Abstract
Analyzing the chemical composition of archaeological glasses can provide an insight into their provenance and raw materials used in their making. However, to the authors’ knowledge, the historical production process itself and melting characteristics of the glasses have not yet been extensively investigated. The main focus of this paper is to describe the melting process of three main types of Bohemian historical glasses: Gothic (14th–1st half of 16th c.); Renaissance (16th–17th c.); and Baroque (end of 17th–18th c.). The model glasses were prepared from natural raw materials and processes that take place during melting were investigated using optical microscopy, SEM-EDS, XRD, and DTA-TG methods. Furthermore, the viscosity of model glasses and thermal dilatation was measured and used to calculate the reference viscosity points. The results illustrate the complexity of historical glass melting, as well as the technological progress between different periods.
1. Introduction
Central European glass production played an important role in Medieval and post-medieval Europe, competing with the Italian glassworks. The glass produced in Central Europe was made with wood ash and wood ash potash (leachate from wood ash rich in K2CO3) as fluxing agents, yielding glass that can be categorized as potassium-calcium type [1,2,3,4], as opposed to the Mediterranean sodium-calcium glass produced with natron or sodium-rich plant ashes [5,6]. In the present day, sodium-calcium glass has been extensively researched, but there is still a huge knowledge gap about their potassium-calcium counterparts and their production technology. This work aims to expand knowledge about the production process of potassium-calcium glasses by analyzing the melting process of model glasses and their properties.
Analytical research on archaeological potassium-calcium glass provides valuable information about the chemical composition, provenance, and, partly, about the raw materials used throughout history. There have been few attempts to reconstruct the historical glassmaking technology using model glasses [1,7,8,9,10] or even replicas of historical glassmaking furnaces [11,12,13,14,15]. These experiments usually aim to prove previous theories and confirm information from surviving historical texts about the materials, recipes, and glassmaking technologies in different historical eras and different areas. However, only a handful of published works discuss the actual production process and melting characteristics of historical glasses [16,17,18,19]. Sometimes, surviving production waste glass from work sites can provide an insight into the production technology too [20,21,22].
To reconstruct historical methods of glassmaking, one must start from the analytical studies of archaeological glasses and raw materials that were available to glassmakers in history in a particular area. The glass recipe (influenced by different raw materials), and therefore the chemical composition of the glass, can vary even in the context of one area, as can be seen in Central European glasses. These are often put into one category, but in fact there are clear differences between glasses from different Central European countries, e.g., Germany, France, or Bohemia [1,4].
The chemical composition of naturally derived materials, especially various types of wood ashes, depends on their area of origin and other factors such as the time of harvest [23,24].
The raw materials and their ratios directly influence the melting process and properties of the melt, and, subsequently, the temperature dependence of viscosity influence the workability options. The effect of different components in the mixture is complex but crucial to the whole glassmaking process [19,25]. The key parameters are the melting temperature, viscosity, and presence of any glass defects. The melting temperature of historical glasses must have been lower or equal to the maximal temperature that could be sustained in the glassmaking furnace. Through experiment, the highest temperature of medieval furnaces was determined to be up to 1300 °C [12,22]. Baroque furnaces were built in a slightly different way, which allowed them to achieve higher temperatures. Glass recipes were formed to respect the temperature limitations, and with the improvement in furnace construction we can also observe an increase in SiO2 content in the archaeological glasses [26,27]. The temperature dependence of melt viscosity determines the workability interval and glass shaping options. In general, additions of SiO2 or Al2O3 cause higher viscosity of the melt and higher melting temperature; alkalis or PbO have the opposite effect [25].
Generally, potassium-calcium glasses have short workability intervals and a steep viscosity curve, which did not allow long shaping of the glass. Sodium glasses with a slow viscosity curve allow a long shaping time. Thinner vessels decorated with intrigue plastic ornaments or engraving can be made from sodium glass, while potassium glass is more suitable for less decorated glass objects with thicker walls that are more suitable for cutting [25,27].
