Carbon Mineralization with North American PGM Mine Tailings—Characterization and Reactivity Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Stillwater Mine
2.2. Characterization Experiments
2.3. Flow-Through Dissolution
2.4. Disk Carbonation
3. Results
3.1. Characterization Experiments
3.1.1. Characteristics of Mine Tailings
3.1.2. Composition of Tailings Storage Facilities
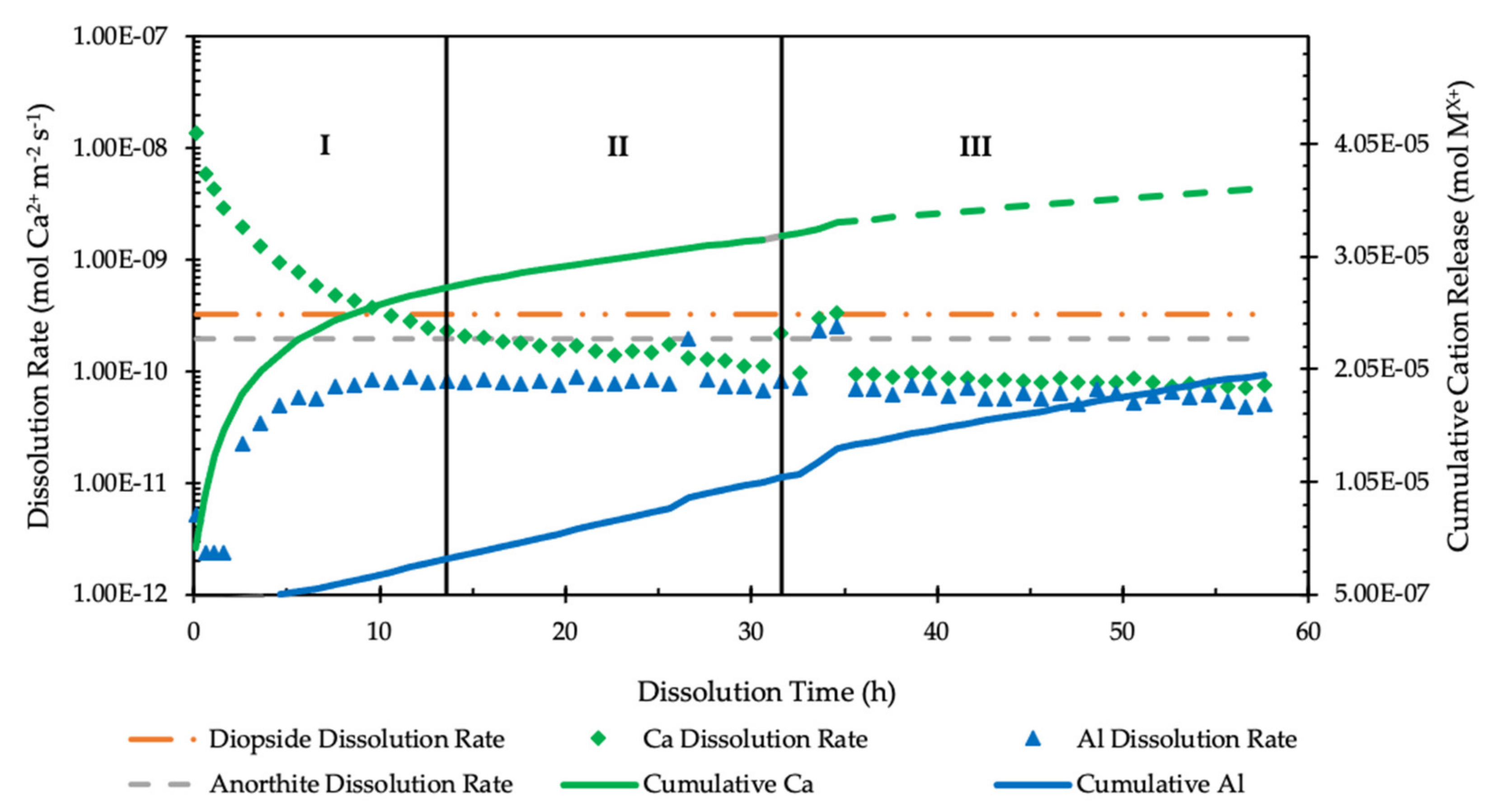
3.2. Flow-Through Dissolution
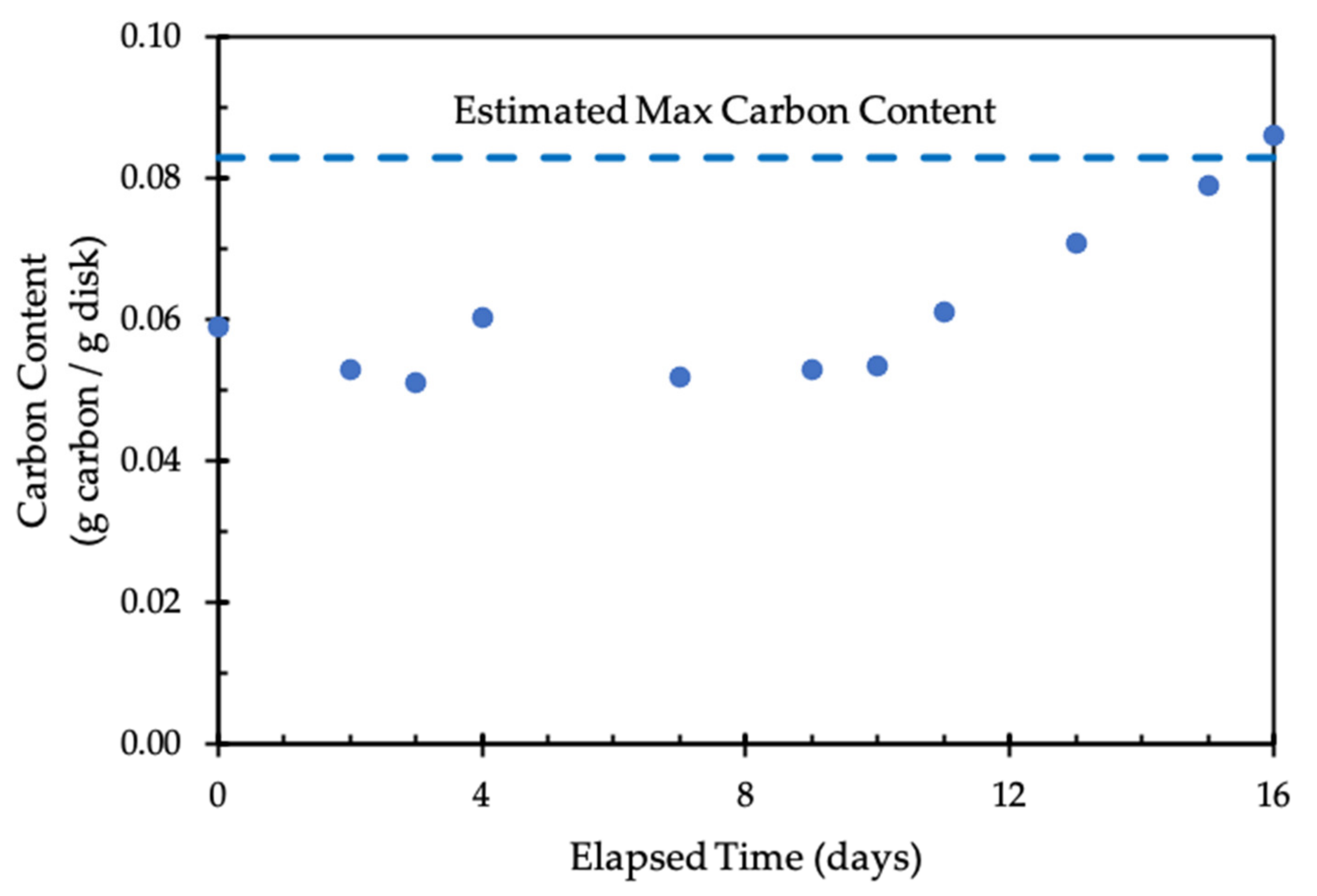
3.3. Disk Carbonation
4. Discussion
4.1. Significance of Results
4.2. Opportunity for Climate Impact
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marcy, C. U.S. Renewable Electricity Generation Has Doubled since 2008 March 2019. Available online: https://www.eia.gov/todayinenergy/detail.php?id=38752 (accessed on 4 August 2021).
- Watari, T.; McLellan, B.C.; Giurco, D.; Dominish, E.; Yamasue, E.; Nansai, K. Total material requirement for the global energy transition to 2050: A focus on transport and electricity. Resour. Conserv. Recycl. 2019, 148, 91–103. [Google Scholar] [CrossRef]
- Pilorgé, H.; McQueen, N.; Maynard, D.; Psarras, P.; He, J.; Rufael, T.; Wilcox, J. Cost Analysis of Carbon Capture and Sequestration of Process Emissions from the U.S. Industrial Sector. Environ. Sci. Technol. 2020, 54, 7524–7532. [Google Scholar] [CrossRef]
- McQueen, N.; Psarras, P.; Pilorgé, H.; Liguori, S.; He, J.; Yuan, M.; Wilcox, J. Cost Analysis of Direct Air Capture and Sequestration Coupled to Low-Carbon Thermal Energy in the United States. Environ. Sci. Technol. 2020, 54, 7542–7551. [Google Scholar] [CrossRef] [PubMed]
- Woodall, C.M.; Piccione, I.; Benazzi, M.; Wilcox, J. Capturing and Reusing CO2 by Converting it to Rocks. Front. Young Minds 2021, 8, 592018. [Google Scholar] [CrossRef]
- Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019.
