Ore and Geochemical Specialization and Substance Sources of the Ural and Timan Carbonatite Complexes (Russia): Insights from Trace Element, Rb–Sr, and Sm–Nd Isotope Data
Abstract
:1. Introduction
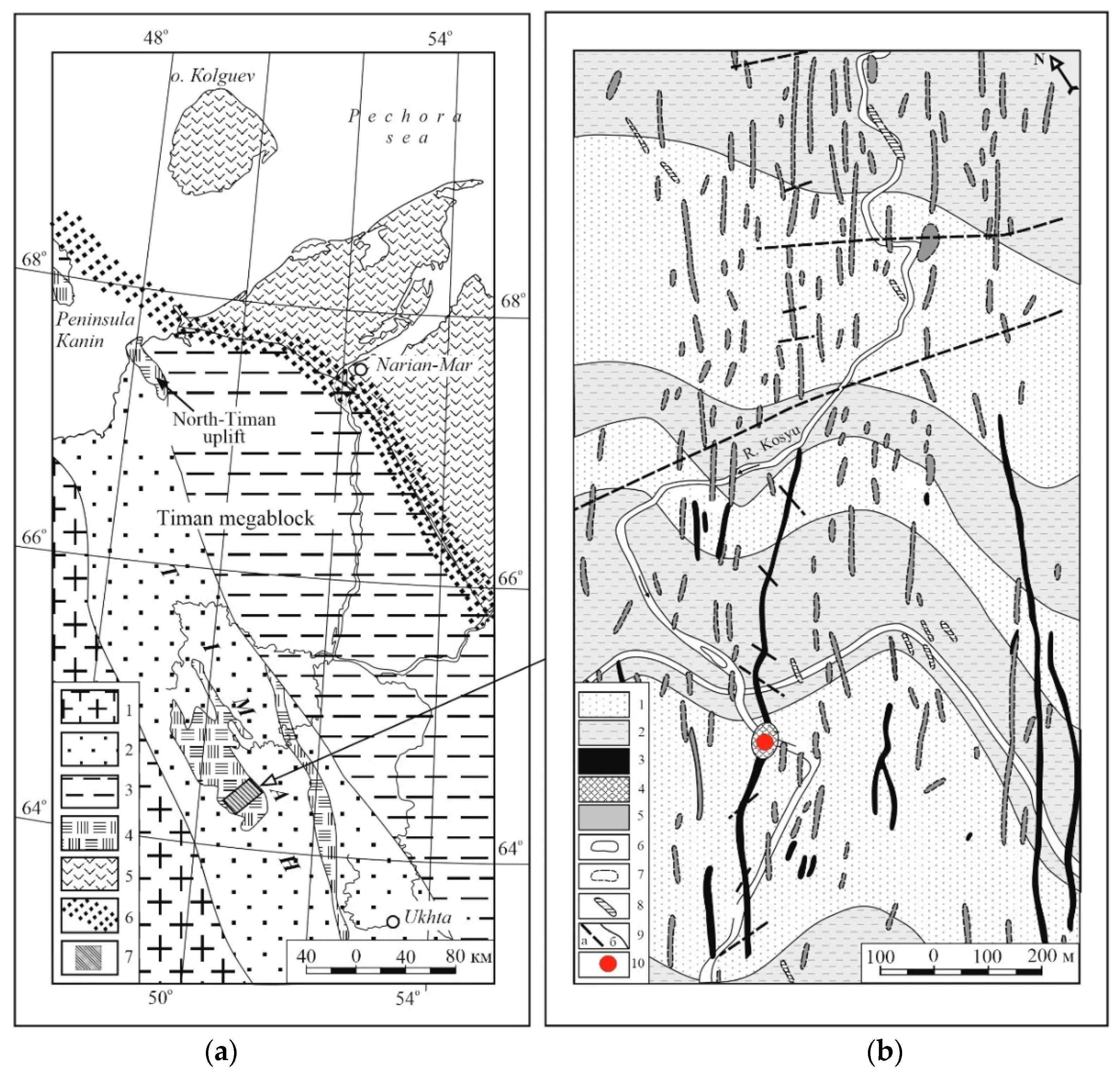
2. Geological Background
2.1. Ilmeno–Vishnevogorsk and Buldym Carbonatite Complexes (Southern Urals)
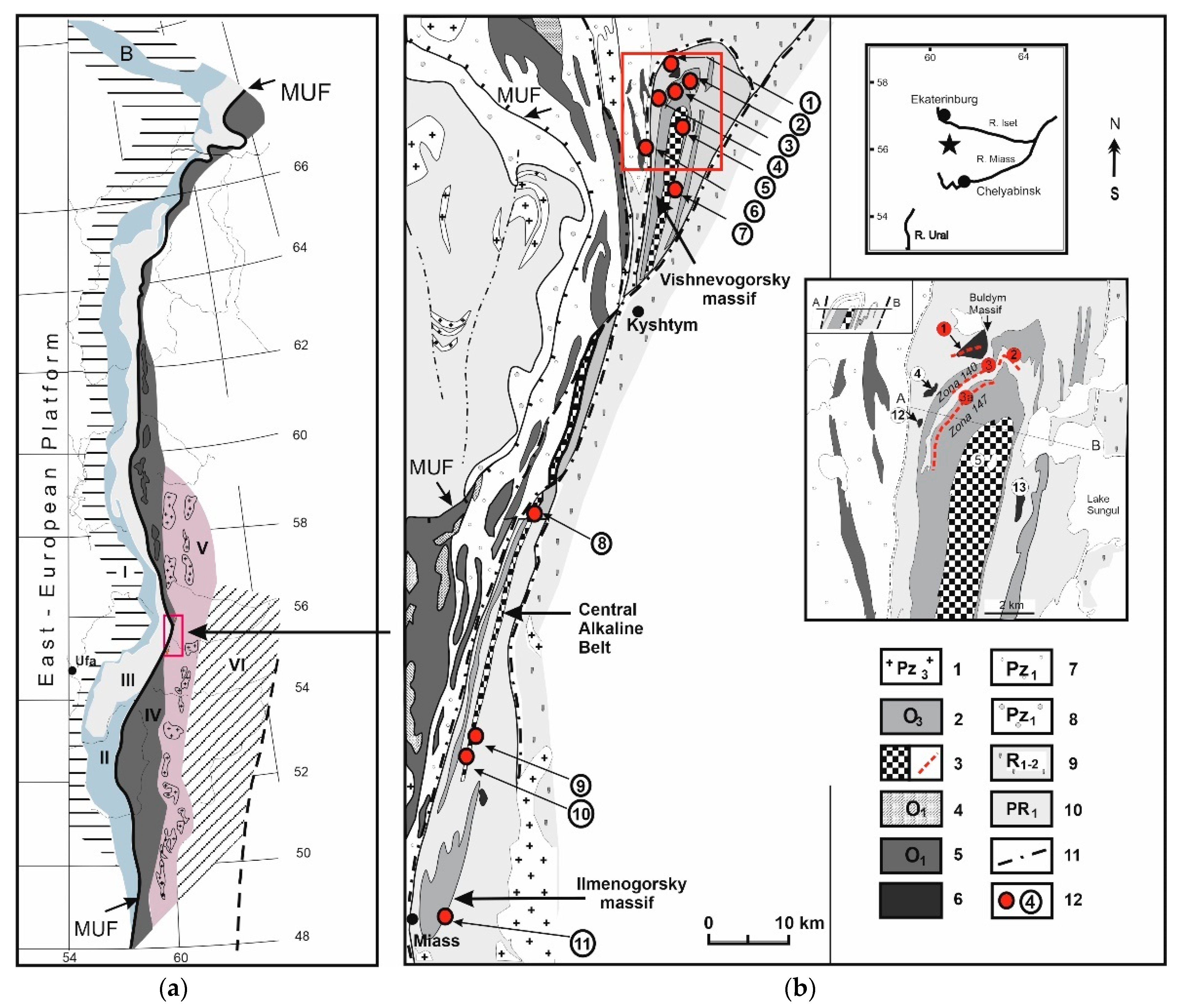
2.2. Chetlassky Carbonatite Complex (Middle Timan)
3. Materials and Methods
4. Results
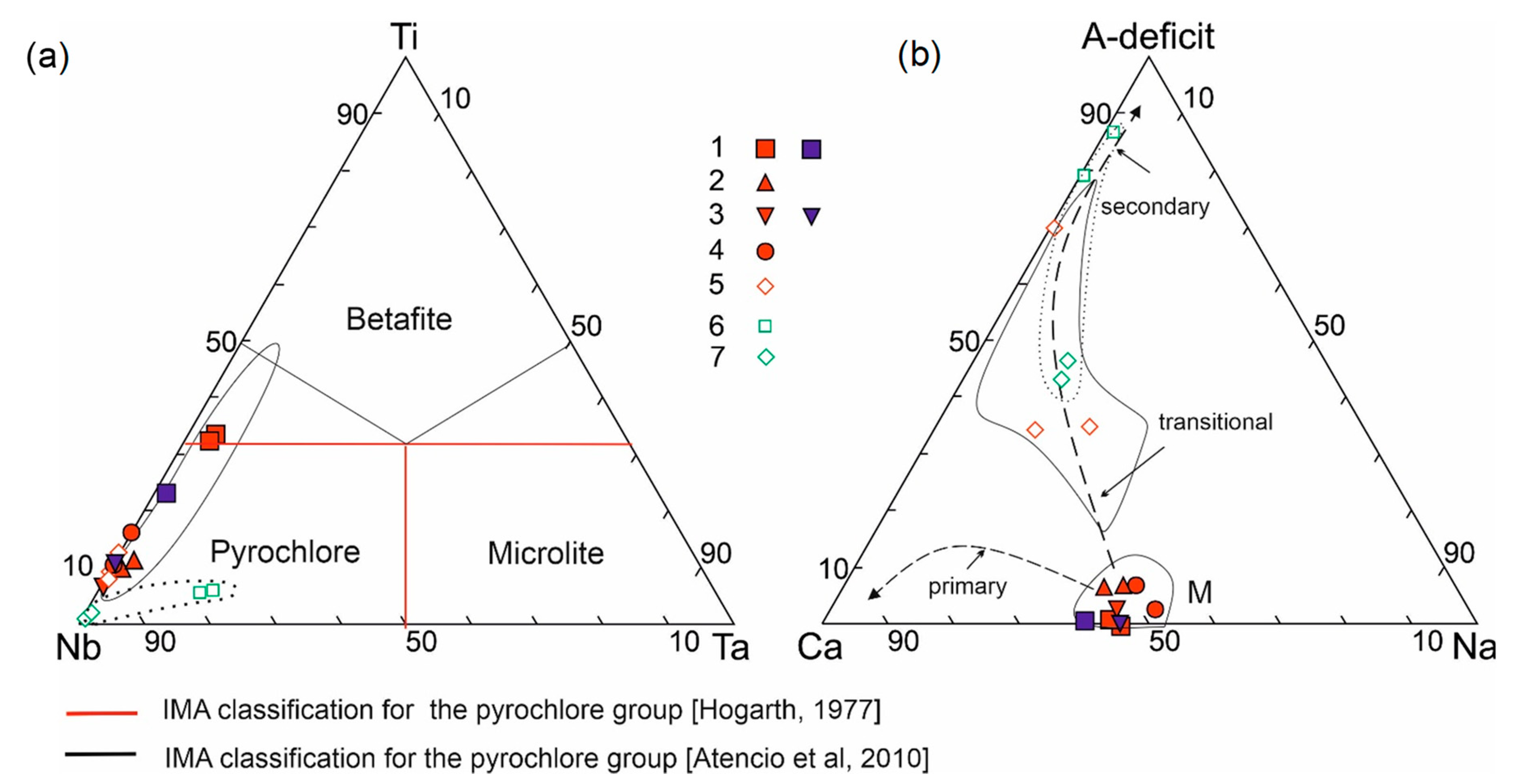
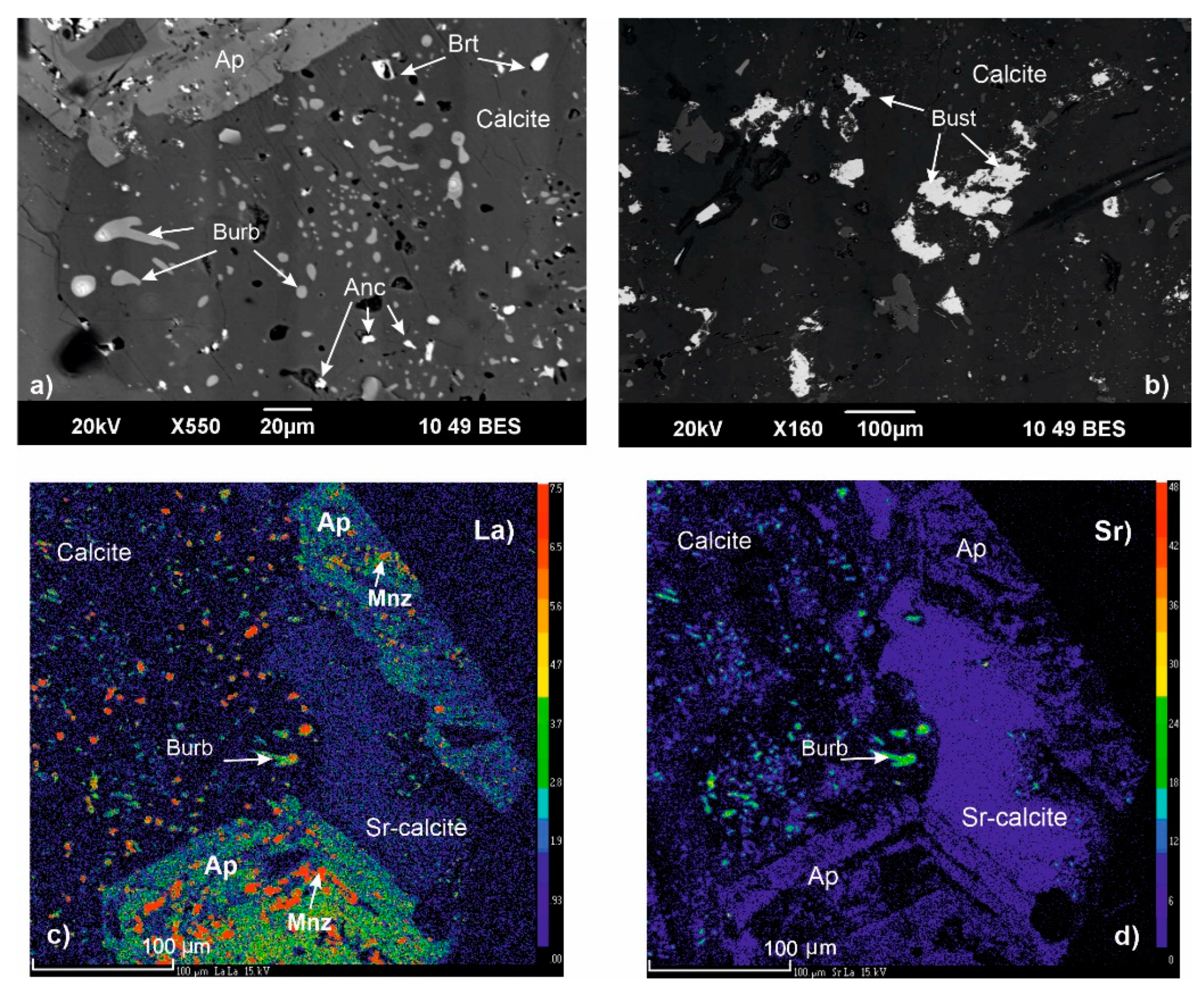
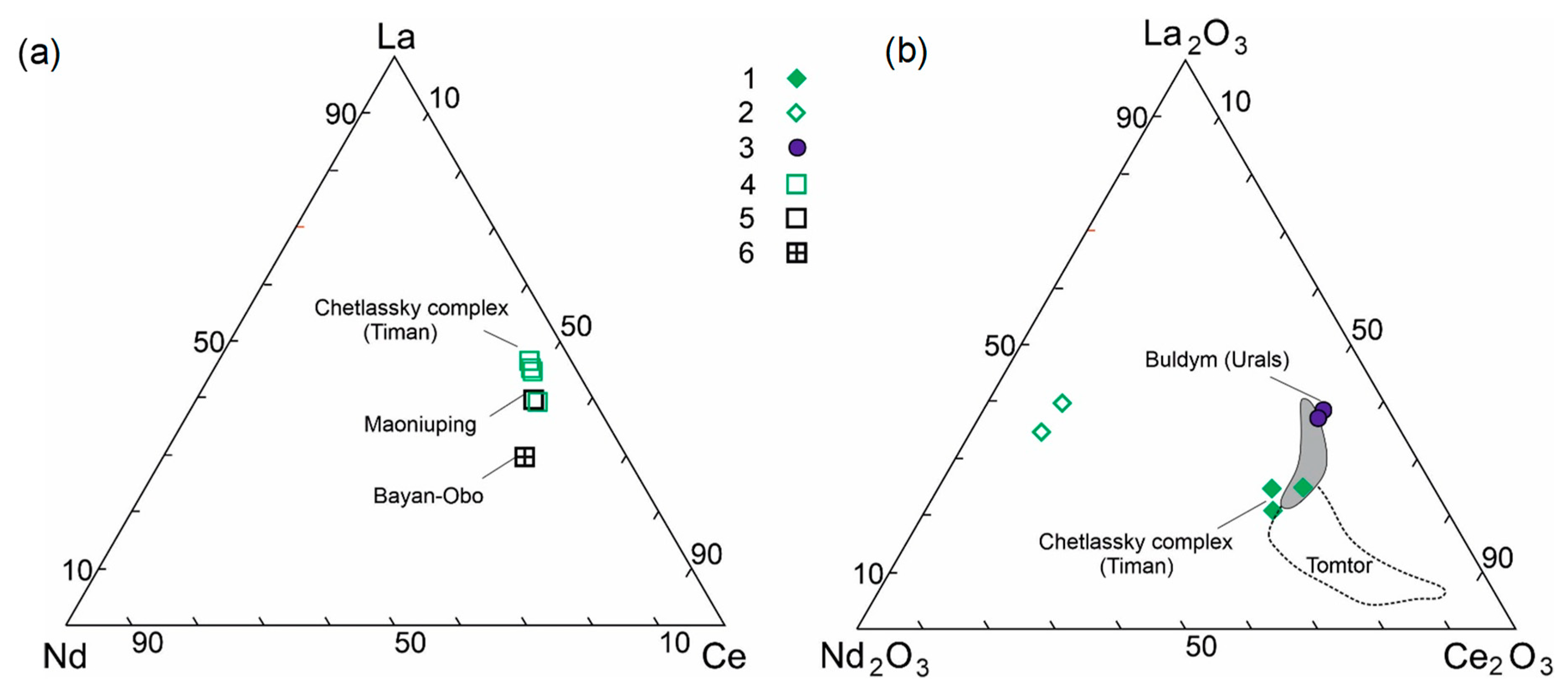
4.1. Rare Metal (Nb–REE) Ore Mineralization
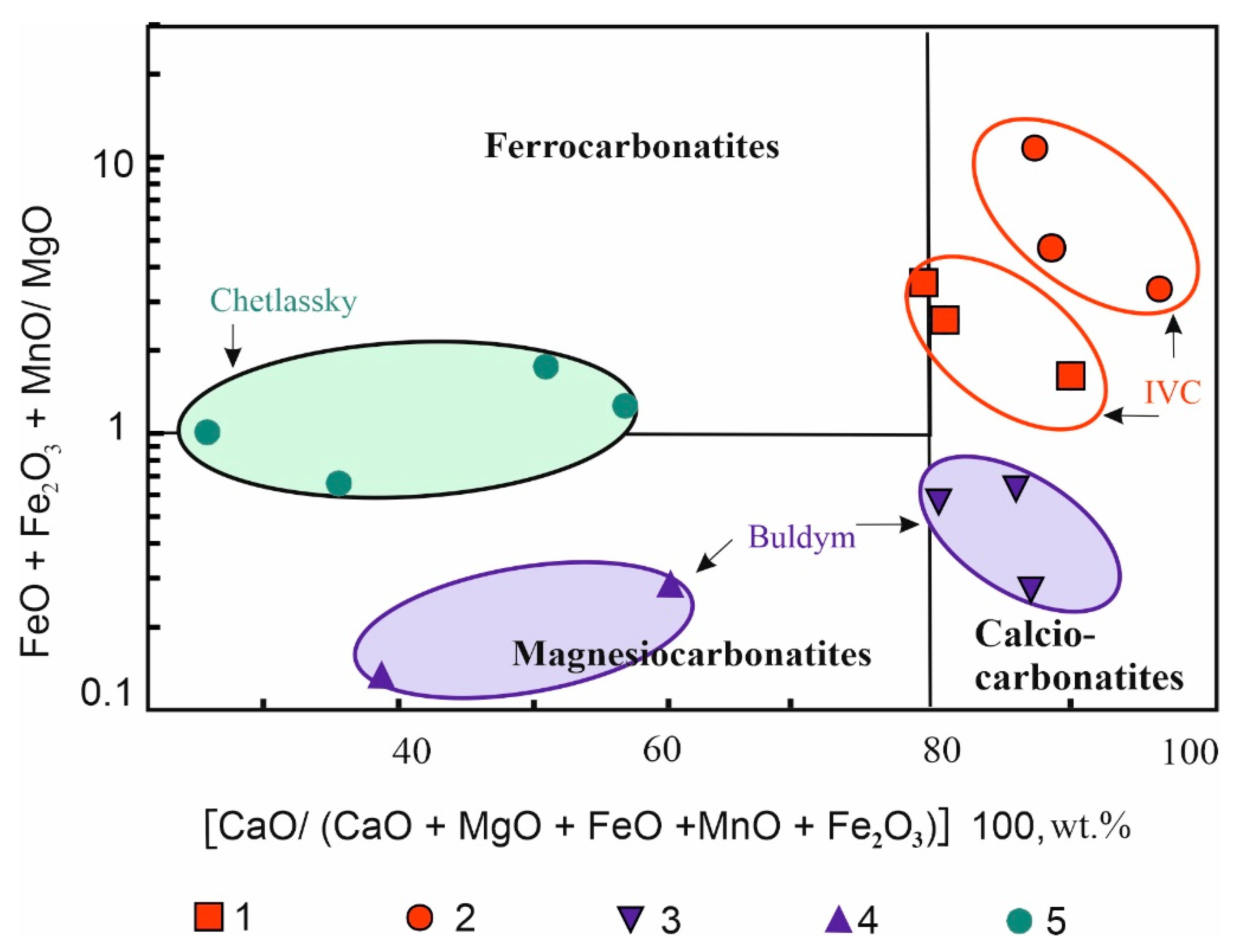
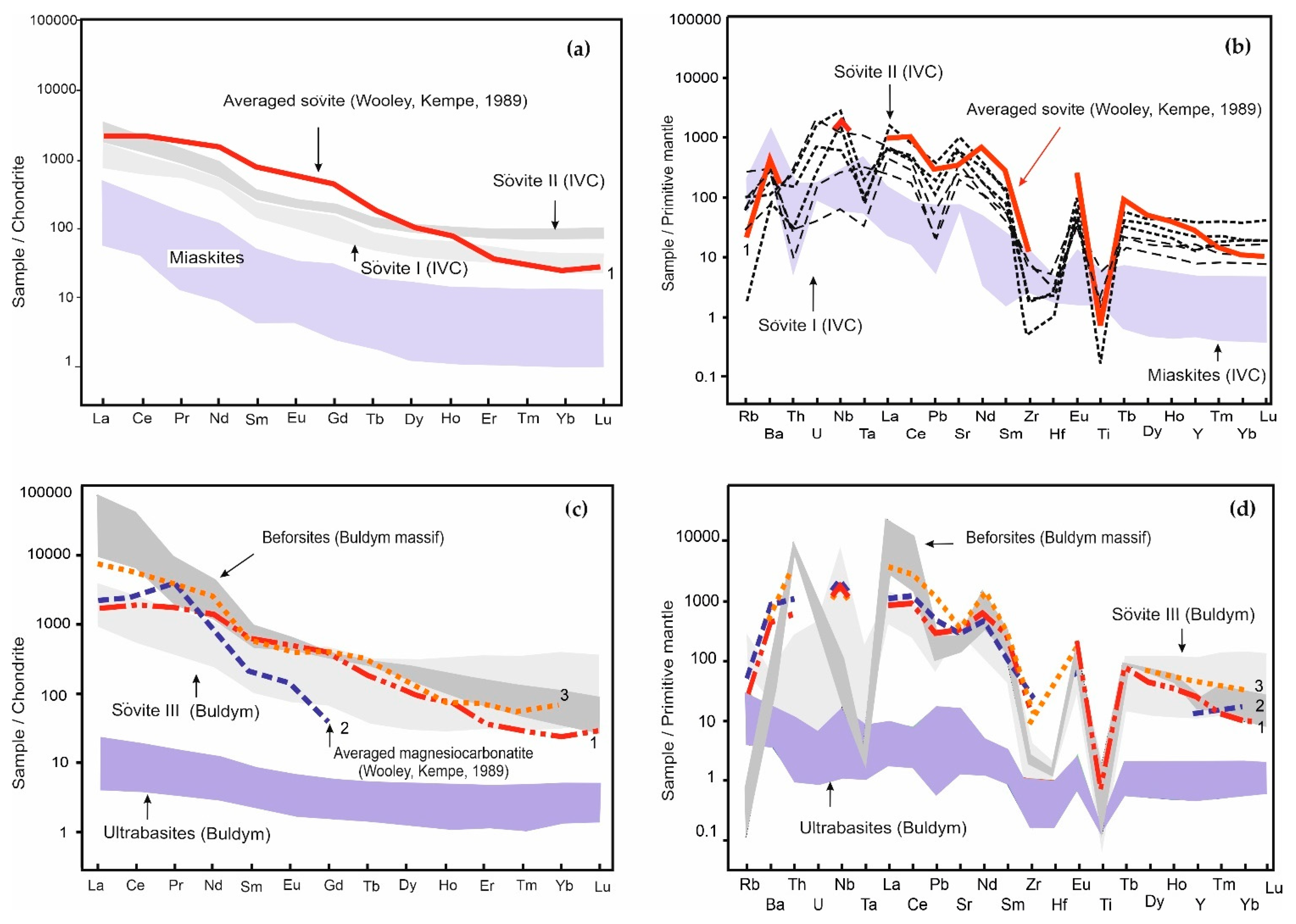
4.2. Bulk-Rock Major and Trace Element Composition
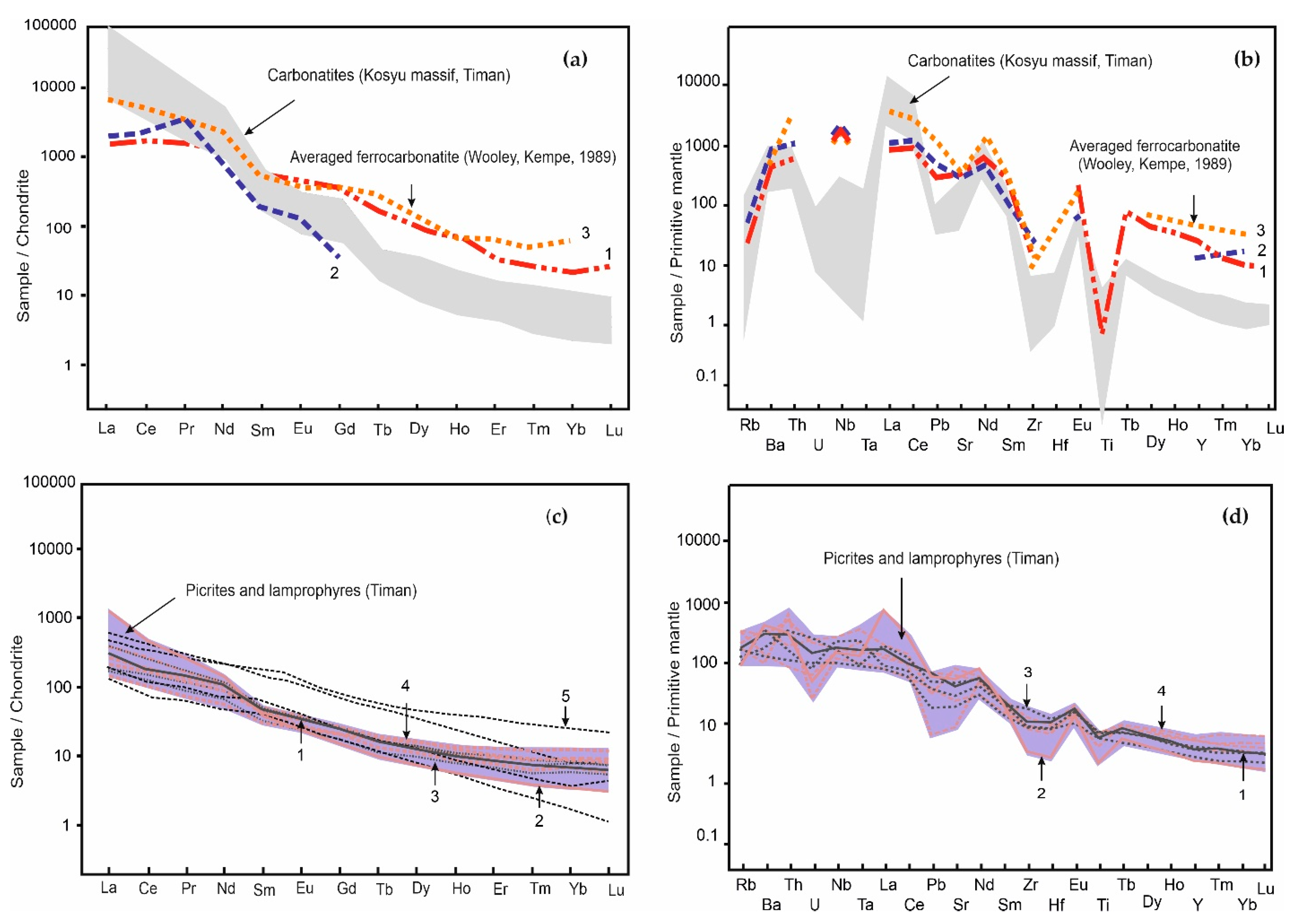
4.3. Rb-Sr and Sm-Nd Isotope Data
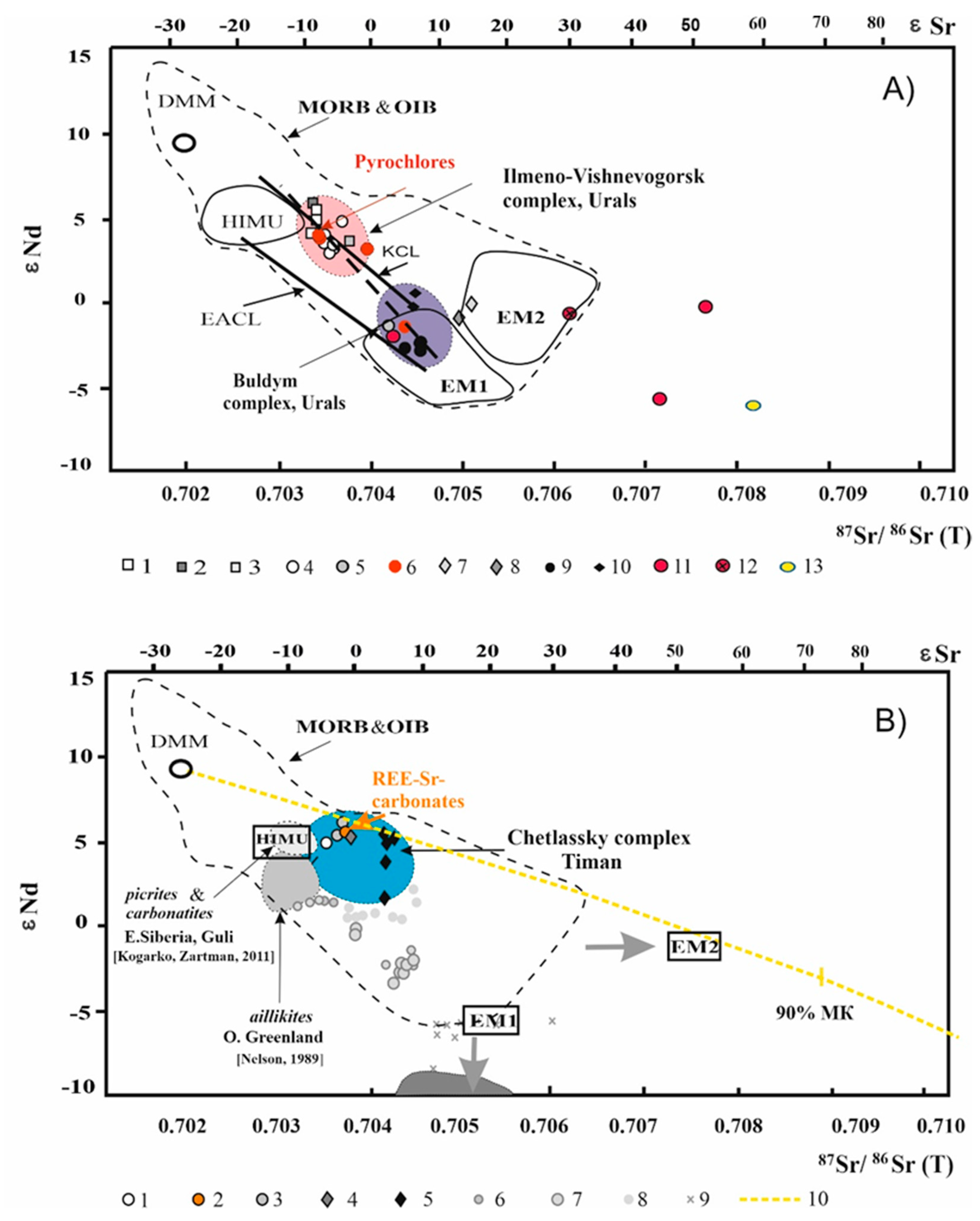
5. Discussion
5.1. Composition, Evolution, and Genesis of Ore Rare-Metal Mineralization
5.2. Evolution of Alkaline–Carbonatite Magmas as a Factor of Ore Specialization
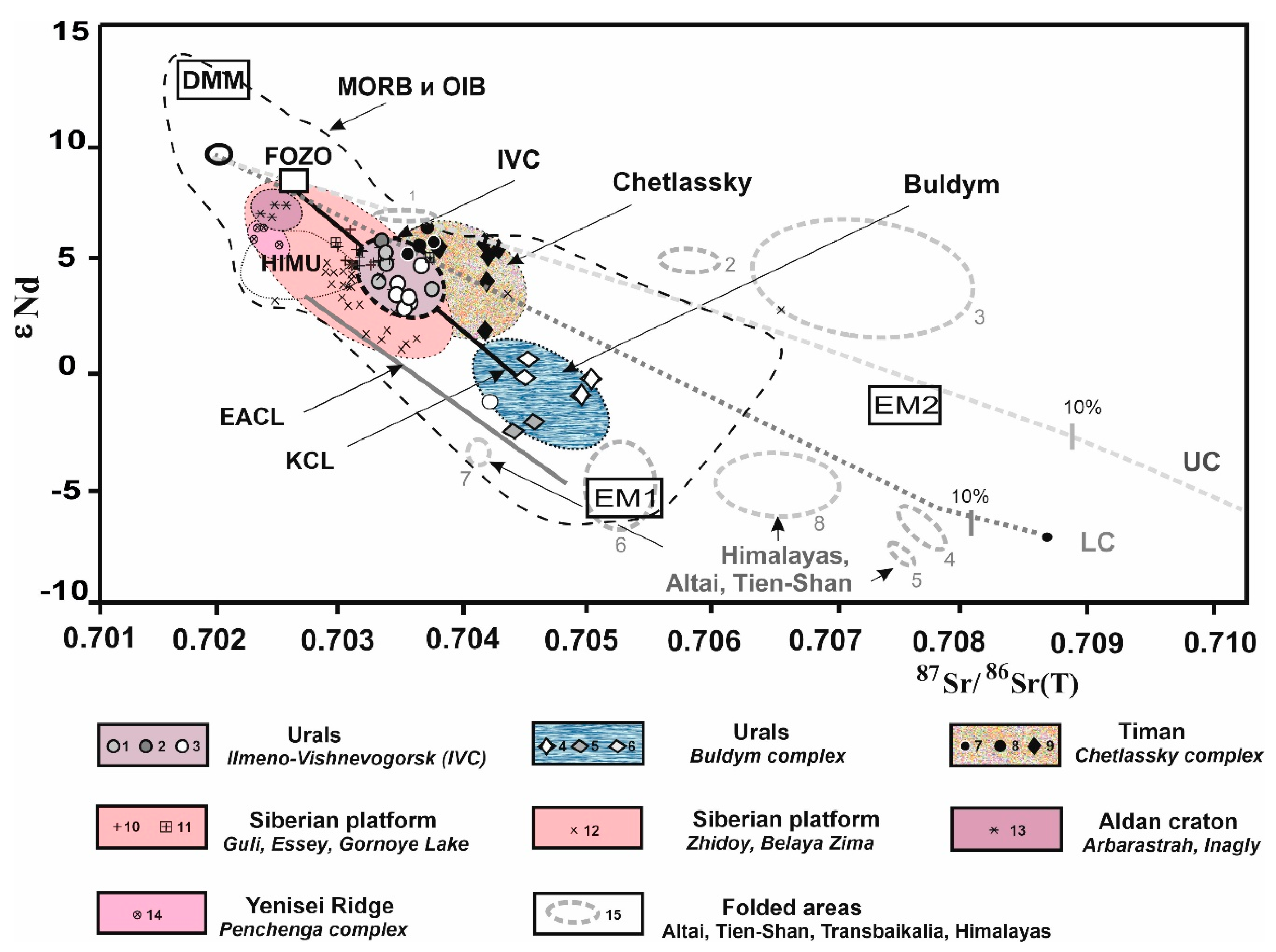
5.3. Mantle Source Characteristics: Rb–Sr and Sm–Nd Isotope Signatures
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, M.; Moore, K.; Kavecsánszki, D.; Finch, A.A.; Kynicky, J.; Wall, F. From mantle to critical zone: A review of large and giant sized deposits of the rare earth elements. Geosci. Front. 2016, 7, 315–334. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.H. Primary and secondary niobium mineral deposits associated with carbonatites. Ore Geol. Rev. 2015, 64, 626–641. [Google Scholar] [CrossRef]
- Bell, K. Carbonatites: Genesis and Evolution; Unwin Hyman: London, UK, 1989; p. 618. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Kononova, V.A.; Orlova, M.P.; Wooley, A.R. Alkaline Rocks and Carbonatites of the World. P. 2. Former USSR; Chapman and Hall: London, UK, 1995; p. 226. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kjarsgaard, B.A. Carbonatite Occurrences of the World: Map and Database. Geological Survey of Canada, Open File Report 5796. Miner. Mag. 2008, 71, 718. [Google Scholar] [CrossRef]
- Frolov, A.A.; Tolstov, A.R.; Belov, S.V. Carbonatite Deposits in Russia; NIA-Priroda: Moscow, Russia, 2003; p. 494. (In Russian) [Google Scholar]
- Tilton, G.R.; Bryce, J.G.; Mateen, A. Pb-Sr-Nd isotope data from 30 and 300 Ma collision zone carbonatites in Northwest Pakistan. J. Petrol. 1998, 39, 1865–1874. [Google Scholar] [CrossRef]
- Vrublevsky, V.V. Petrology of Carbonatite Complexes of Consolidated Folded Areas: The Case of Southern Siberia and the Tien Shan. Doctoral Thesis, Tomsk University, Tomsk, Russia, 2003; p. 303. (In Russian). [Google Scholar]
- Hou, Z.Q.; Tian, S.H.; Yuan, Z.X.; Xie, Y.L.; Yin, S.P.; Yi, L.S.; Fei, H.C.; Yang, Z.M. The Himalayan collision zone carbonatites in western Sichuan, SW China: Petrogenesis, mantle source and tectonic implication. Earth Planet. Sci. Lett. 2006, 244, 234–250. [Google Scholar] [CrossRef]
- Hou, Z.; Cook, N.J. Metallogenesis of the Tibetan collisional orogen: A review and introduction to the special issue. Ore Geol. Rev. 2009, 36, 2–24. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Song, W.; Wu, M. Carbonatites in China: A review for genesis and mineralization. Geosci. Front. 2010, 1, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Doroshkevich, A.G.; Ripp, G.S.; Izbrodin, I.A.; Savatenkov, V.M. Alkaline magmatism of the Vitim province, West Transbaikalia, Russia: Age, mineralogical, geochemical and isotope (O, C, D, Sr and Nd) data. Lithos 2012, 152, 157–172. [Google Scholar] [CrossRef]
- Woodard, J.; Hetherington, C.J. Carbonatite in a post-collisional tectonic setting: Geochronology and emplacement conditions at Naantali, SW Finland. Precambrian Res. 2014, 240, 94–107. [Google Scholar] [CrossRef]
- Moore, M.; Chakhmouradian, A.R.; Mariano, A.N.; Sidhu, R. Evolution of rare-earth mineralization in the Bear Lodge carbonatite, Wyoming: Mineralogical and isotopic evidence. Ore Geol. Rev. 2015, 64, 499–521. [Google Scholar] [CrossRef]
- Xue, S.; Ling, M.-X.; Liuc, Y.L.-L.; Kangd, Q.-Q.; Huange, R.-F.; Zhanga, Z.-K.; Sunf, W. The formation of the giant Huayangchuan U-Nb deposit associated with carbonatite in the Qingling Orogenic Belt. Ore Geol. Rev. 2020, 122, 103498. [Google Scholar] [CrossRef]
- Lapin, A.V.; Tolstov, A.V. Minerageny of Carbonatite Weathering Crusts; Geokart: Geos, Moscow, 2011; p. 308. (In Russian) [Google Scholar]
- Hou, Z.; Liu, Y.; Tian, S.; Yang, Z.; Xie, Y. Formation of carbonatite-related giant rare-earth-element deposits by the recycling of marine sediments. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Huang, Z.; Liu, C.; Qi, L.; Li, W.; Guan, T. Geochemistry of carbonatites in Maoniuping REE deposit, Sichuan Province, China. Sci. China 2004, 46, 246–256. [Google Scholar] [CrossRef]
- Yuhenga, J.; Yan, L. Factors controlling the generation and diversity of giant carbonatite-related rare earth element deposits: Insights from the Mianning–Dechang belt. Ore Geol. Rev. 2020, 121, 103472. [Google Scholar] [CrossRef]
- Vrublevsky, V.V.; Gertner, I.F. Nature of Carbonatite Containing Complexes of Folded Areas: Isotope Evidence of Mantle–Crust Interaction. In Problems of Deep Magmatism and Plume Sources; Innset of Geography of SB RAS: Irkutsk, Russia, 2005; pp. 30–49. [Google Scholar]
- Vrublevskii, V.V. Sources and geodynamic setting of petrogenesis of the Middle Cambrian Upper Petropavlovka alkaline basic pluton (Kuznetsk Alatau, Siberia). Russ. Geol. Geophys. 2015, 56, 379–401. [Google Scholar] [CrossRef]
- Vrublevskii, V.V.; Krupchatnikov, V.I.; Izokh, A.E.; Gertner, I.F. The alkaline and carbonatitic rocks of Gorny Altai (Edel’veis complex) as indicators of Early Paleozoic plume magmatism in the Central Asian Fold Belt. Russ. Geol. Geophys. 2012, 53, 721–735. [Google Scholar] [CrossRef]
- Nikiforov, A.V.; Bolonin, A.V.; Sugorakova, A.M.; Popov, V.A.; Lykhin, D.A. Carbonatites of Central Tuva: Geological structure, mineral and chemical composition. Geol. Ore Depos. 2005, 47, 360–382. [Google Scholar]
- Doroshkevich, A.G.; Ripp, G.S. The Arshan REE carbonatites, Southwestern Transbaikalia, Russia: Mineralogy, paragenesis and evolution. Can. Mineral. 2008, 46, 807–823. [Google Scholar] [CrossRef]
- Ivensen, Y.P. Timan’s Magmatism and the Kanin Peninsula; Nauka: Leningrad, Russia, 1964; p. 124. (In Russian) [Google Scholar]
- Kostyukhin, M.N.; Stepanenko, V.I. Baikal Magmatism of Kanino-Timan Region; Shurkin, K.A., Makhlaev, L.V., Eds.; Nauka: Leningrad, Russia, 1987; p. 232. (In Russian) [Google Scholar]
- Levin, V.Y.; Ronenson, B.M.; Samkov, V.S.; Levina, I.A.; Sergeev, N.S.; Kiselev, A.P. Alkaline and Carbonatite Complexes of the Urals; Uralgeolkom: Ekaterinburg, Russia, 1997; p. 270. (In Russian) [Google Scholar]
- Nedosekova, I.L.; Belousova, E.A.; Sharygin, V.V.; Belyatsky, B.V.; Baynova, T.B. Origin and evolution of the Il’meny–Vishnevogorsky carbonatites (Urals, Russia): Insights from trace–elements compositions, Rb–Sr, Sm–Nd, U–Pb and Lu–Hf isotope data. Mineral. Petrol. 2013, 107, 101–123. [Google Scholar] [CrossRef]
- Vrublevskii, V.V.; Pokrovskii, B.G.; Zhuravlev, D.Z.; Anoshin, G.N. Composition and age of the Penchenga linear carbonatite complex, Yenisei Range. Petrology 2003, 11, 130–146. [Google Scholar]
- Bell, K.; Rukhlov, A.S. Carbonatites from the Kola Alkaline Province: Origin, evolution and source characteristics. In Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; Zaitsev, A., Wall, F., Eds.; The Mineralogical Society of Great Britain and Ireland: London, UK, 2004; pp. 421–455. [Google Scholar]
- Bell, K.; Blenkinsop, J. Neodymium and strontium isotope geochemistry of carbonatites. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 278–300. [Google Scholar]
- Bell, K.; Petersen, T. Nd and Sr Isotope Systematics of Shombole Volcano, East Africa, and the Links between Nephelinite, Phonolites and Carbonatites. Geology 1991, 19, 582–585. [Google Scholar] [CrossRef]
- Kramm, U. Mantle components of carbonatite from the Kola Alkaline Province, Russia and Finland: A Nd–Sr study. Eur. J. Mineral. 1993, 5, 985–989. [Google Scholar] [CrossRef] [Green Version]