In this study we aimed to determine important melting properties of potassium-calcium glasses produced in Bohemia through Gothic (14th–1st half of 16th c.), Renaissance (16th–17th c.), and Baroque (end of 17th–18th c.) eras and characterize their melting process using model glasses. The reasoning behind the chosen glass types and the calculations that led to the model glasses can be found in the paper previously published by the authors [1]. Central European and particularly Bohemian glass production played an important role in historical glassmaking and trade, but the melting process of European potassium-calcium glasses has not been thoroughly investigated and is often extrapolated from the data of sodium-calcium glasses [19]. The findings of this experimental study provide insight into the whole production technology of historical glasses, starting from the raw materials and their changes during the melting, to the final workability characteristics of the glasses.
2. Materials and Methods
2.1. Glassmaking Materials
In order to get the most authentic insight into the melting process and properties of Bohemian historical glasses, natural raw materials were used to mix the model glass batches. These were natural quartz and sand, beech ash, and limestone. Instead of beech ash potash (leachate from beech ash), chemically pure K2CO3 (p.a., Penta, s.r.o.) was used. This change is justified because K2CO3 makes up more than 90 wt.% of beech ash potash, as proved by chemical analyses. For materials that are used in small quantities as additives (NaCl, As2O3), we also chose to use commercially available laboratory materials. NaCl p.a. was supplied by Penta, s.r.o. and As2O3 p.a. by Sigma-Aldrich.
2.2. Model Glass Melting
The melting process and properties were examined on samples that closely represented the three main types of Bohemian historical glasses: Gothic (14th c.–1st half of 16th c.),=; Renaissance (2nd half of 16th c.–3rd quarter of 17th c.); and Baroque (3rd quarter of 17th c.–18th c.). The model glass recipes were calculated from the average composition of Bohemian archaeological glasses from each period. The glasses were melted using natural raw materials that were available in the relevant historical time period. The chemical composition of model glasses, analyzed by XRF, showed a very good correlation with the archaeological finds.
The recipes used for model glasses and their final chemical composition can be found in Table 1 and Table 2. More in-depth information about the particular glasses and their calculated recipes can be found in a previous paper published by the authors [1].

Table 1.
Weight ratios of raw materials used to mix model glass batches [g].

Table 2.
Chemical composition of model and archaeological glasses [wt.%] [1].
The individual samples (5 g batch each) were exposed to temperatures of 800, 900, 1000, 1100, 1200, 1300, and 1400 °C. The glasses were melted in PtRh crucibles in an electric furnace with an oxidizing atmosphere for 180 min. After that, the samples were quenched in water to conserve the phase composition.
For the viscosity and thermal expansion measurements, 300 g samples of model glasses were melted in a porcelain crucible placed inside a PtRh crucible in an electric furnace at 1400 °C for 240 min. The glass was mixed two times during the melting, after 180 and 210 min. The samples were cast into a stainless-steel mold and placed into an annealing furnace at 520 °C for 60 min, then cooled at the rate of 5 °C.
2.3. Optical Microscopy
The overall structure and degree of homogenization of samples were examined using optical microscopy. The samples were fixed into colorless epoxy resin, ground, and polished to form a cross-section. The optical microscope Olympus BX 51, equipped with the Olympus E-600 camera and software QuickPhoto Camera 2.3 was used. The cross-sections were studied in both transmitted and incident light.
2.4. X-ray Diffraction Analysis (XRD)
The XRD analysis was used to obtain the phase composition of prepared samples. Samples were measured in the form of very fine powders. The analysis was performed on the X’PertPRO MPD diffractometer (PANalytical) at the Institute of Inorganic Chemistry of the Czech Academy of Sciences. The measurement used CuKα radiation (40 kV, 30 mA), fast linear detector PIXcel, and reflexive Bragg–Brentano setting. The primary beam was focused using a 10 mm mask and 0.04 rad Soller collimator aperture, the diffracted beam was focused using an anti-scattering collimator on 0.5°, 0.04 rad Soller collimator, and Ni beta-filter. The measurement range was 7–90° 2θ and the time was 800 s per step of 0.013°. The data were evaluated by the HighScore Plus 4.6.1 software, together with the PDF-4 + [28].
2.5. Scanning Electron Microscopy (SEM-EDS)
To identify the structure and different phases better, scanning electron microscopy was used together with energy dispersive spectroscopy. The samples were prepared in the form of polished cross-sections in epoxy resin. To study the samples, the electron microscope JSM 6510 (JEOL Ltd.,Tokyo, Japan) with the EDS and SSD detector Inca (Oxford Instruments, Abingdon, UK) was used. The measurement was conducted in the backscattered electrons (BSE) mode at 20 kV and low pressure of 30 Pa. This allowed us to use the samples without any metal coating.
