Manufacture and Characterization of Fired Bricks from Gold Mine Waste Rocks
Abstract
:1. Introduction
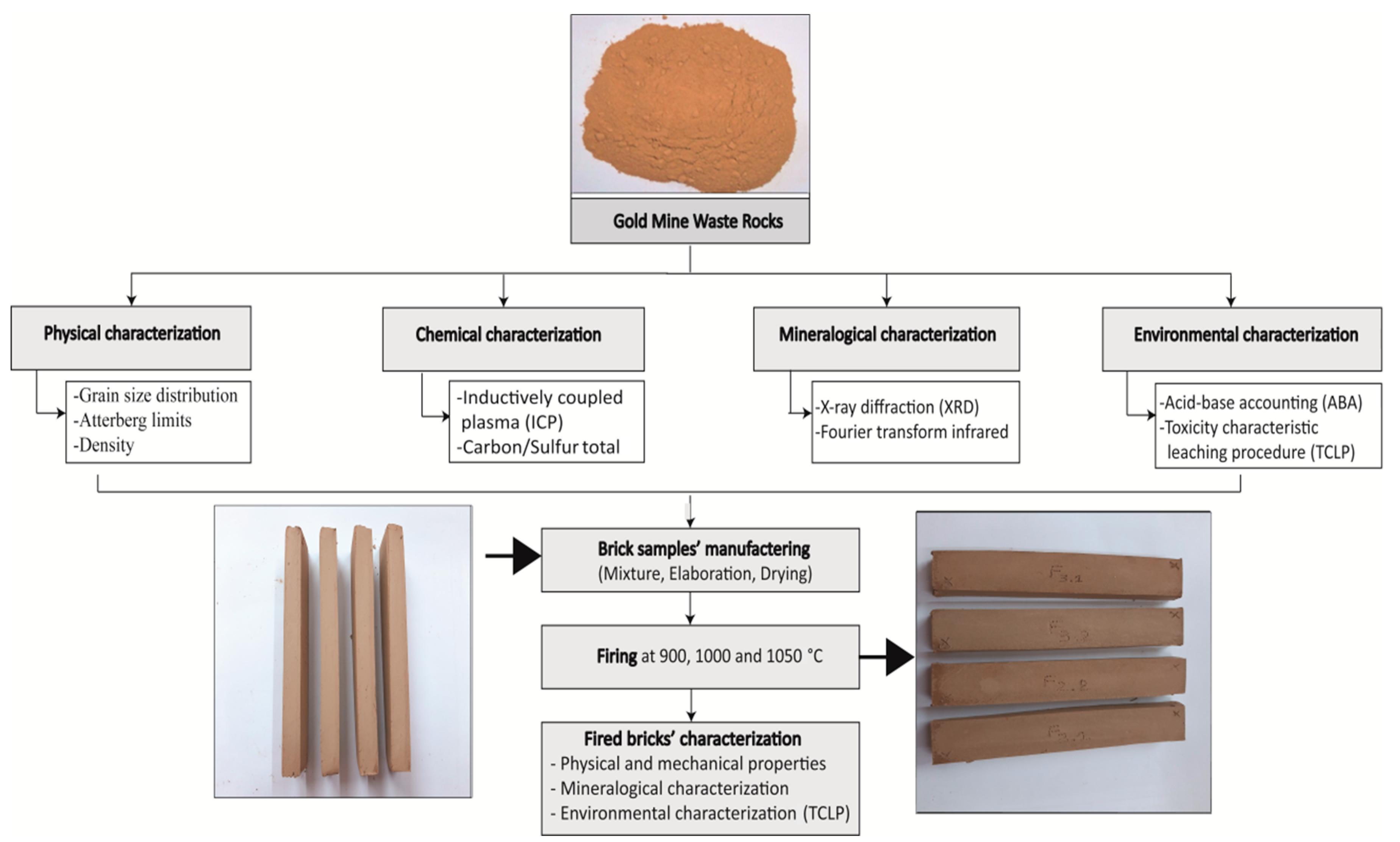
2. Materials and Methods
2.1. Materials and Research Methodology
2.2. Characterization Methods
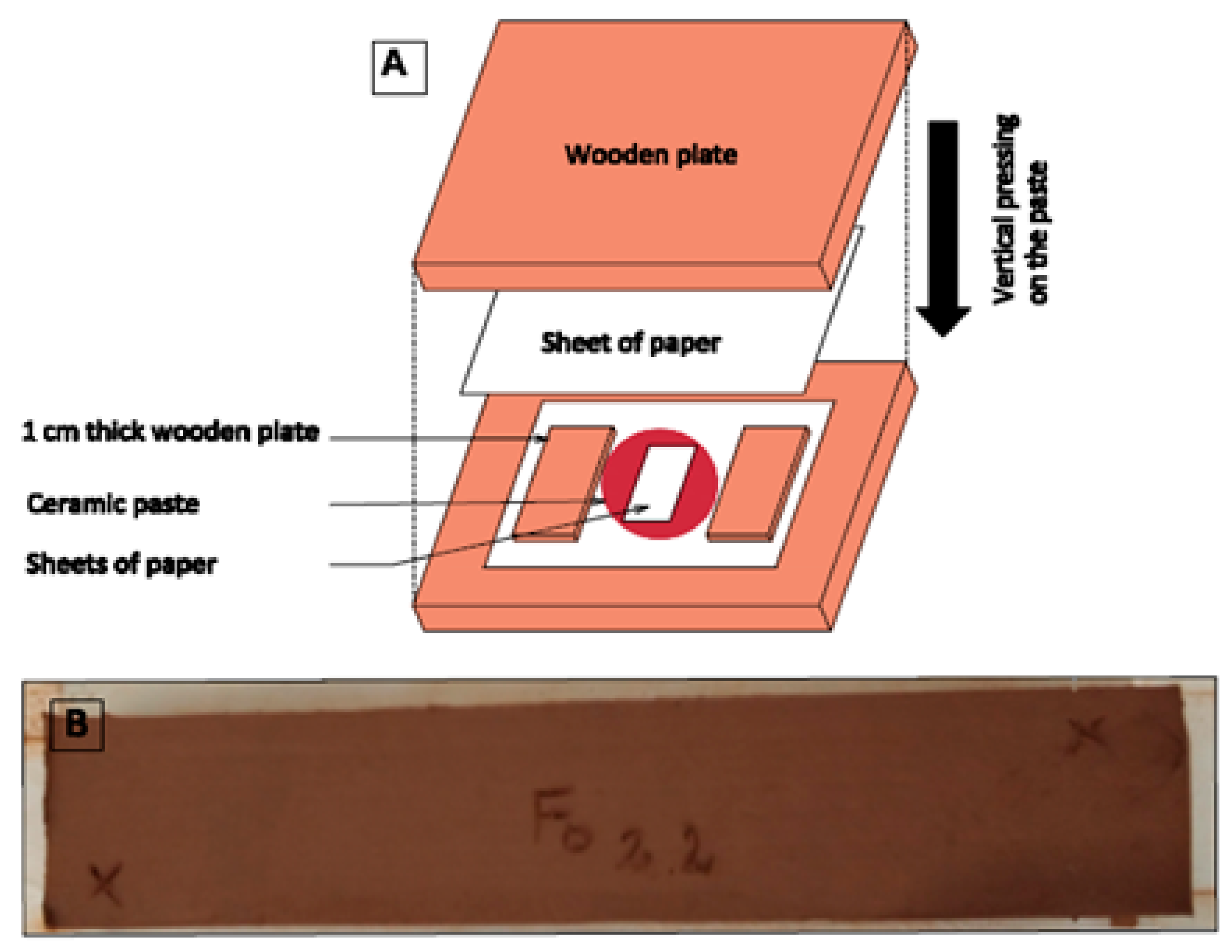
2.3. Manufacture of Laboratory Brick Samples
2.4. Fired Bricks’ Characterization
3. Results and Discussion
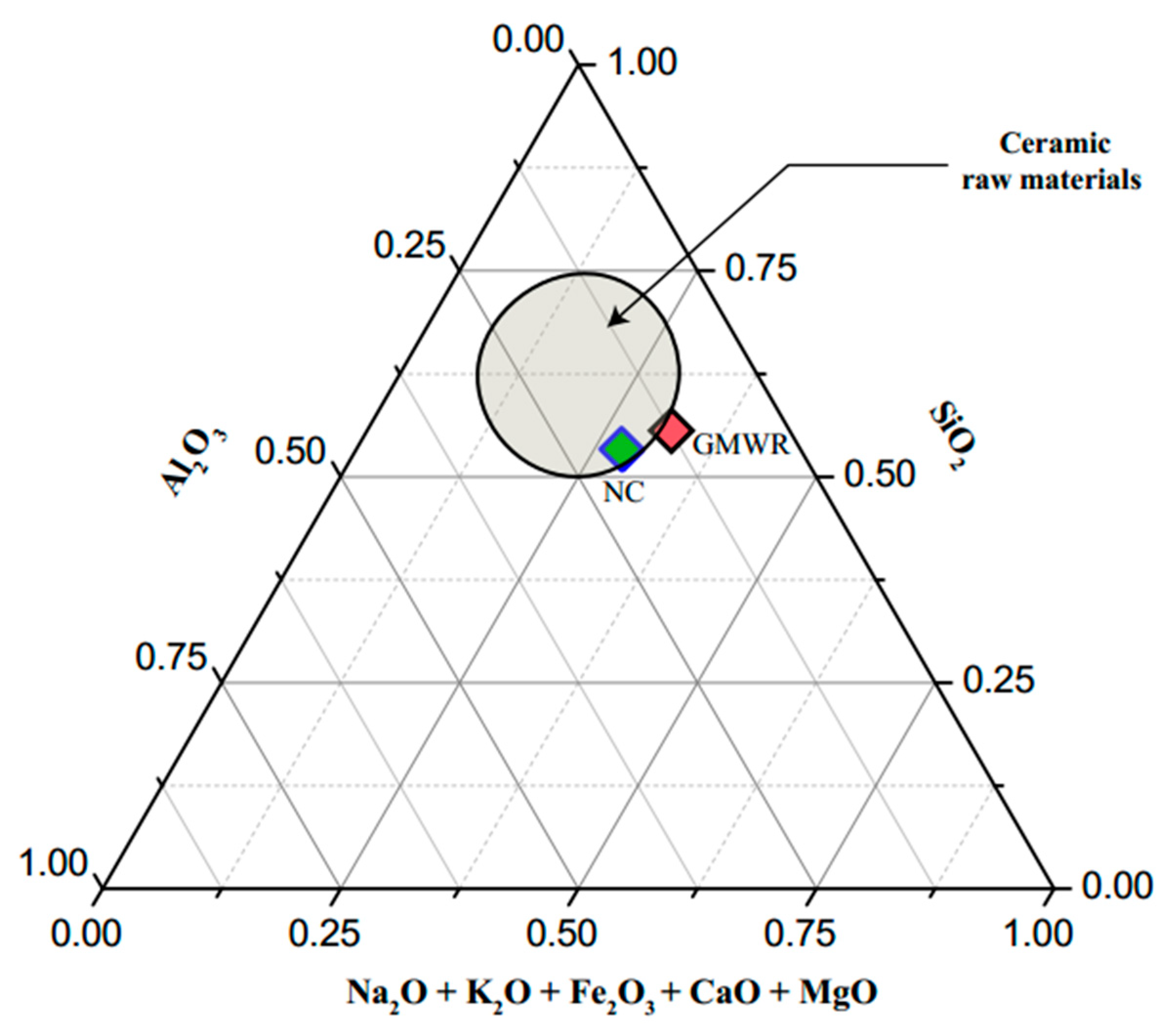
3.1. Raw Materials Characterization
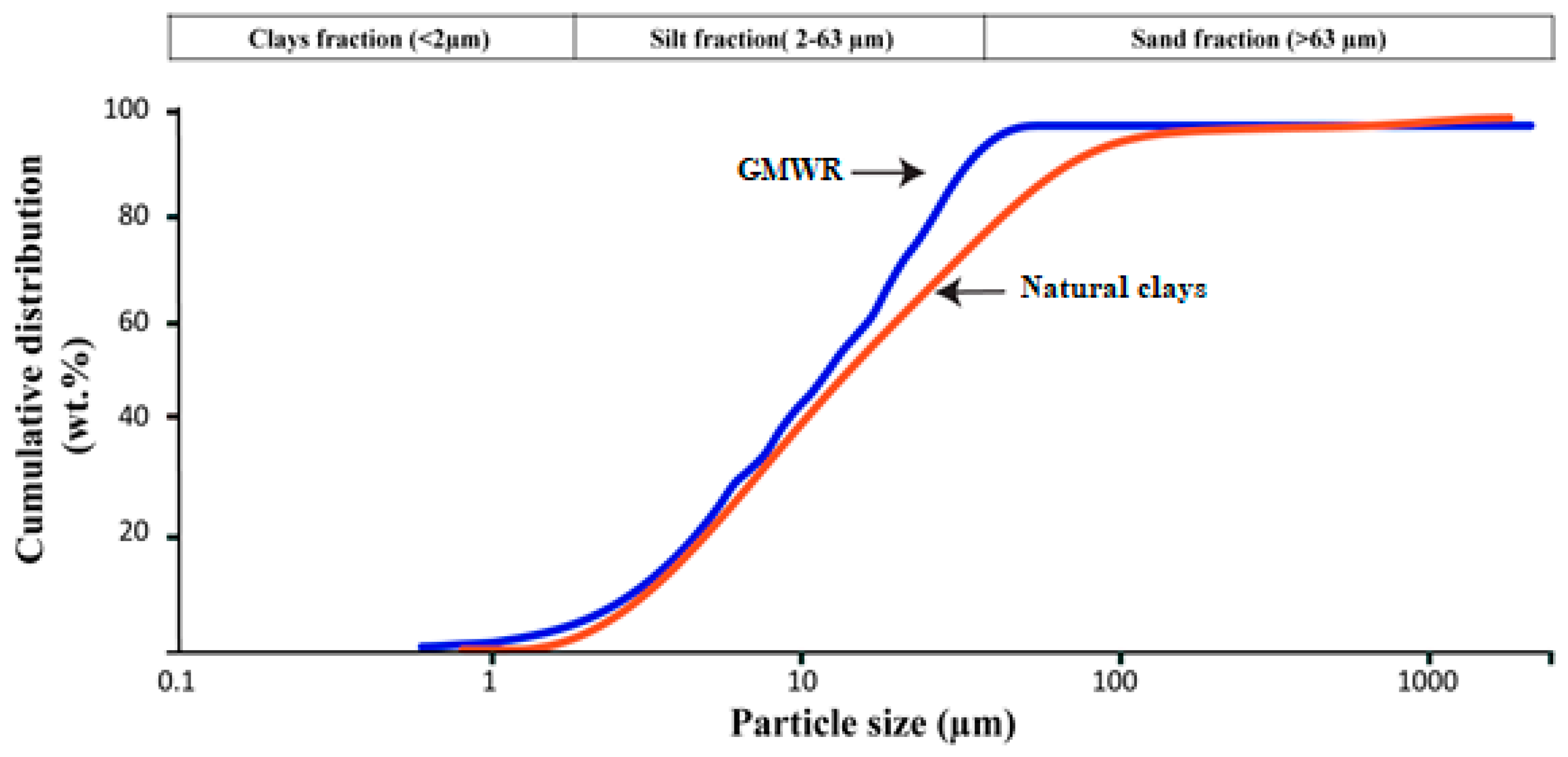
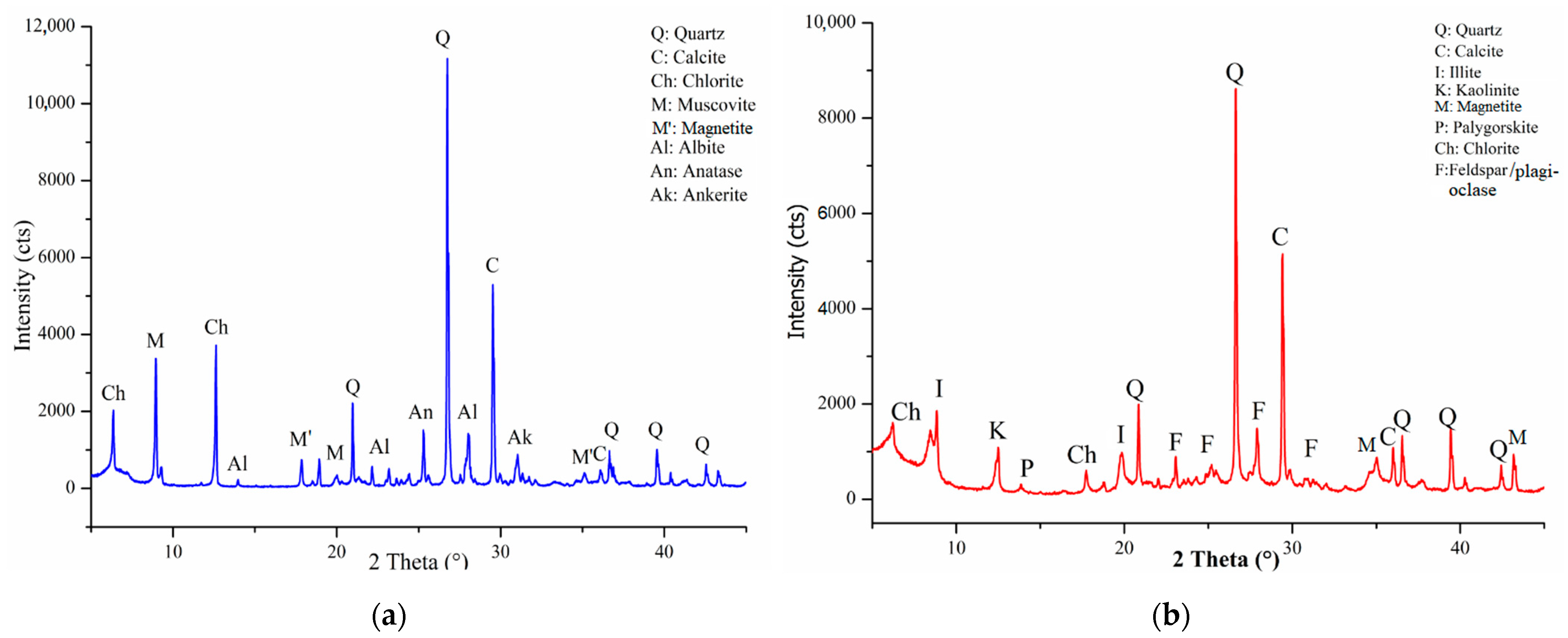
3.1.1. Physical, Chemical and Mineralogical Properties
3.1.2. Thermal Behavior of Raw Materials