- Woodall, C.M.; McQueen, N.; Pilorgé, H.; Wilcox, J. Utilization of Mineral Carbonation Products: Current State and Potential. Greenh. Gases Sci. Technol. 2019, 9, 1096–1113. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Southam, G. Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: Examples from the Clinton Creek and Cassiar Chrysotile deposits, Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; Power, I.M.; Barker, S.L.; Mayer, K.U.; Southam, G. Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. Int. J. Greenh. Gas Control 2014, 25, 121–140. [Google Scholar] [CrossRef]
- Washbourne, C.-L.; Lopez-Capel, E.; Renforth, P.; Ascough, P.L.; Manning, D.A.C. Rapid Removal of Atmospheric CO2 by Urban Soils. Environ. Sci. Technol. 2015, 49, 5434–5440. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Bradshaw, P.M.D.; Harrison, A.L. Prospects for CO2 mineralization and enhanced weathering of ultramafic mine tailings from the Baptiste nickel deposit in British Columbia, Canada. Int. J. Greenh. Gas. Control 2020, 94, 102895. [Google Scholar] [CrossRef]
- Gras, A.; Beaudoin, G.; Molson, J.; Plante, B.; Bussière, B.; Lemieux, J.M.; Dupont, P.P. Isotopic evidence of passive mineral carbonation in mine wastes from the Dumont Nickel Project (Abitibi, Quebec). Int. J. Greenh. Gas. Control 2017, 60, 10–23. [Google Scholar] [CrossRef]
- Vogeli, J.; Reid, D.L.; Becker, M.; Broadhurst, J.; Franzidis, J.P. Investigation of the potential for mineral carbonation of PGM tailings in South Africa. Miner. Eng. 2011, 24, 1348–1356. [Google Scholar] [CrossRef]
- Meyer, N.A.; Vögeli, J.U.; Becker, M.; Broadhurst, J.L.; Reid, D.L.; Franzidis, J.-P. Mineral carbonation of PGM mine tailings for CO2 storage in South Africa: A case study. Miner. Eng. 2014, 59, 45–51. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020.
- Renforth, P. The negative emission potential of alkaline materials. Nat. Commun. 2019, 10, 1401. [Google Scholar] [CrossRef] [Green Version]
- Ncongwane, M.S.; Broadhurst, J.L.; Petersen, J. Assessment of the potential carbon footprint of engineered processes for the mineral carbonation of PGM tailings. Int. J. Greenh. Gas. Control 2018, 77, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Harrison, A.L.; Dipple, G.M.; Power, I.M.; Mayer, K.U. The impact of evolving mineral-water-gas interfacial areas on mineral-fluid reaction rates in unsaturated porous media. Chem. Geol. 2016, 421, 65–80. [Google Scholar] [CrossRef]
- Zarandi, A.E.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Ambient mineral carbonation of different lithologies of mafic to ultramafic mining wastes/tailings—A comparative study. Int. J. Greenh. Gas. Control 2017, 63, 392–400. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. Dynamics of carbon dioxide uptake in chrysotile mining residues—Effect of mineralogy and liquid saturation. Int. J. Greenh. Gas. Control 2013, 12, 124–135. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Wilson, S.A.; Morgan, B.; Harrison, A.L.; Turvey, C.C.; Paterson, D.J.; Southam, G. Accelerating Mineral Carbonation in Ultramafic Mine Tailings via Direct CO2 Reaction and Heap Leaching with Potential for Base Metal Enrichment and Recovery. Econ. Geol. 2020, 115, 303–323. [Google Scholar] [CrossRef]
- Sibanye-Stillwater. Stillwater & East Boulder. 2021. Available online: https://www.sibanyestillwater.com/business/americas/pgm-operations-americas/stillwater-east-boulder/ (accessed on 4 August 2021).
- Jenkins, M.C.; Mungall, J.E.; Zientek, M.L.; Holick, P.; Butak, K. The nature and composition of the J-M reef, Stillwater Complex, Montana, USA. Econ. Geol. 2020, 115, 1799–1826. [Google Scholar] [CrossRef]
- NOAA. Local Climatological Data Annual Summary with Comparative Data; NOAA: Billings, MT, USA, 2019. [Google Scholar]
- Montana Department of Environmental Quality and US Department of Agriculture. Stillwater Mining Company’s Revised Water Management Plans and Boe Ranch LAD; Montana Department of Environmental Quality and US Department of Agriculture: Helena, MT, USA, 2012. [Google Scholar]
- Montana Deparment of Environmental Quality; USFS. Final Environmental Impact Statement for the Stillwater Mine Revised Waste Management Plan and Hertzler Tailings Impoundment; Montana Deparment of Environmental Quality: Helena, MT, USA, 1998. [Google Scholar]
- Bruker AXS. DIFFRACplus EVA 14 [Computer Software]. 2008. Available online: https://www.bruker.com/en/products-and-solutions/diffractometers-and-scattering-systems/x-ray-diffractometers/diffrac-suite-software/diffrac-eva.html (accessed on 4 August 2021).
