Mineralogical Appraisal of Bauxite Overburdens from Brazil
Abstract
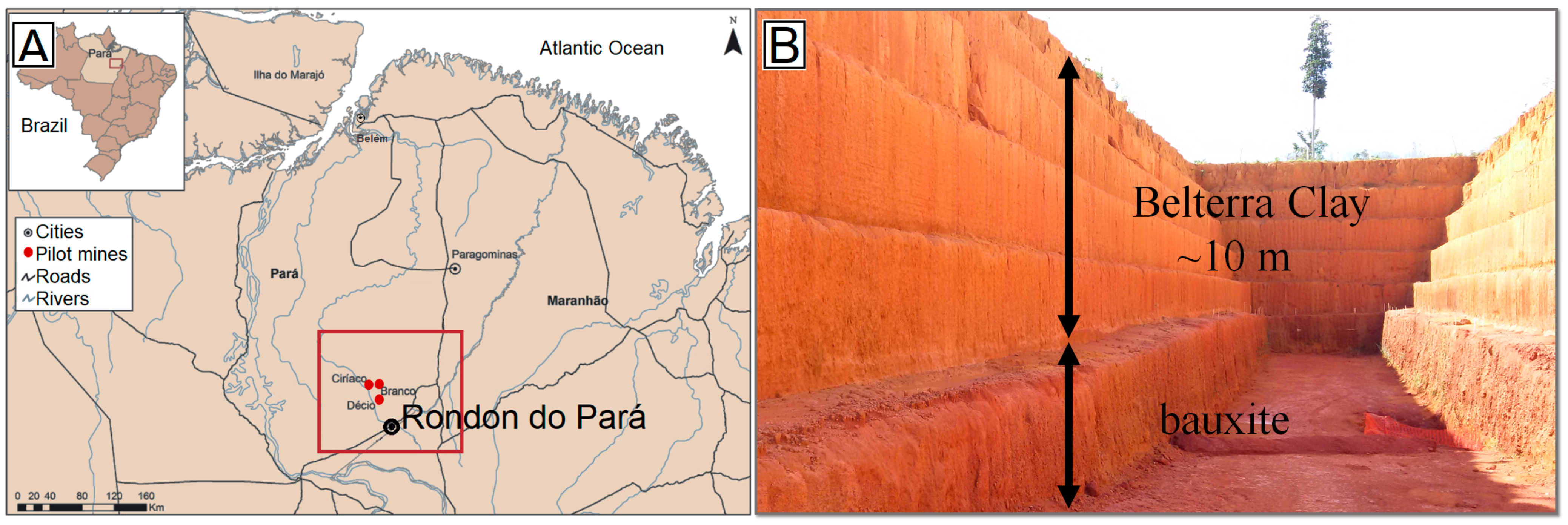
:1. Introduction
2. Materials and Methods
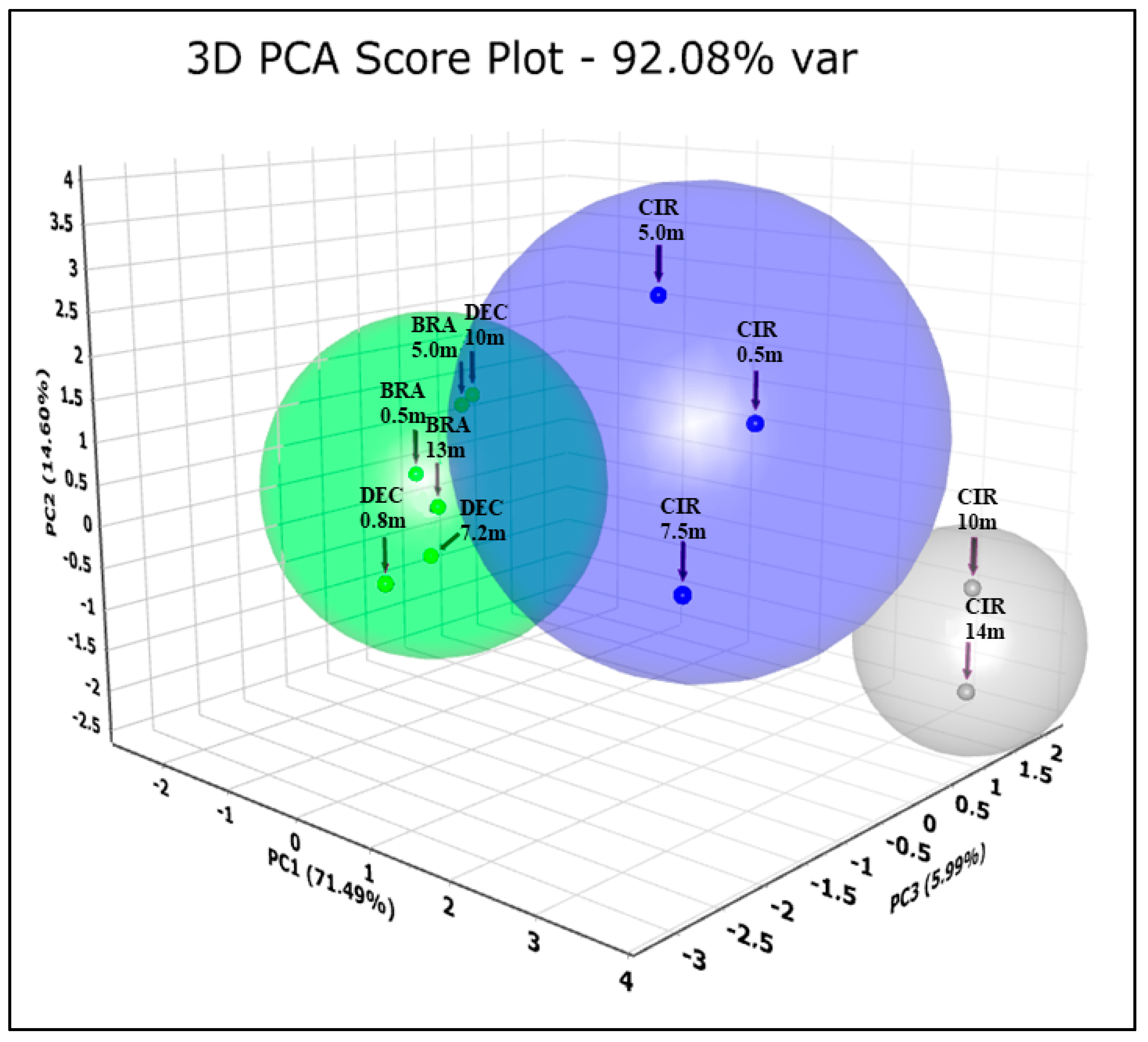
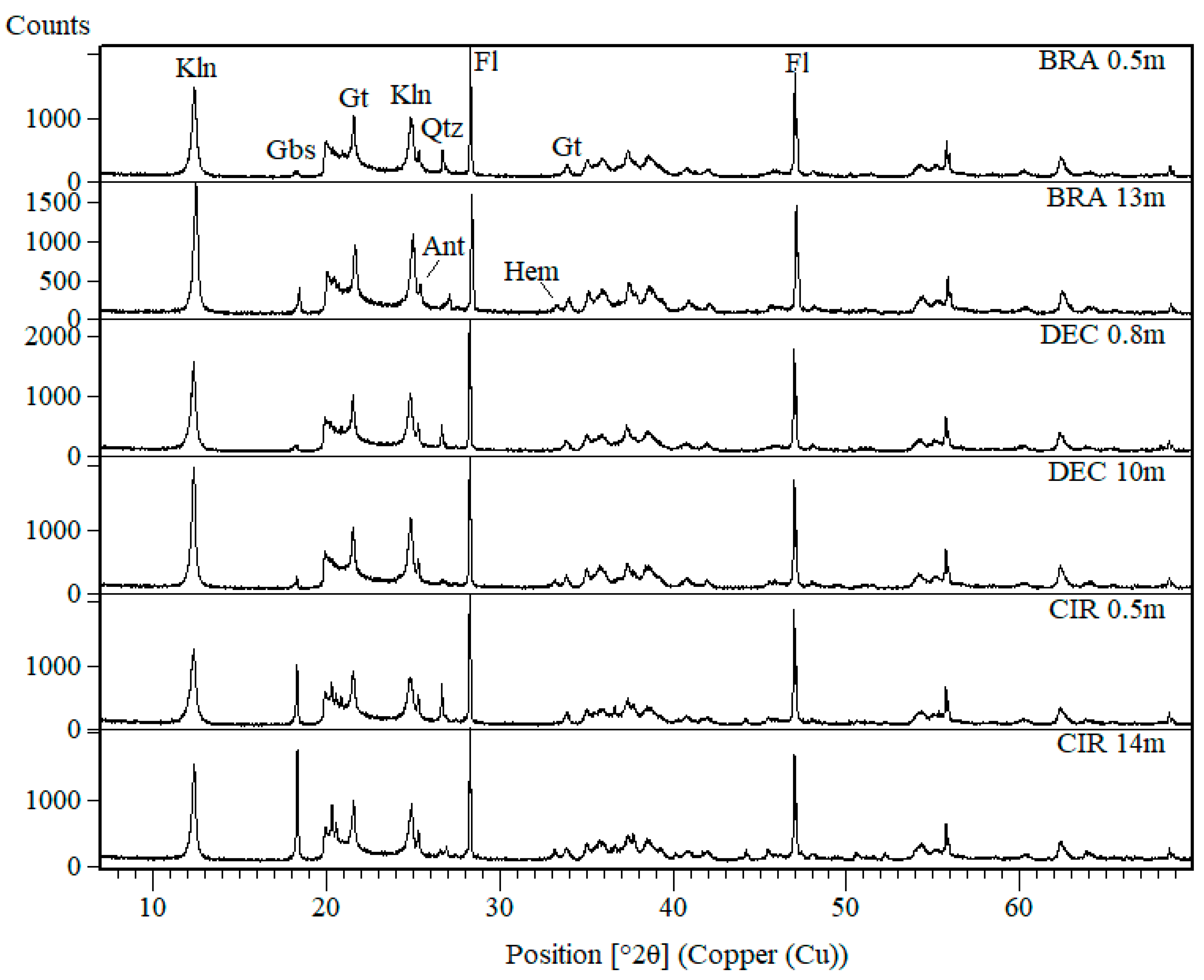
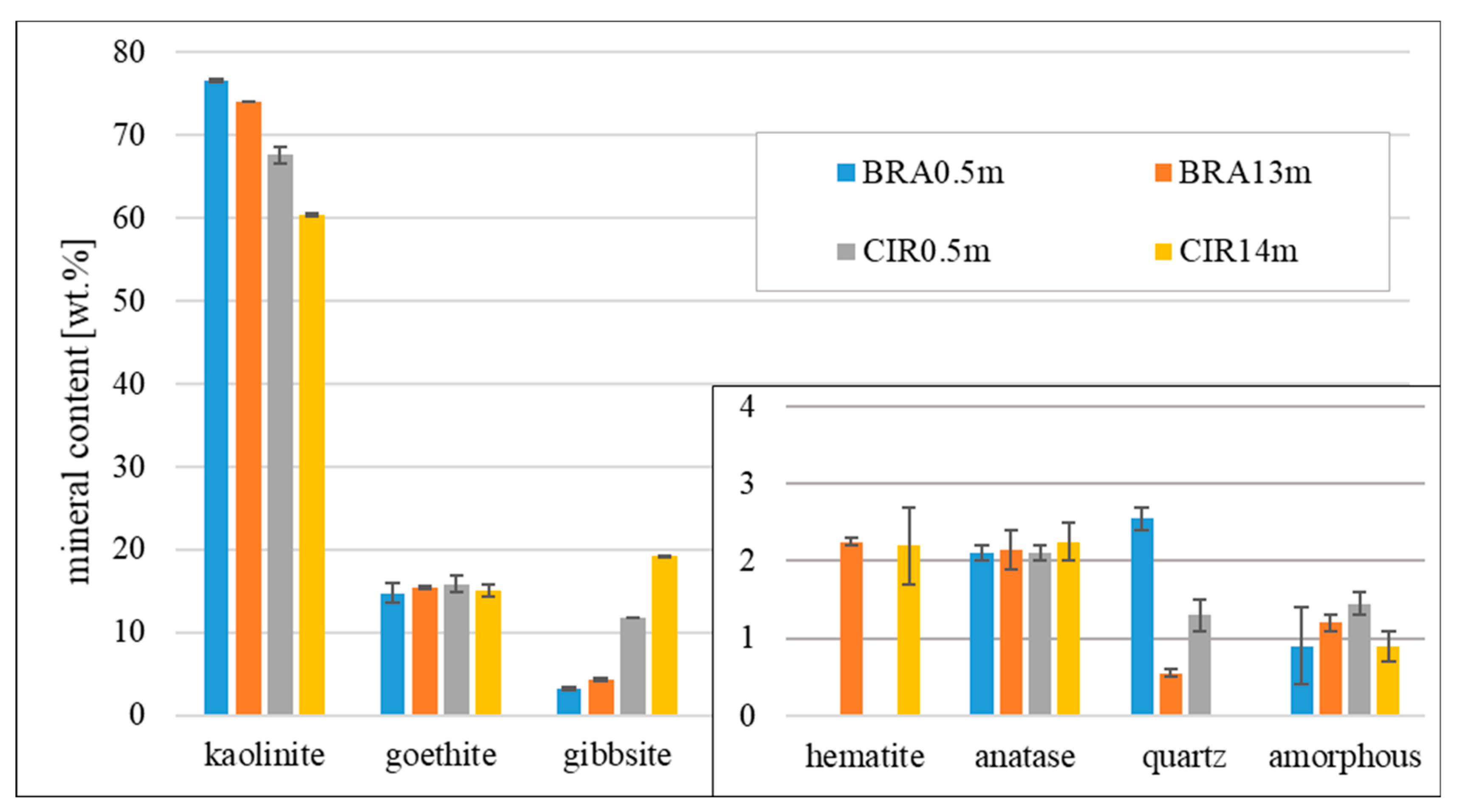
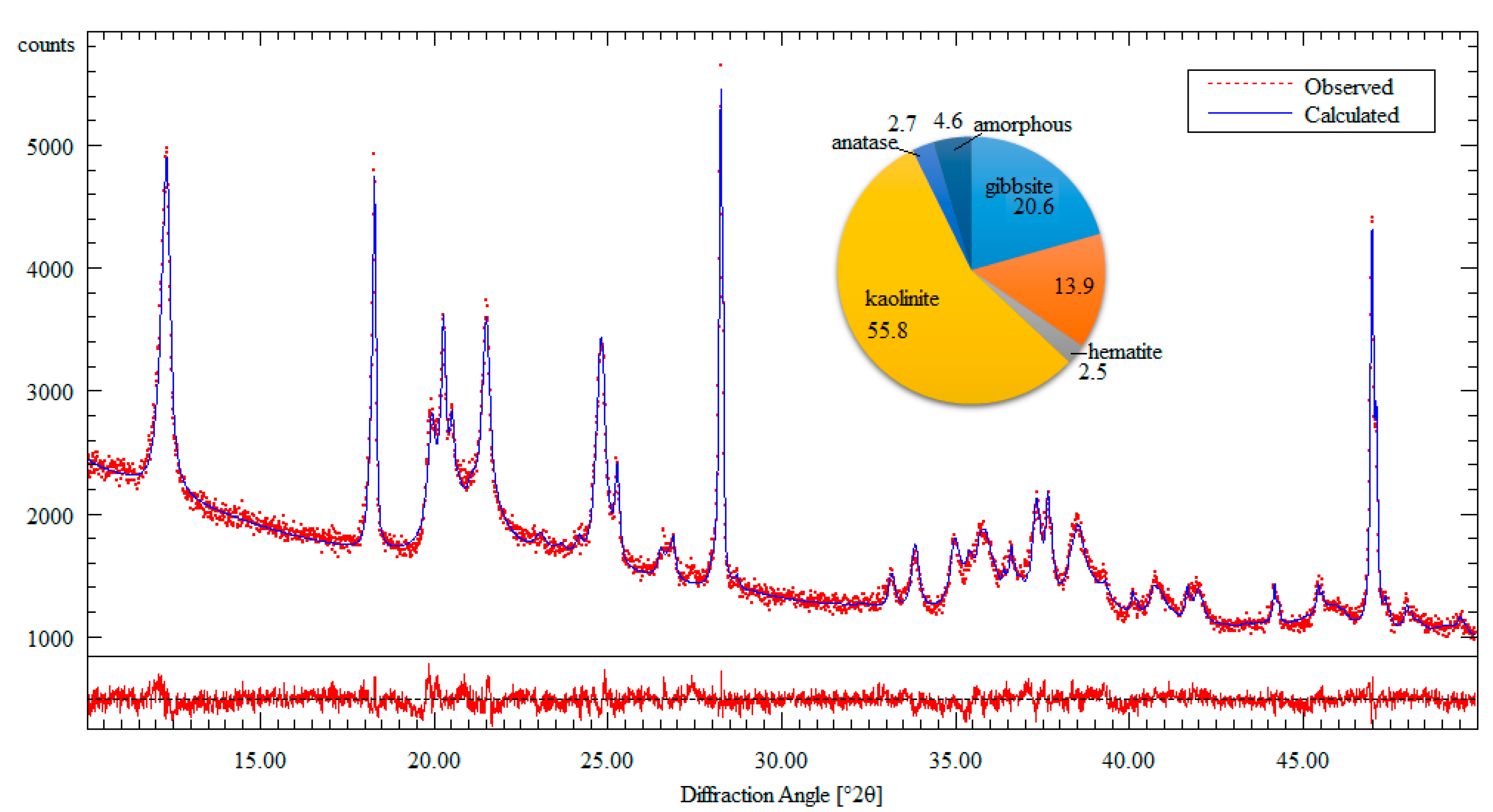
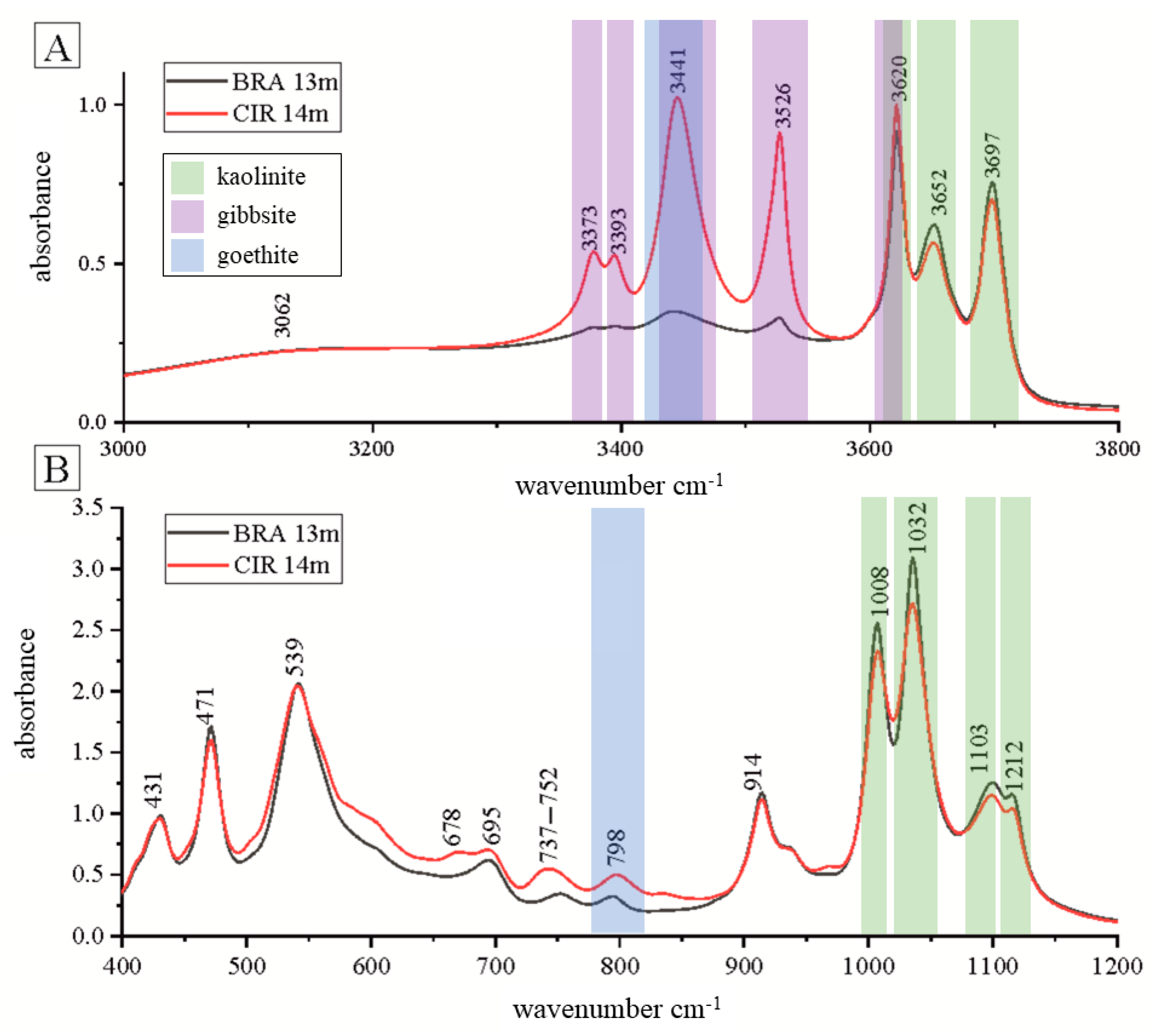
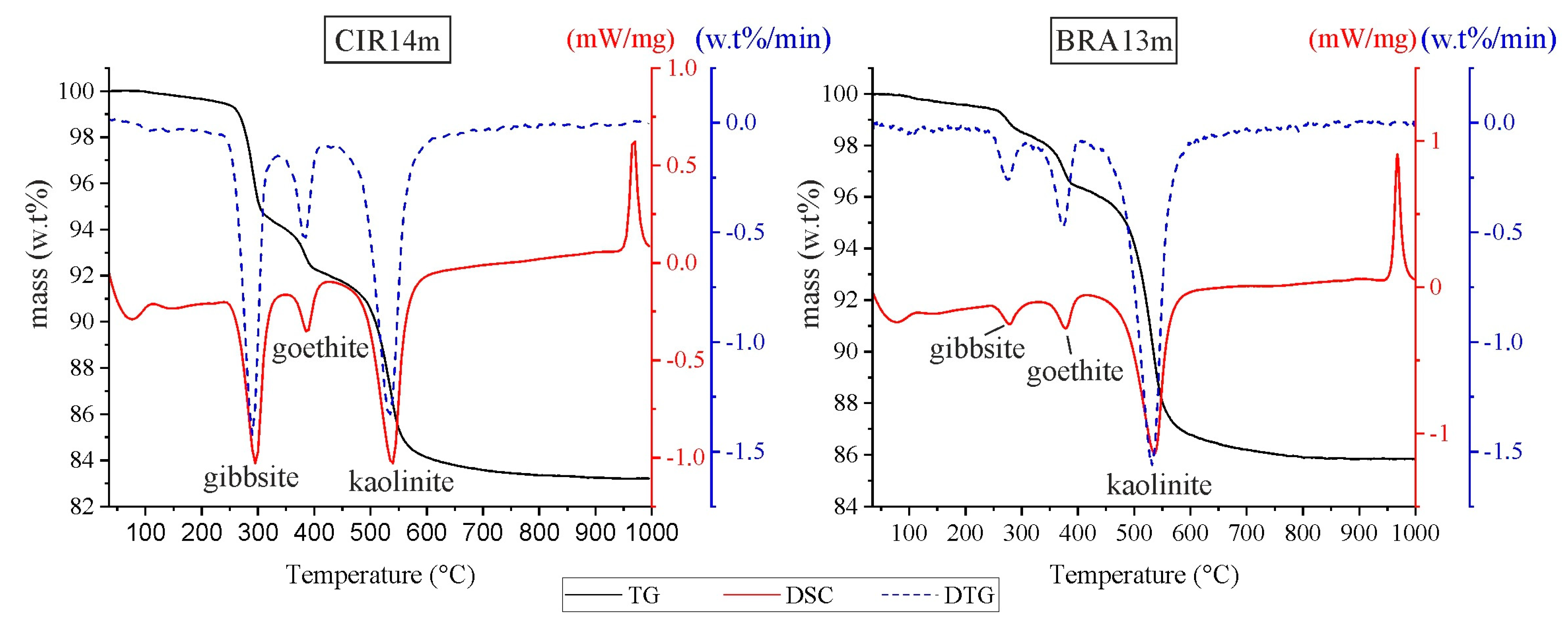
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Streli, C.; Wobrauschek, P.; Kregsamer, P. X-ray Fluorescence Spectroscopy, Applications. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: Amsterdam, The Netherlands, 1999; pp. 2478–2487. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Aranda, M.A.G.; De la Torre, A.G.; León-Reina, L. Powder-diffraction characterization of cements. In International Tables for Crystallography; International Union of Crystallography: Chester, UK, 2019; ISBN 9780470685754. [Google Scholar] [CrossRef]
- Galluccio, S.; Pöllmann, H. Quantifications of Cements Composed of OPC, Calcined Clay, Pozzolanes and Limestone. In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Khelifi, S.; Ayari, F.; Tiss, H.; Hassan Chehimi, D. Ben X-ray fluorescence analysis of Portland cement and clinker for major and trace elements: Accuracy and precision. J. Aust. Ceram. Soc. 2017, 53, 743–749. [Google Scholar] [CrossRef]
- König, U.; Norberg, N.; Gobbo, L. From iron ore to iron sinte-Process Control using X-ray Diffraction. In Proceedings of the 45° Ironmaking/16° Iron Ore/3° Agglomeration, Rio de Janeiro, Brazil, 17–21 August 2015; pp. 146–153. [Google Scholar]
