soils. Sustainability 2014, 6, 9124–9138. [Google Scholar] [CrossRef]
- Sungur, A.; Soylak, M.; Ozcan, H. Investigation of heavy metal mobility and availability by the BCR sequential extraction procedure: Relationship between soil properties and heavy metals availability. Chem Spec. Bioavailab. 2014, 26, 219–230. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Modrzewska, B. Acidity and sorption properties of zinc-contaminated soil following the application of neutralising substances. J. Ecol. Eng. 2016, 17, 63–68. [Google Scholar] [CrossRef]
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Lucas, I.G.; Vidal, M.M.J.; Borras, J.B.; Zorpas, A.A. Trace metal content and availability of essential metals in agricultural soils of Alicante (Spain). Sustainability 2018, 10, 4534. [Google Scholar] [CrossRef]
- Yan, X.; Liu, M.; Zhong, J.; Guo, J.; Wu, W. How human activities affect heavy metal contamination of soil and sediment in a long-term reclaimed area of the Liaohe River Delta, North China. Sustainability 2018, 10, 338. [Google Scholar] [CrossRef]
- Usman, A.R.A. Influence of NaCl-induced salinity and Cd toxicity on respiration activity and Cd availability to barley plants in farmyard manure-amended soil. Appl. Environ. Soil Sci. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Józefowska, A.; Miechówka, A.; Gąsiorek, M.; Zadrożny, P. Content of zinc, lead and cadmium in selected agricultural soils in the area of the Śląskie and Ciężkowickie foothills. J. Ecol. Eng. 2014, 15, 74–80. [Google Scholar]
- Sayadi, M.H.; Rezaei, A.; Sayyed, M.R. Grain size fraction of heavy metals in soil and their relationship with land use. Proc. Int. Acad. Ecol. Environ. Sci. 2017, 7, 1–11. [Google Scholar]
- Verla, E.N.; Verla, A.W.; Osisi, A.F.; Okeke, P.N.; Enyoh, C.E. Finding a relationship between mobility factors of selected heavy metals and soil particle size in soils from children’s playgrounds. Environ. Monit. Assess. 2019, 191, 1–11. [Google Scholar] [CrossRef]
- Aydinalp, C.; Marinova, S. Distribution and forms of heavy metals in some agricultural soils. Pol. J. Environ. Stud. 2003, 12, 629–633. [Google Scholar]
- Różański, S. Fractionation of selected heavy metals in agricultural soils. Ecol. Chem. Eng. S. 2013, 20, 117–125. [Google Scholar]
- Wang, L.; Chen, H.; Wu, J.; Huang, L.; Brookes, P.C.; Rodrigues, M.J.L.; Xu, J.; Liu, X. Effects of magnetic biochar-microbe composite on Cd remediation and microbial responses in paddy soil. J. Hazard. Mater. 2021, 414, 125494. [Google Scholar] [CrossRef] [PubMed]