3.1.3. Evaluation of Acid Generation Potential
3.2. Detailed Fired Bricks Properties
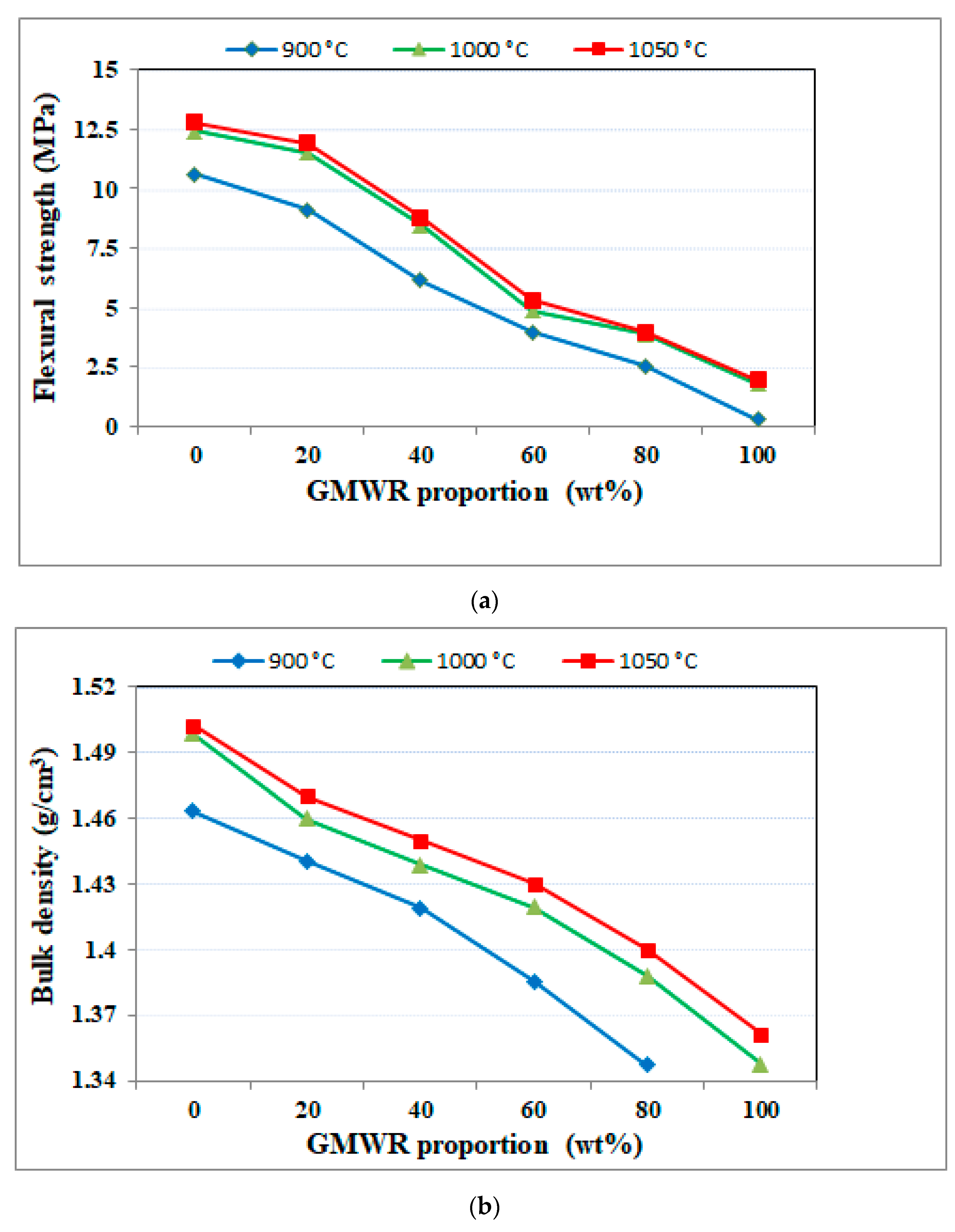
3.2.1. Physical and Mechanical Properties
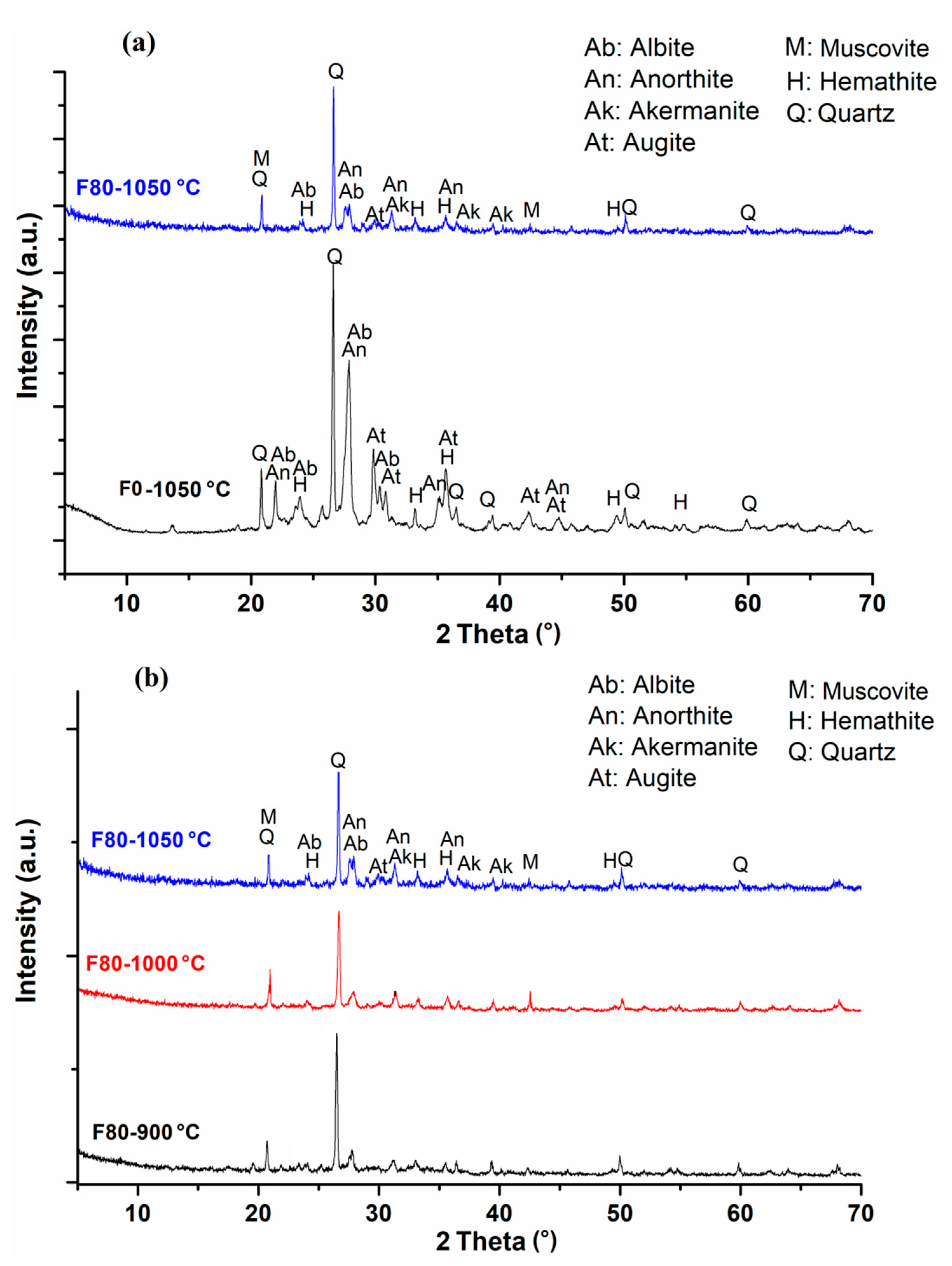
3.2.2. Mineralogical Characterization of Fired Bricks
3.2.3. Environmental Behavior of Fired Bricks
4. Conclusions
- The chemical and mineralogical compositions of GMWRs show compatibility with NCs commonly used in the fabrication of fired bricks. The clay minerals (chlorite) contained in the GMWR favor their reuse in the bricks production.
- The obtained results show that the GMWR cannot be used alone to make good-quality fired bricks.
