The Pb-Zn (Ba) Nonsulfide Mineralizations at Bou Caïd (Ouarsenis, Algeria): Mineralogy, Isotope Geochemistry, and Genetic Inferences
Abstract
1. Introduction
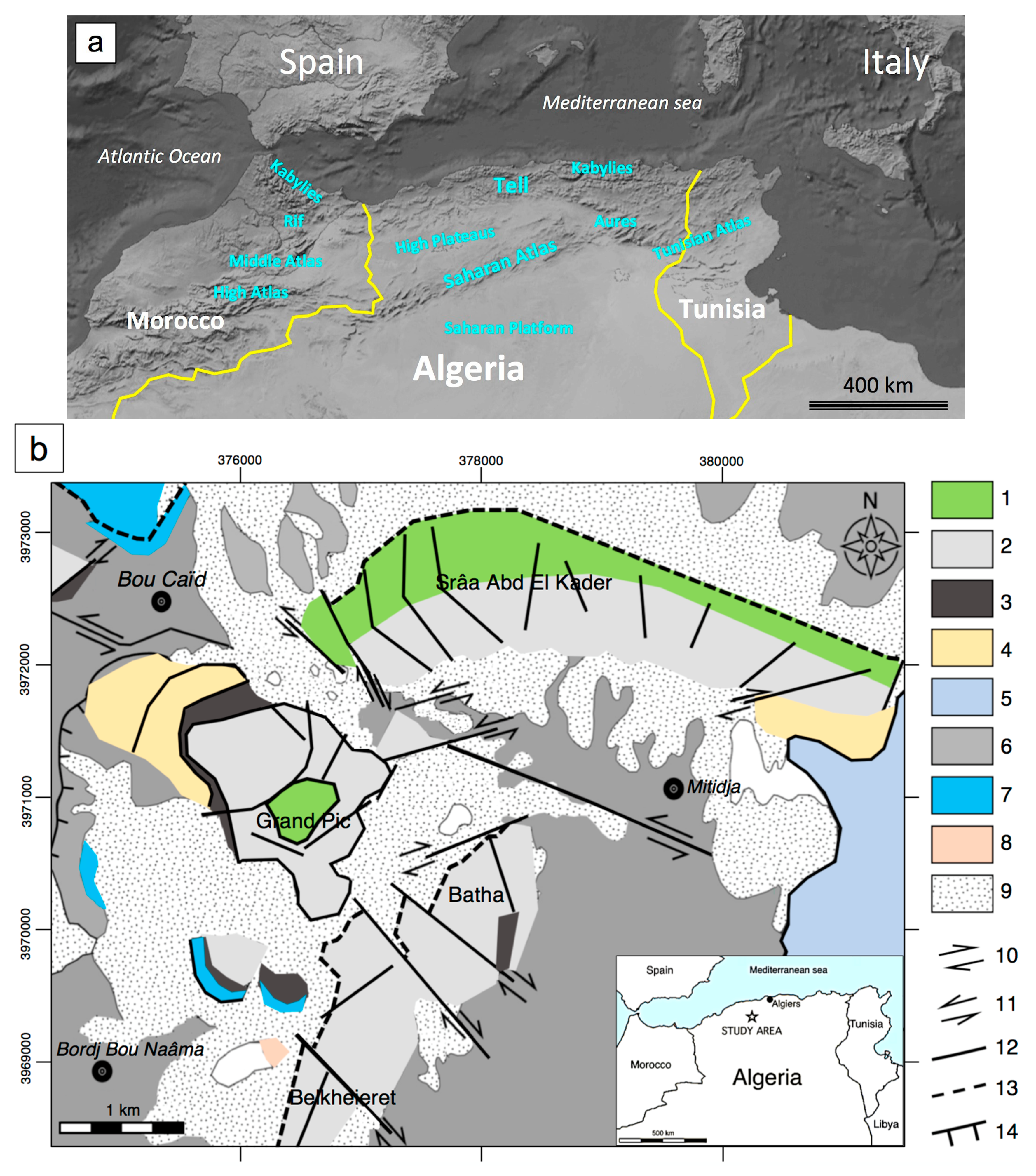
2. Geological Background
2.1. Geology of Northern Algeria and the Ouarsenis Area
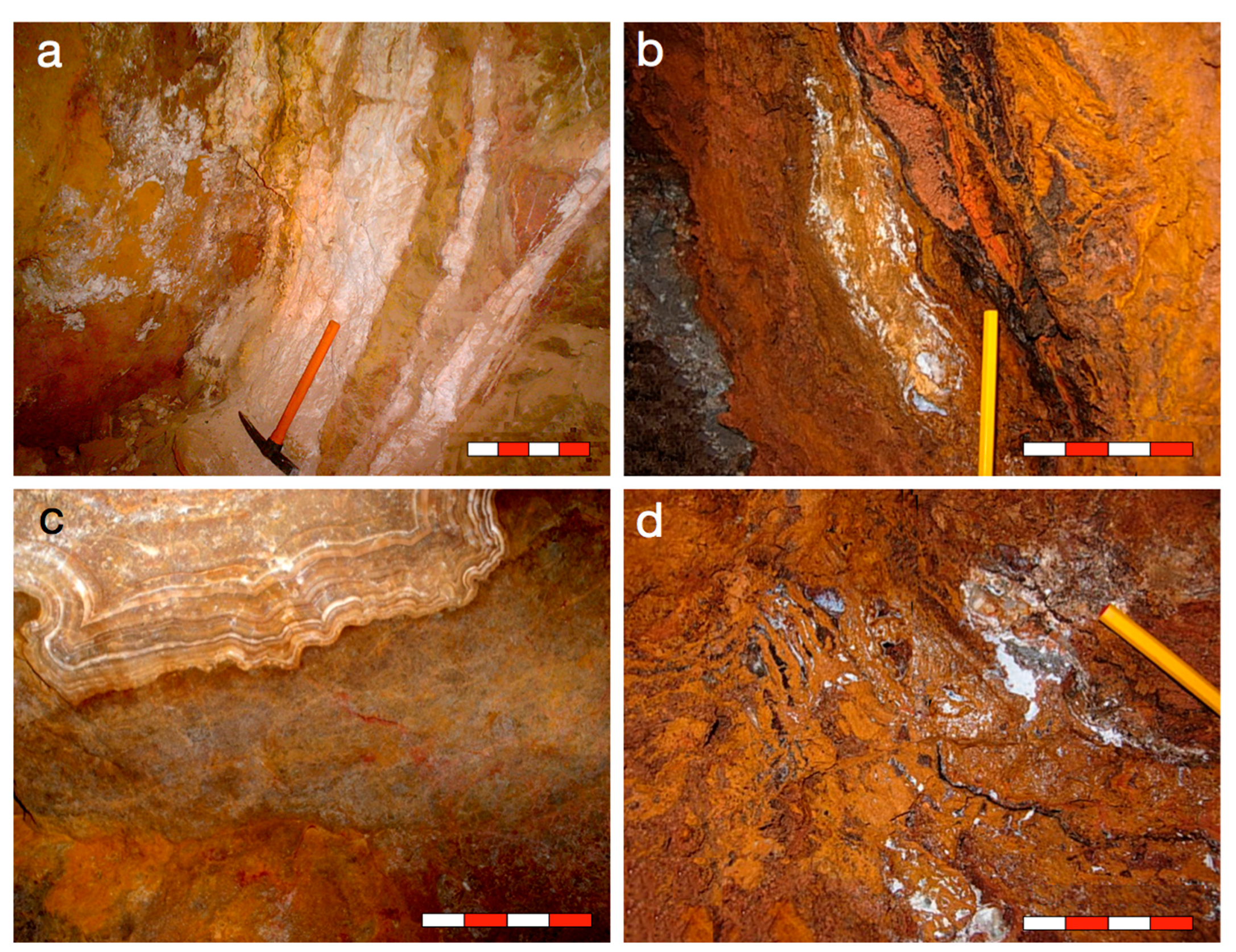
2.2. The ores of the Ouarsenis and Bou Caïd Area
3. Materials and Methods
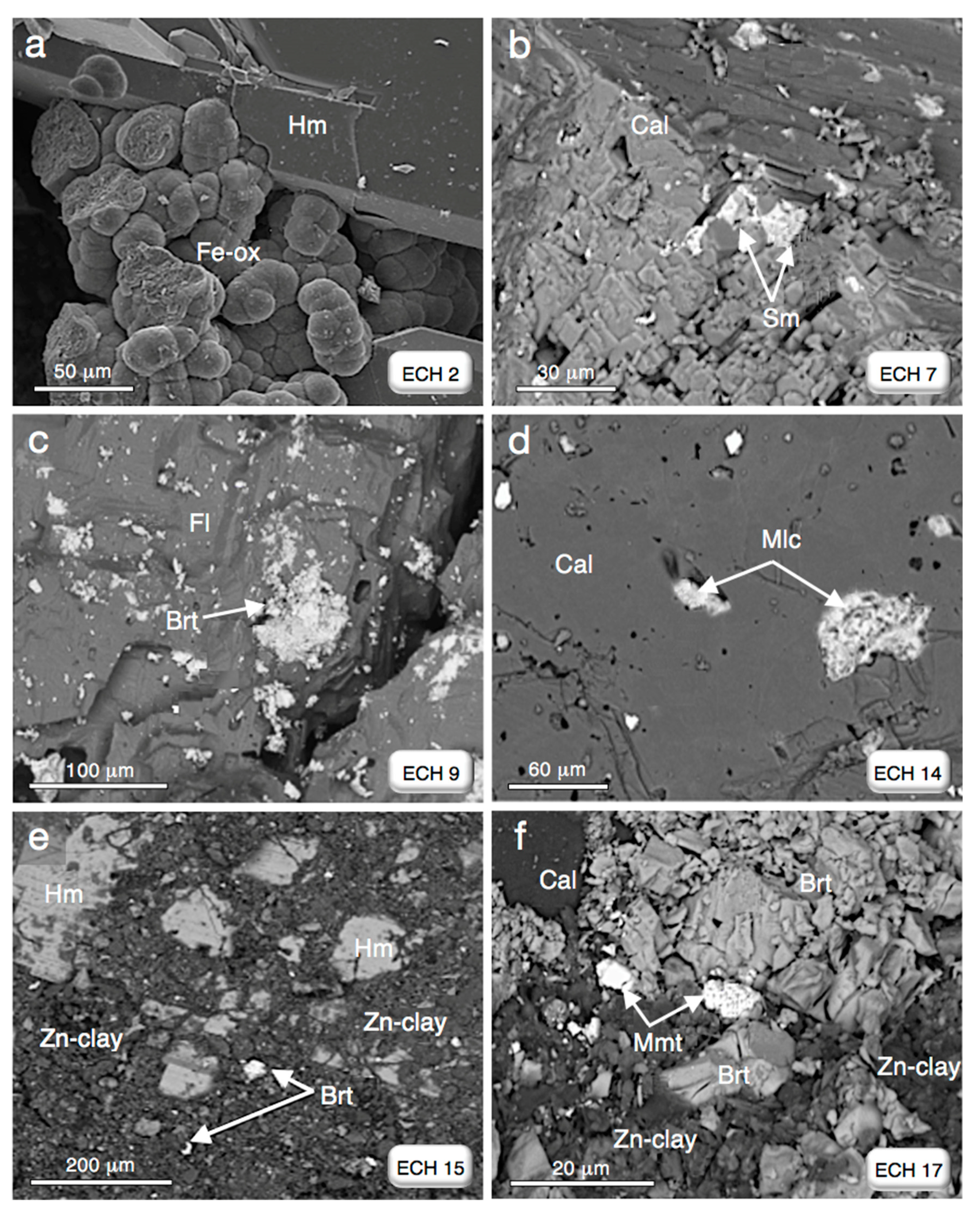
4. Results
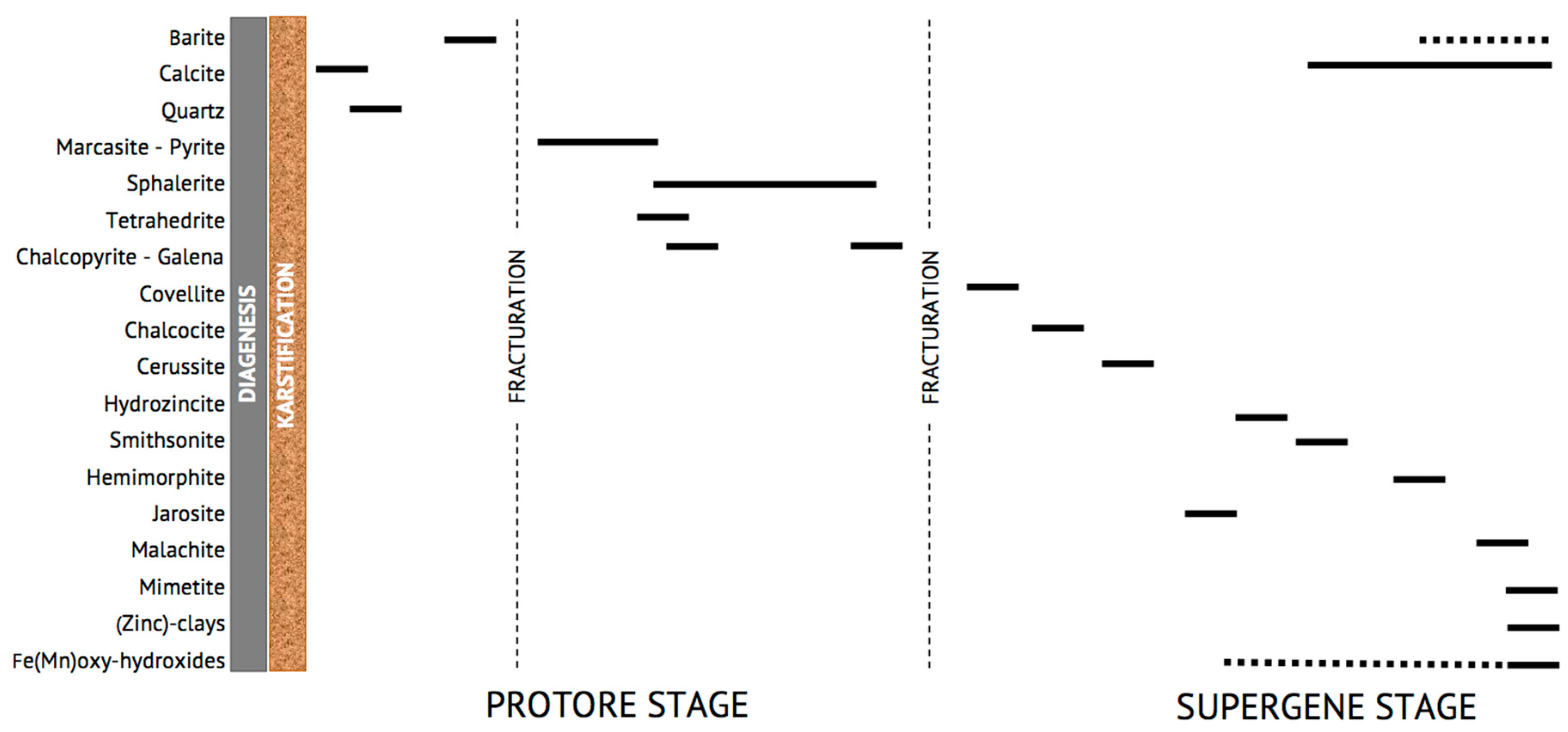
5. Discussion and Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouvier, R.; Perthuisot, V.; Mansouri, A. Pb-Zn deposits and salt bearing diapirs in Southern Europe and North Africa. Econ. Geol. 1985, 80, 666–687. [Google Scholar] [CrossRef]
- Départment Recherches Algérie. Sur les Travaux de Prospection Géologiques Effectués sur le Gisement de l’Ouarsenis, 1968–1970; Internal Report; Départment Recherches Algérie: Algeria, 1971; p. 101. [Google Scholar]
- Bouillin, J.P. Le bassin maghrébin: Une ancienne limite entre l’Europe et l’Afrique à l’Ouest des Alpes. Bulletin Societé Géologique de France 1986, 8, 547–558. [Google Scholar] [CrossRef]
- Leprêtre, R.; Frizon de Lamotte, D.; Combier, V.; Gimeno-Vives, O.; Mohn, G.; Eschard, R. The Tell-Rif orogenic system (Morocco, Algeria, Tunisia) and the structural heritage of the southern Tethys margin. BSGF Earth Sci. Bull. 2018, 189, 10. [Google Scholar] [CrossRef]
- Wildi, W. La chaine tello-rifaine (Algérie, Maroc, Tunisie): Structure, stratigraphie et évolution du Trias au Miocène. Revue de Géologie dynamique et de Géographie Physique 1983, 24, 201–297. [Google Scholar]
- Benaouali-Mebarek, N.; Frizon de Lamotte, D.; Roca, E.; Bracène, R.; Faure, J.L.; Sassi, W.; Roure, F. Post-Cretaceous kinematics of the Atlas and Tell systems in central Algeria: Early foreland folding and subduction-related deformation. Comptes Rendus Geosci. 2006, 338, 115–125. [Google Scholar] [CrossRef]