- Lemanowicz, J.; Bartkowiak, A. Assessment of soil phosphatase activity, phosphorus and heavy metals content depending on the mineral fertilization. Sci. Rev. Eng. Environ. Sci. 2016, 72, 116–129. [Google Scholar]
- Wyszkowski, M.; Modrzewska, B. Effect of neutralising substances on the total content of trace elements in soil contaminated with zinc. J. Elem. 2017, 22, 1439–1451. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R.; Hug, S.M.I.; Kawai, S. Fractionation and mobility of cadmium, lead and zinc in some contaminated and non-contaminated soils of Japan. J. Soil Sci. Environ. Manag. 2011, 3, 241–249. [Google Scholar]
- Osakwe, S.A.; Akpoveta, O.V.; Okoh, B.E.; Ize-Iyamu, O.K. Chemical forms of heavy metals in soils around municipal waste dumpsites in Asaba Metropolis, Delta State, Nigeria. Chem. Spec. Bioavailab. 2012, 24, 23–30. [Google Scholar] [CrossRef]
- Lamb, D.T.; Ming, H.; Megharaj, M.; Naidu, R. Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J. Hazard. Mater. 2009, 171, 1150–1158. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gawliński, A.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plant Properties; Instytut Ochrony Środowiska: Warszawa, Poland, 1991; pp. 1–324. (In Polish) [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchun-gen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extractionsmethoden zur Phospor- und Kaliumbestimmung. Ann. R. Agric. Coll. Swed. 1960, 26, 199–215. [Google Scholar]
- Schlichting, E.; Blume, H.P.; Stahr, K. Bodenkundliches Praktikum; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1995; pp. 1–81. [Google Scholar]
- CEM Corporation. Operation Manual Microwave Reaction System; CEM Corporation: Matthews, NC, USA, 2016. [Google Scholar]
- Kashem, M.A.; Singh, B.R.; Kondo, T.; Hug, S.M.I.; Kawai, S. Comparison of extractability of Cd, Cu, Pb and Zn with sequential extraction in contaminated and non-contaminated soils. Int. J. Environ. Sci. Tech. 2007, 4, 169–176. [Google Scholar] [CrossRef]
- Karczewska, A.; Kabała, C. Methodology of Laboratory Analysis of Soils and Plants; University of Life Sciences Wroclaw: Wrocław, Poland, 2008; pp. 1–45. [Google Scholar]
- Method 3051A Microware Assisted Acid Digestion of Sediment, Sludges, Soils and Oils; US-EPA: Washington, DC, USA, 1994; p. 30.
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 403. [Google Scholar]
- Regulation of Minister of the Environment of 9 September 2002 on the Quality Standards for Soil and Quality Standards for Land. 2002, 1359. (In Polish). Available online: https://static1.money.pl/d/akty_prawne/pdf/DU/2002/165/DU20021651359.pdf (accessed on 22 June 2021).