- The integration of GMWRs in the matrix of fired bricks as secondary raw material led to an increase in water absorption and porosity rates, which resulted in a decrease in flexural strength and bulk density.
- Laboratory bricks with up to 80% GMWRs and fired at 1000 °C and 1050 °C met the requirements of current construction standards. They met the mechanical requirements (ASTM-C674), which require a flexural strength higher than 2.5 MPa. They also were in agreement with the ASTM C373 standards, which require a porosity rate of less than 40% and water absorption of less than 20%.
- The results of the TCLP leaching test showed that the mobility of heavy metals from bricks containing up to 80% GMWRs was below the limits set by the US EPA.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hakkou, R.; Benzaazoua, M. Final Technical Report. Management and Stabilization of Mining and Industrial Wastes; Université Cadi Ayyad: Marrakesh, Morocco; CDRT: Marrakesh, Morocco; Université du Québec en Abitibi-Témiscamingue: Royun-Noranda, QC, Canada, 2015. [Google Scholar]
- Lottermoser, B.G. Recycling, reuse and rehabilitation of mine wastes. Elements 2011, 7, 405–410. [Google Scholar] [CrossRef]
- Aubertin, M.; Bussière, B.; Bernier, L.; Chapuis, R.; Julien, M. Dans un Contexte de Développement Durable et de Protection de l’Environnment. In Proceedings of the Annual Conference of Canadian Society for Civil Engineering, Montréal, QC, Canada, 5–8 June 2002; pp. 1–10. [Google Scholar]
- Bussière, B.; Aubertin, M.; Zagury, G.J.; Potvin, R.; Benzaazoua, M. Principaux Défis et Pistes de Solution Pour la Restauration des Airesd’Entreposage de Rejets Miniers Abandonnés. In Proceedings of the Symposium 2005 Sur L’Environnement les Mines, Rouyn-Noranda, QC, Canada, 15–18 May 2005; pp. 1–29. Available online: https://www.researchgate.net/publication/267855816_PRINCIPAUX_DEFIS_ET_PISTES_DE_SOLUTION_POUR_LA_RESTAURATION_DES_AIRES_D'ENTREPOSAGE_DE_REJETS_MINIERS_ABANDONNEES (accessed on 27 February 2021).