- Frizon de Lamotte, D.; Saint Bezar, B.; Bracène, R. Two main steps of the Atlas building and geodynamics of the Western Mediterranean. Tectonics 2000, 19, 740–761. [Google Scholar] [CrossRef]
- Lillouch, S.; Ait Meziane, Y.; Bendadouche, H. Geotechnical cartographic synthesis of Bejaia City, north east of Algeria. J. Geol. Soc. India 2018, 91, 348–354. [Google Scholar] [CrossRef]
- Bracène, R.; Frizon de Lamotte, D. Origin of intraplate deformation in the Atlas system of western and central Algeria: From Jurassic rifting to Cenozoic-Quaternary inversion. Tectonophysics 2002, 357, 207–226. [Google Scholar] [CrossRef]
- Bracène, R. Géodynamique du Nord de L’Algérie: Impact sur L’exploration Pétrolière. Ph.D. Thesis, Université de Cergy Pontoise, Cergy, France, 2001; 101p. [Google Scholar]
- Aïfa, T.; Zaagane, M. Neotectonic deformation stages in the central Ouarsenis culminating zone, Northwestern Algeria. Arab. J. Geosci. 2015, 8, 2667–2680. [Google Scholar] [CrossRef]
- Calembert, L. Étude Géologique du Massif Culminant de l’Ouarsenis. Service de la Carte Géologique d’Algérie; Bull Serv Carte Géol: Algérie, 1952; 184p. [Google Scholar]
- Mattauer, M. Etude géologique de l’Ouarsenis oriental. Bulletin Service Carte Géologique Algérie 1958, 17, 534. [Google Scholar]
- Tchoumatchenco, P. Brachiopodes jurassiques du Kef Sidi Amar-massif culminant de l’Ouarsenis (Algérie du Nord). Geologica Balcanica 1994, 24, 25–61. [Google Scholar]
- Benhamou, M. Evolution Tectono-Eustatique d’un Bassin de la Téthys Maghrébine: L’Ouarsenis (Algérie) Pendant le Jurassique Inférieur et Moyen. Ph.D. Thesis, Université d’Oran, Es Senia, Algeria, 1996; 434p. [Google Scholar]
- Benhamou, M.; Elmi, S.; Alméras, Y. Age et contexte dynamique des calcaires à brachiopodes téthysiens (Zeilleriidés multiplissés) du Grand Pic de l’Ouarsenis (Tell lgérien). Comptes Rendus Acad. Sci. 2000, 331, 717–723. [Google Scholar]