- Regulation of the Minister of the Environment of 1 September 2016 on the Method of Conducting An Assessment of the Soil Surface Pollution. 2016; (In Polish). Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20160001395/O/D20161395.pdf (accessed on 22 June 2021).
- Burdzy, J. Statistical Tables; Macmillan: New York, NY, USA, 1999; pp. 1–60. [Google Scholar]
- Kirkham, M.B. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Takáč, P.; Szabová, T.; Kozáková, Ľ.; Benková, M. Heavy metals and their bioavailability from soils in the long-term polluted Centarl Spiš region of SR. Plant. Soil Environ. 2009, 55, 167–172. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rosikon, K.; Grobelak, A.; Kacprzak, M. Migration of various chemical compounds in soil solution during inducted phytoremediation. Arch. Environ. Prot. 2011, 37, 49–59. [Google Scholar]
 control (0);
control (0);  first metal dose (I);
first metal dose (I);  second metal dose (II);
second metal dose (II);  third metal dose (III). Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn.
third metal dose (III). Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn.
 control (0);
control (0);  first metal dose (I);
first metal dose (I);  second metal dose (II);
second metal dose (II);  third metal dose (III). Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn.
third metal dose (III). Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn.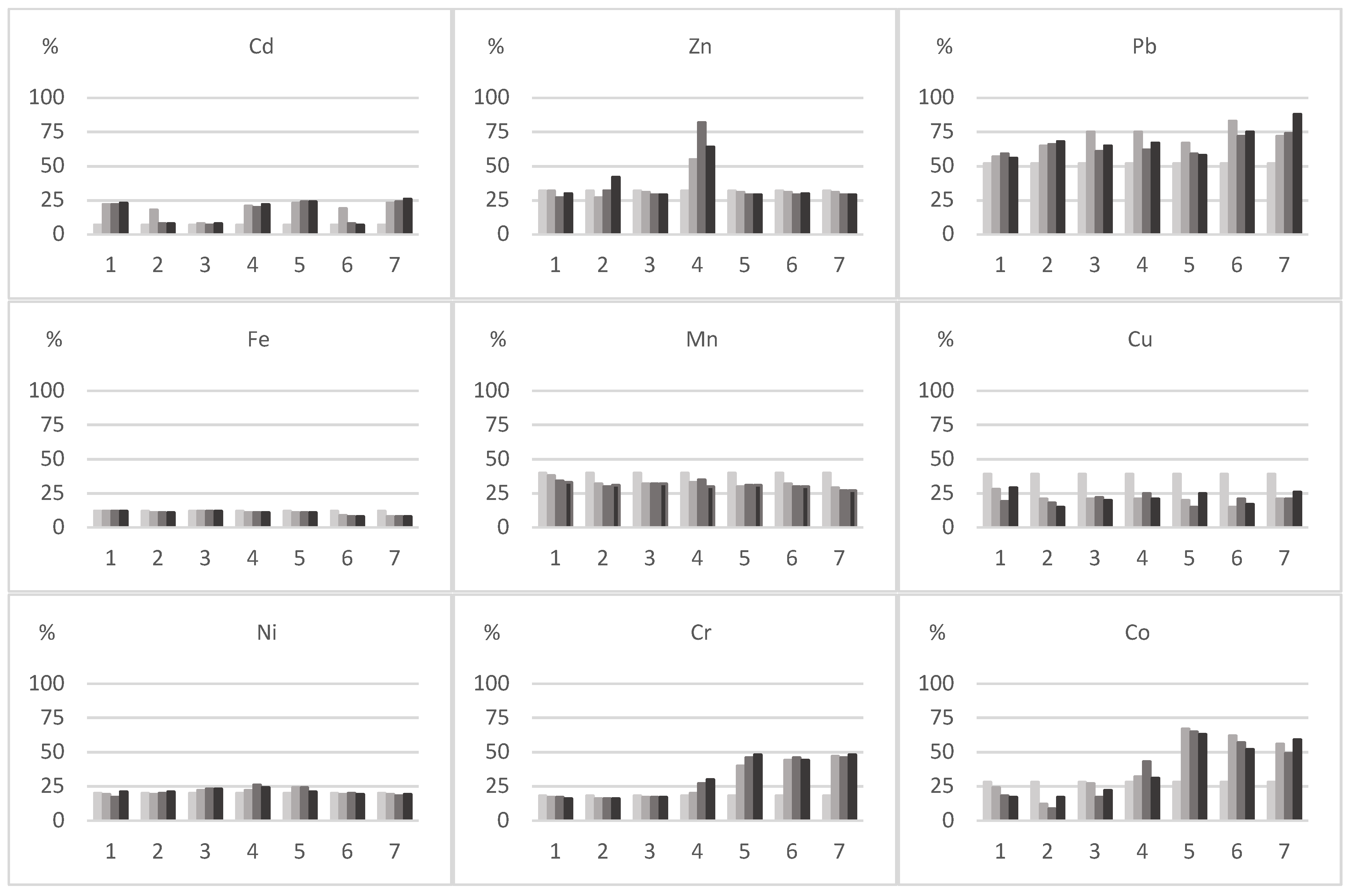
 control (0);
control (0);  first metal dose (I);
first metal dose (I);  second metal dose (II);
second metal dose (II);  third metal dose (III); Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn; correlation coefficient (r) *—significant for p = 0.05; **—highly significant for p = 0.01; n.s.—not significant; n = 12; the letters from “a” to “d” differentiate homogeneous groups, means followed by the same letter do not differ at p = 0.05 by the LSD test.
third metal dose (III); Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn; correlation coefficient (r) *—significant for p = 0.05; **—highly significant for p = 0.01; n.s.—not significant; n = 12; the letters from “a” to “d” differentiate homogeneous groups, means followed by the same letter do not differ at p = 0.05 by the LSD test.
 control (0);
control (0);  first metal dose (I);
first metal dose (I);  second metal dose (II);
second metal dose (II);  third metal dose (III); Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn; correlation coefficient (r) *—significant for p = 0.05; **—highly significant for p = 0.01; n.s.—not significant; n = 12; the letters from “a” to “d” differentiate homogeneous groups, means followed by the same letter do not differ at p = 0.05 by the LSD test.