- Kramm, U.; Kogarko, L.N. Nd and Sr isotope signatures of the Khibina and Lovozero agpaitic centers, Kola alkaline province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Bell, K. Sr and Nd isotope data of apatite, calcite and dolomite as indicators of source, and the relationships of phoscorites and carbonatites from Kovdor massif, Kola peninsula, Russia. Contrib. Mineral. Petrol. 1995, 121, 324–335. [Google Scholar] [CrossRef]
- Dunworth, E.; Bell, K. The Turuy massif, Kola Peninsula, Russia: Isotopic and geochemical evidence for multi-source evolution. J. Petrol. 2001, 42, 377–405. [Google Scholar] [CrossRef]
- Bell, K. Carbonatites: Relationships to mantle plume activity. In Mantle Plumes: Their Identification Through Time; Ernst, R., Buchan, K.L., Eds.; Geological Society of America: Boulder, CO, USA, 2001; Volume 352, pp. 267–290. [Google Scholar]
- Kogarko, L.N.; Lahaye, Y.; Brey, G.P. Plume-related mantle source of super–large rare metal deposits from the Lovozero and Khibina massifs on the Kola Peninsula, Eastern part of Baltic shield: Sr, Nd and Hf isotope systematics. Mineral. Petrol. 2010, 98, 197–208. [Google Scholar] [CrossRef]
- Vladykin, N.V.; Pirajno, F. Types of carbonatites: Geochemistry, genesis and mantle sources. Lithos 2021, 386–387, 105982. [Google Scholar] [CrossRef]
- Vladykin, N.V. Model of nucleation and crystallization of ultrabasic-alkaline carbonatite magmas of Siberian region, problems of their ore content, mantle sources and connection with plume process. Russ. Geol. Geophys. 2016, 57, 889–905. [Google Scholar] [CrossRef]
- Hofmann, A.W. Mantle Geochemistry: The Message from Oceanic Volcanism. Nature 1997, 385, 219–229. [Google Scholar] [CrossRef]
- Vrublevskii, V.V.; Nikiforov, A.V.; Sugorakova, A.M.; Kozulina, T.V. Petrogenesis and tectonic setting of the Cambrian Kharly alkaline–carbonatite complex (Sangilen Plateau, Southern Siberia): Implications for the Early Paleozoic evolution of magmatism in the western Central Asian Orogenic Belt. J. Asian Earth Sci. 2020, 188, 104163. [Google Scholar] [CrossRef]
- Vrublevskii, V.V.; Morova, A.A.; Bukharova, O.V.; Konovalenko, S.I. Mineralogy and geochemistry of Triassic carbonatites in the Matcha alkaline intrusive complex (Turkestan–Alai Ridge, Kyrgyz Southern Tien Shan), SW Central Asian orogenic belt. J. Asian Earth Sci. 2018, 153, 252–281. [Google Scholar] [CrossRef]
- Xu, C.; Chakhmouradian, A.R.; Taylor, R.N.; Kynicky, J.; Li, W.; Song, W.; Fletcher, I.R. Origin of carbonatites in the South Qinling orogen: Implications for crustal recycling and timing of collision between the South and North China Blocks. Geochim. Cosmochim. Acta 2014, 143, 189–206. [Google Scholar] [CrossRef]
- Stoppa, F.; Rukhlov, A.C.; Bell, K.; Schiazza, M.; Vichi, G. Lamprophyres of Italy: Early Cretaceous alkaline lamprophyres of Southern Tuscany, Italy. Lithos 2014, 188, 97–112. [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Vladykin, N.V.; Pribavkin, S.V.; Bayanova, T.B. Ilmeno-Vishnevogorskiy myaskit-carbonatite complex: Origin, ore content, sources of substance (Ural, Russia). Geol. Ore Depos. 2009, 51, 157–181. [Google Scholar] [CrossRef]
- Bell, K.; Tilton, G.R. Nd, Pb and Sr Isotopic Compositions of East African Carbonatites: Evidence for Mantle Mixing and Plume Inhomogeneity. J. Petrol. 2001, 42, 1927–1945. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Sun, W.-D.; Zhang, Y.-X.; Zheng, Y.-F. Geochemical constraints on the genesis of the Bayan Obo Fe-Nb-REE deposit in Inner Mongolia, China. Geochim. Cosmochim. Acta 2009, 73, 1417–1435. [Google Scholar] [CrossRef]
- Castor, S.B. The Mountian Pass rare-earth carbonatite and associated ultrapotassic rocks, California. Can. Mineral. 2008, 46, 779–806. [Google Scholar] [CrossRef]
- Wall, F.; Zaitsev, A.N. Rare earth minerals in Kola carbonatites. In Phoscorites and Carbonatites from Mantle to Mine: The key Example of the Kola Alkaline Province; Zaitsev, A., Wall, F., Eds.; The Mineralogical Society of Great Britain and Ireland: London, UK, 2004; pp. 43–72. [Google Scholar]
- Chakhmouradian, A.; Zaitsev, A.N. Rare Earth Mineralization in Igneous Rocks: Sources and Processes. Elements 2012, 8, 347–353. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y. Occurrence and geochemistry of bastnasite in carbonatite-related REE deposits, Mianning–Dechang REE belt, Sichuan Province, SW China. Ore Geol. Rev. 2019, 107, 266–282. [Google Scholar] [CrossRef]
- Puchkov, V.N. Uralides and Timanides: Their structural relationship and position in the geologic history of the Ural-Mongolian Fold Belt. Rus. Geol. Geophys. 2003, 44, 28–39. (In Russian) [Google Scholar]
- Kuznetsov, N.B.; Soboleva, A.A.; Udoratina, O.V.; Hertseva, M.V.; Andreichev, V.L.; Dorokhov, N.S. Pre-Uralian Tectonic Evolution of the North-East and East Frame of the East European Craton. Part 1. Pre-Uralides, Timanides and Pre-Ordovician granitoid volcano-plutonic associations of the North Urals and Timan-Pechora Region. Lithosphere 2006, 4, 3–22. (In Russian) [Google Scholar]
- Puchkov, V.N. Geology of the Urals and the Trans.-Urals (Topical Issues of Stratigraphy, Tectonics, Geodynamics and Metallogeny); DesignPoligrafService: Ufa, Russia, 2010; p. 280. (In Russian) [Google Scholar]
- Udoratina, O.V.; Travin, A.V. Alkaline picrites of the Chetlas complex of Middle Timan: Ar–Ar data. In Proceedings of the Ore Potential of Alkaline, Kimberlite and Carbonative Magmatism: Materials XXX Internl Conference, Moscow, Russia, 29 September–2 October 2014; pp. 82–84. (In Russian). [Google Scholar]
- Bogdanova, S.V.; Pisarevsky, S.A.; Li, Z.X. Assembly and Breakup of Rodinia (Some Results of IGCP Project 440). Stratigr. Geol. Correl. 2009, 17, 259–274. [Google Scholar] [CrossRef]
- Andreichev, V.L.; Soboleva, A.A.; Udoratina, O.V.; Ronkin, Y.L.; Coble, M.A.; Miller, E.L. Granites of the Northern Timan—probable indicators of Neoproterozoic stages of Rodinia breakup. Geodyn. Tectonophys. 2020, 11, 201–218. [Google Scholar] [CrossRef]
- Kuznetsov, N.B.; Soboleva, A.A.; Udoratina, O.V.; Andreichev, V.L.; Hertseva, M.V. Pre-Ordovician tectonic evolution and volcano-plutonic associations of the Timanides and Northern Pre-Uralides, Northeast part of the East European Craton. Gondwana Res. 2007, 12, 305–323. [Google Scholar] [CrossRef]
- Makeev, A.B.; Lebedev, V.A.; Bryanchaninova, N.I. Magmatites of the Middle Timan; Yushkin, N.P., Ed.; UB RAS: Ekaterinburg, Russia, 2008; p. 348. (In Russian) [Google Scholar]
- Shilov, L.P.; Plyakin, A.M.; Alekseev, V.I. (Eds.) Timan Ridge. T.2. Lithology and Stratigraphy, Geophysical Characteristics of the Earth’s Crust, Tectonics, Mineral Resources; USTU: Ukhta, Russia, 2009; p. 460. (In Russian) [Google Scholar]
- Udoratina, O.V.; Travin, A.V.; Kulikova, K.V.; Varlamov, D.A. Evidence for an Early Permian pulse of ultrapotassic magmatism in the Middle Timan. MOIP 2016, 91, 29–35. (In Russian) [Google Scholar]
- Kononova, V.A.; Dontsova, E.I.; Kuznetsova, L.D. Isotopic composition of oxygen and strontium of the Ilmeno-Vishnevogorsk alkaline complex and questions of the genesis of miaskites. Geochemistry 1979, 12, 1784–1795. [Google Scholar]
- Kramm, U.; Blaxland, A.B.; Kononova, V.A.; Grauert, B. Origin of the Ilmenogorsk–Vishnevogorsk nepheline syenites, Urals, USSR, and their time of emplasement during the history of the Ural fold belt: A Rb–Sr study. J. Geol. 1983, 91, 427–435. [Google Scholar] [CrossRef]
- Kramm, U.; Chernyshev, I.V.; Grauert, B.; Kononova, V.A.; Bröcker, B. Zircon typology and U–Pb systematics: A case study of zircons from nepheline syenite of the Il’meny Mountains, Urals. Petrology 1993, 1, 474–485. [Google Scholar]
- Krasnobaev, A.A.; Rusin, A.I.; Busharina, S.V.; Lepekhina, E.N.; Medvedeva, E.V. Character of zircon distribution on amphibole miaskite of the Ilmenogorsk Massif (Southern Urals). Dokl. Earth Sci. 2010, 430, 76–79. [Google Scholar] [CrossRef]
- Krasnobaev, A.A.; Rusin, A.I.; Valizer, P.M.; Busharina, S.V. Zirconology of calcite carbonatite of the Vishnevogorsk Massif, Southern Urals. Dokl. Earth Sci. 2010, 431, 390–393. [Google Scholar] [CrossRef]
- Krasnobaev, A.A.; Busharina, S.; Valizer, P.M.; Medvedeva, E.V. Zirconology of miaskites of the Ilmeny Mountains (South Urals). Geochem. Intern. 2016, 54, 765–780. [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Belyatsky, B.V. Age and substance sources of the Ilmeno–Vishnevogorsky Alkaline Complex (South Urals): Rb–Sr, Sm–Nd, U–Pb, and Lu–Hf isotope data. Dokl. Earth Sci. 2012, 446, 1071–1076. [Google Scholar] [CrossRef]