2.6. Thermogravimetric Analysis/Differential Thermal Analysis (TG/DTA)
The individual model glass batches were also studied by the thermogravimetric analysis. The batch samples were used in the form of very fine powders. The analysis itself was conducted in the thermal interval of 30–1300 °C with a heating rate of 5 °C per minute. The samples were measured in the oxidating and inert (nitrogen) atmosphere using the STA 449 F1 Jupiter (Netzsch, Germany). The measured data were then evaluated in graphical form.
2.7. Viscosity Measurement
The viscosity curves of the model glass melts were measured in the interval of 102.5 to 104 dPas. The measurement was conducted using an in-house falling ball viscosimeter that was developed at the Department of Glass and Ceramics at UCT Prague. Before the measurement, the viscosimeter was calibrated using a standard glass (Standard Reference Material 710a, Soda-Lime-Silica glass, National Institute of Standards and Technology, Gaithersburg, MD, USA).
A PtRh crucible with about 70 g of glass was placed in the viscosimeter and heated to 1440 °C. At this temperature, PtRh rod with two balls at each end was placed with a lower end 0.5 cm under the surface of the molten glass. Then, the time necessary to submerge the lower ball 2 cm under the surface of the glass was measured using an electrical stopwatch. The measurement was conducted periodically while lowering the temperature (50 °C steps) until the viscosity was too high to allow further measurement.
The data obtained were used for calculating the equation of viscosity-temperature dependence (Andrieu’s formula) using the KING software. In addition, viscosity reference points were calculated from the equation. The A and B constants of Andrieu’s Equation (1) were calculated using the least-squares method.
By using Andrieu’s equation, we can achieve a highly precise extrapolation of the measured values. The constant A is the viscosity limit when temperature T approaches zero, and B represents the steepness of a viscosity curve.
2.8. Thermal Expansion
The thermal expansion of model glasses was measured with the dilatometer DI 24 (Adamel Lhomargy) in the temperature range from 17 to 720 °C. Glass rods (50 mm long with radius 5 mm) were drawn from the model glass melts. The heating rate during the experiment was 3 °C per minute.
The data obtained were analyzed using the LOGIDIL 4.2 software to calculate the expansion coefficient, transformation (Tg), and deformation (TD) temperatures.
3. Results and Discussion
3.1. Phase Composition of the Starting Batches
The raw materials used to prepare the model glass batches according to the calculated recipes were analyzed using XRD to determine their phase composition (see Table 3).

Table 3.
Phase composition of natural raw materials (XRD).
Model Gothic starting glass batch was made up of natural quartz (with 98.9 wt.% of SiO2) with minor impurities of feldspar minerals albite (NaAlSi3O8) and orthoclase ((K0.92Na0.08)(AlSi3O8)). The beech ash contained mostly potassium and calcium salts—fairchildite (K2Ca(CO3)2), a small amount of arcanite (K2SO4), and calcite (CaCO3); quartz and silicious hydroxyapatite were present in minor quantities (both less than 2 wt.%). The originally-used beech ash potash was fully replaced by pure K2CO3.
The Renaissance model glass batch was mixed with natural quartz, beech ash, K2CO3, and an addition of natural limestone. The limestone was mostly made up of calcite (CaCO3) with impurities of dolomite (CaMg(CO3)2), quartz and muscovite (KAl2(Si,Al)4O10(OH)2), all less than 3 wt.%, according to XRF (see Supplementary Material Table S1 or [1]). There was also a small addition of NaCl.
The Baroque model glass was formed from different raw materials to simulate the precise process of using as pure materials as possible to obtain colorless glass. The natural quartz was replaced by glassmaking sand (pure SiO2), K2CO3 to simulate highly purified beech ash potash, and the same limestone as in previous model glasses. The Baroque model glass also contained an addition of As2O3, acting as a refining agent as well as a decolorizer.
3.2. Melting Process and Changes in Phase Composition
Before any solid-phase reactions and first glassy phase formation, decomposition of carbonates took place, starting at 600–650 °C. This process was accompanied by a great loss of mass (around 13 wt.%, see Figure 1, Figure 2 and Figure 3) due to CO2 leaving the samples.