third metal dose (III); Series of experiment: 1—Cd, 2—Pb, 3—Zn, 4—Cd + Pb, 5—Cd + Zn, 6—Pb + Zn, 7—Cd + Pb + Zn; correlation coefficient (r) *—significant for p = 0.05; **—highly significant for p = 0.01; n.s.—not significant; n = 12; the letters from “a” to “d” differentiate homogeneous groups, means followed by the same letter do not differ at p = 0.05 by the LSD test.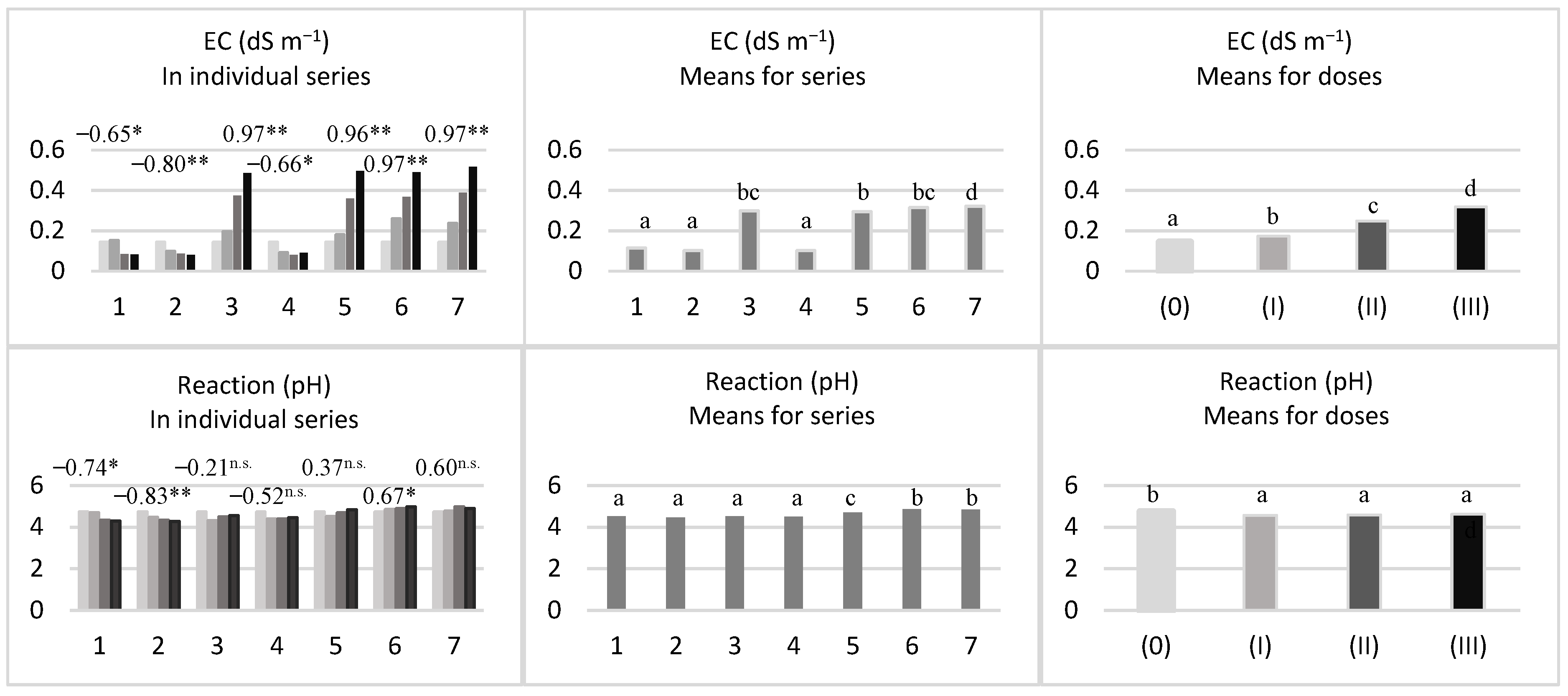
| Metal Doses in Soil ” | Series of Experiment | Means for Doses | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd + Pb | Cd + Zn | Pb + Zn | Cd + Pb + Zn | ||
| Cd | ||||||||
| 0 | 0.012a | 0.012a | 0.012a | 0.012a | 0.012a | 0.012a | 0.012a | 0.012a |
| I | 0.477b | 0.029a | 0.012a | 0.456b | 0.494b | 0.031a | 0.505b | 0.286b |
| II | 0.896d | 0.014a | 0.012a | 0.825c | 0.994e | 0.013a | 1.001e | 0.536c |
| III | 1.446g | 0.013a | 0.012a | 1.337f | 1.495g | 0.011a | 1.579h | 0.842d |
| Means for series | 0.708c | 0.017a | 0.012a | 0.658b | 0.749d | 0.017a | 0.774e | 0.419 |
| r | 0.995** | −0.192n.s. | 0.135n.s. | 0.994** | 1.000** | −0.264n.s. | 0.999** | 0.569** |
| LSD0.05 for: metal dose = 0.019; kind of metal = 0.025; interaction = 0.050 | ||||||||
| Pb | ||||||||
| 0 | 4.82a | 4.82a | 4.82a | 4.82a | 4.82a | 4.82a | 4.82a | 4.82a |
| I | 5.21a | 38.26b | 6.91a | 44.29b,c | 6.16a | 49.32c | 42.87b,c | 27.57b |
| II | 5.41a | 71.68d,e | 5.63a | 68.08d | 5.40a | 79.03e,f | 81.05f | 45.18c |
| III | 5.12a | 107.97g | 6.02a | 106.29g | 5.36a | 120.10h | 139.88i | 70.11d |
| Means for series | 5.14a | 55.68b | 5.84a | 55.87b | 5.44a | 63.32c | 67.15c | 36.92 |
| r | 0.241n.s. | 0.997** | 0.336n.s. | 0.971** | 0.194n.s. | 0.991** | 0.992** | 0.565** |
| LSD0.05 for: metal dose = 2.939; kind of metal = 3.888; interaction = 7.777 | ||||||||
| Zn | ||||||||
| 0 | 9.16a | 9.16a | 9.16a | 9.16a | 9.16a | 9.16a | 9.16a | 9.16a |
| I | 9.04a | 7.67a | 55.26f | 15.60c | 54.87f | 54.94f | 54.48f | 35.98b |
| II | 7.67a | 9.05a | 95.30g | 23.00e | 95.03g | 96.40g | 94.55g | 60.14c |
| III | 8.33a | 11.85b | 140.15h | 18.06d | 140.21h | 142.45h | 140.48h | 85.93d |
| Means for series | 8.55b | 9.43b | 74.97a | 16.46c | 74.82a | 75.74a | 74.67a | 47.80 |
| r | −0.447n.s. | 0.598n.s. | 1.000** | 0.727* | 1.000** | 0.999** | 0.999** | 0.586** |