- Tchoumatchenco, P.; Nikolov, T.; Kozhukharov, D.; Benev, B.; Gochev, P.; Katzkov, N.; Khrischev, K.; Moev, M.; Nicolov, Z.; Slavov, I.; et al. Le Crétacé inférieur dans le massif de l’Ouarsenis et les Monts de Tiaret (Algérie du Nord). Geologica Balcanica 1995, 25, 27–59. [Google Scholar]
- Perrodon, A. Etude géologique des bassins néogènes sublittoraux de l’Algérie occidentale. Bulletin Service Carte Géologique Algérie 1957, 34, 382. [Google Scholar]
- Thomas, G. Géodynamique D’un Bassin Intra-Montagneux, le Bassin du bas Chéliff Occidental (Algérie) Durant le Mio-Plio-Quaternaire. Ph.D. Thesis, Université de Pau, Pau, France, 1985; 594p. [Google Scholar]
- Attoucheik, L.; Jordanova, N.; Bayou, B.; Lagroix, F.; Jordanova, D.; Maouche, S.; Henry, B.; Boutaleb, A. Soil metal pollution from former Zn–Pb mining assessed by geochemical and magnetic investigations: Case study of the Bou Caid area (Tissemsilt, Algeria). Environ. Earth Sci. 2017, 76, 298. [Google Scholar] [CrossRef]
- Louha, H. Contribution à L’étude Gîtologique des Minéralisations du Gisement de Bou Caïd (Ouarsenis-Tissemssilt). Master’s Thesis, Faculté des Sciences de la Terre de la Géographie et L’Aménagement du Territoire, Université des Sciences et de la Technologie Houari Boumediene, Bab Ezzouar, Algeria, 2012; p. 121. [Google Scholar]
- Kim, S.T.; Mucci, A.; Taylor, B.E. Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75 °C, revisited. Chem. Geol. 2007, 246, 135–146. [Google Scholar] [CrossRef]
- Gilg, H.A.; Boni, M.; Hochleitner, R.; Struck, U. Stable isotope geochemistry of carbonate minerals in supergene oxidation zones of Zn-Pb deposits. Ore. Geol. Rev. 2008, 33, 117–133. [Google Scholar] [CrossRef]
- Mondillo, N.; Boni, M.; Balassone, G.; Villa, I.M. The Yanque Prospect (Peru): From Polymetallic Zn-Pb Mineralization to a Nonsulfide Deposit. Econ. Geol. 2014, 109, 1735–1762. [Google Scholar] [CrossRef][Green Version]