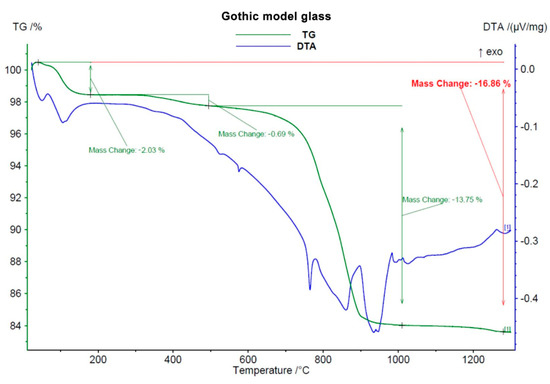
Figure 1.
Course of DTA-TG analysis of Gothic model glass.
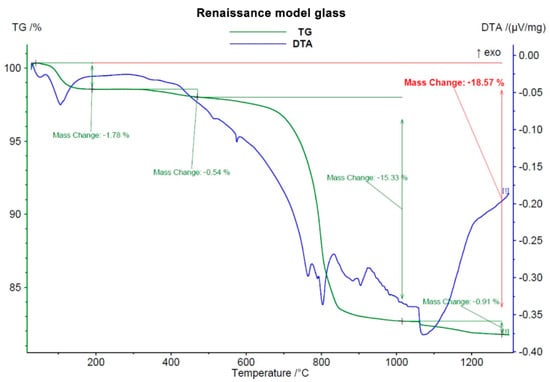
Figure 2.
Course of DTA-TG analysis of Renaissance model glass.
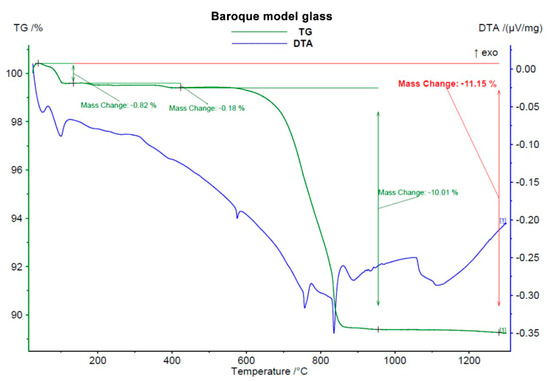
Figure 3.
Course of DTA-TG analysis of Baroque model glass.
The solid-phase reactions started appearing in the glasses at around 700 °C. During this period, various silicates were forming. Mostly, these were potassium and calcium silicates, usually mixed. An interesting phenomenon occurred in Gothic glass. There wwere several layers of bi-product which formed around the quartz core (see Figure 4—SEM picture). The layer closest to the core was made up of potassium silicates, however, further away from the core, the chemical composition changed to calcium silicates. Seemingly, potassium silicates formed first and after that, calcium started incorporating itself into the new layers. A similar effect could be seen in the Renaissance model glass (see Figure 5). In the Baroque model glass, the solid phase reaction products produced only one layer, mostly from mixed potassium-calcium silicates. The solid-phase reactions were finished by 1000–1100 °C when there were no raw materials left to react.
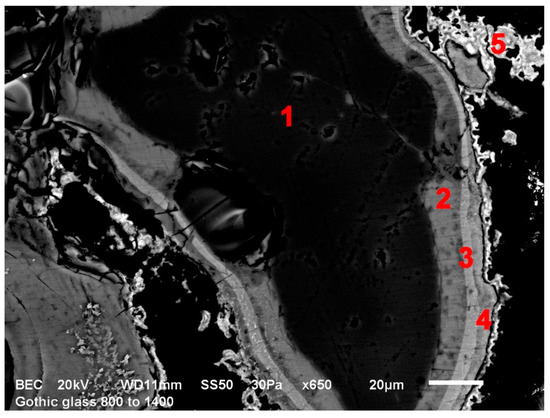
Figure 4.
Model Gothic glass exposed to 800 °C, bi-product layers around SiO2, SEM; 1—SiO2 core, 2—potassium silicates, 3—potassium-calcium silicates, 4—calcium-potassium silicates, 5—calcium silicates.