- Mehta, N.; Dino, G.A.; Passarella, I.; Ajmone-Marsan, F.; Rossetti, P.; de Luca, D.A. Assessment of the possible reuse of extractive waste coming from abandoned mine sites: Case study in Gorno, Italy. Sustainability 2020, 12, 2471. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.; Cipullo, S.; Cocerva, T.; Coulon, F.; Dino, G.A.; Ajmone-Marsan, F.; Padoan, E.; Cox, S.F.; Cave, M.R.; De Luca, D.A. Incorporating oral bioaccessibility into human health risk assessment due to potentially toxic elements in extractive waste and contaminated soils from an abandoned mine site. Chemosphere 2020, 255, 126927. [Google Scholar] [CrossRef] [PubMed]
- Dino, G.A.; Mehta, N.; Rossetti, P.; Ajmone-Marsan, F.; De Luca, D.A. Sustainable approach towards extractive waste management: Two case studies from Italy. Resour. Policy 2018, 59, 33–43. [Google Scholar] [CrossRef]
- Fontes, W.C.; Franco de Carvalho, J.M.; Andrade, L.C.R.; Segadães, A.M.; Peixoto, R.A.F. Assessment of the use potential of iron ore tailings in the manufacture of ceramic tiles: From tailings-dams to “brown porcelain”. Constr. Build. Mater. 2019, 206, 111–121. [Google Scholar] [CrossRef]
- Khaldoun, A.; Ouadif, L.; Baba, K.; Bahi, L. Valorization of mining waste and tailings through paste backfilling solution, Imiter operation, Morocco. Int. J. Min. Sci. Technol. 2016, 26, 511–516. [Google Scholar] [CrossRef]
- Castro, E. Manufacture of Sustainable Clay Bricks Using Waste from Secondary Aluminum Recycling as Raw Material. Materials (Basel) 2018, 11, 2439. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, S.N.; Vieira, C.M.F. On the production of fired clay bricks from waste materials: A critical update. Constr. Build. Mater. 2014, 68, 599–610. [Google Scholar] [CrossRef]
- Raut, S.P.; Ralegaonkar, R.V.; Mandavgane, S.A. Development of sustainable construction material using industrial and agricultural solid waste: A review of waste-create bricks. Constr. Build. Mater. 2011, 25, 4037–4042. [Google Scholar] [CrossRef]
- Chindris, L.; Arad, V.; Arad, S.; Radermacher, L.; Radeanu, C. Valorization of mining waste in the construction industry general considerations. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, Bulgaria, 9 June—5 July 2017; Volume 17, pp. 309–316. [Google Scholar] [CrossRef]
- Aznar-Sánchez, J.; García-Gómez, J.; Velasco-Muñoz, J.; Carretero-Gómez, A. Mining Waste and Its Sustainable Management: Advances in Worldwide Research. Minerals 2018, 8, 284. [Google Scholar] [CrossRef] [Green Version]
- Pappu, A.; Saxena, M.; Asolekar, S.R. Solid wastes generation in India and their recycling potential in building materials. Build. Environ. 2007, 42, 2311–2320. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, J.; Sun, W. Utilization of iron ore tailings as fine aggregate in ultra-high performance concrete. Constr. Build. Mater. 2014, 50, 540–548. [Google Scholar] [CrossRef]
- Filho, J.N.S.; Mendes, J.C.; Peixoto, R.A.F.; Silva, G.C.; Da Silva, S.N. Technical and Environmental Feasibility of Interlocking Concrete Pavers with Iron Ore Tailings from Tailings Dams. J. Mater. Civ. Eng. 2017, 29, 04017104. [Google Scholar] [CrossRef]
- Mendes, J.J.; Araújo, F.G.S.; Castro, C.G.; von Krüger, F.L.; da Silva, F.L. Recycling of Concentration Tailings of Iron Ore for the Production of Concrete Block (Pavers). Mater. Sci. Forum 2014, 775–776, 631–634. [Google Scholar] [CrossRef]
- Khanzadi, M.; Behnood, A. Mechanical properties of high-strength concrete incorporating copper slag as coarse aggregate. Constr. Build. Mater. 2009, 23, 2183–2188. [Google Scholar] [CrossRef]
- Guo, W.J.; Du, G.X.; Zuo, R.F.; Liao, J.H. Utilization of Iron Tailings for High Strength Fired Brick. Adv. Mater. Res. 2012, 550–553, 2373–2377. [Google Scholar] [CrossRef]
- Zhang, G.D.; Zhang, X.Z.; Zhou, Z.H.; Cheng, X. Preparation and Properties of Concrete Containing Iron Tailings/Manufactured Sand as Fine Aggregate. Adv. Mater. Res. 2013, 838–841, 152–155. [Google Scholar] [CrossRef]
- Yellishetty, M.; Karpe, V.; Reddy, E.H.; Subhash, K.N.; Ranjith, P.G. Reuse of iron ore mineral wastes in civil engineering constructions: A case study. Resour. Conserv. Recycl. 2008, 52, 1283–1289. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, T.; Zhao, Y.; Bao, S. Preparation of eco-friendly construction bricks from hematite tailings. Constr. Build. Mater. 2011, 25, 2107–2111. [Google Scholar] [CrossRef]