- De Villiers, J.P.R.; Lu, L. XRD analysis and evaluation of iron ores and sinters. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 85–100. [Google Scholar]
- Santoro, L.; Putzolu, F.; Mondillo, N.; Herrington, R.; Najorka, J.; Boni, M.; Dosbaba, M.; Maczurad, M.; Balassone, G. Quantitative mineralogical evaluation of Ni-Co laterite ores through XRPD-QPA- and automated SEM-based approaches: The Wingellina (Western Australia) case study. J. Geochem. Explor. 2021, 223, 106695. [Google Scholar] [CrossRef]
- Ribeiro, P.P.M.; de Souza, L.C.M.; Neumann, R.; dos Santos, I.D.; Dutra, A.J.B. Nickel and cobalt losses from laterite ore after the sulfation-roasting-leaching processing. J. Mater. Res. Technol. 2020, 9, 12404–12415. [Google Scholar] [CrossRef]
- Ospina-Correa, J.D.; Mejía-Restrepo, E.; Serna-Zuluaga, C.M.; Posada-Montoya, A.; Osorio-Cachaya, J.G.; Tamayo-Sepúlveda, J.A.; Calderón-Gutiérrez, J.A. Process mineralogy of refractory gold ore in thiosulfate solutions. Hydrometallurgy 2018, 182, 104–113. [Google Scholar] [CrossRef]
- Rahfeld, A.; Kleeberg, R.; Möckel, R.; Gutzmer, J. Quantitative mineralogical analysis of European Kupferschiefer ore. Miner. Eng. 2018, 115, 21–32. [Google Scholar] [CrossRef]
- Li, G.; Cheng, H.; Xu, C.; Lu, C.; Lu, X.; Zou, X.; Xu, Q. Mineralogical Analysis of Nickel/Copper Polymetallic Sulfide Ore by X-ray Diffraction Using Rietveld Method. In Characterization of Minerals, Metals, and Materials 2016; Ikhmayies, S.J., Li, B., Carpenter, J.S., Hwang, J.-Y., Monteiro, S.N., Li, J., Firrao, D., Zhang, M., Peng, Z., Escobedo-Diaz, J.P., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 67–74. [Google Scholar]