- Dionex. ICS-2000 Ion. Chromatography System Operator’s Manual. 2006. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/manuals/Man-031857-ICS-2000-Ion-Chromatography-Man031857-EN.pdf (accessed on 4 August 2021).
- Lu, X. Characterization of Ultramafic Mine Tailings Reactivity for Carbon Capture and Storage. Master’s Thesis, The University of British Columbia, Vancouver, BC, Canada, 2020. [Google Scholar]
- De Baere, B.; François, R.; Mayer, K.U. Measuring mineral dissolution kinetics using on-line flow-through time resolved analysis (FT-TRA): An exploratory study with forsterite. Chem. Geol. 2015, 413, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Harrison, A.L.; Dipple, G.M.; Power, I.M.; Mayer, K.U. Influence of surface passivation and water content on mineral reactions in unsaturated porous media: Implications for brucite carbonation and CO2 sequestration. Geochim. Cosmochim. Acta 2015, 148, 477–495. [Google Scholar] [CrossRef]
- Sanna, A.; Steel, L.; Maroto-Valer, M.M. Carbon dioxide sequestration using NaHSO4 and NaOH: A dissolution and carbonation optimisation study. J. Environ. Manag. 2017, 189, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Lavikko, S.; Eklund, O. The role of the Silicate Groups in the Extraction of Mg with the ÅA route method. J. CO2 Util. 2016, 16, 466–473. [Google Scholar] [CrossRef]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Sepp, S. Composition of the Earth’s Crust. Sandatlas. 2012. Available online: https://www.sandatlas.org/composition-of-the-earths-crust/ (accessed on 7 January 2021).
- Yalçin, H.; Bozkaya, Ö. Mineralogy and geochemistry of Paleocene ultramafic- and sedimentary-hosted talc deposits in the southern part of the Sivas Basin, Turkey. Clays Clay Miner. 2006, 54, 333–350. [Google Scholar] [CrossRef]