- Arfè, G.; Mondillo, N.; Boni, M.; Balassone, G.; Joachimski, M.; Mormone, A.; Di Palma, T. The karst-hosted Mina Grande nonsulfide zinc deposit, Bongará district (Amazonas region, Peru). Econ. Geol. 2017, 112, 1089–1110. [Google Scholar] [CrossRef]
- Arfè, G.; Mondillo, N.; Boni, M.; Joachimski, M.; Balassone, G.; Mormone, A.; Santoro, L.; Castro Medrano, E. The Cristal Zn prospect (Amazonas region, Northern Peru). Part II: An example of supergene enrichments in tropical areas. Ore Geol. Rev. 2018, 95, 1076–1105. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Hoefs, J. Carbon and oxygen isotopic variations in hydrothermal calcites. Theoretical modeling on mixing processes and application to Pb-Zn deposits in the Harz Mountains, Germany. Miner. Depos. 1993, 28, 79–89. [Google Scholar]
- Mahboubi, C.N.; Mehadji, A.O.; Chevalier, N. Microfacies and stable isotope features of the Lower–Middle Jurassic carbonate rocks of Western Saharan Atlas (Aïn Ouarka area, Algeria). Geol. J. 2021, 1–16. [Google Scholar] [CrossRef]
- Mondillo, N.; Lupone, F.; Boni, M.; Joachimski, M.; Balassone, G.; De Angelis, M.; Zanin, S.; Granitzio, F. From Alpine-type sulfides to nonsulfides in the Gorno Zn project (Bergamo, Italy). Miner. Depos. 2020, 55, 953–970. [Google Scholar] [CrossRef]
- Boni, M.; Mondillo, N. The “Calamines” and the “Others”: The great family of supergene nonsulfide zinc ore. Ore Geol. Rev. 2015, 68, 208–233. [Google Scholar] [CrossRef]


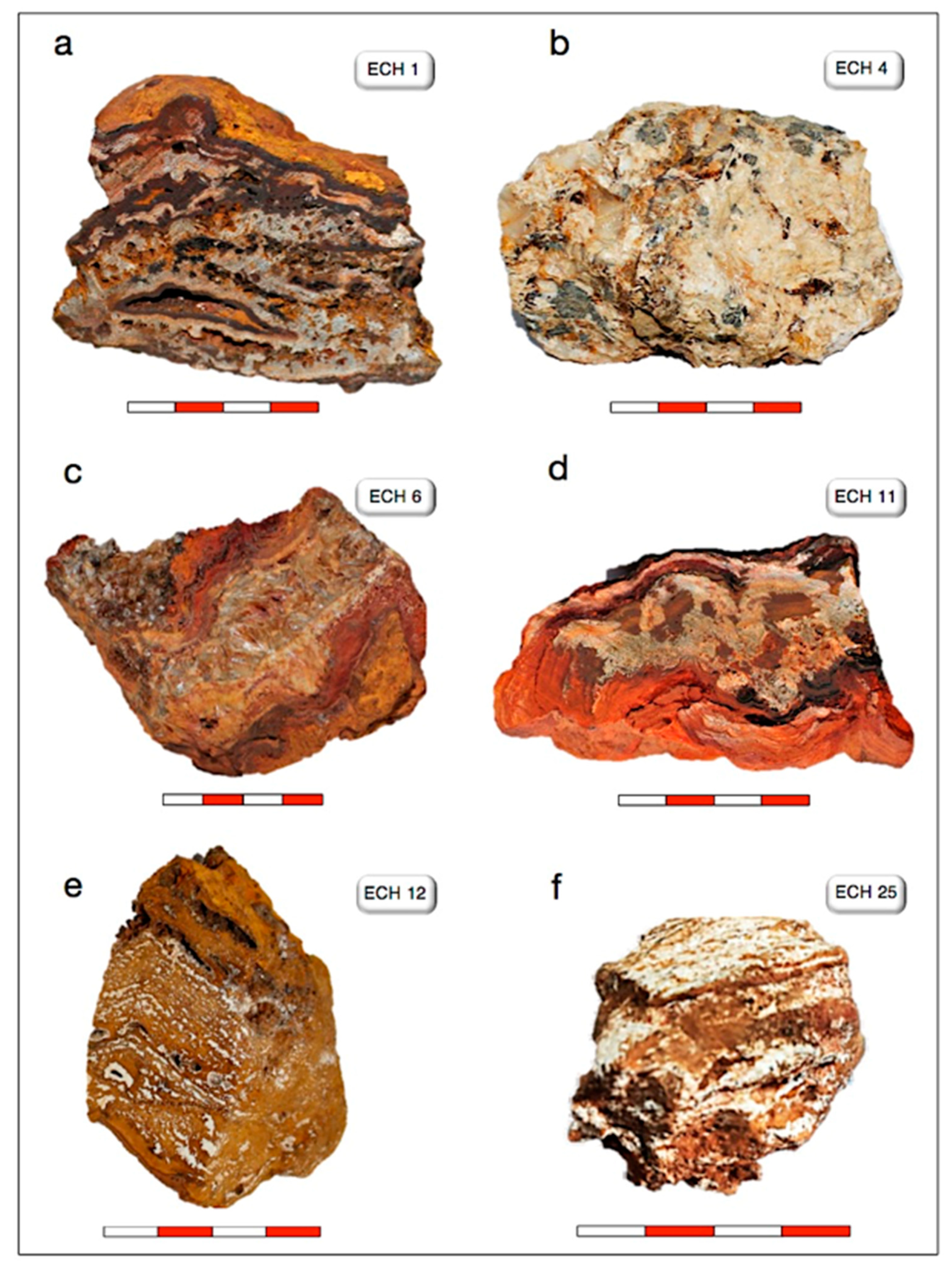

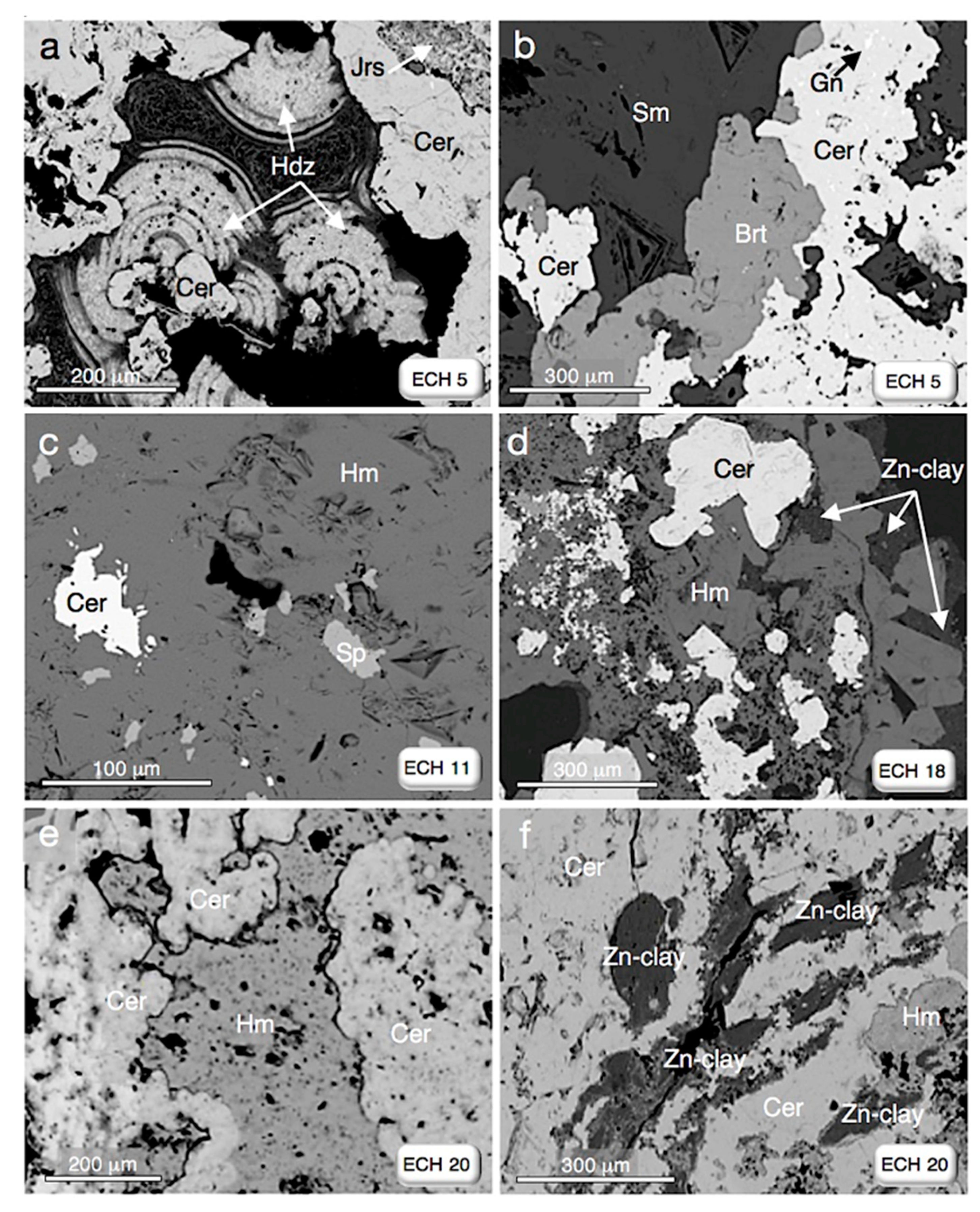
| Sample ID | Sample Location 1 | Main Mineral Assemblage § |
|---|---|---|
| ECH 1 | site 1—Srâa Abd El Kader | hemimorphite, hydrozincite, Fe-(oxy)-hydroxides, and barite |
| ECH 2 | site 1—Srâa Abd El Kader | hemimorphite and Fe-(oxy)-hydroxides |