- Paz, S.P.A.; Angélica, R.S.; Kahn, H. Optimization of the reactive silica quantification method applied to Paragominas-type gibbsitic bauxites. Int. J. Miner. Process. 2017, 162, 48–57. [Google Scholar] [CrossRef]
- Melo, C.C.A.; Angélica, R.S.; Paz, S.P.A. A proposal for rapid grade control of gibbsitic bauxites using multivariate statistics on XRD data. Miner. Eng. 2020, 157, 106539. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; da Costa, M.L.; Pöllmann, H.; Horn, A. An application of the Rietveld refinement method to the mineralogy of a bauxite-bearing regolith in the Lower Amazon. Mineral. Mag. 2018, 82, 413–431. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; da Costa, M.L. Mineralogy and geochemistry of a bauxite-bearing lateritic profile supporting the identification of its parent rocks in the domain of the huge Carajás iron deposits, Brazil. J. South. Am. Earth Sci. 2021, 108, 103164. [Google Scholar] [CrossRef]
- De Ruan, C.; Ward, C.R. Quantitative X-ray powder diffraction analysis of clay minerals in Australian coals using Rietveld methods. Appl. Clay Sci. 2002, 21, 227–240. [Google Scholar] [CrossRef]
- Santini, T.C. Application of the Rietveld refinement method for quantification of mineral concentrations in bauxite residues (alumina refining tailings). Int. J. Miner. Process. 2015, 139, 1–10. [Google Scholar] [CrossRef]
- U.S Geological Survey. Mineral Commodity Summaries; U.S Geological Survey: Reston, VA, USA, 2021. [Google Scholar]