| LSD0.05 for: metal dose = 0.862; kind of metal = 1.140; interaction = 2.280 | ||||||||
| Metal Doses in Soil ” | Series of Experiment | Means for Doses | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd + Pb | Cd + Zn | Pb + Zn | Cd + Pb + Zn | ||
| Fe | ||||||||
| 0 | 728.3h,i | 728.3h,i | 728.3h | 728.3h | 728.3h | 728.3h | 728.3h | 728.3c |
| I | 731.0h | 673.8e–g | 690.1f,g | 664.0d–f | 640.2d,e | 524.1c | 480.1a,b | 629.0b |
| II | 708.7h,i | 646.3d,e | 688.6f,g | 663.3d–f | 645.3d,e | 490.2a,b | 465.1a | 615.4a |
| III | 689.6f,g | 648.5d,e | 691.8f,g,i | 627.4d | 650.5d,e | 502.6b,c | 487.4a,b | 614.0a |
| Means for series | 714.4b | 674.2a | 699.7b | 670.8a | 666.1a | 561.3d | 540.2c | 646.7 |
| r | −0.688* | −0.834** | −0.463n.s. | −0.861** | −0.652* | −0.809** | −0.750* | −0.450** |
| LSD0.05 for: metal dose = 12.031; kind of metal = 15.915; interaction = 31.831 | ||||||||
| Mn | ||||||||
| 0 | 91.26h | 91.26h | 91.26h | 91.26h | 91.26h | 91.26h | 91.26h | 91.26d |
| I | 87.36h | 73.24c–f | 72.93c–f | 75.92e–g | 70.13b–e | 74.27c–g | 65.76a,b | 74.23c |
| II | 78.83f,g | 70.22b–e | 73.53c–f | 79.60g | 71.24b–e | 68.36b,c | 62.70a | 72.07b |
| III | 75.44e–g | 70.78b–e | 74.12c–g | 69.48b–d | 70.47b–e | 68.15b,c | 61.56a | 70.00a |
| Means for series | 83.22d | 76.38a,b | 77.96a,b | 79.07b | 75.78a | 75.51a | 70.32c | 76.89 |
| r | −0.878** | −0.813** | −0.697* | −0.800** | −0.755* | −0.875** | −0.825** | −0.741** |
| LSD0.05 for: metal dose = 1.939; kind of metal = 2.566; interaction = 5.131 | ||||||||
| Cu | ||||||||
| 0 | 1.872e | 1.872e | 1.872e | 1.872e | 1.872e | 1.872e | 1.872e | 1.872b |
| I | 1.352c,d | 1.005a–d | 1.002a–d | 1.007a–d | 0.940a–d | 0.737a | 0.995a–d | 1.005a |
| II | 0.894a–c | 0.859a,b | 1.067a–d | 1.190a–d | 0.736a | 1.002a–d | 1.018a–d | 0.967a |
| III | 1.375d | 0.743a | 0.981a–d | 1.018a–d | 1.188a–d | 0.814a,b | 1.255b–d | 1.053a |
| Means for series | 1.373b | 1.120a | 1.231a,b | 1.272a,b | 1.184a,b | 1.106a | 1.285a,b | 1.224 |
| r | −0.552n.s. | −0.805** | −0.744* | −0.610n.s. | −0.534n.s. | −0.700* | −0.473n.s. | −0.619** |
| LSD0.05 for: metal dose = 0.149; kind of metal = n.s.; interaction = n.s. | ||||||||
| Metal Doses in Soil ” | Series of Experiment | Means for Doses | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd + Pb | Cd + Zn | Pb + Zn | Cd + Pb + Zn | ||