- Tardif-Drolet, M.; Li, L.; Pabst, T.; Zagury, G.J.; Mermillod-Blondin, R.; Genty, T. Revue de la réglementation sur la valorisation des résidus miniers hors site au québec. Environ. Rev. 2020, 28, 32–44. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Mansori, M.; Yvon, J.; Kanari, N.; Hakkou, R. Manufacturing of ceramic products using calamine hydrometallurgical processing wastes. J. Clean. Prod. 2016, 127, 500–510. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Hakkou, R.; Mansori, M. Natural clay substitution by calamine processing wastes to manufacture fired bricks. J. Clean. Prod. 2016, 135, 847–858. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Hakkou, R.; Mansori, M. Coal mine wastes recycling for coal recovery and eco-friendly bricks production. Miner. Eng. 2017, 107, 123–138. [Google Scholar] [CrossRef]
- Loutou, M.; Hajjaji, M.; Mansori, M.; Favotto, C.; Hakkou, R. Phosphate sludge: Thermal transformation and use as lightweight aggregate material. J. Environ. Manag. 2013, 130, 354–360. [Google Scholar] [CrossRef]
- Bayoussef, A.; Loutou, M.; Taha, Y.; Mansori, M.; Benzaazoua, M.; Manoun, B.; Hakkou, R. Use of clays by-products from phosphate mines for the manufacture of sustainable lightweight aggregates. J. Clean. Prod. 2021, 280, 124361. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Bouamrane, A.; Hakkou, R. Valorisation des rejets miniers du district Pb-Zn de Touissit-Boubker (région orientale-Maroc). Déchets Sci. Tech. 2015, 66, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Argane, R.; El Adnan, M.; Benzaazoua, M.; Bouzahzah, H. Geochemical behavior and environmental risks related to the use of abandoned base-metal tailings as construction material in the upperMoulouya district, Morocco. Environ. Sci. Pollut. Res. 2015, 23, 598–611. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Hakkou, R.; Bouamrane, A. A comparative study on the practical use of low sulfide base-metal tailings as aggregates for rendering and masonry mortars. J. Clean. Prod. 2016, 112, 914–925. [Google Scholar] [CrossRef]
- El Machi, A.; Mabroum, S.; Taha, Y.; Tagnit-Hamou, A.; Benzaazoua, M.; Hakkou, R. Valorization of phosphate mine waste rocks as aggregates for concrete. Mater. Construcción 2021, 37, 3840–3846. [Google Scholar] [CrossRef]
- Bahhou, A.; Taha, Y.; El Khessaimi, Y.; Idrissi, H.; Hakkou, R.; Amalik, J.; Benzaazoua, M. Use of phosphate mine by-products as supplementary cementitious materials. Mater. Today Proc. 2020, 37, 3781–3788. [Google Scholar] [CrossRef]
- Moukannaa, S.; Loutou, M.; Benzaazoua, M.; Vitola, L.; Alami, J.; Hakkou, R. Recycling of phosphate mine tailings for the production of geopolymers. J. Clean. Prod. 2018, 185, 891–903. [Google Scholar] [CrossRef]
- Amrani, M.; Taha, Y.; Kchikach, A.; Benzaazoua, M. Valorization of Phosphate Mine Waste Rocks as Materials for Road Construction. Minerals 2019, 9, 237. [Google Scholar] [CrossRef] [Green Version]
- Contreras, M.; Martín, M.I.; Gázquez, M.J.; Romero, M.; Bolívar, J.P. Valorisation of ilmenite mud waste in the manufacture of commercial ceramic. Constr. Build. Mater. 2014, 72, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Onuaguluchi, O.; Eren, Ö. Rheology, strength and durability properties of mortars containing copper tailings as a cement replacement material. Eur. J. Environ. Civ. Eng. 2013, 17, 19–31. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, Ö. Recycling of copper tailings as an additive in cement mortars. Constr. Build. Mater. 2012, 37, 723–727. [Google Scholar] [CrossRef]
- Thomas, B.S.; Damare, A.; Gupta, R.C. Strength and durability characteristics of copper tailing concrete. Constr. Build. Mater. 2013, 48, 894–900. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, T.; Liu, T. Utilization of hematite tailings in non-fired bricks production. In Proceedings of the 2011 5th International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, 10–12 May 2011; Volume 978, pp. 4244–5089. [Google Scholar]
- Shettima, A.U.; Hussin, M.W.; Ahmad, Y.; Mirza, J. Evaluation of iron ore tailings as replacement for fine aggregate in concrete. Constr. Build. Mater. 2016, 120, 72–79. [Google Scholar] [CrossRef]
- Benarchid, Y.; Taha, Y.; Argane, R.; Tagnit-Hamou, A.; Benzaazoua, M. Concrete containing low-sulphide waste rocks as fine and coarse aggregates: Preliminary assessment of materials. J. Clean. Prod. 2019, 221, 419–429. [Google Scholar] [CrossRef]
- Idrissi, H.; Borja, W.; Fagel, N.; Daoudi, L. Simple approach to elaborate test bricks for traditional ceramic studies 1. Preprints 2021. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Flexural Properties of Ceramic Whiteware Materials 1 Designation; ASTM C674–88 Standard; ASTM International: West Conshohocken, PA, USA, 1999. [Google Scholar]
- Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products; ASTM C373-88; ASTM International: West Conshohocken, PA, USA, 1999.