- Horbe, A.M.C.; Anand, R.R. Bauxite on igneous rocks from Amazonia and Southwestern of Australia: Implication for weathering process. J. Geochem. Explor. 2011, 111, 1–12. [Google Scholar] [CrossRef]
- Nyamsari, D.G.; Yalcin, M.G. Statistical analysis and source rock of the Minim-Martap plateau bauxite, Cameroon. Arab. J. Geosci. 2017, 10, 415. [Google Scholar] [CrossRef]
- Sidibe, M.; Yalcin, M.G. Petrography, mineralogy, geochemistry and genesis of the Balaya bauxite deposits in Kindia region, Maritime Guinea, West Africa. J. Afr. Earth Sci. 2019, 149, 348–366. [Google Scholar] [CrossRef]
- Horbe, A.M.C.; da Costa, M.L. Lateritic crusts and related soils in eastern Brazilian Amazonia. Geoderma 2005, 126, 225–239. [Google Scholar] [CrossRef]
- Truckenbrodt, W.; Kotschoubey, B.; Schellmann, W. Composition and origin of the clay cover on North Brazilian laterites. Geol. Rundsch. 1991, 80, 591–610. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; da Costa, M.L.; Pöllmann, H. The Belterra Clay on the bauxite deposits of Rondon do Pará, Eastern Amazon. Braz. J. Geol. 2018, 48, 473–484. [Google Scholar] [CrossRef]
- Sombroek, W.G. Amazon Soils: A Reconnaissance of the Soils Region, the Brazilian Amazonitle; Wageningen University: Wageningen, The Netherlands, 1966. [Google Scholar]
- Barreto, I.A.R.; da Costa, M.L. Sintering of red ceramics from yellow Amazonian latosols incorporated with illitic and gibbsitic clay. Appl. Clay Sci. 2018, 152, 124–130. [Google Scholar] [CrossRef]