| Ni | ||||||||
| 0 | 0.571a–c | 0.571a–c | 0.571a–c | 0.571a–c | 0.571a–c | 0.571a–c | 0.571a–c | 0.571b |
| I | 0.560a–c | 0.554a–c | 0.634b–d | 0.645b–e | 0.703d,e | 0.565a–c | 0.543a,b | 0.601a,b |
| II | 0.487a | 0.594a–d | 0.680c–e | 0.761e | 0.703d,e | 0.571a–c | 0.520a,b | 0.617a |
| III | 0.611a–d | 0.600a–d | 0.680c–e | 0.697d,e | 0.622b–d | 0.543a,b | 0.566a–c | 0.617a |
| Means for series | 0.557a | 0.580a | 0.641b | 0.669b | 0.650b | 0.563a | 0.550a | 0.601 |
| r | 0.075n.s. | 0.259n.s. | 0.702* | 0.612n.s. | 0.236n.s. | −0.151n.s. | −0.074n.s. | 0.210n.s. |
| LSD0.05 for: metal dose = n.s.; kind of metal = 0.054; interaction = n.s. | ||||||||
| Cr | ||||||||
| 0 | 1.864a,b | 1.864a,b | 1.864a,b | 1.864a,b | 1.864a,b | 1.864a,b | 1.864a,b | 1.864a |
| I | 1.782a,b | 1.705a,b | 1.751a,b | 2.102b | 4.200d | 4.562d,e | 4.874e | 2.997b |
| II | 1.766a,b | 1.609a | 1.729a,b | 2.752c | 4.791e | 4.783e | 4.814e | 3.178c |
| III | 1.666a,b | 1.679a,b | 1.745a,b | 3.089c | 4.991e | 4.587d,e | 4.960e | 3.245c |
| Means for series | 1.770a | 1.714a | 1.772a | 2.452c | 3.962b | 3.949b | 4.128b | 2.821 |
| r | −0.516n.s. | −0.504n.s. | −0.288n.s. | 0.874** | 0.873** | 0.765** | 0.781** | 0.354** |
| LSD0.05 for: metal dose = 0.050; kind of metal = 0.066; interaction = 0.131 | ||||||||
| Co | ||||||||
| 0 | 0.346e | 0.346e | 0.346e | 0.346e | 0.346e | 0.346e | 0.346e | 0.346b |
| I | 0.301c–e | 0.154a,b | 0.330d,e | 0.390e | 0.827i | 0.766h,i | 0.683g,h | 0.493a |
| II | 0.224b–d | 0.108a | 0.215a–c | 0.523f | 0.794h,i | 0.699g,h | 0.608f,g | 0.453a |
| III | 0.206a–c | 0.206a–c | 0.272c–e | 0.387e | 0.780h,i | 0.645g | 0.710g,h | 0.458a |
| Means for series | 0.269a | 0.204c | 0.291a | 0.412d | 0.687e | 0.614b | 0.587b | 0.438 |
| r | −0.771** | −0.474n.s. | −0.576n.s. | 0.287n.s. | 0.712* | 0.565n.s. | 0.731* | 0.149n.s. |
| LSD0.05 for: metal dose = 0.039; kind of metal = 0.051; interaction = 0.102 | ||||||||
| Elements | Zn | Cd | Pb | Mn | Fe | Ni | Cr | Cu | Co |
|---|---|---|---|---|---|---|---|---|---|
| Cd | 0.269* | ||||||||
| Pb | 0.311** | 0.235* | |||||||
| Mn | −0.612** | −0.434** | −0.604** | ||||||
| Fe | −0.587** | −0.269* | −0.707** | 0.788** | |||||
| Ni | 0.114n.s. | 0.151n.s. | −0.046n.s. | −0.125n.s. | 0.131n.s. | ||||
