Selective Separation of Chalcopyrite from Galena Using a Green Reagent Scheme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Flotation Experiments
2.3. Zeta Potential Tests
2.4. FT-IR Tests
3. Results and Discussion
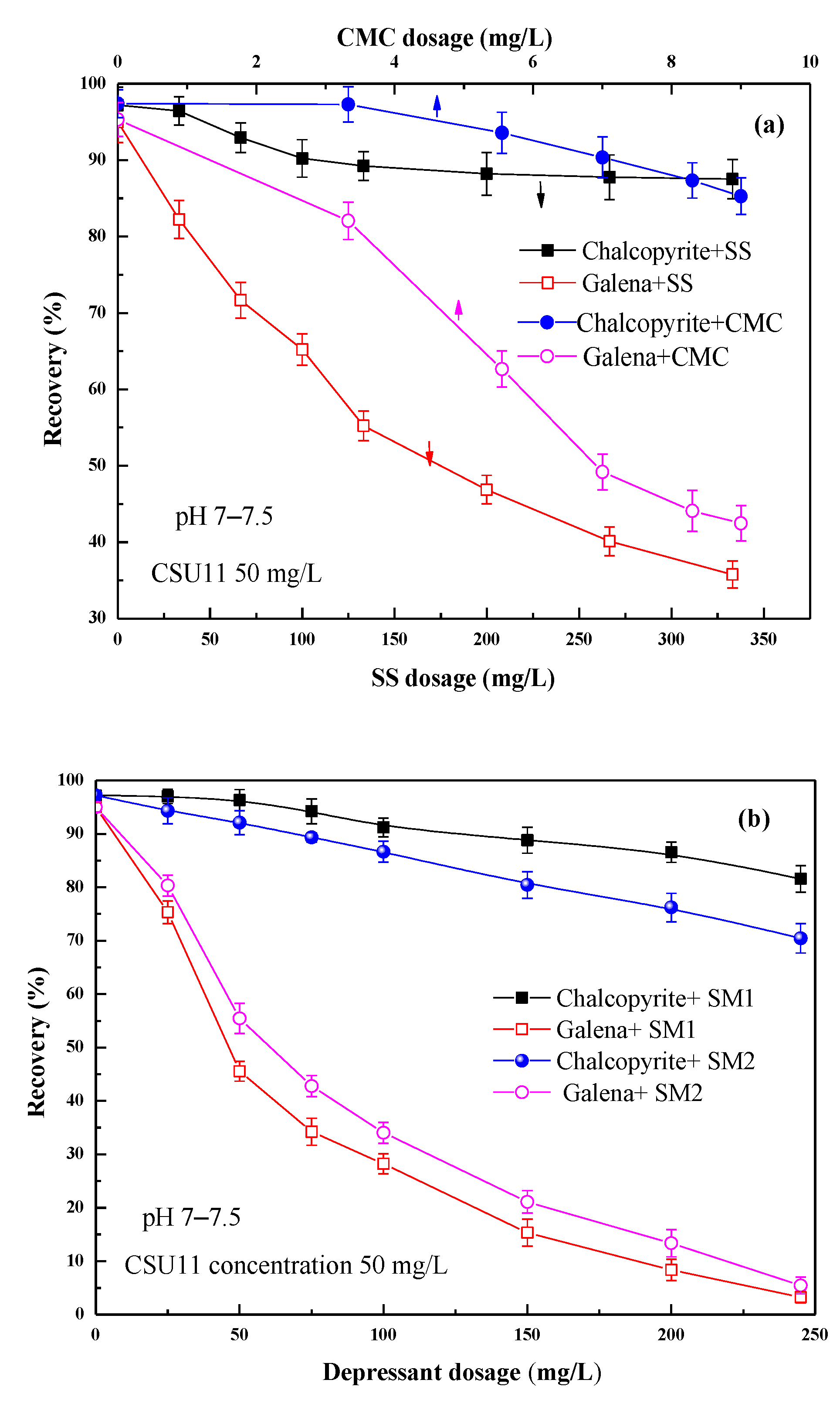
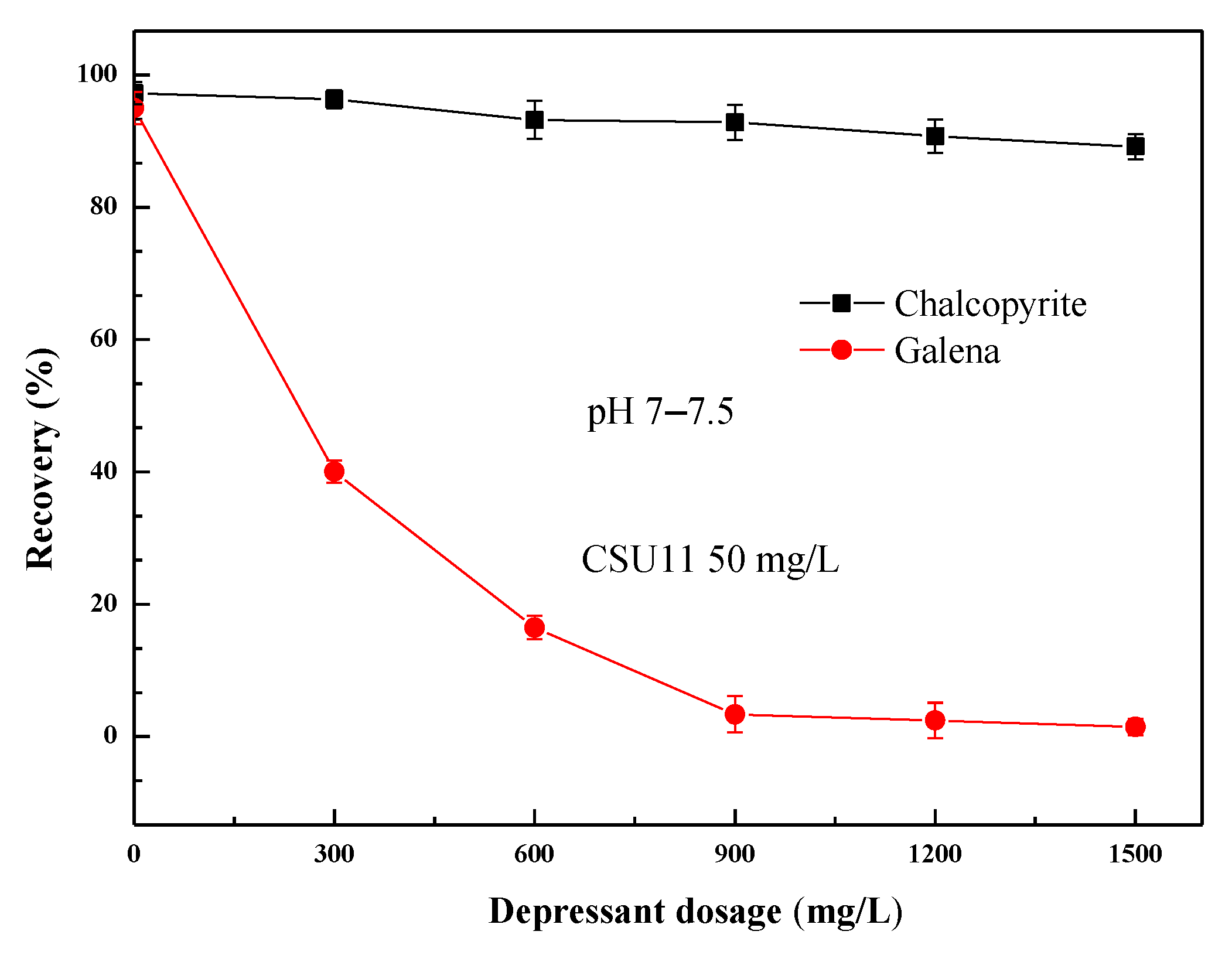
3.1. Micro-Flotation Test Results
3.2. Batch Flotation Test Results
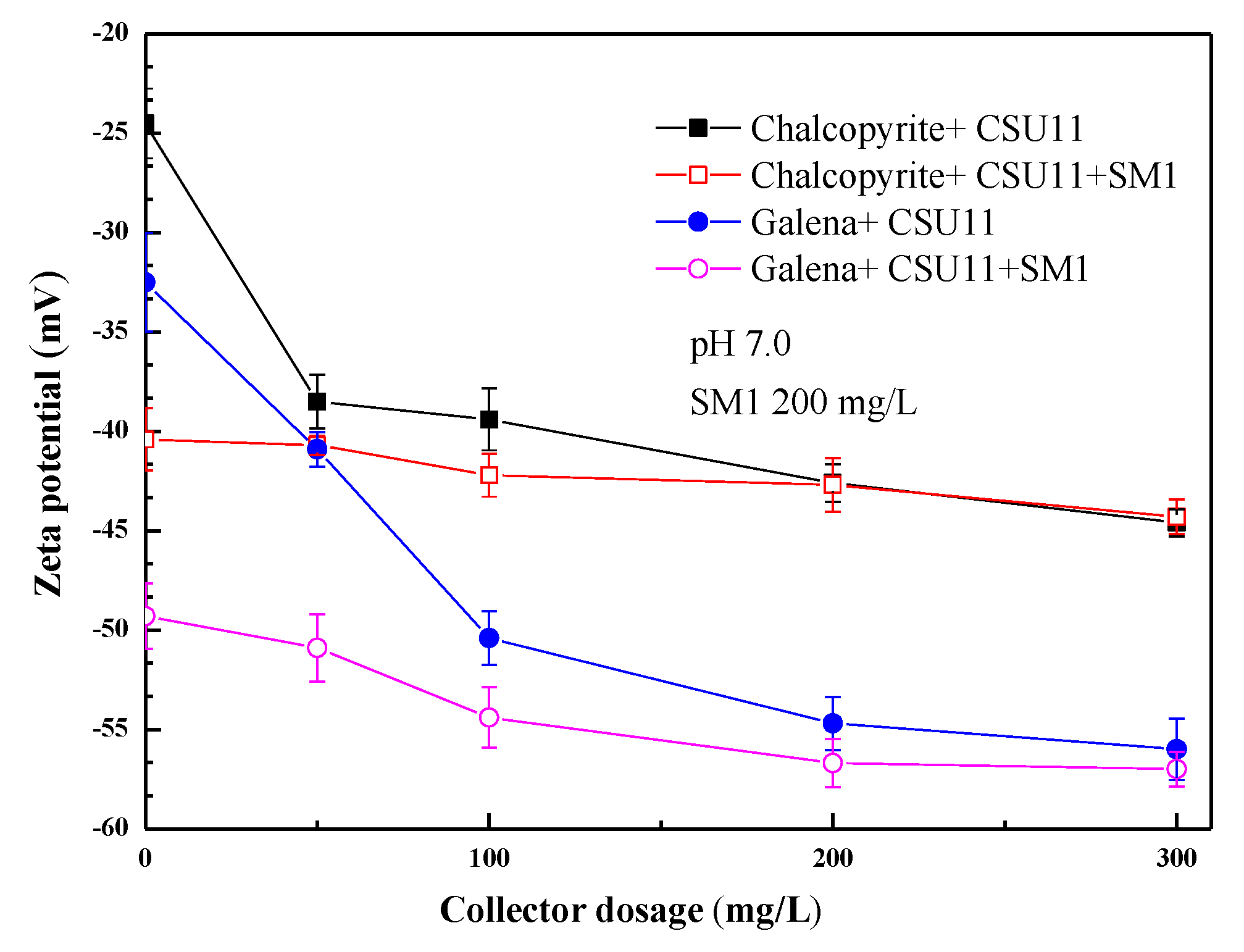
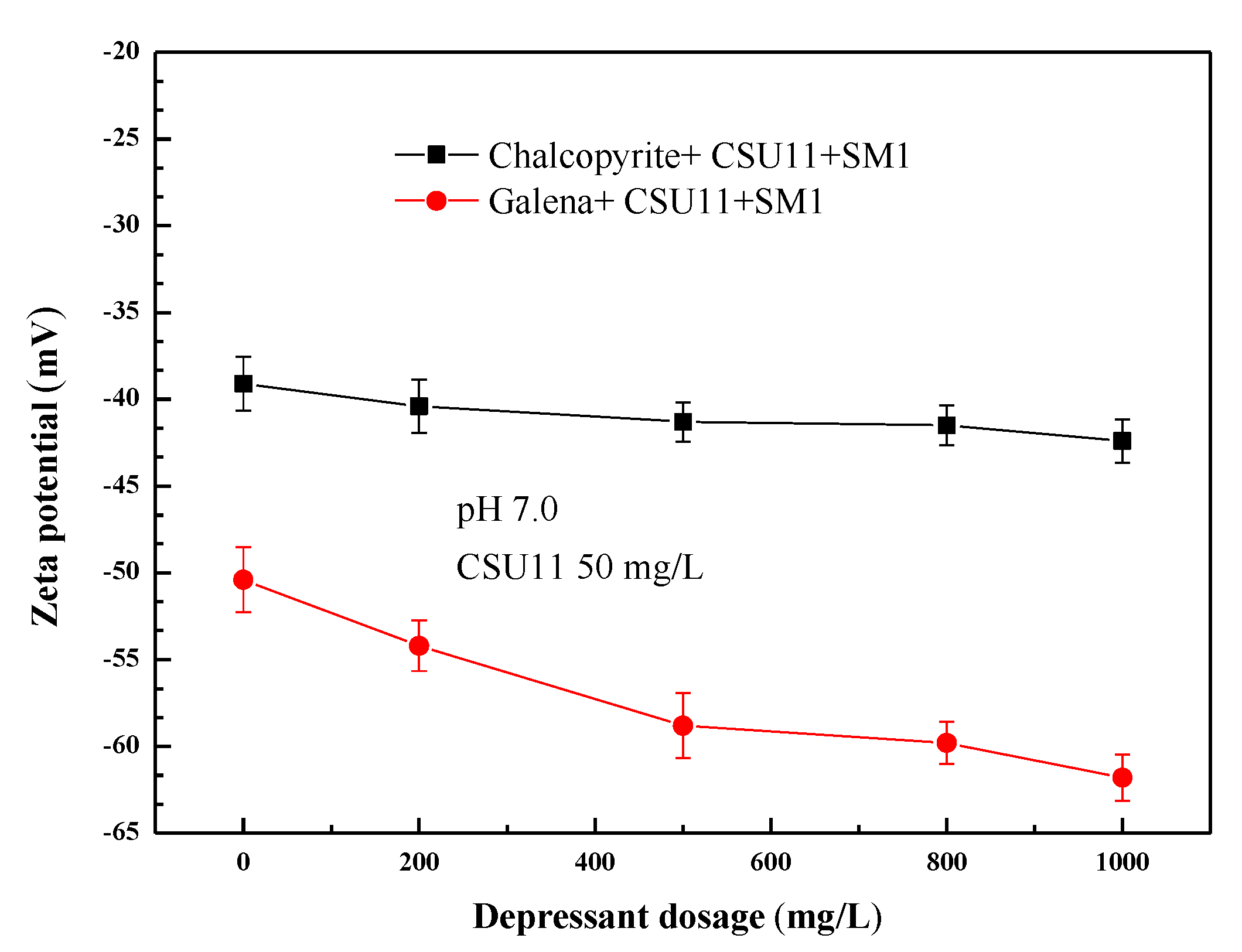
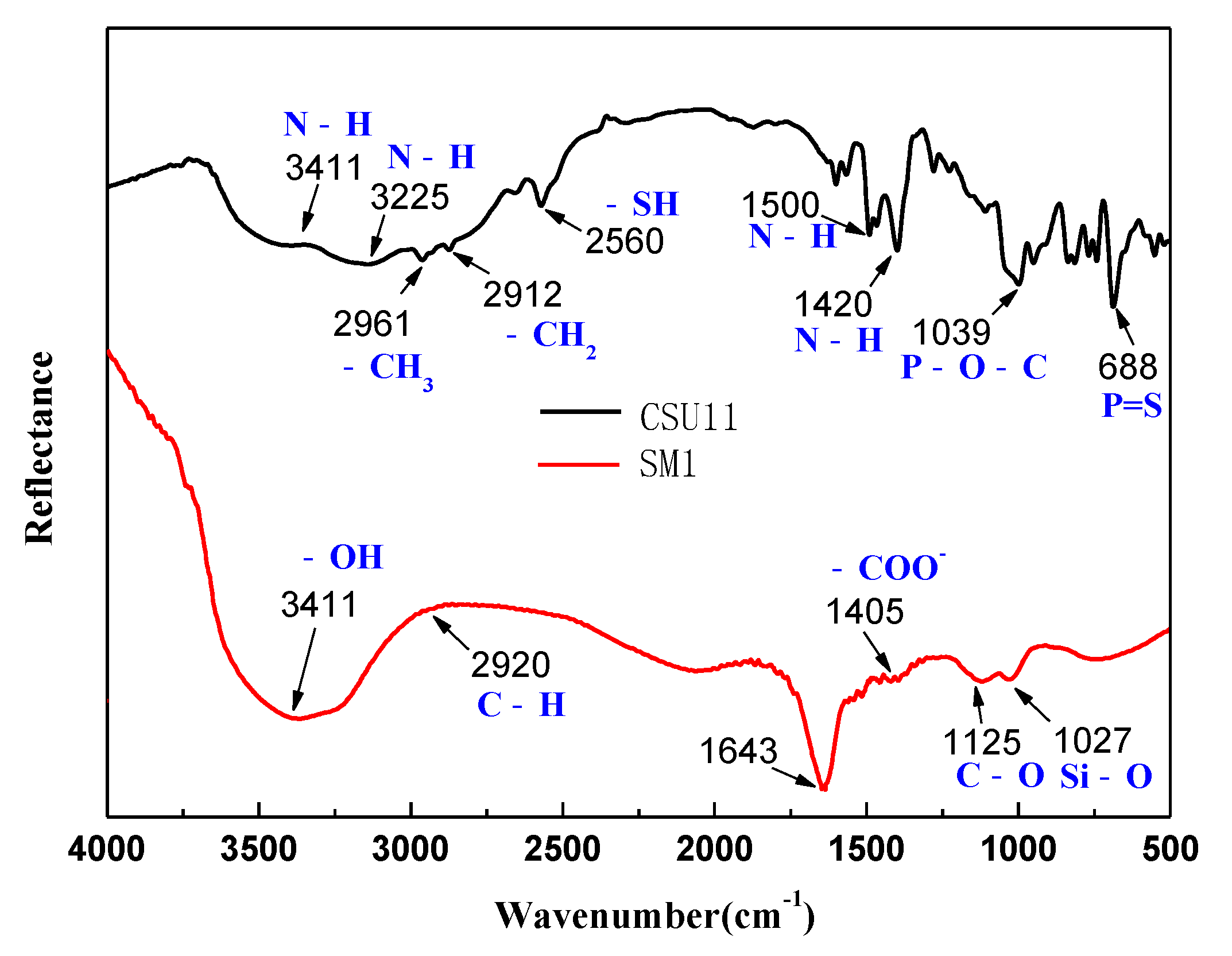
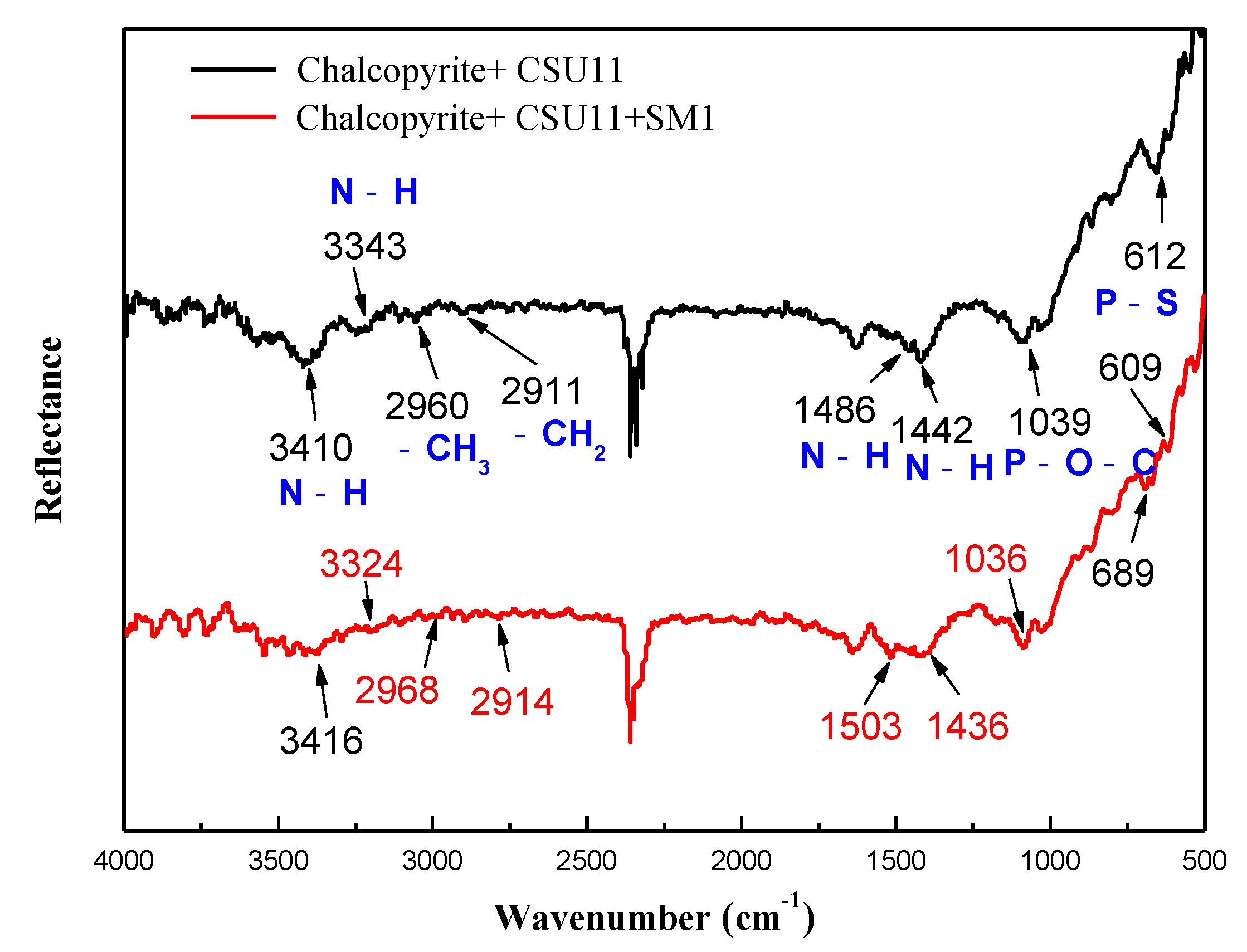
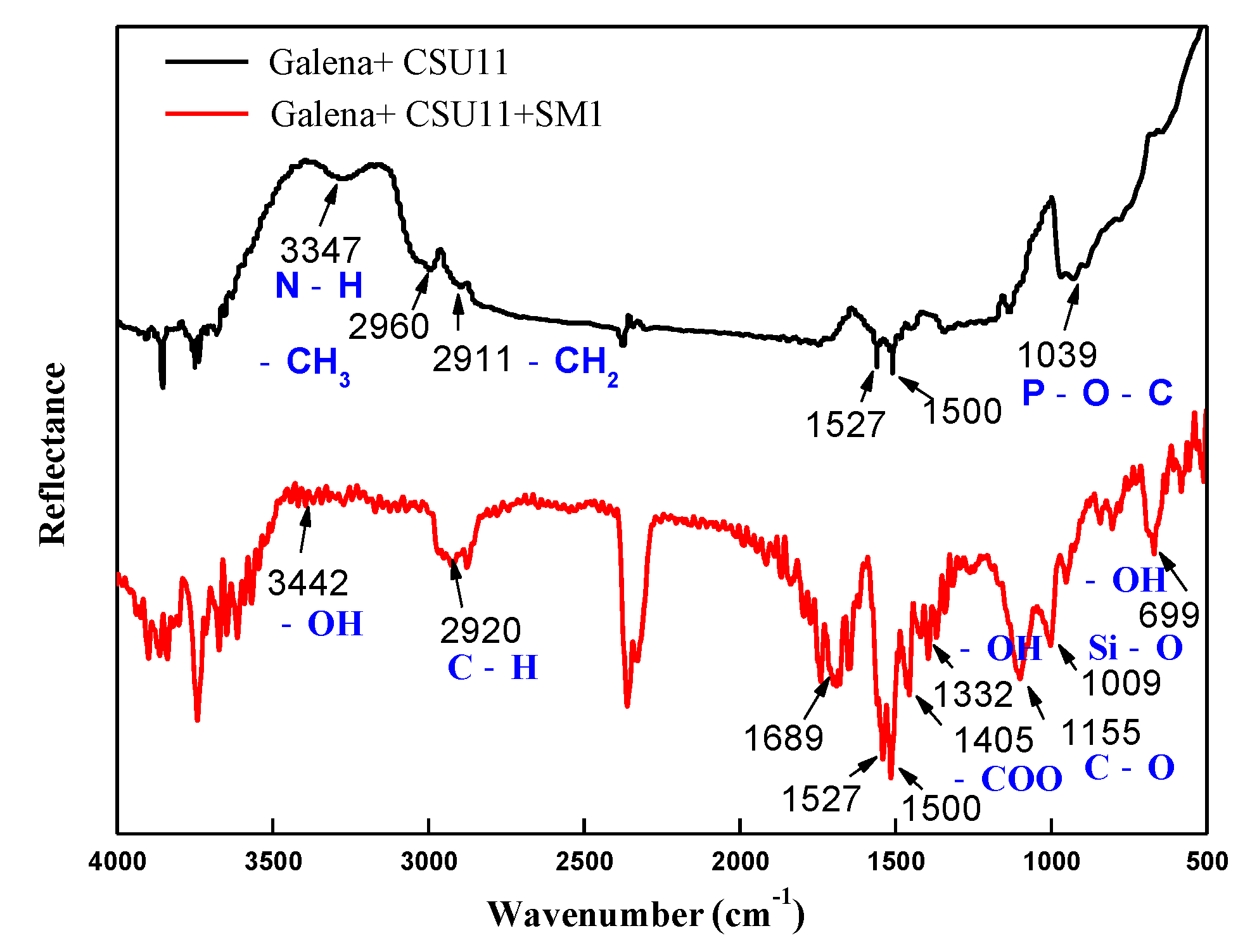
3.3. Selective Adsorption Mechanism of the Mixed Depressant
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hayes, R.A.; Ralston, J. The collectorless flotation and separation of sulphide minerals by Eh control. Int. J. Miner. Process. 1988, 23, 55–84. [Google Scholar] [CrossRef]
- Chen, W.; Chen, T.; Bu, X.Z.; Chen, F.F.; Ding, Y.H.; Zhang, C.H.; Deng, S.; Song, Y.H. The selective flotation of chalcopyrite against galena using alginate as a depressant. Miner. Eng. 2019, 141, 105848. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J.; Barnes, A.R. Classification of the Major Copper Sulphides into semiconductor types, and associated flotation characteristics. Miner. Eng. 2016, 96, 177–184. [Google Scholar] [CrossRef]