- Barreto, I.A.R.; da Costa, M.L. Viability of Belterra clay, a widespread bauxite cover in the Amazon, as a low-cost raw material for the production of red ceramics. Appl. Clay Sci. 2018, 162, 252–260. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; Pöllmann, H.; da Costa, M.L. Production of low-CO2 cements using abundant bauxite overburden “Belterra Clay”. Sustain. Mater. Technol. 2021, e00299. [Google Scholar] [CrossRef]
- Negrão, L.B.A.; Pöllmann, H. The Phase Addition method to evaluate Rietveld mineral quantitative analysis of hydrated cements. Bol. DO Mus. Geociênc. Amaz. 2020, 7, 7. [Google Scholar] [CrossRef]
- Weirich, T.E.; Winterer, M.; Seifried, S.; Hahn, H.; Fuess, H. Rietveld analysis of electron powder diffraction data from nanocrystalline anatase, TiO2. Ultramicroscopy 2000, 81, 263–270. [Google Scholar] [CrossRef]
- Batchelder, D.N.; Simmons, R.O. Lattice constants and thermal expansivities of silicon and of calcium fluoride between 6 and 322 K. J. Chem. Phys. 1964, 41, 2324–2329. [Google Scholar] [CrossRef]
- Saalfeld, H.; Wedde, M. Refinement of the crystal structure of gibbsite, A1(OH)3. Z. Krist. Cryst. Mater. 1974, 139, 129–135. [Google Scholar] [CrossRef]
- Li, D.; O’Connor, B.H.; Low, I.-M.; van Riessen, A.; Toby, B.H. Mineralogy of Al-substituted goethites. Powder Diffr. 2006, 21, 289–299. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Isupova, L.A.; Tsybulya, S.V.; Cherepanova, S.V.; Litvak, G.S.; Burgina, E.B.; Kustova, G.N.; Kolomiichuk, V.N.; Ivanov, V.P.; Paukshtis, E.A.; et al. Effect of mechanical activation on the real structure and reactivity of iron (III) oxide with corundum-type structure. J. Solid State Chem. 1996, 123, 191–202. [Google Scholar] [CrossRef]