| Cr | 0.679** | 0.440** | 0.437** | −0.601** | −0.800** | −0.012n.s. | |||
| Cu | −0.391** | −0.204n.s. | −0.418** | 0.730** | 0.504** | −0.083n.s. | −0.363** | ||
| Co | 0.583** | 0.346** | 0.225* | −0.377** | −0.608** | 0.103n.s. | 0.907** | −0.211n.s. | |
| EC | 0.962** | 0.206n.s. | 0.242* | −0.462** | −0.531** | −0.026n.s. | 0.646** | −0.263* | 0.559** |
| pH | 0.428** | −0.011n.s. | 0.128n.s. | 0.074n.s. | −0.373** | −0.322** | 0.562** | 0.182n.s. | 0.539** |
| HAC | 0.432** | 0.363** | 0.513** | −0.760** | −0.527** | −0.013n.s. | 0.274* | −0.527** | 0.067n.s. |
| TEB | −0.085n.s. | −0.022n.s. | −0.310** | 0.326** | 0.286* | 0.020n.s. | −0.042n.s. | 0.205n.s. | 0.072n.s. |
| CEC | 0.206n.s. | 0.218n.s. | 0.045n.s. | −0.195n.s. | −0.079n.s. | 0.021n.s. | 0.137n.s. | −0.137n.s. | 0.109n.s. |
| BS | −0.282* | −0.201n.s. | −0.485** | 0.619** | 0.483** | 0.022n.s. | −0.184n.s. | 0.411** | 0.003n.s. |
| Metal Doses in Soil ” | Series of Experiment | Means for Doses | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd + Pb | Cd + Zn | Pb + Zn | Cd + Pb + Zn | ||
| Hydrolytic acidity—HAC (mmol kg−1) | ||||||||
| 0 | 37.00a,b | 37.00a,b | 37.00a,b | 37.00a,b | 37.00a,b | 37.00a | 37.00a | 37.00b |
| I | 38.50a–d | 41.50e,f | 40.50d–f | 39.50a–e | 41.00d–f | 40.50d–f | 41.76e–g | 40.47a |
| II | 40.00c–e | 42.00e–g | 42.00e–g | 37.50a–c | 38.50a–d | 39.86b–e | 41.76e–g | 40.23a |
| III | 44.50g,h | 43.00f–h | 42.00e–g | 42.00e–g | 40.50d–f | 43.03f–h | 45.56h | 42.94c |
| Means for series | 40.00a,b | 40.88b,c | 40.38a–c | 39.00a | 39.25a | 40.10a,b | 41.52c | 40.16 |
| r | 0.783** | 0.753* | 0.853** | 0.576 | 0.522 | 0.815** | 0.939** | 0.715** |
| LSD0.05 for: metal dose = 0.94; kind of metal = 1.24; interaction = 2.48 | ||||||||
| Total exchangeable bases—TEB (mmol kg−1) | ||||||||
| 0 | 39.33c–g | 39.33c–g | 39.33c–g | 39.33c–g | 39.33c–g | 39.33c–g | 39.33c–g | 39.33b |
| I | 40.67e–g | 34.67a–f | 37.33a–g | 38.67b–g | 36.00a–g | 41.80g | 32.27a,b | 37.34a,b |
| II | 38.67b–g | 33.33a–c | 36.67a–g | 32.00a | 40.00d–g | 40.80f,g | 34.13a–e | 36.51a |
| III | 38.00a–g | 37.33a–g | 36.67a–g | 39.33c–g | 42.00g | 34.07a–d | 35.60a–g | 37.57a,b |
| Means for series | 39.17a | 36.17b,c | 37.50a–c | 37.33a–c | 39.33a | 39.00a,c | 35.33b | 37.69 |