- Krasnobaev, A.A.; Valizer, P.M.; Rusin, A.I.; Busharina, S.V.; Medvedeva, E.V. Zirconology of ultrabasic rocks of the Buldym Massif (Il’meno–Vishnevogorskii Complex, Southern Urals). Dokl. Earth Sci. 2015, 461, 235–241. [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Belyatsky, B.V.; Belousova, E.A. Trace elements and Hf isotope composition as indicators of zircon genesis in the evolution of the alkaline-carbonatite magmatic system (Ilmeno-Vishnevogorsky complex, Urals, Russia). Rus. Geol. Geophys. 2016, 57, 891–906. [Google Scholar] [CrossRef]
- Baluev, A.S. Continental Rifthogenesis of the North of the Eastern European Platform in Neogee: Geology, History of Development, Comparative Analysis. Doctoral Thesis, Geological Institute RAS, Moscow, Russia, 2013; p. 303. (In Russian). [Google Scholar]
- Nedosekova, I.L.; Koroteev, V.A.; Bayanova, T.B.; Serov, P.A.; Popova, V.I.; Chervyakovskaya, M.V. On the age of pyrochlore carbonatites of the Ilmeno-Vishnevogorsk alkaline complex, South Ural (according to Sm-Nd and Rb-Sr isotope methods). Lithosphere 2020, 20, 486–498. (In Russian) [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Belousova, E.A.; Sharygin, V.V. Sources of matter for the Il’meno-Vishnevogorsky Alkaline Complex: Evidence from Lu-Hf Isotopic data for zircons. Dokl. Earth Sci. 2010, 435, 1487–1491. [Google Scholar] [CrossRef]
- Ivanov, K.S.; Kontorovich, V.A.; Puchkov, V.N.; Fyodorov, Y.N.; Erokhin, Y.V. Tectonics of the Urals and the basement of West Siberia: The main features of the geological structure and development. Reg. Geol. 2014, 2, 22–35. (In Russian) [Google Scholar]
- Zoloev, K.K.; Levin, V.Y.; Moril, S.I.; Shardakova, G.Y. Mineralogy and Deposits of Rare Metals, Molybdenum, Tungsten from the Urals; Ministry of Natural Resources RF, Sverdlovsk Region State Department for Natural Resources, IGG UD RAS, JSC UGSE: Yekaterinburg, Russia, 2004; p. 336. (In Russian) [Google Scholar]
- Golubeva, I.I.; Remizov, D.N.; Burtsev, I.N.; Filippov, V.N.; Shuyskiy, A.S. Fluid-explosion ultramafic rocks of the Middle Timan dyke complex and their paragenetic association with carbonatite. Reg. Geol. Metallog. 2019, 80, 1–15. (In Russian) [Google Scholar]
- Nedosekova, I.L.; Udoratina, O.V.; Vladykin, N.V.; Pribavkin, S.V.; Gulyaeva, T.Y. Petrochemistry and Geochemistry of Dyke Ultrabasites and Carbonatites of the Chetlassky Complex (Middle Timan); Yearbook-2010; IGG UD RAS: Ekaterinburg, Russia, 2011; pp. 122–130. (In Russian) [Google Scholar]
- Makeev, A.B.; Bryanchaninova, N.I. Lamprophyres of Chetlassky Stone, Middle Timan. Reg. Geol. Metallog. 2009, 37, 51–73. (In Russian) [Google Scholar]
- Kovalchuk, N.S.; Shumilova, T.G.; Kozyreva, I.G. Mineralogy of rare–earth phases of Kosyui carbonatites. In Mineralogical Intervention in Micro—and Nanoworld: Mater. International. Mineralogical Seminar; IG KNS UB RAS: Syktyvkar, Russia, 2009; pp. 296–297. (In Russian) [Google Scholar]
- Kovalchuk, N.S. Evolution of the Chemical Composition of Pyrochlore from Carbonatites in the Kosyu Massif. In Structure, Substance, History of Lithosphere of Ural–Timan Segment; Geoprint: Syktyvkar, Russia, 2011; pp. 74–76. (In Russian) [Google Scholar]
- Kovalchuk, N.; Shumilova, T.G.; Stepanenko, V.I. Rare earth mineralization in carbonatite of Kosyu massif (Middle Timan). Zap. RMO 2013, 142, 109–132. (In Russian) [Google Scholar]
- Udoratina, O.V.; Kozyreva, I.V.; Shvetsova, I.V.; Kapitanova, V.A.; Filippov, V.N. Features of rare–metal accessory mineralization of the vein series of carbonatites (Kosyu ore field, Middle Timan). In Crystalline and Solid Non–Crystalline State of Mineral Substance; Geoprint: Syktyvkar, Russia, 2012; pp. 331–333. [Google Scholar]
- Varlamov, D.A.; Udoratina, O.V.; Burakov, N.N. Unusual monazites and Ce Segregation process during alkaline metasomatosis of acid substrates (Kosyu ore field, Middle Timan). In Proceedings of the XXXIV International Confence: Magmatism of the Earth and Related Strategic Metal Deposits, Miass, Russia, 4–9 August 2017; pp. 292–295. [Google Scholar]
- Varlamov, D.A.; Udoratina, O.V. Minerals of rare-metal-rare-earth ore fields of the Chetlassky Kamen of the Middle Timan, Geology and Mineral Resources of the European North-East of Russia. In Proceedings of the XVII Geological Congress of the Komi Republic, Syktyvkar, Russia; IG Komi SC UB RAS: Syktyvkar, Russia, 2019; pp. 160–162. [Google Scholar]
- Khalezova, E.B.; Nazarenko, I.I. About bastnesite the Cherry Mountains. Proc. IMGRE AS USSR 1959, 2, 99–101. (In Russian) [Google Scholar]
- Eskova, E.M.; Zhabin, A.; Muhitdinov, G. Mineralogy and Geochemistry of Rare Elements of the Cherry Mountains; Nauka: Moscow, Russia, 1964; p. 319. (In Russian) [Google Scholar]
- Efimov, A.F.; Eskova, E.M.; Lebedeva, S.I.; Levin, V.Y. Typochimism of accessory pyrochlore in rocks of the Ural alkaline complex. Geochemistry 1985, 2, 202–208. (In Russian) [Google Scholar]
- Polyakov, V.O.; Nedosekova, I.L. Mineralogy of apohyperbasite phenites and carbonatites of the southern part of the Ilmensky mountains. In Minerals of Deposits and Zones of Technogenesis of Ore Regions of the Urals; UB RAS: Sverdlovsk, Russia, 1990; pp. 24–35. (In Russian) [Google Scholar]
- Lebedeva, I.O.; Nedosekova, I.L. About process of eshinitization of pyrochlore from carbonatites of Buldym massif (Cherry Mountains, Ural). Zap. WMO 1993, 2, 69–75. (In Russian) [Google Scholar]
- Kobyashev, Y.S.; Magakonov, E.P.; Nikandrov, S.N. Minerals Vishnyevye Potanino Mountains; Ilmensky State Nature Reserve of the UB RAS: Miass, Russia, 1998; p. 77. (In Russian) [Google Scholar]
- Popov, V.A.; Popova, V.I. Mineralogy of Pegmatites in the Ilmensky Mountains. Mineralogical Almanac; Ecost: Moscow, Russia, 2006; Volume 9, p. 151. (In Russian) [Google Scholar]
- Popova, V.I.; Popov, V.A.; Blinov, I.A.; Kotlyarov, V.A.; Kasatkin, A.V.; Škoda, R.; Lebedeva, S.M. New findings of rare minerals in pegmatites of Vishnevye Mountains in the Southern Urals. Mineralogy 2019, 1, 1–14. (In Russian) [Google Scholar]
- Popova, V.I.; Popov, V.A.; Blinov, I.A.; Kotlyarov, V.A. New data on pyrochlore of alkali pegmatites and ore zones of the Vishnevye Mountains (Southern Urals). Mineralogy 2018, 4, 46–60. (In Russian) [Google Scholar]
- Popova, V.I.; Popov, V.A.; Kasatkin, A.V.; Kuznetsov, A.M. Aeschynite group minerals from Vishnevye Mountains (South Urals). Mineralogy 2019, 5, 16–25. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Popov, V.A. Titanite of the Vishnevogorsky Alkaline Complex (South Ural). Mineralogy 2019, 1, 29–35. (In Russian) [Google Scholar]
- Cherednichenko, S.V.; Kotlyarov, V.A. Mineralogy of zirconium and niobium in calcite-nepheline-feldspar pegmatite of the Ilmeno-Vishnevogorsk complex (South Urals). Zap. RMO 2019, 2, 87–99. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Kasatkin, A.V.; Škoda, R.; Nestola, F.; Kuznetsov, A.M.; Belogub, E.V.; Agakhanov, A.A. Röentgenite-(Ce) and other ree fluorcarbonates from vein No. 35, Vishnevye Mountains, Southern Urals. Mineralogy 2019, 5, 10–22. (In Russian) [Google Scholar] [CrossRef]
- Popov, V.A.; Rassomakhin, M.A.; Kolisnichenko, S.V. A unique ore locality of polyakovite-(Ce) in the Ilmeny Mountains, South Urals—new finds. Mineralogy 2020, 6, 17–32. (In Russian) [Google Scholar] [CrossRef]
- Atencio, D.; Andrade, M.B.; Christy, A.G.; Giere, R.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature. Can. Mineral. 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kempe, D.R.C. Carbonatites: Nomenclature, average compositions, and element distribution. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 1–14. [Google Scholar]
- Le Maitre, R.W. Igneous Rocks: A Classification and Glossary of Terms; Cambridge University Press: Cambridge, UK, 2002; p. 236. [Google Scholar]
- Bagdasarov, Y.A. Rare–metal ore potential of igneous and hydrothermal metasomatic carbonatites. Geol. Ore Depos. 1994, 36, 326–335. [Google Scholar]
- Arzamastsev, A.A.; Bea, F.; Glaznev, V.N.; Arzamastseva, L.V.; Montero, P. Kola alkaline province in the Palaeozoic: Evaluation of primary mantle magma composition and magma generation conditions. Rus. J. Earth Sci. 2001, 3, 1–32. [Google Scholar] [CrossRef]
- Nedosekova, I.L. New data on carbonatites of the Il’mensky–Vishnevogorsky alkaline complex, the Southern Urals, Russia. Geol. Ore Depos. 2007, 49, 129–146. [Google Scholar] [CrossRef]