- Turvey, C.C.; Hamilton, J.L.; Wilson, S.A. Comparison of Rietveld-compatible structureless fitting analysis methods for accurate quantification of carbon dioxide fixation in ultramafic mine tailings. Am. Mineral. 2018, 103, 1649–1662. [Google Scholar] [CrossRef]
- Casas, C.C.; Schaschke, C.J.; Akunna, J.C.; Jorat, M.E. Dissolution experiments on dolerite quarry fines at low liquid to solid ratio: A source of calcium for microbial- induced calcite precipitation. Environ. Geotech. 2019, 1–9, accepted manuscript. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. In U.S. Geological Survey Techniques and Methods, Book 6, Chapter A43; U.S. Geologic Survey: Denver, CO, USA, 2013; pp. 6–43A. [Google Scholar]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral. Interaction Kinetics for Application to Geochemical Modeling; U.S. Geologic Survey: Denver, CO, USA, 2004. [Google Scholar]
- Murakami, T.; Kogure, T.; Kadohara, H.; Ohnuki, T. Formation of secondary minerals and its effect on anorthite dissolution. Am. Mineral. 1998, 83, 1209–1219. [Google Scholar] [CrossRef]
- Stillings, L.L.; Brantley, S.L. Feldspar dissolution at 25 °C and pH 3: Reaction stoichiometry and the effect of cations. Geochim. Cosmochim. Acta 1995, 59, 1483–1496. [Google Scholar] [CrossRef]
- Shotyk, W.; Nesbitt, H.W. Incongruent and congruent dissolution of plagioclase feldspar: Effect of feldspar composition and ligand complexation. Geoderma 1992, 55, 55–78. [Google Scholar] [CrossRef]
- Thom, J.G.M.; Dipple, G.M.; Power, I.M.; Harrison, A.L. Chrysotile dissolution rates: Implications for carbon sequestration. Appl. Geochem. 2013, 35, 244–254. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part II Application of a new theory to silicates, aluminosilicates and quartz. Hydrometallurgy 2014, 149, 265–275. [Google Scholar] [CrossRef]