- Bu, Y.J.; Hu, Y.H.; Sun, W.; Gao, Z.Y.; Liu, R.Q. Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector. Minerals 2018, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.W.; Han, Y.X.; Zhu, Y.M.; Li, Y.J.; Liu, H. Flotation behaviors and mechanisms of chalcopyrite and galena after cyanide treatment. Trans. Nonferrous Met. Soc. 2016, 26, 3245–3252. [Google Scholar] [CrossRef]
- Öztürk, Y.; Bıçak, Ö.; Özdemir, E.; Ekmekçi, Z. Mitigation negative effects of thiosulfate on flotation performance of a Cu-Pb-Zn sulfide ore. Miner. Eng. 2018, 122, 142–147. [Google Scholar] [CrossRef]
- Sehlotho, N.; Sindane, Z.; Bryson, M.; Lindvelt, L. Flowsheet development for selective Cu-Pb-Zn recovery at Rosh Pinah concentrator. Miner. Eng. 2018, 122, 10–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.Q.; Sun, W.; Wang, L.; Dong, Y.H.; Wang, C.T. Electrochemical mechanism and flotation of chalcopyrite and galena in the presence of sodium silicate and sodium sulfite. Trans. Nonferrous Met. Soc. 2020, 30, 1091–1101. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. An electrochemical and electron spectroscopic investigation of the galena/diethyl dithiophosphate system. Colloid Surf. 1991, 59, 307–319. [Google Scholar] [CrossRef]
- Güler, T.; Hiçyilmaz, C.; Gökagˇaç, G.; Ekmeçi, Z. Adsorption of dithiophosphate and dithiophosphinate on chalcopyrite. Miner. Eng. 2005, 19, 62–71. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, Y.M.; Liu, G.Y.; Liu, S.; Liu, J.; Yang, X.L. Separation of chalcopyrite from galena with 3-amyl-4-amino-1, 2, 4-triazole-5-thione collector: Flotation behavior and mechanism. J. Ind. Eng. Chem. 2020, 92, 210–217. [Google Scholar] [CrossRef]