- Petrov, O.V.; Sharpenok, L.N. (Eds.) Petrographic Code of Russia: Magmatic, Metamorphic, Metasomatic, Impact Formations; VSEGEI: Saint Petersburg, Russia, 2008; p. 198. (In Russian) [Google Scholar]
- Frolov, A.A.; Lapin, A.V.; Tolstov, A.V.; Zinchuk, N.N.; Belov, S.V.; Burmistrov, A.A. Carbonatites and Kimberlites; NIA–Nature: Moscow, Russia, 2005; p. 540. (In Russian) [Google Scholar]
- Rock, N.M.S. The nature and origin of Ultramafic Lamprophyres: Alnoites and Allied Rocks. J. Petrol. 1986, 27, 155–196. [Google Scholar] [CrossRef]
- Vladykin, N.V. Formation types of carbonatites, their geochemistry and genesis. In Depth Magmatism, its Sources and Plumes. Proceed of VIII Intern Workshop; IG SD RAS: Irkutsk, Russia, 2008; pp. 45–58. (In Russian) [Google Scholar]
- Mitchell, R.H. Carbonatites and carbonatites and carbonatites. Can. Mineral. 2005, 43, 2049–2068. [Google Scholar] [CrossRef]
- Stoppa, F.; Pirajno, F.; Schiazza, M.; Vladykin, N.V. State of the art: Italian carbonatites and their potential for critical–metal deposits. Gondwana Res. 2016, 37, 152–171. [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Vladykin, N.V.; Udoratina, O.V. Carbonatites of the Chetlassky Complex (Middle Timan): Geochemical and Isotope Data. In Yearbook-2012; IGG UD RAS: Ekaterinburg, Russia, 2013; Volume 160, pp. 150–158. (In Russian) [Google Scholar]
- Bryanchaninova, N.I.; Makeev, A.B.; Larionova, Y.O. Sm–Nd isotope systematics of Timan’s lamprophyre. In Proceedings of the Vseros Confence, IGEM RAS, Moscow, Russia, 8–11 November 2010; pp. 414–415. [Google Scholar]
- Hanyu, T.; Nakamura, E. Constraints on HIMU and EM by Sr and Nd isotopes re–examined. Earth Planets Space 2000, 52, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Zindler, A.; Hart, S.R. Chemical geodynamics. Ann. Rev. Earth Planet. Sci. 1986, 14, 493–571. [Google Scholar] [CrossRef]
- Hart, S.R.; Hauri, E.; Oschmann, L.A.; Whitehead, J.A. Mantle plumes and entrainment: Isotopic evidence. Science 1992, 256, 517–520. [Google Scholar] [CrossRef]
- Salters, V.J.M.; Stracke, A. Composition of the depleted mantle. J. Earth Sci. 2004, 5. [Google Scholar] [CrossRef]
- Stracke, A.; Hofmann, A.W.; Stan, R.; Hart, S.R. FOZO, HIMU, and the rest of the mantle zoo. J. Earth Sci. 2005, 6. [Google Scholar] [CrossRef]
- Tappe, S.; Foley, S.F.; Kjarsgaard, B.A.; Romer, R.L.; Heaman, L.M.; Stracke, A.; Jenner, G.A. Between carbonatite and lamproite–Diamondiferous Torngat ultramafic lamprophyres formed by carbonate–fluxed melting of cratonic MARID–type metasomes. Geochim. Cosmochim Acta 2008, 72, 3258–3286. [Google Scholar] [CrossRef] [Green Version]
- Tappe, S.; Foley, S.F.; Stracke, A.; Romer, R.L.; Kjarsgaard, B.A.; Heaman, L.M.; Joyce, N. Craton reactivation on the Labrador Sea margins: 40Ar/39Ar age and Sr–Nd–Hf–Pb isotope constraints from alkaline and carbonatite intrusives. Earth Planet. Sci. Lett. 2007, 256, 433–454. [Google Scholar] [CrossRef]
- Kogarko, L.; Zartman, R.E. New Data on the age of the Guli Intrusion and Implications for the relationships between Alkaline Magmatism in the Maymecha–Kotuy Province and the Siberian Superplume: U–Th–Pb Isotopic Systematics. Geochem. Intern. 2011, 49, 439–448. [Google Scholar] [CrossRef]
- Nelson, D.R. Isotopic characteristics and petrogenesis of the lamproites and kimberlites of central West Greenland. Lithos 1989, 22, 265–274. [Google Scholar] [CrossRef]
- Goldstein, S.J.; Jacobsen, S.B. Nd and Sr isotopic systematics of river water suspended material implications for crystal evolution. Earth Plan. Sci. Lett. 1988, 87, 249–265. [Google Scholar] [CrossRef]
- Hogarth, D.D. Classification and nomenclature of the pyrochlore group. Am. Mineral. 1977, 62, 403–410. [Google Scholar]
- Möller, P. REE (Y), Nb, and Ta enrichment in pegmatites and carbonatite–alkalic rock complexes. In Lanthanides, Tantalum and Niobium; Möller, P., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 38–67. [Google Scholar] [CrossRef]
- Walter, B.F.; Parsapoor, A.; Braunger, S.; Marks, M.A.W.; Wenzel, T.; Martin, M.; Markl, G. Pyrochlore as a monitor for magmatic and hydrothermal processes in carbonatites from the Kaiserstuhl volcanic complex (SW Germany). Chem. Geol. 2018, 498, 1–16. [Google Scholar] [CrossRef]
- Bambi, A.C.J.M.; Costanzo, A.; Goncë Alves, A.O.; Melgarejo, J.C. Tracing the chemical evolution of primary pyrochlore from plutonic to volcanic carbonatites: The role of fluorine. Mineral. Mag. 2012, 76, 377–392. [Google Scholar] [CrossRef]
- Hogarth, D.D.; Williams, C.T.; Jones, P. Primary Zoning in Pyrochlore Group Minerals from Carbonatites. Mineral. Mag. 2000, 64, 683–697. [Google Scholar] [CrossRef]
- Torro, L.; Villanova, C.; Castillo, M.; Campeny, M.; Gonçalves, A.O.; Melgarejo, J.C. Niobium and rare earth minerals from the Virulundo carbonatite, Namibe, Angola. Mineral. Mag. 2012, 76, 393–409. [Google Scholar] [CrossRef] [Green Version]
- Boniface, N. Crystal chemistry of pyrochlore from the Mesozoic Panda Hill carbonatite deposit, western Tanzania. J. Afr. Earth Sci. 2017, 126, 33–44. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Doroshkevich, A.G. Mineralogy of secondary olivine-hosted inclusions in calcite carbonatites of the Belaya Zima alkaline complex, Eastern Sayan, Russia: Evidence for late-magmatic Na-Ca-rich carbonate composition. J. Geol. Soc. India 2017, 90, 524–530. [Google Scholar] [CrossRef]
- Jago, B.C.; Gittins, J. Pyrochlore crystallization in carbonatites: The role of fluorine. S. Afr. J. Geol. 1993, 96, 149–159. [Google Scholar]
- Nasraoui, M.; Bilal, E. Pyrochlores from the Lueshe carbonatite complex (Democratic Republic of Congo): A geochemical record of different alteration stages. J. Asian Earth Sci. 2000, 18, 237–251. [Google Scholar] [CrossRef]
- Deditius, A.P.; Smith, F.N.; Utsunomiya, S.; Ewing, R.C. Role of vein phases in nanoscale sequestration of U, Nb, Ti, and Pb during the alteration of pyrochlore. Geochim. Cosmochim. Acta 2015, 150, 226–252. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V. Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia). Geosciences 2018, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Nedosekova, I.L.; Pribavkin, S.V. Ore niobium Mineralization of Rare Metal Deposits and Ore Occurrences of the Ilmeno–Vishnevogorsk Alkaline–Carbonatite Complex (South Urals); Yearbook-2014; IGG UD RAS: Ekaterinburg, Russia, 2015; pp. 175–183. (In Russian) [Google Scholar]
- Nedosekova, I.L.; Pribavkin, S.V. Ore niobium minerals of the pyrochlore group of carbonatite complexes of the Urals: Compositional features and geochemical evolution. Izv. USGU 2019, 3, 46–57. (In Russian) [Google Scholar] [CrossRef]
- Melgarejo, J.C.; Costanzo, A.; Bambi, A.C.J.M.; Gonçalves, A.O.; Neto, A.B. Subsolidus processes as a key factor on the distribution of Nb species in plutonic carbonatites: The Tchivira case, Angola. Lithos 2012, 152, 187–201. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. Lueshite, pyrochlore and monazite-(Ce) from apatite-dolomite carbonatite, Lesnaya Varaka complex, Kola Peninsula, Russia. Mineral. Mag. 1998, 62, 769–782. [Google Scholar] [CrossRef]
- Zheng, L.; Gu, X.; Zhang, Y. Pyrochlore Chemistry from the Bonga Carbonatite-type Nb Deposit, Huila Province, Angola: Implications for Magmatic-Hydrothermal Processes of Carbonatite. Acta Geol. Sin. Engl. 2014, 88, 487–488. [Google Scholar] [CrossRef]
- Khromova, E.A.; Doroshkevich, A.G.; Sharygin, V.V.; Izbrodin, L.A. Compositional Evolution of Pyrochlore-Group Minerals in Carbonatites of the Belaya Zima Pluton, Eastern Sayan. Geol. Ore. Depos. 2017, 59, 752–764. [Google Scholar] [CrossRef]
- Tremblay, J.; Bédard, L.P.; Matton, G. Columbitization of fluorcalciopyrochlore by hydrothermalism at the Saint-Honoré alkaline complex, Québec (Canada): New insights on halite in carbonatites. Ore Geol. Rev. 2017, 91, 695–707. [Google Scholar] [CrossRef]
- Chebotarev, D.A.; Doroshkevich, A.G.; Klemd, R.; Karmanov, N.S. Evolution of Nb-mineralization in the Chuktukon carbonatite massif, Chadobets upland (Krasnoyarsk Territory, Russia). Period. Mineral. 2017, 86, 99–118. [Google Scholar] [CrossRef]