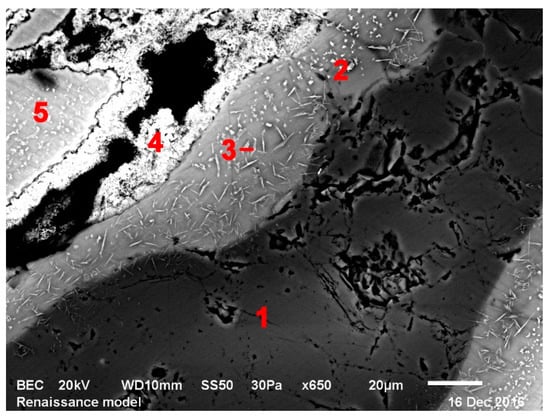
Figure 5.
Model Renaissance glass exposed to 800 °C, bi-product layers around SiO2, SEM; 1—SiO2 core, 2, 3, 5—potassium-calcium silicates, 4—calcium-potassium silicates.
At 900 °C, the first glassy phase appeared in the Gothic model glass. The formation of mixed silicates continued and their chemical composition shifted to more calcium-based ones.
First, the glassy phase of Renaissance and Baroque model glasses formed at 1000 °C (although TG/DTA analysis of Baroque model glass puts it at 1150 °C, it was visually detected at 1000 °C). This temperature also marks the formation of wollastonite (CaSiO3) crystals in both glasses (Figure 6). Wollastonite was observed even by the optical microscope (Figure 7) in the form of needle-shaped crystals in the glass mass. These were more prevalent in Renaissance glass, as it contains the highest amount of calcium of the three and were not detected in the Gothic glass at all, caused by the low amount of calcium in these. The presence of Wollastonite was previously observed in archaeological production waste glasses [19].
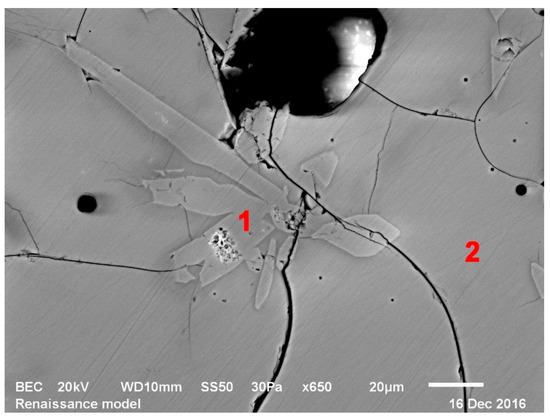
Figure 6.
Wollastonite crystals in Renaissance model glass, 1000 °C, SEM; 1—wollastonite, 2—glass.
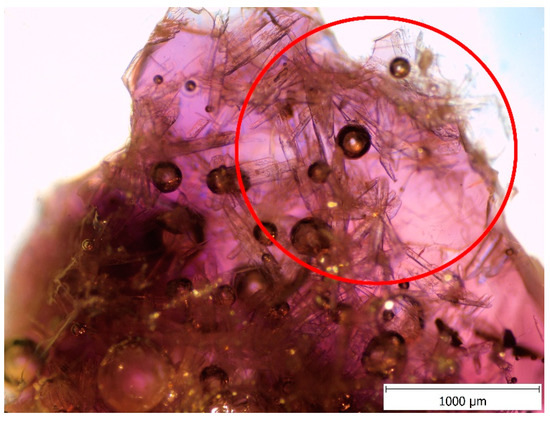
Figure 7.
Wollastonite crystals in Renaissance model glass, OM.
All three modifications of SiO2—quartz, cristobalite, and tridymite—were present together in the Gothic and Baroque model glass at 1100 °C. They were still in the form of solid grains. In Renaissance and Baroque model glasses, the temperature of 1100 °C signified the first appearance of visible bubbles in the glass matrix.
At the higher temperatures of 1200 and 1300 °C, there were no new phases. The melting of the remaining SiO2 particles continued. The last crystals of wollastonite disappear from Renaissance and Baroque glasses, but bubbles remained. At 1400 °C, all glasses were completely melted. Gothic and Renaissance glasses were homogeneous; Baroque glass still showed some bubbles that would need a longer melting time to disappear. An overview of all processes can be found in Table 4.

Table 4.
Overview of the most important changes in phase composition and homogeneity during melting of model glasses.
3.3. Viscosity
During melting, Gothic and Renaissance glassmelts behaved very similarly. They are potassium-calcium glasses, so a short workability interval and higher viscosity of melt were expected (as opposed to sodium glasses). The Baroque glass showed much higher viscosity than the previous two, therefore it was difficult to homogenize and cast this glass. The measured viscosity curves in Figure 8 illustrate this very well.