| ECH 3 | site 1—Srâa Abd El Kader | hemimorphite, goethite, hematite, and barite |
| ECH 4 | site 3—Srâa Abd El Kader | barite, galena, calcite, quartz, cerussite, Fe-(oxy)-hydroxides magnetite, and sulfur |
| ECH 5 | site 3—Srâa Abd El Kader | smithsonite, hydrozincite, cerussite, barite, galena, and dolomite |
| ECH 6 | site 1—Srâa Abd El Kader | hemimorphite and Fe-(oxy)-hydroxides |
| ECH 7 | site 2—Grand Pic | calcite, smithsonite, and Fe-(oxy)-hydroxides |
| ECH 8 | site 2—Grand Pic | calcite, barite, and Fe-(oxy)-hydroxides |
| ECH 9 | site 2—Grand Pic | barite, and fluorite |
| ECH 10 | site 2—Grand Pic | calcite, barite, cerussite, Mn(Fe)-(oxy)-hydroxides, and (Zn)clay minerals |
| ECH 11 | site 1—Srâa Abd El Kader | hemimorphite, goethite, barite, cerussite, and sphalerite |
| ECH 12 | site 1—Srâa Abd El Kader | hemimorphite, hydrozincite, goethite, calcite, barite, and (Zn)clay minerals |
| ECH 14 | site 2—Grand Pic | calcite, quartz, barite, Fe-(oxy)-hydroxides, and malachite |
| ECH 15 | site 1—Srâa Abd El Kader | hemimorphite, barite, and (Zn)clay minerals |
| ECH 16 | site 2—Grand Pic | calcite, Fe-(oxy)-hydroxides, and cerussite |
| ECH 17 | site 1—Srâa Abd El Kader | calcite, barite, (Zn)clay minerals, and mimetite |
| ECH 18 | site 1—Srâa Abd El Kader | hemimorphite, cerussite, barite, galena, and (Zn)clay minerals |
| ECH 19 | site 1—Srâa Abd El Kader | hemimorphite, Fe-(oxy)-hydroxides, and (Zn)clay minerals |
| ECH 20 | site 1—Srâa Abd El Kader | hemimorphite, Fe-(oxy)-hydroxides, quartz, Mn(Zn)-(oxy)-hydroxides, barite, cerussite, and (Zn)clays |
| ECH 21 | site 3—Srâa Abd El Kader | calcite |
| ECH 22 | site 3—Srâa Abd El Kader | calcite, quartz, and barite |
| ECH 23 | site 3—Srâa Abd El Kader | calcite and sphalerite |
| ECH 24 | site 2—Grand Pic | hydrozincite and barite |
| ECH 25 | site 2—Grand Pic | smithsonite, hydrozincite, cerussite, calcite and quartz |
| ECH 30 | site 3—Srâa Abd El Kader | calcite |