- Wall, F.; Williams, C.T.; Woolley, A.R.; Nasraoui, M. Pyrochlore from Weathered Carbonatite at Lueshe, Zaire. Mineral. Mag. 1996, 60, 731–750. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, J.I.; Garcia, D.; Moutte, J.; Williams, C.T.; Wall, F.; Kim, Y. Pyrochlore chemistry from the Sokli phoscorite-carbonatite complex, Finland: Implications for the genesis of phoscorite and carbonatite association. Geochem. J. 2006, 40, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chakhmouradian, A.R.; Zaitsev, A.N. Calcite-amphibole-clinopyroxene rock from the Afrikanda Complex, Kola Peninsula, Russia; mineralogy and a possible link to carbonatites; I, Oxide minerals. Canad. Mineral. 1999, 37, 177–198. [Google Scholar]
- Chakhmouradian, A.R.; Williams, C.T. Mineralogy of high-field-strength elements (Ti, Nb, Zr, Ta, Hf) in phoscoritic and carbonatitic rocks of the Kola Peninsula, Russia. In Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; The Mineralogical Society of Great Britain and Ireland: London, UK, 2004; Volume 10, pp. 293–340. [Google Scholar]
- Chakhmouradian, A.R. High-field-strength elements in carbonatitic rocks: Geochemistry, crystal chemistry and significance for constraining the sources of carbonatites. Chem. Geol. 2006, 235, 138–160. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. The mineralogy of Ba-and Zr-rich alkaline pegmatites from Gordon Butte, Crazy Mountains (Montana, USA): Comparisons between potassic and sodic agpaitic pegmatites. Contrib. Mineral. Petrol. 2002, 143, 93–114. [Google Scholar] [CrossRef]
- Simonov, V.A. Mineral. Formation Conditions in Nongranite Pegmatites; Science: Novosibirsk, Russia, 1981; p. 169. (In Russian) [Google Scholar]
- Andrsen, T. Composition variation of some rare metal minerals from the Fen compex (Telemark, SE, Norway): Implications for the mobility of rare earth in a carbonatite system. Miner. Mag. 1986, 50, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.P.; Henderson, P.; Campbell, L.S. Fractionation of the REE during hydrothermal processes: Constraints from the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 2000, 64, 3141–3160. [Google Scholar] [CrossRef]
- Xu, C.; Campbell, I.H.; Kynicky, J.; Allen, C.M.; Chen, Y.; Huang, Z.; Qi, L. Comparison of the Daluxiang and Maoniuping carbonatitic REE deposits with Bayan Obo REE deposit, China. Lithos 2008, 106, 12–24. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.; Chakhmouradian, A.R.; Campbell, I.H.; Allen, C.M. Trace element modeling of the magmatic evolution of rare-earth-rich carbonatite from the Miaoya deposit, Central China. Lithos 2010, 118, 145–155. [Google Scholar] [CrossRef]
- Lapin, A.V.; Tolstov, A.V.; Kulikova, I.M. Distribution of REE, Y, Sc, and Th in the unique complex rare-metal ores of the Tomtor deposit. Geochem. Int. 2016, 54, 1061–1078. [Google Scholar] [CrossRef]
- Yang, X.M.; Le Bas, M.J. Chemical compositions of carbonate minerals from Bayan Obo, Inner Mongolia, China: Implications for petrogenesis. Lithos 2004, 72, 97–116. [Google Scholar] [CrossRef]
- Wall, F.; Mariano, A.N. Rare earth minerals in carbnatites: A discussion centred on the Kangankunde carbonatite, Malawi. In Rare Earths Minerals: Chemistry, Origin and Ore Deposits; Jones, A.P., Wall, F., Williams, C.T., Eds.; Chapman & Hall: London, UK, 1996; Volume 7, pp. 193–225. [Google Scholar]
- Wang, Z.-Y.; Fan, H.-R.; Zhou, L.; Yang, K.-F.; She, H.-D. Carbonatite-related REE deposits: An overview. Minerals 2020, 10, 965. [Google Scholar] [CrossRef]
- Fournier, A. Magmatic and Hydrothermal Controls of LREE Mineralization of the St.—Honoré Carbonatite; McGill: Québec, QC, Canada, 1993; pp. 1–95. [Google Scholar]
- Andrade, F.R.D.; Möller, P.; Lüders, V.; Dulski, P.; Gilg, H.A. Hydrothermal rare earth elements mineralization in the Barra do Itapirapuã carbonatite, southern Brazil: Behaviour of selected trace elements and stable isotopes (C, O). Chem. Geol. 1999, 155, 91–113. [Google Scholar] [CrossRef]
- Szucs, A.M.; Stavropoulou, A.; O’Donnell, C.; Davis, S.; Rodriguez-Blanco, J.D. Reaction Pathways toward the Formation of Bastnäsite: Replacement of Calcite by Rare Earth Carbonates. Cryst. Growth Des. 2021, 21, 512–527. [Google Scholar] [CrossRef]
- Boyarko, G.Y. Dynamics of global production and commodity flows of niobium raw materials. Bull. Tomsk. Polytech. Univ. Geo. Assets Eng. 2019, 330, 216–229. [Google Scholar] [CrossRef]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A Detailed Assessment of Global Rare Earth Element Resources: Opportunities and Challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Jones, A.P.; Genge, M.; Carmody, L. Carbonate Melts and Carbonatites. Rev. Mineral. Geochem. 2013, 75, 289–322. [Google Scholar] [CrossRef] [Green Version]
- Samoilov, V.S. Geochemistry of Carbonatites; Nedra: Moscow, Russia, 1984; p. 190. (In Russian) [Google Scholar]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Chakhmouradian, A.R.; Kynicky, J.; Li, Y.; Song, W.; Chen, W.A. Paleoproterozoic mantle source modified by subducted sediments under the North China craton. Geochim. Cosmochim. Acta 2019, 245, 222–239. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Kressall, R.D.; Crozier, J.; Pisiak, L.K.; Sidhu, R.; Yang, P. Carbonatite-Hosted Niobium Deposit at Aley, Northern British Columbia (Canada): Mineralogy, Geochemistry and Petrogenesis. Ore Geol. Rev. 2015, 64, 642–666. [Google Scholar] [CrossRef]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lantanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Talantsev, A.S.; Petrova, G.A. Conditions and Mechanism of Formation of Carbonatites of the Ilmenogorsk-Vishnevogorsk Alkaline Complex; IGG UD RAS: Sverdlovsk, Russia, 1991; p. 70. (In Russian) [Google Scholar]
- Levin, V.Y. Alkaline Province of the Il’Meny-Vishnevye Mountains (Nepheline Syenites of the Urals); Nauka: Moscow, Russia, 1974; p. 221. (In Russian) [Google Scholar]
- Kogarko, L.N.; Henderson, M.; Foland, K. Evolution and isotopic sources of Guli ultrabasic alkaline massif. Dokl. Earth Sci. 1999, 364, 235–247. [Google Scholar]
- Vladykin, N.V. Geochemistry of Sr and Nd isotopes of alkaline and carbonatite complexes of Siberia and Mongolia and some geodynamic consequences. In Problems of Sources of Deep Magmatism and Plumes; IG SD RAS: Irkutsk, Russia, 2005; pp. 13–29. [Google Scholar]
- Sazonov, A.M.; Vrublevsky, V.V.; Gertner, I.F.; Fedorova, A.V.; Gavrilenko, V.V.; Zvyagina, E.A.; Leont’ev, S.I. The Transangara Alkaline Pluton, Yenisei Range: Rb–Sr and Sm–Nd isotope ages and sources of feldspathoid magmas in Late Precambrian. Dokl. Earth Sci. 2007, 413, 469–473. [Google Scholar] [CrossRef]
- Vrublevskii, V.V.; Bukharova, O.V.; Nebera, T.S.; Sveshnikova, V.L. Composition and origin of rare–metal (Nb–Ta, REE) and sulfide mineralization in magnesiocarbonatites from the Yenisei Ridge, Central Siberia. Ore Geol. Rev. 2019, 111, 1–26. [Google Scholar] [CrossRef]
- Khromova, E.A.; Doroshkevich, A.G.; Izbrodin, I.A. Geochemical and Sr-Nd-Pb isotope characteristics of alkaline rocks and carbonaites of the Beloziminsky Massif (East Sayan). Geospheric. Res. 2020, 1, 33–55. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Kempton, P.D.; Harmon, R.S.; Hawkeswortth, C.J. Petrology and geochemistry of lower crustal granulites from the Geronimo volcanic field, Southeastern Arizona. Geochim. Cosmochim. Acta 1990, 54, 3401–3426. [Google Scholar] [CrossRef]
- Yang, K.-F.; Fan, H.-R.; Santosh, M.; Hu, F.-F.; Wang, K.-Y. Mesoproterozoic carbonatitic magmatism in the Bayan Obo deposit, Inner Mongolia, North China: Constraints for the mechanism of super accumulation of rare earth elements. Ore Geol. Rev. 2011, 40, 122–131. [Google Scholar] [CrossRef]
- Ying, J.-F.; Zhou, X.-H.; Zhang, H.-F. Geochemical and isotopic investigation of the Laiwu–Zibo carbonatites from western Shandong Province, China, and implications for their petrogenesis and enriched mantle source. Lithos 2004, 75, 413–426. [Google Scholar] [CrossRef]
- Demeny, A.; Ahijado, R.; Casillas, T.W.; Vennemann, M. Crustal contamination and fluid rock interaction in the carbonatites of Fuerteventura-Canary Islands, Spain: A C, O, H isotope study. Lithos 1998, 44, 101–115. [Google Scholar] [CrossRef]
- Faure, G. Principles of Isotope Geology; Wiley: Hoboken, NJ, USA, 1989; p. 590. [Google Scholar]
- Kalt, A.; Hegner, E.; Satir, M. Nd, Sr, and Pb isotopic evidence for diverse lithospheric mantle sources of East African Rift carbonatites. Tectonophysics 1997, 278, 31–45. [Google Scholar] [CrossRef] [Green Version]