- Validation, D. Standard Operating Procedures: Toxicity Characteristic Leaching Procedure (TCLP); SERAS: Arlington, VA, USA, 2005; pp. 1–12. [Google Scholar]
- CEAEQ. Protocole de Lixiviation Pour les Espèces Inorganiques; MA. 100–Lix.com.1.1, Rév. 1; Ministère du Développement Durable, L’Environnement, la Faune des Parcs du Québec: Québec City, QC, Canada, 2012; Volume 7, pp. 1–17. [Google Scholar]
- Taha, Y. Valorisation des Rejetes Miniers Dans la Fabrication de Briques Cuites: Évaluations Technique et Environnementale. Ph.D. Thesis, Université du Québec en Abitibi-Témiscamingue, Rouyn-Noranda, QC, USA, 2017. [Google Scholar]
- Bouzahzah, H.; Benzaazoua, M.; Bussière, B.; Plante, B. Revue de littérature détaillée sur les tests statiques et les essais cinétiques comme outils de prédiction du drainage minier acide. Déchets Sci. Tech. 2014, 14–31. [Google Scholar] [CrossRef] [Green Version]
- Sobek, A.A.; Schuller, W.A.; Freeman, J.R.; Smith, R.M. Field and Laboratory Methods Applicable to Overburdens and Minesoils; BiblioGov: Grandview Heights, OH, USA, 1978; EPA-600/2-78-054. [Google Scholar]
- Lawrence, R.W.; Scheske, M. A method to calculate the neutralization potential of mining wastes. Environ. Geol. 1997, 32, 100–106. [Google Scholar] [CrossRef]
- Bouzahzah, H.; Benzaazoua, M.; Bussiere, B.; Plante, B. Prediction of Acid Mine Drainage: Importance of Mineralogy and the Test Protocols for Static and Kinetic Tests. Mine Water Environ. 2014, 33, 54–65. [Google Scholar] [CrossRef]
- Skousen, J. Geoderma A methodology for geologic testing for land disturbance: Acid-Base Accounting for surface mines. Geoderma 2017. [Google Scholar] [CrossRef]




| Chemical Compositions (%) | Physical Properties | Mixture Designs (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| DL * | NC | GMWRs | NC | GMWRs | NC | GMWRs | ||
| SiO2 0.1 | 50.8 ± 1.5 | 43.26 ± 1 | Moisture (%) | 10.4 | 11.5 | F0 | 100 | 0 |
| Al2O3 0.1 | 16.4 ± 1 | 10.13 ± 0.5 | Plasticity limits | 25 | 19 | F20 | 80 | 20 |
| TiO2 0.1 | 1.1 ± 0.7 | 1.1 ± 0.2 | (%) | F40 | 60 | 40 | ||
| Fe2O3 0.1 | 5.9 ± 0.9 | 8.59 ± 1 | Plasticity index | 27 | 12 | F60 | 40 | 60 |
| MnO 0.01 | 0.1 ± 0.05 | 0.156 ± 0.02 | (%) | F80 | 20 | 80 | ||
| MgO 0.1 | 3.5 ± 0.5 | 2.65 ± 0.2 | Specific gravity | 2.6 | 2.61 | F100 | 0 | 100 |
| CaO 0.1 | 8.5 ± 1 | 11.23 ± 1.2 | (g·cm−3) | |||||
| Na2O 0.03 | 0.3 ± 0.01 | -- | Bulk density | 1.5 | 1.5 | |||
| K2O 0.01 | 3.0 ± 0.1 | 1.19 ± 0.3 | (g·cm−3) | |||||
| P2O5 0.1 | -- | 0.16 ± 0.01 | LOI (%) | 11.5 | 21.53 | |||
| Sample | C% | S% | NP | AP | NNP | NP/AP |
|---|---|---|---|---|---|---|
| GMWR | 2.28 | 0.14 | 189.9 | 4.37 | 185.53 | 42.45 |
| Sample | As | Ba | Cd | Cr | Cu | Mo | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| µg·L−1 | µg·L−1 | µg·L−1 | µg·L−1 | µg·L−1 | µg·L−1 | µg·L−1 | µg·L−1 | |
| GMWRs | 86.74 | 2612.13 | ND | 43.14 | 212.62 | ND | 231.92 | 243.4 |
| F0 | 3.66 | 114.14 | 0.22 | 10.56 | 10.7 | ND | ND | 280.48 |
| F100 | 8014.33 | 15.94 | 0.28 | 291.87 | 1.66 | 25.33 | ND | 6.6 |
| F80 | 2741.54 | 18.51 | 0.3 | 251.24 | 1.17 | 16.48 | ND | 8.04 |
| Limits (US EPA) | 5000 | 100,000 | 1000 | 5000 | - | - | 5000 | 2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benahsina, A.; Taha, Y.; Bouachera, R.; Elomari, M.; Bennouna, M.A. Manufacture and Characterization of Fired Bricks from Gold Mine Waste Rocks. Minerals 2021, 11, 695. https://doi.org/10.3390/min11070695
Benahsina A, Taha Y, Bouachera R, Elomari M, Bennouna MA. Manufacture and Characterization of Fired Bricks from Gold Mine Waste Rocks. Minerals. 2021; 11(7):695. https://doi.org/10.3390/min11070695
Chicago/Turabian StyleBenahsina, Azzeddine, Yassine Taha, Rachida Bouachera, Mohamed Elomari, and Mohammed Abdouh Bennouna. 2021. "Manufacture and Characterization of Fired Bricks from Gold Mine Waste Rocks" Minerals 11, no. 7: 695. https://doi.org/10.3390/min11070695
APA StyleBenahsina, A., Taha, Y., Bouachera, R., Elomari, M., & Bennouna, M. A. (2021). Manufacture and Characterization of Fired Bricks from Gold Mine Waste Rocks. Minerals, 11(7), 695. https://doi.org/10.3390/min11070695