- Bish, D.L.; Von Dreele, R.B. Rietveld refinement of non-hydrogen atomic positions in kaolinite. Clays Clay Miner. 1989, 37, 289–296. [Google Scholar] [CrossRef]
- D’Amour, H.; Denner, W.; Schulz, H. Structure determination of α-quartz up to 68 × 10 8 Pa. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, J.; Kleeberg, R. Rietveld Analysis of Disordered Layer Silicates. Mater. Sci. Forum 1998, 278–281, 300–305. [Google Scholar] [CrossRef]
- Ufer, K.; Kleeberg, R.; Monecke, T. Quantification of stacking disordered Si-Al layer silicates by the Rietveld method: Application to exploration for high-sulphidation epithermal gold deposits. Powder Diffr. 2015, 30, S111–S118. [Google Scholar] [CrossRef]
- Leonardi, A.; Bish, D.L. Understanding Powder X-ray Diffraction Profiles from Layered Minerals: The Case of Kaolinite Nanocrystals. Inorg. Chem. 2020, 59, 5357–5367. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. IR Spectra of Minerals and Related Compounds, and Reference Samples’ Data. In Principles and Applications of Well Logging; Springer: Berlin/Heidelberg, Germany, 2016; pp. 51–1047. [Google Scholar]
- Balan, E.; Saitta, A.M.; Mauri, F.; Calas, G. First-principles modeling of the infrared spectrum of kaolinite. Am. Mineral. 2001, 86, 1321–1330. [Google Scholar] [CrossRef]
- Brindley, G.W.; Kao, C.C.; Harrison, J.L.; Lipsicas, M.; Raythatha, R. Relation between structural disorder and other characteristics of kaolinites and dickites. Clays Clay Miner. 1986, 34, 239–249. [Google Scholar] [CrossRef]