- Da Silva, G.R.; Waters, K.E. The effects of microwave irradiation on the floatability of chalcopyrite, pentlandite and pyrrhotite. Adv. Powder Technol. 2018, 29, 3049–3061. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Ralston, J.; Smart, R.S.C. The role of cyanide in the interaction of ethyl xanthate with galena. Colloid Surf. A-Physicochem. Eng. Asp. 1993, 81, 103–119. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice. Flotation of Tantalum/Niobium Ores; Elsevier: Amsterdam, The Netherlands, 2007; pp. 127–149. [Google Scholar]
- Kocabağ, D.; Güler, T. Two-liquid flotation of sulphides: An electrochemical approach. Miner Eng. 2007, 20, 1246–1254. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Liu, Q. Depressant function of high molecular weight polyacrylamide in the xanthate flotation of chalcopyrite and galena. Int. J. Miner. Process. 2014, 128, 6–15. [Google Scholar] [CrossRef]
- Qiu, X.M.; Yang, H.Y.; Chen, G.B.; Zhong, S.P.; Cai, C.K.; Lan, B.B. Inhibited mechanism of carboxymethyl cellulose as a galena depressant in chalcopyrite and galena separation flotation. Miner. Eng. 2020, 150, 106273. [Google Scholar]
- Zhang, X.R.; Qian, Z.B.; Zheng, G.B.; Zhu, Y.G.; Wu, W.G. The design of a macromolecular depressant for galena based on DFT studies and its application. Miner. Eng. 2017, 112, 50–56. [Google Scholar] [CrossRef]