| Pcl I | Pcl II | Pcl III | Pcl IV | Pcl V | Aesh | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Sample | 37–95 | Soln-1 | K2-18 | Do-21 | 43–62 | 140-1 | 3296 | 92 | B-331 | 31-6c | 31-6m | 31-6r | 2–23 |
| Nb2O5 | 38.72 | 40.58 | 46.29 | 64.76 | 61.56 | 65.64 | 66.95 | 59.97 | 64.66 | 60.33 | 52.08 | 59.26 | 44.65 |
| Ta2O5 | 4.02 | 3.90 | 0.84 | 2.21 | 3.21 | 0.19 | - | - | 0.22 | 0.31 | - | - | 0.01 |
| SiO2 | - | - | 0.04 | - | - | - | 0.02 | - | - | 1.58 | - | - | - |
| TiO2 | 12.50 | 12.42 | 8.52 | 4.32 | 4.85 | 4.78 | 2.96 | 6.89 | 4.50 | 4.45 | 4.42 | 3.51 | 14.58 |
| UO2 | 22.11 | 23.75 | 17.57 | 0.07 | 1.81 | 0.07 | - | 0.42 | 2.78 | 3.72 | 3.72 | 0.85 | 0.04 |
| ThO2 | 0.78 | 0.45 | 0.69 | 0.45 | 1.78 | 0.27 | 0.51 | 1.01 | 0.41 | 0.30 | 0.53 | 0.59 | 9.34 |
| Fe2O3 | 0.00 | 0.19 | 1.54 | 0.02 | 0.11 | 0.02 | 0.01 | 0.07 | - | 1.52 | 1.69 | 1.50 | 2.20 |
| Y2O3 | 0.12 | - | 0.11 | - | - | - | - | 0.10 | - | 0.10 | - | 0.03 | 0.48 |
| La2O3 | 0.31 | 0.00 | 0.18 | 0.32 | 0.12 | 0.61 | 0.05 | 1.15 | 0.55 | 0.37 | 0.70 | 1.08 | 4.29 |
| Ce2O3 | 0.72 | 0.00 | 0.40 | 1.10 | 0.73 | 1.27 | 0.20 | 2.87 | 1.45 | 1.52 | 2.42 | 2.98 | 11.28 |
| Nd2O3 | 0.94 | 0.19 | 0.84 | 0.22 | 0.24 | 0.31 | 0.06 | 0.96 | 0.39 | n.a. | n.a. | n.a. | 3.48 |
| MnO | - | 0.09 | 0.11 | - | - | - | 0.01 | 0.02 | 0.03 | 0.21 | 0.42 | 0.50 | 0.08 |
| CaO | 11.06 | 12.02 | 13.91 | 15.08 | 15.48 | 16.06 | 16.21 | 13.19 | 13.74 | 11.68 | 6.04 | 12.24 | 6.26 |
| BaO | - | - | - | - | - | - | - | - | - | 0.77 | 2.39 | 0.73 | 0.20 |
| SrO | 0.28 | - | 0.17 | 1.00 | 0.51 | 0.56 | 0.67 | 0.92 | 1.29 | 1.97 | 5.01 | 3.27 | 0.06 |
| PbO | 0.91 | 1.00 | 0.95 | - | - | 0.25 | 0.04 | 0.21 | 0.29 | 0.48 | 0.31 | 0.05 | 0.00 |
| Na2O | 5.11 | 5.16 | 4.88 | 6.99 | 6.37 | 7.23 | 7.50 | 7.56 | 6.94 | 3.64 | 0.05 | 2.09 | 0.12 |
| F | 1.00 | 1.02 | 1.76 | 4.72 | 4.42 | 4.59 | 4.33 | 5.13 | 3.75 | 2.76 | 0.24 | 2.13 | - |
| Total | 98.56 | 100.77 | 98.79 | 101.24 | 101.20 | 101.85 | 99.51 | 100.46 | 101.00 | 95.71 | 80.02 | 90.82 | 97.07 |
| O = F2 | 0.42 | 0.43 | 0.74 | 1.99 | 1.86 | 1.93 | 1.82 | 2.16 | 1.58 | 1.16 | 0.10 | 0.90 | 0.00 |
| Total | 98.14 | 100.34 | 98.05 | 99.27 | 99.34 | 99.92 | 97.69 | 98.30 | 99.42 | 94.55 | 79.92 | 89.92 | 97.07 |
| Nb, apfu | 1.250 | 1.270 | 1.455 | 1.767 | 1.716 | 1.780 | 1.861 | 1.676 | 1.789 | 1.632 | 1.673 | 1.753 | 1.231 |
| Ta | 0.078 | 0.073 | 0.016 | 0.036 | 0.054 | 0.003 | - | - | 0.004 | 0.005 | - | - | - |
| Ti | 0.672 | 0.647 | 0.445 | 0.196 | 0.225 | 0.216 | 0.137 | 0.320 | 0.207 | 0.200 | 0.236 | 0.173 | 0.668 |
| Fe3+ | - | 0.010 | 0.081 | 0.001 | 0.005 | 0.001 | 0.001 | 0.003 | 0.000 | 0.068 | 0.090 | 0.074 | 0.101 |
| Si | - | - | 0.003 | - | - | - | 0.001 | - | - | 0.095 | - | - | - |
| Sum B | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| Ca | 0.847 | 0.892 | 1.036 | 0.975 | 1.023 | 1.032 | 1.068 | 0.874 | 0.901 | 0.749 | 0.460 | 0.858 | 0.409 |
| Mn | - | 0.005 | 0.007 | - | - | - | 0.001 | 0.001 | 0.002 | 0.011 | 0.025 | 0.028 | 0.004 |
| Ba | - | - | 0.000 | - | - | - | - | - | 0.018 | 0.067 | 0.019 | 0.005 | |
| Sr | 0.011 | 0.007 | 0.035 | 0.018 | 0.019 | 0.024 | 0.033 | 0.046 | 0.068 | 0.206 | 0.124 | 0.002 | |
| Pb | 0.018 | 0.019 | 0.018 | - | - | 0.004 | 0.001 | 0.003 | 0.005 | 0.008 | 0.006 | 0.001 | - |
| Na | 0.708 | 0.693 | 0.657 | 0.818 | 0.761 | 0.841 | 0.894 | 0.906 | 0.824 | 0.422 | 0.007 | 0.265 | 0.015 |
| Y | 0.004 | - | 0.004 | - | - | - | - | 0.003 | 0.000 | 0.003 | 0.000 | 0.001 | 0.016 |
| LREE | 0.051 | 0.005 | 0.036 | 0.036 | 0.025 | 0.048 | 0.007 | 0.112 | 0.053 | 0.041 | 0.081 | 0.097 | 0.424 |
| U | 0.351 | 0.366 | 0.272 | 0.001 | 0.025 | 0.001 | - | 0.006 | 0.038 | 0.050 | 0.059 | 0.012 | 0.001 |
| Th | 0.013 | 0.007 | 0.011 | 0.006 | 0.025 | 0.004 | 0.007 | 0.014 | 0.006 | 0.004 | 0.009 | 0.009 | 0.130 |
| Sum A | 2.003 | 1.986 | 2.047 | 1.871 | 1.877 | 1.949 | 2.001 | 1.953 | 1.874 | 1.374 | 0.920 | 1.414 | 1.004 |
| A-deficit | −0.003 | 0.014 | −0.047 | 0.129 | 0.123 | 0.051 | −0.001 | 0.047 | 0.126 | 0.626 | 1.080 | 0.586 | |
| F | 0.225 | 0.223 | 0.388 | 0.900 | 0.861 | 0.871 | 0.843 | 1.004 | 0.726 | 0.522 | 0.054 | 0.441 | - |
| Monazites | Bastnäsites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Sample | Pol-8 | Pol-9 | 3-15 | 2-1 | 2-2 | 3-14 | 3-19 | 1385(5) | 1385(3) | 1385(4) | 1385(11) |
| CaO | 2.5 | 1.5 | 0.15 | 0.42 | - | 2.31 | 2.2 | 1.29 | 2.36 | 1.18 | 1.29 |
| La2O3 | 21.09 | 23.4 | 11.2 | 12.09 | 14.53 | 18.31 | 20.45 | 32.51 | 26.46 | 32.75 | 31.44 |
| Ce2O3 | 30.20 | 32.31 | 29.72 | 25.95 | 33.79 | 5.89 | 6.15 | 34.43 | 35.93 | 34.51 | 33.92 |
| Pr2O3 | 2.37 | 2.86 | 3.59 | 2.59 | 3.68 | 6.03 | 6.46 | n.a. | n.a | n.a. | n.a. |
| Nd2O3 | 6.24 | 5.85 | 14.5 | 12.3 | 11.61 | 29.29 | 25.53 | 3.72 | 5.90 | 3.93 | 4.59 |
| Sm2O3 | 1.00 | 0.26 | 2.01 | 3.94 | 1.54 | 4.01 | 3.11 | n.a. | n.a. | n.a. | n.a. |
| Gd2O3 | 0.81 | 0.26 | 1 | 3.3 | 1.25 | 1.01 | 0.5 | n.a. | n.a. | n.a. | n.a. |
| ThO2 | n.a. | n.a. | 3.42 | 9.27 | 0.31 | 0.82 | - | n.a. | n.a. | n.a. | n.a. |
| PbO | n.a. | n.a. | 0.49 | 0.61 | 0 | 1.24 | 2.79 | n.a. | n.a. | n.a. | n.a. |
| P2O5 | 32.20 | 30.09 | 33.39 | 28.36 | 31.45 | 29.92 | 29.54 | - | - | - | - |
| F | - | - | - | - | - | - | - | 4.33 | 4.42 | 3.34 | 1.47 |
| CO2 * | - | - | - | - | - | - | - | 20.00 | 20.17 | 20.05 | 19.8 |
| H2O * | - | - | - | - | - | - | - | 2.04 | 2.03 | 2.52 | 3.35 |
| O = F2 | - | - | - | - | - | - | - | 1.82 | 1.86 | 1.41 | 0.62 |
| Total | 96.41 | 96.53 | 99.47 | 98.83 | 98.16 | 98.83 | 96.73 | 96.50 | 95.42 | 96.88 | 95.25 |
| Ca | 0.106 | 0.063 | 0.007 | 0.018 | 0.000 | 0.094 | 0.184 | 0.051 | 0.092 | 0.046 | 0.05 |
| La | 0.308 | 0.340 | 0.175 | 0.181 | 0.220 | 0.256 | 0.294 | 0.439 | 0.354 | 0.441 | 0.429 |
| Ce | 0.437 | 0.466 | 0.460 | 0.385 | 0.509 | 0.082 | 0.088 | 0.462 | 0.477 | 0.461 | 0.459 |
| Pr | 0.034 | 0.041 | 0.055 | 0.038 | 0.055 | 0.083 | 0.092 | n.a. | n.a. | n.a. | n.a. |
| Nd | 0.088 | 0.082 | 0.219 | 0.178 | 0.171 | 0.397 | 0.356 | 0.049 | 0.076 | 0.051 | 0.061 |
| Sm | 0.014 | 0.004 | 0.029 | 0.055 | 0.022 | 0.052 | 0.042 | n.a. | n.a. | n.a. | n.a. |
| Gd | 0.013 | 0.004 | 0.017 | 0.053 | 0.020 | 0.015 | 0.008 | n.a. | n.a. | n.a. | n.a. |
| Th | n.a. | n.a. | 0.033 | 0.085 | 0.003 | 0.007 | - | n.a. | n.a. | n.a. | n.a. |
| Pb | n.a. | n.a. | 0.006 | 0.007 | 0.000 | 0.013 | 0.029 | n.a. | n.a. | n.a. | n.a. |
| Total | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| F | - | - | - | - | - | - | - | 0.501 | 0.507 | 0.386 | 0.172 |
| OH | - | - | - | - | - | - | - | 0.499 | 0.493 | 0.614 | 0.828 |
| Miaskites | Sövite I | Sövite II | Ultrabasites | Sövite III | Beforsite IV | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| Sample | 324 | 337 | 338 | Sav4 | Po-4 | LPo-1 | 354 | D11-3 | LPo2 | 331 | V348 | 43332 | 505-27 | 15-22 | T-1б | K97-8 | 3296 | 1-54 | 10-21 |
| SiO2, wt% | 53.62 | 57.69 | 53.62 | 57.51 | 53.63 | 13.02 | 22.88 | 36.12 | 12.86 | 6.44 | 0.90 | 41.39 | 37.71 | 40.40 | 7.4 | 3.08 | 3.51 | 0.72 | 30.5 |
| TiO2 | 0.58 | 0.40 | 0.67 | 1.24 | 0.43 | 2.10 | 0.38 | 0.49 | 0.16 | 0.51 | 0.02 | 0.02 | 0.02 | 0.04 | 0.07 | 0.03 | 0.02 | 0.18 | 0.11 |
| Al2O3 | 20.98 | 21.55 | 18.45 | 17.55 | 20.29 | 4.57 | 8.71 | 11.96 | 3.38 | 2.00 | 0.03 | 0.73 | 0.76 | 1.24 | 1.4 | 0.56 | 0.74 | 0.02 | 1.29 |
| Fe2O3 | 1.64 | 1.25 | 1.43 | 1.90 | 1.42 | 0.01 | 0.17 | 1.02 | 0.70 | n.a. | 0.2 | 4.19 | 2.56 | 2.50 | 0.79 | 0.29 | 0.33 | 0.34 | 1.96 |
| FeO | 1.95 | 1.05 | 2.10 | 3.2 | 1.4 | 7.00 | 2.00 | 3.00 | 4.50 | 6.50 | 1.30 | 3.70 | 6.00 | 5.55 | 2.10 | 1.10 | 1.60 | 3.00 | 1.00 |
| MnO | 0.16 | 0.05 | 0.09 | 0.21 | 0.07 | 0.31 | 0.25 | 0.42 | 0.36 | 0.32 | 0.28 | 0.14 | 0.19 | 0.15 | 1.10 | 0.27 | 1.20 | 1.40 | 0.21 |
| MgO | 0.96 | 0.55 | 1.03 | 1.57 | 0.72 | 2.93 | 1.54 | 1.3 | 1.23 | 0.65 | 0.55 | 36.81 | 42.89 | 42.20 | 7.27 | 6.2 | 5.12 | 17.68 | 25.5 |
| CaO | 2.21 | 1.02 | 3.95 | 2.80 | 3.11 | 41.26 | 33.36 | 21.00 | 49.00 | 48.66 | 54.66 | 1.41 | 0.15 | 1.01 | 44.00 | 50.33 | 48.01 | 33.22 | 17.91 |
| Na2O | 6.60 | 6.20 | 6.00 | 5.8 | 9.7 | 0.90 | 2.50 | 5.20 | 2.20 | 0.50 | 0.4 | 0.20 | 0.4 | 0.60 | 0.60 | 0.40 | 0.20 | 0.15 | 0.7 |
| K2O | 7.97 | 7.14 | 7.62 | 5.66 | 5.15 | 3.36 | 4.32 | 4.90 | 1.69 | 1.48 | 0.4 | 0.02 | 0.12 | 0.40 | 1.09 | 0.54 | 0.68 | 0.01 | 0.01 |