- Gadikota, G.; Matter, J.; Kelemen, P.; Brady, P.V.; Park, A.H.A. Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage. Fuel 2020, 277, 117900. [Google Scholar] [CrossRef]
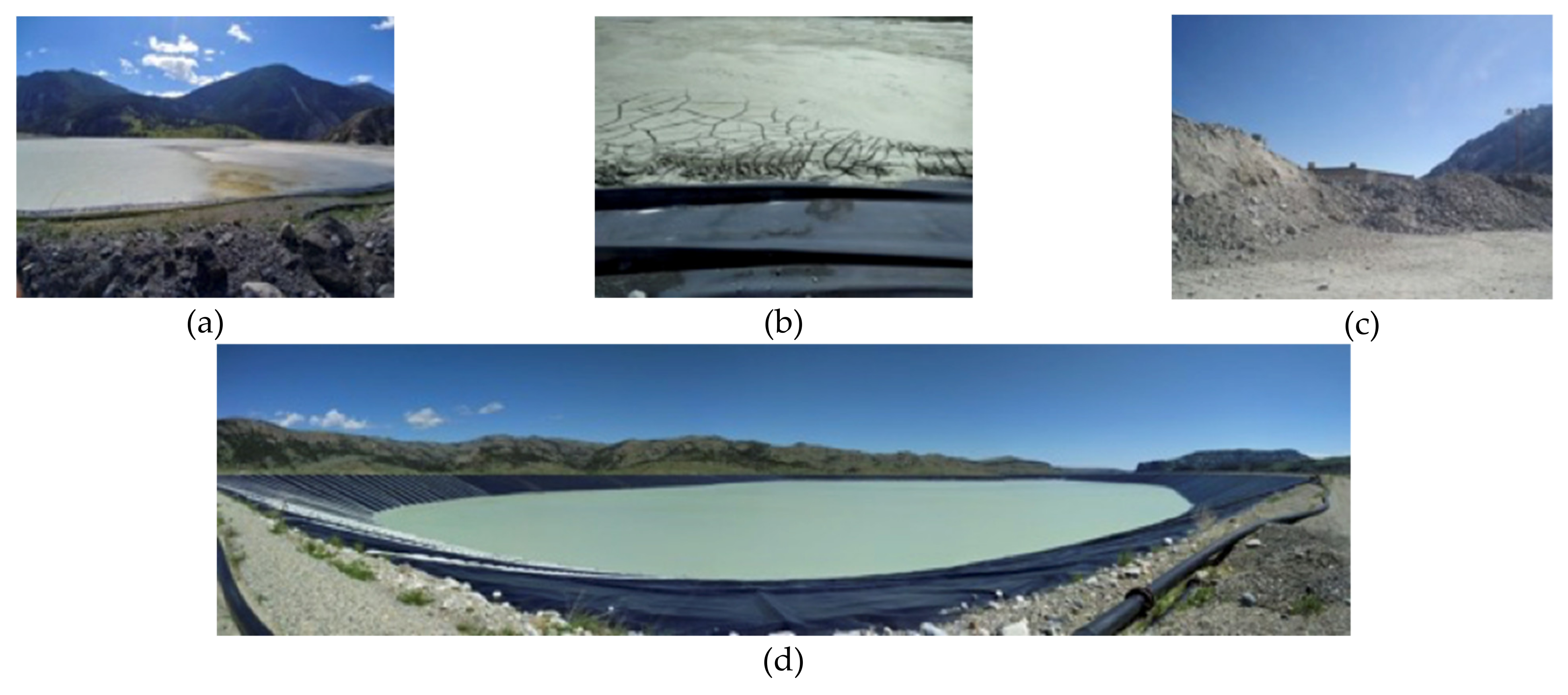
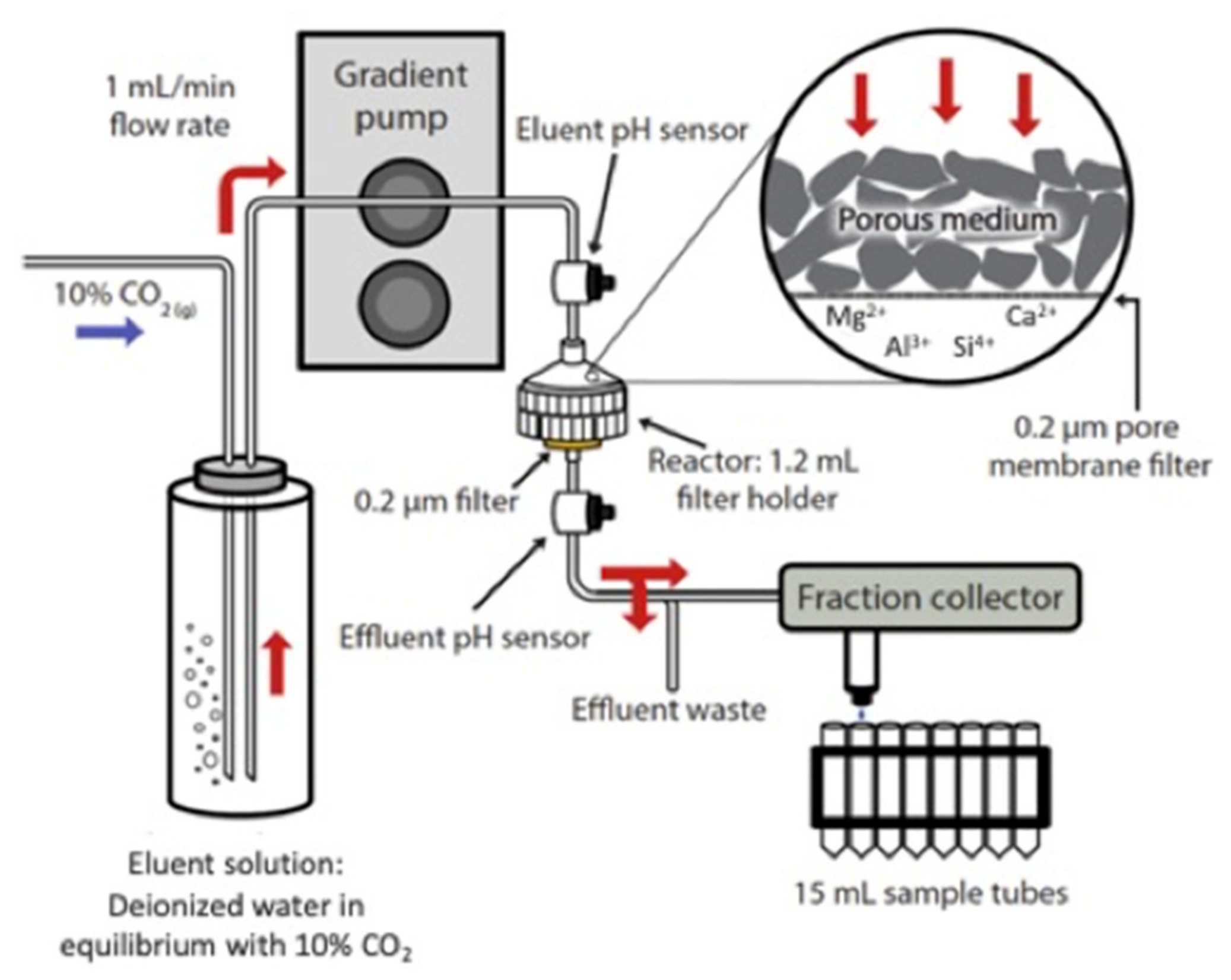
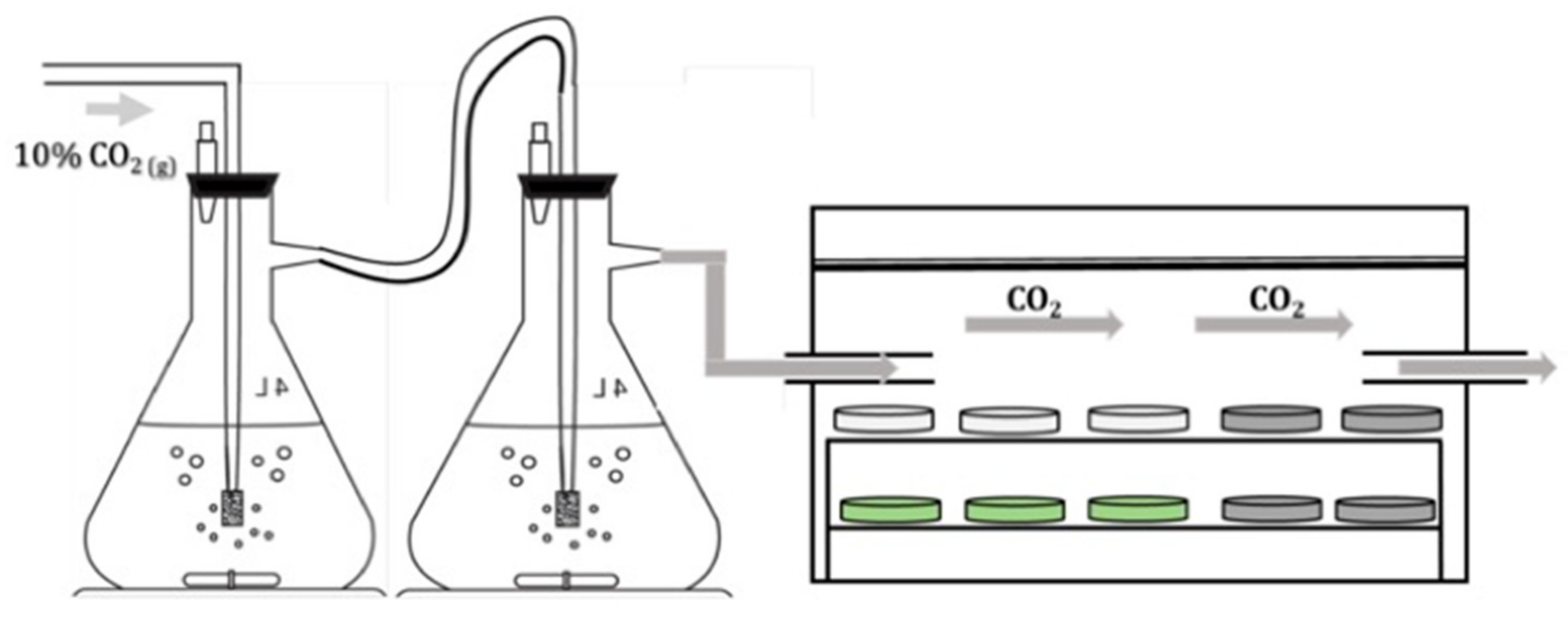
| Silicate Group | Mineral Type | Mineral | Tail 1 | Tail 2a | Tail 2b | Tail 2c |
|---|---|---|---|---|---|---|
| Tecto- (3-D framework) | Plagioclase Feldspar | Anorthite—CaAl2Si2O8 | 36.7 | 54.3 | 54.3 | 35.9 |
| Albite—NaAlSi3O8 | 3.2 | 3.9 | 3.7 | 5.6 | ||
| Ino- (chain) | Pyroxene | Enstatite—(Mg,Fe)Si2O6 | 10.1 | 11.9 | 14.7 | 4.5 |
| Diopside—CaMgSi2O6 | 11.5 | 9.7 | 9.3 | 6.5 | ||
| Amphibole | Tremolite—Ca2Mg5(Si8O22)(OH)2 | 3.0 | 2.2 | 2.6 | 2.4 | |
| Cummingtonite—Mg7(Si8O22)(OH)2 | 0.4 | 1.3 | 1.3 | 1.1 | ||
| Phyllo- (sheet) | Serpentine | Lizardite—Mg3Si2O5(OH)4 | 2.2 | 8.4 | 6.5 | 12.3 |
| Clay | Talc—Mg3Si4O10(OH)2 | 8.1 | 2.3 | 2.8 | 8.9 | |
| Mica | Clinochlore—(Mg,Fe)5Al2Si3O10(OH) | 21.7 | 6.0 | 4.9 | 20.6 | |
| N/A | Carbonate | Calcite—CaCO3 | 2.9 | - | - | - |
| Hydromagnesite—Mg5(CO3)4(OH)2-4H2O | - | - | - | 2.1 | ||
| TIC (% C) | 0.25 | 0.07 | 0.06 | 0.13 | ||
| d(0.9) (µm) | 20.79 | 51.17 | 60.71 | 9.75 | ||
| BET Reactive Surface Area (m2/g) | 3.8 | 1.29 | 0.69 | 11.15 | ||
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | LOI |
|---|---|---|---|---|---|---|---|---|
| Tail 1 | 45.07 | 18.34 | 5.93 | 11.50 | 11.40 | 1.07 | 0.14 | 5.95 |
| Tail 2a | 45.58 | 22.98 | 5.14 | 13.30 | 8.55 | 1.30 | 0.08 | 2.29 |
| Tail 2b | 46.44 | 22.91 | 5.12 | 13.55 | 8.43 | 1.29 | 0.06 | 1.90 |
| Tail 2c | 43.52 | 19.00 | 6.78 | 9.27 | 13.90 | 1.12 | 0.11 | 5.76 |
| Sample | BaO | Cr2O3 | MnO | P2O5 | SO3 | SrO | TiO2 | Total |
| Tail 1 | 0.02 | 0.16 | 0.10 | 0.01 | 0.14 | 0.01 | 0.12 | 99.96 |
| Tail 2a | 0.01 | 0.09 | 0.08 | <0.01 | 0.09 | 0.01 | 0.09 | 99.59 |