- Houot, R.; Duhamet, D. The use of sodium sulphite to improve the flotation selectivity between chalcopyrite and galena in a complex sulphide ore. Miner. Eng. 1992, 5, 343–355. [Google Scholar] [CrossRef]
- Sui, C.; Finch, J.A.; Nesset, J.E.; Kim, J.; Lajoie, S. Characterisation of the surfaces of galena and sphalerite in the presence of dithionite. Dev. Miner. Process. 2000, 13, C8b–15–C8b–22. [Google Scholar]
- Multani, R.S.; Waters, K.E. Pyrrhotite depression studies with DETA and SMBS on a Ni-Cu sulphide ore. Can. J. Chem. Eng. 2019, 97, 2121–2130. [Google Scholar] [CrossRef]
- Qin, W.Q.; Wei, Q.; Jiao, F.; Wang, L.; Wang, P.P.; Ke, L.F. Effect of sodium pyrophosphate on the flotation separation of chalcopyrite from galena. Int. J. Min. Sci. Technol. 2012, 22, 345–349. [Google Scholar] [CrossRef]
- Yoon, R.H. Collectorless flotation of chalcopyrite and sphalerite ores by using sodium sulfide. Int. J. Miner. Process. 1981, 8, 31–48. [Google Scholar] [CrossRef]
- Qin, W.Q.; Wei, Q.; Jiao, F.; Yang, C.G.; Liu, R.Z.; Wang, P.P.; Ke, L.F. Utilization of polysaccharides as depressants for the flotation separation of copper/lead concentrate. Int. J. Min. Sci. Technol. 2013, 23, 191–198. [Google Scholar] [CrossRef]
- Liu, R.Z.; Qin, W.Q.; Jiao, F.; Wang, X.J.; Pei, B.; Yang, Y.J.; Lai, C.H. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate. Trans. Nonferrous Met. Soc. 2016, 26, 265–271. [Google Scholar] [CrossRef]