| P2O5 | 0.23 | 0.02 | 0.10 | 0.24 | 0.04 | 2.45 | 0.79 | 0.42 | 1.21 | 2.37 | 1.22 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | 0.18 | 1.35 |
| S | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.80 | 1.31 | 1.24 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| LOI | 2.5 | 2.0 | 3.5 | 1.8 | 2.7 | 19.80 | 19.40 | 12.70 | 23.10 | 28.50 | 38.30 | 12.20 | 7.20 | 4.60 | 34.5 | 38.45 | 38.4 | 43.25 | 17.8 |
| Σ | 99.45 | 98.91 | 98.61 | 99.48 | 98.67 | 97.71 | 96.30 | 100.25 | 101.70 | 99.17 | 98.26 | 100.82 | 98.02 | 98.71 | 100.33 | 101.27 | 99.82 | 100.15 | 98.34 |
| Li, ppm | 9 | 2.14 | 5 | 32 | 2.2 | 10 | 2.8 | 13 | 8 | 9 | 12 | 13 | 8 | 14 | 18 | 16 | 50 | 0.09 | 3.48 |
| Rb | 59 | 34 | 42 | 114 | 55 | 120 | 52 | 14 | 64 | 29 | 17 | 2.27 | 3.8 | 18 | 52 | 42 | 170 | 0.4 | 0.12 |
| Sr | 2317 | 1661 | 2530 | 1405 | 1647 | 3953 | 9247 | 4051 | 11,527 | 21,982 | 16,498 | 309 | 26 | 56 | 9547 | 6336 | 5600 | 6611 | 3796 |
| Ba | 1816 | 5667 | 9857 | 1589 | 2406 | 3405 | 3054 | 380 | 793 | 282 | 542 | 28 | 24 | 42 | 484 | 198 | 140 | 233 | 302 |
| Sc | 1.61 | 1.25 | 1.36 | 2.54 | 0.96 | 4.04 | 3.14 | - | 5 | 3 | 1.2 | 5.2 | 6.0 | 8.2 | 5.8 | 0.57 | 2.0 | 1.15 | 1.78 |
| V | 179 | 194 | 100 | 78 | 69 | 239 | 61 | 56 | 108 | 134 | 13 | 22 | 19 | 29 | 67 | 1.18 | 20 | 8 | 51 |
| Cr | 93 | 84 | 70 | 8 | 4 | 53 | 25 | 7 | 14 | 5 | 11 | 1019 | 1451 | 911 | 138 | 23 | 50 | 23 | 35 |
| Co | 0.41 | 1.6 | 3.7 | 9 | 7 | 20 | 4.3 | 6 | 13 | 10 | 7 | 83 | 121 | 112 | 7 | 3.6 | 12 | 16 | 7 |
| Ni | 1.07 | 16 | 1.8 | 9 | 7 | 16 | 11 | 5 | 22 | 2.69 | 33 | 1519 | 1570 | 2363 | 13 | 39 | 190 | 13 | 30 |
| Cu | 0.33 | 7 | 8 | 34 | 30 | 21 | 15 | 8 | 25 | 8 | 15 | 19 | 26 | 18 | 24 | 32 | 3.8 | 20 | 24 |
| Zn | 61 | 22 | 64 | 87 | 25 | 174 | 32 | 59 | 36 | 85 | 13 | 39 | 50 | 52 | 88 | 14 | 110 | 47 | 8 |
| Y | 10 | 2.07 | 11 | 20 | 6 | 61 | 98 | 47 | 88 | 73 | 138 | 2.80 | 1.94 | 8 | 62 | 523 | 50 | 93 | 74 |
| Nb | 57 | 45 | 51 | 188 | 54 | 123 | 57 | 660 | 1598 | 98 | 872 | 0.73 | 5.6 | 12 | 930 | 8 | 5800 | 88 | 15 |
| Ta | 2.4 | 2.1 | 3.6 | 20 | 4.3 | 11 | 16.7 | 34 | 7 | 0.07 | 3.25 | 0.07 | 0.33 | 0.04 | 1.24 | 0.27 | 7 | 0.08 | 0.14 |
| Zr | 94 | 109 | 144 | 77 | 40 | 21 | 109 | 57 | 32 | 7 | 4.7 | 11 | 1.69 | 5.0 | 42 | 0.64 | 4.0 | 24 | 37 |
| Hf | 1.52 | 0.78 | 0.95 | 1.30 | 0.71 | 0.83 | 1.19 | 1.20 | 1.03 | 0.22 | 0.26 | 0.28 | 0.05 | 0.12 | 0.51 | 0.40 | - | 0.43 | 0.46 |
| Mo | 4 | 6 | 1.55 | 1.47 | 16 | 0.11 | 0.32 | 54 | 0.65 | 1.55 | 0.5 | 0.50 | 0.58 | 0.07 | 0.62 | 0.07 | 0.90 | 1.20 | 0.00 |
| Pb | 2.56 | 0.94 | 1.45 | 12 | 2.07 | 4.2 | 4.89 | 7 | 34 | 12 | 60 | 2.34 | 0.10 | 2.84 | 23 | 38 | 11 | 23 | 59 |
| Th | 1.66 | 0.32 | 0.85 | 13 | 1.43 | 1.94 | 3.07 | 19 | 19 | 14 | 2.81 | 0.87 | 0.08 | 0.77 | 22 | 0.38 | 21 | 681 | 1418 |
| U | n.a. | n.a. | n.a. | 3.2 | 2.4 | 0.4 | 1.01 | 32 | 27 | n.a. | 5 | 0.12 | 0.02 | 0.03 | n.a. | 0.04 | 9 | n.a. | n.a. |
| La | 105 | 15 | 40 | 80 | 23 | 191 | 370 | 373 | 388 | 926 | 849 | 6.01 | 1.12 | 5.78 | 577 | 1003 | 260 | 2285 | 18,959 |
| Ce | 162 | 28 | 77 | 142 | 35 | 394 | 641 | 618 | 731 | 1513 | 1176 | 9.21 | 2.82 | 12.6 | 1022 | 1822 | 400 | 4092 | 25,500 |
| Pr | 15 | 1.30 | 8.05 | 17 | 4.51 | 56 | 70 | 66 | 71 | 121 | 141 | 0.79 | 0.37 | 1.51 | 58 | 168 | 40 | 180 | 913 |
| Nd | 41 | 4.35 | 27 | 57 | 15 | 211 | 221 | 198 | 239 | 385 | 433 | 2.76 | 1.58 | 6.11 | 180 | 550 | 130 | 543 | 2273 |
| Sm | 4.76 | 0.66 | 3.92 | 7.62 | 2.17 | 31 | 31 | 22 | 41 | 58 | 50 | 0.49 | 0.37 | 1.33 | 38 | 100 | 18 | 70 | 168 |
| Eu | 1.27 | 0.26 | 1.91 | 2.01 | 0.87 | 9.57 | 8.95 | 5.84 | 11.7 | 16 | 12 | 0.11 | 0.11 | 0.40 | 10 | 26 | 5.0 | 34 | 29 |
| Gd | 3.16 | 0.55 | 2.95 | 6.14 | 1.75 | 29 | 25 | 14 | 39 | 43 | 43 | 0.41 | 0.36 | 1.21 | 28 | 110 | 16 | 65 | 87 |
| Tb | 0.40 | 0.07 | 0.40 | 0.69 | 0.21 | 2.70 | 3.04 | 1.81 | 4.03 | 6.0 | 4.93 | 0.07 | 0.06 | 0.20 | 4.3 | 13 | 1.60 | 10 | 11 |
| Dy | 1.93 | 0.35 | 1.99 | 4.17 | 1.26 | 14 | 15 | 10.7 | 23 | 31 | 26 | 0.46 | 0.35 | 1.35 | 23 | 86 | 9 | 60 | 46 |
| Ho | 0.35 | 0.07 | 0.40 | 0.79 | 0.25 | 2.48 | 3.10 | 2.13 | 4.33 | 5.82 | 5.85 | 0.10 | 0.07 | 0.29 | 5.0 | 20 | 1.90 | 11 | 6.6 |
| Er | 0.96 | 0.20 | 1.05 | 2.25 | 0.69 | 5.95 | 7.97 | 6.25 | 11.9 | 16 | 16 | 0.28 | 0.22 | 0.81 | 14 | 65 | 6 | 23 | 12 |
| Tm | 0.13 | 0.03 | 0.14 | 0.33 | 0.10 | 0.72 | 1.08 | 0.94 | 1.67 | 2.10 | 2.34 | 0.05 | 0.03 | 0.13 | 2.20 | 10 | 0.90 | 3.0 | 1.31 |
| Yb | 0.80 | 0.18 | 0.85 | 2.15 | 0.69 | 4.11 | 6.66 | 6.21 | 10.9 | 14 | 15 | 0.33 | 0.25 | 0.91 | 15 | 71 | 6.0 | 16 | 6.2 |
| Lu | 0.12 | 0.03 | 0.13 | 0.31 | 0.11 | 0.56 | 0.97 | 0.95 | 1.63 | 2.21 | 2.39 | 0.05 | 0.04 | 0.13 | 2.37 | 10 | 1.10 | 2.04 | 0.77 |
| ΣREE+Y | 348 | 53 | 178 | 342 | 93 | 1013 | 1504 | 1373 | 1666 | 3212 | 2918 | 23.9 | 9.69 | 40.8 | 2043 | 4576 | 946 | 7487 | 48,087 |
| Picrites | Lamprophyres | Carbonate-Bearing Lamprophyres | Carbonatites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Sample | 1217 | 13857a | 1290 | 13205 | 1284 | 13422 | 12701 | 1328 | 12242 | 1374 | T-3 | 13855 | 1389 |
| SiO2, wt% | 37.9 | 34.02 | 38.34 | 37.92 | 41.58 | 30.42 | 32.62 | 35.64 | 33.10 | 13.88 | 12.17 | 28.96 | 28.76 |
| TiO2 | 1.18 | 0.69 | 1.67 | 1.74 | 1.39 | 1.45 | 1.41 | 1.61 | 1.13 | 0.10 | 0.03 | 1.10 | 1.21 |
| A12O3 | 7.9 | 5.42 | 11.45 | 10.05 | 9.54 | 9.11 | 9.05 | 9.04 | 7.14 | 0.10 | 0.47 | 6.66 | 7.69 |
| Fe2O3 | 6.1 | 6.57 | 4.84 | 6.59 | 4.43 | 3.35 | 5.59 | 3.2 | 5.73 | 1.95 | 4.28 | 5.93 | 8.04 |
| FeO | 3.59 | 3.63 | 5.46 | 6.21 | 4.87 | 5.75 | 3.81 | 11.3 | 2.97 | 7.45 | 8.44 | 3.77 | 6.56 |
| MnO | 0.15 | 0.3 | 0.23 | 0.27 | 0.18 | 0.18 | 0.19 | 0.34 | 0.17 | 1.87 | 1.98 | 0.68 | 1.08 |
| MgO | 21.23 | 26.32 | 14.27 | 16.66 | 17.01 | 12.66 | 13.8 | 12.72 | 15.97 | 9.22 | 8.65 | 16.13 | 15.76 |
| CaO | 12.05 | 6.51 | 14.69 | 10.75 | 12.61 | 16.23 | 17.06 | 7.76 | 9.72 | 26.68 | 24.04 | 14.58 | 10.75 |
| Na2O | 0.52 | 0.49 | 1.23 | 1.47 | 0.66 | 0.9 | 0.59 | 0.18 | 3.68 | 0.31 | 0.1 | 1.48 | 0.34 |
| K2O | 1.4 | 2.58 | 2.3 | 1.76 | 3.14 | 5.19 | 3.72 | 4.24 | 3.67 | 0.05 | 0.4 | 4.39 | 1.38 |
| P2O5 | 0.28 | 0.51 | 0.70 | 0.17 | n.a. | 0.78 | 0.75 | 0.52 | 0.15 | 6.10 | 3.76 | 2.97 | 3.28 |
| CO2 | 1.90 | 3.98 | 2.06 | 2.26 | 1.01 | 10.78 | 6.84 | 10.57 | 13.34 | 30.67 | 32.37 | 9.46 | 9.66 |
| LOI | 7.19 | 11.73 | 4.25 | 4.96 | 3.1 | 12.54 | 9.99 | 12.6 | 16.38 | 30.69 | 31.09 | 11.95 | 13.78 |
| Total | 99.49 | 98.77 | 99.43 | 98.55 | 98.51 | 98.56 | 98.58 | 99.15 | 99.81 | 98.40 | 96.69 | 98.60 | 98.63 |
| Rb, ppm | 106 | 53 | 77 | 57 | 104 | 191 | 118 | 185 | 99 | 0.45 | 4 | 127 | 54 |
| Sr | 822 | 1085 | 993 | 612 | 407 | 1169 | 1519 | 165 | 447 | 7043 | 4232 | 1310 | 1063 |
| Ba | 1994 | 2725 | 1186 | 2428 | 884 | 1780 | 2244 | 858 | 613 | 1596 | 9607 | 2994 | 2518 |
| Sc | 15 | 6.4 | 30 | 21 | 27 | 22 | 27 | 22 | 15 | 0.35 | 4 | 22 | 12 |
| V | 149 | 57 | 209 | 185 | 195 | 222 | 256 | 229 | 135 | 4 | 5 | 122 | 185 |
| Cr | 686 | 621 | 514 | 532 | 1511 | 484 | 504 | 352 | 895 | 22 | 9 | 493 | 187 |
| Co | 52 | 84 | 52 | 59 | 50 | 51 | 40 | 37 | 45 | 8 | 24 | 39 | 44 |
| Ni | 393 | 649 | 222 | 382 | 364 | 235 | 130 | 119 | 466 | 8 | 10 | 145 | 110 |
| Cu | 58 | 40 | 78 | 100 | 61 | 80 | 128 | 39 | 46 | 4 | 45 | 27 | 78 |
| Zn | 132 | 152 | 69 | 85 | 46 | 53 | 52 | 33 | 50 | 106 | 25 | 69 | 124 |
| Y | 16 | 10 | 20 | 18 | 14 | 22 | 22 | 17 | 23 | 9 | 12 | 20 | 20 |
| Nb | 120 | 100 | 116 | 156 | 71 | 110 | 169 | 96 | 75 | 3 | 22 | 196 | 262 |
| Ta | 6 | 5 | 4 | 9 | 3 | 5 | 13 | 8 | 3.7 | 0.06 | 0.08 | 9 | 10 |
| Zr | 114 | 37 | 104 | 203 | 100 | 82 | 128 | 90 | 92 | 8 | 5 | 82 | 96 |
| Hf | 3.1 | 0.81 | 2.7 | 3.8 | 2.6 | 1.9 | 2.6 | 2.14 | 2.3 | 0.94 | 0.37 | 1.9 | 3 |
| Mo | 2.3 | 35 | 1.9 | 4.2 | 1.8 | 2.6 | 1.9 | 1.4 | 0.34 | 65 | - | 37 | 53 |
| Pb | 12 | 10 | 9 | 7 | 3 | 11 | 5 | 1.10 | 6 | 18 | 9 | 12 | 26 |
| Th | 24 | 25 | 28 | 14 | 9 | 37 | 14 | 6.5 | 45 | 36 | 112 | 22 | 82 |
| U | 2.9 | 0.99 | 5 | 1.6 | 2 | 4 | 4 | 1.2 | 0.48 | 0.21 | 0.3 | 0.54 | 2.7 |
| La | 110 | 476 | 129 | 62 | 54 | 84 | 122 | 74 | 49 | 3727 | 14,573 | 2610 | 3397 |
| Ce | 166 | 471 | 218 | 132 | 107 | 156 | 222 | 153 | 91 | 4126 | 17,212 | 3131 | 4227 |
| Pr | 20 | 39 | 22 | 15 | 11 | 17 | 22 | 16 | 10 | 277 | 1013 | 228 | 305 |
| Nd | 72 | 104 | 73 | 57 | 41 | 62 | 73 | 58 | 36 | 768 | 2286 | 579 | 764 |
| Sm | 10 | 9 | 10 | 9 | 6 | 8 | 10 | 9 | 6 | 39 | 100 | 35 | 47 |