| ECH 33 | site 2—Grand Pic | hydrozincite |
| Hm | Hm | Hm | Hm | Hm | Hm | Hm | Hm | Ilt | Ilt | Ilt | Ilt | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECH 1 | ECH 1 | ECH 1 | ECH 5 | ECH 11 | ECH 12 | ECH 12 | ECH 18 | ECH 18 | ECH 18 | ECH 20 | ECH 20 | |
| Si2O | 24.50 | 25.73 | 24.73 | 25.40 | 25.67 | 26.02 | 26.53 | 25.45 | 49.04 | 47.57 | 49.13 | 44.37 |
| Al2O3 | 0.22 | 0.09 | 0.21 | 0.09 | 0.21 | 0.33 | 0.34 | 0.54 | 23.04 | 23.92 | 22.82 | 24.66 |
| K2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 8.14 | 8.83 | 8.04 | 8.61 |
| ZnO | 67.42 | 65.10 | 66.69 | 66.00 | 66.11 | 65.56 | 65.43 | 65.53 | 1.22 | 1.33 | 0.96 | 1.39 |
| CaO | 0.07 | 0.00 | 0.13 | 0.16 | 0.02 | 0.00 | 0.00 | 0.10 | 0.20 | 0.24 | 0.12 | 0.16 |
| FeO | 0.10 | 0.52 | 0.39 | 0.23 | 0.38 | 0.27 | 0.26 | 0.75 | 1.26 | 1.46 | 2.09 | 1.88 |
| PbO | 0.20 | 0.20 | 0.00 | 0.32 | 0.18 | 0.00 | 0.28 | 0.00 | 0.00 | 0.10 | 0.00 | 0.17 |
| MgO | 0.00 | 0.00 | 0.08 | 0.25 | 0.13 | 0.00 | 0.00 | 0.00 | 2.71 | 2.11 | 2.84 | 2.08 |
| MnO | 0.00 | 0.00 | 0.06 | 0.00 | 0.10 | 0.67 | 0.04 | 0.02 | 0.23 | 0.38 | 0.45 | 0.2 |
| CdO | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| As2O5 | 0.00 | 0.00 | 0.00 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 92.60 | 91.64 | 92.29 | 92.75 | 92.80 | 93.06 | 92.88 | 92.39 | 85.84 | 85.94 | 86.45 | 83.52 |
| Sm | Sm | Sm | Sm | Sm | Sm | Sm | Hdz | Hdz | Cer | Cer | Cer | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECH 1 | ECH 1 | ECH 5 | ECH 5 | ECH 5 | ECH 12 | ECH 12 | ECH 5 | ECH 5 | ECH 5 | ECH 18 | ECH 18 | |
| Si2O | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.68 | 0.00 | 0.00 | 0.00 | 0.57 |
| Al2O3 | 0.00 | 0.00 | 0.00 | 0.03 | 0.21 | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 |
| ZnO | 64.59 | 63.14 | 64.12 | 63.17 | 62.34 | 64.07 | 64.40 | 73.10 | 73.44 | 0.11 | 0.42 | 0.00 |