| Tail 2b | 0.01 | 0.12 | 0.08 | <0.01 | 0.07 | 0.01 | 0.09 | 100.08 |
| Tail 2c | 0.01 | 0.09 | 0.10 | 0.02 | 0.14 | 0.01 | 0.08 | 99.91 |
| Tailings Sample | Tailings Pond | Composition (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Al | Ca | Fe | K | Mg | Na | Si | ||
| Tail 1 | 1 | 0.65 | 156.47 | 0.44 | 7.04 | 53.58 | 189.20 | 11.20 |
| Tail 2a | 2 | 0.02 | 33.58 | 0.00 | 7.41 | 10.05 | 151.50 | 5.90 |
| ~Ratio Pond 1:2 | 31 | 5 | 113 | 1 | 5 | 1 | 2 | |
| Tailings Sample | Tailings Pond | Anions (mmol/L) | Cations (mmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl − | SO42− | NO3− | Al3+ | Ca2+ | Fe3+ | K+ | Mg2+ | Na+ | Si4+ | ||
| Tail 1 | 1 | 10.61 | 8.22 | 1.55 | 2.06 | 4.80 | 0.42 | 90.46 | 3.77 | 16.45 | 1.67 |
| Tail 2a | 2 | 3.56 | 3.78 | 3.46 | 0.01 | 0.87 | 0.00 | 15.86 | 0.41 | 12.10 | 0.19 |
| ~Ratio Pond 1:2 | 3 | 2 | 0 | 167 | 6 | 365 | 6 | 9 | 1 | 9 | |
| Tailings Sample | Tailings Pond | Charge Balance (meq/L) | ||
|---|---|---|---|---|
| Anion | Cation | Difference | ||
| Tail 1 | Pond 1 | 28.60 | 138.27 | 109.67 |
| Tail 2a | Pond 2 | 14.57 | 31.33 | 16.76 |
| ~Ratio Pond 1:2 | 2 | 4 | 7 | |
| Tailings Sample | Tailings Pond | pH | Alkalinity (meq/L) | |||
|---|---|---|---|---|---|---|
| Total | OH− | HCO3− | CO32− | |||
| Tail 1 | 1 | 9.68 | 96.91 | 0.06 | 37.02 | 35.59 |
| Tail 2a | 2 | 8.70 | 4.06 | 0.01 | 3.54 | 0.26 |
| Cation Ratio | Stoichiometric Ratio | Region II | Region III, Jump | Region III, Post-Jump |
|---|---|---|---|---|
| (14–32 h) | (33–36 h) | (37–58 h) | ||
| Ca2+/Al3+ | 0.5 | 0.6 | 1.1 | 0.5 |
| Ca2+/Si4+ | 0.5 | 1.4 | 0.7 | 1.3 |
| Al3+/Si4+ | 1 | 2.3 | 0.7 | 2.9 |
| Cation | Tail 2a Composition (Weight %) | Mass in Sample (mg) | Leached from Sample (mg) | Leached from Carbonate * (mg) | Leached from Silicate (mg) | Labile (Total) + (Weight %) | Labile (Silicate) ++ (Weight %) |