| Eu | 2.8 | 2.1 | 2.7 | 2.7 | 1.8 | 2.5 | 2.9 | 2.2 | 2.0 | 7 | 19 | 7.3 | 9 |
| Gd | 7.2 | 5 | 7 | 7 | 5 | 7 | 7 | 6 | 6 | 22 | 58 | 23 | 23 |
| Tb | 0.86 | 0.58 | 0.79 | 0.80 | 0.54 | 0.93 | 0.82 | 0.70 | 0.91 | 0.95 | 1.8 | 1.08 | 1.67 |
| Dy | 4.5 | 3 | 5 | 5 | 3 | 5 | 5 | 4 | 6 | 3 | 6 | 6 | 7 |
| Ho | 0.81 | 0.52 | 0.83 | 0.78 | 0.58 | 0.93 | 0.87 | 0.70 | 1.08 | 0.48 | 0.7 | 0.93 | 1.04 |
| Er | 2.02 | 1.24 | 2.25 | 1.90 | 1.48 | 2.23 | 2.25 | 1.84 | 3.01 | 1.13 | 1.2 | 2.12 | 2.38 |
| Tm | 0.27 | 0.16 | 0.30 | 0.26 | 0.19 | 0.31 | 0.31 | 0.23 | 0.43 | 0.15 | 0.11 | 0.28 | 0.3 |
| Yb | 1.58 | 0.90 | 1.81 | 1.71 | 1.24 | 1.85 | 2.13 | 1.59 | 2.84 | 0.57 | 1.15 | 1.50 | 1.33 |
| Lu | 0.22 | 0.12 | 0.24 | 0.26 | 0.18 | 0.26 | 0.32 | 0.21 | 0.42 | 0.09 | 0.13 | 0.21 | 0.18 |
| REE + Y | 414 | 1123 | 493 | 313 | 247 | 370 | 493 | 344 | 238 | 8981 | 35,284 | 6645 | 8806 |
| № | Sample | Rock | Mineral | Rb, ppm | Sr, ppm | (87Sr/86Sr)t | ε Sr (T) | Sm, ppm | Nd, ppm | 147Sm/144Nd | 143Nd/144Nd | (143Nd/144Nd)t | ε Nd (T) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ilmeno–Vishnevogorsk miaskite–carbonatite complex, Southern Urals | |||||||||||||
| 1 | 338W | Miaskite | 79 | 2095 | 0.70341 | −8.2 | 3.9 | 27 | 0.08635 | 0.512569 | 0.512320 | 4.9 | |
| 2 | 324W | Miaskite | 122 | 1994 | 0.70342 | −7.9 | 4.8 | 42 | 0.06878 | 0.512549 | 0.512351 | 5.5 | |
| 3 | Dol-2W | Miaskite | 181 | 4525 | 0.70336 | −8.8 | 8.3 | 75 | 0.09310 | 0.512550 | 0.512282 | 4.1 | |
| 4 | Po-1W | Miaskite | 157 | 2791 | 0.70380 | −8.5 | 13.8 | 94 | 0.08839 | 0.512511 | 0.512256 | 3.6 | |
| 5 | 330–1 | Syenite | 105 | 383 | 0.70338 | −8.6 | 18.0 | 133 | 0.08211 | 0.512609 | 0.512372 | 5.9 | |
| 6 | LPo-1_Ca | Sövite I | Calcite | 0.06 | 12,231 | 0.70371 | −8.5 | 50.1 | 328 | 0.09232 | 0.512584 | 0.512318 | 4.8 |
| 7 | 37-95_Pcl | Sövite I | U-Pyrochlore | 9.4 | 8140 | 0.70343 | −7.8 | 37 | 280 | 0.08067 | 0.512501 | 0.512268 | 3.8 |
| 8 | 354W | Sövite I | 65 | 8576 | 0.70350 | −6.8 | 27.4 | 1984 | 0.08359 | 0.512491 | 0.512250 | 3.5 | |
| 9 | 354_Ca | Sövite I | Calcite | - | 9247 | 0.70356 | −6.0 | 50.9 | 368 | 0.08510 | 0.512460 | 0.512219 | 2.9 |
| 10 | 329_Ca | Sövite I | Calcite | 45 | 12,340 | 0.70361 | −5.3 | 8.0 | 47 | 0.09972 | 0.512533 | 0.512235 | 3.2 |
| 11 | D11-3W | Sövite I | 124 | 4971 | 0.70350 | −6.9 | 19.2 | 170 | 0.06811 | 0.512447 | 0.512251 | 3.5 | |
| 12 | 331_Ca | Sövite II | Calcite | 29 | 21,982 | 0.70359 | −5.6 | 58.3 | 385 | 0.09160 | 0.512507 | 0.512243 | 3.4 |
| 13 | B331W | Sövite II | n.a. | n.a. | n.a. | n.a. | 52 | 429 | 0.07368 | 0.512486 | 0.512274 | 3.9 | |
| 14 | B331_Ca | Sövite II | Calcite | n.a. | n.a. | n.a. | n.a. | 47.5 | 358 | 0.08022 | 0.512506 | 0.512275 | 4.0 |
| 15 | B331_Pcl | Sövite II | Pyrochlore | 7.1 | 3731 | 0.70341 | −8.1 | 381 | 3876 | 0.05954 | 0.512447 | 0.512275 | 4.0 |
| 16 | LPo-2W | Sövite II | n.a. | n.a. | n.a. | n.a. | 33.6 | 255 | 0.07973 | 0.512398 | 0.512168 | 1.9 | |
| 17 | Dol-21 | Ne-pegmatite | Pyrochlore | n.a. | n.a. | 0.70399 | −2.8 | 458 | 5324 | 0.05200 | 0.512380 | 0.512233 | 3.1 |
| 18 | 140-1_Ca | Sövite II * | Calcite | 0.55 | 16,718 | 0.70421 | 3.2 | 51.9 | 392 | 0.08001 | 0.512230 | 0.511999 | −1.4 |
| 19 | 140-1_Pcl | Sövite II * | Pyrochlore | 1.48 | 8332 | 0.70438 | 5.7 | 104 | 930 | 0.0677 | 0.512187 | 0.511992 | −1.5 |
| 20 | 330-2W | Fenite * | n.a. | n.a. | n.a. | n.a. | 14.8 | 109 | 0.08243 | 0.511564 | 0.511326 | −14.5 | |
| Host metamorphic rocks, Vishnevogorsk, and Ilmenogorsk suites (PR1), South Urals | |||||||||||||
| 21 | Mo-1_Ca | Calciphyre | Calcite | 0.99 | 1202 | 0.70817 | 59.4 | 4.36 | 32 | 0.08291 | 0.511992 | 0.511753 | −6.2 |
| 22 | Vish-2W | Plagiogneiss | 90 | 328 | 0.72550 | 305 | 20.1 | 127 | 0.09521 | 0.511069 | 0.510795 | −24.9 | |
| Buldym ultrabasite-carbonatite complex, Southern Urals | |||||||||||||
| 23 | 43332W | Metaperidotite | 2.27 | 309 | 0.70497 | 12.1 | 0.35 | 2.1 | 0.100140 | 0.512310 | 0.512021 | −0.97 | |
| 24 | 505-27W | Metadunite | 3.52 | 21.2 | 0.70513 | 16.3 | 0.37 | 1.4 | 0.162076 | 0.512495 | 0.512028 | −0.16 | |
| 25 | 503-27W | Olivinite | n.a. | n.a. | n.a. | n.a. | 5.2 | 58 | 0.054602 | 0.512213 | 0.512055 | −0.30 | |
| 26 | 15-22W | Olivinite | n.a. | n.a. | n.a. | n.a. | 1.6 | 8.8 | 0.112044 | 0.512293 | 0.511970 | −2.0 | |
| 27 | 3311W | Sövite III | n.a. | n.a. | n.a. | n.a. | 42.0 | 314 | 0.08091 | 0.512168 | 0.511935 | −2.7 | |
| 28 | 3311_Ca | Sövite III | Calcite | - | 8373 | 0.70455 | 8.0 | 52.1 | 391 | 0.08048 | 0.512172 | 0.511940 | −2.6 |
| 29 | 3311_Rch | Sövite III | Richterite | n.a. | n.a. | n.a. | n.a. | 0.46 | 3 | 0.09476 | 0.512187 | 0.511914 | −3.1 |
| 30 | 3311_Do | Sövite III | Dolomite | n.a. | n.a. | 0.70455 | 8.0 | 8.10 | 64 | 0.07611 | 0.512166 | 0.511947 | −2.4 |
| 31 | 915_Ca | Sövite III | Calcite | - | 10,279 | 0.70440 | 5.9 | 39.3 | 292 | 0.08138 | 0.512164 | 0.511929 | −2.8 |
| 32 | 3296_Pcl | Sövite III | Pyrochlore | 8.7 | 7058 | 0.70425 | 3.9 | 127 | 117 | 0.068640 | 0.512160 | 0.511962 | −2.1 |
| 33 | 154_Do | Beforsite IV | Dolomite | - | 9097 | 0.70447 | 6.9 | 24.5 | 181 | 0.08150 | 0.512292 | 0.512057 | −0.3 |
| 34 | 1021_Do | Beforsite IV | Dolomite | - | 5334 | 0.70450 | 7.3 | 12.6 | 93 | 0.08190 | 0.512341 | 0.512105 | 0.7 |
| 35 | K18_Pcl | Phlog-Do | U-Pyrochlore | 41 | 3772 | 0.70766 | 52 | 391 | 2603 | 0.090700 | 0.512315 | 0.512054 | −0.3 |
| 36 | K21_Pcl ** | Phlog-Do | Pyrochlore | 1.26 | 2990 | 0.70715 | 45 | 2928 | 11,154 | 0.15865 | 0.512232 | 0.511775 | −5.8 |
| 37 | K23_Ash | Phlog-Do | Aeschynite | 1.02 | 1156 | 0.70617 | 31 | 4762 | 37,700 | 0.076342 | 0.512254 | 0.512034 | −0.7 |
| Chetlassky complex of dike ultramafic-mafic rocks and carbonatites, Middle Timan | |||||||||||||
| 38 | 1374K | Carbonatite | Dolomite | n.a. | n.a. | 0.70348 | −4.6 | 1.2 | 50 | 0.01468 | 0.512194 | 0.512137 | 5.1 |
| 39 | 1387/2 | Carbonatite | Apatite | n.a. | n.a. | 0.70300 | −11.3 | 5.2 | 80 | 0.0392 | 0.512326 | 0.512174 | 5.8 |
| 40 | 1385-5 | Carbonatite | REE-carbonate | 90 | 1272 | 0.70369 | −1.6 | 1.3 | 52 | 0.01524 | 0.512227 | 0.512168 | 5.7 |
| 41 | 1385-5 | Carbonatite | 129 | 1262 | 0.70367 | −1.8 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| 42 | T-450 | Lamprophyre | Dolomite | n.a. | n.a. | 0.70364 | −6.2 | 0.04 | 5.5 | 0.00462 | 0.512183 | 0.512165 | 5.6 |
| 43 | 1270-1 | Lamprophyre | 116 | 1535 | 0.70365 | −2.2 | 0.11 | 6.1 | 0.01048 | 0.512235 | 0.512194 | 6.2 | |
| 44 | 1284 | Lamprophyre | 104 | 432 | 0.70376 | −0.6 | 5.2 | 75 | 0.04190 | 0.512317 | 0.512155 | 5.4 | |
| 45 | 55/46.3 | Lamprophyre | 121 | 901 | 0.70554 | 24.6 | 8.1 | 56 | 0.0875 | 0.512310 | 0.511972 | 1.8 | |
| 46 | 55/38.7 | Lamprophyre | 100 | 575 | 0.70589 | 29.7 | 7.3 | 45 | 0.0982 | 0.512365 | 0.511985 | 2.1 | |
| 47 | 55/172 | Lamprophyre | 87 | 1127 | 0.70489 | 15,5 | 13 | 93 | 0.0853 | 0.512314 | 0.511984 | 2.1 | |
| Host carbonate-sedimentary strata of the Bystrinskaya Group (Rf3), Middle Timan | |||||||||||||
| 48 | 1445 | Dolomite | 0.5 | 77 | 0.71380 | 142 | 0.19 | 0.89 | 0.12621 | 0.512084 | 0.511596 | −5.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedosekova, I.; Vladykin, N.; Udoratina, O.; Belyatsky, B. Ore and Geochemical Specialization and Substance Sources of the Ural and Timan Carbonatite Complexes (Russia): Insights from Trace Element, Rb–Sr, and Sm–Nd Isotope Data. Minerals 2021, 11, 711. https://doi.org/10.3390/min11070711
Nedosekova I, Vladykin N, Udoratina O, Belyatsky B. Ore and Geochemical Specialization and Substance Sources of the Ural and Timan Carbonatite Complexes (Russia): Insights from Trace Element, Rb–Sr, and Sm–Nd Isotope Data. Minerals. 2021; 11(7):711. https://doi.org/10.3390/min11070711
Chicago/Turabian StyleNedosekova, Irina, Nikolay Vladykin, Oksana Udoratina, and Boris Belyatsky. 2021. "Ore and Geochemical Specialization and Substance Sources of the Ural and Timan Carbonatite Complexes (Russia): Insights from Trace Element, Rb–Sr, and Sm–Nd Isotope Data" Minerals 11, no. 7: 711. https://doi.org/10.3390/min11070711
APA StyleNedosekova, I., Vladykin, N., Udoratina, O., & Belyatsky, B. (2021). Ore and Geochemical Specialization and Substance Sources of the Ural and Timan Carbonatite Complexes (Russia): Insights from Trace Element, Rb–Sr, and Sm–Nd Isotope Data. Minerals, 11(7), 711. https://doi.org/10.3390/min11070711