Figure 8.
Dependence of viscosity on temperature for model glasses.
Following viscosity equations were obtained:
Gothic model glass:
Renaissance model glass:
Baroque model glass:
Reference viscosity temperatures calculated from the equations are given in Table 5.

Table 5.
Reference viscosity points for model glasses [°C].
The Gothic and Renaissance model glasses displayed similar behaviors. Both viscosity curves are quite steep, which correlates with their chemical composition. For Gothic model glass, the theoretical melting temperature was calculated for 1437 °C and the workability interval at 1211–798 °C. The Renaissance model glass had a theoretical melting point at 1365 °C and a workability interval between 1169–802 °C. The lower melting temperature for Renaissance glass is caused by a different CaO/K2O ratio. The intervals for both glasses are relatively short, which would make it difficult to form thin-walled objects. This fact can also be seen in archaeological glasses, where Bohemian glasses show medium to thick walls, especially when compared with soda-type glasses from Italy.
The steepness of the viscosity curves decreases from Renaissance glass to Baroque glass. The reason is the decrease in CaO concentration. The Baroque model glass shows a much higher theoretical melting temperature of 1690 °C. It is necessary to mention that this temperature was obtained by extrapolation. The model Baroque glass has previously been successfully melted at 1400 °C; therefore, the actual melting temperature was probably lower than the extrapolated one. The workability interval between 1379–837 °C would provide more time for glass forming. The overall high viscosity of this type of glass makes it suitable for thick-walled objects decorated with cutting, which it was mostly used for in history.
In general, the viscosity of glass melts decreases when K2O and/or CaO increases. This is caused by decreasing the concentration of bridging and increasing the concentration of non-bridging oxygen atoms. On the other hand, SiO2 strongly increases viscosity because it increases the concentration of bridging and decreases the concentration of non-bridging oxygen atoms. Therefore, the viscosity of Renaissance glass melts is the lowest because of the highest content of CaO and high content of K2O, and the highest viscosity of Baroque glass is caused by the highest content of SiO2 and the lowest content of K2O and CaO.
3.4. Thermal Expansion
The behavior at lower temperatures is also similar for Gothic and Renaissance model glasses (see Figure 9). The Baroque model glass shows lower thermal dilatation than the other two.
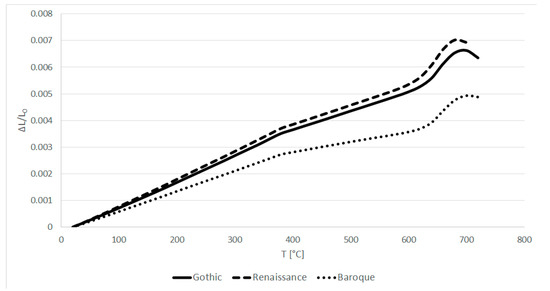
Figure 9.
Thermal expansion curves of model glasses.
The glass transformation temperature Tg and deformation temperature (TD) of model glasses are given in Table 6 together with the calculated dilatation coefficient α.

Table 6.
Thermal expansion data for model glasses.
Similar to the viscosity, when K2O and/or CaO increase or SiO2 decreases the transformation temperature of glass decreases, and the thermal expansion coefficient increases. Therefore, Renaissance model glass has both the lowest transformation temperature and the highest thermal expansion coefficient. On the other hand, Baroque model glass has the highest transformation point and the lowest expansion coefficient. This correlates with the viscosity data, where Renaissance glass shows the lowest viscosity in this temperature interval, and the viscosity of Baroque glass is much higher.
4. Conclusions
This study was the first step to successfully reconstruct the historical production technology of Bohemian glasses. The experimental melting shows that the model glasses would theoretically need temperatures higher than 1400 °C to completely melt. However, the melting temperatures can be lowered by prolonging the melting times, making it possible to produce homogeneous glasses of this chemical composition in history. The addition of a glass cullet or recycled glass can also lower the temperature and is highly probable. The time-temperature dependence of melting is most important in the case of Baroque glass, with a calculated melting temperature above 1600 °C. This value is theoretical and even though we can expect better furnace constructions, and therefore higher achievable temperatures than in the Gothic or Renaissance periods, the glasses were probably melted at temperatures not higher than 1400 °C. However, the melting process in history was not always perfect, as can often be seen in archaeological glasses containing various bubbles or the residuals of solid particles.