| r | −0.282 | −0.264 | −0.445 | −0.185 | 0.488 | −0.472 | −0.198 | −0.179 |
| LSD0.05 for: metal dose = n.s.; kind of metal = 2.67; interaction = 5.35 | ||||||||
| Cation exchange capacity—CEC (mmol kg−1) | ||||||||
| 0 | 76.33b–d | 76.33b–d | 76.33b–d | 76.33b–d | 76.33b–d | 76.33b–d | 76.33b–d | 76.33a |
| I | 79.17b–d | 76.17b–d | 77.83b–d | 78.17d | 77.00b–d | 82.30b–d | 74.03a,b | 77.81a |
| II | 78.67b–d | 75.33a–c | 78.67b–d | 69.50a | 78.50b–d | 80.66b–d | 75.89b–d | 76.75a |
| III | 82.50d | 80.33b–d | 78.67b–d | 81.33c,d | 82.50d | 77.09b–d | 81.16c,d | 80.51b |
| Means for series | 79.17a | 77.04a | 77.88a | 76.33a | 78.58a | 79.10a | 76.85a | 77.85 |
| r | 0.502 | 0.440 | 0.368 | 0.113 | 0.813** | −0.006 | 0.332 | 0.302** |
| LSD0.05 for: metal dose = 2.13; kind of metal = n.s.; interaction = n.s. | ||||||||
| Base saturation—BS (%) | ||||||||
| 0 | 51.50g | 51.50g | 51.50g | 51.50g | 51.50g | 51.50g | 51.50g | 51.50b |
| I | 51.41f,g | 45.46a–c | 47.92a–g | 49.48c–g | 46.73a–g | 50.71d–g | 43.38a | 47.87a |
| II | 49.05b–g | 44.23a,b | 46.60a–g | 45.85a–d | 50.94e–g | 50.53d–g | 44.93a–c | 47.45a |
| III | 46.10a–e | 46.48a–f | 46.58a–g | 48.38b–g | 50.89e–g | 44.16a,b | 43.37a | 46.57a |
| Means for series | 49.52a | 46.92b,c | 48.15a,b | 48.80a,b | 50.02a | 49.23a | 45.80c | 48.35 |
| r | −0.760* | −0.529 | −0.740* | −0.524 | 0.111 | −0.694* | −0.536 | −0.481** |
| LSD0.05 for: metal dose = 1.56; kind of metal = 2.07; interaction = 4.14 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolka, E.; Wyszkowski, M. Availability of Trace Elements in Soil with Simulated Cadmium, Lead and Zinc Pollution. Minerals 2021, 11, 879. https://doi.org/10.3390/min11080879
Rolka E, Wyszkowski M. Availability of Trace Elements in Soil with Simulated Cadmium, Lead and Zinc Pollution. Minerals. 2021; 11(8):879. https://doi.org/10.3390/min11080879
Chicago/Turabian StyleRolka, Elżbieta, and Mirosław Wyszkowski. 2021. "Availability of Trace Elements in Soil with Simulated Cadmium, Lead and Zinc Pollution" Minerals 11, no. 8: 879. https://doi.org/10.3390/min11080879
APA StyleRolka, E., & Wyszkowski, M. (2021). Availability of Trace Elements in Soil with Simulated Cadmium, Lead and Zinc Pollution. Minerals, 11(8), 879. https://doi.org/10.3390/min11080879