| CaO | 0.14 | 0.11 | 0.22 | 0.23 | 0.57 | 0.12 | 0.00 | 0.19 | 0.65 | 0.38 | 0.24 | 0.32 |
| FeO | 0.10 | 0.56 | 0.09 | 0.83 | 0.92 | 0.24 | 0.29 | 0.00 | 0.32 | 0.25 | 0.00 | 0.00 |
| PbO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.00 | 1.71 | 0.98 | 80.77 | 81.57 | 82.38 |
| MgO | 0.14 | 0.16 | 0.31 | 0.23 | 0.32 | 0.14 | 0.00 | 0.00 | 0.18 | 0.64 | 0.16 | 0.00 |
| MnO | 0.00 | 0.09 | 0.26 | 0.27 | 0.21 | 0.09 | 0.00 | 0.24 | 0.13 | 0.18 | 0.79 | 0.00 |
| CdO | 0.30 | 0.24 | 0.00 | 0.00 | 0.22 | 0.00 | 0.00 | 0.04 | 0.12 | 0.00 | 0.00 | 0.00 |
| As2O5 | 0.00 | 0.00 | 0.23 | 0.30 | 0.00 | 0.00 | 0.00 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 |
| CuO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.75 | 0.84 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CO2* | 35.36 | 34.89 | 35.40 | 35.27 | 35.28 | 35.10 | 35.01 | 16.19 | 16.42 | 17.25 | 17.16 | 16.49 |
| Total | 100.63 | 99.19 | 100.63 | 100.37 | 100.07 | 100.69 | 100.54 | 92.57 | 92.24 | 99.58 | 100.34 | 99.76 |
| Gth | Gth | Gth | Gth | Gth | Gth | Mmt | |
|---|---|---|---|---|---|---|---|
| ECH 1 | ECH 1 | ECH 6 | ECH 11 | ECH 12 | ECH 20 | ECH 17 | |
| Si2O | 4.51 | 4.67 | 2.30 | 3.44 | 2.00 | 5.56 | 0.00 |
| Al2O3 | 0.15 | 1.04 | 0.40 | 0.23 | 0.16 | 0.33 | 0.00 |
| ZnO | 1.16 | 1.47 | 1.34 | 2.19 | 1.95 | 2.16 | 72.73 |
| CaO | 0.46 | 0.03 | 0.27 | 0.11 | 0.08 | 0.17 | 0.34 |
| FeO | 87.45 | 86.40 | 88.40 | 83.49 | 83.76 | 84.25 | 0.18 |
| PbO | 1.88 | 1.29 | 0.98 | 1.00 | 1.23 | 3.64 | 0.45 |
| MgO | 0.20 | 0.19 | 0.00 | 0.32 | 0.15 | 0.11 | 0.12 |
| As2O5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 22.68 |
| Cl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.12 |
| Total | 95.81 | 95.09 | 93.69 | 90.78 | 89.33 | 96.22 | 98.62 |
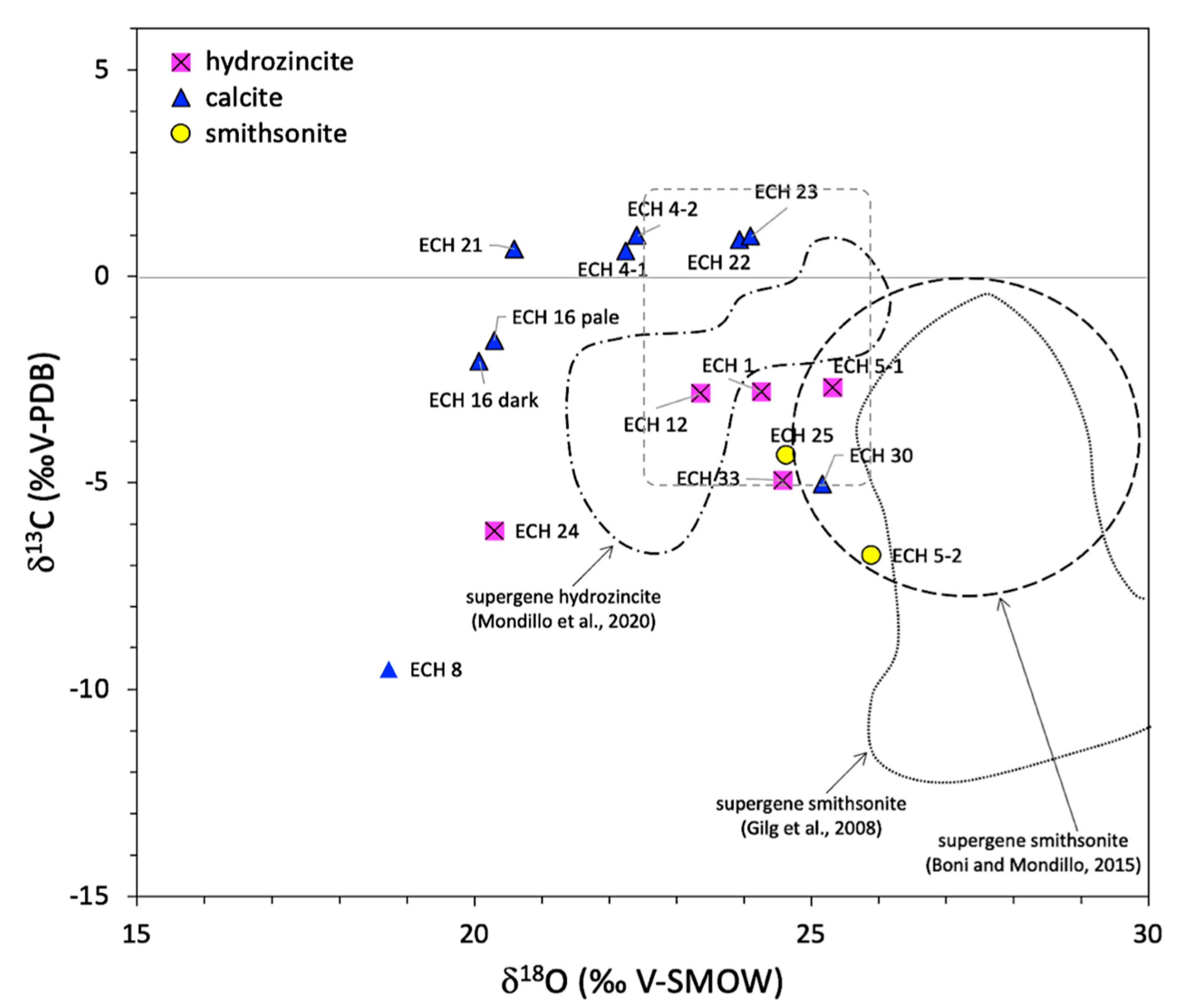
| Sample # | Description | δ13C (‰ V-PDB) | δ18O (‰ V-SMOW) |
|---|---|---|---|
| ECH 1 | hydrozincite | −2.80 | 24.26 |