The viscosity of Bohemian historical glass melts is influenced by their chemical composition, making them more viscous than soda-type glasses. These glasses were suitable for the production of glass objects decorated by cutting or engraving, but not very suitable for the plastic decorations known from, e.g., Venetian glasses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/min11080829/s1, Figure S1: XRD data of quartz, Figure S2: XRD data for limestone, Figure S3: XRD data for beech ash, Figure S4: XRD data for Gothic model glass, Figure S5: XRD data for Renaissance model glass, Figure S6: XRD data for Baroque model glass, Table S1: XRF analysis of raw materials, Table S2: Viscosity data obtained during the experiment.
Author Contributions
Conceptualization and methodology, K.P. and D.R.; validation, D.R.; formal analysis, K.P., D.R., F.L., D.G. and M.M.; investigation, K.P. and K.J.; data curation, K.P. and D.R.; writing—original draft preparation, K.P.; writing—review and editing, K.P., D.R. and K.J.; visualization, K.P.; supervision, D.R.; project administration, D.R.; funding acquisition, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant of the Czech Science Foundation No. GA 19-05677S: Glass in the Czech Lands from the Gothic to the Baroque, in the Light of Finds from Chrudim and Brno. The importance of regional production in the European context.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its Supplementary files.
Acknowledgments
The authors would like to thank Petr Bezdička for the XRD measurement. This paper is also a part of a dissemination of the activities arising from the FunGlass project. This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 739566.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pánová, K.; Rohanová, D.; Randáková, S. Modeling of Bohemian and Moravian glass recipes from Gothic to Baroque periods. Heritage Sci. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Wedepohl, K.H.; Simon, K. The chemical composition of medieval wood ash glass from Central Europe. Geochemistry 2010, 70, 89–97. [Google Scholar] [CrossRef]
- Schalm, O.; Janssens, K.; Wouters, H.; Caluwé, D. Composition of 12–18th century window glass in Belgium: Non-figurative windows in secular buildings and stained-glass windows in religious buildings. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 663–668. [Google Scholar] [CrossRef]
- Adlington, L.; Freestone, I.; Kunicki-Goldfinger, J.; Ayers, T.; Scott, H.G.; Eavis, A. Regional patterns in medieval European glass composition as a provenancing tool. J. Archaeol. Sci. 2019, 110, 104991. [Google Scholar] [CrossRef]
- Verità, M.; Zecchin, S. Thousand years of Venetian glass: The evolution of chemical composition from the origins to the 18th century. Ann. L’association Int. pour L’histoire Verre 2009, 17, 602e613. [Google Scholar]
- Cagno, S.; Janssens, K.; Mendera, M. Compositional analysis of Tuscan glass samples: In search of raw material fingerprints. Anal. Bioanal. Chem. 2008, 391, 1389–1395. [Google Scholar] [CrossRef]
- Cílová, Z.; Woitsch, J. Potash—A key raw material of glass batch for Bohemian glasses from 14th–17th centuries? J. Archaeol. Sci. 2012, 39, 371–380. [Google Scholar] [CrossRef]
- Stern, W.B.; Gerber, Y. Potassium-Calcium Glass: New Data and Experiments. Archaeometry 2004, 46, 137–156. [Google Scholar] [CrossRef]
- Jackson, C.; Booth, C.A.; Smedley, J.W. Glass by Design? Raw Materials, Recipes and Compositional Data. Archaeometry 2005, 47, 781–795. [Google Scholar] [CrossRef]
- Paynter, S.; Dungworth, D. Recognising Frit: Experiments Reproducing Post-Mediev. Plant Ash Glass. 2010, 133–138. [Google Scholar] [CrossRef]