- Li, J.M.; Song, K.W.; Liu, D.W.; Zhang, X.L.; Lan, Z.Y.; Sun, Y.L.; Wen, S.M. Hydrolyzation and adsorption behaviors of SPH and SCT used as combined depressants in the selective flotation of galena from sphalerite. J. Mol. Liq. 2017, 231, 485–490. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.H. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin. Miner. Eng. 2000, 13, 1405–1416. [Google Scholar] [CrossRef]
- Qi, L.; Laskowski, J.S. The role of metal hydroxides at mineral surfaces in dextrin adsorption, II. Chalcopyrite-galena separations in the presence of dextrin. Int. J. Miner. Process. 1989, 27, 147–155. [Google Scholar]
- Jin, S.Z.; Shi, Q.; Li, Q.; Ou, L.M.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Liu, M.F.; Zhang, C.Y.; Hu, B.; Sun, Z.M.; Xu, Q.H.; Wen, J.; Xiao, J.; Dong, Y.H.; Gan, M.; Sun, W.; et al. Enhancing flotation separation of chalcopyrite and galena by the surface synergism between sodium sulfite and sodium lignosulfonate. Appl. Surf. Sci. 2020, 507, 145042. [Google Scholar] [CrossRef]
- Gonzalez, M.S.; Fornasiero, D. Understanding the effect of sulphate in mining-process water on sulphide flotation. Miner. Eng. 2021, 165, 106865. [Google Scholar] [CrossRef]
- Jin, S.Z.; Zhang, P.Y.; Ou, L.M. Study on the depression mechanism of zinc sulfate on talc in chalcopyrite flotation. Colloid Surf. A-Phys. Eng. 2020. [Google Scholar] [CrossRef]
- Zhao, K.L.; Wang, X.H.; Yan, W.; Gu, G.H.; Wang, C.Q.; Wang, Z.; Xu, L.H.; Peng, T.F. Depression mechanism of pyrophyllite by a novel polysaccharide xanthan gum. Miner. Eng. 2019, 132, 134–141. [Google Scholar] [CrossRef]