- Balan, E.; Lazzeri, M.; Morin, G.; Mauri, F. First-principles study of the OH-stretching modes of gibbsite. Am. Mineral. 2006, 91, 115–119. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Infrared spectroscopy of goethite dehydroxylation. II. Effect of aluminium substitution on the behaviour of hydroxyl units. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 479–491. [Google Scholar] [CrossRef]
- Schulze, D.G.; Schwertmann, U. The influence of aluminium on iron oxides: X. properties of Al-substituted goethites. Clay Miner. 1984, 19, 521–539. [Google Scholar] [CrossRef]
- Colombo, C.; Violante, A. Effect of time and temperature on the chemical composition and crystallization of mixed iron and aluminum species. Clays Clay Miner. 1996, 44, 113–120. [Google Scholar] [CrossRef]
- Yeskis, D.; Van Groos, A.F.K.; Guggenheim, S. The dehydroxylation of kaolinite. Am. Mineral. 1985, 70, 159–164. [Google Scholar]
- Chen, Y.F.; Wang, M.C.; Hon, M.H. Phase transformation and growth of mullite in kaolin ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- Aparicio, P.; Galán, E.; Ferrell, R.E. A new kaolinite order index based on XRD profile fitting. Clay Miner. 2006, 41, 811–817. [Google Scholar] [CrossRef]
- Plançon, A.; Giese, R.F.; Snyder, R. The Hinckley index for kaolinites. Clay Miner. 1988, 23, 249–260. [Google Scholar] [CrossRef]
- Bish, D.L.; Howard, S.A. Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Djangang, C.N.; Kamseu, E.; Ndikontar, M.K.; Nana, G.L.L.; Soro, J.; Melo, U.C.; Elimbi, A.; Blanchart, P.; Njopwouo, D. Sintering behaviour of porous ceramic kaolin-corundum composites: Phase evolution and densification. Mater. Sci. Eng. A 2011, 528, 8311–8318. [Google Scholar] [CrossRef]