| ECH 4–1 | hydrothermal calcite | 0.61 | 22.25 |
| ECH 4–2 | hydrothermal calcite | 0.98 | 22.42 |
| ECH 5–1 | hydrozincite | −2.70 | 25.32 |
| ECH 5–2 | smithsonite | −6.76 | 25.89 |
| ECH 8 | calcite | −9.52 | 18.74 |
| ECH 12 | hydrozincite | −2.84 | 23.36 |
| ECH 16 dark | calcite | −2.07 | 20.08 |
| ECH 16 pale | calcite | −1.56 | 20.30 |
| ECH 21 | hydrothermal calcite | 0.66 | 20.60 |
| ECH 22 | hydrothermal calcite | 0.88 | 23.94 |
| ECH 23 | hydrothermal calcite | 0.96 | 24.10 |
| ECH 24 | hydrozincite | −6.19 | 20.30 |
| ECH 25 | smithsonite with hydrozincite | −4.34 | 24.64 |
| CH 30 | calcite | −5.04 | 25.17 |
| ECH 33 | hydrozincite | −4.95 | 24.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louha, H.; Balassone, G.; Boutaleb, A.; Boni, M.; Joachimski, M.M.; Mondillo, N. The Pb-Zn (Ba) Nonsulfide Mineralizations at Bou Caïd (Ouarsenis, Algeria): Mineralogy, Isotope Geochemistry, and Genetic Inferences. Minerals 2021, 11, 687. https://doi.org/10.3390/min11070687
Louha H, Balassone G, Boutaleb A, Boni M, Joachimski MM, Mondillo N. The Pb-Zn (Ba) Nonsulfide Mineralizations at Bou Caïd (Ouarsenis, Algeria): Mineralogy, Isotope Geochemistry, and Genetic Inferences. Minerals. 2021; 11(7):687. https://doi.org/10.3390/min11070687
Chicago/Turabian StyleLouha, Hassina, Giuseppina Balassone, Abdelhak Boutaleb, Maria Boni, Michael M. Joachimski, and Nicola Mondillo. 2021. "The Pb-Zn (Ba) Nonsulfide Mineralizations at Bou Caïd (Ouarsenis, Algeria): Mineralogy, Isotope Geochemistry, and Genetic Inferences" Minerals 11, no. 7: 687. https://doi.org/10.3390/min11070687
APA StyleLouha, H., Balassone, G., Boutaleb, A., Boni, M., Joachimski, M. M., & Mondillo, N. (2021). The Pb-Zn (Ba) Nonsulfide Mineralizations at Bou Caïd (Ouarsenis, Algeria): Mineralogy, Isotope Geochemistry, and Genetic Inferences. Minerals, 11(7), 687. https://doi.org/10.3390/min11070687