- Zhang, W.J.; Sun, W.; Hu, Y.H.; Cao, J.; Gao, Z.Y. Selective Flotation of Pyrite from Galena Using Chitosan with Different Molecular Weights. Minerals 2019, 9, 549. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Huang, X.P.; Huang, K.H.; Wang, S.; Cao, Z.F.; Zhong, H. Synthesis, flotation performance and adsorption mechanism of 3-(ethylamino)-N-phenyl-3-thioxopropanamide onto galena/sphalerite surfaces. J. Ind. Eng. Chem. 2019, 77, 416–425. [Google Scholar] [CrossRef]
- Xiao, J.J.; Liu, G.Y.; Zhong, H. The adsorption mechanism of N -butoxypropyl- S -[2-(hydroxyimino) propyl] dithiocarbamate ester to copper minerals flotation. Int. J. Miner. Process. 2017, 166, 53–61. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Y.; Zhong, H.; Wang, S.; Liu, G.Y.; Zhao, G. A novel surfactant S-benzoyl-N, N-diethyldithiocarbamate synthesis and its flotation performance to galena. Appl. Surf. Sci. 2016, 365, 342–351. [Google Scholar] [CrossRef]
- Zhang, W.J.; Feng, Z.T.; Yang, Y.H.; Sun, W.; Pooley, S.; Cao, J.; Gao, Z.Y. Bi-functional hydrogen and coordination bonding surfactant: A novel and promising collector for improving the separation of calcium minerals. J. Colloid Inter. Sci. 2021, 585, 787–799. [Google Scholar] [CrossRef]
- Zhang, W.J.; Feng, Z.T.; Mulenga, H.; Sun, W.; Cao, J.; Gao, Z.Y. Synthesis of a novel collector based on selective nitrogen coordination for improved separation of galena and sphalerite against pyrite. Chem. Eng. Sci. 2020, 226, 115860. [Google Scholar] [CrossRef]