|---|---|---|---|---|---|---|---|
| Calcium | 9.50 | 47.65 | 1.35 | 1.07 | 0.28 | 2.83 | 0.59 |
| Magnesium | 5.16 | 25.86 | 0.03 | 0.03 | 0.00 | 0.11 | 0.00 |
| Sample | Initial TIC (Weight %) | Final TIC (Weight %) | Carbon Uptake (ΔTIC, Weight %) | Carbon Uptake (% Difference) |
|---|---|---|---|---|
| Tail 1 | 0.211 | 0.334 | 0.123 | 58 |
| Tail 2a | 0.059 | 0.086 | 0.027 | 46 |
| Tail 2b | 0.051 | 0.047 | −0.004 | −7 |
| Tail 2c | 0.110 | 0.163 | 0.053 | 48 |
| Efficiency Achieved | Carbonation Extent (%) | kg-CO2 per t-Tailings | Total t CO2/Year | |
|---|---|---|---|---|
| Stillwater | World * | |||
| (1 Mt Tailings/Yr) | (3.5 Gt Tailings/Yr) | |||
| This work | 1.0 | 1.79 | 1792 | 6,273,599 |
| Ref. [47] | 9.0 | 16.13 | 16,132 | 56,462,389 |
| Ref. [47] | 19.0 | 34.06 | 34,057 | 119,198,377 |
| Potential target | 50.0 | 89.62 | 89,623 | 313,679,938 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodall, C.M.; Lu, X.; Dipple, G.; Wilcox, J. Carbon Mineralization with North American PGM Mine Tailings—Characterization and Reactivity Analysis. Minerals 2021, 11, 844. https://doi.org/10.3390/min11080844
Woodall CM, Lu X, Dipple G, Wilcox J. Carbon Mineralization with North American PGM Mine Tailings—Characterization and Reactivity Analysis. Minerals. 2021; 11(8):844. https://doi.org/10.3390/min11080844
Chicago/Turabian StyleWoodall, Caleb M., Xueya Lu, Gregory Dipple, and Jennifer Wilcox. 2021. "Carbon Mineralization with North American PGM Mine Tailings—Characterization and Reactivity Analysis" Minerals 11, no. 8: 844. https://doi.org/10.3390/min11080844
APA StyleWoodall, C. M., Lu, X., Dipple, G., & Wilcox, J. (2021). Carbon Mineralization with North American PGM Mine Tailings—Characterization and Reactivity Analysis. Minerals, 11(8), 844. https://doi.org/10.3390/min11080844