- Jedlička, J.; Knápek, A. Stavba repliky středověké sklářské a pomocné fritovací pece v Havlíčkově Brodě—Východiska, kon-strukce, stavba (Construction of a replica of Medieval glass and auxiliary frit furnaces in Havlíčkův Brod—Bases, construc-tion, process of constructing). Sklář Keram. 2017, 67, 100–104. [Google Scholar]
- Jedlička, J.; Knápek, A.; Rohanová, D. Experimentální středověká sklářská a fritovací pec v Havlíčkově Brodě—Příprava dřeva, výroba frity a tavba skla (Construction of Medieval glass and frit furnace in Havlíčkův Brod—Wood preparation, frit production and glass melting). Sklář Keram. 2018, 68, 58–63. [Google Scholar]
- Wiesenberg, F. Experimentelle Archoeologie: Roemische Glasoefen. Rekonstruktion und Betrieb Einer Glashuette Nach Roemischem Vorbild in der Villa Borg. Borg Furnace Project 2013; Archäologieparks Römische Villa Borg: Perl, Germany, 2014. [Google Scholar]
- Erná, E. Experimentální stavba středověké sklářské pece na otop dřevem (Experimental construction of Medieval glass fur-nace heated by wood). Archaeol. Hist. 1993, 18, 419–424. [Google Scholar]
- Erná, E.; Kirsch, R.; Štrojsa, J. Třetí experimentální tavba skla v rekonstruované středověké peci—Moldava 1994 (Third ex-perimental glass melting in the reconstructed Medieval glass furnace). Sklář Keram. 1995, 45, 229–234. [Google Scholar]
- Smedley, J.W.; Jackson, C.M.; Booth, C.A. Back to the roots: The raw materials, glass recipes, and glassmaking practices of Theophilus. In The Prehistory and History of Glassmaking Technology. Ceramics and Civilization 8; McCray, P., Kingery, W.D., Eds.; American Ceramic Society: Westerville, OH, USA, 1998; pp. 145–165. ISBN 978-1574980417. [Google Scholar]
- Jackson, C.M.; Smedley, J.W. Medieval and post-medieval glass technology: Melting characteristics of some glasses melted from vegetable ash and sand mixtures. Glass Technol. 2004, 45, 36–42. [Google Scholar]
- Tanimoto, S.; Rehren, T. Interactions between silicate and salt melts in LBA glassmaking. J. Archaeol. Sci. 2008, 35, 2566–2573. [Google Scholar] [CrossRef]
- Hunault, M.O.J.Y.; Vinel, V.; Cormier, L.; Calas, G. Thermodynamic insight into the evolution of medieval glassworking properties. J. Am. Ceram. Soc. 2017, 75, 57–2367. [Google Scholar] [CrossRef]
- Riccardi, M.P.; Marchesi, V.; Messiga, B. Melting path-ways of medieval glass from Certosa di Pavia (Italy). Thermochim. Acta 2005, 425, 127–130. [Google Scholar] [CrossRef]
- Messiga, B.; Riccardi, M.P. A petrological approach to the study of ancient glass. Period. Mineral. 2001, 70, 57–70. [Google Scholar]
- Messiga, B.; Riccardi, M.P.; Rebay, G.; Basso, E.; Lerma, S. Microtextures recording melting-history of a medieval glass cake. J. Non-Cryst. Solids 2004, 342, 116–124. [Google Scholar] [CrossRef]
- Stern, W.B.; Gerber, Y. Ancient potassium-calcium glass and its raw materials (wood-ash, fern-ash, potash) in Central Europe. Mitt. Nat. Ges. Beider Basel 2009, 11, 107–122. [Google Scholar]
- Jackson, C.M.; Smedley, J.W. Medieval and post-medieval glass technology: Seasonal changes in the composition of bracken ashes from different habitats through a growing season. Glass Technol. Eur. J. Glass Sci. Technol. A 2008, 49, 240–245. [Google Scholar]
- Heis, R. Vývoj českého křišťálového skla z pohledu sklářské technologie (Od baroka do 19. století) (Development of Czech Crystal Glass in Terms of Technology—From the Baroque to the 19th Century). Sklář Keram. 2017, 67, 51–58. [Google Scholar]
- Hlaváč, J. The Technology of Glass and Ceramics; Elsevier: Amsterdam, The Netherlands, 1983; ISBN 978-0444996886. [Google Scholar]
- Sedláčková, H.; Rohanová, D. Renaissance and Baroque Glass from the Central Danube Region; Archaia Brno: Brno, Czech Republic, 2016; ISBN 978-80-905546-5-8. [Google Scholar]
- Kabekkodu, S. (Ed.) Powder Diffraction File, International Centre for Diffraction Data; ICDD: Newton Square, PA, USA, 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).