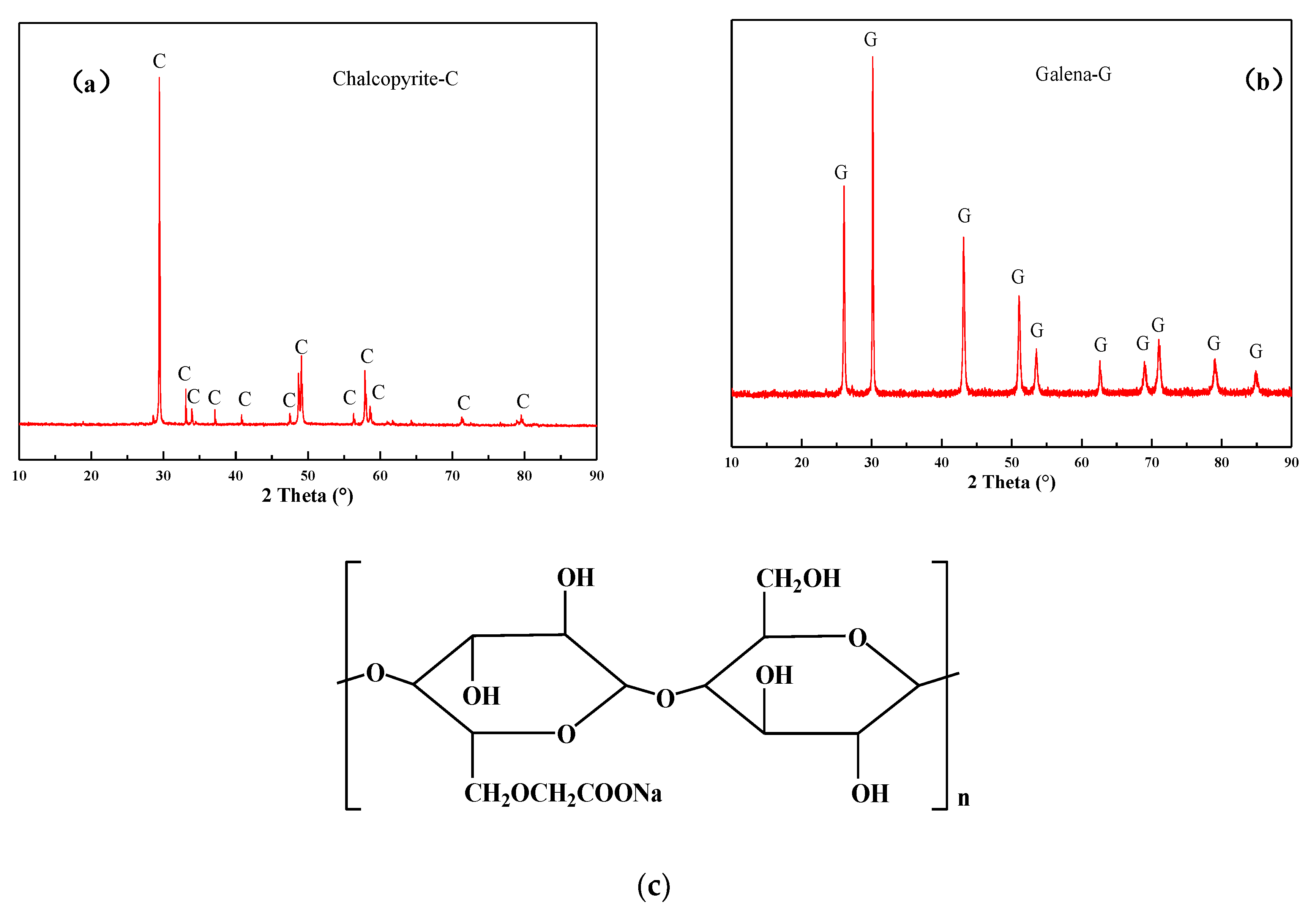




| Element | Cu | Pb | Zn | S | Fe | As | Al2O3 | SiO2 | CaO | MgO | Ag |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 3.53 | 65.84 | 2.11 | 16.63 | 5.32 | 0.021 | 0.41 | 2.89 | 1.66 | 0.73 | 752.5 (g/t) |
| Chemical Phase of Copper | Primary Copper Sulfide | Secondary Copper Sulfide | Free Copper Oxide | Conjunction Copper Oxide | Total | |
|---|---|---|---|---|---|---|
| wt % | 3.26 | 0.078 | 0.16 | 0.032 | 3.53 | |
| % of Total Cu | 92.35 | 2.21 | 4.53 | 0.91 | 100.00 | |
| Chemical Phase of Lead | Lead Sulfide | Lead Oxide | Plumbojarosite | Total | ||
| wt % | 64.81 | 0.62 | 0.41 | 65.84 | ||
| % of Total Pb | 98.44 | 0.94 | 0.62 | 100.00 | ||
| Mineral | wt % | Mineral | wt % |
|---|---|---|---|
| Galena | 75.1 | Tetrahedrite | 1.8 |
| Chalcopyrite | 9.4 | Quartz | 4.3 |
| Sphalerite | 3.2 | Calcite | 0.7 |
| Pyrite | 2.2 | Feldspar | 0.7 |
| Chalcocite | 2.2 | Chlorite | 0.4 |
| Flotation Process | Locked-Cycle Test Reagent Concentrations (g/t) | |
|---|---|---|
| Experimental System Non-Toxic Depressant (SM1: Sodium Sulfite: Zinc Sulfate = 1:2:2) | Comparison System Potassium Dichromate | |
| Rougher | CSU11 40, DFinal 850 | CSU11 40, potassium dichromate 100 |
| Scavenger | CSU11 15 | CSU11 15 |
| 1st cleaner | DFinal 400 | Potassium dichromate 50 |