- Da Costa, M.L.; da Cruz, G.S.; de Almeida, H.D.F.; Poellmann, H. On the geology, mineralogy and geochemistry of the bauxite-bearing regolith in the lower Amazon basin: Evidence of genetic relationships. J. Geochemical Explor. 2014, 146, 58–74. [Google Scholar] [CrossRef]

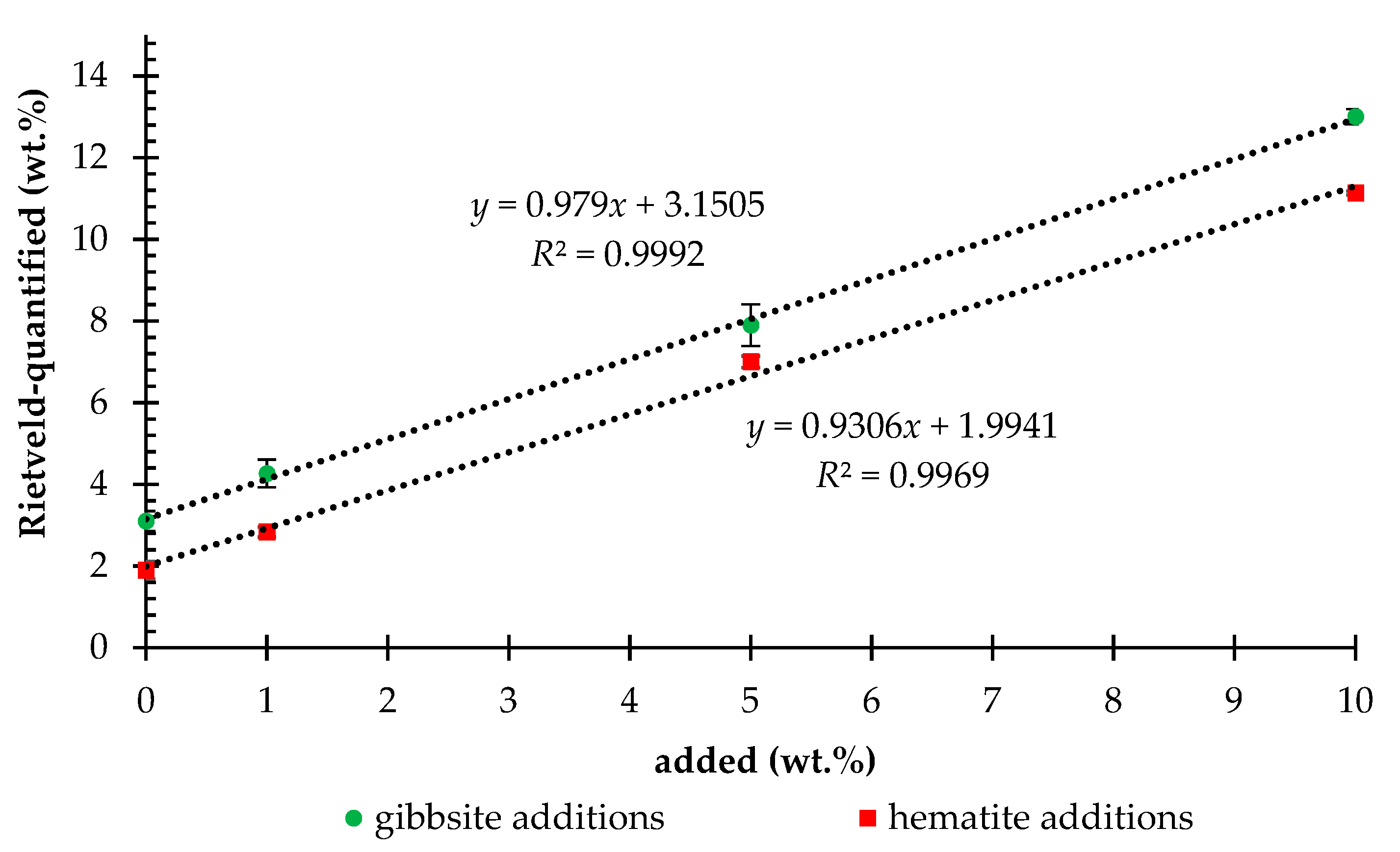

| Phase | Chemical Formula | Space Group | ICSD Code | Reference | |
|---|---|---|---|---|---|
| Anatase | TiO2 | I41/amd | 92363 | [31] | |
| Fluorite | CaF2 | Fm-3m | 60368 | [32] | |
| Gibbsite | Al(OH)3 | P21 | 6162 | [33] | |
| Goethite | (Fe,Al)O(OH) | Pnma | 109411 | [34] | |
| Hematite | Fe2O3 | R-3c | 82137 | [35] | |
| Kaolinite | Al2Si2O5(OH)4 | P1 | 63192 | [36] | |
| Quartz | SiO2 | P3121 | 16331 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrão, L.B.A.; Pöllmann, H.; Cortinhas Alves, T.K. Mineralogical Appraisal of Bauxite Overburdens from Brazil. Minerals 2021, 11, 677. https://doi.org/10.3390/min11070677
Negrão LBA, Pöllmann H, Cortinhas Alves TK. Mineralogical Appraisal of Bauxite Overburdens from Brazil. Minerals. 2021; 11(7):677. https://doi.org/10.3390/min11070677
Chicago/Turabian StyleNegrão, Leonardo Boiadeiro Ayres, Herbert Pöllmann, and Tiago Kalil Cortinhas Alves. 2021. "Mineralogical Appraisal of Bauxite Overburdens from Brazil" Minerals 11, no. 7: 677. https://doi.org/10.3390/min11070677
APA StyleNegrão, L. B. A., Pöllmann, H., & Cortinhas Alves, T. K. (2021). Mineralogical Appraisal of Bauxite Overburdens from Brazil. Minerals, 11(7), 677. https://doi.org/10.3390/min11070677