| 2nd cleaner | DFinal 260 | Potassium dichromate 20 |
| 3rd cleaner | DFinal 100 | Potassium dichromate 10 |
| Flotation System | Products | Ratio (wt %) | Grades (%) | Recovery (%) | ||
|---|---|---|---|---|---|---|
| Cu | Pb | Cu | Pb | |||
| Experimental system (Non-toxic depressant, DFinal) | Cu concentrate | 14.36 | 21.88 | 7.96 | 89.07 | 1.74 |
| Pb concentrate | 85.64 | 0.45 | 75.53 | 10.93 | 98.26 | |
| Feed | 100.00 | 3.53 | 65.83 | 100.00 | 100.00 | |
| Comparison system (Potassium dichromate) | Cu concentrate | 13.93 | 22.80 | 7.12 | 90.00 | 1.51 |
| Pb concentrate | 86.07 | 0.41 | 75.34 | 10.00 | 98.49 | |
| Feed | 100.00 | 3.52 | 65.84 | 100.00 | 100.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Ma, C.; Gu, G.; Gao, Z. Selective Separation of Chalcopyrite from Galena Using a Green Reagent Scheme. Minerals 2021, 11, 796. https://doi.org/10.3390/min11080796
Zhao K, Ma C, Gu G, Gao Z. Selective Separation of Chalcopyrite from Galena Using a Green Reagent Scheme. Minerals. 2021; 11(8):796. https://doi.org/10.3390/min11080796
Chicago/Turabian StyleZhao, Kaile, Chao Ma, Guohua Gu, and Zhiyong Gao. 2021. "Selective Separation of Chalcopyrite from Galena Using a Green Reagent Scheme" Minerals 11, no. 8: 796. https://doi.org/10.3390/min11080796
APA StyleZhao, K., Ma, C., Gu, G., & Gao, Z. (2021). Selective Separation of Chalcopyrite from Galena Using a Green Reagent Scheme. Minerals, 11(8), 796. https://doi.org/10.3390/min11080796







